Abstract
In cell biology, the stem cell niche is the dynamic microenvironment in which stem cells reside and receive signals that determine their behavior and fate. The stem cell niche has largely been a theoretical construct due to the difficulty in identifying and manipulating individual stem cells and their surroundings. Recent technical advances have made it possible to characterize the niches that maintain and control stem cell activity in several organs, including the small intestine. Although the small intestine has a relatively simple architecture, it has an extraordinary capacity for fast self-renewal. Thus, the organ is a unique model for studying intestinal stem cells (ISCs) and their niche. The intestinal epithelium maintains the intestine, enabling it to perform its absorption, secretion, and barrier functions. ISCs reside at the base of crypts adjacent to Paneth cells. In vivo, ISCs are surrounded by the microenvironment that makes up the niche, which provides a variety of stimuli that determine the fate of the cells. Research on stem cell niches is beginning to deepen our understanding of ISC regulation at the cellular and molecular levels and is expected to provide insights that can be applied to ISC therapy. Intestinal organoids originate from a group of crypt base ISCs. These organoids possess a three-dimensional (3D) cell structure made up of the lumen facing inward. Therefore, 3D intestinal organoids are often digested and seeded in a two-dimensional (2D) manner to form confluent organoid monolayers. Here, we not only review our current understanding of ISC niches with a focus on systems that are well-characterized at the cellular and mechanistic levels, but we also summarize the current applications of intestinal organoids.
1. Introduction
The epithelia of the small and large intestines of mammals are extremely versatile; they have important roles in food digestion, nutrient uptake, immune modulation, and the front-line defense against pathogenic bacteria and toxins in the lumen. To maintain the epithelial integrity of these organs, under healthy conditions, intestinal epithelial cells (IECs) are continuously replaced by proliferating and undifferentiated progenitors that are derived from multipotent intestinal stem cells (ISCs) with a turnover time of 3 to 5 days. The epithelium of the small intestine is organized into two morphologically and functionally distinct structures: villus and crypt (Figure 1A). During early embryonic development (E14.5), a sheet of epithelium thickens to form the beginning of the intestinal epithelium [1]. Subsequently, the sheet invaginates to create crypts, and signals from the niche enter the crypts to support the self-renewal of cells and to prevent the differentiation of the proliferative stem cells in the compartment [1]. The ISC niche is the dynamic microenvironment in which ISCs reside and receive signals that determine their behavior and fate; it comprises the mesenchymal cell niche and the epithelial cell niche.
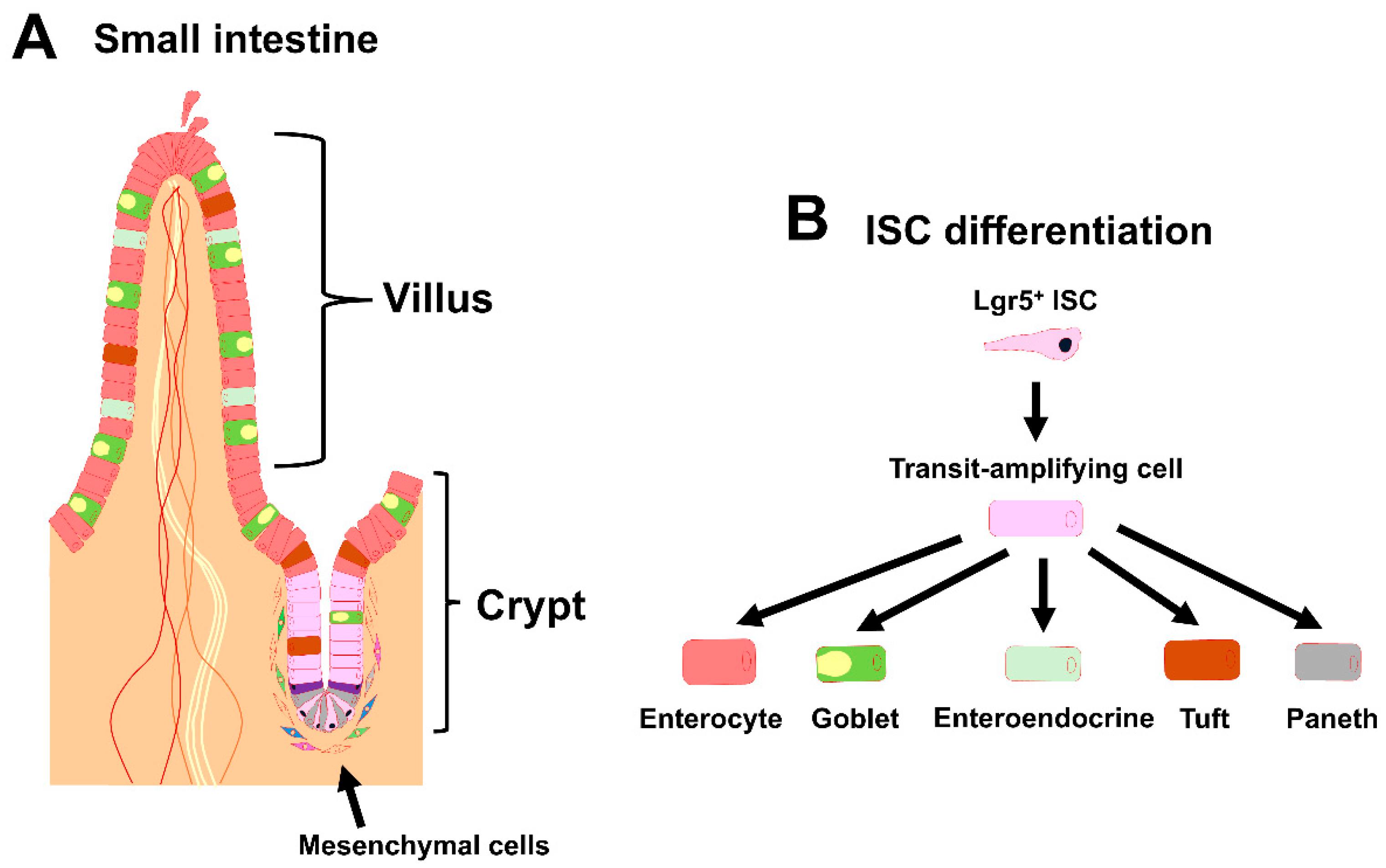
Figure 1.
Overview of the intestinal stem cell (ISC) niche. (A) Schematic representation of the small intestinal crypt–villus axis and its surrounding mesenchymal cells. ISCs are found at the base of the crypt. Neighboring niche cells include Paneth cells and mesenchymal cells. (B) Schematic representation of ISC differentiation in the small intestine.
In early studies, the ISCs in the adult small intestine were meticulously defined based on their columnar morphology and multipotency [2]. At the appropriate time, leucine-rich repeat-containing G-protein-coupled receptor (Lgr5) is expressed by ISCs, and Lgr5 is, thus, considered to be a specific marker of the compartment [3]. Once Lgr5+ ISCs exit the compartment, they rapidly give rise to highly proliferative and short-lived progenitors in the transit-amplifying (TA) zone located in the upper crypts. Lgr5 is necessary to ensure proper cell turnover and to confine cell division to crypts to avoid malignant overgrowth. After exiting the TA zone, the progenitor cells enter the post-mitotic phase and differentiate into various mature IEC lineages that have a variety of intestinal homeostatic functions [4,5]. The cell lineages include absorptive enterocytes, mucus-secreting goblet cells, hormone-producing enteroendocrine cells, chemosensory tuft cells, and Paneth cells (Figure 1B), and they are all involved in the immune response against infections by pathogens and parasites [6,7,8]. These cells migrate toward the villi, except for the terminally differentiated Paneth cells, which migrate toward the crypt base in the small intestine and produce anti-microbial agents, including cryptidin, as well as niche factors for supporting ISCs [9]. In addition to Lgr5+ ISCs, the existence of functionally distinct populations of ISCs has long been a hotly debated topic with many research groups dedicating themselves to this question [10,11,12,13,14,15,16,17]. The highly proliferative Lgr5+ ISCs can also be divided into sub-populations [11].
It is generally considered that adult somatic cells cannot be cultured for a long time without senescence or transformation. Organoids derived from small intestinal crypts were first reported in 2009 [18] as powerful tools for studying intestinal development and diseases. The organoids are three-dimensional (3D) mini guts with intact crypt and villus structural domains. The organoids can be cultured from a crypt or a single Lgr5+ ISC in R-spondin-based 3D cultures [18]. The proliferation of adult intestinal organoids is restricted to the crypt structural domain, and the crypts of proliferating organoids extend outward to form branching structures in a process known as budding.
Single-cell RNA sequencing (scRNA-seq) profiles can provide a coherent view of the compartmental and functional anatomy of the small intestine [19,20,21,22,23], enabling better understanding of the precise anatomical and cell population structures. Subpopulations and novel cellular entities can now be molecularly defined and identified based on differences or similarities in gene expression profiles [24,25,26,27,28,29], providing an unprecedented resolution of the cellular makeup of the intestinal epithelium. It has been established that many cell types share the ability to re-acquire stem cell potential under challenging conditions and functionally regenerate the Lgr5+ stem cell pool. In this review, we describe the mechanistic principles and key players involved in intestinal homeostasis.
2. The Intestinal Stem Cell Niche
2.1. The Mesenchymal Niche
In the past, it was believed that it is impossible to establish long-term cultures from primary adult tissues without any genetic transformation [30]. However, in 2009, Sato and colleagues established an in vitro culture system for growing crypt–villus organoids in the absence of a mesenchymal niche [18]; this system has since been used extensively in stem cell research.
The intestinal mesenchymal niche includes a variety of cell types located beneath crypts that act as a critical source of signaling molecules that are needed to maintain ISCs. It is well-known that canonical Wnt/β-catenin signaling is indispensable for the ISC’s self-renewal [31]. Genetic and pharmacological evidence indicates that canonical Wnt/beta-catenin signaling is a pivotal role for intestinal homeostasis and ISC proliferation [32,33,34,35,36]. Non-canonical members of the Wnt family, including Wnt4, Wnt5a, and Wnt5b, are also expressed in mesenchymal cells in the intestine [37]. Additionally, R-spondin (Rspo) proteins, which are the ligands for Lgr5, are also expressed in mesenchymal cells [38].
Mesenchymal cells are heterogeneous, and specific subsets of mesenchymal cells exert niche functions. The subtypes of mesenchymal cells can be determined from single-cell RNA profiles according to their activities in regulating ISCs. Together, the different mesenchymal cell subtypes contribute to homeostasis and regeneration. Several representative mesenchymal cell subtypes that can be identified by marker genes, and their contributions to ISC regulation, are described below (Figure 2).
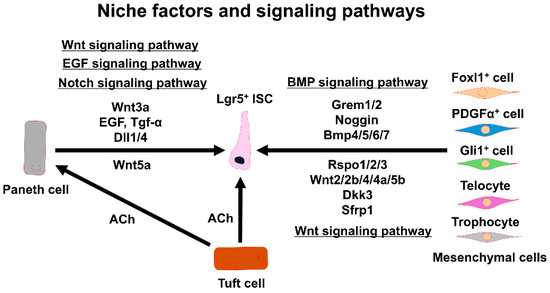
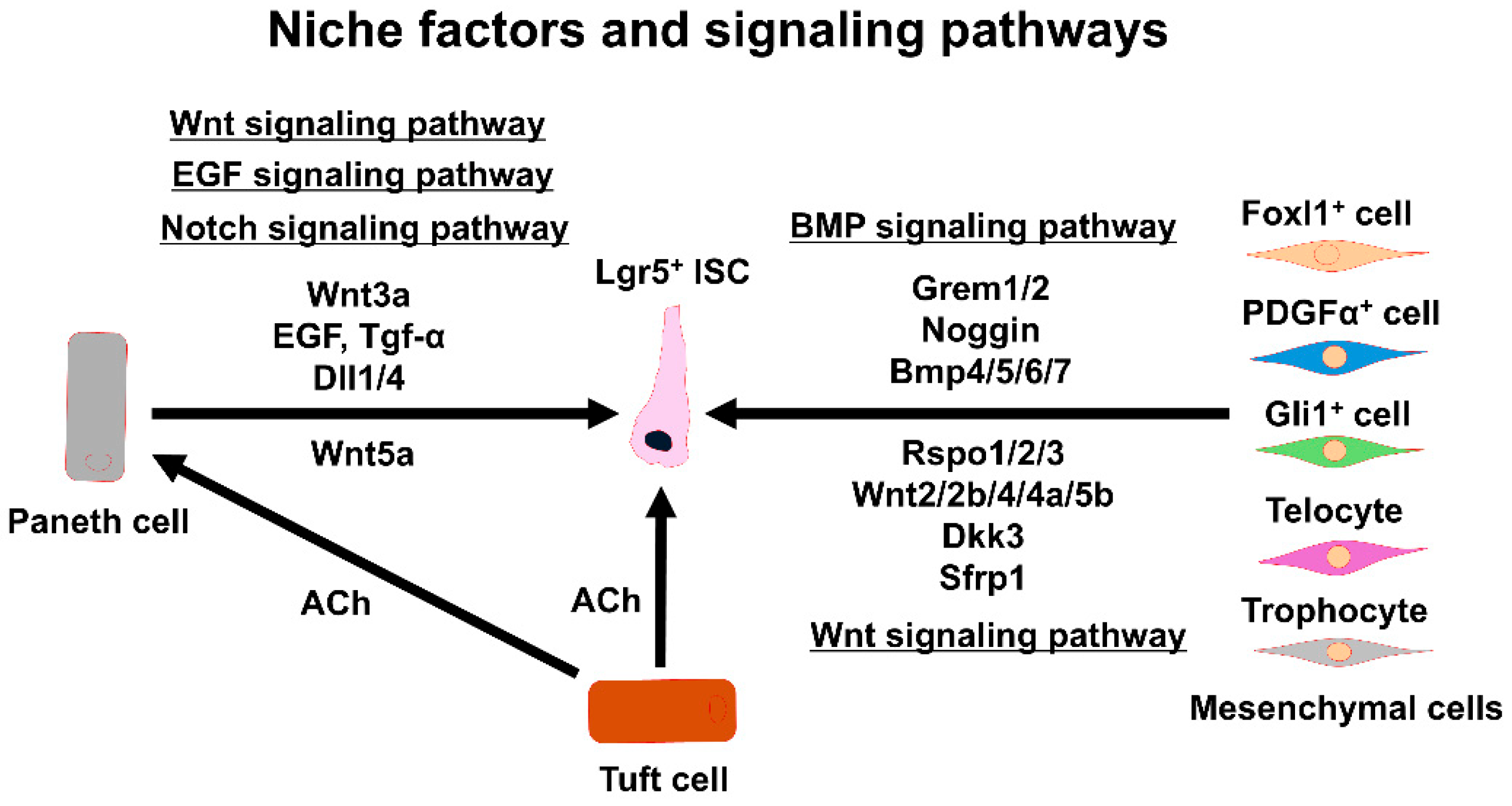
Figure 2.
Signaling factors and pathways sustaining Lgr5+ ISC functions. The interactions between Lgr5+ ISCs and various niche components are shown. ISC: intestinal stem cell, EGF: epidermal growth factor, Tgf-α: transforming growth factor α, Dll1/4: delta-like protein 1/4, Grem1/2: gremlin 1/2, Bmp4/5/6/7: bone morphogenetic protein 4/5/6/7, Rspo1/2/3: R-spondin 1/2/3, ACh: acetylcholine, Dkk3: dickkopf Wnt signaling pathway inhibitor 3, Sfrp1: secreted frizzled-related protein 1.
Winged-helix transcription factor forkhead box L1 (Foxl1)-expressing mesenchymal cells are important cells for the ISC niche. A gene expression analysis of Foxl1+ cells revealed that they express multiple niche signaling molecules, including Wnt2b and Rspo3, indicating that they produce Wnt signals required in the niche for the self-renewal of ISCs [39]. Additionally, the expression of Foxl1 overlaps with the expression of platelet-derived growth factor receptor α (PDGFα) on telocytes, which are named for their characteristic long cytoplasmic extensions that were suggested to play a role in cell-to-cell communication within the crypt [40]. Additionally, PDGFα+ cells also express the transcription factor glioma-associated oncogene1 (Gli1), which mediates hedgehog (Hh) signaling. Gli1+ mesenchymal cells are found within a subset of mesenchymal cells that are located right beneath the epithelium, and they show an expression pattern similar to that of Foxl1+ cells [20,40]. Gli1+ cells are found in the colonic epithelium, but not in the small intestinal epithelium, and they produce Wnt2, Wnt2b, and Wnt4 for ISC maintenance in the colon. It has been suggested that the Gli1+ cell and Foxl1+ cell populations partially overlap with each other [20].
The Gli1+ cell population can be divided into eight subclusters, and CD34 expression is enriched in two of the subclusters. Gli1+/CD34+ mesenchymal cells express multiple Wnt ligands. Pericryptal cells are a subset of CD34+ mesenchymal cells that surround the crypt region in the small and large intestines [41]. In cultured organoids, the addition of pericryptal CD34+ mesenchymal cells to the culture causes the organoids to become spherically shaped due to increased ISC proliferation and impaired intestinal epithelial differentiation [41]. Thus, it has been suggested that pericryptal CD34+ mesenchymal cells may have pivotal roles in regulating ISC proliferation during normal homeostasis.
An analysis of scRNA-seq data from a mouse’s small-intestine mesenchymal cells revealed that the classical pericyte markers chondroitin sulfate proteoglycan 4 (Cspg4) and neuron-glial antigen 2 (Ng2) are broadly expressed among resident mesenchymal cells [21]. Although Cspg4+ mesenchymal cells (pericytes) express Wnt2b and Wnt4, the blocking of the Wnt ligands in the pericytes of mice does not appear to affect the differentiation of the epithelial cells [21], suggesting that Cspg4+ pericytes, which are rare, are not strongly associated with the mesenchymal niche.
PDGFα+ mesenchymal cells represent a large population of mesenchymal cells in the intestinal sub-epithelium, and they have been extensively investigated for niche functions in normal mice [19,40,41,42]. Porcupine O-acyltransferase (Porcn) excision in the PDGFα+ cells blocked intestinal crypt formation, indicating that PDGFα+ cells provide Wnt signals for supporting the ISC niche [42]. Additionally, mice with Rspo3 excision in the PDGFα+ cells showed decreased intestinal crypt Wnt/β-catenin signaling and Paneth cell differentiation and were hypersensitive when stressed with dextran sodium sulfate [42]; this suggested that Rspo3 is essential for epithelial repair after tissue damage. Consensus mRNA signatures can be used to distinguish two subpopulations of PDGFα+ cells, i.e., PDGFαlow cells and PDGFαhigh cells. These cells can be further divided into two subtypes marked by CD81 using flow cytometry. Cells positive for both PDGFα and CD81 are called trophocytes [19]. The PDGFα+/CD81+ trophocytes can support organoid growth without the addition of trophic factors in the culture medium [19]. In contrast, PDGFαlow/CD81− cells cannot support organoid growth without the addition of Noggin [19]. Details on the heterogeneity of these PDGFα+ cells remain unclear, but it may reflect the ultrastructural heterogeneity seen in the small intestine, but not myofibroblasts [43].
In addition to Wnt-RSPO signaling from mesenchymal cells in the ISC niche, bone morphogenetic protein (BMP) signaling is also important for antagonizing Wnt activity and promoting ISC differentiation [44,45]. BMP signaling occurs predominantly in PDGFα+ cells. BMP ligands activate BMP receptors, and the signals maintain ISC stemness and promote ISC differentiation through phosphorylated Smad transcription factors [46]. Graded BMP signaling formed by PDGFα+ cells in the intestinal crypt–villus axis guides ISC differentiation and stemness maintenance. BMP signaling is low in the crypt base by PDGFαlow cells and higher towards the top of the villi by PDGFαhigh cells [47]. The gradient inhibits proliferation of the Lgr5+ ISCs via a Smad-mediated repression of genes including Lgr5. Eventually, BMP signaling promotes ISC differentiation [47]. The activities of BMP and Wnt signaling produce opposite effects in the crypt–villus axis. Additionally, to balance the intestinal crypts, BMP inhibitors, such as noggin and gremlin 1 (Grem1), ectopically induce proliferative crypts in post-mitotic villi [48,49,50]. BMP inhibitors that promote ISCs’ self-renewal are secreted from intestinal sub-epithelial myofibroblasts and smooth muscle cells near the ISC zone at crypt bottoms [45]. Fine-tuning of the BMP pathway occurs in the crypts of the small intestine. For example, the inappropriate expression of Grem1 in intestinal villi leads to the formation of an ectopic niche and the formation of new crypts [50].
Foxl1+ mesenchymal cells express BMP-related signaling molecules, including chordin-like1, Grem1/2 [39], and Bmp4/5/6/7 [40]. The Foxl1+ mesenchymal cells also express the Wnt inhibitor dickkopf3 (Dkk3) and secrete frizzled-related protein 1 (SFRP1) [40]. Foxl1+ mesenchymal cells control ISCs’ self-renewal through these complex signaling pathways. The Foxl1+ mesenchymal cells belong to a subset of PDGFα+ cells. PDGFα+ telocytes represent the richest source of BMP ligands, and they express the BMP inhibitor chordin [19]. On the other hand, PDGFα+/CD81+ trophocytes abundantly express Grem1 as well as Noggin [19]. The subcryptal production of BMPs and BMP inhibitors by each telocyte and trophocyte may generate a polarity [19,45]. Of note, it was found that PDGFα+/CD81+ trophocytes isolated by flow cytometry with antibodies against PDGFα and CD81 supported organoid growth without the addition of any tropic factors in the culture medium [19]. In contrast, isolated PDGFαlow/CD81− cells did not support organoid growth. Thus, the BMP inhibitor Noggin secreted from trophocytes appears to be a major signaling molecule for organoid growth. Trophocytes mainly localize near the crypt’s bottom, as shown by in situ hybridization of the Grem1 gene [19]. The ablation of the Grem1 gene in mice causes the loss of ISCs in the crypt [19], suggesting that Grem1+ trophocytes contribute to the ISC niche by producing the BMP inhibitor required for ISC maintenance. Collectively, it appears that there are at least two distinct mesenchymal niche populations of cells, i.e., telocytes and trophocytes, that are critical for regulating ISCs’ self-renewal and differentiation (Figure 2).
2.2. The Epithelial Niche
The intestinal epithelial niche includes a variety of epithelial cell types in the intestine that have barrier and absorption functions and provide signaling molecules that are needed to maintain ISCs. Wnt3a-producing Paneth cells [9] that are adjacent to ISCs at the crypt base are indispensable for ISCs’ self-renewal. They are surrounded by the mesenchymal cell niche, and collectively, the mesenchymal cell niche and the epithelial cell niche constitute the ISC niche. Wnt3a-producing Paneth cells produce epidermal growth factor (EGF), transforming growth factor α (Tgf-α), and notch ligands (delta-like protein 1/4; Dll1/4) that support ISC functions [51,52,53] (Figure 2). In mice with the loss of Paneth cells or the disruption of Wnt production, ISCs’ self-renewal is ensured through compensatory Wnt production by the surrounding mesenchymal cells [39,54]. Indeed, it was reported that Wnt ligands, including Wnt2b, Wnt4, and Wnt5a, are secreted by the mesenchymal cells surrounding the crypt base [37]. Additionally, Rspo ligands for Lgr5 are also expressed by the mesenchymal cells [38]. Nevertheless, ISC niche factors provided by epithelial cells are still required in the niche environment for maintaining ISCs.
Homeostatic ISC populations are maintained by the cellular niche. Upon injury, the ISC niche adapts to not only maintain homeostasis, but also to induce epithelial regeneration, which is mediated by the surviving Lgr5+ ISCs or other mature cell types, such as enterocytes, enteroendocrine cells, and Paneth cells that have the ability to convert back into Lgr5+ ISCs [55]. Thus, epithelial cell plasticity appears to play a central role in regeneration in the intestine. Such elucidation of the underlying mechanisms at the molecular level provides important new insights into intestinal injury and repair that may facilitate the development of potential new therapeutic approaches for human intestinal disorders.
A quiescent population of stem cells residing at position +4 from the base of the crypt has been described as ISCs [56,57]. These cells were identified to be label-retaining cells marked by the expression of polycomb ring finger oncogene B-cell-specific Moloney leukemia virus insertion site 1 (Bmi1), and they elicit an ISC-like response in vivo [58]. Bmi1+ cells, which show Wnt-insensitivity and radiation resistance, are functionally distinct from Lgr5+ ISCs [58,59]. Upon radiation exposure, Bmi1+ cells give rise to Lgr5+ ISCs to sustain intestinal homeostasis for post-radiation epithelial repair [58,59]. Similar to Bmi1+ cells, slowly cycling homeodomain-only protein (Hopx)-expressing cells also give rise to Lgr5+ ISCs [59]. Intriguingly, Lgr5+ ISCs also exhibit heterogeneity that contributes to intestinal epithelial regeneration upon injury. Slowly cycling Lgr5+ subsets, including label-retaining cells and RNA-binding protein mex-3 RNA-binding family member A (Mex3a)-expressing cells, contribute to regeneration after chemotherapy and radiation injury [11,24]. Therefore, active Lgr5+ ISCs are highly sensitive to injury, and slowly cycling Lgr5+ cells regenerate damaged crypts through an Lgt5-independent pathway. Although it has been shown that interconversion occurs between slowly and rapidly cycling ISCs, the underlying mechanisms of cellular plasticity remain unclear.
As mentioned above, Paneth cells also act as niche cells. It has been reported that Paneth cells can dedifferentiate to join the ISC pool upon the depletion of fast-cycling Lgr5+ ISCs [60,61]. Furthermore, following Lgr5+ ISC ablation, alkaline phosphatase-expressing intestinal Alpi+ enterocyte daughter cells dedifferentiate into Lgr5+ ISCs [62]. As another example, a combined analysis of flow cytometry, scRNA-seq, and histology data showed that the ablated Paneth cells are replaced by enteroendocrine and tuft cells in the niche, and that these cells physically occupy the Paneth cell positions next to Lgr5+ ISCs [63]. These enteroendocrine and tuft cells, which express the Dll1 gene, serve as an alternative source of notch signals that are essential for Lgr5+ ISC maintenance. Recently, it has been shown that isthmus progenitor cells expressing the fibroblast growth factor binding protein 1 (fgfbp1) gene contribute to homeostatic cellular turnover and support regeneration upon intestinal injury [64,65]. These results provide an alternative model of the intestinal epithelial organization and suggest that the ISC stemness potential is not restricted to Lgr5+ ISCs, and that neither dedifferentiation nor reserve stem cells are drivers of intestinal regeneration [64].
Murine tuft cells can be identified by the expression of the doublecortin-like kinase 1 (DCLK1) gene, and they exist as rare epithelial cells in the small intestine [66,67,68]. Intriguingly, tuft cells are putative quiescent stem cells [69]. Tuft cells express the cytokine interleukin (IL)-25, which triggers type 2 innate lymphoid cells to secrete IL-13. IL-13 is a signaling molecule involved in Lgr5+ ISC differentiation into goblet and tuft cells, which occurs in a positive-feedback loop [7,70]. Finally, goblet and tuft-2 cells contribute to the clearance of pathogens, and they provide immunity against further infections in the intestine [71]. Collectively, the plasticity and dedifferentiation of tuft cells contribute to the prevention of infections in combination with type 2 innate lymphoid cells. The trinity of tuft, immune, and IECs provides a new approach to controlling and potentially preventing intestinal infections.
In mice, two distinct subsets of tuft cells have been identified: tuft-1 and tuft-2. Recently, Huang and coworkers identified four distinct subsets of human tuft cells: tuft-1, tuft-2, tuft-3, and tuft-4 [72]. Two of them overlap with the murine tuft cells (tuft-1 and tuft-2) at a steady state [72]. Exposure to the cytokine IL-4 shifts the human tuft cell substrate balance, triggering the appearance of cycling tuft-3 cells and the expansion of tuft-4 cells [72]. Human tuft-4 cells may be involved in the immune response and/or intestinal epithelial renewal. Since it appeared that tuft cells can expand, Huang and coworkers subsequently examined their organoid-forming potential. As expected, single tuft cells gave rise to spheroid-like organoids when cultured in an expansion medium, and the spheroids could be passaged [72]. Therefore, tuft cells have stem cell-like properties and can act as reserve and/or revival stem cells upon intestinal injury, such as irradiation damage [72].
The biosynthesis of acetylcholine (ACh) by choline acetyltransferase (ChAT) has been well established in neurons. The biosynthesis and release of ACh in tuft cells are considered to be part of the non-neuronal cholinergic system [73,74]. We revealed that non-neuronal ACh synthesized in the epithelium regulates Lgr5+ ISC maintenance via a muscarinic ACh receptor M3 (mAChR-M3) [75]. Furthermore, RNA sequencing of the crypts in M3-knockout mice revealed the upregulation of EphB/ephrin-B signaling as well as mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) signaling that is downstream of EphB/ephrin-B signaling [76]. Collectively, the mAChR-M3, EphB/ephrin-B, and MAPK/ERK signaling cascade maintains the homeostasis of IEC growth and differentiation in response to changes in the cholinergic intestinal niche [76]. Middelhoff and coworkers also confirmed the importance of tuft cells in intestinal epithelial homeostasis [77].
Apart from the ACh-M3-Lgr5+ ISC axis, tuft cells can modulate IEC homeostasis via nicotinic ACh receptors (nAChRs). It has been revealed that nAChRs also play a pivotal role in the regulation of intestinal epithelial growth and differentiation in mice and humans [78,79]. Pharmacological studies have shown that nicotine enhances intestinal organoid growth and differentiation, and that the nAChR antagonist mecamylamine has the opposite effect [79]. Results from immunohistochemical and flow cytometry analyses provided the first descriptions of the α2β4 subtype of nAChR (nAChR-α2β4) in Paneth cells, suggesting that this subtype is involved in the regulation of ISC proliferation and differentiation [79]. RNA-sequencing results have also revealed that non-canonical Wnt5a gene expression is dramatically upregulated by treatment with nicotine, and that recombinant Wnt5a rescues the intestinal organoid growth and differentiation suppressed by mecamylamine [79]. As mentioned above, non-canonical Wnt5a is secreted from mesenchymal telocyts. Paneth cells may also secrete Wnt5a proteins. Collectively, the coordinated activities of nAChR-α2β4 and Wnt5a signaling maintain Lgr5+ ISC activity and balanced differentiation.
Signaling via both mAChR-M3 and nAChR-α2β4 appears to be needed to maintain the homeostasis of intestinal epithelial growth and differentiation, in addition to the fine-tuned regulation from the cholinergic intestinal niche [80] (Figure 2). However, the mechanism by which tuft cells control ACh synthesis and release remains to be elucidated. Further studies are needed to clarify the exact role of tuft cells in maintaining intestinal epithelial homeostasis via the intestinal cholinergic niche.
3. Organoid Formation in the Small Intestine
The intestinal stem niche is like a cradle of ISCs for maintaining and controlling the activity in the gut. The specialized functions required to ensure proper ISC functions are provided by neighboring differentiated cells including Paneth and mesenchymal cells [81]. Through signaling pathways and intercellular interactions, these cells influence the behavior of adjacent ISCs, which themselves exhibit relatively limited specialization. Consequently, ISC identity is defined more by spatial localization within the niche rather than by distinct gene expression patterns [82]. To characterize ISCs within their niche, researchers analyze the surrounding cellular environment, including neighboring cell types, signaling molecule expression, and extracellular matrix components [81,82,83,84]. Once commonalities are found, the niche can be perturbed to learn which aspects affect ISC behavior. A better understanding of niches and their signals will provide valuable insights into why most of the characterized niche regulatory mechanisms and signals also function in developing embryos [85,86,87]. Unlike embryonic cells, ISCs can operate in a steady state. They can generate on average one replacement ISC and one tissue cell at each division with no apparent limit. For over a decade, intestinal organoids have been utilized to mimic villus and crypt development in vitro [18]. Most research works have been developed by culturing organoids in three-dimensional organoids that form crypt-like structures that grow stochastically and uncontrollably. When cultured on two-dimensional flat elastic substrates, organoids only form flat crypt-like structures [88]. In this section, we describe the generation and recent utilization of intestinal organoids.
3.1. Three-Dimensional (3D) Intestinal Organoids
Organoids are simple tissue-engineered cell-based in vitro models that recapitulate a variety of aspects of the complex structure and function of the corresponding in vivo tissue. The Matrigel-based culture system was established based on the growth factors derived from mesenchymal and epithelial niches to enable the formation of 3D intestinal organoids in vitro from crypts or a single Lgr5+ ISC [18]. An essential component of the culture system is the Wnt-RSPO-Noggin-EGF cocktail [18]. Murine intestinal organoids, unlike their human, can be cultured without the addition of exogenous Wnt3a [18,72]. However, intestinal organoids derived from Wntless-deficient mice, which lack the ability to secrete Wnt ligands extracellularly, cannot proliferate in the absence of Wnt3a supplementation [89]. This finding suggests that in mice, Wnt ligands secreted by IECs, primarily Paneth cells, are sufficient to sustain organoid growth by providing the necessary Wnt signaling. Co-culture systems have been employed to examine the interactions between mesenchymal cells and crypts as well as the factors expressed by each cell. As these systems cannot completely and faithfully replicate the complicated nature of organoids and their microenvironment, results from mesenchyme–crypt co-cultures need to be validated separately after the experiments.
Air–liquid interface (ALI) intestinal organoids that contain both epithelial cells and mesenchymal cells are extremely useful for examining mesenchymal ISC niches [90]. Murine ALI intestinal organoids can grow without the addition of niche factors but are inhibited by Wnt inhibitors, such as DKK1 and ipafricept (Fzd8-Fc) [90]. In co-culture systems, induced pluripotent stem cell (iPSC)-derived human intestinal organoids are composed of both epithelial cells and mesenchymal cells [91,92]. Like Foxl1+ and PDGFα+ mesenchymal cells, the addition of Gli+/CD34+ mesenchymal cells into the co-culture system induces organoid formation with a spherical shape, suggesting increased ISC proliferation and impaired epithelial cell differentiation [40]. Mesenchyme–crypt co-culture systems are promising as surrogate models. However, the Matrigel matrix used in the systems contains yet-unidentified factors.
Thus far, we have described mesenchymal signaling to IECs. However, communication from IECs to mesenchymal cells likely also occurs. For example, endoderm-derived Hh and PDGF signals modulate mesenchymal cells [93]. New insights into mesenchymal cell populations may provide a framework for future research in this area.
As the emergence of cutting-edge technologies including 3D bioprinting or microfluidic chips raises the prospect of closely mimicking the microenvironment [94], current research frequently focuses on combinations of IECs or mesenchymal cells. Multi-organ chips containing vascularization and immunization that connect multiple organ components appear to be better models for studying drug transport and metabolism [95,96].
3.2. Two-Dimensional (2D) Epithelial Monolayers
The development of the 3D intestinal organoid culture method has significantly advanced our understanding of ISCs and their niche environment. However, it should be noted that 3D organoids are not a universal experimental model for intestinal research. One major limitation is the orientation of epithelial polarity. In 3D organoids, the apical surface of epithelial cells, which interacts with luminal factors such as dietary components and microbes, faces inward toward the organoid lumen, rendering direct access to this interface experimentally challenging. Additionally, these structures lack distinct villus formations, which are critical for modeling intestinal physiology. To overcome these constraints, various experimental approaches have been explored. For example, methods of injecting substances into the lumen of a 3D organoid or turning the organoid inside out have been reported [97,98]. However, these methods are difficult to perform and inefficient. Additionally, monolayer cultures derived from human colorectal cancer cell lines (Caco-2) have been utilized as analytical systems [99,100]. However, Caco-2 monolayers have been reported to exhibit gene expression and physiological characteristics distinct from those of the native small intestinal epithelium [100,101]. Given these challenges, recent research has focused on developing 2D epithelial monolayers derived from 3D intestinal organoids as an alternative experimental system to overcome these limitations.
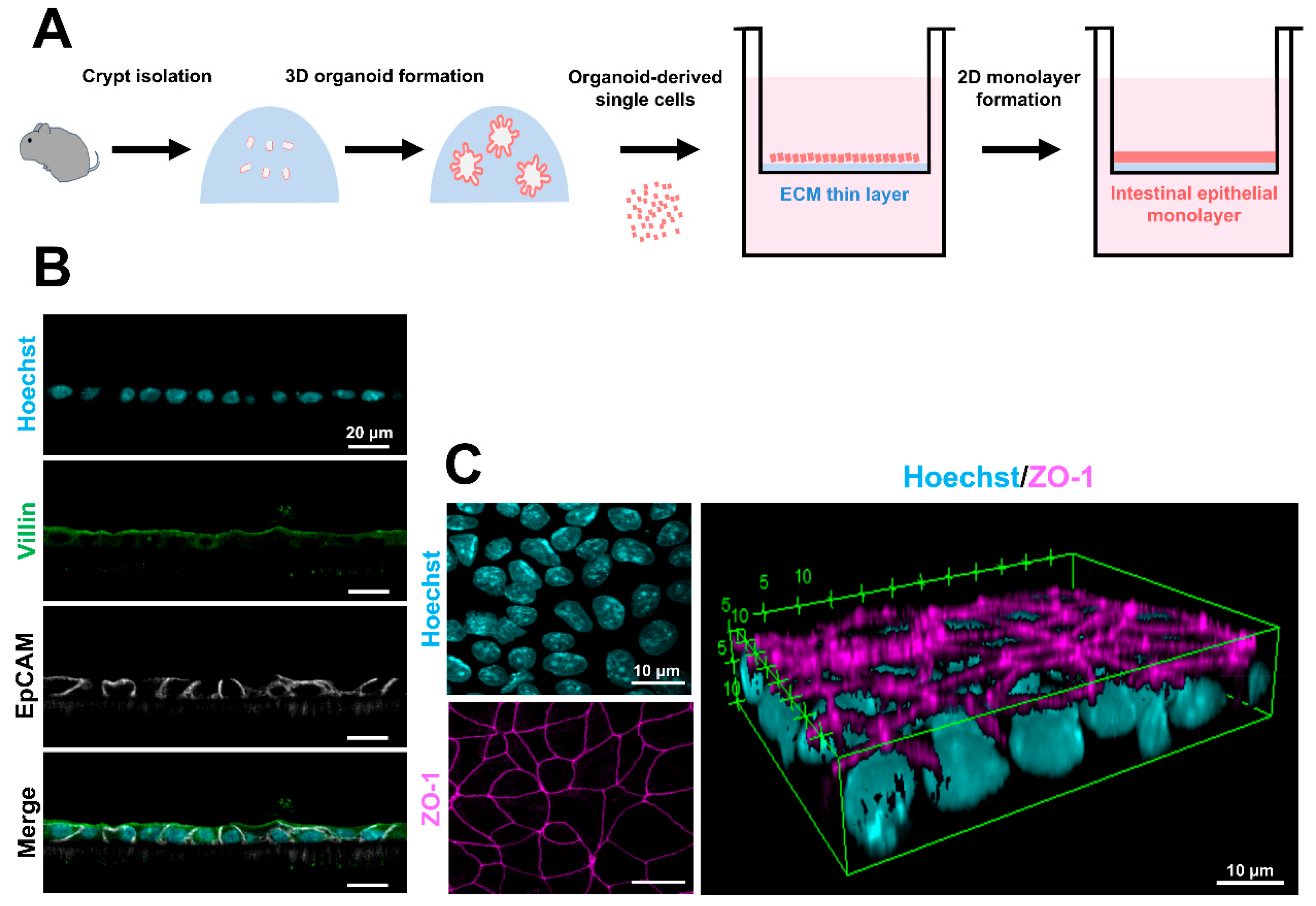
The generation of 2D small intestinal epithelial monolayers has been attempted in multiple species, including humans, mice, pigs, and bovines [101,102,103,104,105,106,107,108,109,110,111]. The utility of these 2D monolayers has been demonstrated in several studies, particularly in IgA transcytosis and pathogen–epithelial interactions [102,103]. In particular, for human-derived monolayers, several research groups have reported methods using 3D intestinal organoids or iPSC-derived IECs [101,103,104,105,106,107]. In contrast, generating 2D monolayers from mouse 3D intestinal organoids has been challenging due to the rapid turnover of IECs. In some cases, methods have been reported that utilize 3D organoids derived from the colon, where IEC turnover is slower, or directly from living intestinal epithelium to produce monolayers [102,105]. Additionally, approaches that require primary tissue (myofibroblast or enteric nervous system)-derived culture medium or long culture periods have also been reported [108,109]. Recently, we developed a method for efficiently forming 2D epithelial monolayers from murine 3D small intestinal organoids using only commercially available reagents and media [112] (Figure 3A). The 2D epithelial monolayers have stable intercellular junctions and contain ISCs and mature IECs [112] (Figure 3B,C). Moreover, the transepithelial electric resistance values of the monolayer were within the expected physiological range [112,113]. This new method is expected to be useful in physiology and pharmaceutical research.
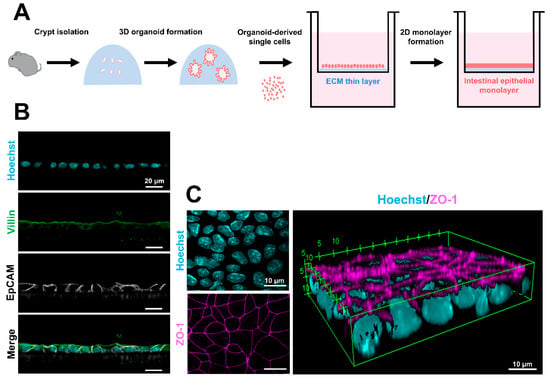
Figure 3.
Procedure for 2D epithelial monolayer formation and the epithelial characteristics. (A) Schematic diagram of 2D epithelial monolayer formation from murine 3D intestinal organoids. (B) Confocal images of EpCAM (white) and villin (green) in the 2D epithelial monolayers. The nuclei were stained with Hoechst 33342 (cyan). Scale bars: 20 μm. (C) A 3D reconstruction of ZO-1-stained (magenta) images. The nuclei were stained with Hoechst 33342 (cyan). Scale bars: 10 μm. The fluorescent images were compiled from our published data [112].
Additionally, 2D intestinal epithelial monolayers, when combined with various experimental approaches, have the potential to provide novel insights into the mechanisms underlying intestinal epithelial morphogenesis. During development, the intestinal epithelium initially forms a flat structure, which subsequently gives rise to characteristic villus and crypt structures through interactions with surrounding tissues [1,114,115]. Recent studies have reported that human 2D intestinal monolayers, when cultured under ALI conditions or subjected to shear forces via a shaking culture, which mimics intestinal luminal flow, can induce the formation of villus-like protrusions [116,117]. The intestinal epithelium with its specialized cell organization is intricately folded into arrays of crypts and villi. While the mechanisms driving villi formation are well described, the processes regulating crypt formation remain largely unknown. Pérez-González and coworkers found that the size of an ISC compartment depends on the extracellular-matrix stiffness and endogenous cellular forces [88]. Additionally, computational modeling revealed that the crypt shape and force distribution rely on cell surface tensions following cortical actomyosin density. They will clarify crypt morphogenesis using 2D intestinal monolayers in the near future. Eicher and colleagues successfully established a co-culture system by separately differentiating endoderm and mesoderm from human iPS cells to generate gastrointestinal tissue-like structures [118]. More recently, Deguchi and colleagues developed a micro-small intestinal system by simultaneously differentiating definitive endoderm and mesoderm from human iPS cells on a microfluidic device that replicates intestinal flow. This system enabled the formation of a 3D small-intestine-like tissue with villus-like epithelium and an aligned mesenchymal layer [119]. However, it has not yet fully recapitulated the mature crypt structure. Analysis systems employing microfluidic devices and stiffness-controlled artificial hydrogels to constrain the shape of 2D monolayers and 3D organoids have provided valuable insights into the mechanical properties of ISCs and their niche during intestinal morphogenesis [120,121,122]. While none of these experimental models perfectly recapitulate the in vivo situation, they continue to advance our understanding of various aspects of intestinal biology.
4. Conclusions and Future Directions
The small intestine has a striking homeostatic capacity due to its epithelium that is organized into a large number of self-renewing crypt–villus units. The ISC niche is central to its homeostatic capacity. It is well-known that multiple mesenchymal and epithelial cell populations collectively produce diverse niche signals to regulate ISCs’ self-renewal and differentiation in the normal homeostatic state. On the other hand, niche cells also regulate ISCs during repair. The adaptive and homeostatic niches can interconvert through cell reverse differentiation (dedifferentiation) and interconversion with operating niche factors and cells, such as Wnts, Rspos, notch, BMP, Hh, and Foxl1+ and PDGFα+ mesenchymal cells. It is unclear how the homeostatic niche and injury-adapted ISC niche differentially regulate the equilibrium between active and quiescent/reserve ISCs. Since dedifferentiation in response to intestinal injury does not occur solely for tissue repair, the cells capable of dedifferentiation are considered to be facultative stem cells [123]. Further research on the interconversion between active and injury-inducible ISCs is expected to help us better understand stem cell plasticity and to provide more insights into the relationship between these two stem cell populations, into disease mechanisms, and into therapeutic approaches for intestinal disorders. For instance, the technique for conversion from native colonic epithelium into small intestinalized colon using ileum-derived organoids is transformative for the development of tissue engineering applications for diseases such as short bowel syndrome [117]. Additionally, many intestinal diseases show changes in crypt morphology including inflammatory bowel disease and irritable bowel disease. These diseases represent an important health burden affecting a significant percentage of the population. The understanding of the mechanisms that regulate crypt malformations in the intestinal stem cell niche may provide critical information to progress in disease treatment and drug development.
We discussed ISC-derived organoid culture including their methodology, application, and weakness of intestinal organoids and engineered organoids compared to cell lines. The appropriate applications, advantages, and disadvantages of the model are articulated in Section 3. The development of 2D epithelial monolayers as well as intestinal 3D organoids provides an unprecedented tool for humans to study diseases. By combining microfluidic technology, innovative biological support materials such as extracellular matrices, and automated detection methods using AI technologies, we have a more faithful promise that intestinal organoid technology will greatly accelerate the drug discovery process for intestinal diseases and the innovation of treatment methods.
Author Contributions
Conceptualization, T.T.; writing of the original draft, T.T. and Y.T.; preparation of the scientific illustrations, T.T. and Y.T. All authors have read and agreed to the published version of the manuscript.
Funding
Japan Society for the Promotion of Science: JP17K07495, JP20K06751, JP23K05862.
Data Availability Statement
No new data were produced in this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Shyer, A.E.; Huycke, T.R.; Lee, C.; Mahadevan, L.; Tabin, C.J. Bending gradients: How the intestinal stem cell gets its home. Cell 2015, 161, 569–580. [Google Scholar] [PubMed]
- Cheng, H.; Leblond, C.P. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the origin of the four epithelial cell types. Am. J. Anat. 1974, 141, 537–561. [Google Scholar] [PubMed]
- Barker, N.; van Es, J.H.; Kuipers, J.; Kujala, P.; van den Born, M.; Cozijnsen, M.; Haegebarth, A.; Korving, J.; Begthel, H.; Peters, P.J.; et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007, 449, 1003–1007. [Google Scholar]
- Barker, N. Adult intestinal stem cells: Critical drivers of epithelial homeostasis and regeneration. Nat. Rev. Mol. Cell Biol. 2014, 15, 19–33. [Google Scholar]
- Clevers, H. The intestinal crypts, a prototype stem cell compartment. Cell 2013, 154, 274–284. [Google Scholar]
- Gerbe, F.; Legraverend, C.; Jay, P. The intestinal epithelium tuft cells: Specification and function. Cell. Mol. Life Sci. 2012, 69, 2907–2917. [Google Scholar]
- Von Moltke, J.; Ji, M.; Liang, H.E.; Locksley, R.M. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 2016, 529, 221–225. [Google Scholar]
- Luo, X.-C.; Chen, Z.-H.; Xue, J.-B.; Zhao, D.-X.; Lu, C.; Li, Y.-H.; Li, S.-M.; Du, Y.-W.; Liu, Q.; Wang, P.; et al. Infection by the parasitic helminth Trichinella spiralis activates a Tas2r-mediated signaling pathway in intestinal tuft cells. Proc. Natl. Acad. Sci. USA 2019, 116, 5564–5569. [Google Scholar]
- Sato, T.; van Es, J.H.; Snippert, H.J.; Stange, D.E.; Vries, R.G.; van den Born, M.; Barker, N.; Shroyer, N.F.; van de Wetering, M.; Clevers, H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 2011, 469, 415–418. [Google Scholar]
- Ayyaz, A.; Kumar, S.; Sangiorgi, B.; Ghoshal, B.; Gosio, J.; Ouladan, S.; Fink, M.; Barutcu, S.; Trcka, D.; Shen, J.; et al. Single-cell transcriptomes of the regenerating intestine reveal a revival stem cell. Nature 2019, 569, 121–125. [Google Scholar]
- Barriga, F.M.; Montagni, E.; Mana, M.; Mendez-Lago, M.; Hernando-Momblona, X.; Sevillano, M.; Guillaumet-Adkins, A.; Rodriguez-Esteban, G.; Buczacki, S.J.A.; Gut, M.; et al. Mex3a markes a slowly dividing subpopulation of Lgr5+ intestinal stem cells. Cell Stem Cell 2017, 20, 801–816.e7. [Google Scholar] [PubMed]
- Li, N.; Yousefi, M.; Nakauka-Ddamba, A.; Jain, R.; Tobias, J.; Epstein, J.A.; Jensen, S.T.; Lengner, C.J. Single-Cell Analysis of Proxy Repor ter Allele-Marked Epithelial Cells Establishes Intestinal Stem Cell Hierarchy. Stem Cell Rep. 2014, 3, 876–891. [Google Scholar]
- May, R.; Sureban, S.M.; Hoang, N.; Riehl, T.E.; Lightfoot, S.A.; Ramanujam, R.; Wyche, J.H.; Anant, S.; Houchen, C.W. Doublecortin and CaM kinase-like-1 and leucine-rich-repeat-containing G-protein-coupled receptor mark quiescent and cycling intestinal stem cells, respectively. Stem Cells 2009, 27, 2571–2579. [Google Scholar] [PubMed]
- Metcalfe, C.; Kljavin, N.M.; Ybarra, R.; de Sauvage, F.J. Lgr5+ stem cells are indispensable for radiation-induced intestinal regeneration. Cell Stem Cell 2014, 14, 149–159. [Google Scholar] [PubMed]
- Montgomery, R.K.; Carlone, D.L.; Richmond, C.A.; Farilla, L.; Kranendonk, M.E.G.; Henderson, D.E.; Baffour-Awuah, N.Y.; Ambruzs, D.M.; Fogli, L.K.; Algra, S.; et al. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc. Natl. Acad. Sci. USA 2011, 108, 179–184. [Google Scholar]
- Roche, K.C.; Gracz, A.D.; Liu, X.F.; Newton, V.; Akiyama, H.; Magness, S.T. SOX9 maintains reserve stem cells and preserves radioresistance in mouse small intestine. Gastroenterology 2015, 149, 1553–1563.e10. [Google Scholar]
- Yan, K.S.; Chia, L.A.; Li, X.; Ootani, A.; Su, J.; Lee, J.Y.; Su, N.; Luo, Y.; Heilshorn, S.C.; Amieva, M.R. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc. Natl. Acad. Sci. USA 2012, 109, 466–471. [Google Scholar]
- Sato, T.; Vries, R.G.; Snippert, H.J.; van de Wetering, M.; Baker, N.; Stange, D.E.; van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, 262–265. [Google Scholar]
- McCarthy, N.; Manieri, E.; Storm, E.E.; Saadatpour, A.; Luoma, A.M.; Kapoor, V.N.; Madha, S.; Gaynor, L.T.; Cox, C.; Keerthivasan, S.; et al. Distinct mesenchymal cell populations generate the essential intestinal BMP signaling gradient. Cell Stem Cell 2020, 26, 391–402.e5. [Google Scholar]
- Degirmenci, B.; Valenta, T.; Dimitrieva, S.; Hausmann, G.; Basler, K. GLI1-expressing mesenchymal cells form the essential Wnt-secreting niche for colon stem cells. Nature 2018, 558, 449–453. [Google Scholar]
- Kim, J.-E.; Fei, L.; Yin, W.-C.; Coquenlorge, S.; Rao-Bhatia, A.; Zhang, X.; Shi, S.S.W.; Lee, J.H.; Hahn, N.A.; Rizvi, W.; et al. Single cell and genetic analyses reveal conserved populations and signaling mechanisms of gastrointestinal stromal niches. Nat. Commun. 2020, 11, 334. [Google Scholar] [CrossRef] [PubMed]
- Kinchen, J.; Chen, H.H.; Parikh, K.; Antanaviciute, A.; Jagielowicz, M.; Fawkner-Corbett, D.; Ashley, N.; Cubitt, L.; Mellado-Gomez, E.; Attar, M.; et al. Structural remodeling of the human colonic mesenchyme in inflammatory bowel disease. Cell 2018, 175, 372–386.e7. [Google Scholar] [CrossRef] [PubMed]
- Roulis, M.; Nikolaou, C.; Kotsaki, E.; Kaffe, E.; Karagianni, N.; Koliaraki, V.; Salpea, K.; Ragoussis, J.; Aidinis, V.; Martini, E.; et al. Intestinal myofibroblast-specific Tpl2-Cox-2-PGE2 pathway links innate sensing to epithelial homeostasis. Proc. Natl. Acad. Sci. USA 2014, 111, E4658–E4667. [Google Scholar] [CrossRef]
- Buczacki, S.J.; Zecchini, H.I.; Nicholson, A.M.; Russell, R.; Vermeulen, L.; Kemp, R.; Winton, D.J. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature 2013, 495, 65–69. [Google Scholar] [CrossRef]
- Grün, D.; Lyubimova, A.; Kester, L.; Wiebrands, K.; Basak1, O.; Sasaki, N.; Clevers, H.; van Oudenaarden, A. Single-cell messenger RNA sequencing reveals rare intestinal cell types. Nature 2015, 525, 251–255. [Google Scholar] [CrossRef]
- Haber, A.L.; Biton, M.; Rogel, N.; Herbst, R.H.; Shekhar, K.; Smillie, C.; Burgin, G.; Delorey, T.M.; Howitt, M.R.; Katz, Y.; et al. A single-cell survey of the small intestinal epithelium. Nature 2017, 551, 333–339. [Google Scholar] [CrossRef]
- Li, N.; Nakauka-Ddamba, A.; Tobias, J.; Jensen, S.T.; Lengner, C.J. Mouse label-retaining cells are molecularly and functionally distinct from reserve intestinal stem cells. Gastroenterology 2016, 151, 298–310. [Google Scholar] [CrossRef]
- Moor, A.E.; Harnik, Y.; Ben-Moshe, S.; Massasa, E.E.; Rozenberg, M.; Eilam, R.; Halpern, K.B.; Itzkovitz, S. Spatial reconstruction of single enterocytes uncovers broad zonation along the intestinal villus axis. Cell 2018, 175, 1156–1167. [Google Scholar] [CrossRef]
- Yan, K.S.; Gevaert, O.; Zheng, G.X.Y.; Anchang, B.; Probert, C.S.; Larkin, K.A.; Davies, P.S.; Cheng, Z.-F.; Kaddis, J.S.; Han, A.; et al. Intestinal enteroendocrine lineage cells possess homeostatic and injury-inducible stem cell activity. Cell Stem Cell 2017, 21, 78–90. [Google Scholar] [CrossRef]
- Hayflick, L. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 1965, 37, 614–636. [Google Scholar] [CrossRef]
- Clevers, H.; Loh, K.M.; Nusse, R. An integral for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 2014, 346, 1248012. [Google Scholar] [PubMed]
- Van Es, J.H.; Haegebarth, A.; Kujala, P.; Itzkovitz, S.; Koo, B.-K.; Boj, S.F.; Korving, J.; van den Born, M.; van Oudenaarden, A.; Robine, S.; et al. A critical role for the Wnt effector Tcf4 in adult intestinal homeostatic self-renewal. Mol. Cell Biol. 2012, 32, 1918–1927. [Google Scholar] [CrossRef]
- Kabiri, Z.; Greicius, G.; Madan, B.; Biechele, S.; Zhong, Z.; Zaribafzadeh, H.; Edison; Aliyev, J.; Wu, Y.; Bunte, R.; et al. Stroma provides an intestinal stem cell niche in the absence of epithelial Wnts. Development 2014, 141, 2206–2215. [Google Scholar]
- San Roman, A.K.; Jayewickreme, C.D.; Murtaugh, L.C.; Ramesh, A.; Shivdasani, R.A. Wnt secretion from epithelial cells and subepithelial myofibroblasts is not required in the mouse intestinal stem cell niche in vivo. Stem Cell Rep. 2014, 2, 127–134. [Google Scholar]
- Kuhnert, F.; Davis, C.R.; Wang, H.-T.; Chu, P.; Lee, M.; Yuan, J.; Nusse, R.; Kuo, C.J. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc. Natl. Acad. Sci. USA 2004, 101, 266–271. [Google Scholar] [PubMed]
- Pinto, D.; Gregorieff, A.; Begthel, H.; Clevers, H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003, 17, 1709–1713. [Google Scholar]
- Gregorieff, A.; Pinto, D.; Begthel, H.; Destrée, O.; Kielman, M.; Clevers, H. Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology 2005, 129, 626–638. [Google Scholar] [CrossRef]
- Aoki, R.; Shoshkes-Carmel, M.; Gao, N.; Shin, S.; May, C.L.; Golson, M.L.; Zahm, A.M.; Ray, M.; Wiser, C.L.; Wright, C.V.E.; et al. Foxl1-expressing mesenchymal cells constitute the intestinal stem cell niche. Cell Mol. Gastroenterol. Hepatol. 2016, 2, 175–188. [Google Scholar] [CrossRef]
- Shoshkes-Carmel, M.; Wang, Y.J.; Wangensteen, K.J.; Tóth, B.; Kondo, A.; Massasa, E.E.; Itzkovitz, S.; Kaestner, K.H. Subepithelial telocytes are an important source of Wnts that supports intestinal crypts. Nature 2018, 557, 242–246. [Google Scholar]
- Stzepourginski, I.; Nigro, G.; Jacob, J.-M.; Dulauroy, S.; Sansonetti, P.J.; Eberl, G.; Peduto, L. CD34+ mesenchymal cells are a major component of the intestinal stem cells niche at homeostasis and after injury. Proc. Natl. Acad. Sci. USA 2017, 114, E506–E513. [Google Scholar] [CrossRef]
- Greicius, G.; Kabiri, Z.; Sigmundsson, K.; Liang, C.; Bunte, R.; Singh, M.K.; Virshup, D.M. PDGFRα+ pericryptal stromal cells are the critical source of Wnts and RSPO3 for murine intestinal stem cells in vivo. Proc. Natl. Acad. Sci. USA 2018, 115, E3173–E3181. [Google Scholar] [PubMed]
- Eyden, B.; Currt, A.; Wang, G. Stromal cell in the human gut show ultrastructural features of fibroblasts and smooth muscle cells but not myofibroblasts. J. Cell Mol. Med. 2011, 15, 1483–1491. [Google Scholar] [PubMed]
- Auclair, B.A.; Benoit, Y.D.; Rivard, N.; Mishina, Y.; Perreault, N. Bone morphogenetic protein signaling is essential for terminal differentiation of the intestinal secretory cell lineage. Gastroenterology 2007, 133, 887–896. [Google Scholar] [PubMed]
- Kosinski, C.; Li, V.S.W.; Chan, A.S.Y.; Zhang, J.; Ho, C.; Tsui, W.Y.; Chan, T.L.; Mifflin, R.C.; Powell, D.W.; Yuen, S.T.; et al. Gene expression patterns of human colon tops and basal crypts and BMP antagonists as intestinal stem cell niche factors. Proc. Natl. Acad. Sci. USA 2007, 104, 15418–15423. [Google Scholar]
- Kretzschmar, M.; Liu, F.; Hata, A.; Doody, J.; Massagué, J. The TGF-β family mediator Smadl is phosphorylated directly and activated functionally by the BMP receptor kinase. Genes Dev. 1997, 11, 984–995. [Google Scholar]
- Batts, L.E.; Polk, D.B.; Dubois, R.N.; Kulessa, H. Bmp signaling is required for intestinal growth and morphogenesis. Dev. Dyn. 2006, 235, 1563–1570. [Google Scholar]
- Kraiczy, J.; McCarthy, N.; Malagola, E.; Tie, G.; Madha, S.; Boffelli, D.; Wagner, D.E.; Wang, T.C.; Shivdasani, R.A. Graded BMP signaling within intestinal crypt architecture directs self-organization of the Wnt-secreting stem cell niche. Cell Stem Cell 2023, 30, 433–449. [Google Scholar]
- Haramis, A.-P.G.; Begthel, H.; van den Born, M.; van Es, J.; Jonkheer, S.; Offerhaus, G.J.A.; Clevers, H. De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science 2004, 303, 1684–1686. [Google Scholar]
- Davis, H.; Irshad, S.; Bansal, M.; Rafferty, H.; Boitsova, T.; Bardella, C.; Jaeger, E.; Lewis, A.; Freeman-Mills, L.; Giner, F.C.; et al. Aberrant epithelial GREM1 expression initiates colonic tumorigenesis from cells outside of the crypt base stem cell niche. Nat. Med. 2015, 21, 62–70. [Google Scholar]
- Fevr, T.; Robine, S.; Louvard, D.; Huelsken, J. Wnt/β-catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol. Cell Biol. 2007, 27, 7551–7559. [Google Scholar]
- Pellegrinet, L.; Rodilla, V.; Liu, Z.; Chen, S.; Koch, U.; Espinosa, L.; Kaestner, K.H.; Kopan, R.; Lewis, J.; Radtke, F. Dll1- and Dll4-mediated Notch signaling is required for homeostasis of intestinal stem cells. Gastroenterology 2011, 140, 1230–1240. [Google Scholar] [PubMed]
- Van Es, J.H.; van Gijn, M.E.; Riccio2, O.; van den Born, M.; Vooijs, M.; Begthel, H.; Cozijnsen, M.; Robine, S.; Winton, D.J.; Freddy Radtke, F.; et al. Notch/γ-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature 2005, 435, 959–963. [Google Scholar] [PubMed]
- Chee, Y.C.; Pahnke, J.; Bunte, R.; Adsool, V.A.; Madan, B.; Virshup, D.M. Intrinsic xenobiotic resistance of the intestinal stem cell niche. Dev. Cell 2018, 46, 681–695. [Google Scholar] [PubMed]
- Beumer, J.; Clevers, H. Regulation and plasticity of intestinal stem cells during homeostasis and regeneration. Development 2016, 143, 3639–3649. [Google Scholar]
- Hendry, J.H.; Potten, C.S. Cryptogenic cells and proliferative cells in intestinal epithelium. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1974, 25, 583–588. [Google Scholar]
- Potten, C.S. Extreme sensitivity of some intestinal crypt cells to X and gamma irradiation. Nature 1977, 269, 518–521. [Google Scholar]
- Sangiorgi, E.; Capecchi, M.R. Bmi1 is expressed in vivo in intestinal stem cells. Nat. Genet. 2008, 40, 915–920. [Google Scholar]
- Tian, H.; Biehs, B.; Warming, S.; Leong, K.G.; Rangell, L.; Klein, O.D.; de Sauvage, F.J. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature 2011, 478, 255–259. [Google Scholar]
- Takeda, N.; Jain, R.; LeBoeuf, M.R.; Wang, Q.; Lu, M.M.; Epstein, J.A. Inter-conversion between intestinal stem cell populations in distinct niches. Science 2011, 334, 1420–1424. [Google Scholar]
- Schmitt, M.; Schewe, M.; Sacchetti, A.; Feijtel, D.; van de Geer, W.S.; Teeuwssen, M.; Sleddens, H.F.; Joosten, R.; van Royen, M.E.; van de Werken, H.J.G.; et al. Paneth cells respond to inflammation and contribute to tissue regeneration by acquiring stem-like features through SCF/c-Kit signaling. Cell Rep. 2018, 24, 2312–2328. [Google Scholar]
- Yu, S.; Tong, K.; Zhao, Y.; Balasubramanian, I.; Yap, G.S.; Ferraris, R.P.; Bonder, E.M.; Verzi, M.P.; Gao, N. Paneth cell multipotency induced by notch activation following injury. Cell Stem Cell 2018, 23, 46–59. [Google Scholar] [PubMed]
- Tetteh, P.W.; Basak, O.; Farin, H.F.; Wiebrands, K.; Kretzschmar, K.; Begthel, H.; van den Born, M.; Korving, J.; de Sauvage, F.; van Es, J.H.; et al. Replacement of lost Lgr5-positive stem cells through plasticity of their enterocyte-lineage daughters. Cell Stem Cell 2016, 18, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Van Es, J.H.; Wiebrands, K.; López-Iglesiasc, C.; van de Wetering, M.; Zeinstra, L.; van den Born, M.; Korving, J.; Sasaki, N.; Peters, P.J.; van Oudenaarden, A.; et al. Enteroendocrine and tuft cells support Lgr5 stem cells on Paneth cell depletion. Proc. Natl. Acad. Sci. USA 2019, 116, 26599–26605. [Google Scholar] [PubMed]
- Capdevila, C.; Miller, J.; Cheng, L.; Komberg, A.; George, J.J.; Lee, H.; Botella, T.; Moon, C.S.; Murray, J.W.; Lam, S.; et al. Time-resolved fate mapping identifies the intestinal upper crypt zone as an origin of Lgr5+ crypt base columnar cells. Cell 2024, 187, 3039–3055. [Google Scholar] [CrossRef]
- Malagola, E.; Vasciaveo, A.; Ochiai, Y.; Kim, W.; Zheng, B.; Zanella, L.; Wang, A.L.E.; Middelhoff, M.; Nienhüser, H.; Deng, L.; et al. Isthmus progenitor cells contribute to homeostatic cellular turnover and support regeneration following intestinal injury. Cell 2024, 187, 3056–3071. [Google Scholar]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef]
- Meyerhof, W.; Batram, C.; Kuhn, C.; Brockhoff, A.; Chudoba, E.; Bufe, B.; Appendino, G.; Behrens, M. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem. Senses 2010, 35, 157–170. [Google Scholar]
- Gerbe, F.; van Es, J.H.; Makrini, L.; Brulin, B.; Mellitzer, G.; Robine, S.; Romagnolo, B.; Shroyer, N.F.; Bourgaux, J.-F.; Pignodel, C.; et al. Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. J. Cell Biol. 2011, 192, 767–780. [Google Scholar] [CrossRef]
- Westphalen, C.B.; Asfaha, S.; Hayakawa, Y.; Takemoto, Y.; Lukin, D.J.; Nuber, A.H.; Brandtner, A.; Setlik, W.; Remotti, H.; Muley, A.; et al. Long-lived intestinal tuft cells serve as colon cancer–initiating cells. J. Clin. Investig. 2014, 124, 1283–1295. [Google Scholar]
- Gerbe, F.; Sidot, E.; Smyth, D.J.; Ohmoto, M.; Matsumoto, I.; Dardalhon, V.; Cesses, P.; Garnier, L.; Bruschi, M.; Harcus, Y.; et al. Intestinal epithelial tuft cells regulate type 2 mucosal responses required for expulsion of helminth parasites. Nature 2016, 529, 226–230. [Google Scholar]
- Howitt, M.R.; Lavoie, S.; Michaud, M.; Blum, A.M.; Tran, S.V.; Weinstock, J.V.; Gallini, C.A.; Redding, K.; Margolskee, R.F.; Osborne, L.C.; et al. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science 2016, 351, 1329–1333. [Google Scholar] [CrossRef] [PubMed]
- Lulu Huang, L.; Bernink, J.H.; Giladi, A.; Krueger, D.; van Son, G.J.F.; Geurts, M.H.; Busslinger, G.; Lin, L.; Begthel, H.; Zandvliet, M.; et al. Tuft cells act as regenerative stem cells in the human intestine. Nature 2024, 634, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Ting, H.A.; von Moltke, J. The immune function of tuft cells at gut mucosal surfaces and beyond. J. Immunol. 2019, 202, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Hollenhorst, M.I.; Jurastow, I.; Nandigama, R.; Appenzeller, S.; Li, L.; Vogel, J.; Wiederhold, S.; Althaus, M.; Empting, M.; Altmüller, J.; et al. Tracheal brush cells release acetylcholine in response to bitter tastants for paracrine and autocrine signaling. FASEB J. 2020, 34, 316–332. [Google Scholar] [CrossRef]
- Takahashi, T.; Ohnishi, H.; Sugiura, Y.; Honda, K.; Suematsu, M.; Kawasaki, T.; Deguchi, T.; Fujii, T.; Orihashi, K.; Hippo, Y.; et al. Non-neuronal acetylcholine as an endogenous regulator of proliferation and diffusion of lgr5-positive stem cells in mice. FEBS J. 2014, 281, 4672–4690. [Google Scholar] [CrossRef]
- Takahashi, T.; Shiraishi, A.; Murata, J.; Matsubara, S.; Nakaoka, S.; Kirimoto, S.; Osawa, M. Muscarinic receptor M3 contributes to intestinal stem cell maintenance via EphB/ephrin-B signaling. Life Sci. Alliance 2021, 4, e202000962. [Google Scholar] [CrossRef]
- Middelhoff, M.; Nienhüser, H.; Valenti, G.; Maurer, C.; Hayakawa, Y.; Takahashi, R.; Kim, W.; Jiang, Z.; Malagola, E.; Cuti, K.; et al. Prox1-positive cells monitor and sustain the murine intestinal epithelial cholinergic niche. Nat. Commun. 2020, 11, 111. [Google Scholar] [CrossRef]
- Wessler, I.; Kirkpatrick, C.J. Acetylcholine beyond neurons: The non-neuronal cholinergic system in humans. Br. J. Pharmacol. 2008, 154, 1558–1571. [Google Scholar] [CrossRef]
- Takahashi, T.; Shiraishi, A.; Murata, J. The coordinated activities of nAChR and Wnt signaling regulate intestinal stem cell function in mice. Int. J. Mol. Sci. 2018, 19, 738. [Google Scholar] [CrossRef]
- Takahashi, T. Multiple roles for cholinergic signaling from the perspective of stem cell function. Int. J. Mol. Sci. 2021, 22, 666. [Google Scholar] [CrossRef]
- Santos, A.J.M.; Lo, Y.-H.; Mah, A.T.; Kuo, C.J. The intestinal stem cell niche: Homeostasis and adaptations. Trends Cell Biol. 2018, 28, 1062–1078. [Google Scholar] [PubMed]
- Spradling, A.; Drummond-Barbosa, D.; Kai, T. Stem cells find their niche. Nature 2001, 414, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Elosegui-Artola, A. The extracellular matrix viscoelasticity as a regulator of cell and tissue dynamics. Curr. Opin. Cell Biol. 2021, 72, 10–18. [Google Scholar]
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A.; Shenoy, V.B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 2020, 584, 535–546. [Google Scholar]
- Briscoe, J.; Small, S. Morphogen rules: Design principles of gradient-mediated embryo patterning. Development 2015, 142, 3996–4009. [Google Scholar]
- Romanova-Michaelides, M.; Hadjivasiliou, Z.; Aguilar-Hidalgo, D.; Basagiannis, D.; Seum, C.; Dubois, M.; Jülicher, F.; Gonzalez-Gaitan, M. Morphogen gradient scaling by recycling of intracellular Dpp. Nature 2022, 602, 287–293. [Google Scholar]
- Kicheva, A.; Bollenbach, T.; Wartlick, O.; Jülicher, F.; Gonzalez-Gaitan, M. Investigating the principles of morphogen gradient formation: From tissue to cells. Curr. Opin. Genet. Dev. 2012, 22, 527–532. [Google Scholar]
- Pérez-González, C.; Ceada, G.; Greco, F.; Matejčić, M.; Gómez-González, M.; Castro, N.; Menendez, A.; Kale, S.; Krndija, D.; Clark, A.G. Mechanical compartmentalization of the intestinal organoid enables crypt folding and collective cell migration. Nat. Cell Biol. 2021, 23, 745–757. [Google Scholar]
- Gao, G.; Wei, G.; Liu, S.; Chen, J.; Zeng, Z.; Zhang, X.; Chen, F.; Zhuo, L.; Hsu, W.; Li, D.; et al. Epithelial Wntless is dispensable for intestinal tumorigenesis in mouse models. Biochem. Biophys. Res. Commun. 2019, 519, 754–760. [Google Scholar]
- Ootani, A.; Li, X.; Sangiorgi, E.; Ho, Q.T.; Ueno, H.; Toda, S.; Sugihara, H.; Fujimoto, K.; Weissman, I.W.; Capecchi, M.R.; et al. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat. Med. 2009, 15, 701–706. [Google Scholar] [CrossRef]
- McCracken, K.W.; Howell, J.C.; Wells, J.M.; Spence, J.R. Generating human intestinal tissue from pluripotent stem cells in vitro. Nat. Protoc. 2011, 6, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Münera, J.O.; Sundaram, N.; Rankin, S.A.; Hill, D.; Watson, C.; Mahe, M.; Vallance, J.E.; Shroyer, N.F.; Sinagoga, K.L.; Zarzoso-Lacoste, A.; et al. Differentiation of human pluripotent stem cells into colonic organoids via transient activation of BMP signaling. Cell Stem Cell 2017, 21, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Chin, A.M.; Hill, D.R.; Aurora, M.; Spence, J.R. Morphogenesis and maturation of the embryonic and postnatal intestine. Semin. Cell Dev. Biol. 2017, 66, 81–93. [Google Scholar] [CrossRef]
- Radhakrishnan, J.; Varadaraj, S.; Dash, S.K.; Sharma, A.; Verma, R.S. Organotypic cancer tissue models for drug screening: 3D constructs, bioprinting and microfluidic chips. Drug Discov. Today 2020, 25, 879–890. [Google Scholar] [CrossRef]
- Shirure, V.S.; Hughes, C.C.W.; George, S.C. Engineering vascularized organoid-on-a-chip models. Annu. Rev. Biomed. Eng. 2021, 23, 141–167. [Google Scholar] [CrossRef]
- Schafer, S.T.; Mansour, A.A.; Schlachetzki, J.C.M.; Pena, M.; Ghassemzadeh, S.; Mitchell, L.; Mar, A.; Quang, D.; Stumpf, S.; Ortiz, I.S.; et al. An in vivo neuroimmune organoid model to study human microglia phenotypes. Cell 2023, 186, 2111–2126.e2120. [Google Scholar] [CrossRef]
- Wilson, S.S.; Tocchi, A.; Holly, M.K.; Parks, W.C.; Smith, J.G.A. Small Intestinal Organoid Model of Non-Invasive Enteric Pathogen-Epithelial Cell Interactions. Mucosal Immunol. 2015, 8, 352–361. [Google Scholar] [CrossRef]
- Co, J.Y.; Margalef-Català, M.; Li, X.; Mah, A.T.; Kuo, C.J.; Monack, D.M.; Amieva, M.R. Controlling Epithelial Polarity: A Human Enteroid Model for Host-Pathogen Interactions. Cell Rep. 2019, 26, 2509–2520.e4. [Google Scholar] [CrossRef]
- Sambuy, Y.; De Angelis, I.; Ranaldi, G.; Scarino, M.L.; Stammati, A.; Zucco, F. The Caco-2 Cell Line as a Model of the Intestinal Barrier: Influence of Cell and Culture-Related Factors on Caco-2 Cell Functional Characteristics. Cell Biol. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef]
- Sun, H.; Chow, E.C.Y.; Liu, S.; Du, Y.; Pang, K.S. The Caco-2 Cell Monolayer: Usefulness and Limitations. Expert Opin. Drug Metab. Toxicol. 2008, 4, 395–411. [Google Scholar] [CrossRef]
- Negoro, R.; Takayama, K.; Kawai, K.; Harada, K.; Sakurai, F.; Hirata, K.; Mizuguchi, H. Efficient Generation of Small Intestinal Epithelial-like Cells from Human IPSCs for Drug Absorption and Metabolism Studies. Stem Cell Rep. 2018, 11, 1539–1550. [Google Scholar]
- Moon, C.; Vandussen, K.L.; Miyoshi, H.; Stappenbeck, T.S. Development of a Primary Mouse Intestinal Epithelial Cell Monolayer Culture System to Evaluate Factors That Modulate IgA Transcytosis. Mucosal Immunol. 2014, 7, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Ettayebi, K.; Crawford, S.E.; Murakami, K.; Broughman, J.R.; Karandikar, U.; Tenge, V.R.; Neill, F.H.; Blutt, S.E.; Zeng, X.L.; Qu, L.; et al. Replication of Human Noroviruses in Stem Cell-Derived Human Enteroids. Science 2016, 353, 1387–1393. [Google Scholar] [PubMed]
- VanDussen, K.L.; Marinshaw, J.M.; Shaikh, N.; Miyoshi, H.; Moon, C.; Tarr, P.I.; Ciorba, M.A.; Stappenbeck, T.S. Development of an Enhanced Human Gastrointestinal Epithelial Culture System to Facilitate Patient-Based Assays. Gut 2015, 64, 911–920. [Google Scholar]
- Kozuka, K.; He, Y.; Koo-McCoy, S.; Kumaraswamy, P.; Nie, B.; Shaw, K.; Chan, P.; Leadbetter, M.; He, L.; Lewis, J.G.; et al. Development and Characterization of a Human and Mouse Intestinal Epithelial Cell Monolayer Platform. Stem Cell Rep. 2017, 9, 1976–1990. [Google Scholar]
- Sasaki, N.; Miyamoto, K.; Maslowski, K.M.; Ohno, H.; Kanai, T.; Sato, T. Development of a Scalable Coculture System for Gut Anaerobes and Human Colon Epithelium. Gastroenterology 2020, 159, 388–390.e5. [Google Scholar] [CrossRef]
- Takahashi, Y.; Noguchi, M.; Inoue, Y.; Sato, S.; Shimizu, M.; Kojima, H.; Okabe, T.; Kiyono, H.; Yamauchi, Y.; Sato, R. Organoid-Derived Intestinal Epithelial Cells Are a Suitable Model for Preclinical Toxicology and Pharmacokinetic Studies. iScience 2022, 25, 104542. [Google Scholar]
- Puzan, M.; Hosic, S.; Ghio, C.; Koppes, A. Enteric Nervous System Regulation of Intestinal Stem Cell Differentiation and Epithelial Monolayer Function. Sci. Rep. 2018, 8, 6313. [Google Scholar]
- Altay, G.; Larrañaga, E.; Tosi, S.; Barriga, F.M.; Batlle, E.; Fernández-Majada, V.; Martínez, E. Self-Organized Intestinal Epithelial Monolayers in Crypt and Villus-like Domains Show Effective Barrier Function. Sci. Rep. 2019, 9, 10140. [Google Scholar]
- Hoffmann, P.; Schnepel, N.; Langeheine, M.; Kunnemann, K.; Grassl, G.A.; Brehm, R.; Seeger, B.; Mazzuoli-Weber, G.; Breves, G. Intestinal Organoid-Based 2D Monolayers Mimic Physiological and Pathophysiological Properties of the Pig Intestine. PLoS ONE 2021, 16, e0256143. [Google Scholar]
- Kawasaki, M.; Ambrosini, Y.M. Accessible Luminal Interface of Bovine Rectal Organoids Generated from Cryopreserved Biopsy Tissues. PLoS ONE 2024, 19, e0301079. [Google Scholar]
- Takase, Y.; Takahashi, T. Method for two-dimensional epithelial monolayer formation derived from mouse three-dimensional small intestinal organoids. Methods Mol. Biol. 2024, 2749, 73–84. [Google Scholar] [PubMed]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [PubMed]
- Zhao, L.Z.; Song, W.L.; Chen, Y.G. Mesenchymal-epithelial interaction regulates gastrointestinal tract development in mouse embryos. Cell Rep. 2022, 40, 111053. [Google Scholar]
- Felsenthal, N.; Vignjevic, D.M. Stand by Me: Fibroblasts Regulation of the Intestinal Epithelium during Development and Homeostasis. Curr. Opin. Cell Biol. 2022, 78, 102116. [Google Scholar]
- Onozato, D.; Ogawa, I.; Kida, Y.; Mizuno, S.; Hashita, T.; Iwao, T.; Matsunaga, T. Generation of Budding-Like Intestinal Organoids from Human Induced Pluripotent Stem Cells. J. Pharm. Sci. 2021, 110, 2637–2650. [Google Scholar]
- Sugimoto, S.; Kobayashi, E.; Fujii, M.; Ohta, Y.; Arai, K.; Matano, M.; Ishikawa, K.; Miyamoto, K.; Toshimitsu, K.; Takahashi, S.; et al. An Organoid-Based Organ-Repurposing Approach to Treat Short Bowel Syndrome. Nature 2021, 592, 99–104. [Google Scholar]
- Eicher, A.K.; Kechele, D.O.; Sundaram, N.; Berns, H.M.; Poling, H.M.; Haines, L.E.; Sanchez, J.G.; Kishimoto, K.; Krishnamurthy, M.; Han, L.; et al. Functional human gastrointestinal organoids can be engineered from three primary germ layers derived separately from pluripotent stem cells. Cell Stem Cell 2022, 29, 36–51. [Google Scholar]
- Deguchi, S.; Kosugi, K.; Takeishi, N.; Watanabe, Y.; Morimoto, S.; Negoro, R.; Yokoi, F.; Futatsusako, H.; Nakajima-Koyama, M.; Iwasaki, M.; et al. Construction of multilayered small intestine-like tissue by reproducing interstitial flow. Cell Stem Cell 2024, 31, 1315–1326. [Google Scholar]
- Pentinmikko, N.; Lozano, R.; Scharaw, S.; Andersson, S.; Englund, J.I.; Castillo-Azofeifa, D.; Gallagher, A.; Broberg, M.; Song, K.Y.; Carvajal, A.S.; et al. Cellular Shape Reinforces Niche to Stem Cell Signaling in the Small Intestine. Sci. Adv. 2022, 8, eabm1847. [Google Scholar]
- Yavitt, F.M.; Kirkpatrick, B.E.; Blatchley, M.R.; Speckl, K.F.; Mohagheghian, E.; Moldovan, R.; Wang, N.; Dempsey, P.J.; Anseth, K.S. In Situ Modulation of Intestinal Organoid Epithelial Curvature through Photoinduced Viscoelasticity Directs Crypt Morphogenesis. Sci. Adv. 2023, 9, eadd5668. [Google Scholar] [PubMed]
- Baghdadi, M.B.; Houtekamer, R.M.; Perrin, L.; Rao-Bhatia, A.; Whelen, M.; Decker, L.; Bergert, M.; Pérez-Gonzàlez, C.; Bouras, R.; Gropplero, G.; et al. PIEZO-Dependent Mechanosensing Is Essential for Intestinal Stem Cell Fate Decision and Maintenance. Science 2024, 386, eadj7615. [Google Scholar] [PubMed]
- Shivdasani, R.A.; Clevers, H.; Sauvage, F.J. Tissue regeneration: Reserve or reverse? Science 2021, 371, 784–786. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).