Rapid Onset of Innate Response, Cytokine Signaling and Humoral Immunity in Inactivated LPAI-H9N2-Vaccinated Broilers
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Experimental Study and Sampling
2.3. Serum Sampling and Serological Indices of Immune Mediators
2.3.1. Phagocytic Index Assay
2.3.2. Nitric Oxide (NO)
2.3.3. Lysozyme (LYZ)
2.3.4. Interleukin-1β (IL-1β) and Interleukin-8 (IL-8)
2.3.5. Interleukin-6 (IL-6)
2.3.6. Humoral Immunity Evaluation Through Hemagglutination Inhibition Test
2.4. Gene Expression Analysis of Immune Cell Signaling (IFN-γ and TLR-21) Using Quantitative Reverse Transcriptase–Polymerase Chain Reaction (qRT-PCR)
2.5. Statistical Analysis
3. Results
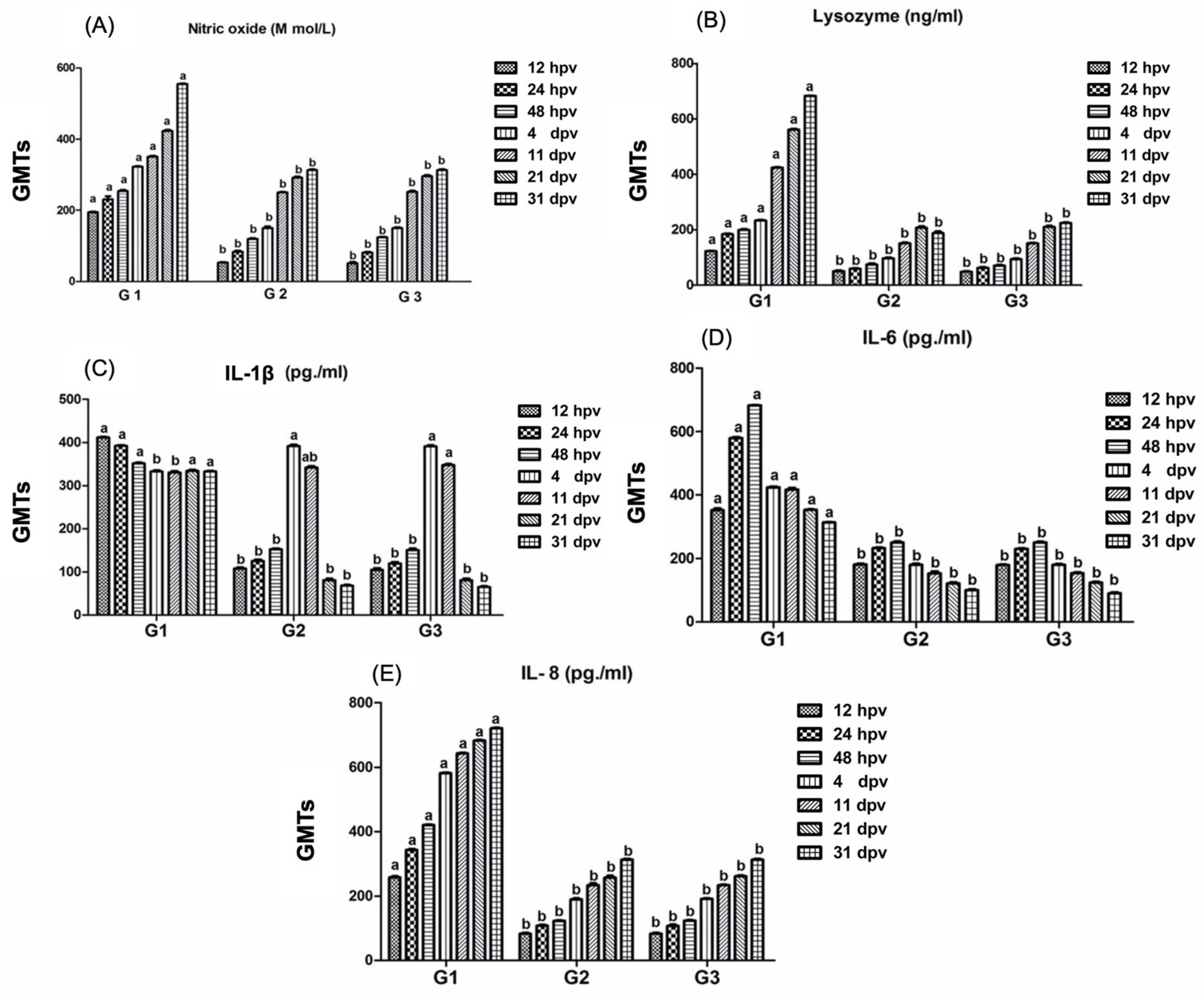
3.1. Detection of Phagocytic Activity Mediators (Nitric Oxide, Lysozyme, Interleukin-1β, Interleukin-6, Interleukin-8
3.2. Detection of Humoral Immunity Evaluation Through Hemagglutination Inhibition Test
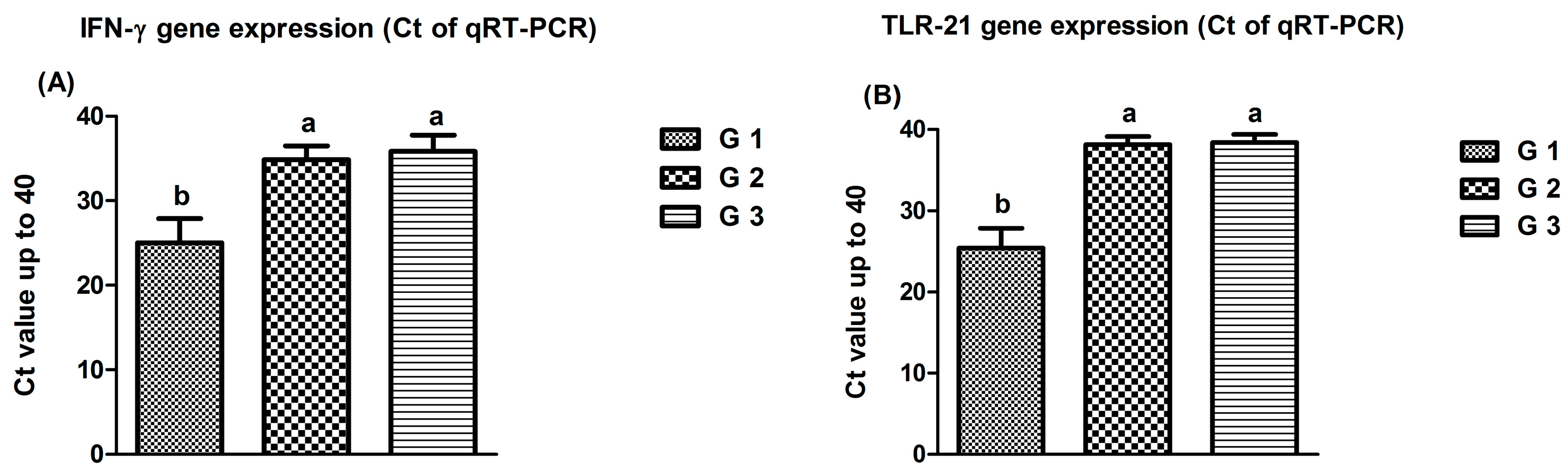
3.3. Results of Gene Expression of Immune Cells Signaling (IFN-γ and TLR-21)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iwasaki, A.; Medzhitov, R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015, 16, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R.; Janeway, C.A., Jr. Innate immunity: The virtues of a nonclonal system of recognition. Cell 1997, 91, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Signaling pathways activated by microorganisms. Curr. Opin. Cell Biol. 2007, 19, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Kogut, M.H. Issues and consequences of using nutrition to modulate the avian immune response. J. Appl. Poult. Res. 2017, 26, 605–612. [Google Scholar] [CrossRef]
- Schokker, D.; De Koning, D.-J.; Rebel, J.M.J.; Smits, M.A. Shift in chicken intestinal gene association networks after infection with Salmonella. Comp. Biochem. Physiol. Part D Genom. Proteom. 2011, 6, 339–347. [Google Scholar] [CrossRef]
- Li, X.; Zhang, P.; Jiang, X.; Du, H.; Yang, C.; Zhang, Z.; Men, S.; Zhang, Z.; Jiang, W.; Wang, H. Differences in expression of genes in the MyD88 and TRIF signaling pathways and methylation of TLR4 and TRIF in Tibetan chickens and DaHeng S03 chickens infected with Salmonella enterica serovar enteritidis. Vet. Immunol. Immunopath. 2017, 189, 28–35. [Google Scholar] [CrossRef]
- Carvajal, B.G.; Methner, U.; Pieper, J.; Berndt, A. Effects of Salmonella enterica serovar Enteritidis on cellular recruitment and cytokine gene expression in caecum of vaccinated chickens. Vaccine 2008, 26, 5423–5433. [Google Scholar] [CrossRef]
- Lee, D.H.; Fusaro, A.; Song, C.S.; Suarez, D.L.; Swayne, D.E. Poultry vaccination directed evolution of H9N2 low pathogenicity avian influenza viruses in Korea. Virology 2016, 488, 225–231. [Google Scholar] [CrossRef]
- Zhu, Y.C.; Zhang, B.; Sun, Z.H.; Wang, X.J.; Fan, X.H.; Gao, L.X.; Liang, Y.; Chen, X.Y.; Zhang, Z.F. Replication and pathology of duck influenza virus subtype H9N2 in Chukar. Biomed. Environ. Sci. 2018, 31, 306–310. [Google Scholar]
- El-Zoghby, E.F.; Arafa, A.S.; Hassan, M.K.; Aly, M.M.; Selim, A.; Kilany, W.H.; Selim, U.; Nasef, S.; Aggor, M.G.; Abdelwhab, E.M.; et al. Isolation of H9N2 avian influenza virus from bobwhite quail (Colinus virginianus) in Egypt. Arch. Virol. 2012, 157, 1167–1172. [Google Scholar] [CrossRef]
- Li, R.; Adel, A.; Bohlin, J.; Lundkvist, Å.; Olsen, B.; Pettersson, J.H.; Naguib, M.M. Phylogeographic Dynamics of Influenza A(H9N2) Virus Crossing Egypt. Front. Microbiol. 2020, 11, 392. [Google Scholar] [CrossRef] [PubMed]
- Naeem, K.; Siddique, N. Use of Strategic Vaccination for the Control of Avian Influenza in Pakistan. Dev. Biol. 2006, 124, 145–150. [Google Scholar]
- Bahari, P.; Pourbakhsh, S.A.; Shoushtari, H.; Bahmaninejad, M.A. Molecular characterization of H9N2 avian influenza viruses isolated from vaccinated broiler chickens in northeast Iran. Trop. Anim. Health Prod. 2015, 47, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Tang, Y.; Liu, X.; Peng, D.; Liu, W.; Liu, H.; Lu, S.; Liu, X. Characterization of H9N2 influenza viruses isolated from vaccinated flocks in an integrated broiler chicken operation in eastern China during a 5 year period (1998–2002). J. Gen. Virol. 2008, 89, 3102–3112. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Song, C.S. H9N2 avian influenza virus in Korea: Evolution and vaccination. Clin. Exp. Vaccine Res. 2013, 2, 26–33. [Google Scholar] [CrossRef]
- Sultan, H.A.; Abd El-Razik, A.G.; Allam, T.S.; El-Deeb, H.A. Inactivated oil emulsion H9N2 vaccine in broiler chickens: Pathogenesis and Clinicopathological studies. Appalach. Justice Res. Cent. 2015, 3, 38–53. [Google Scholar]
- Lau, S.Y.; Joseph, S.; Chan, K.H.; Chen, H.; Patteril, N.A.G.; Elizabeth, S.K.; Muhammed, R.; Baskar, V.; Lau, S.K.P.; Kinne, J. Complete Genome Sequence of Influenza Virus H9N2 Associated with a Fatal Outbreak among Chickens in Dubai. Genome Announc. 2016, 4, e00752-16. [Google Scholar] [CrossRef]
- Alqazlan, N.; Astill, J.; Raj, S.; Sharif, S. Strategies for enhancing immunity against avian influenza virus in chickens: A review. Avian Pathol. 2022, 51, 211–235. [Google Scholar] [CrossRef]
- Yang, W.; Liu, X.; Wang, X. The immune system of chicken and its response to H9N2 avian influenza virus. Vet. Q. 2023, 43, 1–14. [Google Scholar] [CrossRef]
- Bastamy, M.; Raheel, I.; Elbestawy, A.; Diab, M.; Hammad, E.; Elebeedy, L.; El-Barbary, A.M.; Albadrani, G.M.; Abdel-Daim, M.M.; Abdel-Latif, M.A.; et al. Postbiotic, anti-inflammatory, and immunomodulatory effects of aqueous microbial lysozyme in broiler chickens. Anim. Biotechnol. 2024, 35, 2309955. [Google Scholar] [CrossRef]
- Yu, Z.; Ono, C.; Aiba, S.; Kikuchi, Y.; Sora, I.; Matsuoka, H.; Tomita, H. Therapeutic concentration of lithium stimulates complement C3 production in dendritic cells and microglia via GSK-3 inhibition. Glia 2015, 63, 257–270. [Google Scholar] [CrossRef]
- Yang, M.D.; Sun, Q.; Asim, M.B.R.; Jiang, X.; Zhong, B.; Shahzad, M.; Zhang, F.; Han, Y.; Lu, S. Nitric oxide in both bronchoalveolar lavage fluid and serum is associated with pathogenesis and severity of antigen-induced pulmonary inflammation in rats. J. Asthma 2010, 47, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Bos, H.; de Souza, W. Phagocytosis of yeast: A method for concurrent quantification of binding and internalization using differential interference contrast microscopy. J. Immunol. Methods 2000, 238, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Truong, A.D.; Tran, H.T.; Nguyen, H.T.; Chu, N.T.; Hong, Y.H.; Lillehoj, H.S.; Dang, H.V.; Song, K.-D. Molecular and functional characterization of chicken interleukin 1 receptor 2 (chIL-1R2). Poult. Sci. 2023, 102, 102399. [Google Scholar] [CrossRef] [PubMed]
- Shini, S.; Shini, A.; Kaiser, P. Cytokine and chemokine gene expression profiles in heterophils from chickens treated with corticosterone. Stress 2010, 13, 185–194. [Google Scholar] [CrossRef] [PubMed]
- World Organization for Animal Health (WOAH). Avian Influenza (Including Infection with High Pathogenicity Avian Influenza Viruses). In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; OIE: Paris, France, 2021; Chapter 3.3.4. [Google Scholar]
- Adams, S.C.; Xing, Z.; Li, J.; Cardona, C.J. Immune-related gene expression in response to H11N9 low pathogenic avian influenza virus infection in chicken and Pekin duck peripheral blood mononuclear cells. Mol. Immunol. 2009, 46, 1744–1749. [Google Scholar] [CrossRef]
- Jie, H.; Lian, L.; Qu, L.J.; Zheng, J.X.; Hou, Z.C.; Xu, G.Y.; Song, J.Z.; Yang, N. Differential expression of Toll-like receptor genes in lymphoid tissues between Marek’s disease virus-infected and noninfected chickens. Poult. Sci. 2013, 92, 645–654. [Google Scholar] [CrossRef]
- Yuan, J.S.; Reed, A.; Chen, F.; Stewart, C.N., Jr. Statistical analysis of real-time PCR data. BMC Bioinform. 2006, 22, 85. [Google Scholar] [CrossRef]
- Abd El-Hamid, H.S.; Ellakany, H.F.; Hussien, H.A.; Elbestawy, A.R.; Abdel Baky, K.M. Pathogenicity of an Avian Influenza H9N2 Virus isolated from Broiler Chickens in Egypt. Alex. J. Vet. Sci. 2014, 51, 90–100. [Google Scholar] [CrossRef]
- Kandeil, A.; El-Shesheny, R.; Maatouq, A.M.; Moatasim, Y.; Shehata, M.M.; Bagato, O.; Rubrum, A.; Shanmuganatham, K.; Webby, R.J.; Ali, M.A.; et al. Genetic and antigenic evolution of H9N2 avian influenza viruses circulating in Egypt between 2011 and 2013. Arch. Virol. 2014, 159, 2861–2876. [Google Scholar] [CrossRef]
- Marinova-Petkova, A.; Shanmuganatham, K.; Feeroz, M.M.; Jones-Engel, L.; Hasan, M.K.; Akhtar, S.; Turner, J.; Walker, D.; Seiler, P.; Franks, J.; et al. The continuing evolution of H5N1 and H9N2 influenza viruses in Bangladesh between 2013 and 2014. Avian Dis. 2016, 60, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Yaqub, T.; Mukhtar, N.; Imran, M.; Ghafoor, A.; Shahid, M.F.; Yaqub, S.; Smith, G.J.D.; Su, Y.C.F.; Naeem, M. Prevalence and phylogenetics of H9n2 in backyard and commercial poultry in Pakistan. Avian Dis. 2018, 62, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Song, H.; Ye, J.; Shao, H.; Padmanabhan, R.; Sutton, T.C.; Perez, D.R. Improved hatchability and efficient protection after in ovo vaccination with live-attenuated H7N2 and H9N2 avian influenza viruses. Virol. J. 2011, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Hajam, I.A.; Kim, J.; Lee, J.H. Salmonella Gallinarum delivering M2eCD40L in protein and DNA formats acts as a bivalent vaccine against fowl typhoid and H9N2 infection in chickens. Vet. Res. 2018, 49, 99. [Google Scholar] [CrossRef]
- Xu, X.; Xue, C.; Liu, X.; Li, J.; Fei, Y.; Liu, Z.; Mu, J.; Bi, Y.; Qian, J.; Yin, R.; et al. A novel recombinant attenuated Newcastle disease virus expressing H9 subtype hemagglutinin protected chickens from challenge by genotype VII virulent Newcastle disease virus and H9N2 avian influenza virus. Vet. Microbiol. 2019, 228, 173–180. [Google Scholar] [CrossRef]
- Shrestha, A.; Sadeyen, J.-R.; Lukosaityte, D.; Chang, P.; Smith, A.; Van Hulten, M.; Iqbal, M. Selectively targeting haemagglutinin antigen to chicken CD83 receptor induces faster and stronger immunity against avian influenza. NPJ Vaccines 2021, 6, 90. [Google Scholar] [CrossRef]
- Kaiser, L.; Fritz, R.S.; Straus, S.E.; Gubareva, L.; Hayden, F.G. Symptom pathogenesis during acute influenza: Interleukin-6 and other cytokine responses. J. Med. Virol. 2001, 64, 262–268. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Innate immunity to virus infection. Immunol. Rev. 2009, 227, 75–86. [Google Scholar] [CrossRef]
- Herold, S.; von Wulffen, W.; Steinmueller, M.; Pleschka, S.; Kuziel, W.A.; Mack, M.; Srivastava, M.; Seeger, W.; Maus, U.A.; Lohmeyer, J. Alveolar epithelial cells direct monocyte transepithelial migration upon influenza virus infection: Impact of chemokines and adhesion molecules. J. Immunol. 2006, 177, 1817–1824. [Google Scholar] [CrossRef]
- Hiscott, J. Triggering the innate antiviral response through IRF-3 activation. J. Biol. Chem. 2007, 282, 15325–15329. [Google Scholar] [CrossRef]
- Pulendran, B.; Maddur, M.S. Innate immune sensing and response to influenza. In Influenza Pathogenesis and Control-Vol. II; Oldstone, M.B.A., Compans, R.W., Eds.; Springer International Publishing: Cham, Switzerland, 2012; pp. 139–157. [Google Scholar]
- Cheng, Y.; Sun, Y.; Wang, H.; Yan, Y.; Ding, C.; Sun, J. Chicken STING Mediates Activation of the IFN Gene Independently of the RIG-I Gene. J. Immunol. 2015, 195, 3922–3936. [Google Scholar] [CrossRef] [PubMed]
- Nang, N.T.; Lee, J.S.; Song, B.M.; Kang, Y.M.; Kim, H.S.; Seo, S.H. Induction of inflammatory cytokines and toll-like receptors in chickens infected with avian H9N2 influenza virus. Vet. Res. 2011, 42, 64. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tang, C.; Wang, Q.; Li, R.; Chen, Z.; Han, X.; Wang, J.; Xu, X. Apoptosis induction and release of inflammatory cytokines in the oviduct of egg-laying hens experimentally infected with H9N2 avian influenza virus. Vet. Microbiol. 2015, 12, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.M.; Alkie, T.N.; Hodgins, D.C.; Nagy, É.; Shojadoost, B.; Sharif, S. Systemic immune responses to an inactivated, whole H9N2 avian influenza virus vaccine using class B CpG oligonucleotides in chickens. Vaccine 2015, 33, 3947–3952. [Google Scholar] [CrossRef] [PubMed]
- Raj, S.; Alizadeh, M.; Shoojadoost, B.; Hodgins, D.; Nagy, É.; Mubareka, S.; Karimi, K.; Behboudi, S.; Sharif, S. Determining the Protective Efficacy of Toll-Like Receptor Ligands to Minimize H9N2 Avian Influenza Virus Transmission in Chickens. Viruses 2023, 15, 238. [Google Scholar] [CrossRef]
- Sharma, J.M.; Tizard, I. Avian cellular immune effector mechanisms—A review. Avian Pathol. 1984, 13, 357–376. [Google Scholar] [CrossRef]
- Gado, H.A.; Ghanem, I.A.; Selim, A.A.; Elsafty, M.M.; Soliman, R.A.; Eid, A.A.M. Efficacy of commercial vaccines against H9N2 avian influenza challenge in chickens. Adv. Anim. Vet. Sci. 2022, 10, 35–48. [Google Scholar] [CrossRef]
- Pan, X.; Su, X.; Ding, P.; Zhao, J.; Cui, H.; Yan, D.; Teng, Q.; Li, X.; Beerens, N.; Zhang, H.; et al. Maternal-derived antibodies hinder the antibody response to H9N2 AIV inactivated vaccine in the field. Anim. Dis. 2022, 2, 9. [Google Scholar] [CrossRef]
- Ibrahim, H.H.; Seioudy, M. Comparison of the efficacy of local and imported inactivated combined H9-ND virus vaccines in protection of broiler flocks against H9N2 infection in Egypt. Benha Vet. Med. J. 2020, 83, 52–56. [Google Scholar] [CrossRef]
- Lee, D.; Kwon, J.S.; Lee, H.J.; Lee, Y.N.; Hur, W.; Hong, Y.H.; Lee, J.B.; Park, S.Y.; Choi, I.S.; Song, C.S. Inactivated H9N2 avian influenza virus vaccine with gel-primed and mineral oil boosted regimen could produce improved immune response in broiler breeders. Poult. Sci. 2011, 90, 1020–1022. [Google Scholar] [CrossRef]
- Elbestawy, A.; Ellakany, H.; Sedeik, M.; Gado, A.; Abdel-Latif, M.; Noreldin, A.; Orabi, A.; Radwan, I.; El-Ghany, W.A. Superior Efficacy of Apathogenic Genotype I (V4) over Lentogenic Genotype II (LaSota) Live Vaccines against Newcastle Disease Virus Genotype VII.1.1 in Pathogen-Associated Molecular Pattern-H9N2 Vaccinated Broiler Chickens. Vaccines 2023, 11, 1638. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raheel, I.A.; Elbestawy, A.R.; Diab, M.S.; Abdel-Latif, M.A.; Tag, N.; Orabi, A. Rapid Onset of Innate Response, Cytokine Signaling and Humoral Immunity in Inactivated LPAI-H9N2-Vaccinated Broilers. Poultry 2024, 3, 420-429. https://doi.org/10.3390/poultry3040032
Raheel IA, Elbestawy AR, Diab MS, Abdel-Latif MA, Tag N, Orabi A. Rapid Onset of Innate Response, Cytokine Signaling and Humoral Immunity in Inactivated LPAI-H9N2-Vaccinated Broilers. Poultry. 2024; 3(4):420-429. https://doi.org/10.3390/poultry3040032
Chicago/Turabian StyleRaheel, Ismail A., Ahmed R. Elbestawy, Mohamed S. Diab, Mervat A. Abdel-Latif, Nehal Tag, and Ahmed Orabi. 2024. "Rapid Onset of Innate Response, Cytokine Signaling and Humoral Immunity in Inactivated LPAI-H9N2-Vaccinated Broilers" Poultry 3, no. 4: 420-429. https://doi.org/10.3390/poultry3040032
APA StyleRaheel, I. A., Elbestawy, A. R., Diab, M. S., Abdel-Latif, M. A., Tag, N., & Orabi, A. (2024). Rapid Onset of Innate Response, Cytokine Signaling and Humoral Immunity in Inactivated LPAI-H9N2-Vaccinated Broilers. Poultry, 3(4), 420-429. https://doi.org/10.3390/poultry3040032









