Abstract
Azadirachta indica (Neem tree) has become widely naturalised and invasive across many countries and regions including northern Australia. To aid management of A. indica where it has become a weed, a series of studies were undertaken to determine its potential soil seed bank persistence. In a field trial, packets of seeds were buried, retrieved periodically over two years and the seed viability assessed. Viability declined rapidly, with a single viable seed retrieved after 12 months burial and none thereafter. Burial depth, soil type, and pasture cover (present and excluded) significantly influenced viability (%) at 3- and 6-month retrievals. Similar data were obtained from repeated runs of a controlled ageing laboratory experiment, which categorized seeds as forming a ‘transient’ seed bank. In a third trial, fresh fruits were placed on the soil surface in replicated field enclosures over two consecutive years and seedling emergence monitored fortnightly. In both years there was no emergence from pasture excluded soil plots and emergence ceased after 2.3 and 8.4 months in plots with pasture present. A fourth (glasshouse) trial found most seeds will emerge from the soil when buried from 1 to 4 cm. However, more fatal germination than successful emergence was recorded for seeds buried at 8 cm. Seed desiccation and fatal germination are factors in A. indica developing a transient soil seed bank, and infestations require shorter-term control programs where seed input is prevented.
1. Introduction
Azadirachta indica A. Juss (neem tree) belongs to the mahogany family ‘Meliaceae’. It is native to parts of the Indian subcontinent and Myanmar but naturalised across warmer tropical areas throughout the Indo-Malaysia region, the Sahel region of Africa, Central America, the Caribbean, South America and Australia [1]. There are over 72 countries [2] where A. indica has been grown and is widely cultivated due to its importance as a traditional medicine and other commercial and environmental benefits. The tree is well established as a productive plant with a variety of uses as a medicine, pesticide, fungicide, windbreak, timber, firewood, and as a source of lipid oils, tannins and gums [1,2,3,4,5].
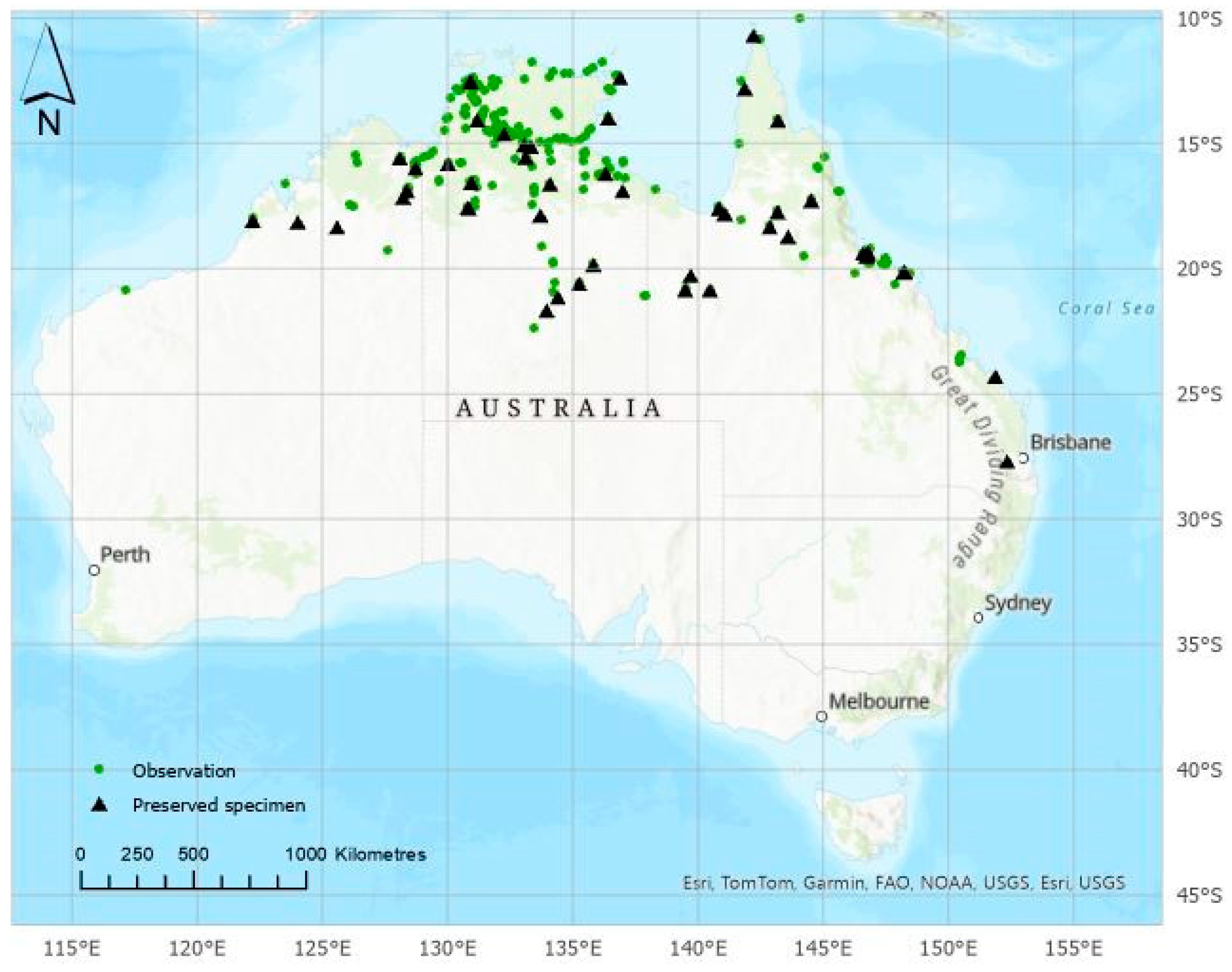
Whilst undoubtably a useful plant, there are 18 countries where A. indica is introduced and invasive and a further 85 where it was introduced but the invasive status was not recorded [6]. An earlier list had an exotic range of 87 countries [3]. They include 13 states within Brazil [6], where A. indica spread from cultivation from 1986 onwards [7] and poses an invasive threat across humid coastal and semi-arid areas of north-eastern Brazil. In Australia, A. indica was not considered naturalized in 1998 [8] but was a potential environmental weed due to its establishment overseas. Unfortunately, A. indica has become more prominent in herbarium records over the last 20 years and is now widely naturalised across three states and territories in northern Australia (Figure 1), where is it considered a major environmental threat to the native riparian flora [9]. Following an introduction from horticultural and domestic sources around 1990, A. indica had invaded at least 7100 ha of the Kununurra region of Western Australia by 2008 [9]. By 2013, it was the highest priority for regional control due to agricultural, environmental and cultural damage [10]. Recently A. indica was listed as a declared weed in Western Australia due to damage to riparian habitats, threats to endangered animals, reducing access to agricultural infrastructure and impeding mustering [11]. It is an invasive weed (Class B) in the Northern Territory where there are dense infestations around Katherine and many other towns and rivers [12]. The Gilbert River in Queensland and the Wickham River in the Northern Territory have also been heavily invaded [9,13] (Figure 1).

Figure 1.
Distribution of Azadirachta indica across northern Australia. Data from preserved specimens (64) and observations (7283) between 1987 and 2024 [13]. Not all points may have naturalised plants, and some plants may have been removed. Some observations may not be accurately identified, although most observations were from government or corporate sources.
Azadirachta indica is an evergreen but sometimes deciduous, fast-growing tree, up to 25 m tall, and with a large crown up to 20 m in diameter [1]. It may live over 200 years in native areas receiving 450 to 1200 mm of annual rainfall but has been introduced into areas receiving as little as 150 mm of annual rain [2]. Large trees are prolific fruit producers [1] with individual plants from the age of three years producing more than 9 kg of fruit per young tree, with averages around 20 kg and up to 55 kg from older trees, with more than 1800 seeds per kg [4]. Fruit is dispersed by birds, flying foxes (Pteropus spp.) and other domestic and native animals [5,14]. The fruit, an ellipsoid drupe consists of a thin leathery exocarp, a fleshy mesocarp hereafter referred to as pulp, and a cartilaginous endocarp which encloses the embryo [1], usually with one seed but sometimes up to three seeds [4]. Unripe fruit is green with white milky sap, while ripe fruit is yellow to brown. The change in fruit colour from green to yellow begins after approximately 12 weeks of seed development [15].
Optimal seed germination occurs at 26 °C but is adversely influenced by temperatures greater than 30 °C and lower than 7 °C [16,17]. Seeds may germinate after five days at temperatures of between 20 °C and 30 °C [18] and have limited desiccation tolerance [15] losing viability within 1 to 4 months of ex situ conservation [16,19]. Although A. indica seeds preserved at 10–15 °C with a moisture content ≥ 10% survived up to two years, seeds originating from mature yellow fruits lived longer than seeds from green or brown fruits [20]. There may be a broad correlation between ex situ and in situ seed longevity [21].
The feasibility of managing weed incursions can hinge on biological traits such as dispersal vectors, time to maturity and persistence in the soil seed bank [22]. It has been observed that A. indica seed only appears viable during the first wet season after maturation [9] but no detailed studies of the persistence of A. indica soil seed banks have been identified. Research into the field seed persistence will guide management of this weed which is at high risk of establishing from cultivation and further spread from naturalised infestations. A series of experiments were undertaken across field and controlled environments to investigate seed persistence, particularly correlating the results for buried packet trials with controlled ageing tests [23]. To assist in explaining the field application of the persistence data, seedling emergence was also investigated from varying burial depths and from fruit placed in field enclosures.
2. Materials and Methods
2.1. Study Area and Baseline Seed Treatments
For each experiment, fresh A. indica seeds in fruits were collected from the branches of adult trees located in the vicinity of Charters Towers, Australia (20°09′ S, 146°26′ E; elevation 318 m), between January and March in multiple years. Mature yellow fruit was preferred as initial testing showed lower and quicker moisture loss, and fruit was de-pulped for trials 1, 2 and 4 [15,16,20]. Germination testing was conducted under a 30/20 °C, 12-h diurnal regime in (Thermoline® Scientific, Fairfield, Australia) incubators. Seeds that germinated (identified by radicle emergence) were counted and removed. Seeds that did not germinate were subject to a physical test of viability (pressed for solidity or collapse), then ‘solid’ seeds were checked for dormancy using the tetrazolium method [24]. Trial dates and variations in these methods are documented within each experiment. Untreated seed lots served as ‘controls’ to determine pre-treatment viabilities. Statistical analyses for all trials were conducted in Genstat® V22 or 24 (VSN International Ltd., Hemel Hempstead, UK).
2.2. Experiment 1 Buried Seed Longevity
A buried seed packet field trial (experiment 1) investigated A. indica seed persistence at 20°09′ S, 146°26′ E; elevation 318 m. The trial location, design and treatments have been documented [25]. Local ripe fruits of A. indica were collected in March 2009. Six hundred and forty sub-samples of 50 de-pulped seeds (endocarp on) were randomly selected and placed in bags of shade cloth (4 cm × 4 cm × 0.5 cm; 1.1 mm × 2.4 mm mesh size). On the 17 March 2009, a multi-factor split-split plot design with four replications and nine retrievals was established. The factors were two soil types [alluvial river loam and black cracking clay (whole plot)], two levels of pasture cover [pasture present or pasture excluded (split plot)]. The split-split plot factor was four seed burial depths with packets buried at 0, 2.5, 10 and 20 cm within single PVC pipes filled with the soil type matching the whole plot treatment [25]. Retrievals were scheduled for 3, 6 and 12 months then annually or until no viable seeds were recorded for two consecutive retrievals.
To determine initial seed viability prior to burial, 64 × 50 de-pulped seeds were placed on absorbent paper towels in rectangular plastic trays (11 cm × 17 cm × 7 cm), kept moist with distilled water and germinated in incubators. Germinated seeds were counted and removed daily for 17 days, and viability included those that did not germinate but were ‘solid’ and considered viable after tetrazolium testing. At each retrieval time, seeds were removed from retrieved field packets, washed and similarly tested for viability. The percentage of viable seed retrieved was analysed separately for the 3- and 6-month retrievals using a split-split-plot design. At the 3-month retrieval, the number of seeds that germinated in the bags prior to retrieval was also recorded by counting emerged seedlings; this was not possible in later retrievals due to desiccation of seedlings.
2.3. Experiment 2—Controlled Ageing Test
Three controlled ageing tests (experiments 2.1, 2.2 and 2.3) were conducted under laboratory conditions to determine if the results from the field burial trial, aligned with the weed seed persistence categories [23]. Fresh fruit was collected on 8 and 15 January 2020, and on the 13 February 2024. Fruit was de-pulped under running water, dried with a paper towel and stored at laboratory temperature, then sorted into 24 lots of 30 fruits for testing. Lots were placed in individual open glass vials, which were evenly split between two replicate electrical boxes (labelled A and B) sealed to IP67. Vials were subjected to a 14 day ‘hydration’ phase with a 47% relative humidity over a lithium chloride solution (320 g L−1 H2O) [26] in a dark 20 °C Thermoline® incubator. After the hydration phase, reference (day 0) samples of 30 de-pulped fruits were removed from both boxes, the endocarp removed, seeds counted and germinated. Then an ‘ageing’ phase was conducted in the same dark incubator set at 45 °C and with 60% relative humidity from a lithium chloride of 370 g L−1 H2O [26]. A seed lot was removed from each box in the ageing environment after the days shown in Table 1.

Table 1.
Commencement and retrieval schedule of Azadirachta indica seeds from the ageing environment in experiment 2.
The hydration and ageing phases were repeated as experiment 2.2 with another 24 lots of 30 fresh de-pulped fruit, except the retrieval intervals were shortened (Table 1). In experiments 2.1 and 2.2 the endocarp was removed and the seed counted (range 30–34 seeds) after retrieval and prior to germination testing. Experiment 2.3 also used de-pulped fruit, collected 13 February 2024, but the seed was removed from the endocarp, counted into 24 lots of 30 seeds prior to the hydration phase.
Once removed from the ageing environment, seeds were placed on moistened filter paper over an inverted watch glass in a 90 mm Petri dish and incubated to assess germinability [27]. Ungerminated seeds were non-viable as they collapsed when subjected to physical tests of viability. Germination of the total of seeds removed from the ageing environment was calculated as a proportion of the un-aged day 0 germination (proportion germinable = 1) per box. The proportion germinable data over time in the A and B boxes were used to chart negative logistic regression curves [28] for each experiment.
2.4. Experiment 3—Seedling Emergence Cages
To reflect the natural fall of fruits, seedling emergence from intact fruit on the soil surface was followed within field enclosures from placements over consecutive years (experiment 3). Soil type and ground cover experimental factors were the same as experiment 1. It was a split plot design with four replicates, the main treatment was soil type (alluvial river loam and black cracking clay), and sub treatments where the pasture ground cover was present and excluded [25]. Each of the 16 plots consisted of a square enclosure (cage) (Figure 2).

Figure 2.
A subdivided field enclosure with pasture excluded treatment used in experiment 3, after Azadirachta indica fruit was added to the right subdivision on 21 February 2017. Cage was made of 5 mm galvanized mesh of 4 sides (0.9 m wide × 0.9 m long × 0.46 m high), buried approximately 4 cm deep and with a meshed hinged lid and no bottom mesh.
Mature A. indica fruits were collected on the 28 January 2016. Approximately 500 fresh mature seeds within intact fruits were placed on the ground per plot on the 11 February 2016. This process was repeated on the 20 February 2017 after low numbers of seedling emergence and after several rain events (discussed in the results). In 2017, each enclosure was halved by installing mesh through the centre of each cage (Figure 2). Any remaining 2016 fruit placement was carefully moved into one of the half sections of each plot. On the 21 February 2017, a fresh batch of mature fruits was collected, counted and 500 were spread on the ground within a cleared half section of each of the 16 original enclosures. All seedling emergence within each plot or half plot was recorded and seedlings carefully removed every two weeks. The trial concluded in February 2019 after no emergence was recorded over consecutive summer wet seasons.
Reference samples of seeds (10 × 25 seeds, endocarp off) from de-pulped fruit were tested for initial germinability and viability on the 24 April 2016 and the 24 February 2017. Seeds were placed in petri dishes on top of filter paper and moistened with distilled water and germinated. Solid, ungerminated seed was subject to tetrazolium testing. An additional sample of 50 seeds was retrieved from each half plot on 20 February 2019 and tested for germinability/viability.
The data are presented as the cumulative seedling emergence summed across the four replicates of each combination of cover and soil treatments (maximum 2000 seeds). The total recorded seedlings per plot to the 20 February 2019 were analysed as a split-plot design with placement date, soil type, and pasture present and excluded as factors. Daily rainfall, minimum and maximum temperatures were extracted for grid mid-point at Latitude −20.10 and Longitude 146.25 [29] and converted to fortnightly temperature means and total rainfall to match the plot monitoring frequency.
2.5. Experiment 4, Depth of Seedling Emergence
To assist in explaining the results from the packets buried in experiment 1, the fate of seedlings from seed buried between 0 and 8 cm was investigated in a glasshouse trial. The trial was conducted four times using de-pulped seed from fruit collected on 13 February 2024. Seed was retained in the fibrous endocarp for two runs and removed from the endocarp for two further runs (Table 2). Each treatment in each run had six replicates. A locally sourced clay-based soil was sieved to 3 mm and dried at 80 °C for at least three days. Square nursery seedling tubes (155 × 65 mm) were carefully filled with uncompacted soil and 10 A. indica seeds were placed at depths in Table 2, one depth per tube. Tubes were placed in a glasshouse with overhead mist sprinklers for 5 min every 6 h. In run 2 (endocarp off) it was thought to be more informative to replace the 5 mm treatment with a 60 mm treatment (Table 2). Reference batches of 2 × 20 seeds were germinated for both endocarp off runs, ungerminated seeds were non-viable as they collapsed when subjected to physical tests of viability.

Table 2.
Start dates, endocarp and depth treatment details for Azadirachta indica fruit experiment 4.
Emergent seedlings were counted and carefully removed to avoid disturbing the soil. Each run concluded after three weeks of no recorded emergence, then each seed was carefully excavated and classified as successfully emerged, unsuccessfully emerged (fatal germination), viable (physically solid) or unviable (failed physical test). The surface seeds of run 2 (endocarp off) suffered a fungal attack which limited emergence to 4 of 60 seeds, and they were excluded from further analysis. The data were analysed as an unbalanced accumulated ANOVA using regression with run, depth, endocarp and block factors, for the fates of successful emergence, fatal emergence and non-viable seeds. A separate linear polynomial regression analysis was conducted for all values (0–10) of seedlings emerging from depths for the endocarp and run factors.
3. Results
3.1. Seed Responses over Time
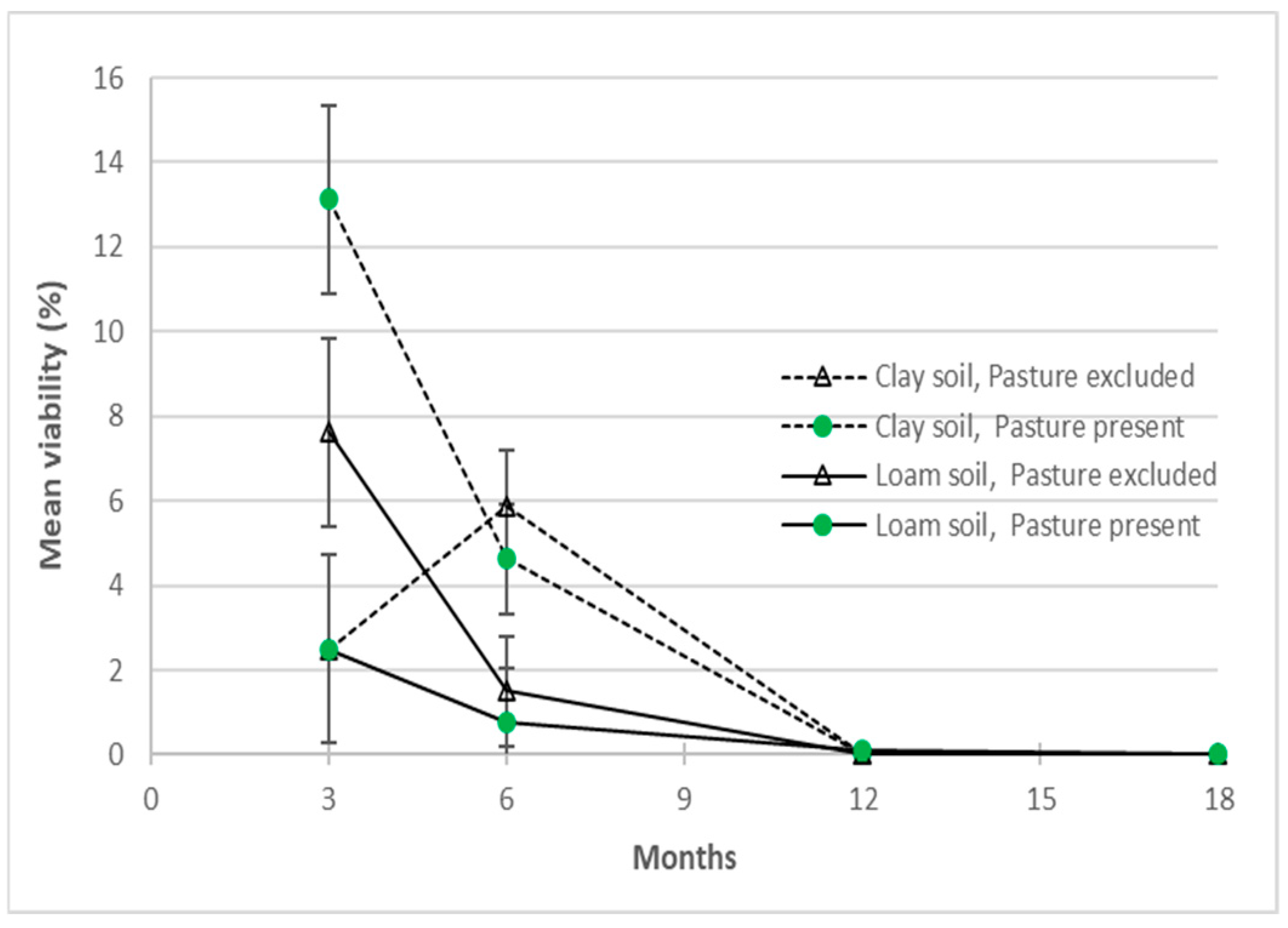
The viability of A. indica seed was assessed over time under the different test conditions in experiments 1, 2 and 3. In experiment 1, the initial germination and viability of unburied, de-pulped, endocarp on A. indica seeds averaged 15.2% (±1.6) (Standard Error Means SEM) and 39.7% (±2.1), respectively. Viability amongst the 64 unburied seed lots ranged greatly from 6 to 80% and endocarp off was preferred for germinating reference batches in later experiments. Across all the buried packet treatments, viability declined to 6.4% at 3 months, 3.2% at 6 months and 0.03% at 12 months (Appendix A Table A1). After 12 months burial a single seed germinated from a loam soil, pasture present, surface packet. No viable seed was recovered after 18 and 24 months of burial and the trial concluded.
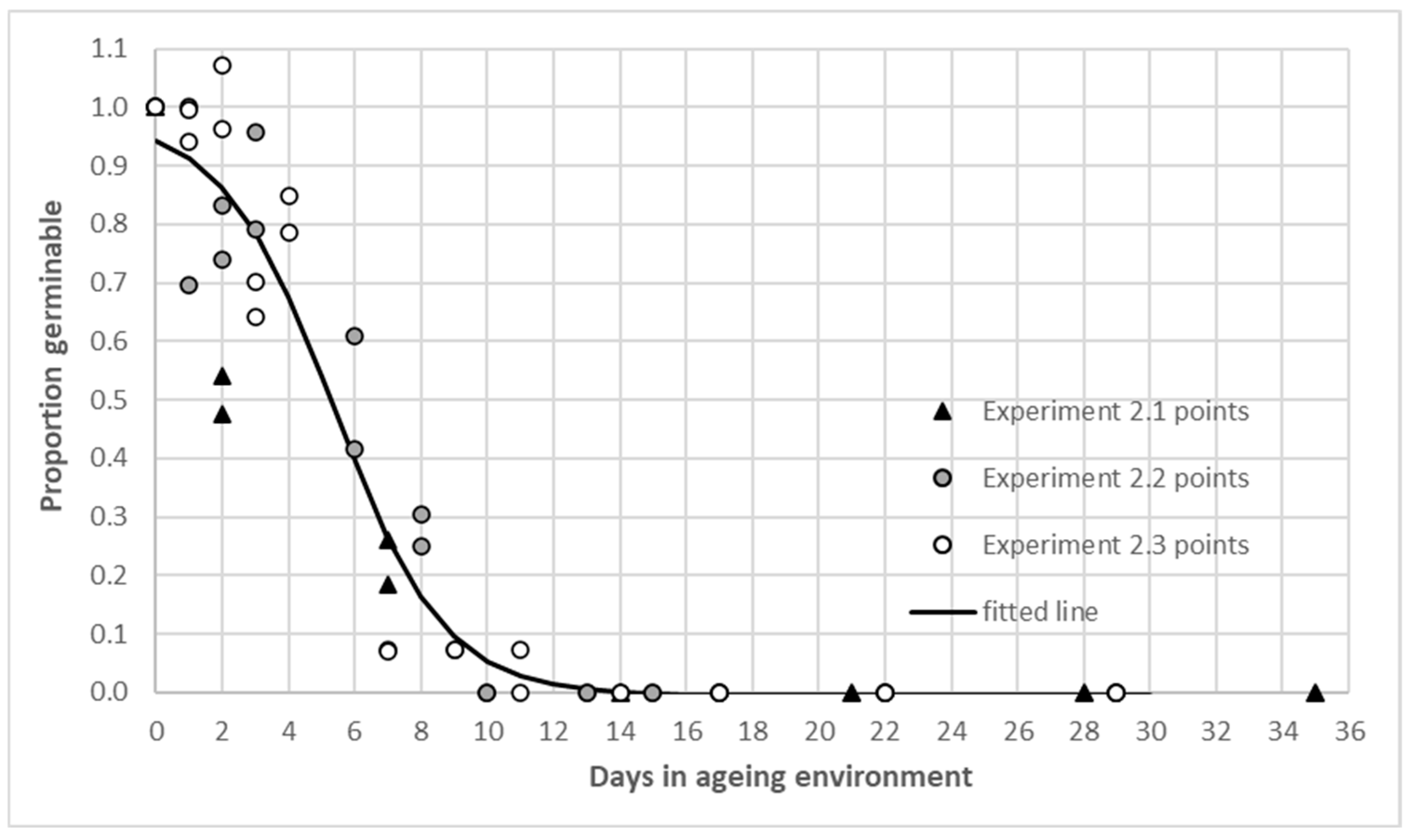
In experiment 2, the relative proportion of viable seed over time in the ageing environment was calculated from the un-aged germinability for retrievals from each of the boxes in each of the 3 runs. The mean and per box reference germinability after the hydration phase of each experiment is shown in Appendix A Table A2. The fitted curves for both boxes in experiments 2.1 and 2.2 were influenced by a rapid decline and lacked a positive inflection point (‘M’ parameter, equation 1) [28]. Compared with the effects of time in the ageing environment, the influence of endocarp on or off in the hydration phase was minimal in this test. The fitted line in Figure 3 is for data pooled from the boxes in three experiments and the P50 value was 5.35 days, (‘M’ parameter Appendix A Table A3). The results place A. indica in a transient seed longevity category [23].
where G represents the germinable proportion of seeds remaining at time x (days); C is the fitted initial viability; B the slope decay; A represents the vertical shift/asymptote of the sigmoid curve; and M = P50, the time taken for the germinable proportion to fall to 50%.
G = A + C/[1+ e − B(x − M)]
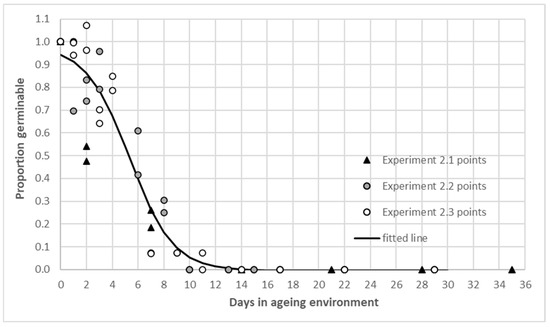
Figure 3.
Proportion of germinable Azadirachta indica seed from experiment 2.1, 2.2 and 2.3 raw data and fitted curve over days in the ageing environment. Estimated parameters of fitted line (Equation (1)) are in Appendix A Table A3.
Across all retrievals the proportion germinable was below 0.5 after two to six days of ageing. Two seeds germinated after ageing for 11 days in experiment 2.3 box A, no other seed germinated after nine days of ageing (Figure 3). The seed quickly lost viability under the high temperature and humidity environment of experiment 2.
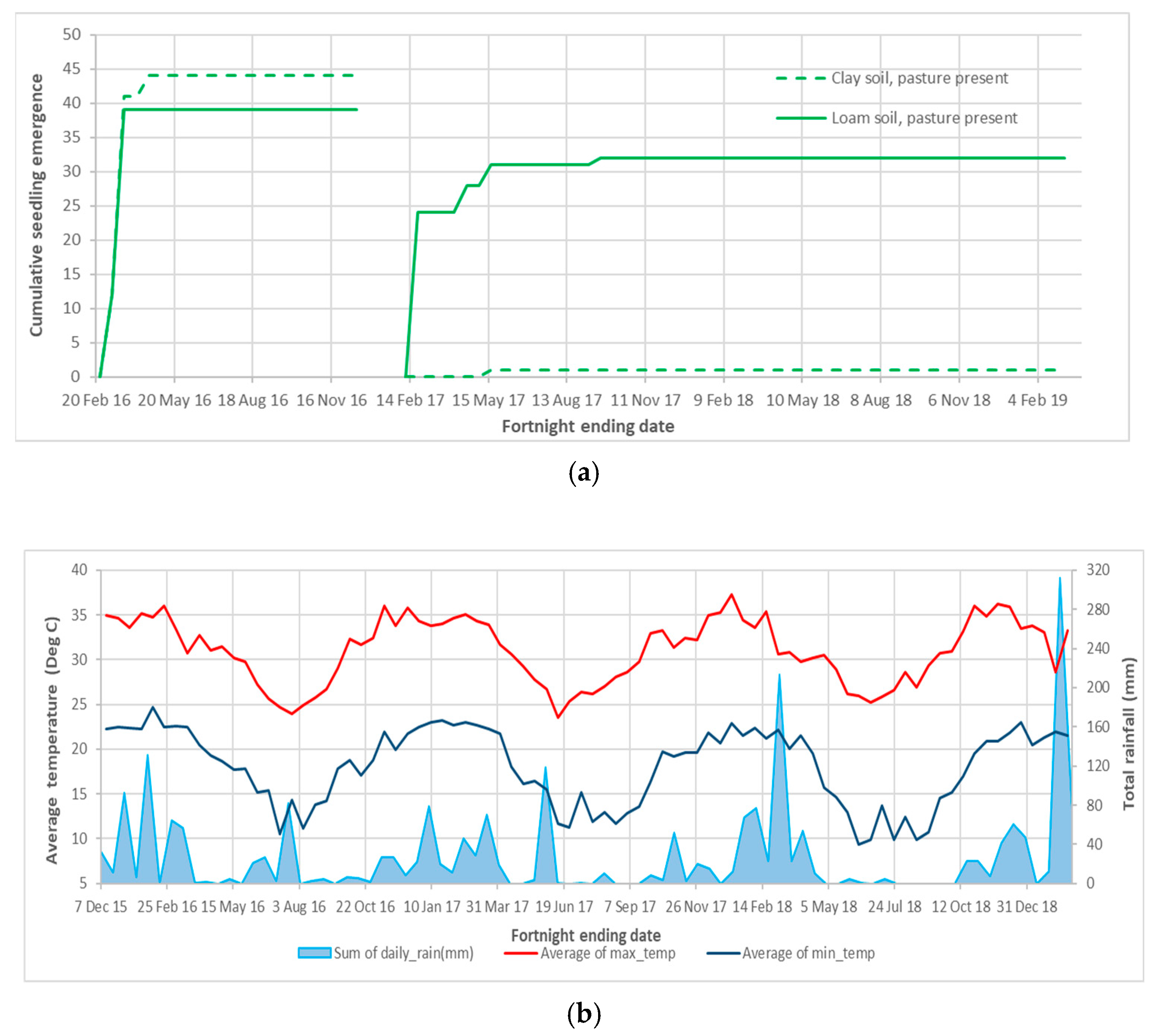
After fresh fruit was placed in the experiment 3 enclosures during the 2016 and 2017 wet seasons, the last seedlings were recorded after 2.3 and 8.4 months, respectively. Due to small and variable numbers of seedling emergence the data are presented as the total emergence from four replicates of each pasture and soil combination of treatments (Figure 4a). The treatment effects on the mean total emergence of each plot are presented in Appendix A Table A4.
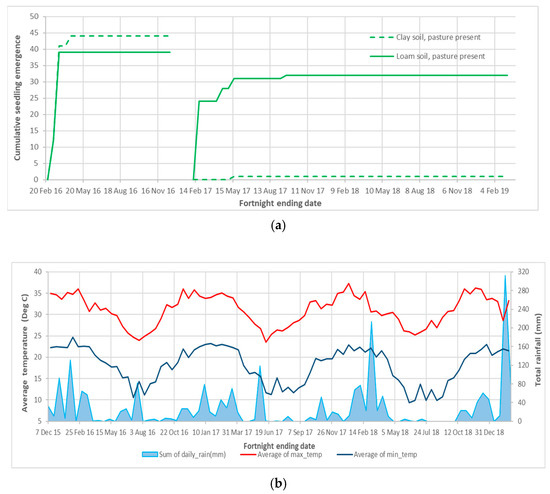
Figure 4.
Azadirachta indica seedling emergence and weather details for experiment 3: (a) Total seedling emergence from four replicates and two placement dates of fruit in field cages; and (b) Local fortnightly rainfall and temperature maximums and minimums [29] corresponding to monitoring dates.
Pre-testing found 46.8% (±4.09) or 936 of the 2000 A. indica seeds per soil and pasture cover treatment combination that were placed in 16 plots on 11 February 2016 were viable. Seedling emergence was recorded in the pasture present plots on 10 and 24 of March 2016, following over 100 mm of rain in February and March 2016 (Figure 4b). The last recorded emergence (three seedlings) was in the pasture present, clay soil plots on the 21 April 2016, 70 days after placement (Figure 4a). None of the following events resulted in further seedling emergence; 82 mm of rain for the fortnight ending 25 July 2016, the next rainfall over 40 mm per fortnight in early January 2017 (Figure 4b) and the concentration of the remaining seed in half plots in February 2017.
The average viability of the 2017 batch of unplaced seeds was 75.9% (+3.76), which equates to a viable pool of 1518 seeds from approximately 2000 seeds per soil and pasture cover treatment. After fruit was placed in the cages in 16 half plots (21 February), 31 seedlings emerged from two loam soil pasture covered plots, 24 on 6 April and 7 in June 2017, with 164 mm of rain recorded across the 8 weeks after placement. One seedling was recorded in a pasture covered, clay soil plot on 29 June 2017 (118 mm of rain a month prior). The last seedling emerged in a pasture present, loam soil plot on the 2 November 2017, which was 8.4 months after placement and followed 64 mm of rain in October 2017 (Figure 4a,b).
Following a large rainfall event and mild temperatures in March 2018, the authors recorded mass emergence from other weed seeds placed in different cages in the same trial area in March and April 2018. No A. indica emergence was recorded after November 2017. No viable seed was recovered from any of the plots on the 20 February 2019 and plots were monitored for fortnightly emergence after a different weed species was placed in the plots on the 19 August 2021. Emergence was concentrated in the first 8 weeks after placement.
The viability of A. indica seed dropped quickly in two field trials and in a laboratory test. The results from experiments 1, 2 and 3 all showed a lack of seed persistence, and that seed desiccates quickly, so field seed banks are likely to be transient.
3.2. Seed Responses to Burial Depth
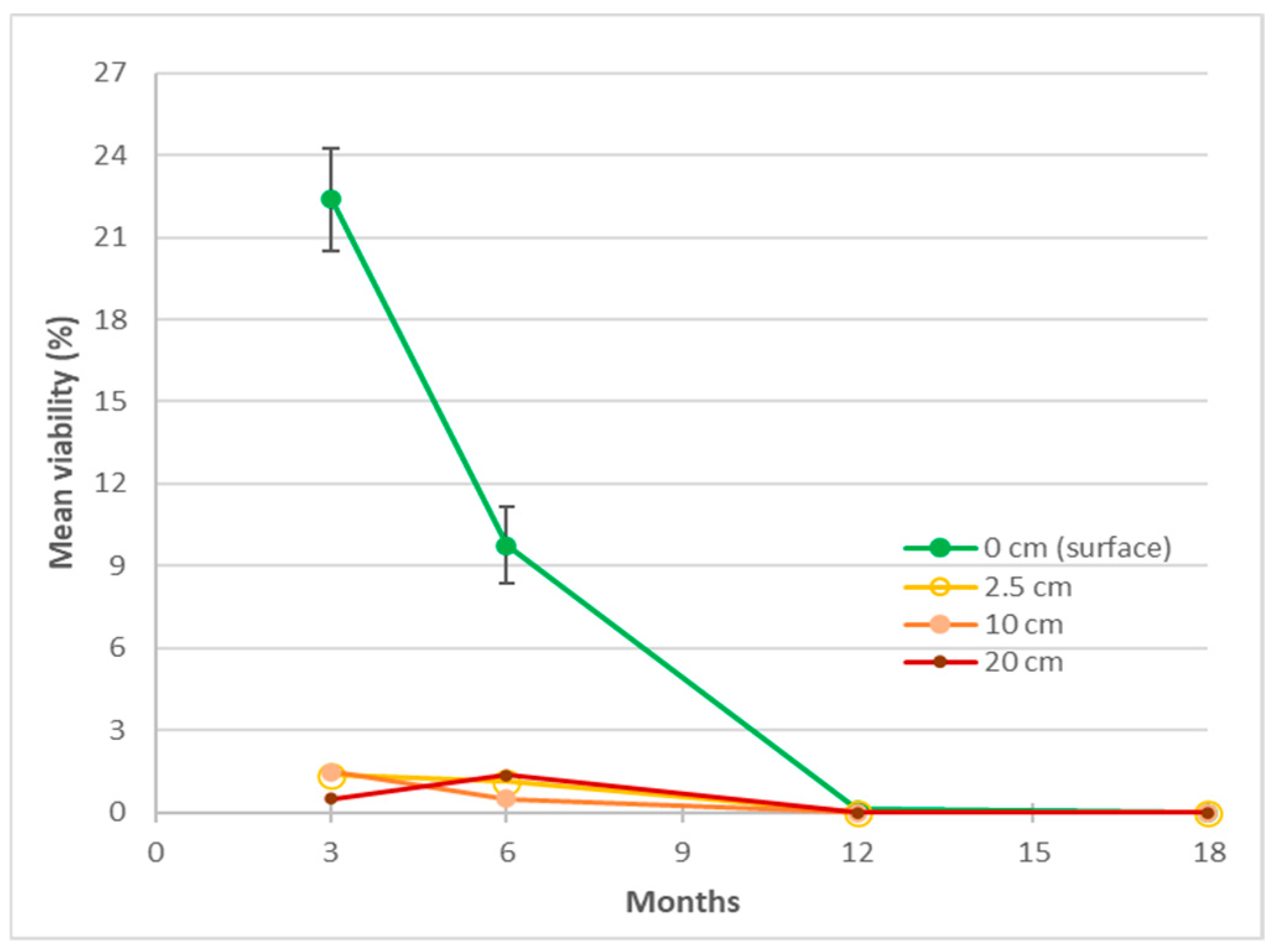
In experiment 1, burial depth was a significant factor (p < 0.001) in the viability of the seeds at 3 and 6 months, higher viability in the 0 cm (surface) packets compared to the buried packets (Figure 5, Appendix A Table A1). The overall viability of surface-located seeds decreased initially from 22% to <1% within 12 months, then to nil at 18 months after burial (Figure 5). When the packets were retrieved after 3 months, within packet germination was noted and was unevenly distributed between the retrieval depths, 13 seeds at 0 cm, 97 at 2.5 cm, 287 at 10 cm, and 106 at 20 cm. Viability decreased within in 3 months for seed lots buried at 2.5, 10 and 20 cm, with overall means between 0 and 1.5% and the effects of those depths were inseparable at all retrieval times. Pre-retrieval, in-packet germination may have contributed to the lower viability of the sub-surface packets with seeds attempting to establish from packets as deep as 20 cm underground.
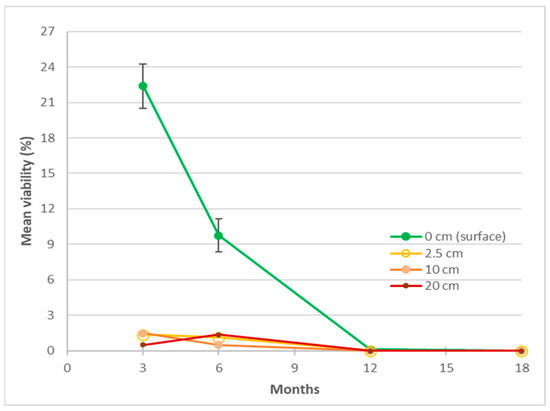
Figure 5.
The effect of burial depth on Azadirachta indica seed viability in retrieved packets buried for up to 18 months in a seed longevity trial (experiment 1). Pooled standard errors of means (SEM) shown for depth treatment at 3 and 6 months for the surface packets only.
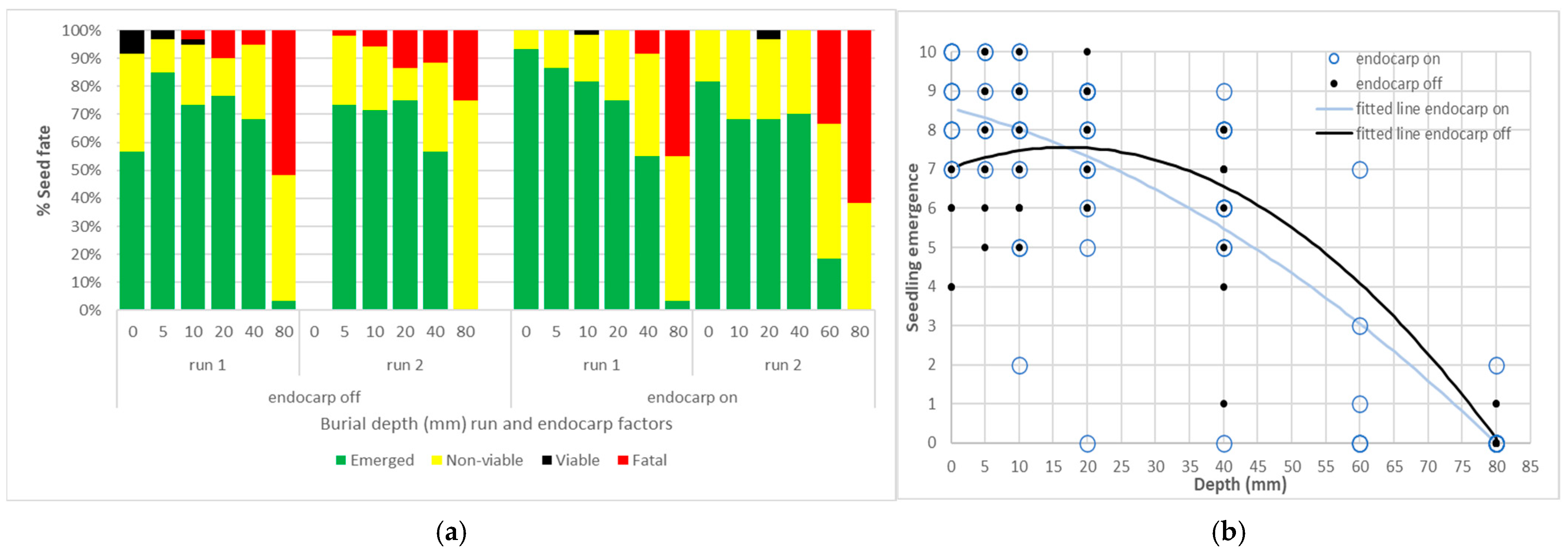
Given the observations from the 3-month retrieval of buried packets in experiment 1, the fate of seeds buried at different depths was assessed in experiment 4. The endocarp off reference germination fell between run 1 and run 2, averaging 87.5% and 67.5%, respectively, (SEM ± 3.54) (Table 1). Burial depth also significantly influenced seedling emergence in both runs, irrespective of whether the endocarp was on or off (p < 0.05). Average seed fate over six replicates of ten seeds were converted to a percentage (Figure 6a). Over 50% of seeds buried at 0 to 40 mm successfully emerged, while emergence was limited to two seeds at 80 mm in run 1. Run 2 emergence and viability was lower reflecting the later start date and older seed. Surface emergence was greater where the endocarp was retained (Figure 6b), even with the 0 mm, run 2, endocarp off treatment combination excluded. The fitted lines for both endocarp treatments crossed the x axis at 80 mm in Figure 6b. The endocarp on treatment is more reflective of field dispersed seed and there was a more consistent decline in emergence means and trendline from 0 to 40 mm (Figure 6b) across both runs.
where E represents seedling emergence from depth x (mm), A is the fitted initial emergence, B and C are constants. Values in Appendix A Table A5.
E = A + Bx + Cx2
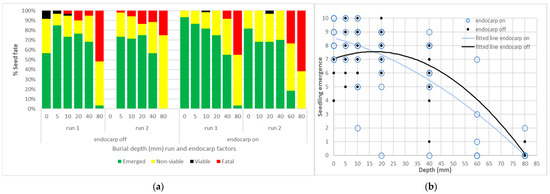
Figure 6.
Fate and emergence from Azadirachta indica seed buried at different depths in experiment 4: (a) Categorisation of seed fate by depth (mm) and run and endocarp on or off. Each value is a mean of 6 replicates of 10 seeds and displayed as a percentage; (b) Seedling emergence from burial depths (mm), individual values from 6 replicates and 2 runs to a maximum of 10. Duplicate values are obscured. Fitted lines from equation 2, parameters in Appendix A Table A5.
There may be some differences between non-viable seed and those that were allocated to ‘fatal emergence’ that were not evident when each run concluded, and (non-emerged) seed was recovered. However, depth was a significant factor in the ‘fatal’ fate as more seeds attempted to emerge from 60 and 80 mm than shallower depths. Run was not significant in the fatal fate analysis, however fatal emergence was slightly higher at 10, 20 and 40 mm amongst endocarp off replicates. At the end of experiment 4 only eight seeds were viable after burial at 0 to 20 mm, and no further analysis was conducted on this category.
3.3. Seed Responses to Soil and Pasture Treatments
Within the field experiments (1 and 3), there were differences and interactions with the pasture and soil treatments. In experiment 1, more consistent 6-monthly differences (p < 0.05) in soil, depth and soil*depth factors reflect the higher viability of A. indica seeds from the surface and clay soil treatments (Figure 7), than at 3 months. After 6 months burial, soil type had a significant influence on seed viability with more seeds remaining viable in clay soil (5.3%) than in river loam (1.1%). The presence or absence of pasture cover did not have a significant effect (p > 0.05) on seed viability. More viable seed was recovered from the surface loam soil packets at 3, 6 and 12 months, whereas sub-surface packets in loam soil plots only contained viable seed at the 3-month retrieval (Appendix A Table A1). Also, at 3 months, higher levels of viable seed in the sub-surface clay soil + pasture present, and loam soil + pasture excluded treatment combinations (Figure 7) contributed to significant soil*pasture and soil*pasture*depth interactions (p < 0.05). These differences may reflect variations in initial seed viability, in packet germination and treatment effects, and were inconsistent with the 6-monthly data.
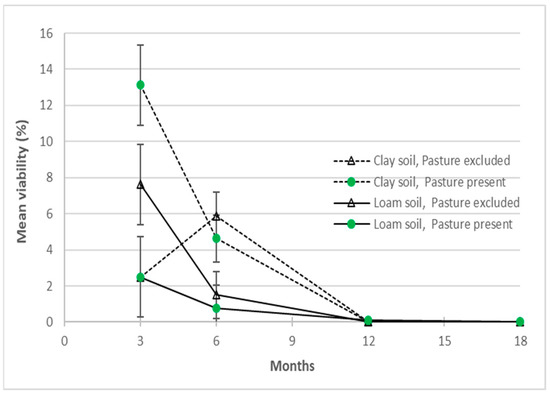
Figure 7.
The effects of soil and pasture cover treatment on Azadirachta indica seed buried for up to 18 months in a seed longevity trial (experiment 1). Pooled standard errors of means (SEM) shown for depth treatment at 3 and 6 months.
Experiments 1 and 3 were conducted at the same site, with soil and pasture treatments common to the field experiments. From the 2016 placement in experiment 3, 44 seedlings were recorded in pasture covered, clay soil plots, and 39 seedlings were recorded in pasture present loam soil plots, which equates to 2.08% of the estimated viable pool of seed emerging in pasture present plots (Figure 4a). Pasture present plots recorded between 1 and 23 seedlings, no seedlings were recorded in plots with pasture excluded, and pasture cover was the only significant factor (p < 0.05) in the mean plot emergence of the first placement data (Appendix A Table A4). From the 2017 fruit placement in experiment 3, a total of 32 seedlings emerged in two pasture present, loam soil plots, (31 in one plot) which is 0.83% of the viable pool across the pasture present, loam soil plots (Figure 4a). With low overall emergence in the second placement, the mean plot emergence for the soil and pasture factors were not significantly different (Appendix A Table A4). Although seedling emergence was low, all emergent seedlings were recorded in pasture present plots, and there were no significant differences found in mean plot emergence across clay and loam soil types. Overall, 2% or less of the viable pool of seed resulted in seedling emergence where pasture cover was present. Pasture presence was required for A. indica seedling emergence.
4. Discussion
4.1. Seed Persistence
Azadirachta indica seeds were effectively moribund less than 12 months after burial in a field trial. Results of three trials found that A. indica lacks seed bank persistence and forms a transient seed bank [30]. Only one viable seed was recovered from the buried packet trial after 12 months and the data from experiment 1 may have been further informed by a 9-month retrieval. More viable seed was recovered from surface packets than buried packets at 3 and 6 months and from clay soil packets at 6 months. Burial depth and time had larger and more consistent effects on seed persistence in experiment 1, than soil type and pasture cover. Utilising the same soil types in the field trials in experiment 4 may have aided the explanation of the depth trends.
Emergence from surface sown fruit was heavily concentrated in the two months after sowing and limited until the final recorded emergent seedling 8.4 months after a second placement. Any minor soil effects in experiment 3 were not evident amongst the small, variable emergence data and larger effect of pasture cover.
Fresh A. indica seed quickly lost viability under the high temperature/humidity conditions of an artificial ageing laboratory environment. A P50 value below 20 days suggests a ‘transient’ seed bank of less than 1 year [23]. When exposed to an ageing environment of 41 °C and 100% humidity a high initial germinability (~90%) of A. indica seed fell below 50% after 6 days and 0% after 11 days [31]. Reduced vigour was also recorded in seedlings from aged seed after one day and collected seed should be sown immediately in nurseries [31]. As researchers seek to correlate the results of buried packet trials and controlled ageing tests [23,27] it is encouraging to get complimentary indications in the trials presented. Rapid seed ageing, evident in the laboratory test, may contribute to the physical decline and loss of viability in field situations.
Germination is a mechanism of soil seed bank depletion [32]. Buried A. indica seeds attempted to establish when buried under 80 mm of soil, but few successfully emerged from that depth and more fatal germination was recorded amongst the deeper buried seed. Seed that germinated in field packets buried at 10 or 20 cm was unlikely to successfully emerge and resulted in fatal germination. The materials used in experiments 1, 3 and 4 should prevent seed bank loss due to emigration and macro-invertebrate predation. So, the seed bank loss may be attributed to combinations of detected and undetected fatal germination, seed ageing, and micro-predation [32], singularly or in unison.
4.2. Seedling Emergence
Azadirachta indica seed will attempt to germinate or rot when exposed to moisture even when buried to 80 mm. However, attempts to establish are more likely to be successful from the top 40 mm of the soil profile and (glasshouse) seedling emergence was highest from seeds buried between 0 and 20 mm deep.
The placement of intact fruit on the soil surface of field enclosures reflects fruit falling to the soil surface and is an additional method in the buried packet trial area [25,33]. The cages prevent seed loss through emigration [32]. The rapid breakdown of the fruit pulp and endocarp in this field environment enabled most seedlings to emerge within two months of placement. Emergence was linked to rainfall events and establishment may reflect the need for adequate soil moisture in the months after fruit fall, though the effects of moisture stress on germination were not assessed in this study. Although local conditions are drier in winter and spring, there was no evidence of fruit and seed persisting until the next summer wet season and larger rainfall events. While there may have been small germinant seedlings that perished before reaching a detectable size, there was no emergence from any pasture excluded plots in experiment 3. Field seedling emergence was limited to plots with ground-level vegetation and leaf litter, which was a pre-requisite for A. indica seedling emergence. Authors have observed A. indica seedlings in leaf litter under parent trees from fruit falling from the plant canopy. Seedlings that were recorded as emerged and removed may not have gone on to establish as trees, but further growth and survival data were not assessed in experiment 3. The contrasting emergence totals between the pasture cover factors were not consistently reflected in the viability of the seed retrieved from the buried packets in the same pasture treatments.
4.3. Seed Storage and Treatment
The removal of the endocarp improves germination considerably and suggested that the endocarp provides a physical barrier for water, gases, enzymes and inhibitors and for metabolism of fat [34,35]. Endocarp removal/presence had little effect in response to the ageing environment (experiment 3). However, the emergence of seedlings was lower in the shallower depths of experiment 4 when the endocarp was removed, which would support the suggestion of seed protection. Across experiments there were substantial differences in the reference (untreated) germinability of the local seed samples studied. In trial 1 the endocarp protecting the seed embryo was not removed and germination was low. Higher germination was achieved in the reference samples for trials 2, 3 and 4 with the endocarp removed. For faster and higher germination rates, tests investigating germination in controlled environments and viability of reference batches should be conducted with the endocarp removed.
Issues with A. indica seed storage are well documented [16,36,37]. The method used in experiment 3 was adapted from studying the persistence of seeds stored for conservation [23,26], and the low P50 value from controlled ageing test reflects the issues with storing fresh (undried) seed. Storage time may also have been a factor in the later and lower reference germination tests in experiments 2 and 4. Azadirachta indica is another case of comparable in situ and ex situ seed longevity, characters which are positively correlated and may have a genetic basis [21].
4.4. Weed Management Implications
The trials indicate that the chance of an A. indica seedling establishing after a year is negligible. The eradication of local infestations is more feasible with a transient soil seed bank [22] and three experiments supported an earlier anecdote [9] that seeds produced early in the calendar year over the northern Australian (summer) wet season are exhausted, and unlikely to remain viable into the next wet season. However, seed bank persistence may vary in different field situations if conditions are mild, dry, vegetated or there is fresh seed input.
Azadirachta indica has been widely planted across northern Australia and much of the risk of further invasion comes from its prolific seed production and potential dispersal. Seed sources and dispersal vectors should be considered when managing A. indica on a broader landscape scale. Even with the control of local plants, endozoochory, dispersal by animals such as frugivores and hydrochory, dispersal along riparian corridors [38] can cause re-infestation. To establish, seed must arrive in a suitable habitat and be subjected to suitable germination and emergence conditions. In a field trial, A. indica seedlings require ground cover to establish. This may contribute to the local observations of seedlings establishing at the base of other trees (presumably frugivorous dispersal) where leaf litter or grass are present. It may also limit the establishment of seedlings where seed is dispersed onto the surface of denuded ground, although seed incorporated into the top 4 cm of dampened soil (or potentially sand) may establish a seedling.
Time to maturity research [22,39] can inform local weed eradication efforts but has not been investigated under local field conditions. Observations from Brazil indicated A. indica seedlings appear five years after planting and naturalised with a third generation ten years after planting [7]. Initial fruit production occurs in three to six years [2,40]. The multiple years taken to produce fruit will exceed the time the seed can persist in the soil. This increases the feasibility of effectively controlling infestations with short-term survey and control operations [41] where fresh seed input is prevented.
5. Conclusions
A buried packet found the viability of seed of A. indica was 0.03% after one year, a viability half-life of 5.35 days in a controlled ageing environment and field seedling emergence ceased less than 8.4 months after placement. The mechanisms of seed bank depletion include a low tolerance of physical stress in fresh seed and fruit, and a higher likelihood of fatal germination from seed incorporated more than 2 cm into the soil profile. The soil seed bank of A. indica is transient, unlikely to persist more than a year, and requires shorter-term weed management plans in field situations.
Author Contributions
Different experiments were conceived and implemented by different combinations of authors. The trials were designed by S.D.C., F.F.B. and S.J.B. and were implemented by F.F.B., D.A.B. and K.L.G. Data, results and method text were validated by S.J.B., F.F.B., D.A.B. and K.L.G. Formal analysis was by S.J.B. An original manuscript for experiment 1 was written by F.F.B.; this was re-drafted with the three later trials added by S.J.B. The first author did most of the editing, but text was reviewed and edited by the other authors depending on their involvement in the trials. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
We also thank J. Scanlan and W. Vogler for reviewing the manuscript. The technical assistance of C. Crowley, K. Thorp, R. Stevenson, K. Risdale, C. Andersen and C. Warren is also acknowledged.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Experiment 1. Viability (%) of retrieved Azadirachta indica seed for each depth, clay or loam soil and pasture present or excluded at 3, 6 and 12 months after a 3-, 6- and 12-month burial. Each value is a mean of 4 replicates and 39.7% of seed viable in unburied samples. Significant 1-way and 2-way interactions are shaded grey (p < 0.05) *.
Table A1.
Experiment 1. Viability (%) of retrieved Azadirachta indica seed for each depth, clay or loam soil and pasture present or excluded at 3, 6 and 12 months after a 3-, 6- and 12-month burial. Each value is a mean of 4 replicates and 39.7% of seed viable in unburied samples. Significant 1-way and 2-way interactions are shaded grey (p < 0.05) *.
| Clay Soil | Loam Soil | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Months | Depth | Excluded | Present | Total | Excluded | Present | Total | Total | Factors * |
| 3 | 0 | 10.0 | 40.0 | 25.0 | 29.5 | 10.0 | 19.8 | 22.4 | Depth |
| 2.5 | 0.0 | 5.5 | 2.8 | 0.0 | 0.0 | 0.0 | 1.4 | Soil × pasture | |
| 10 | 0.0 | 5.0 | 2.5 | 1.0 | 0.0 | 0.5 | 1.5 | Depth × soil × pasture | |
| 20 | 0.0 | 2 | 1 | 0.0 | 0.0 | 0.0 | 0.5 | ||
| Total | 2.5 | 13.1 | 7.8 | 7.6 | 2.5 | 5.1 | 6.4 | ||
| 6 | 0 | 13.0 | 17.0 | 15.0 | 6.0 | 3.0 | 4.5 | 9.8 | Depth |
| 2.5 | 3.5 | 1.0 | 2.3 | 0.0 | 0.0 | 0.0 | 1.1 | Soil | |
| 10 | 1.5 | 0.5 | 1.0 | 0.0 | 0.0 | 0.0 | 0.5 | Depth × soil | |
| 20 | 1.5 | 0.0 | 2.8 | 0.0 | 0.0 | 0.0 | 1.4 | ||
| Total | 5.9 | 4.6 | 5.3 | 1.5 | 0.8 | 1.1 | 3.2 | ||
| 12 | 0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.5 | 0.3 | 0.1 | 1 seed |
| 2.5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||
| 10 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||
| 20 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||
| Total | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.03 |

Table A2.
Azadirachta indica seed germination at the end of the hydration phase, prior to ageing (day 0) in Experiment 2. The per box germination is the basis for comparing the relative germinability after days in the ageing environment.
Table A2.
Azadirachta indica seed germination at the end of the hydration phase, prior to ageing (day 0) in Experiment 2. The per box germination is the basis for comparing the relative germinability after days in the ageing environment.
| Experiment | Mean Germination (±Standard Error) at the End of the Hydration Phase (%) | Box A Germinable Seed Counts | Box B Germinable Seed Counts |
|---|---|---|---|
| 2.1 | 76.15 ± 10.52 | 21 of 30 | 26 of 30 |
| 2.2 | 91.83 ± 1.51 | 28 of 31 | 28 of 30 |
| 2.3 | 71.67 ± 1.67 | 22 of 30 | 21 of 30 |

Table A3.
Parameters for the response of the proportion of germinable Azadirachta indica seed over days in the ageing environment in Experiment 2. Fitted parameters for Equation 1, traced in Figure 3.
Table A3.
Parameters for the response of the proportion of germinable Azadirachta indica seed over days in the ageing environment in Experiment 2. Fitted parameters for Equation 1, traced in Figure 3.
| Parameter | Estimate | Standard Error |
|---|---|---|
| B | −0.595 | 0.110 |
| M | 5.350 | 0.418 |
| C | 0.988 | 0.0681 |
| A | −0.006 | 0.021 |

Table A4.
Means of total Azadirachta indica seedling emergence for four replicates of each soil and pasture cover type and each placement date in experiment 3. * Pasture present and excluded was significantly different (p < 0.05) across the first and both placement dates. Pooled standard errors of the means shown for pasture present treatments only.
Table A4.
Means of total Azadirachta indica seedling emergence for four replicates of each soil and pasture cover type and each placement date in experiment 3. * Pasture present and excluded was significantly different (p < 0.05) across the first and both placement dates. Pooled standard errors of the means shown for pasture present treatments only.
| Placement | Soil | Pasture Excluded | Pasture Present | Total |
|---|---|---|---|---|
| 2016 | Clay | 0.00 | 11.00 ± 4.98 | |
| Loam | 0.00 | 9.75 ± 4.98 | ||
| Total | 0.00 * | 10.38 * ± 3.52 | 5.19 ± 2.15 | |
| 2017 | Clay | 0.00 | 0.25 ± 4.98 | |
| Loam | 0.00 | 8.00 ± 4.98 | ||
| Total | 0.00 | 4.13 ± 3.52 | 2.06 ± 2.15 | |
| Combined | 0.00 * | 7.25 * ± 2.79 | 3.63 |

Table A5.
Response of the Azadirachta indica seedlings (maximum 10) emerging from different burial depths in experiment 4. Fitted parameters (±standard error) for Equation 2, traced in Figure 6b.
Table A5.
Response of the Azadirachta indica seedlings (maximum 10) emerging from different burial depths in experiment 4. Fitted parameters (±standard error) for Equation 2, traced in Figure 6b.
| Treatment | A | B | C | R2 |
|---|---|---|---|---|
| Endocarp on | 8.57 ± 0.45 | −0.0467 ± 0.033 | −0.0008 ± 0.00039 | 69.1 |
| Endocarp off | 7.03 ± 0.38 | 0.0632 ± 0.029 | −0.0019 ± 0.00033 | 57.7 |
References
- Azadirachta indica (Neem Tree)—Invasive Species Compendium. Available online: http://www.cabi.org/isc/datasheet/8112 (accessed on 22 November 2016).
- Ogbuewu, I.P.; Odoemenam, V.U.; Obikaonu, H.O.; Opara, M.N.; Emenalom, O.O.; Uchegbu, M.C.; Okoli, I.C.; Esonu, B.O.; and Iloeje, M.U. The growing importance of Neem (Azadirachta indica A. Juss.) in Agriculture, Industry, Medicine and Environment: A review. Res. J. Med. Plants 2011, 5, 230–245. [Google Scholar] [CrossRef]
- Agroforestree Database: A Tree Reference and Selection Guide Version 4.0. Azadirachta indica. Available online: https://apps.worldagroforestry.org/treedb2/speciesprofile.php?Spid=271 (accessed on 18 September 2024).
- Schmutterer, H. The Neem tree Azadirachta indica a. Juss. and Other Meliaceous Plants: Sources of Unique Natural Products for Integrated Pest Management, Medicine, Industry and Other Purposes; VCH Verlagschaft: Weinheim, Germany, 1995; 696p. [Google Scholar]
- Vietmeyer, N.D. Neem: A tree for solving global problems. In Report of an ad hoc Panel of the Board on Science and Technology for International Development, National Research Council; National Academies Press: Washington, DC, USA, 1992; 141p. [Google Scholar]
- Azadirachta indica (Neem Tree)–Region and Country Distribution and Status. csv. Available online: https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.8112#sec-12 (accessed on 28 July 2024).
- Moro, M.F.; Westerkamp, C.; Martins, F.R. Naturalization and potential impact of the exotic tree Azadirachta indica in Northeastern Brazil. Check List 2013, 9, 153–156. [Google Scholar] [CrossRef][Green Version]
- Csurhes, S.; Edwards, R. Potential Environmental Weeds in Australia: Candidate Species for Preventative Control; Environment Australia: Canberra, Australia, 1998; 140p. [Google Scholar]
- Pasfield, D. Neem—A new threat to northern rivers. In Proceedings of the 16th Australasian Weeds Conference, Cairns, Australia, 18 May 2008. [Google Scholar]
- Miller, J.; Beams, L. A magic bullet for the neem nightmare? In Proceedings of the 21st Australasian Weeds Conference, Sydney, Australia, 9 September 2018. [Google Scholar]
- Neem: Declared Pest|Agriculture and Food. Available online: https://www.agric.wa.gov.au/declared-plants/neem-declared-pest (accessed on 9 August 2024).
- Weed Management Plan for Neem 2015–2025, 2022 Revision. Available online: https://hdl.handle.net/10070/869735 (accessed on 9 August 2024).
- Atlas of Living Australia Occurrence Download for Azadirachta indica. Available online: https://doi.ala.org.au/doi/387d553d-c771-48ad-bc6d-6947dd0d784f (accessed on 20 August 2024).
- Csurhes, S. Neem Tree Azadirachta Indica—Invasive Plant Risk Assessment; Department of Agriculture and Fisheries, Queensland Government: Brisbane, Australia, 2016; 14p.
- Sacandé, M.; Groot, S.P.C.; Hoekstra, F.A.; De Castro, R.D.; Bino, R.J. Cell cycle events in developing neem seeds: Are they related to intermediate storage behaviour? Seed Sci. Res. 1997, 7, 161–168. [Google Scholar] [CrossRef]
- Ezumah, B.S. Germination and storage of neem (Azadirachta indica A. Juss.) seeds. Seed Sci. Technol. 1986, 14, 593–600. [Google Scholar]
- Sacandé, M.; Golovina, E.A.; van Aelst, A.C.; Hoekstra, F.A. Viability loss of neem (Azadirachta indica) seeds associated with membrane phase behaviour. J. Exp. Bot. 2001, 52, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Sacande, M.; van Pijlen, J.G.; de Vos, C.H.R.; Hoekstra, F.A.; Bino, R.J.; Groot, S.P.C. Intermediate storage behaviour of neem tree (Azadirachta indica) seeds from Burkina Faso. In Improved Methods for the Handling and Storage of Intermediate/Recalcitrant Tropical Forest Tree Seeds; IPGRI: Rome, Italy, 1996; pp. 101–104. [Google Scholar]
- Berjak, P.; Campbell, G.K.; Farrant, J.M.; Omondi Oloo, W.; Pammenter, N.W. Responses of seeds of Azadirachta indica (neem) to short-term storage under ambient or chilled conditions. Seed Sci. Technol. 1995, 23, 779–792. [Google Scholar]
- Sacandé, M.; Hoekstra, F.A.; van Pijlen, J.G.; Groot, S.P.C. A multifactorial study of conditions influencing longevity of neem (Azadirachta indica) seeds. Seed Sci. Res. 1998, 8, 473–482. [Google Scholar] [CrossRef]
- Bekker, R.M.; Bakker, J.P.; Ozinga, W.A.; Thompson, K. Seed traits: Essential for understanding seed longevity. Asp. Appl. Biol. 2003, 69, 1–9. [Google Scholar]
- Panetta, F.D.; Timmins, S.M. Evaluating the feasibility of eradication for terrestrial weed incursions. Plant Prot. Q 2004, 19, 5–11. [Google Scholar]
- Long, R.L.; Panetta, F.D.; Steadman, K.J.; Probert, R.; Bekker, R.; Brooks, S.J.; Adkins, S.W. Seed persistence in the field may be predicted by laboratory-controlled aging. Weed. Sci. 2008, 56, 523–528. [Google Scholar] [CrossRef]
- Moore, R.P. Handbook of Tetrazolium Testing; International Seed Testing Association: Zurich, Germany, 1985; 99p. [Google Scholar]
- Bebawi, F.F.; Campbell, S.D.; Mayer, R.J. Seed bank longevity and age to reproductive maturity of Calotropis procera (Aiton) W.T. Aiton in the dry tropics of northern Queensland. Rangel. J. 2015, 37, 239–247. [Google Scholar] [CrossRef]
- Hay, F.; Klin, K.; Probert, R.J. Can a post-harvest ripening treatment extend the longevity of Rhododendron, L. seeds? Sci. Hortic. 2006, 111, 80–83. [Google Scholar] [CrossRef]
- Brooks, S.J.; Brazier, D.A.; Warren, C. Estimating tropical weed seed longevity with a laboratory test. In Proceedings of the 2022 Australasian Weeds Conference, Adelaide, Australia, 25 September 2022. [Google Scholar]
- Panetta, F.D.; Campbell, S.; Brooks, S.; Brazier, D.; Chauhan, B.S. Germination responses of the invasive hedge cactus (Cereus uruguayanus) to environmental factors. Weed Sci. 2024, 72, 241–246. [Google Scholar] [CrossRef]
- Silo Long Paddock Point Data Set. Available online: https://www.longpaddock.qld.gov.au/silo/point-data/ (accessed on 20 July 2023).
- Thompson, K.; Bakker, J.P.; Bekker, R.M. Soil Seed Banks of NW Europe: Methodology, Density and Longevity; Cambridge University Press: Cambridge, UK, 1997; 276p. [Google Scholar]
- Vanangamudi, K.; Venkatesh, A.; Balaji, B.; Vanangamudi, M.; Rai, R.S.V. Prediction of seed storability in neem (Azadirachta indica) and jamun (Syzygium cuminii) through accelerated ageing test. J. Trop. For. Sci. 2000, 12, 270–275. [Google Scholar]
- Forcella, F. Debiting the seedbank: Priorities and predictions. Asp. Appl. Biol. 2003, 69, 151–162. [Google Scholar]
- Bebawi, F.F.; Campbell, S.D.; Mayer, R.J. Seed bank persistence and germination of chinee apple (Ziziphus mauritiana Lam.). Rangel. J. 2016, 38, 17–25. [Google Scholar] [CrossRef]
- Chaney, W.R.; Knudson, D.M. Germination of seeds of Azadirachta indica enhanced by endocarp removal. Int. Tree Crop. J. 1988, 5, 230–233. [Google Scholar]
- Radhamani, J.; Chaudhury, R.; Chandel, K.P.S. Inhibition of seed germination by the endocarp in Neem (Azadirachta indica A. Juss.). Indian J Plant Genet. Res. 1990, 3, 35–40. [Google Scholar]
- Kumar, D. Assessment of seed viability and vigour in neem (Azadirachta indica A. Juss.). J. For. Sci. 2013, 29, 282–291. [Google Scholar] [CrossRef]
- Kumar, D. Storage response of neem (Azadirachta indica A. Juss.) seed under different moisture and temperature regime. Global J. Sci. Frontier Res. 2015, 15, 7–17. [Google Scholar]
- Setter, S.D.; Setter, M.J.; Vogler, W.D. Survival of tropical weed species propagules after immersion in fresh, brackish and saltwater. In Proceedings of the 22nd Australasian Weeds Conference, Adelaide, Australia, 25 September 2022. [Google Scholar]
- Brooks, S.J.; Setter, S.D. Issues and solutions for researching weed eradication target species. In Proceedings of the 19th Australasian Weeds Conference, Hobart, Australia, 1 September 2014. [Google Scholar]
- Gupta, P.K.; Pal, B.S.; Emmanuel, C.J.S.K. Initial flowering and fruiting of neem in national provenance trials. Indian For. 1995, 121, 1063–1068. [Google Scholar]
- Cacho, O.J.; Spring, D.; Pheloung, P.; Hester, S. Evaluating the feasibility of eradicating an invasion. Biol. Invasions 2006, 8, 903–917. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).