Utilizing Plant Growth-Promoting Rhizobacteria (PGPR) to Advance Sustainable Agriculture
Abstract
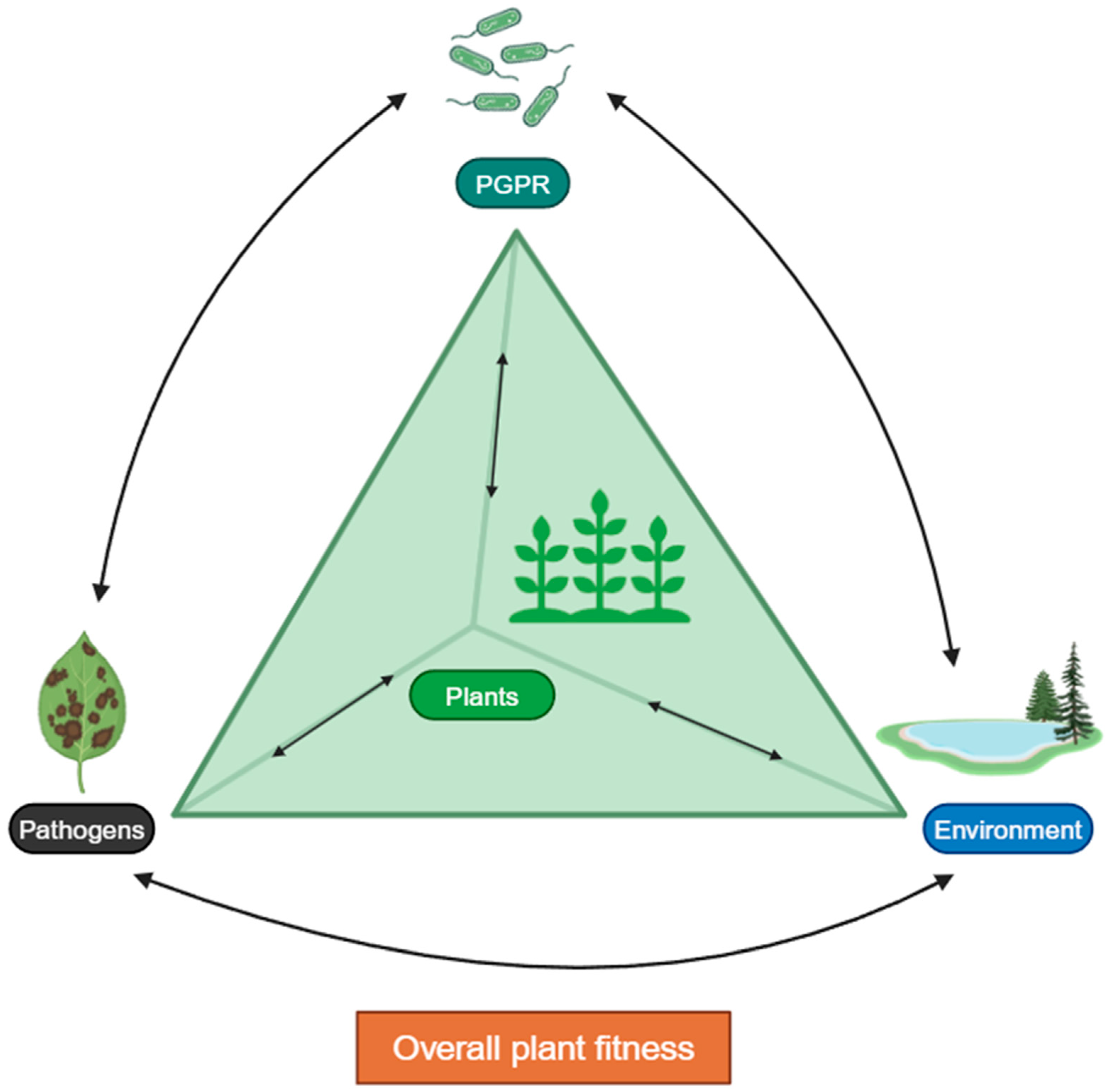
1. Introduction
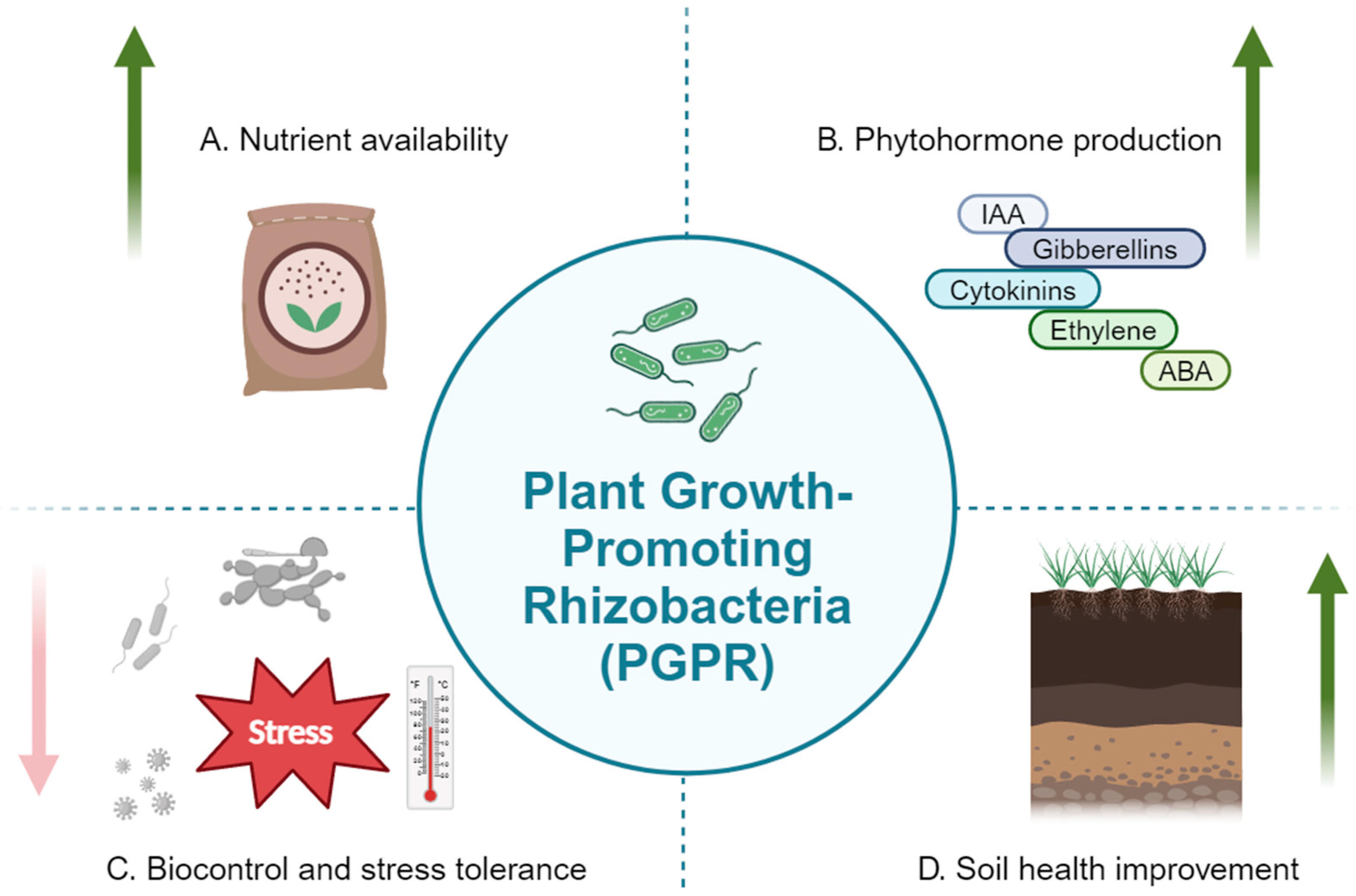
2. Mechanisms of Action
2.1. Nutrient Solubilization and Fixation
2.2. Phytohormone Production
2.3. Biocontrol of Pathogens
2.4. Abiotic Stress Tolerance
3. Applications in Agriculture
3.1. Biofertilizers
3.2. Seed Treatments and Soil Amendments
4. Challenges and Limitations
4.1. Consistency and Survival
4.2. Compatibility and Interaction
4.3. Commercialization and Adoption
5. Advances and Future Directions
5.1. Nano-Encapsulation Technology
5.2. Biotechnological Approaches
5.3. Integrated Plant Management (IPM)
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mohanty, P.; Singh, P.K.; Chakraborty, D.; Mishra, S.; Pattnaik, R. Insight Into the Role of PGPR in Sustainable Agriculture and Environment. Front. Sustain. Food Syst. 2021, 5, 667150. [Google Scholar] [CrossRef]
- Yang, P.; Bokros, N.; Debolt, S.; Zhao, Z.; Xia, Y. Genome Sequence Source of Bacillus amyloliquefaciens Strain GD4a, a Bacterial Endophyte Associated with Switchgrass Plants. Phytobiomes J. 2022, 6, 354–357. [Google Scholar] [CrossRef]
- Sahib, M.R.; Yang, P.; Bokros, N.; Shapiro, N.; Woyke, T.; Kyrpides, N.C.; Xia, Y.; DeBolt, S. Improved Draft Genome Sequence of Microbacterium sp. Strain LKL04, a Bacterial Endophyte Associated with Switchgrass Plants. Microbiol. Resour. Announc. 2019, 8, 10-1128. [Google Scholar] [CrossRef]
- Yang, P.; Yuan, P.; Liu, W.; Zhao, Z.; Bernier, M.C.; Zhang, C.; Adhikari, A.; Opiyo, S.O.; Zhao, L.; Banks, F.; et al. Plant Growth Promotion and Plant Disease Suppression Induced by Bacillus amyloliquefaciens Strain GD4a. Plants 2024, 13, 672. [Google Scholar] [CrossRef]
- Yang, P.; Liu, W.; Yuan, P.; Zhao, Z.; Zhang, C.; Opiyo, S.O.; Adhikari, A.; Zhao, L.; Harsh, G.; Xia, Y. Plant Growth Promotion and Stress Tolerance Enhancement through Inoculation with Bacillus proteolyticus OSUB18. Biology 2023, 12, 1495. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Zhao, Z.; Fan, J.; Liang, Y.; Bernier, M.C.; Gao, Y.; Zhao, L.; Opiyo, S.O.; Xia, Y. Bacillus proteolyticus OSUB18 Triggers Induced Systemic Resistance against Bacterial and Fungal Pathogens in Arabidopsis. Front. Plant Sci. 2023, 14, 1078100. [Google Scholar] [CrossRef]
- Zhu, Y.-G.; Peng, J.; Chen, C.; Xiong, C.; Li, S.; Ge, A.; Wang, E.; Liesack, W. Harnessing Biological Nitrogen Fixation in Plant Leaves. Trends Plant Sci. 2023, 12, 1391–1405. [Google Scholar] [CrossRef]
- Feng, J.; Lee, T.; Schiessl, K.; Oldroyd, G.E.D. Processing of NODULE INCEPTION Controls the Transition to Nitrogen Fixation in Root Nodules. Science 2021, 374, 629–632. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, L.A.; Santos, C.H.B.; Frezarin, E.T.; Sales, L.R.; Rigobelo, E.C. Plant Growth-Promoting Rhizobacteria for Sustainable Agricultural Production. Microorganisms 2023, 11, 1088. [Google Scholar] [CrossRef]
- Wang, D.; Wang, C.; Chen, Y.; Xie, Z. Developing Plant-Growth-Promoting Rhizobacteria: A Crucial Approach for Achieving Sustainable Agriculture. Agronomy 2023, 13, 1835. [Google Scholar] [CrossRef]
- Vejan, P.; Abdullah, R.; Khadiran, T.; Ismail, S.; Nasrulhaq Boyce, A. Role of Plant Growth Promoting Rhizobacteria in Agricultural Sustainability—A Review. Molecules 2016, 21, 573. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, P.N.; Jha, D.K. Plant Growth-Promoting Rhizobacteria (PGPR): Emergence in Agriculture. World J. Microbiol. Biotechnol. 2012, 28, 1327–1350. [Google Scholar] [CrossRef]
- Katiyar, D.; Singh, B. Plant Growth Promoting Rhizobacteria-an Efficient Tool for Agriculture Promotion. Adv. Plants Agric. Res. 2016, 4, 426–434. [Google Scholar] [CrossRef]
- Hasan, A.; Tabassum, B.; Hashim, M.; Khan, N. Role of Plant Growth Promoting Rhizobacteria (PGPR) as a Plant Growth Enhancer for Sustainable Agriculture: A Review. Bacteria 2024, 3, 59–75. [Google Scholar] [CrossRef]
- Mekonnen, H.; Kibret, M. The Roles of Plant Growth Promoting Rhizobacteria in Sustainable Vegetable Production in Ethiopia. Chem. Biol. Technol. Agric. 2021, 8, 15. [Google Scholar] [CrossRef]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate Solubilizing Microbes: Sustainable Approach for Managing Phosphorus Deficiency in Agricultural Soils. SpringerPlus 2013, 2, 587. [Google Scholar] [CrossRef]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial Phosphorus Solubilization and Its Potential for Use in Sustainable Agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef] [PubMed]
- Oleńska, E.; Małek, W.; Wójcik, M.; Swiecicka, I.; Thijs, S.; Vangronsveld, J. Beneficial Features of Plant Growth-Promoting Rhizobacteria for Improving Plant Growth and Health in Challenging Conditions: A Methodical Review. Sci. Total Environ. 2020, 743, 140682. [Google Scholar] [CrossRef]
- Liang, J.; Klingl, A.; Lin, Y.-Y.; Boul, E.; Thomas-Oates, J.; Marín, M. A Subcompatible Rhizobium Strain Reveals Infection Duality in Lotus. J. Exp. Bot. 2019, 70, 1903–1913. [Google Scholar] [CrossRef]
- Ahemad, M.; Kibret, M. Mechanisms and Applications of Plant Growth Promoting Rhizobacteria: Current Perspective. J. King Saud. Univ.-Sci. 2014, 26, 1–20. [Google Scholar] [CrossRef]
- Poveda, J.; Eugui, D.; Abril-Urías, P.; Velasco, P. Endophytic Fungi as Direct Plant Growth Promoters for Sustainable Agricultural Production. Symbiosis 2021, 85, 1–19. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, J.-J.; Zhang, J.-Z. Aux/IAA Gene Family in Plants: Molecular Structure, Regulation, and Function. Int. J. Mol. Sci. 2018, 19, 259. [Google Scholar] [CrossRef] [PubMed]
- Grobelak, A.; Napora, A.; Kacprzak, M. Using Plant Growth-Promoting Rhizobacteria (PGPR) to Improve Plant Growth. Ecol. Eng. 2015, 84, 22–28. [Google Scholar] [CrossRef]
- Nett, R.S.; Montanares, M.; Marcassa, A.; Lu, X.; Nagel, R.; Charles, T.C.; Hedden, P.; Rojas, M.C.; Peters, R.J. Elucidation of Gibberellin Biosynthesis in Bacteria Reveals Convergent Evolution. Nat. Chem. Biol. 2017, 13, 69–74. [Google Scholar] [CrossRef]
- Rizza, A.; Jones, A.M. The Makings of a Gradient: Spatiotemporal Distribution of Gibberellins in Plant Development. Curr. Opin. Plant Biol. 2019, 47, 9–15. [Google Scholar] [CrossRef]
- Adeleke, B.S.; Babalola, O.O. The Endosphere Microbial Communities, a Great Promise in Agriculture. Int. Microbiol. 2021, 24, 1–17. [Google Scholar] [CrossRef]
- Guo, Y.; Ren, G.; Zhang, K.; Li, Z.; Miao, Y.; Guo, H. Leaf Senescence: Progression, Regulation, and Application. Mol. Hortic. 2021, 1, 5. [Google Scholar] [CrossRef]
- Hönig, M.; Plíhalová, L.; Husičková, A.; Nisler, J.; Doležal, K. Role of Cytokinins in Senescence, Antioxidant Defence and Photosynthesis. Int. J. Mol. Sci. 2018, 19, 4045. [Google Scholar] [CrossRef]
- Mekureyaw, M.F.; Pandey, C.; Hennessy, R.C.; Nicolaisen, M.H.; Liu, F.; Nybroe, O.; Roitsch, T. The Cytokinin-Producing Plant Beneficial Bacterium Pseudomonas Fluorescens G20-18 Primes Tomato (Solanum Lycopersicum) for Enhanced Drought Stress Responses. J. Plant Physiol. 2022, 270, 153629. [Google Scholar] [CrossRef]
- Lobhi, D.; Patil, N.P.; Sansinenea, E.; Sayyed, R.Z. Plant Growth-Promoting Rhizobacteria (PGPR): An Overview. In Secondary Metabolites and Volatiles of PGPR in Plant-Growth Promotion; Sayyed, R.Z., Uarrota, V.G., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 1–19. ISBN 978-3-031-07559-9. [Google Scholar]
- Yang, P.; Zhao, L.; Gao, Y.G.; Xia, Y. Detection, Diagnosis, and Preventive Management of the Bacterial Plant Pathogen Pseudomonas syringae. Plants 2023, 12, 1765. [Google Scholar] [CrossRef]
- Wang, H.; Liu, R.; You, M.P.; Barbetti, M.J.; Chen, Y. Pathogen Biocontrol Using Plant Growth-Promoting Bacteria (PGPR): Role of Bacterial Diversity. Microorganisms 2021, 9, 1988. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Takishita, Y.; Zhou, G.; Smith, D.L. Plant Associated Rhizobacteria for Biocontrol and Plant Growth Enhancement. Front. Plant Sci. 2021, 12, 634796. [Google Scholar] [CrossRef] [PubMed]
- Al-Turki, A.; Murali, M.; Omar, A.F.; Rehan, M.; Sayyed, R.Z. Recent Advances in PGPR-Mediated Resilience toward Interactive Effects of Drought and Salt Stress in Plants. Front. Microbiol. 2023, 14, 1214845. [Google Scholar] [CrossRef]
- Bhat, M.A.; Kumar, V.; Bhat, M.A.; Wani, I.A.; Dar, F.L.; Farooq, I.; Bhatti, F.; Koser, R.; Rahman, S.; Jan, A.T. Mechanistic Insights of the Interaction of Plant Growth-Promoting Rhizobacteria (PGPR) With Plant Roots Toward Enhancing Plant Productivity by Alleviating Salinity Stress. Front. Microbiol. 2020, 11, 1952. [Google Scholar] [CrossRef]
- Ilangumaran, G.; Smith, D.L. Plant Growth Promoting Rhizobacteria in Amelioration of Salinity Stress: A Systems Biology Perspective. Front. Plant Sci. 2017, 8, 1768. [Google Scholar] [CrossRef]
- Kour, D.; Rana, K.L.; Sheikh, I.; Kumar, V.; Yadav, A.N.; Dhaliwal, H.S.; Saxena, A.K. Alleviation of Drought Stress and Plant Growth Promotion by Pseudomonas libanensis EU-LWNA-33, a Drought-Adaptive Phosphorus-Solubilizing Bacterium. Proc. Natl. Acad. Sci., India Sect. B Biol. Sci. 2020, 90, 785–795. [Google Scholar] [CrossRef]
- Jochum, M.D.; McWilliams, K.L.; Borrego, E.J.; Kolomiets, M.V.; Niu, G.; Pierson, E.A.; Jo, Y.-K. Bioprospecting Plant Growth-Promoting Rhizobacteria That Mitigate Drought Stress in Grasses. Front. Microbiol. 2019, 10, 2106. [Google Scholar] [CrossRef]
- Vurukonda, S.S.K.P.; Vardharajula, S.; Shrivastava, M.; SkZ, A. Enhancement of Drought Stress Tolerance in Crops by Plant Growth Promoting Rhizobacteria. Microbiol. Res. 2016, 184, 13–24. [Google Scholar] [CrossRef]
- Nawaz, A.; Shahbaz, M.; Asadullah; Imran, A.; Marghoob, M.U.; Imtiaz, M.; Mubeen, F. Potential of Salt Tolerant PGPR in Growth and Yield Augmentation of Wheat (Triticum aestivum L.) Under Saline Conditions. Front. Microbiol. 2020, 11, 2019. [Google Scholar] [CrossRef]
- Abbas, R.; Rasul, S.; Aslam, K.; Baber, M.; Shahid, M.; Mubeen, F.; Naqqash, T. Halotolerant PGPR: A Hope for Cultivation of Saline Soils. J. King Saud. Univ.-Sci. 2019, 31, 1195–1201. [Google Scholar] [CrossRef]
- Dehghani Bidgoli, R.; Azarnezhad, N.; Akhbari, M.; Ghorbani, M. Salinity Stress and PGPR Effects on Essential Oil Changes in Rosmarinus officinalis L. Agric. Food Secur. 2019, 8, 2. [Google Scholar] [CrossRef]
- Goswami, M.; Deka, S. Plant Growth-Promoting Rhizobacteria—Alleviators of Abiotic Stresses in Soil: A Review. Pedosphere 2020, 30, 40–61. [Google Scholar] [CrossRef]
- Numan, M.; Bashir, S.; Khan, Y.; Mumtaz, R.; Shinwari, Z.K.; Khan, A.L.; Khan, A.; AL-Harrasi, A. Plant Growth Promoting Bacteria as an Alternative Strategy for Salt Tolerance in Plants: A Review. Microbiol. Res. 2018, 209, 21–32. [Google Scholar] [CrossRef]
- Yashavantha Rao, H.C.; Chandra Mohana, N.; Satish, S. Biocommercial Aspects of Microbial Endophytes for Sustainable Agriculture. In Microbial Endophytes; Kumar, A., E.k, R., Eds.; Woodhead Publishing: Cambridge, UK, 2020; pp. 323–347. ISBN 978-0-12-819654-0. [Google Scholar]
- García-López, J.V.; Redondo-Gómez, S.; Flores-Duarte, N.J.; Zunzunegui, M.; Rodríguez-Llorente, I.D.; Pajuelo, E.; Mateos-Naranjo, E. Exploring through the Use of Physiological and Isotopic Techniques the Potential of a PGPR-Based Biofertilizer to Improve Nitrogen Fertilization Practices Efficiency in Strawberry Cultivation. Front. Plant Sci. 2023, 14, 1243509. [Google Scholar] [CrossRef]
- Zhang, T.; Jian, Q.; Yao, X.; Guan, L.; Li, L.; Liu, F.; Zhang, C.; Li, D.; Tang, H.; Lu, L. Plant Growth-Promoting Rhizobacteria (PGPR) Improve the Growth and Quality of Several Crops. Heliyon 2024, 10, e31553. [Google Scholar] [CrossRef] [PubMed]
- Aloo, B.N.; Tripathi, V.; Makumba, B.A.; Mbega, E.R. Plant Growth-Promoting Rhizobacterial Biofertilizers for Crop Production: The Past, Present, and Future. Front. Plant Sci. 2022, 13, 1002448. [Google Scholar] [CrossRef]
- Kumar, M.; Giri, V.P.; Pandey, S.; Gupta, A.; Patel, M.K.; Bajpai, A.B.; Jenkins, S.; Siddique, K.H.M. Plant-Growth-Promoting Rhizobacteria Emerging as an Effective Bioinoculant to Improve the Growth, Production, and Stress Tolerance of Vegetable Crops. Int. J. Mol. Sci. 2021, 22, 12245. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Turgay, O.C.; Farooq, M.; Hayat, R. Seed Biopriming with Plant Growth Promoting Rhizobacteria: A Review. FEMS Microbiol. Ecol. 2016, 92, fiw112. [Google Scholar] [CrossRef]
- Romero-Estonllo, M.; Ramos-Castro, J.; San Miguel del Río, Y.; Rodríguez-Garrido, B.; Prieto-Fernández, Á.; Kidd, P.S.; Monterroso, C. Soil Amendment and Rhizobacterial Inoculation Improved Cu Phytostabilization, Plant Growth and Microbial Activity in a Bench-Scale Experiment. Front. Microbiol. 2023, 14, 1184070. [Google Scholar] [CrossRef]
- Yang, P. Exploring Plant-Microbe Interactions through the Lens of Beneficial Bacteria. Doctoral Dissertation, The Ohio State University, Columbus, OH, USA, 2023. [Google Scholar]
- Ikiz, B.; Dasgan, H.Y.; Gruda, N.S. Utilizing the Power of Plant Growth Promoting Rhizobacteria on Reducing Mineral Fertilizer, Improved Yield, and Nutritional Quality of Batavia Lettuce in a Floating Culture. Sci. Rep. 2024, 14, 1616. [Google Scholar] [CrossRef]
- Samain, E.; Duclercq, J.; Ait Barka, E.; Eickermann, M.; Ernenwein, C.; Mazoyon, C.; Sarazin, V.; Dubois, F.; Aussenac, T.; Selim, S. PGPR-Soil Microbial Communities’ Interactions and Their Influence on Wheat Growth Promotion and Resistance Induction against Mycosphaerella graminicola. Biology 2023, 12, 1416. [Google Scholar] [CrossRef] [PubMed]
- Haskett, T.L.; Tkacz, A.; Poole, P.S. Engineering Rhizobacteria for Sustainable Agriculture. ISME J. 2021, 15, 949–964. [Google Scholar] [CrossRef]
- Zipfel, C.; Oldroyd, G.E.D. Plant Signalling in Symbiosis and Immunity. Nature 2017, 543, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Rosier, A.; Medeiros, F.H.V.; Bais, H.P. Defining Plant Growth Promoting Rhizobacteria Molecular and Biochemical Networks in Beneficial Plant-Microbe Interactions. Plant Soil. 2018, 428, 35–55. [Google Scholar] [CrossRef]
- Vacheron, J.; Desbrosses, G.; Bouffaud, M.-L.; Touraine, B.; Moënne-Loccoz, Y.; Muller, D.; Legendre, L.; Wisniewski-Dyé, F.; Prigent-Combaret, C. Plant Growth-Promoting Rhizobacteria and Root System Functioning. Front. Plant Sci. 2013, 4, 356. [Google Scholar] [CrossRef] [PubMed]
- Thakur, R.; Dhar, H.; Mathew, S. PGPR Strategies for Climate-Resilient Agriculture. July 2024. Available online: https://www.the-microbiologist.com/features/pgpr-strategies-for-climate-resilient-agriculture/3389.article (accessed on 25 August 2024).
- Wang, C.; Kuzyakov, Y. Rhizosphere Engineering for Soil Carbon Sequestration. Trends Plant Sci. 2024, 29, 447–468. [Google Scholar] [CrossRef]
- Wang, B.; Wang, C.; Xue, R. Improvement of Rhizosphere—Rhizoplane—Endosphere Bacterial Network on Salt Tolerance of Maize after Biological Enhancement of Earthworm and Mycorrhizal Fungi. Plant Soil. 2024, 7, 1–7. [Google Scholar] [CrossRef]
- Li, Y.; Narayanan, M.; Shi, X.; Chen, X.; Li, Z.; Ma, Y. Biofilms Formation in Plant Growth-Promoting Bacteria for Alleviating Agro-Environmental Stress. Sci. Total Environ. 2024, 907, 167774. [Google Scholar] [CrossRef]
- Khoso, M.A.; Wagan, S.; Alam, I.; Hussain, A.; Ali, Q.; Saha, S.; Poudel, T.R.; Manghwar, H.; Liu, F. Impact of Plant Growth-Promoting Rhizobacteria (PGPR) on Plant Nutrition and Root Characteristics: Current Perspective. Plant Stress. 2024, 11, 100341. [Google Scholar] [CrossRef]
- Igiehon, B.C.; Babalola, O.O.; Hassen, A.I. Rhizosphere Competence and Applications of Plant Growth-Promoting Rhizobacteria in Food Production—A Review. Sci. Afr. 2024, 23, e02081. [Google Scholar] [CrossRef]
- Agbodjato, N.A.; Babalola, O.O. Promoting Sustainable Agriculture by Exploiting Plant Growth-Promoting Rhizobacteria (PGPR) to Improve Maize and Cowpea Crops. PeerJ 2024, 12, e16836. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Yadav, V.K.; Chundawat, R.S.; Soltane, R.; Awwad, N.S.; Ibrahium, H.A.; Yadav, K.K.; Vicas, S.I. Enhancing Plant Growth Promoting Rhizobacterial Activities through Consortium Exposure: A Review. Front. Bioeng. Biotechnol. 2023, 11, 1099999. [Google Scholar] [CrossRef] [PubMed]
- Barratt, B.I.P.; Moran, V.C.; Bigler, F.; van Lenteren, J.C. The Status of Biological Control and Recommendations for Improving Uptake for the Future. BioControl 2018, 63, 155–167. [Google Scholar] [CrossRef]
- Begum, M.; Lees, E.; Ampt, P.; Mansfield, S. Development of Australian Commercial Producers of Invertebrate Biological Control Agents from 1971 to 2014. BioControl 2017, 62, 525–533. [Google Scholar] [CrossRef]
- Bashan, Y.; de-Bashan, L.E.; Prabhu, S.R.; Hernandez, J.-P. Advances in Plant Growth-Promoting Bacterial Inoculant Technology: Formulations and Practical Perspectives (1998–2013). Plant Soil. 2014, 378, 1–33. [Google Scholar] [CrossRef]
- Heimpel, G.E.; Yang, Y.; Hill, J.D.; Ragsdale, D.W. Environmental Consequences of Invasive Species: Greenhouse Gas Emissions of Insecticide Use and the Role of Biological Control in Reducing Emissions. PLoS ONE 2013, 8, e72293. [Google Scholar] [CrossRef]
- Balla, A.; Silini, A.; Cherif-Silini, H.; Chenari Bouket, A.; Alenezi, F.N.; Belbahri, L. Recent Advances in Encapsulation Techniques of Plant Growth-Promoting Microorganisms and Their Prospects in the Sustainable Agriculture. Appl. Sci. 2022, 12, 9020. [Google Scholar] [CrossRef]
- Ravichandran, M.; Samiappan, S.C.; Rangaraj, S.; Murugan, K.; Al-Dhabi, N.A.; Karuppiah, P. Chapter 13—Nanoemulsion Formulations with Plant Growth Promoting Rhizobacteria (PGPR) for Sustainable Agriculture. In Bio-Based Nanoemulsions for Agri-Food Applications; Abd-Elsalam, K.A., Murugan, K., Eds.; Nanobiotechnology for Plant Protection; Elsevier: Amsterdam, The Netherlands, 2022; pp. 207–223. ISBN 978-0-323-89846-1. [Google Scholar]
- Pour, M.M.; Saberi-Riseh, R.; Mohammadinejad, R.; Hosseini, A. Nano-Encapsulation of Plant Growth-Promoting Rhizobacteria and Their Metabolites Using Alginate-Silica Nanoparticles and Carbon Nanotube Improves UCB1 Pistachio Micropropagation. J. Microbiol. Biotechnol. 2019, 29, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Pour, M.M.; Riseh, R.S.; Ranjbar-Karimi, R.; Hassanisaadi, M.; Rahdar, A.; Baino, F. Microencapsulation of Bacillus velezensis Using Alginate-Gum Polymers Enriched with TiO2 and SiO2 Nanoparticles. Micromachines 2022, 13, 1423. [Google Scholar] [CrossRef]
- Yang, K.; Wang, Q.; Wang, Y.; Li, S.; Gu, Y.; Gao, N.; Zhang, F.; Lei, P.; Wang, R.; Xu, H. Poly(γ-Glutamic Acid) Nanocoating To Enhance the Viability of Pseudomonas stutzeri NRCB010 through Cell Surface Engineering. ACS Appl. Mater. Interfaces 2021, 13, 39957–39966. [Google Scholar] [CrossRef]
- Fahde, S.; Boughribil, S.; Sijilmassi, B.; Amri, A. Rhizobia: A Promising Source of Plant Growth-Promoting Molecules and Their Non-Legume Interactions: Examining Applications and Mechanisms. Agriculture 2023, 13, 1279. [Google Scholar] [CrossRef]
- Chieb, M.; Gachomo, E.W. The Role of Plant Growth Promoting Rhizobacteria in Plant Drought Stress Responses. BMC Plant Biol. 2023, 23, 407. [Google Scholar] [CrossRef] [PubMed]
- Paterson, J.; Jahanshah, G.; Li, Y.; Wang, Q.; Mehnaz, S.; Gross, H. The Contribution of Genome Mining Strategies to the Understanding of Active Principles of PGPR Strains. FEMS Microbiol. Ecol. 2017, 93, fiw249. [Google Scholar] [CrossRef]
- Khan, M.; Ali, S.; Al Azzawi, T.N.I.; Saqib, S.; Ullah, F.; Ayaz, A.; Zaman, W. The Key Roles of ROS and RNS as a Signaling Molecule in Plant–Microbe Interactions. Antioxidants 2023, 12, 268. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Wang, Y.; Li, X.; Ji, H. Genome-Wide Identification of the Soybean LysM-RLK Family Genes and Its Nitrogen Response. Int. J. Mol. Sci. 2023, 24, 13621. [Google Scholar] [CrossRef]
- Agrawal, R.; Satlewal, A.; Varma, A. Characterization of Plant Growth-Promoting Rhizobacteria (PGPR): A Perspective of Conventional Versus Recent Techniques. In Heavy Metal Contamination of Soils: Monitoring and Remediation; Sherameti, I., Varma, A., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 471–485. ISBN 978-3-319-14526-6. [Google Scholar]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; El Enshasy, H. Plant Growth Promoting Rhizobacteria (PGPR) as Green Bioinoculants: Recent Developments, Constraints, and Prospects. Sustainability 2021, 13, 1140. [Google Scholar] [CrossRef]
- Shah, A.; Nazari, M.; Antar, M.; Msimbira, L.A.; Naamala, J.; Lyu, D.; Rabileh, M.; Zajonc, J.; Smith, D.L. PGPR in Agriculture: A Sustainable Approach to Increasing Climate Change Resilience. Front. Sustain. Food Syst. 2021, 5, 667546. [Google Scholar] [CrossRef]
- Stassinos, P.M.; Rossi, M.; Borromeo, I.; Capo, C.; Beninati, S.; Forni, C. Amelioration of Salt Stress Tolerance in Rapeseed (Brassica napus) Cultivars by Seed Inoculation with Arthrobacter globiformis. Plant Biosyst.-An. Int. J. Deal. All Asp. Plant Biol. 2022, 156, 370–383. [Google Scholar] [CrossRef]
- Ultanbekova, G.; Mukhatayeva, K.; Mamytova, N.; Kanalbek, G.; Kazizmurat, M. Isolation and Selection of Nodulating Bacteria from the Rhizosphere of Legume (Mash - Vigna Radiata) and Bean (Phaseolus Lunatus) Plants and to Enhance Specific Properties through Induced Mutagenesis Methods. BIO Web Conf. 2024, 100, 02033. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, P.; Xue, J.; Du, Y.; Yan, H.; Wang, L.; Yi, X.; Sun, J.; Zhang, X.; Gao, J. Arthrobacter wenxiniae sp. Nov., a Novel Plant Growth-Promoting Rhizobacteria Species Harbouring a Carotenoids Biosynthetic Gene Cluster. Antonie Van Leeuwenhoek 2022, 115, 353–364. [Google Scholar] [CrossRef]
- Spaepen, S.; Bossuyt, S.; Engelen, K.; Marchal, K.; Vanderleyden, J. Phenotypical and Molecular Responses of Arabidopsis thaliana Roots as a Result of Inoculation with the Auxin-Producing Bacterium Azospirillum brasilense. New Phytol. 2014, 201, 850–861. [Google Scholar] [CrossRef] [PubMed]
- El-Ballat, E.M.; Elsilk, S.E.; Ali, H.M.; Ali, H.E.; Hano, C.; El-Esawi, M.A. Metal-Resistant PGPR Strain Azospirillum brasilense EMCC1454 Enhances Growth and Chromium Stress Tolerance of Chickpea (Cicer arietinum L.) by Modulating Redox Potential, Osmolytes, Antioxidants, and Stress-Related Gene Expression. Plants 2023, 12, 2110. [Google Scholar] [CrossRef] [PubMed]
- Abdel Latef, A.A.H.; Abu Alhmad, M.F.; Kordrostami, M.; Abo–Baker, A.-B.A.-E.; Zakir, A. Inoculation with Azospirillum lipoferum or Azotobacter chroococcum Reinforces Maize Growth by Improving Physiological Activities Under Saline Conditions. J. Plant Growth Regul. 2020, 39, 1293–1306. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; Omer, A.M.; Badawy, A.A.; Osman, M.S.; Ragaey, M.M. Strategy of Salt Tolerance and Interactive Impact of Azotobacter chroococcum and/or Alcaligenes faecalis Inoculation on Canola (Brassica napus L.) Plants Grown in Saline Soil. Plants 2021, 10, 110. [Google Scholar] [CrossRef]
- Zalila-Kolsi, I.; Ben-Mahmoud, A.; Al-Barazie, R. Bacillus amyloliquefaciens: Harnessing Its Potential for Industrial, Medical, and Agricultural Applications—A Comprehensive Review. Microorganisms 2023, 11, 2215. [Google Scholar] [CrossRef]
- Qi, H.-Y.; Wang, D.; Han, D.; Song, J.; Ali, M.; Dai, X.-F.; Zhang, X.-J.; Chen, J.-Y. Unlocking Antagonistic Potential of Bacillus amyloliquefaciens KRS005 to Control Gray Mold. Front. Microbiol. 2023, 14, 1189354. [Google Scholar] [CrossRef]
- Luo, Z.; Yan, Y.; Du, S.; Zhu, Y.; Pan, F.; Wang, R.; Xu, Z.; Xu, X.; Li, S.; Xu, H. Recent Advances and Prospects of Bacillus amyloliquefaciens as Microbial Cell Factories: From Rational Design to Industrial Applications. Crit. Rev. Biotechnol. 2023, 43, 1073–1091. [Google Scholar] [CrossRef]
- Xu, F.; Liao, H.; Zhang, Y.; Yao, M.; Liu, J.; Sun, L.; Zhang, X.; Yang, J.; Wang, K.; Wang, X.; et al. Coordination of Root Auxin with the Fungus Piriformospora indica and Bacterium Bacillus cereus Enhances Rice Rhizosheath Formation under Soil Drying. ISME J. 2021, 16, 801–811. [Google Scholar] [CrossRef]
- Yu, S.; Yu, P.; Wang, J.; Li, C.; Guo, H.; Liu, C.; Kong, L.; Yu, L.; Wu, S.; Lei, T.; et al. A Study on Prevalence and Characterization of Bacillus cereus in Ready-to-Eat Foods in China. Front. Microbiol. 2020, 10, 3043. [Google Scholar] [CrossRef]
- Kulkova, I.; Dobrzyński, J.; Kowalczyk, P.; Bełżecki, G.; Kramkowski, K. Plant Growth Promotion Using Bacillus cereus. Int. J. Mol. Sci. 2023, 24, 9759. [Google Scholar] [CrossRef]
- Zhou, C.; Zhu, J.; Qian, N.; Guo, J.; Yan, C. Bacillus subtilis SL18r Induces Tomato Resistance Against Botrytis cinerea, Involving Activation of Long Non-Coding RNA, MSTRG18363, to Decoy miR1918. Front. Plant Sci. 2021, 11, 634819. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Sun, B.; Shi, H.; Gao, T.; He, Y.; Li, Y.; Liu, Y.; Li, X.; Zhang, L.; Li, S.; et al. Sucrose Triggers a Novel Signaling Cascade Promoting Bacillus subtilis Rhizosphere Colonization. ISME J. 2021, 15, 2723–2737. [Google Scholar] [CrossRef] [PubMed]
- Pohare, M.B.; Wagh, S.G.; Udayasuriyan, V. Bacillus thuringiensis as Potential Biocontrol Agent for Sustainable Agriculture. In Current Trends in Microbial Biotechnology for Sustainable Agriculture; Yadav, A.N., Singh, J., Singh, C., Yadav, N., Eds.; Environmental and Microbial Biotechnology; Springer: Singapore, 2021; pp. 439–468. ISBN 9789811569494. [Google Scholar]
- Kahn, T.W.; Duck, N.B.; McCarville, M.T.; Schouten, L.C.; Schweri, K.; Zaitseva, J.; Daum, J. A Bacillus thuringiensis Cry Protein Controls Soybean Cyst Nematode in Transgenic Soybean Plants. Nat. Commun. 2021, 12, 3380. [Google Scholar] [CrossRef]
- Malik, L.; Sanaullah, M.; Mahmood, F.; Hussain, S.; Shahzad, T. Co-Application of Biochar and Salt Tolerant PGPR to Improve Soil Quality and Wheat Production in a Naturally Saline Soil. Rhizosphere 2024, 29, 100849. [Google Scholar] [CrossRef]
- de Almeida, J.R.; Bonatelli, M.L.; Batista, B.D.; Teixeira-Silva, N.S.; Mondin, M.; dos Santos, R.C.; Bento, J.M.S.; de Almeida Hayashibara, C.A.; Azevedo, J.L.; Quecine, M.C. RZ2MS9, a Tropical Plant Growth-Promoting Rhizobacterium, Colonizes Maize Endophytically and Alters the Plant’s Production of Volatile Organic Compounds during Co-Inoculation with Ab-V5. Environ. Microbiol. Rep. 2021, 13, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Madala, N.E.; Molinaro, A.; Dubery, I.A. Distinct Carbohydrate and Lipid-Based Molecular Patterns within Lipopolysaccharides from Burkholderia cepacia Contribute to Defense-Associated Differential Gene Expression in Arabidopsis thaliana. Innate Immun. 2012, 18, 140–154. [Google Scholar] [CrossRef]
- Heo, A.Y.; Koo, Y.M.; Choi, H.W. Biological Control Activity of Plant Growth Promoting Rhizobacteria Burkholderia contaminans AY001 against Tomato Fusarium Wilt and Bacterial Speck Diseases. Biology 2022, 11, 619. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, K.; Mitra, S.; Sarkar, A.; Maiti, T.K. Alleviation of Phytotoxic Effects of Cadmium on Rice Seedlings by Cadmium Resistant PGPR Strain Enterobacter Aerogenes MCC 3092. J. Hazard. Mater. 2018, 351, 317–329. [Google Scholar] [CrossRef]
- Yue, H.; Sun, S.; Wang, R.; Ma, X.; Shen, S.; Luo, Y.; Ma, X.; Wu, T.; Li, S.; Yang, Z.; et al. Study on the Mechanism of Salt Relief and Growth Promotion of Enterobacter cloacae on Cotton. BMC Plant Biol. 2023, 23, 656. [Google Scholar] [CrossRef]
- Seo, H.; Kim, J.H.; Lee, S.-M.; Lee, S.-W. The Plant-Associated Flavobacterium: A Hidden Helper for Improving Plant Health. Plant Pathol. J. 2024, 40, 251–260. [Google Scholar] [CrossRef]
- Kraut-Cohen, J.; Shapiro, O.H.; Dror, B.; Cytryn, E. Pectin Induced Colony Expansion of Soil-Derived Flavobacterium Strains. Front. Microbiol. 2021, 12, 651891. [Google Scholar] [CrossRef] [PubMed]
- Sommer, A.; Wenig, M.; Knappe, C.; Kublik, S.; Foesel, B.U.; Schloter, M.; Vlot, A.C. A Salicylic Acid-Associated Plant-Microbe Interaction Attracts Beneficial Flavobacterium sp. to the Arabidopsis thaliana Phyllosphere. Physiol. Plant. 2024, 176, e14483. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, K.; Mitra, S.; Sarkar, A.; Soren, T.; Maiti, T.K. Characterization of Cadmium-Resistant Klebsiella pneumoniae MCC 3091 Promoted Rice Seedling Growth by Alleviating Phytotoxicity of Cadmium. Environ. Sci. Pollut. Res. 2017, 24, 24419–24437. [Google Scholar] [CrossRef] [PubMed]
- Kusale, S.P.; Attar, Y.C.; Sayyed, R.Z.; Malek, R.A.; Ilyas, N.; Suriani, N.L.; Khan, N.; El Enshasy, H.A. Production of Plant Beneficial and Antioxidants Metabolites by Klebsiella variicola under Salinity Stress. Molecules 2021, 26, 1894. [Google Scholar] [CrossRef]
- Dubey, A.; Kumar, A.; Khan, M.L.; Payasi, D.K. Plant Growth-Promoting and Bio-Control Activity of Micrococcus luteus Strain AKAD 3-5 Isolated from the Soybean (Glycine max (L.) Merr.) Rhizosphere. Open Microbiol. J. 2021, 15. [Google Scholar] [CrossRef]
- Badawy, I.H.; Hmed, A.A.; Sofy, M.R.; Al-Mokadem, A.Z. Alleviation of Cadmium and Nickel Toxicity and Phyto-Stimulation of Tomato Plant L. by Endophytic Micrococcus luteus and Enterobacter cloacae. Plants 2022, 11, 2018. [Google Scholar] [CrossRef]
- Kabiraj, A.; Halder, U.; Chitikineni, A.; Varshney, R.K.; Bandopadhyay, R. Insight into the Genome of an Arsenic Loving and Plant Growth-Promoting Strain of Micrococcus luteus Isolated from Arsenic Contaminated Groundwater. Environ. Sci. Pollut. Res. 2024, 31, 39063–39076. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; Zaid, A.; Abo-Baker, A.-B.A.-E.; Salem, W.; Abu Alhmad, M.F. Mitigation of Copper Stress in Maize by Inoculation with Paenibacillus polymyxa and Bacillus circulans. Plants 2020, 9, 1513. [Google Scholar] [CrossRef]
- E, Y.; Yuan, J.; Yang, F.; Wang, L.; Ma, J.; Li, J.; Pu, X.; Raza, W.; Huang, Q.; Shen, Q. PGPR Strain Paenibacillus polymyxa SQR-21 Potentially Benefits Watermelon Growth by Re-Shaping Root Protein Expression. AMB Expr. 2017, 7, 104. [Google Scholar] [CrossRef]
- Langendries, S.; Goormachtig, S. A Jack of All Trades. Environ. Microbiol. 2021, 23, 5659–5669. [Google Scholar] [CrossRef]
- Liu, H.; Li, Y.; Ge, K.; Du, B.; Liu, K.; Wang, C.; Ding, Y. Interactional Mechanisms of Paenibacillus polymyxa SC2 and Pepper (Capsicum annuum L.) Suggested by Transcriptomics. BMC Microbiol. 2021, 21, 70. [Google Scholar] [CrossRef]
- Paulsen, I.T.; Press, C.M.; Ravel, J.; Kobayashi, D.Y.; Myers, G.S.A.; Mavrodi, D.V.; DeBoy, R.T.; Seshadri, R.; Ren, Q.; Madupu, R.; et al. Complete Genome Sequence of the Plant Commensal Pseudomonas fluorescens Pf-5. Nat. Biotechnol. 2005, 23, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.H.; Trotel-Aziz, P.; Villaume, S.; Rabenoelina, F.; Schwarzenberg, A.; Nguema-Ona, E.; Clément, C.; Baillieul, F.; Aziz, A. Bacillus Subtilis and Pseudomonas Fluorescens Trigger Common and Distinct Systemic Immune Responses in Arabidopsis thaliana Depending on the Pathogen Lifestyle. Vaccines 2020, 8, 503. [Google Scholar] [CrossRef] [PubMed]
- Costa-Gutierrez, S.B.; Adler, C.; Espinosa-Urgel, M.; de Cristóbal, R.E. Pseudomonas putida and Its Close Relatives: Mixing and Mastering the Perfect Tune for Plants. Appl. Microbiol. Biotechnol. 2022, 106, 3351–3367. [Google Scholar] [CrossRef]
- Vives-Peris, V.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Salt Stress Alleviation in Citrus Plants by Plant Growth-Promoting Rhizobacteria Pseudomonas putida and Novosphingobium sp. Plant Cell Rep. 2018, 37, 1557–1569. [Google Scholar] [CrossRef]
- Sun, X.; Xu, Z.; Xie, J.; Hesselberg-Thomsen, V.; Tan, T.; Zheng, D.; Strube, M.L.; Dragoš, A.; Shen, Q.; Zhang, R.; et al. Bacillus velezensis Stimulates Resident Rhizosphere Pseudomonas stutzeri for Plant Health through Metabolic Interactions. ISME J. 2021, 16, 774–787. [Google Scholar] [CrossRef]
- Lami, M.J.; Adler, C.; Caram-Di Santo, M.C.; Zenoff, A.M.; de Cristóbal, R.E.; Espinosa-Urgel, M.; Vincent, P.A. Pseudomonas stutzeri MJL19, a Rhizosphere-colonizing Bacterium That Promotes Plant Growth under Saline Stress. J. Appl. Microbiol. 2020, 129, 1321–1336. [Google Scholar] [CrossRef]
- Ahmad, A.; Mushtaq, Z.; Nazir, A.; Jaffar, M.T.; Asghar, H.N.; Alzuaibr, F.M.; Alasmari, A.; Alqurashi, M. Growth Response of Cowpea (Vigna unguiculata L.) Exposed to Pseudomonas fluorescens, Pseudomonas stutzeri, and Pseudomonas gessardii in Lead Contaminated Soil. Plant Stress. 2023, 10, 100259. [Google Scholar] [CrossRef]
- Jiang, S.; Li, J.; Wang, Q.; Yin, C.; Zhan, Y.; Yan, Y.; Lin, M.; Ke, X. Maize Growth Promotion by Inoculation with an Engineered Ammonium-Excreting Strain of Nitrogen-Fixing Pseudomonas stutzeri. Microorganisms 2022, 10, 1986. [Google Scholar] [CrossRef]
- Ma, W.; Guinel, F.C.; Glick, B.R. Rhizobium leguminosarum Biovar Viciae 1-Aminocyclopropane-1-Carboxylate Deaminase Promotes Nodulation of Pea Plants. Appl. Environ. Microbiol. 2003, 69, 4396–4402. [Google Scholar] [CrossRef]
- Yadav, J.; Verma, J.P. Effect of Seed Inoculation with Indigenous Rhizobium and Plant Growth Promoting Rhizobacteria on Nutrients Uptake and Yields of Chickpea (Cicer Arietinum L.). Eur. J. Soil. Biol. 2014, 63, 70–77. [Google Scholar] [CrossRef]
- Zahir, Z.A.; Zafar-ul-Hye, M.; Sajjad, S.; Naveed, M. Comparative Effectiveness of Pseudomonas and Serratia sp. Containing ACC-Deaminase for Coinoculation with Rhizobium leguminosarum to Improve Growth, Nodulation, and Yield of Lentil. Biol. Fertil. Soils 2011, 47, 457–465. [Google Scholar] [CrossRef]
- Suárez-Moreno, Z.R.; Vinchira-Villarraga, D.M.; Vergara-Morales, D.I.; Castellanos, L.; Ramos, F.A.; Guarnaccia, C.; Degrassi, G.; Venturi, V.; Moreno-Sarmiento, N. Plant-Growth Promotion and Biocontrol Properties of Three Streptomyces Spp. Isolates to Control Bacterial Rice Pathogens. Front. Microbiol. 2019, 10, 290. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Babalola, O.O. Streptomyces: Implications and Interactions in Plant Growth Promotion. Appl. Microbiol. Biotechnol. 2019, 103, 1179–1188. [Google Scholar] [CrossRef]
- Dev, A.S.R.; Harish, S.; Karthikeyan, G.; Nivedha, M.; Sangeetha, C. Consortia of Streptomyces Spp. Triggers Defense/PAMP Genes during the Interaction of Groundnut budnecrosis Orthotospovirus in Tomato. 3 Biotech. 2024, 14, 196. [Google Scholar] [CrossRef]
- Lu, L.; Liu, N.; Fan, Z.; Liu, M.; Zhang, X.; Tian, J.; Yu, Y.; Lin, H.; Huang, Y.; Kong, Z. A Novel PGPR Strain, Streptomyces lasalocidi JCM 3373T, Alleviates Salt Stress and Shapes Root Architecture in Soybean by Secreting Indole-3-Carboxaldehyde. Plant Cell Environ. 2024, 47, 1941–1956. [Google Scholar] [CrossRef]
- Wang, M.; Xue, J.; Ma, J.; Feng, X.; Ying, H.; Xu, H. Streptomyces lydicus M01 Regulates Soil Microbial Community and Alleviates Foliar Disease Caused by Alternaria alternata on Cucumbers. Front. Microbiol. 2020, 11, 942. [Google Scholar] [CrossRef]
- Devi, S.; Sharma, M.; Manhas, R.K. Investigating the Plant Growth Promoting and Biocontrol Potentiality of Endophytic Streptomyces SP. SP5 against Early Blight in Solanum lycopersicum Seedlings. BMC Microbiol. 2022, 22, 285. [Google Scholar] [CrossRef]
- Antar, M.; Gopal, P.; Msimbira, L.A.; Naamala, J.; Nazari, M.; Overbeek, W.; Backer, R.; Smith, D.L. Inter-Organismal Signaling in the Rhizosphere. In Rhizosphere Biology: Interactions Between Microbes and Plants; Gupta, V.V.S.R., Sharma, A.K., Eds.; Springer: Singapore, 2021; pp. 255–293. ISBN 9789811561252. [Google Scholar]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant Growth-Promoting Rhizobacteria: Context, Mechanisms of Action, and Roadmap to Commercialization of Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef]
- Leontidou, K.; Genitsaris, S.; Papadopoulou, A.; Kamou, N.; Bosmali, I.; Matsi, T.; Madesis, P.; Vokou, D.; Karamanoli, K.; Mellidou, I. Plant Growth Promoting Rhizobacteria Isolated from Halophytes and Drought-Tolerant Plants: Genomic Characterisation and Exploration of Phyto-Beneficial Traits. Sci. Rep. 2020, 10, 14857. [Google Scholar] [CrossRef]
- Kumar, A.; Patel, J.S.; Meena, V.S.; Ramteke, P.W. Plant Growth-Promoting Rhizobacteria: Strategies to Improve Abiotic Stresses under Sustainable Agriculture. J. Plant Nutr. 2019, 42, 1402–1415. [Google Scholar] [CrossRef]
- Campos, E.V.R.; Pereira, A.D.E.S.; Aleksieienko, I.; Do Carmo, G.C.; Gohari, G.; Santaella, C.; Fraceto, L.F.; Oliveira, H.C. Encapsulated Plant Growth Regulators and Associative Microorganisms: Nature-Based Solutions to Mitigate the Effects of Climate Change on Plants. Plant Sci. 2023, 331, 111688. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Ilyas, N.; Meraj, T.A.; Pour-Aboughadareh, A.; Sayyed, R.Z.; Mashwani, Z.-R.; Poczai, P. Improvement of Plant Responses by Nanobiofertilizer: A Step towards Sustainable Agriculture. Nanomaterials 2022, 12, 965. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Song, I.-G.; Yoon, H.; Park, J.-W. Sub-Micron Microplastics Affect Nitrogen Cycling by Altering Microbial Abundance and Activities in a Soil-Legume System. J. Hazard. Mater. 2023, 460, 132504. [Google Scholar] [CrossRef] [PubMed]
- Enebe, M.C.; Babalola, O.O. The Influence of Plant Growth-Promoting Rhizobacteria in Plant Tolerance to Abiotic Stress: A Survival Strategy. Appl. Microbiol. Biotechnol. 2018, 102, 7821–7835. [Google Scholar] [CrossRef]
- Saeed, Q.; Xiukang, W.; Haider, F.U.; Kučerik, J.; Mumtaz, M.Z.; Holatko, J.; Naseem, M.; Kintl, A.; Ejaz, M.; Naveed, M.; et al. Rhizosphere Bacteria in Plant Growth Promotion, Biocontrol, and Bioremediation of Contaminated Sites: A Comprehensive Review of Effects and Mechanisms. Int. J. Mol. Sci. 2021, 22, 10529. [Google Scholar] [CrossRef]
- Fry, W.E. Principles of Plant Disease Management; Academic Press: Cambridge, MA, USA, 2012; ISBN 978-0-08-091830-3. [Google Scholar]

| Functional PGPR Species | Source Isolated From | Beneficial Roles | Mechanisms of Action | Applicable Plants | References |
|---|---|---|---|---|---|
| Arthrobacter globiformis | Soil, rhizosphere | Nutrient solubilization, pathogen control | Phosphate solubilization, biocontrol of pathogens | Vegetables, cereals | [84,85,86] |
| Azospirillum brasilense | Soil, plant roots | Enhanced root growth, nitrogen supply | Nitrogen fixation, phytohormone production (auxins) | Cereals (e.g., maize, wheat), grasses | [87,88] |
| Azotobacter chroococcum | Soil, rhizosphere | Nitrogen fixation, soil fertility improvement | Nitrogen fixation, produces growth-promoting substances | Cereals, vegetables | [89,90] |
| Bacillus amyloliquefaciens | Soil, plant roots | Pathogen suppression, growth enhancement | Antifungal activity, induced systemic resistance | Fruits, vegetables | [4,91,92,93] |
| Bacillus cereus | Soil, plant roots | Disease control, plant vigor improvement | Biocontrol of pathogens, induced systemic resistance | Vegetables, cereals | [94,95,96] |
| Bacillus subtilis | Soil, plant roots | Disease suppression, nutrient uptake enhancement | Produces antibiotics, induces systemic resistance, solubilizes phosphorus | Vegetables, cereals, legumes | [97,98] |
| Bacillus thuringiensis | Soil, plant roots | Insect pest suppression, plant protection | Pest control, biocontrol of insects | Vegetables, fruits | [99,100,101,102] |
| Burkholderia cepacia | Soil, rhizosphere | Pathogen control, plant growth promotion | Produces antifungal compounds, competes with pathogens | Vegetables, ornamentals | [103,104] |
| Enterobacter cloacae | Soil, plant roots | Disease suppression, nutrient enhancement | Phosphate solubilization, biocontrol of pathogens | Vegetables, cereals | [105,106] |
| Flavobacterium johnsoniae | Soil, rhizosphere | Disease suppression, growth enhancement | Biocontrol of pathogens, growth promotion | Vegetables, cereals | [107,108,109] |
| Klebsiella pneumoniae | Soil, rhizosphere | Nitrogen supply, growth enhancement | Nitrogen fixation, growth promotion | Vegetables, cereals | [110,111] |
| Micrococcus luteus | Soil, plant roots | Nutrient availability, plant growth support | Phosphate solubilization, growth promotion | Vegetables, cereals | [112,113,114] |
| Paenibacillus macerans | Soil, plant roots | Nutrient availability, growth promotion | Produces enzymes, solubilizes phosphorus | Vegetables, cereals | [115,116] |
| Paenibacillus polymyxa | Soil, plant roots | Nutrient solubilization, disease suppression | Nitrogen fixation, phosphate solubilization | Cereals, vegetables | [117,118] |
| Pseudomonas fluorescens | Soil, plant roots, rhizosphere | Disease resistance, improved nutrient acquisition | Produces siderophores and antibiotics, induces systemic resistance | Vegetables, fruits, cereals | [119,120] |
| Pseudomonas putida | Soil, rhizosphere | Enhanced nutrient uptake, disease control | Siderophore production, biocontrol of pathogens | Vegetables, ornamentals | [121,122] |
| Pseudomonas stutzeri | Soil, rhizosphere | Nitrogen fixation, disease suppression | Nitrogen fixation, biocontrol of pathogens | Vegetables, cereals | [123,124,125,126] |
| Rhizobium leguminosarum | Soil, legume roots | Symbiotic nitrogen fixation, plant growth promotion | Nitrogen fixation, nodule formation | Legumes (e.g., peas, beans) | [127,128,129] |
| Streptomyces griseoviridis | Soil, plant roots | Disease control, enhanced plant health | Produces antibiotics, competes with pathogens | Vegetables, ornamentals | [130,131,132] |
| Streptomyces lydicus | Soil, rhizosphere | Disease control, enhanced plant health | Antifungal activity, biocontrol of pathogens | Vegetables, ornamentals | [133,134,135] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, P.; Condrich, A.; Scranton, S.; Hebner, C.; Lu, L.; Ali, M.A. Utilizing Plant Growth-Promoting Rhizobacteria (PGPR) to Advance Sustainable Agriculture. Bacteria 2024, 3, 434-451. https://doi.org/10.3390/bacteria3040030
Yang P, Condrich A, Scranton S, Hebner C, Lu L, Ali MA. Utilizing Plant Growth-Promoting Rhizobacteria (PGPR) to Advance Sustainable Agriculture. Bacteria. 2024; 3(4):434-451. https://doi.org/10.3390/bacteria3040030
Chicago/Turabian StyleYang, Piao, Abraham Condrich, Sean Scranton, Camina Hebner, Ling Lu, and Muhammad Azam Ali. 2024. "Utilizing Plant Growth-Promoting Rhizobacteria (PGPR) to Advance Sustainable Agriculture" Bacteria 3, no. 4: 434-451. https://doi.org/10.3390/bacteria3040030
APA StyleYang, P., Condrich, A., Scranton, S., Hebner, C., Lu, L., & Ali, M. A. (2024). Utilizing Plant Growth-Promoting Rhizobacteria (PGPR) to Advance Sustainable Agriculture. Bacteria, 3(4), 434-451. https://doi.org/10.3390/bacteria3040030







