Interaction of Chicken Heterophils and Eimeria tenella Results in Different Phenotypes of Heterophil Extracellular Traps (HETs)
Abstract
1. Introduction
2. Materials and Methods
2.1. Parasite Maintenance and Excystation
2.2. Purification of Poultry Heterophils
2.3. Live Cell Imaging of Eimeria tenella Sporozoite-Heterophil Interactions Using 3D-Holotomographic Microscopy
2.4. Characterization of Eimeria tenella-Triggered HET Formation
2.5. Quantification of Extracellular Heterophil DNA
2.6. Statistical Analysis
3. Results
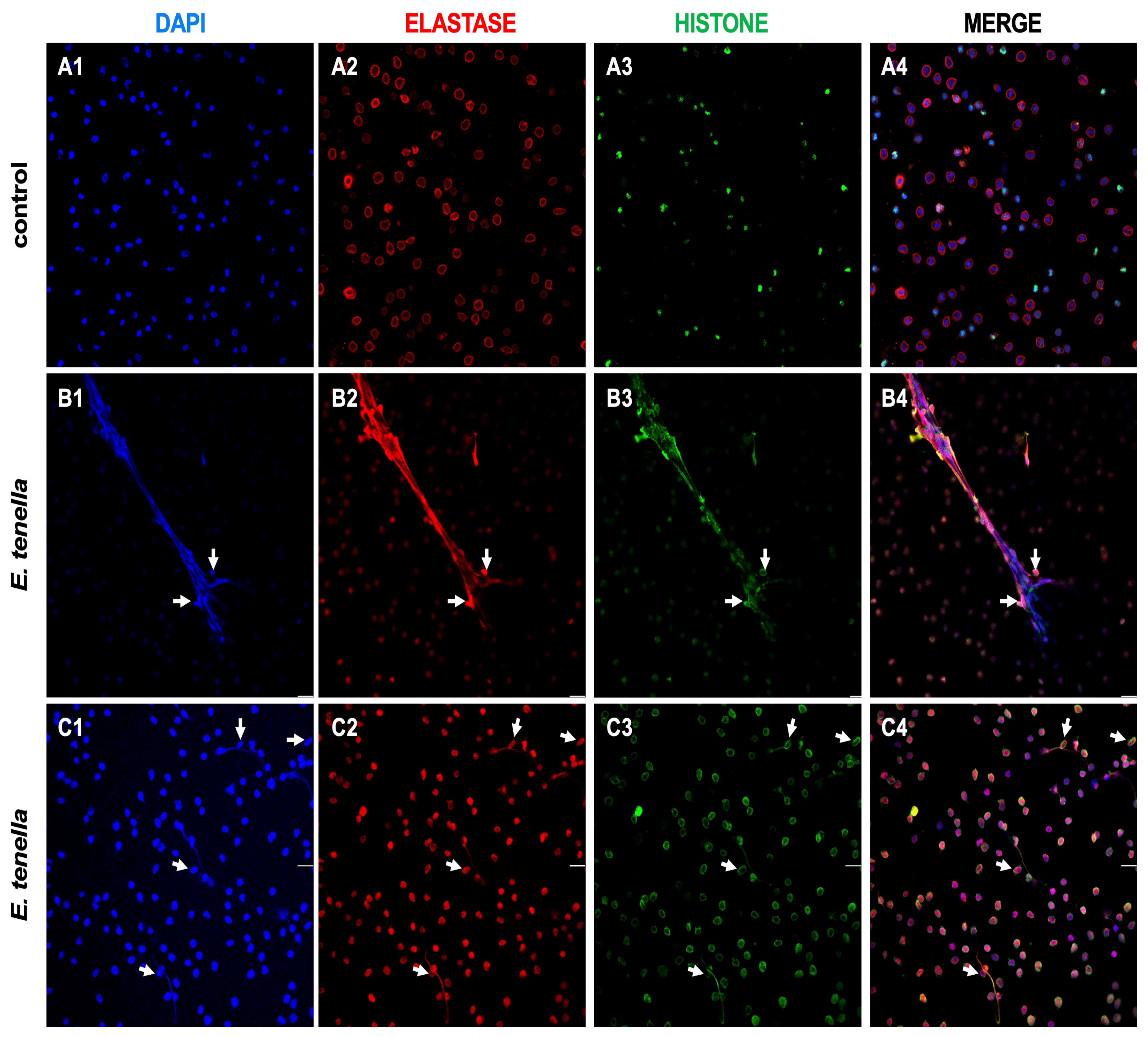
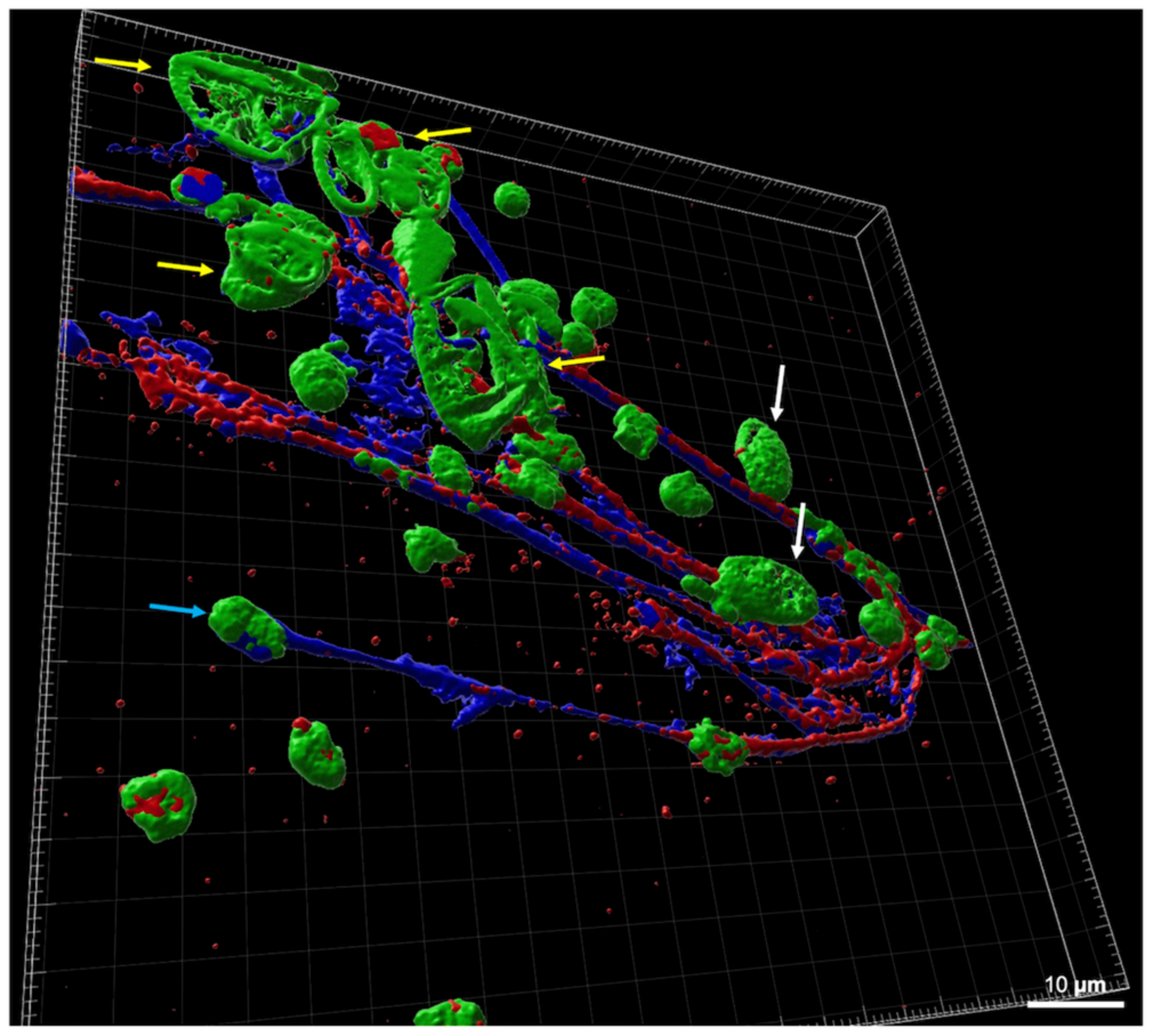
3.1. Visualization of Eimeria tenella–Heterophils Interactions and HET Formation
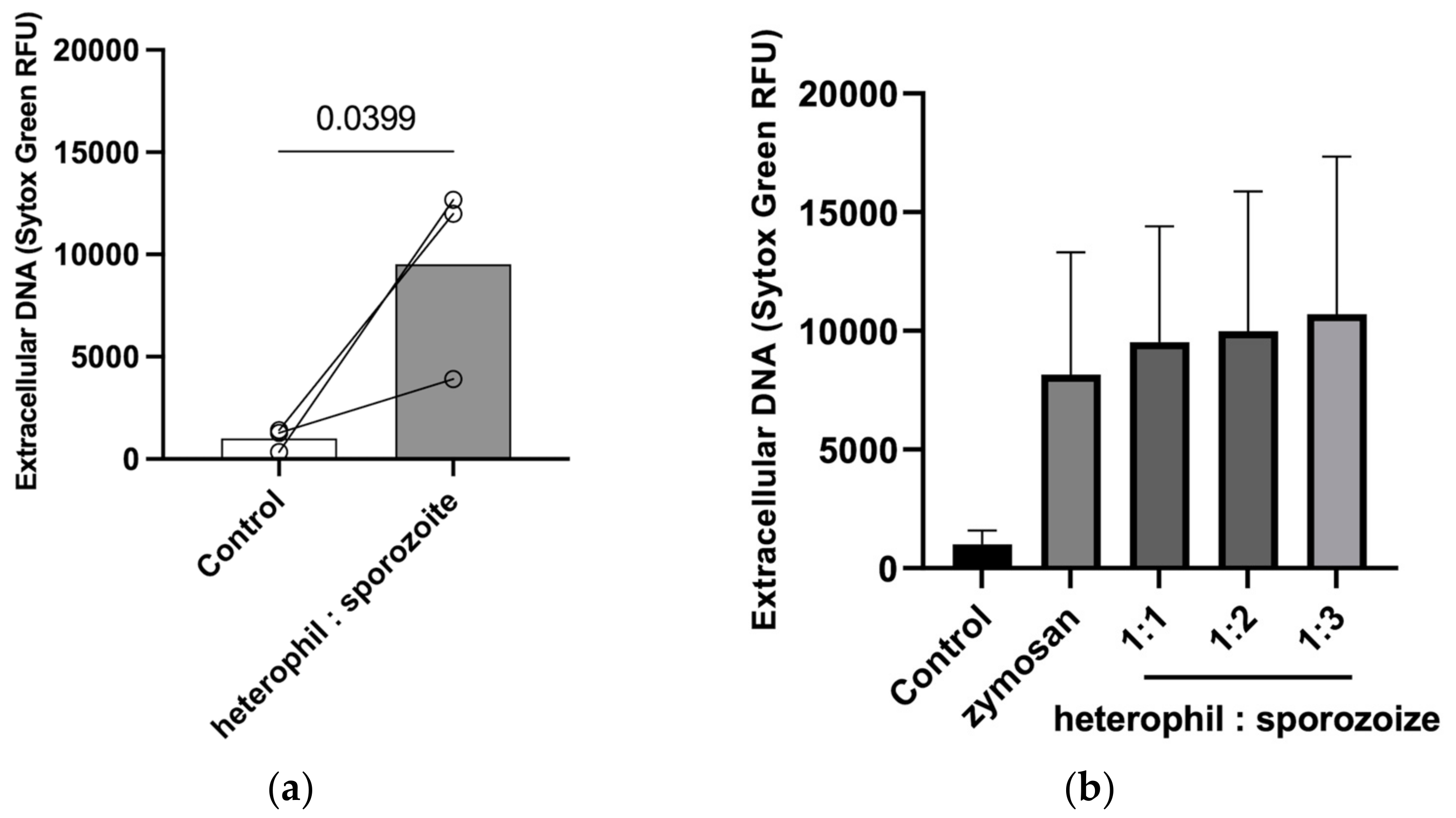
3.2. Quantification of Eimeria tenella-Triggered HETs
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blake, D.P.; Knox, J.; Dehaeck, B.; Huntington, B.; Rathinam, T.; Ravipati, V.; Ayoade, S.; Gilbert, W.; Adebambo, A.O.; Jatau, I.D.; et al. Re-Calculating the Cost of Coccidiosis in Chickens. Vet. Res. 2020, 51, 115. [Google Scholar] [CrossRef]
- Shirley, M.W.; Smith, A.L.; Tomley, F.M. The Biology of Avian Eimeria with an Emphasis on Their Control by Vaccination. In Advances in Parasitology; Elsevier: Amsterdam, The Netherlands, 2005; Volume 60, pp. 285–330. ISBN 978-0-12-031760-8. [Google Scholar]
- Deplazes, P.; Eckert, J.; Mathis, A.; von Samson-Himmelstjerna, G.; Zahner, H. Parasitology in Veterinary Medicine; Wageningen Academic Publishers: Wageningen, The Netherlands, 2016. [Google Scholar]
- López-Osorio, S.; Chaparro-Gutiérrez, J.J.; Gómez-Osorio, L.M. Overview of Poultry Eimeria Life Cycle and Host-Parasite Interactions. Front. Vet. Sci. 2020, 7, 384. [Google Scholar] [CrossRef]
- Blake, D.P.; Vrba, V.; Xia, D.; Jatau, I.D.; Spiro, S.; Nolan, M.J.; Underwood, G.; Tomley, F.M. Genetic and Biological Characterisation of Three Cryptic Eimeria Operational Taxonomic Units That Infect Chickens (Gallus gallus Domesticus). Int. J. Parasitol. 2021, 51, 621–634. [Google Scholar] [CrossRef]
- Tadesse, C.; Feyissa, B.D. Poultry Coccidiosis: Prevalence and Associated Risk Factors in Extensive and Intensive Farming Systems in Jimma Town, Jimma, Ethiopia. J. Vet. Med. Anim. Health 2016, 8, 223–227. [Google Scholar] [CrossRef][Green Version]
- Blake, D.P.; Marugan-Hernandez, V.; Tomley, F.M. Spotlight on Avian Pathology: Eimeria and the Disease Coccidiosis. Avian Pathol. 2021, 50, 209–213. [Google Scholar] [CrossRef]
- Badri, M.; Olfatifar, M.; Hayati, A.; Bijani, B.; Samimi, R.; Abdoli, A.; Nowak, O.; Diaz, D.; Eslahi, A.V. The Global Prevalence and Associated Risk Factors of Eimeria Infection in Domestic Chickens: A Systematic Review and Meta-analysis. Vet. Med. Sci. 2024, 10, e1469. [Google Scholar] [CrossRef]
- Macdonald, S.E.; Nolan, M.J.; Harman, K.; Boulton, K.; Hume, D.A.; Tomley, F.M.; Stabler, R.A.; Blake, D.P. Effects of Eimeria tenella Infection on Chicken Caecal Microbiome Diversity, Exploring Variation Associated with Severity of Pathology. PLoS ONE 2017, 12, e0184890. [Google Scholar] [CrossRef] [PubMed]
- Blake, D.P.; Tomley, F.M. Securing Poultry Production from the Ever-Present Eimeria Challenge. Trends Parasitol. 2014, 30, 12–19. [Google Scholar] [CrossRef]
- Boulton, K.; Nolan, M.J.; Wu, Z.; Riggio, V.; Matika, O.; Harman, K.; Hocking, P.M.; Bumstead, N.; Hesketh, P.; Archer, A.; et al. Dissecting the Genomic Architecture of Resistance to Eimeria Maxima Parasitism in the Chicken. Front. Genet. 2018, 9, 528. [Google Scholar] [CrossRef]
- Chapman, H.D. Biochemical, Genetic and Applied Aspects of Drug Resistance in Eimeria Parasites of the Fowl. Avian Pathol. 1997, 26, 221–244. [Google Scholar] [CrossRef] [PubMed]
- Heams, T.; Bed’hom, B.; Rebours, E.; Jaffrezic, F.; Pinard-van Der Laan, M.-H. Insights into Gene Expression Profiling of Natural Resistance to Coccidiosis in Contrasting Chicken Lines. BMC Proc. 2011, 5, S26. [Google Scholar] [CrossRef]
- Chuammitri, P.; Ostojić, J.; Andreasen, C.B.; Redmond, S.B.; Lamont, S.J.; Palić, D. Chicken Heterophil Extracellular Traps (HETs): Novel Defense Mechanism of Chicken Heterophils. Vet. Immunol. Immunopathol. 2009, 129, 126–131. [Google Scholar] [CrossRef]
- Swaggerty, C.L.; Ferro, P.J.; Pevzner, I.Y.; Kogut, M.H. Heterophils Are Associated with Resistance to Systemic Salmonella enteritidis Infections in Genetically Distinct Chicken Lines. FEMS Immunol. Med. Microbiol. 2005, 43, 149–154. [Google Scholar] [CrossRef]
- Silva, L.M.R.; Muñoz-Caro, T.; Burgos, R.A.; Hidalgo, M.A.; Taubert, A.; Hermosilla, C. Far beyond Phagocytosis: Phagocyte-Derived Extracellular Traps Act Efficiently against Protozoan Parasites In Vitro and In Vivo. Mediat. Inflamm. 2016, 2016, 1–13. [Google Scholar] [CrossRef]
- Ramos-Martínez, E.; Hernández-González, L.; Ramos-Martínez, I.; Pérez-Campos Mayoral, L.; López-Cortés, G.I.; Pérez-Campos, E.; Mayoral Andrade, G.; Hernández-Huerta, M.T.; José, M.V. Multiple Origins of Extracellular DNA Traps. Front. Immunol. 2021, 12, 621311. [Google Scholar] [CrossRef]
- Omar, M.; Abdelal, H. NETosis in Parasitic Infections: A Puzzle That Remains Unsolved. Int. J. Mol. Sci. 2023, 24, 8975. [Google Scholar] [CrossRef]
- Schauer, C.; Janko, C.; Munoz, L.E.; Zhao, Y.; Kienhöfer, D.; Frey, B.; Lell, M.; Manger, B.; Rech, J.; Naschberger, E.; et al. Aggregated Neutrophil Extracellular Traps Limit Inflammation by Degrading Cytokines and Chemokines. Nat. Med. 2014, 20, 511–517. [Google Scholar] [CrossRef]
- Salinas, C.; Barriga, K.; Albornoz, A.; Alarcon, P.; Quiroga, J.; Uberti, B.; Sarmiento, J.; Henriquez, C.; Ehrenfeld, P.; Burgos, R.A.; et al. Tamoxifen Triggers the In Vitro Release of Neutrophil Extracellular Traps in Healthy Horses. Front. Vet. Sci. 2023, 9, 1025249. [Google Scholar] [CrossRef]
- Muñoz-Caro, T.; Conejeros, I.; Zhou, E.; Pikhovych, A.; Gärtner, U.; Hermosilla, C.; Kulke, D.; Taubert, A. Dirofilaria Immitis Microfilariae and Third-Stage Larvae Induce Canine NETosis Resulting in Different Types of Neutrophil Extracellular Traps. Front. Immunol. 2018, 9, 968. [Google Scholar] [CrossRef]
- Pieper, J.; Locke, M.; Ruzaike, G.; Voigt, S.; Methner, U.; Berndt, A. In Vitro and in Vivo Generation of Heterophil Extracellular Traps after Salmonella Exposure. Vet. Immunol. Immunopathol. 2017, 188, 1–11. [Google Scholar] [CrossRef]
- Wu, D.; Li, S.; Li, P.; Jiang, A.; Liu, Z.; Zhang, Y.; Wang, J.; Yang, Z.; Wei, Z. Diacetoxyscirpenol-Induced Heterophil Extracellular Traps Contribute to the Immune Toxicity of Liver Injury in Chickens. Food Chem. Toxicol. 2021, 148, 111926. [Google Scholar] [CrossRef]
- Wei, Z.; Zhao, Y.; Zhang, N.; Han, Z.; Liu, X.; Jiang, A.; Zhang, Y.; Wang, C.; Gong, P.; Li, J.; et al. Eimeria tenella Induces the Release of Chicken Heterophil Extracellular Traps. Vet. Parasitol. 2019, 275, 108931. [Google Scholar] [CrossRef]
- Han, Z.; Zhang, Y.; Wang, C.; Liu, X.; Jiang, A.; Liu, Z.; Wang, J.; Yang, Z.; Wei, Z. Ochratoxin A-Triggered Chicken Heterophil Extracellular Traps Release through Reactive Oxygen Species Production Dependent on Activation of NADPH Oxidase, ERK, and P38 MAPK Signaling Pathways. J. Agric. Food Chem. 2019, 67, 11230–11235. [Google Scholar] [CrossRef]
- Rentería-Solís, Z.; Zhang, R.; Taha, S.; Daugschies, A. A Modified Method for Purification of Eimeria tenella Sporozoites. Parasitol. Res. 2020, 119, 1429–1432. [Google Scholar] [CrossRef]
- Andreasen, C.B.; Frank, D.E. The Effects of Ascorbic Acid on in Vitro Heterophil Function. Avian Dis. 1999, 43, 656–663. [Google Scholar] [CrossRef]
- Kogut, M.H.; Genovese, K.J.; Lowry, V.K. Differential Activation of Signal Transduction Pathways Mediating Phagocytosis, Oxidative Burst, and Degranulation by Chicken Heterophils in Response to Stimulation with Opsonized Salmonella enteritidis. Inflammation 2001, 25, 7–15. [Google Scholar] [CrossRef]
- He, H.; Farnell, M.B.; Kogut, M.H. Inflammatory Agonist Stimulation and Signal Pathway of Oxidative Burst in Neonatal Chicken Heterophils. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2003, 135, 177–184. [Google Scholar] [CrossRef]
- Jiang, A.; Zhang, Y.; Wu, D.; Li, S.; Liu, Z.; Yang, Z.; Wei, Z. Sodium Molybdate Induces Heterophil Extracellular Traps Formation in Chicken. Ecotoxicol. Environ. Saf. 2021, 210, 111886. [Google Scholar] [CrossRef]
- Nascimento, M.T.C.; Silva, K.P.; Garcia, M.C.F.; Medeiros, M.N.; Machado, E.A.; Nascimento, S.B.; Saraiva, E.M. DNA Extracellular Traps Are Part of the Immune Repertoire of Periplaneta americana. Dev. Comp. Immunol. 2018, 84, 62–70. [Google Scholar] [CrossRef]
- Branzk, N.; Lubojemska, A.; Hardison, S.E.; Wang, Q.; Gutierrez, M.G.; Brown, G.D.; Papayannopoulos, V. Neutrophils Sense Microbe Size and Selectively Release Neutrophil Extracellular Traps in Response to Large Pathogens. Nat. Immunol. 2014, 15, 1017–1025. [Google Scholar] [CrossRef]
- Ehrens, A.; Lenz, B.; Neumann, A.-L.; Giarrizzo, S.; Reichwald, J.J.; Frohberger, S.J.; Stamminger, W.; Buerfent, B.C.; Fercoq, F.; Martin, C.; et al. Microfilariae Trigger Eosinophil Extracellular DNA Traps in a Dectin-1-Dependent Manner. Cell Rep. 2021, 34, 108621. [Google Scholar] [CrossRef] [PubMed]
- Abi Abdallah, D.S.; Lin, C.; Ball, C.J.; King, M.R.; Duhamel, G.E.; Denkers, E.Y. Toxoplasma gondii Triggers Release of Human and Mouse Neutrophil Extracellular Traps. Infect. Immun. 2012, 80, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, C.; McMaster, W.R.; Girard, D.; Descoteaux, A. Leishmania donovani Promastigotes Evade the Antimicrobial Activity of Neutrophil Extracellular Traps. J. Immunol. 2010, 185, 4319–4327. [Google Scholar] [CrossRef]
- Morrissette, N.S.; Sibley, L.D. Cytoskeleton of Apicomplexan Parasites. Microbiol. Mol. Biol. Rev. 2002, 66, 21–38. [Google Scholar] [CrossRef]
- Striepen, B.; Jordan, C.N.; Reiff, S.; Van Dooren, G.G. Building the Perfect Parasite: Cell Division in Apicomplexa. PLoS Pathog. 2007, 3, e78. [Google Scholar] [CrossRef]
- Knopf, J.; Leppkes, M.; Schett, G.; Herrmann, M.; Muñoz, L.E. Aggregated NETs Sequester and Detoxify Extracellular Histones. Front. Immunol. 2019, 10, 2176. [Google Scholar] [CrossRef] [PubMed]
- Neumann, A.; Brogden, G.; Von Köckritz-Blickwede, M. Extracellular Traps: An Ancient Weapon of Multiple Kingdoms. Biology 2020, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Ševčíková, Z.; Asheg, A.A.; Kolodzieyski, L.; Cigánková, V.; Komorová, T.; Levkut, M. Heterophils and Macrophage-like Cells in the Caeca Chicks’ Caeca after Experimental Infection with Salmonella enteritidis PT4. Acta Vet. Brno 2003, 72, 565–570. [Google Scholar] [CrossRef][Green Version]
- Rada, B. Neutrophil Extracellular Traps. In NADPH Oxidases; Knaus, U.G., Leto, T.L., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2019; Volume 1982, pp. 517–528. ISBN 978-1-4939-9423-6. [Google Scholar]
- Yildiz, K.; Gokpinar, S.; Gazyagci, A.N.; Babur, C.; Sursal, N.; Azkur, A.K. Role of NETs in the Difference in Host Susceptibility to Toxoplasma gondii between Sheep and Cattle. Vet. Immunol. Immunopathol. 2017, 189, 1–10. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rentería-Solís, Z.; Silva, L.M.R.; Grochow, T.; Zhang, R.; Nguyen-Ho-Bao, T.; Daugschies, A.; Taubert, A.; Conejeros, I.; Hermosilla, C. Interaction of Chicken Heterophils and Eimeria tenella Results in Different Phenotypes of Heterophil Extracellular Traps (HETs). Poultry 2024, 3, 318-329. https://doi.org/10.3390/poultry3030024
Rentería-Solís Z, Silva LMR, Grochow T, Zhang R, Nguyen-Ho-Bao T, Daugschies A, Taubert A, Conejeros I, Hermosilla C. Interaction of Chicken Heterophils and Eimeria tenella Results in Different Phenotypes of Heterophil Extracellular Traps (HETs). Poultry. 2024; 3(3):318-329. https://doi.org/10.3390/poultry3030024
Chicago/Turabian StyleRentería-Solís, Zaida, Liliana M. R. Silva, Thomas Grochow, Runhui Zhang, Tran Nguyen-Ho-Bao, Arwid Daugschies, Anja Taubert, Iván Conejeros, and Carlos Hermosilla. 2024. "Interaction of Chicken Heterophils and Eimeria tenella Results in Different Phenotypes of Heterophil Extracellular Traps (HETs)" Poultry 3, no. 3: 318-329. https://doi.org/10.3390/poultry3030024
APA StyleRentería-Solís, Z., Silva, L. M. R., Grochow, T., Zhang, R., Nguyen-Ho-Bao, T., Daugschies, A., Taubert, A., Conejeros, I., & Hermosilla, C. (2024). Interaction of Chicken Heterophils and Eimeria tenella Results in Different Phenotypes of Heterophil Extracellular Traps (HETs). Poultry, 3(3), 318-329. https://doi.org/10.3390/poultry3030024







