Oxidant and Antioxidant Profiling in Viscaria alpina Seed Populations Following the Temporal Dynamics of an Alpine Climate
Abstract
1. Introduction
2. Materials and Methods
2.1. Seed Material and Climate Data
2.2. Measurement of Reactive Oxygen Species
2.3. Determination of Malondialdehyde
2.4. Determination of Tocopherols
2.5. Determination of Quercetin and Quercetin-3-Rutinoside
2.6. Statistical Analyses
3. Results and Discussion
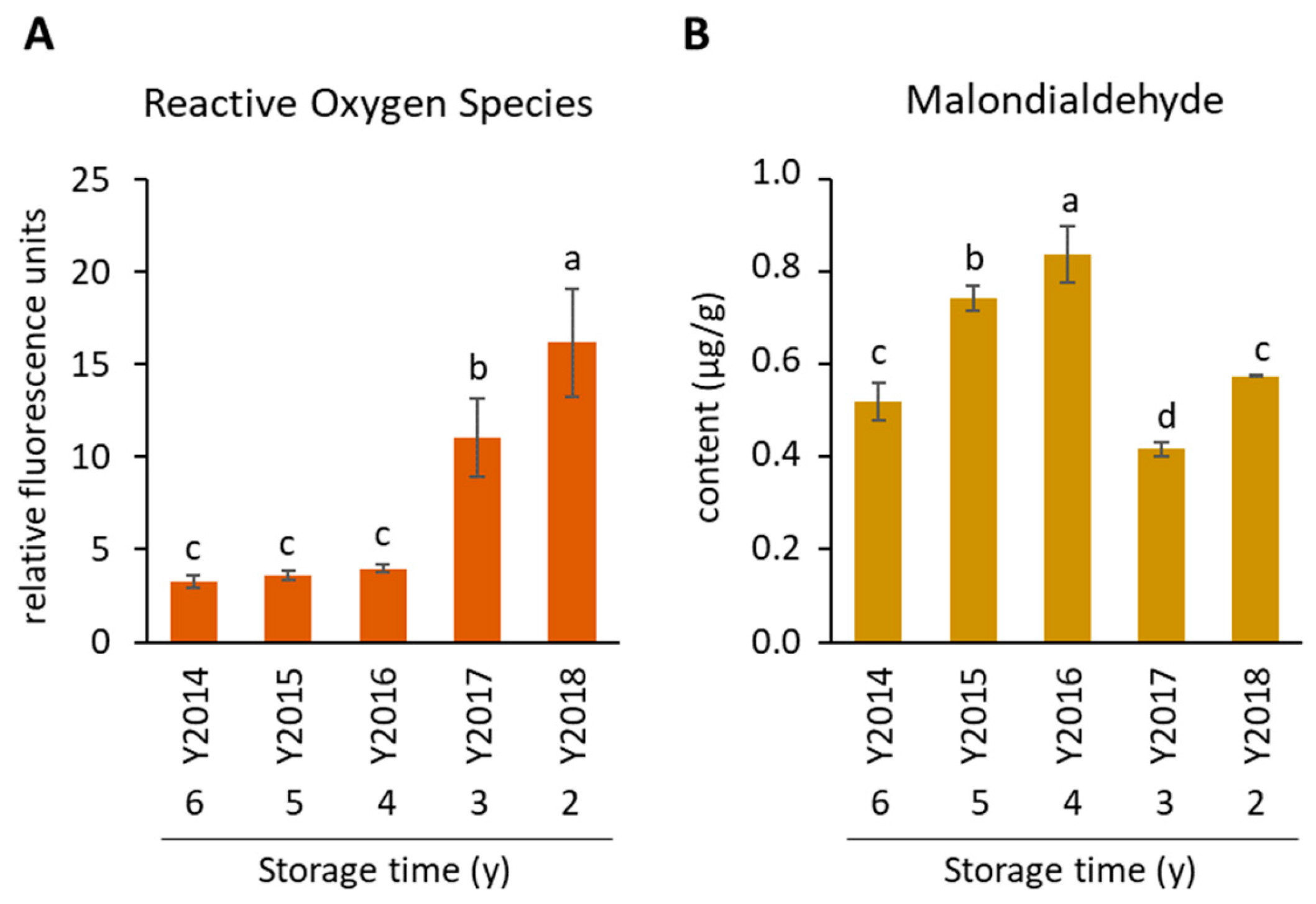
3.1. Different ROS and MDA Accumulation Patterns in V. alpina Seed Accessions
3.2. Tocopherols and Quercetin Content Have Comparable Patterns in V. alpina Seed Accessions
3.3. Correlation Analyses Point at Climate-Related Patterns in Oxidant and Antioxidant Profiles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Sixth Assessment Report. Climate Change 2023: Synthesis Report. 2023. Available online: https://www.ipcc.ch/assessment-report/ar6/ (accessed on 1 June 2023).
- Hock, R.; Rasul, G.; Adler, C.; Cáceres, B.; Gruber, S.; Hirabayashi, Y.; Jackson, M.; Kääb, A.; Kang, S.; Kutuzov, S.; et al. High Mountain Areas. In IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; Pörtner, H.O., Roberts, D.C., Masson-Delmotte, V., Zhai, P., Tignor, M., Poloczanska, E., Mintenbeck, K., Alegría, A., Nicolai, M., Okem, A., et al., Eds.; IPCC: New York, NY, USA, 2019; pp. 131–202. [Google Scholar]
- Scherrer, S.C.; Hirschi, M.; Spirig, C.; Maurer, F.; Kotlarski, S. Trends and drivers of recent summer drying in Switzerland. Environ. Res. Commun. 2022, 4, 025004. [Google Scholar] [CrossRef]
- Kotlarski, S.; Gobiet, A.; Morin, S.; Olefs, M.; Rajczak, J.; Samacoïts, R. 21st Century alpine climate change. Clim. Dyn. 2023, 60, 65–86. [Google Scholar] [CrossRef]
- Matiu, M.; Crespi, A.; Bertoldi, G.; Carmagnola, C.M.; Marty, C.; Morin, S.; Schöner, W.; Cat Berro, D.; Chiogna, G.; de Gregorio, L.; et al. Observed snow depth trends in the European Alps: 1971 to 2019. Cryosphere 2021, 15, 1343–1382. [Google Scholar] [CrossRef]
- Briceno, V.F.; Hoyle, G.L.; Nicotra, A.B. Seeds at risk: How will a changing alpine climate affect regeneration from seeds in alpine areas? Alpine Bot. 2015, 125, 59–68. [Google Scholar] [CrossRef]
- Vázquez-Ramírez, J.; Venn, S.E. Seeds and seedlings in a changing world: A systematic review and meta-analysis from high altitude and high latitude ecosystems. Plants 2021, 10, 768. [Google Scholar] [CrossRef]
- Mondoni, A.; Jimenez-Alfaro, B.; Cavieres, L.A. Effect of climate change on plant regeneration from seeds in the arctic and alpine biome. In Plant Regeneration from Seeds; A Global Warming Perspective; Baskin, C., Baskin, J., Eds.; Academic Press: London, UK, 2022. [Google Scholar] [CrossRef]
- Walck, J.L.; Hidayati, S.N.; Dixon, K.W.; Thompson, K.; Poschlod, P. Climate change and plant regeneration from seed. Glob. Change Biol. 2011, 17, 2145–2161. [Google Scholar] [CrossRef]
- Fenner, M.; Thompson, K. The Ecology of Seeds; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar] [CrossRef][Green Version]
- Waterworth, W.M.; Bray, C.M.; West, C.E. The importance of safeguarding genome integrity in germination and seed longevity. J. Exp. Bot. 2015, 66, 3549–3558. [Google Scholar] [CrossRef]
- Treep, J.; de Jager, M.; Bartumeus, F.; Soons, M.B. Seed dispersal as a search strategy: Dynamic and fragmented landscapes select for multi-scale movement strategies in plants. Mov. Ecol. 2021, 9, 4. [Google Scholar] [CrossRef]
- Mattana, E.; Ulian, T.; Pritchard, H.W. Seeds as natural capital. Trends Plant Sci. 2022, 27, 139–146. [Google Scholar] [CrossRef]
- Milivojević, M.; Ripka, Z.; Petrovi’c, T. ISTA rules changes in seed germination testing at the beginning of the 21st century. J. Process. Energy Agric. 2018, 22, 40–45. [Google Scholar] [CrossRef]
- Pagano, A.; Forti, C.; Gualtieri, C.; Balestrazzi, A.; Macovei, A. Oxidative stress and antioxidant defense in germinating seeds: A Q&A session. In Reactive Oxygen, Nitrogen and Sulfur Species in Plants: Production, Metabolism, Signaling and Defense Mechanisms; Hasanuzzaman, M., Fotopoulos, V., Nahar, K., Fujita, M., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019; pp. 267–289. [Google Scholar]
- Pagano, A.; Folini, G.; Pagano, P.; Sincinelli, F.; Rossetto, A.; Macovei, A.; Balestrazzi, A. ROS accumulation as a hallmark of dehydration stress in primed and overprimed Medicago truncatula seeds. Agronomy 2022, 12, 268. [Google Scholar] [CrossRef]
- Griffo, A.; Bosco, N.; Pagano, A.; Balestrazzi, A.; Macovei, A. Noninvasive methods to detect reactive oxygen species as a proxy of seed quality. Antioxidants 2023, 12, 626. [Google Scholar] [CrossRef] [PubMed]
- Jeevan Kumar, S.P.; Rajendra Prasad, S.; Banerjee, R.; Thammineni, C. Seed birth to death: Dual functions of reactive ox-ygen species in seed physiology. Ann. Bot. 2015, 116, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chu, P.; Zhou, Y.; Li, Y.; Liu, J.; Ding, Y.; Tsang, E.W.; Jiang, L.; Wu, K.; Huang, S. Overexpression of AtOGG1, a DNA glycosylase/AP lyase, enhances seed longevity and abiotic stress tolerance in Arabidopsis. J. Exp. Bot. 2012, 63, 4107–4121. [Google Scholar] [CrossRef]
- Rehmani, M.S.; Aziz, U.; Xian, B.; Shu, K. Seed dormancy and longevity: A mutual dependence or a trade-off? Plant Cell Physiol. 2022, 63, 1029–1037. [Google Scholar] [CrossRef]
- Donà, M.; Balestrazzi, A.; Mondoni, A.; Rossi, G.; Ventura, L.; Buttafava, A.; Macovei, A.; Sabatini, M.E.; Valassi, A.; Carbonera, D. DNA profiling, telomere analysis and antioxidant properties as tools for monitoring ex situ seed longevity. Ann. Bot. 2013, 111, 987–998. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, C.; Wang, X.; Shen, H.; Yang, L. Exogenous ethylene alleviates the inhibition of Sorbus pohuashanensis embryo germination in a saline-alkali environment (NaHCO3). Int. J. Mol. Sci. 2023, 24, 4244. [Google Scholar] [CrossRef]
- Richards, S.L.; Wilkins, K.A.; Swarbreck, S.M.; Anderson, A.A.; Habib, N.; Smith, A.G.; McAinsh, M.; Davies, J.M. The hydroxyl radical in plants: From seed to seed. J. Exp. Bot. 2015, 66, 37–46. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Yuasa, T.; Iwaya-Inoue, M. Mechanisms of maturation and germination in crop seeds exposed to environmental stresses with a focus on nutrients, water status, and reactive oxygen species. Adv. Exp. Med. Biol. 2018, 1081, 233–257. [Google Scholar]
- Farooq, M.A.; Ma, W.; Shen, S.; Gu, A. Underlying biochemical and molecular mechanisms for seed germination. Int. J. Mol. Sci. 2022, 23, 8502. [Google Scholar] [CrossRef]
- Macovei, A.; Pagano, A.; Leonetti, P.; Carbonera, D.; Balestrazzi, A.; Araújo, S.S. Systems biology and genome-wide approaches to unveil the molecular players involved in the pre-germinative metabolism: Implications on seed technology traits. Plant Cell Rep. 2017, 36, 669–688. [Google Scholar] [CrossRef] [PubMed]
- Kurek, K.; Plitta-Michalak, B.; Ratajczak, E. Reactive oxygen species as potential drivers of the seed aging process. Plants 2019, 8, 174. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C.; El-Maarouf-Bouteau, H.; Corbineau, F. From intracellular signaling networks to cell death: The dual role of ReactiveOxygen Species in seed physiology. C. R. Biol. 2008, 331, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Doria, E.; Pagano, A.; Ferreri, C.; Larocca, A.V.; Macovei, A.; Araújo, S.S.; Balestrazzi, A. How does the seed pre-germinative metabolism fight against imbibition damage? Emerging roles of fatty acid cohort and antioxidant defence. Front. Plant Sci. 2019, 10, 1505. [Google Scholar] [CrossRef]
- Wilson, D.O.; McDonald, M.B. The lipid peroxidation model of seed aging. Seed Sci. Technol. 1986, 14, 269–300. [Google Scholar]
- Murthy, U.N.; Sun, W.Q. Protein modification by Amadori and Maillard reactions during seed storage: Roles of sugar hydrolysis and lipid peroxidation. J. Exp. Bot. 2000, 51, 1221–1228. [Google Scholar] [CrossRef]
- Rajjou, L.; Debeaujon, I. Seed longevity: Survival and maintenance of high germination ability of dry seeds. C. R. Biol. 2008, 331, 796–805. [Google Scholar] [CrossRef]
- Debeaujon, I.; Leon-Kloosterziel, K.M.; Koornneef, M. Influence of the testa on seed dormancy, germination, and longevity in Arabidopsis. Plant Physiol. 2000, 122, 403–414. [Google Scholar] [CrossRef]
- Sano, N.; Rajjou, L.; North, H.M.; Debeaujon, I.; Marion-Poll, A.; Seo, M. Staying alive: Molecular aspects of seed longevity. Plant Cell Physiol. 2016, 57, 660–674. [Google Scholar] [CrossRef]
- Sattler, S.E.; Gilliland, L.U.; Magallanes-Lundback, M.; Pollard, M.; DellaPenna, D. Vitamin E is essential for seed longevity and for preventing lipid peroxidation during germination. Plant Cell 2004, 16, 1419–1432. [Google Scholar] [CrossRef]
- Nagy, L. Biological flora of the British Isles: Silene suecica. J. Ecol. 2013, 101, 532–544. [Google Scholar] [CrossRef]
- Abeli, T.; Gentili, R.; Rossi, G.; Bedini, G.; Foggi, B. Can the IUCN criteria be effectively applied to peripheral isolated plant populations? Biodivers. Conserv. 2009, 18, 3877. [Google Scholar] [CrossRef]
- White, F.J.; Hay, F.R.; Abeli, T.; Mondoni, A. Two decades of climate change alters seed longevity in an alpine herb: Implications for ex situ seed conservation. Alpine Bot. 2022, 133, 11–20. [Google Scholar] [CrossRef]
- Newton, R.; Hay, F.; Probert, R. Protocol for Comparative Seed Longevity Testing: Technical Information Sheet_01; Royal Botanic Gardens: Kew, UK, 2014. [Google Scholar]
- Mondoni, A.; Orsenigo, S.; Müller, J.V.; Carlsson-Graner, U.; Jiménez-Alfaro, B.; Abeli, T. Seed dormancy and longevity in subarctic and alpine populations of Silene suecica. Alp. Botany 2018, 128, 71–81. [Google Scholar] [CrossRef]
- Probert, R.J.; Daws, M.I.; Hay, F.R. Ecological correlates of ex situ seed longevity: A comparative study on 195 species. Ann. Bot. 2009, 104, 57–69. [Google Scholar] [CrossRef]
- Antolini, G.; Auteri, L.; Pavan, V.; Tomei, F.; Tomozeiu, R.; Marletto, V. A daily high-resolution gridded climatic data set for Emilia-Romagna, Italy, during 1961–2010. Int. J. Climatol. 2016, 36, 1970–1986. [Google Scholar] [CrossRef]
- Sari, A.; Kursat, M.; Civelek, S. Determination of MDA levels in the plant (some Salvia L. Taxa growing in Turkey). J. Drug Metabol. Toxicol. 2012, 20123, 3. [Google Scholar] [CrossRef]
- Zeb, A.; Ullah, F. A simple spectrophotometric method for the determination of thiobarbituric acid reactive substances in fried fast foods. J. Anal. Methods Chem. 2016, 2016, 9412767. [Google Scholar] [CrossRef]
- Kurilich, C.; Juvik, J. Quantification of carotenoid and tocopherol antioxidants in Zea mays. J. Agric. Food Chem. 1999, 47, 1948–1955. [Google Scholar] [CrossRef]
- Doria, E.; Galleschi, L.; Calucci, L.; Pinzino, C.; Pilu, R.; Cassani, E.; Nielsen, E. Phytic acid prevents oxidative stress in seeds: Evidence from a maize (Zea mays L.) low phytic acid mutant. J. Exp. Bot. 2009, 60, 967–978. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C. The signalling role of ROS in the regulation of seed germination and dormancy. Biochem. J. 2019, 476, 3019–3032. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Vivancos, P.; Barba-Espín, G.; Hernández, J.A. Elucidating hormonal/ROS networks during seed germination: Insights and perspectives. Plant Cell Rep. 2013, 32, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, A.; Kumar, A.; Kaur, N. ROS and oxidative burst: Roots in plant development. Plant Divers 2019, 42, 33–43. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Y.; Sun, J.; Meng, J.; Tao, J. Deterioration of orthodox seeds during ageing: Influencing factors, physiological alterations and the role of reactive oxygen species. Plant Physiol. Biochem. 2021, 158, 475–485. [Google Scholar] [CrossRef]
- Wiebach, J.; Nagel, M.; Börner, A.; Altmann, T.; Riewe, D. Age-dependent loss of seed viability is associated with increased lipid oxidation and hydrolysis. Plant Cell Environ. 2020, 43, 303–314. [Google Scholar] [CrossRef]
- Cakmak, T.; Atici, Ö.; Agar, G. The natural aging-related biochemical changes in the seeds of two legume varieties stored for 40 years. Acta Agric. Scand. Sect. B—Plant Soil Sci. 2010, 60, 353–360. [Google Scholar] [CrossRef]
- Ratajczak, E.; Małecka, A.; Bagniewska-Zadworna, A.; Kalemba, E.M. The production, localization and spreading of reactive oxygen species contributes to the low vitality of long-term stored common beech (Fagus sylvatica L.) seeds. J. Plant Physiol. 2015, 174, 147–156. [Google Scholar] [CrossRef]
- Trusiak, M.; Plitta-Michalak, B.P.; Michalak, M. Choosing the right path for the successful storage of seeds. Plants 2022, 12, 72. [Google Scholar] [CrossRef]
- Li, J.; Wei, Z.; Min, X.; Zhao, P.; Yang, L.; Liu, N. Physiological and biochemical changes in the seeds of naturally aged wenling medic (Medicago polymorpha) with its recovery of viability. Agronomy 2023, 13, 787. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Q.; Karagić, Đ.; Liu, X.; Cui, J.; Gui, J.; Gu, M.; Gao, W. Effects of ultrasonication on increased germination and improved seedling growth of aged grass seeds of tall fescue and Russian wildrye. Sci. Rep. 2016, 6, 22403. [Google Scholar] [CrossRef]
- Paravar, A.; Maleki Farahani, S.; Rezazadeh, A. Morphological, physiological and biochemical response of Lallemantia species to elevated temperature and light duration during seed development. Heliyon 2023, 9, e15149. [Google Scholar] [CrossRef]
- Morales, M.; Munné-Bosch, S. Malondialdehyde: Facts and artifacts. Plant Physiol. 2019, 180, 1246–1250. [Google Scholar] [CrossRef]
- Biswas, M.S.; Mano, J. Lipid peroxide-derived reactive carbonyl species as mediators of oxidative stress and signaling. Front. Plant Sci. 2021, 12, 720867. [Google Scholar] [CrossRef]
- Chen, D.; Li, Y.; Fang, T.; Shi, X.; Chen, X. Specific roles of tocopherols and tocotrienols in seed longevity and germination tolerance to abiotic stress in transgenic rice. Plant Sci. 2016, 244, 31–39. [Google Scholar] [CrossRef]
- Gianella, M.; Doria, E.; Dondi, D.; Milanese, C.; Gallotti, L.; Börner, A.; Zannino, L.; Macovei, A.; Pagano, A.; Guzzon, F.; et al. Physiological and molecular aspects of seed longevity: Exploring intra-species variation in eight Pisum sativum L. accessions. Physiol. Plant. 2022, 174, e13698. [Google Scholar] [CrossRef]
- Boca, S.; Koestler, F.; Ksas, B.; Chevalier, A.; Leymarie, J.; Fekete, A.; Mueller, M.J.; Havaux, M. Arabidopsis lipocalins AtCHL and AtTIL have distinct but overlapping functions essential for lipid protection and seed longevity. Plant Cell Environ. 2014, 37, 368–381. [Google Scholar] [CrossRef]
- Gerna, D.; Arc, E.; Holzknecht, M.; Roach, T.; Jansen-Dürr, P.; Weiss, A.K.H.; Kranner, I. AtFAHD1a: A new player influencing seed longevity and dormancy in Arabidopsis? Int. J. Mol. Sci. 2021, 22, 2997. [Google Scholar] [CrossRef]
- Yang, W.; Xu, X.; Li, Y.; Wang, Y.; Li, M.; Wang, Y.; Ding, X.; Chu, Z. Rutin-mediated priming of plant resistance to three bacterial pathogens initiating the early SA signal pathway. PLoS ONE 2016, 11, e0146910. [Google Scholar] [CrossRef]
- Amorati, R.; Baschieri, A.; Cowden, A.; Valgimigli, L. The antioxidant activity of quercetin in water solution. Biomimetics 2017, 2, 9. [Google Scholar] [CrossRef]
- Xu, H.Y.; Zheng, H.C.; Zhang, H.W.; Zhang, J.Y.; Ma, C.M. Comparison of antioxidant constituents of Agriophyllum squarrosum seed with conventional crop seeds. J. Food Sci. 2018, 83, 1823–1831. [Google Scholar] [CrossRef]
- Singh, P.; Arif, Y.; Bajguz, A.; Hayat, S. The role of quercetin in plants. Plant Physiol. Biochem. 2021, 166, 10–19. [Google Scholar] [CrossRef]
- Bai, C.Z.; Feng, M.L.; Hao, X.L.; Zhong, Q.M.; Tong, L.G.; Wang, Z.H. Rutin, quercetin, and free amino acid analysis in buckwheat (Fagopyrum) seeds from different locations. Genet. Mol. Res. 2015, 14, 19040–19048. [Google Scholar] [CrossRef]
- Cui, M.; Wu, D.; Bao, K.; Wen, Z.; Hao, Y.; Luo, L. Dynamic changes of phenolic compounds during artificial aging of soybean seeds identified by high-performance liquid chromatography coupled with transcript analysis. Anal. Bioanal. Chem. 2019, 411, 3091–3101. [Google Scholar] [CrossRef]
- Ghotbzadeh Kermani, S.; Saeidi, G.; Sabzalian, M.R.; Gianinetti, A. Drought stress influenced sesamin and sesamolin content and polyphenolic components in sesame (Sesamum indicum L.) populations with contrasting seed coat colors. Food Chem. 2019, 289, 360–368. [Google Scholar] [CrossRef]
- Gabr, A.M.M.; Fayek, N.M.; Mahmoud, H.M.; El-Bahr, M.K.; Ebrahim, H.S.; Sytar, O.; El-Halawany, A.M. Effect of light quality and media components on shoot growth, rutin, and quercetin production from common buckwheat. ACS Omega 2022, 7, 26566–26572. [Google Scholar] [CrossRef]
- Forti, C.; Ottobrino, V.; Doria, E.; Bassolino, L.; Toppino, L.; Rotino, G.L.; Pagano, A.; Macovei, A.; Balestrazzi, A. Hydropriming applied on fast germinating Solanum villosum Miller seeds: Impact on pre-germinative metabolism. Front. Plant Sci. 2021, 12, 639336. [Google Scholar] [CrossRef]
- Liang, Y.; Kang, K.; Gan, L.; Ning, S.; Xiong, J.; Song, S.; Xi, L.; Lai, S.; Yin, Y.; Gu, J.; et al. Drought-responsive genes, late embryogenesis abundant group3 (LEA3) and vicinal oxygen chelate, function in lipid accumulation in Brassica napus and Arabidopsis mainly via enhancing photosynthetic efficiency and reducing ROS. Plant Biotechnol. J. 2019, 17, 2123–2142. [Google Scholar] [CrossRef]
- Kodad, O.; Socias, I.; Company, R.; Alonso, J.M. Genotypic and environmental effects on tocopherol content in almond. Antioxidants 2018, 7, 6. [Google Scholar] [CrossRef]


| Accession | Storage Time (y) | Seed Mass (mg) | p50 (d ± s.e.) | Maximal Germination (% ± C.I.) | |
|---|---|---|---|---|---|
| 15 °C | 20 °C | ||||
| Y2014 | 6.00 | 7.65 | 7.77 ± 0.5831 | 41.67 ± 8.82 | 55.96 ± 3.04 |
| Y2015 | 5.00 | 7.48 | 12.28 ± 0.689 | 5.48 ± 0.26 | 60.09 ± 7.46 |
| Y2016 | 4.00 | 7.03 | 10.07 ± 0.7256 | 20.18 ± 5.62 | 59.20 ± 7.64 |
| Y2017 | 3.00 | 6.94 | 13.01 ± 0.7697 | 58.52 ± 5.96 | 50.26 ± 8.21 |
| Y2018 | 2.00 | 5.94 | 10.88 ± 0.7938 | 32.95 ± 5.89 | 65.00 ± 10.13 |
| Accession | Average Temperature (°C ± s.e.) | Total Precipitations (mm) | ||||||
|---|---|---|---|---|---|---|---|---|
| Current Year | Previous Year | Current Year | Previous Year | |||||
| Annual | Growth | Annual | Growth | Annual | Growth | Annual | Growth | |
| Y2014 | 7.19 ± 0.29 | 13.38 ± 0.31 | 6.40 ± 0.38 | 16.44 ± 0.39 | 2659.00 | 217.90 | 2209.40 | 67.00 |
| Y2015 | 7.65 ± 0.36 | 14.79 ± 0.36 | 7.19 ± 0.29 | 13.38 ± 0.31 | 1369.30 | 349.30 | 2659.00 | 217.90 |
| Y2016 | 6.85 ± 0.34 | 15.15 ± 0.39 | 7.65 ± 0.36 | 14.79 ± 0.36 | 1927.20 | 139.80 | 1369.30 | 349.30 |
| Y2017 | 7.32 ± 0.39 | 16.50 ± 0.35 | 6.85 ± 0.34 | 15.15 ± 0.39 | 1741.90 | 97.10 | 1927.20 | 139.80 |
| Y2018 | 7.13 ± 0.39 | 15.64 ± 0.28 | 7.32 ± 0.39 | 16.50 ± 0.35 | 1689.00 | 152.20 | 1741.90 | 97.10 |
| Time (min) | 5% v/v Acetic Acid | 5% v/v Pure Methanol |
|---|---|---|
| 1 | 90 | 10 |
| 5 | 90 | 10 |
| 7 | 80 | 20 |
| 8 | 80 | 20 |
| 10 | 75 | 25 |
| 15 | 70 | 30 |
| 20 | 20 | 80 |
| 25 | 50 | 50 |
| 28 | 70 | 30 |
| 30 | 90 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagano, A.; Doria, E.; Mondoni, A.; White, F.J.; Balestrazzi, A.; Macovei, A. Oxidant and Antioxidant Profiling in Viscaria alpina Seed Populations Following the Temporal Dynamics of an Alpine Climate. Seeds 2023, 2, 357-369. https://doi.org/10.3390/seeds2030027
Pagano A, Doria E, Mondoni A, White FJ, Balestrazzi A, Macovei A. Oxidant and Antioxidant Profiling in Viscaria alpina Seed Populations Following the Temporal Dynamics of an Alpine Climate. Seeds. 2023; 2(3):357-369. https://doi.org/10.3390/seeds2030027
Chicago/Turabian StylePagano, Andrea, Enrico Doria, Andrea Mondoni, Fiona Jane White, Alma Balestrazzi, and Anca Macovei. 2023. "Oxidant and Antioxidant Profiling in Viscaria alpina Seed Populations Following the Temporal Dynamics of an Alpine Climate" Seeds 2, no. 3: 357-369. https://doi.org/10.3390/seeds2030027
APA StylePagano, A., Doria, E., Mondoni, A., White, F. J., Balestrazzi, A., & Macovei, A. (2023). Oxidant and Antioxidant Profiling in Viscaria alpina Seed Populations Following the Temporal Dynamics of an Alpine Climate. Seeds, 2(3), 357-369. https://doi.org/10.3390/seeds2030027









