Polyelectrolytes and Polyelectrolyte Complexes as Future Antibacterial Agents
Abstract
:1. Introduction
2. Classification and Physicochemical Properties of PEs
2.1. Natural Polyelectrolytes
- Chitosan (CS) is a polysaccharide derived from chitin by deacetylation reactions, and thus, some authors consider it pseudo-natural. It consists of N-acetyl-D-glucosamine and D-glucosamine. It is the second most abundant natural polymer after cellulose. It is found in several marine creatures but is mainly extracted from shrimp and crabs, since it is found in their exoskeleton. It is also found in the cell walls of yeast and fungi, imparting strength to their structures [41,42,43].
- CS is characterized by its positive charge and is used in wound healing and drug delivery systems thanks to its excellent pharmaceutical properties, such as mucoadhesion, biocompatibility, biodegradability, and antimicrobial efficacy [44,45]. CS turns into a PE only in acidic conditions due to the protonation of amino groups; thus, it is very sensitive to pH [43]. In addition, its properties vary accordingly to the degree of acetylation and charge distribution, as well as the Mw [43].
- Alginate (Alg) is a linear polysaccharide derived mainly from brown seaweeds, such as Laminaria species, Macrocystis pyrifera, Saccharina japonica, and Ascophyllum nodosum. It is a polyacid that consists of alternating β-D-mannuronic acid and α-L-guluronic acid residues linked by 1,4-glycosidic bonds [46,47]. Alginate is well-known for its gelling properties (mainly in the presence of divalent cations), which, coupled with its remarkable biodegradability and biocompatibility, makes it a cornerstone in pharmaceutical technology and biotechnology [48,49]. Importantly, alginate also possesses antimicrobial properties, minimal immunogenicity, and both hygroscopic and humectant qualities [49]. These features not only enhance its role in drug delivery, tissue engineering, and wound healing, but also highlight its transformative impact on the pharmaceutical industry and its potential for pioneering research applications [50].
- Carboxymethylcellulose (CMC) is a cellulose-derived PE found in plant cell walls, such as those in wood pulp and cotton. Recent research has explored alternative, sustainable sources of cellulose, including sago palm, corn husk, rice husk, rice stubble, and waste materials like paper sludge and cotton gin waste [48,51]. CMC is produced by chemically modifying cellulose with carboxymethyl groups, enhancing its functionality. CMC is known for its water solubility and hemostatic properties; it has applications in wound dressings and drug delivery systems. Furthermore, its strong hydrophilicity, bioadhesive properties, and low toxicity make it an excellent candidate for various applications [52,53].
- Chondroitin Sulfate (ChS), is an anionic glycosaminoglycan, widely found in vertebrates, invertebrates, and bacteria. Structurally, ChS consists of repeating disaccharide units of D-glucuronic acid and N-acetyl galactosamine, with sulfate groups at various carbon positions. It is categorized into subgroups like ChS-A, ChS-B, ChS-C, ChS-D, and ChS-E, based on the sulfate group’s position [54]. ChS has valued biomedical applications thanks to its chondroprotective and anti-atherogenic effects [10,55]. Antibacterial activity was also reported [11].
- Hyaluronic Acid (HA) is a natural, linear glycosaminoglycan and an important component of the extracellular matrix. Structurally, HA comprises repeating disaccharide units of D-glucuronic acid and N-acetyl-D-glucosamine, linked by alternating β-1,3 and β-1,4 glycosidic bonds [56]. This biopolymer exists predominantly as high Mw, typically over 1000 kDa, in healthy tissues, where it exhibits antiangiogenic and immunosuppressive properties. Conversely, low Mw HA fragments, resulting from degradation, are linked to inflammatory responses and angiogenesis [57]. Due to its superior biodegradability, biocompatibility, and “hydration capacity” HA is widely utilized in tissue engineering and wound healing applications [56,58].
- Carrageenans (CRGs) are naturally occurring anionic polysaccharides, being extracted from red algae (Rhodophyta); their corresponding to sulfated esters of polygalactose of high Mw. β-carrageen is an exception, since it is deprived of sulfate ester moieties [59]. Depending on the degree of sulfation and the positions of the sulfate groups, CRGs are classified into five main groups: λ, κ, ι, ε, and μ. κ-CRG, the most common in pharmaceutical technology, is composed of alternating units of 1,3-linked β-D-galactose where a sulfate group is bound to C4, and 1,4 linked anhydogalactose units [60,61]. It has several biological activities, including those antiviral against herpes simplex virus (HSV) and human papilloma virus (HPV) [62]. Studies have proven that i-CRG is effective against a strain of influenza virus, and its effect is similar to that of oseltamivir. Furthermore, CRGs have shown antioxidant, antitumor, and immunomodulatory properties [62,63,64].
- Fucoidan (Fuc) is a sulfated polyanion and polysaccharide derived from brown seaweeds, such as Fucus vesiculosus. It is composed primarily of L-fucose and sulfated ester groups, with minor components including D-xylose, glucuronic acid, D-galactose, and D-mannose [65,66]. Its biological activities—anticoagulant, anti-inflammatory, antibacterial, antiviral, and anticancer properties—are influenced by Mw, sulfation levels, and monosaccharides composition [66]. Fuc typically features two types of chains: Type I, consisting of α (1→3) linked fucose, and Type II, characterized by alternating α (1→3) and α (1→4) linked fucose [65,66].
- Heparin (Hep) is a highly sulfated, heterogeneous linear glycosaminoglycan predominantly sourced from animal tissues, such as porcine intestinal mucosa and bovine lung. It consists of 1,4-glycosidically linked D-glucosamine and uronic acid. Uronic acid units can either be α-L-iduronic acid or β-D-glucuronic acid, which can be sulfated at several positions [67,68]. Beyond its well-documented anticoagulant and anti-inflammatory properties, Hep has demonstrated significant efficacy in combating bacterial infections. It also showed activity against malaria and Lyme disease [68,69,70].
- Pectin is a complex, weak polyanionic heteropolysaccharide found in plant cell walls, widely used for its gelling and thickening properties. It consists mainly of (1 → 4)-α-D-galacturonic acid (Gal A) residues, branched with neutral sugars [71]. Pectin is known for its ability to form stable complexes with positively charged molecules. This property makes it valuable in electrochemistry and biocompatible film formation [49,72].
- Poly-amino acids are increasingly valued in drug delivery systems [73]. Among these, γ-poly (glutamic acid) (γ-PGA) stands out as a biodegradable, non-toxic, and water-soluble biopolymer, synthesized through bacterial fermentation. Interestingly, its resistance to proteolytic degradation makes it a strong contender for a variety of biomedical applications, as it can be used in drug carriers or as an antibacterial agent. High Mw γ-PGA (800–1000 kDa) is particularly notable for its exceptional water retention capability, absorbing up to 5000 times its weight, although its water solubility diminishes with increased Mw [73,74].
- Gelatin, a denatured collagen derivative used in food, pharmaceuticals, and biomedical fields for its ability to form thermoreversible gels. It is zwitterionic, has a positive or negative charge according to pH, and is produced through partial hydrolysis of collagen. Gelatin has a Mw ranging from 15 to 250 kDa. Gelatin’s gelation occurs as the denatured polypeptides partially reform collagen-like triple helices upon cooling, stabilized by hydrogen bonds, electrostatic, and hydrophobic interactions [75,76].
- Others such as cyclodextrin-based macrocyclic oligosaccharides are derived from starch. Cyclodextrins are categorized into α-, β-, or γ-cyclodextrins based on the number of glucopyranose units in their cyclic structure, but their derivatives may be synthesized. The hydrophobic cavity within cyclodextrins facilitates the formation of inclusion complexes with a variety of compounds, thereby enhancing drug solubility, stability, and permeability through biological barriers [77,78]. Additionally, xanthan gum, a polysaccharide produced through the fermentation of Xanthomonas campestris, is widely used in pharmaceutical formulations. Its high Mw and anionic nature enable it to significantly increase viscosity, stabilize emulsions, and form protective films around active ingredients, thereby enhancing the bioavailability and controlled release of drugs [79].
| Polyelectrolyte | Functional Group 1 | Properties | Source | Refs. |
|---|---|---|---|---|
| Alginate | -COOH | Gel-forming with divalent cations |
| [48,51] |
| Carboxymethylcellulose | -COOH | High hydrophilicity, biodegradable |
| [51,52] |
| Carrageenan | -SO42⁻ | Gel-forming, thickening properties |
| [64,80] |
| Chondroitin sulfate | -SO42⁻ | Biocompatible, chondroprotective |
| [10,55] |
| Chitosan | -NH2 | pH-sensitive, biodegradable |
| [44,45] |
| Fucoidan | -SO42⁻ | Interacts with proteins and cells |
| [65,81] |
| Gelatins | -NH2 | Thermoreversible gels |
| [75,76] |
| Heparin | -SO42⁻ | Interacts selectively with multiple proteins |
| [67,68] |
| Hyaluronic acid | -COOH | Biodegradable, enhances drug specificity |
| [56,57] |
| Pectin | -COOH | Anionic, forms stable complexes |
| [49,71] |
| Poly-amino acids | -NH2 | Biodegradable, hydrophilic |
| [73,74] |
| Xanthan Gum | -COOH | High viscosity, gel-forming |
| [79] |
2.2. Synthetic Polyelectrolytes
- Dextran sulfate (DS) is an anionic PE derived from dextran, a complex polysaccharide composed of α-1,6-linked glucose units. It is widely employed in biomedical applications, notably for its anticoagulant properties, due to its ability to inhibit blood clotting by interacting with specific biological proteins. Additionally, DS has demonstrated potential as an antibacterial agent, contributing to its versatility in therapeutic applications [82,83].
- Polyacrylic acid (PAA) is an anionic polyelectrolyte characterized by its remarkable water solubility and high-water absorption capacity. Synthesized from acrylic acid monomers, PAA features ionizable carboxyl groups that facilitate its gelation and thickening properties [84,85]. These attributes make PAA valuable in pharmaceutical applications, particularly in controlled drug release systems and gel formulations. Its capacity to form cross-linked networks, achieved through radiation or chemical cross-linking, enhances its stability and versatility. It is biodegradable and recent studies indicate that it also exhibits antibacterial properties [85].
- Polyvinylsulfonic acid sodium salt (PVSNa) is a sulfonic acid-based polyanion with remarkable physicochemical properties, including high water solubility and the ability to form complexes with cations, due to its sulfonic acid functional groups. It is synthesized through the polymerization of vinyl sulfonic acid, resulting in a strong aliphatic sulfonic acid polymer that is soluble in water and lower alcohols [88,89].
- Polystyrene carboxylic acid (PSA) is an anionic PE synthesized by introducing carboxylic acid groups onto polystyrene chains [90,91]. This polymer is recognized for its high water solubility and ability to form complexes with various cations. PSA is utilized in drug delivery systems and industrial formulations due to its capacity to modulate solution viscosity and enhance formulation stability [90,91].
- Polyacrylamide acid (PAM) is a polyanion with key physicochemical properties, including the formation of three-dimensional networks with high water retention. Its structure can be tailored through co-polymerization with anionic monomers like acrylate or 2-acrylamido-2-methylpropane sulfonate (AMPS), affecting its solubility and viscoelastic properties. These modifications enhance PAM’s effectiveness in drug delivery systems by improving drug encapsulation and controlled release [92,93].
3. Polyelectrolyte Complexes (PECs)
3.1. Formation of PECs
3.2. Antibacterial Application
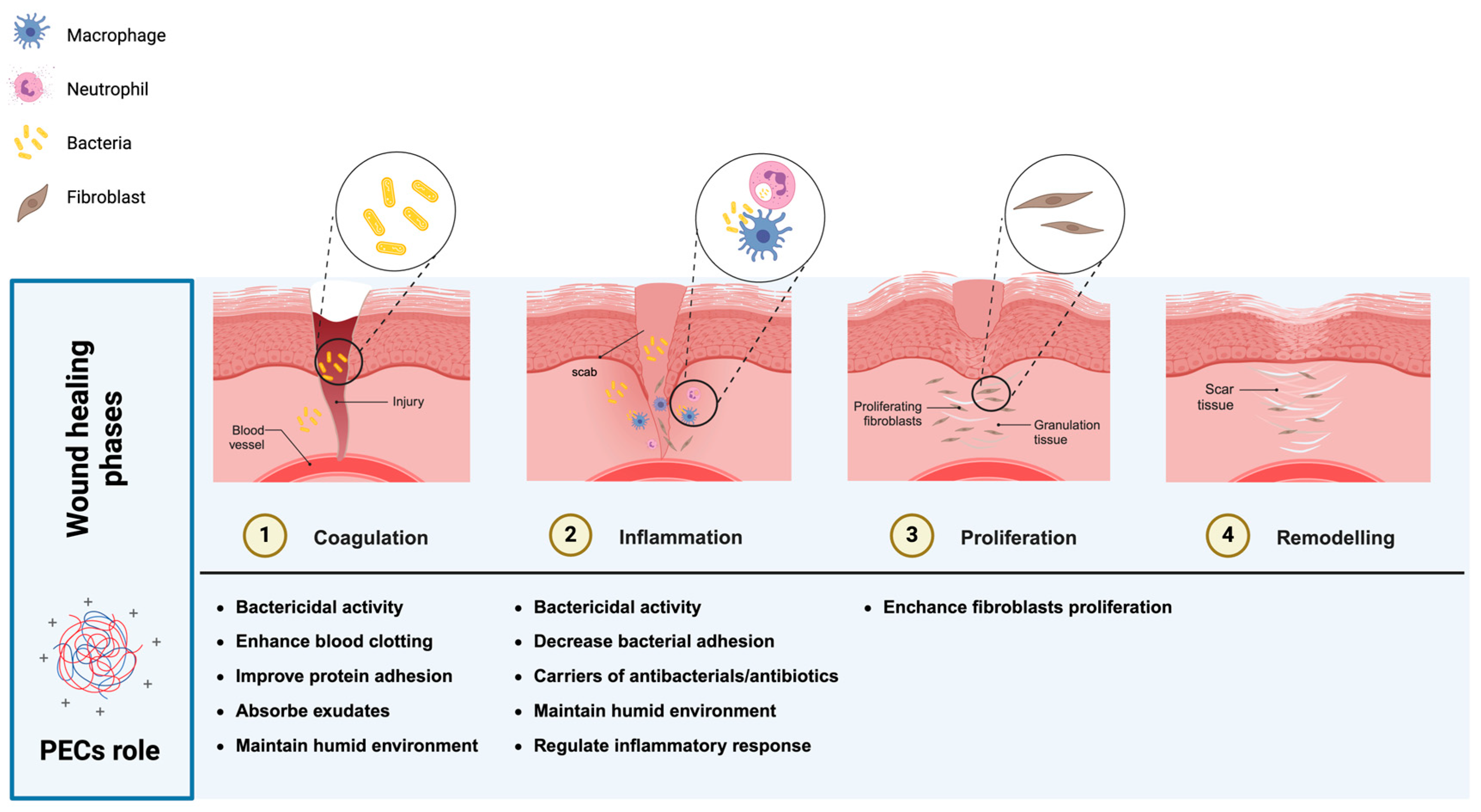
3.2.1. Wound Healing
3.2.2. Implants
3.2.3. Skin and Ocular Products
3.2.4. Oral Cavity and Lung Infections
3.2.5. Controlled Release
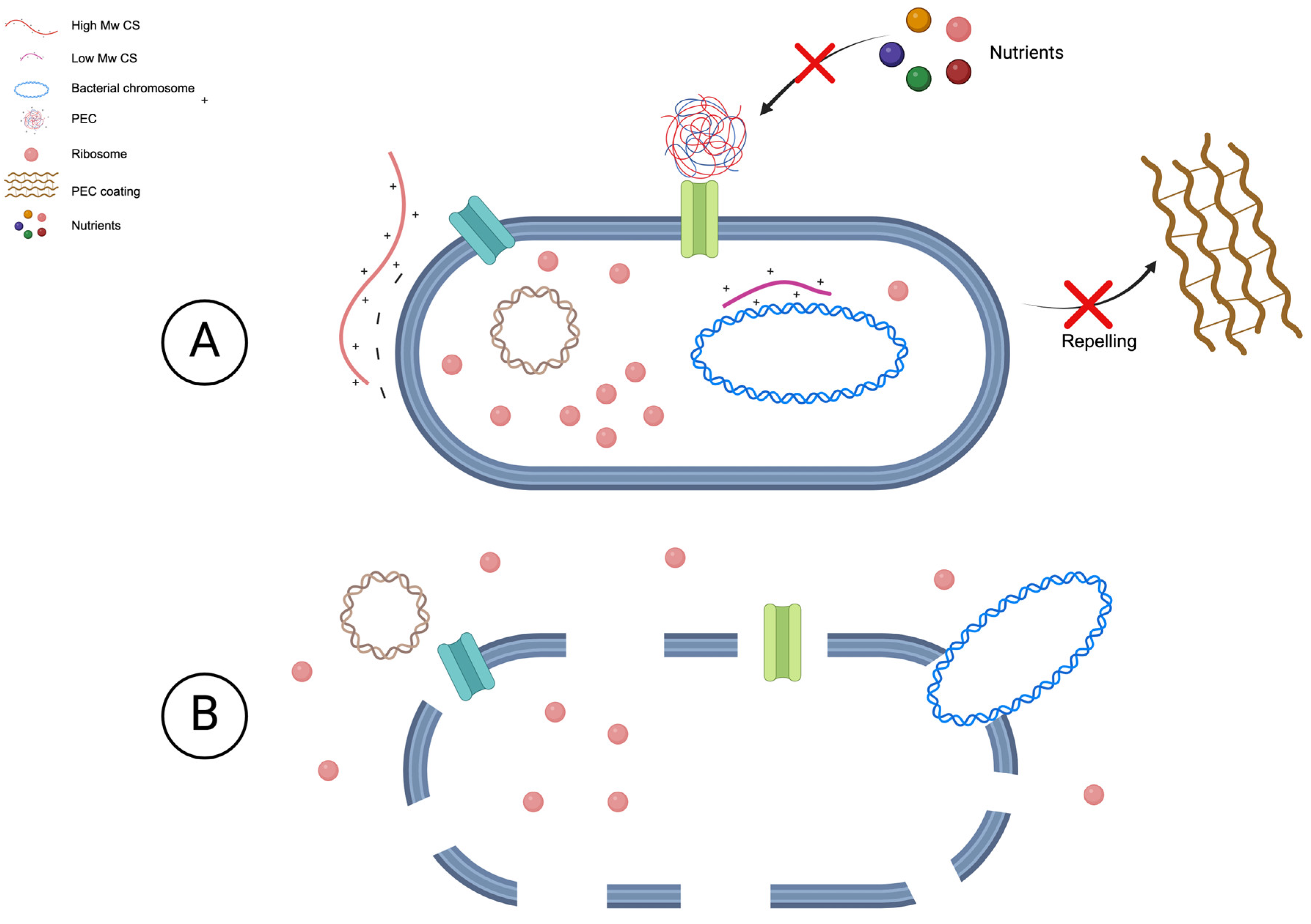
4. PECs Mechanism of Action
4.1. Against Procaryotic Bacteria
4.2. Against Biofilms
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2024; Volume 898, pp. 1–72. [Google Scholar]
- Ribeiro, A.; Alsayyed, R.; Oliveira, D.; Loureiro, R.; Cabral-Marques, H. Cannabinoids from C. Sativa L.: Systematic Review on Potential Pharmacological Effects against Infectious Diseases Downstream and Multidrug-Resistant Pathogens. Future Pharmacol. 2024, 4, 590–625. [Google Scholar] [CrossRef]
- Hurlow, J.; Bowler, P.G. Acute and Chronic Wound Infections: Microbiological, Immunological, Clinical and Therapeutic Distinctions. J. Wound Care 2022, 31, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Pulingam, T.; Parumasivam, T.; Gazzali, A.M.; Sulaiman, A.M.; Chee, J.Y.; Lakshmanan, M.; Chin, C.F.; Sudesh, K. Antimicrobial Resistance: Prevalence, Economic Burden, Mechanisms of Resistance and Strategies to Overcome. Eur. J. Pharm. Sci. 2022, 170, 106103. [Google Scholar] [CrossRef] [PubMed]
- Healthcare-Associated Infections 2024. Available online: https://www.ecdc.europa.eu/en/healthcare-associated-infections (accessed on 30 July 2024).
- Hibbard, R.; Mendelson, M.; Page, S.W.; Ferreira, J.P.; Pulcini, C.; Paul, M.C.; Faverjon, C. Antimicrobial Stewardship: A Definition with a One Health Perspective. NPJ Antimicrob. Resist. 2024, 2, 15. [Google Scholar] [CrossRef]
- Tran, T.-T.; Hadinoto, K. A Potential Quorum-Sensing Inhibitor for Bronchiectasis Therapy: Quercetin-Chitosan Nanoparticle Complex Exhibiting Superior Inhibition of Biofilm Formation and Swimming Motility of Pseudomonas Aeruginosa to the Native Quercetin. Int. J. Mol. Sci. 2021, 22, 1541. [Google Scholar] [CrossRef]
- McConoughey, S.J.; Howlin, R.; Granger, J.F.; Manring, M.M.; Calhoun, J.H.; Shirtliff, M.; Kathju, S.; Stoodley, P. Biofilms in Periprosthetic Orthopedic Infections. Future Microbiol. 2014, 9, 987–1007. [Google Scholar] [CrossRef]
- Donelli, G. Vascular Catheter-Related Infection and Sepsis. Surg. Infect. 2006, 7 (Suppl. S2), S25–S27. [Google Scholar] [CrossRef]
- Sharma, S.; Swetha, K.L.; Roy, A. Chitosan-Chondroitin Sulfate Based Polyelectrolyte Complex for Effective Management of Chronic Wounds. Int. J. Biol. Macromol. 2019, 132, 97–108. [Google Scholar] [CrossRef]
- Karakurt, I.; Ozaltin, K.; Pištěková, H.; Vesela, D.; Michael-Lindhard, J.; Humpolícek, P.; Mozetič, M.; Lehocky, M. Effect of Saccharides Coating on Antibacterial Potential and Drug Loading and Releasing Capability of Plasma Treated Polylactic Acid Films. Int. J. Mol. Sci. 2022, 23, 8821. [Google Scholar] [CrossRef]
- Cheng, D.; Hurst, J.R. Chronic Obstructive Pulmonary Disease: Aetiology, Pathology, Physiology and Outcome. Medicine 2023, 51, 737–741. [Google Scholar] [CrossRef]
- Davies, J.C. Pseudomonas Aeruginosa in Cystic Fibrosis: Pathogenesis and Persistence. Paediatr. Respir. Rev. 2002, 3, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Ul Haq, I.; Khan, T.A.; Krukiewicz, K. Etiology, Pathology, and Host-Impaired Immunity in Medical Implant-Associated Infections. J. Infect. Public Health 2024, 17, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef] [PubMed]
- Muthukumar, M. 50th Anniversary Perspective: A Perspective on Polyelectrolyte Solutions. Macromolecules 2017, 50, 9528–9560. [Google Scholar] [CrossRef]
- Buriuli, M.; Verma, D. Polyelectrolyte Complexes (PECs) for Biomedical Applications. In Advanced Structured Materials; Öchsner, A., da Silva, L.F.M., Altenbach, H., Eds.; Springer: Singapore, 2017; pp. 45–93. [Google Scholar]
- Hassan, M.A.; Omer, A.M.; Abbas, E.; Baset, W.M.A.; Tamer, T.M. Preparation, Physicochemical Characterization and Antimicrobial Activities of Novel Two Phenolic Chitosan Schiff Base Derivatives. Sci. Rep. 2018, 8, 11416. [Google Scholar] [CrossRef]
- Goy, R.C.; Morais, S.T.B.; Assis, O.B.G. Evaluation of the Antimicrobial Activity of Chitosan and Its Quaternized Derivative on E. Coli and S. Aureus Growth. Rev. Bras. Farmacogn. 2016, 26, 122–127. [Google Scholar] [CrossRef]
- Li, X.; Shang, L.; Li, D.; Wang, W.; Chen, S.; Zhong, H.; Huang, Y.; Long, S. High-Strength, Strong-Adhesion, and Antibacterial Polyelectrolyte Complex Hydrogel Films from Natural Polysaccharides. Polym. Test. 2022, 109, 107547. [Google Scholar] [CrossRef]
- Fu, J.; Ji, J.; Yuan, W.; Shen, J. Construction of Anti-Adhesive and Antibacterial Multilayer Films via Layer-by-Layer Assembly of Heparin and Chitosan. Biomaterials 2005, 26, 6684–6692. [Google Scholar] [CrossRef]
- Hernandez-Montelongo, J.; Lucchesi, E.G.; Gonzalez, I.; Macedo, W.A.A.; Nascimento, V.F.; Moraes, A.M.; Beppu, M.M.; Cotta, M.A. Hyaluronan/Chitosan Nanofilms Assembled Layer-by-Layer and Their Antibacterial Effect: A Study Using Staphylococcus Aureus and Pseudomonas Aeruginosa. Colloids Surf. B Biointerfaces 2016, 141, 499–506. [Google Scholar] [CrossRef]
- Lamch, Ł.; Wilk, K.A.; Dékány, I.; Deák, Á.; Hornok, V.; Janovák, L. Rational Mitomycin Nanocarriers Based on Hydrophobically Functionalized Polyelectrolytes and Poly(Lactide-Co-Glycolide). Langmuir 2022, 38, 5404–5417. [Google Scholar] [CrossRef]
- Saini, S.; Kukrety, A.; Patel, P.A.; Kumar, U.; Senthilkumar, T. Synthesis of Polycationic Nanoparticles for Microbial Inhibition and Killing. Nanotheranostics 2023, 7, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Škugor Rončević, I.; Krivić, D.; Buljac, M.; Vladislavić, N.; Buzuk, M. Polyelectrolytes Assembly: A Powerful Tool for Electrochemical Sensing Application. Sensors 2020, 20, 3211. [Google Scholar] [CrossRef] [PubMed]
- Vilsinski, B.H.; de Oliveira, A.C.; Souza, P.R.; Martins, A.F. Polysaccharide-Based Polyelectrolyte Multilayers Fabricated via Layer-by-Layer Approach: From Preparation to Applications. Prog. Org. Coat. 2024, 196, 108720. [Google Scholar] [CrossRef]
- Cabral-Marques, H.; Almeida, R. Optimisation of Spray-Drying Process Variables for Dry Powder Inhalation (DPI) Formulations of Corticosteroid/Cyclodextrin Inclusion Complexes. Eur. J. Pharm. Biopharm. 2009, 73, 121–129. [Google Scholar] [CrossRef]
- Sharma, R.; Kumar, S.; Malviya, R.; Prajapati, B.G.; Puri, D.; Limmatvapirat, S.; Sriamornsak, P. Recent Advances in Biopolymer-Based Mucoadhesive Drug Delivery Systems for Oral Application. J. Drug Deliv. Sci. Technol. 2024, 91, 105227. [Google Scholar] [CrossRef]
- Ribeiro, A.; Serrano, R.; Da Silva, I.B.M.; Pinto, J.F.; Silva, O. Development of an Adhesive Tablet for the Delivery of Diospyros Villosa Root for the Treatment of Oral Cavity Diseases. J. Drug Deliv. Sci. Technol. 2023, 90, 105148. [Google Scholar] [CrossRef]
- Haglund, E.; Seale-Goldsmith, M.-M.; Leary, J.F. Design of Multifunctional Nanomedical Systems. Ann. Biomed. Eng. 2009, 37, 2048–2063. [Google Scholar] [CrossRef]
- Hartig, S.M.; Greene, R.R.; Dikov, M.M.; Prokop, A.; Davidson, J.M. Multifunctional Nanoparticulate Polyelectrolyte Complexes. Pharm. Res. 2007, 24, 2353–2369. [Google Scholar] [CrossRef]
- Gindy, M.E.; Prud’homme, R.K. Multifunctional Nanoparticles for Imaging, Delivery and Targeting in Cancer Therapy. Expert. Opin. Drug Deliv. 2009, 6, 865–878. [Google Scholar] [CrossRef]
- Meka, V.S.; Sing, M.K.G.; Pichika, M.R.; Nali, S.R.; Kolapalli, V.R.M.; Kesharwani, P. A Comprehensive Review on Polyelectrolyte Complexes. Drug Discov. Today 2017, 22, 1697–1706. [Google Scholar] [CrossRef]
- Chen, H.; Song, Y.; Liu, N.; Wan, H.; Shu, G.; Liao, N. Effect of Complexation Conditions on Microcapsulation of Lactobacillus Acidophilus in Xanthan-Chitosan Polyelectrolyte Complex Gels. Acta Sci. Pol. Technol. Aliment. 2015, 14, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Jonas, A.M.; Glinel, K.; Behrens, A.; Anselmo, A.C.; Langer, R.S.; Jaklenec, A. Controlling the Growth of Staphylococcus Epidermidis by Layer-By-Layer Encapsulation. ACS Appl. Mater. Interfaces 2018, 10, 16250–16259. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Song, Y.; Liu, N.; Wan, H.; Liao, N.; Shu, G. Effect of Complexation Conditions on Microcapsulation of B. Bifidum BB01 in Xanthan-Chitosan Polyelectrolyte Complex Gels. J. Chem. Pharm. Res. 2014, 6, 1355–1360. [Google Scholar]
- Kulig, D.; Zimoch-Korzycka, A.; Kró, Z.; Oziembłowski, M.; Jarmoluk, A. Effect of Film-Forming Alginate/Chitosan Polyelectrolyte Complex on the Storage Quality of Pork. Molecules 2017, 22, 98. [Google Scholar] [CrossRef]
- Lai, W.-F.; Zhao, S.; Chiou, J. Antibacterial and Clusteroluminogenic Hypromellose-Graft-Chitosan-Based Polyelectrolyte Complex Films with High Functional Flexibility for Food Packaging. Carbohydr. Polym. 2021, 271, 118447. [Google Scholar] [CrossRef]
- Van der Gucht, J.; Spruijt, E.; Lemmers, M.; Cohen Stuart, M.A. Polyelectrolyte Complexes: Bulk Phases and Colloidal Systems. J. Colloid. Interface Sci. 2011, 361, 407–422. [Google Scholar] [CrossRef]
- Kulkarni, A.D.; Vanjari, Y.H.; Sancheti, K.H.; Patel, H.M.; Belgamwar, V.S.; Surana, S.J.; Pardeshi, C.V. Polyelectrolyte Complexes: Mechanisms, Critical Experimental Aspects, and Applications. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1615–1625. [Google Scholar] [CrossRef]
- Zhao, L.; Skwarczynski, M.; Toth, I. Polyelectrolyte-Based Platforms for the Delivery of Peptides and Proteins. ACS Biomater. Sci. Eng. 2019, 5, 4937–4950. [Google Scholar] [CrossRef]
- Shah, S.; Eyler, A.; Tabandeh, S.; Leon, L. Electrostatically Driven Self-Assembled Nanoparticles and Coatings. In Nanoparticles for Biomedical Applications; Chung, E.J., Leon, L., Rinaldi, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 349–370. ISBN 978-0-12-816662-8. [Google Scholar]
- Rinaudo, M. Chitin and Chitosan: Properties and Applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Amrutkar, J.R.; Gattani, S.G. Chitosan–Chondroitin Sulfate Based Matrix Tablets for Colon Specific Delivery of Indomethacin. AAPS Pharm. Sci. Tech. 2009, 10, 670–677. [Google Scholar] [CrossRef]
- Wu, H.-D.; Ji, D.-Y.; Chang, W.-J.; Yang, J.-C.; Lee, S.-Y. Chitosan-Based Polyelectrolyte Complex Scaffolds with Antibacterial Properties for Treating Dental Bone Defects. Mater. Sci. Eng. C 2012, 32, 207–214. [Google Scholar] [CrossRef]
- Smidsrod, O.; Skjakbrk, G. Alginate as Immobilization Matrix for Cells. Trends Biotechnol. 1990, 8, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Gomez, C.G.; Pérez Lambrecht, M.V.; Lozano, J.E.; Rinaudo, M.; Villar, M.A. Influence of the Extraction–Purification Conditions on Final Properties of Alginates Obtained from Brown Algae (Macrocystis pyrifera). Int. J. Biol. Macromol. 2009, 44, 365–371. [Google Scholar] [CrossRef]
- Gao, S.-K.; Yin, R.; Wang, X.-C.; Jiang, H.-N.; Liu, X.-X.; Lv, W.; Ma, Y.; Zhou, Y.-X. Structure Characteristics, Biochemical Properties, and Pharmaceutical Applications of Alginate Lyases. Mar. Drugs 2021, 19, 628. [Google Scholar] [CrossRef] [PubMed]
- Martău, G.A.; Mihai, M.; Vodnar, D.C. The Use of Chitosan, Alginate, and Pectin in the Biomedical and Food Sector—Biocompatibility, Bioadhesiveness, and Biodegradability. Polymers 2019, 11, 1837. [Google Scholar] [CrossRef] [PubMed]
- Tønnesen, H.H.; Karlsen, J. Alginate in Drug Delivery Systems. Drug Dev. Ind. Pharm. 2002, 28, 621–630. [Google Scholar] [CrossRef]
- Ramakrishnan, R.; Tae Kim, J.; Roy, S.; Jayakumar, A. Recent Advances in Carboxymethyl Cellulose-Based Active and Intelligent Packaging Materials: A Comprehensive Review. Int. J. Biol. Macromol. 2024, 259, 129194. [Google Scholar] [CrossRef]
- Javanbakht, S.; Shaabani, A. Carboxymethyl Cellulose-Based Oral Delivery Systems. Int. J. Biol. Macromol. 2019, 133, 21–29. [Google Scholar] [CrossRef]
- Ohta, S.; Nishiyama, T.; Sakoda, M.; Machioka, K.; Fuke, M.; Ichimura, S.; Inagaki, F.; Shimizu, A.; Hasegawa, K.; Kokudo, N.; et al. Development of Carboxymethyl Cellulose Nonwoven Sheet as a Novel Hemostatic Agent. J. Biosci. Bioeng. 2015, 119, 718–723. [Google Scholar] [CrossRef]
- Sharma, R.; Kuche, K.; Thakor, P.; Bhavana, V.; Srivastava, S.; Kumar Mehra, N.; Jain, S. Chondroitin Sulfate: Emerging Biomaterial for Biopharmaceutical Purpose and Tissue Engineering. Carbohydr. Polym. 2022, 286, 119305. [Google Scholar] [CrossRef]
- Yu, D.-G.; Lin, W.-C.; Lin, C.-H.; Yang, M.-C. Cytocompatibility and Antibacterial Activity of a PHBV Membrane with Surface-Immobilized Water-Soluble Chitosan and Chondroitin-6-Sulfate. Macromol. Biosci. 2006, 6, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Álvarez, L.; Ruiz-Rubio, L.; Azua, I.; Benito, V.; Bilbao, A.; Vilas-Vilela, J.L. Development of Multiactive Antibacterial Multilayers of Hyaluronic Acid and Chitosan onto Poly(Ethylene Terephthalate). Eur. Polym. J. 2019, 112, 31–37. [Google Scholar] [CrossRef]
- Marinho, A.; Nunes, C.; Reis, S. Hyaluronic Acid: A Key Ingredient in the Therapy of Inflammation. Biomolecules 2021, 11, 1518. [Google Scholar] [CrossRef] [PubMed]
- ter Boo, G.-J.A.; Richards, R.G.; Moriarty, T.F.; Grijpma, D.W.; Eglin, D. Hyaluronic Acid Derivatives and Its Polyelectrolyte Complexes with Gentamicin as a Delivery System for Antibiotics. Polym. Adv. Technol. 2017, 28, 1325–1333. [Google Scholar] [CrossRef]
- Beta-Carrageenan. Available online: https://pubchem.ncbi.nlm.nih.gov/substance/275324378 (accessed on 7 September 2024).
- Olatunji, O.; Kalia, S. Aquatic Biopolymers: Understanding Their Industrial Significance and Environmental Implications, 1st ed.; Springer: Cham, Switzerland, 2020; pp. 124–132. ISBN 978-3-030-34708-6. [Google Scholar]
- Borsani, B.; De Santis, R.; Perico, V.; Penagini, F.; Pendezza, E.; Dilillo, D.; Bosetti, A.; Zuccotti, G.V.; D’Auria, E. The Role of Carrageenan in Inflammatory Bowel Diseases and Allergic Reactions: Where Do We Stand? Nutrients 2021, 13, 3402. [Google Scholar] [CrossRef]
- Necas, J.; Bartosikova, L. Carrageenan: A Review. Veterinární Medicína 2013, 58, 187–205. [Google Scholar] [CrossRef]
- Leibbrandt, A.; Meier, C.; König-Schuster, M.; Weinmüllner, R.; Kalthoff, D.; Pflugfelder, B.; Graf, P.; Frank-Gehrke, B.; Beer, M.; Fazekas, T.; et al. Iota-Carrageenan Is a Potent Inhibitor of Influenza A Virus Infection. PLoS ONE 2010, 5, 14320. [Google Scholar] [CrossRef]
- Pacheco-Quito, E.-M.; Ruiz-Caro, R.; Veiga, M.-D. Carrageenan: Drug Delivery Systems and Other Biomedical Applications. Mar. Drugs 2020, 18, 583. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Q.; Ahmad Farooqi, A.; Wang, J.; Yue, Y.; Geng, L.; Wu, N. Opportunities and Challenges of Fucoidan for Tumors Therapy. Carbohydr. Polym. 2024, 324, 121555. [Google Scholar] [CrossRef]
- Fitton, J.H.; Stringer, D.N.; Park, A.Y.; Karpiniec, S.S. Therapies from Fucoidan: New Developments. Mar. Drugs 2019, 17, 571. [Google Scholar] [CrossRef]
- Baytas, S.N.; Linhardt, R.J. Advances in the Preparation and Synthesis of Heparin and Related Products. Drug Discov. Today 2020, 25, 2095–2109. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Chi, L.; Zhang, Z.; Zhao, H.; Zhang, F.; Linhardt, R.J. Heparin: An Old Drug for New Clinical Applications. Carbohydr. Polym. 2022, 295, 119818. [Google Scholar] [CrossRef] [PubMed]
- Vlcek, J.R.; Hedayati, M.; Melvin, A.C.; Reynolds, M.M.; Kipper, M.J. Blood-Compatible Materials: Vascular Endothelium-Mimetic Surfaces That Mitigate Multiple Cell-Material Interactions. Adv. Healthc. Mater. 2021, 10, e202001748. [Google Scholar] [CrossRef]
- Lin, W.-C.; Liu, T.-Y.; Yang, M.-C. Hemocompatibility of Polyacrylonitrile Dialysis Membrane Immobilized with Chitosan and Heparin Conjugate. Biomaterials 2004, 25, 1947–1957. [Google Scholar] [CrossRef]
- Thibault, J.-F.; Ralet, M.-C. Physico-Chemical Properties of Pectins in the Cell Walls and After Extraction. In Advances in Pectin and Pectinase Research; Voragen, F., Schols, H., Visser, R., Eds.; Springer: Dordrecht, The Netherlands, 2003; pp. 91–105. [Google Scholar]
- Ciriminna, R.; Fidalgo, A.; Meneguzzo, F.; Presentato, A.; Scurria, A.; Nuzzo, D.; Alduina, R.; Ilharco, L.M.; Pagliaro, M. Pectin: A Long-Neglected Broad-Spectrum Antibacterial. Chem. Med. Chem. 2020, 15, 2228–2235. [Google Scholar] [CrossRef]
- Boddu, S.H.S.; Bhagav, P.; Karla, P.K.; Jacob, S.; Adatiya, M.D.; Dhameliya, T.M.; Ranch, K.M.; Tiwari, A.K. Polyamide/Poly(Amino Acid) Polymers for Drug Delivery. J. Funct. Biomater. 2021, 12, 58. [Google Scholar] [CrossRef]
- Elbanna, K.; Alsulami, F.S.; Neyaz, L.A.; Abulreesh, H.H. Poly (γ) Glutamic Acid: A Unique Microbial Biopolymer with Diverse Commercial Applicability. Front. Microbiol. 2024, 15, 1348411. [Google Scholar] [CrossRef]
- Ijaz Ahmad, M.; Li, Y.; Pan, J.; Liu, F.; Dai, H.; Fu, Y.; Huang, T.; Farooq, S.; Zhang, H. Collagen and Gelatin: Structure, Properties, and Applications in Food Industry. Int. J. Biol. Macromol. 2024, 254, 128037. [Google Scholar]
- Antezana, P.E.; Municoy, S.; Orive, G.; Desimone, M.F. Design of a New 3D Gelatin—Alginate Scaffold Loaded with Cannabis Sativa Oil. Polymers 2022, 14, 4506. [Google Scholar] [CrossRef]
- Cabral Marques, H. The Pharmacist and the Research and Development of the Medicinal Product; Faculdade de Ciências e Tecnologia da Universidade do Algarve: Lisbon, Portugal, 2024; pp. 417–451. [Google Scholar]
- Davis, M.E.; Brewster, M.E. Cyclodextrin-Based Pharmaceutics: Past, Present and Future. Nat. Rev. Drug. Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef]
- Nsengiyumva, E.M.; Alexandridis, P. Xanthan Gum in Aqueous Solutions: Fundamentals and Applications. Int. J. Biol. Macromol. 2022, 216, 583–604. [Google Scholar] [CrossRef] [PubMed]
- Neamtu, B.; Barbu, A.; Negrea, M.O.; Berghea-Neamțu, C.Ș.; Popescu, D.; Zăhan, M.; Mireșan, V. Carrageenan-Based Compounds as Wound Healing Materials. Int. J. Mol. Sci. 2022, 23, 9117. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.-T.; Huang, G.-Y.; Chang, W.-J.; Lu, T.-W.; Huang, T.-W.; Ho, M.-H.; Mi, F.-L. Modification of Chitosan Nanofibers with CuS and Fucoidan for Antibacterial and Bone Tissue Engineering Applications. Carbohydr. Polym. 2022, 281, 119035. [Google Scholar] [CrossRef] [PubMed]
- Cheow, W.S.; Hadinoto, K. Green Preparation of Antibiotic Nanoparticle Complex as Potential Anti-Biofilm Therapeutics via Self-Assembly Amphiphile-Polyelectrolyte Complexation with Dextran Sulfate. Colloids Surf. B Biointerfaces 2012, 92, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Madkhali, O.A.; Sivagurunathan Moni, S.; Sultan, M.H.; Bukhary, H.A.; Ghazwani, M.; Alhakamy, N.A.; Meraya, A.M.; Alshahrani, S.; Alqahtani, S.S.; Bakkari, M.A.; et al. Formulation and Evaluation of Injectable Dextran Sulfate Sodium Nanoparticles as a Potent Antibacterial Agent. Sci. Rep. 2021, 11, 9914. [Google Scholar] [CrossRef]
- Savaskan Yilmaz, S.; Yildirim, N.; Misir, M.; Misirlioglu, Y.; Celik, E. Synthesis, Characterization of a New Polyacrylic Acid Superabsorbent, Some Heavy Metal Ion Sorption, the Adsorption Isotherms, and Quantum Chemical Investigation. Materials 2020, 13, 4390. [Google Scholar] [CrossRef]
- Chang, Y.-Z.; Lin, J.-T.; Prasannan, A.; Chen, P.-C.; Ko, C.-Y.; Tsai, H.-C. Evaluation of the Bacterial Anti-Adhesive Properties of Polyacrylic Acid, Chitosan and Heparin-Modified Medical Grade Silicone Rubber Substrate. J. Polym. Res. 2015, 22, 131. [Google Scholar] [CrossRef]
- Huang, X.; Xu, L.; Qian, H.; Wang, X.; Tao, Z. Polymalic Acid for Translational Nanomedicine. J. Nanobiotechnol 2022, 20, 295. [Google Scholar] [CrossRef]
- Zou, X.; Li, S.; Wang, P.; Li, B.; Feng, Y.; Yang, S. Sustainable Production and Biomedical Application of Polymalic Acid from Renewable Biomass and Food Processing Wastes. Crit. Rev. Biotechnol. 2021, 41, 216–228. [Google Scholar] [CrossRef]
- Ekbote, S.S.; Panda, A.G.; Bhor, M.D.; Bhanage, B.M. Polyvinylsulfonic Acid as a Novel Brønsted Acid Catalyst for Michael Addition of Indoles to α,β-Unsaturated Ketones. Catal. Commun. 2009, 10, 1569–1573. [Google Scholar] [CrossRef]
- Earl, C.C.; Smith, M.T.; Lease, R.A.; Bundy, B.C. Polyvinylsulfonic Acid: A Low-Cost RNase Inhibitor for Enhanced RNA Preservation and Cell-Free Protein Translation. Bioengineered 2018, 9, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Madani, A.; Nessark, B.; Brayner, R.; Elaissari, H.; Jouini, M.; Mangeney, C.; Chehimi, M.M. Carboxylic Acid-Functionalized, Core–Shell Polystyrene@polypyrrole Microspheres as Platforms for the Attachment of CdS Nanoparticles. Polymer 2010, 51, 2825–2835. [Google Scholar] [CrossRef]
- Gao, B.; Shi, N.; Qiao, Z. Structure and Luminescent Property of Complexes of Aryl Carboxylic Acid-Functionalized Polystyrene with Eu(III) and Tb(III) Ions. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 150, 565–574. [Google Scholar] [CrossRef]
- Xiong, B.; Loss, R.D.; Shields, D.; Pawlik, T.; Hochreiter, R.; Zydney, A.L.; Kumar, M. Polyacrylamide Degradation and Its Implications in Environmental Systems. NPJ Clean. Water 2018, 1, 17. [Google Scholar] [CrossRef]
- Huang, Y.; Zhong, M.; Shi, F.; Liu, X.; Tang, Z.; Wang, Y.; Huang, Y.; Hou, H.; Xie, X.; Zhi, C. An Intrinsically Stretchable and Compressible Supercapacitor Containing a Polyacrylamide Hydrogel Electrolyte. Angew Chem. Int. Ed. Engl. 2017, 56, 9141–9145. [Google Scholar] [CrossRef] [PubMed]
- Cheow, W.S.; Hadinoto, K. Self-Assembled Amorphous Drug–Polyelectrolyte Nanoparticle Complex with Enhanced Dissolution Rate and Saturation Solubility. J. Colloid. Interface Sci. 2012, 367, 518–526. [Google Scholar] [CrossRef]
- Bhattarai, A. Tribhuvan University A Review on Polyelectrolytes (PES) and Polyelectrolyte Complexes (PECs). Int. J. Eng. Tech. Res. 2020, 9, 876–889. [Google Scholar]
- Costa, R.R.; Mano, J.F. Polyelectrolyte Multilayered Assemblies in Biomedical Technologies. Chem. Soc. Rev. 2014, 43, 3453. [Google Scholar] [CrossRef]
- Martin, A.; Tabary, N.; Chai, F.; Leclercq, L.; Junthip, J.; Aubert-Viard, F.; Neut, C.; Weltrowski, M.; Blanchemain, N.; Martel, B. Build-up of an Antimicrobial Multilayer Coating on a Textile Support Based on a Methylene Blue-Poly(Cyclodextrin) Complex. Biomed. Mater. 2013, 8, 065006. [Google Scholar] [CrossRef]
- Li, S.; Wang, L.; Zhang, J.; Zhao, Z.; Yu, W.; Tan, Z.; Gao, P.; Chen, X. Combination of Natural Polyanions and Polycations Based on Interfacial Complexation for Multi-Functionalization of Wound Dressings. Front. Bioeng. Biotechnol. 2022, 10, 1006584. [Google Scholar] [CrossRef]
- Boas, M.; Gradys, A.; Vasilyev, G.; Burman, M.; Zussman, E. Electrospinning Polyelectrolyte Complexes: pH-Responsive Fibers. Soft Matter 2015, 11, 1739–1747. [Google Scholar] [CrossRef] [PubMed]
- Tziveleka, L.-A.; Pippa, N.; Georgantea, P.; Ioannou, E.; Demetzos, C.; Roussis, V. Marine Sulfated Polysaccharides as Versatile Polyelectrolytes for the Development of Drug Delivery Nanoplatforms: Complexation of Ulvan with Lysozyme. Int. J. Biol. Macromol. 2018, 118, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Solomevich, S.O.; Dmitruk, E.I.; Bychkovsky, P.M.; Salamevich, D.A.; Kuchuk, S.V.; Yurkshtovich, T.L. Biodegradable Polyelectrolyte Complexes of Chitosan and Partially Crosslinked Dextran Phosphate with Potential for Biomedical Applications. Int. J. Biol. Macromol. 2021, 169, 500–512. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.T.; Chang, C.H.; Lin, Y.Y.; Wu, M.F.; Wang, J.-L.; Han, J.L.; Hsieh, K.H. Antibacterial Activity and Biocompatibility of a Chitosan-γ- Poly(Glutamic Acid) Polyelectrolyte Complex Hydrogel. Carbohydr. Res. 2010, 345, 1774–1780. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Chen, X.-G.; Park, H.-J.; Liu, C.-G.; Liu, C.-S.; Meng, X.-H.; Yu, L.-J. Effect of MW and Concentration of Chitosan on Antibacterial Activity of Escherichia Coli. Carbohydr. Polym. 2006, 64, 60–65. [Google Scholar] [CrossRef]
- Kalinov, K.; Ignatova, M.; Manolova, N.; Rashkov, I.; Markova, N.; Momekova, D. N,N,N-Trimethylchitosan Iodide Complexes with a Weak or a Strong Polyacid and Nanoparticles Thereof. Colloid. Polym. Sci. 2014, 292, 2899–2912. [Google Scholar] [CrossRef]
- Ramirez, C.A.B.; Carriero, M.M.; Leomil, F.S.C.; Moro de Sousa, R.L.; de Miranda, A.; Mertins, O.; Mathews, P.D. Complexation of a Polypeptide-Polyelectrolytes Bioparticle as a Biomaterial of Antibacterial Activity. Pharmaceutics 2022, 14, 2746. [Google Scholar] [CrossRef]
- Birch, N.P.; Schiffman, J.D. Characterization of Self-Assembled Polyelectrolyte Complex Nanoparticles Formed from Chitosan and Pectin. Langmuir 2014, 30, 3441–3447. [Google Scholar] [CrossRef]
- Shu, M.; Long, S.; Huang, Y.; Li, D.; Li, H.; Li, X. High Strength and Antibacterial Polyelectrolyte Complex CS/HS Hydrogel Films for Wound Healing. Soft. Matter 2019, 15, 7686–7694. [Google Scholar] [CrossRef]
- Chen, X.; Yan, Y.; Li, H.; Wang, X.; Tang, S.; Li, Q.; Wei, J.; Su, J. Evaluation of Absorbable Hemostatic Agents of Polyelectrolyte Complexes Using Carboxymethyl Starch and Chitosan Oligosaccharide Both: In Vitro and in Vivo. Biomater. Sci. 2018, 6, 3332–3344. [Google Scholar] [CrossRef]
- Phoeung, T.; Spanedda, M.V.; Roger, E.; Heurtault, B.; Fournel, S.; Reisch, A.; Mutschler, A.; Perrin-Schmitt, F.; Hemmerlé, J.; Collin, D.; et al. Alginate/Chitosan Compact Polyelectrolyte Complexes: A Cell and Bacterial Repellent Material. Chem. Mater. 2017, 29, 10418–10425. [Google Scholar] [CrossRef]
- Yu, S.-H.; Mi, F.-L.; Wu, Y.-B.; Peng, C.-K.; Shyu, S.-S.; Huang, R.-N. Antibacterial Activity of Chitosan-Alginate Sponges Incorporating Silver Sulfadiazine: Effect of Ladder-Loop Transition of Interpolyelectrolyte Complex and Ionic Crosslinking on the Antibiotic Release. J. Appl. Polym. Sci. 2005, 98, 538–549. [Google Scholar] [CrossRef]
- Mogrovejo-Valdivia, A.; Maton, M.; Garcia-Fernandez, M.J.; Tabary, N.; Chai, F.; Neut, C.; Martel, B.; Blanchemain, N. In Vitro Microbiological and Drug Release of Silver/Ibuprofen Loaded Wound Dressing Designed for the Treatment of Chronically Infected Painful Wounds. Antibiotics 2021, 10, 805. [Google Scholar] [CrossRef] [PubMed]
- Batista, M.P.; Gonçalves, V.S.S.; Gaspar, F.B.; Nogueira, I.D.; Matias, A.A.; Gurikov, P. Novel Alginate-Chitosan Aerogel Fibres for Potential Wound Healing Applications. Int. J. Biol. Macromol. 2020, 156, 773–782. [Google Scholar] [CrossRef]
- Arısoy, S.; Bux, K.; Herwig, R.; Şalva, E. Development, Evaluation, and Molecular Dynamics Study of Ampicillin-Loaded Chitosan-Hyaluronic Acid Films as a Drug Delivery System. ACS Omega 2024, 9, 19805–19815. [Google Scholar] [CrossRef]
- Pérez-Anes, A.; Gargouri, M.; Laure, W.; Van Den Berghe, H.; Courcot, E.; Sobocinski, J.; Tabary, N.; Chai, F.; Blach, J.-F.; Addad, A.; et al. Bioinspired Titanium Drug Eluting Platforms Based on a Poly-β-Cyclodextrin-Chitosan Layer-by-Layer Self-Assembly Targeting Infections. ACS Appl. Mater. Interfaces 2015, 7, 12882–12893. [Google Scholar] [CrossRef]
- Ouerghemmi, S.; Degoutin, S.; Maton, M.; Tabary, N.; Cazaux, F.; Neut, C.; Blanchemain, N.; Martel, B. Core-Sheath Electrospun Nanofibers Based on Chitosan and Cyclodextrin Polymer for the Prolonged Release of Triclosan. Polymers 2022, 14, 1955. [Google Scholar] [CrossRef]
- Gauzit Amiel, A.; Palomino-Durand, C.; Maton, M.; Lopez, M.; Cazaux, F.; Chai, F.; Neut, C.; Foligné, B.; Martel, B.; Blanchemain, N. Designed Sponges Based on Chitosan and Cyclodextrin Polymer for a Local Release of Ciprofloxacin in Diabetic Foot Infections. Int. J. Pharm. 2020, 587, 119677. [Google Scholar] [CrossRef]
- Puppi, D.; Migone, C.; Grassi, L.; Pirosa, A.; Maisetta, G.; Batoni, G.; Chiellini, F. Integrated Three-Dimensional Fiber/Hydrogel Biphasic Scaffolds for Periodontal Bone Tissue Engineering. Polym. Int. 2016, 65, 631–640. [Google Scholar] [CrossRef]
- Novoskoltseva, O.A.; Litmanovich, E.A.; Loiko, N.G.; Nikolaev, Y.A.; Yaroslavov, A.A. Biodegradable Water-Soluble Matrix for Immobilization of Biocidal 4-Hexylresorcinol Int. J. Mol. Sci. 2023, 24, 14717. [Google Scholar] [CrossRef]
- Potaś, J.; Wach, R.A.; Rokita, B.; Wróblewska, M.; Winnicka, K. Evaluation of the Impact of Tragacanth/Xanthan Gum Interpolymer Complexation with Chitosan on Pharmaceutical Performance of Gels with Secnidazole as Potential Periodontal Treatment. Eur. J. Pharm. Sci. 2024, 192, 106657. [Google Scholar] [CrossRef] [PubMed]
- Ignatova, M.; Labaye, D.; Lenoir, S.; Strivay, D.; Jérôme, R.; Jérôme, C. Immobilization of Silver in Polypyrrole/Polyanion Composite Coatings: Preparation, Characterization, and Antibacterial Activity. Langmuir 2003, 19, 8971–8979. [Google Scholar] [CrossRef]
- Xu, X.-Y.; Chen, Y.-F.; Tan, Q.-G.; Chen, Z.-J.; Li, Y.; Wu, W.-G.; Wang, X.-F.; Liu, Y.-B. Construction of Multilayer Films with Bactericidal and Long-Term Antitumor Drug Release Properties as a Non-Vascular Stent Coating for Therapy in Obstruction. J. Mater. Chem. B 2019, 7, 4963–4972. [Google Scholar] [CrossRef] [PubMed]
- Tiyaboonchai, W.; Rodleang, I.; Ounaroon, A. Mucoadhesive Polyethylenimine-Dextran Sulfate Nanoparticles Containing Punica Granatum Peel Extract as a Novel Sustained-Release Antimicrobial. Pharm. Dev. Technol. 2015, 20, 426–432. [Google Scholar] [CrossRef]
- Broughton, G.; Janis, J.E.; Attinger, C.E. Wound Healing: An Overview. Plast. Reconstr. Surg. 2006, 117, 1e-S–32e-S. [Google Scholar] [CrossRef]
- Farahani, M.; Shafiee, A. Wound Healing: From Passive to Smart Dressings. Adv Healthcare Materials 2021, 10, 2100477. [Google Scholar] [CrossRef]
- Caldwell, M.D. Bacteria and Antibiotics in Wound Healing. Surg. Clin. North Am. 2020, 100, 757–776. [Google Scholar] [CrossRef]
- Serena, T.E.; Gould, L.; Ousey, K.; Kirsner, R.S. Reliance on Clinical Signs and Symptoms Assessment Leads to Misuse of Antimicrobials: Post Hoc Analysis of 350 Chronic Wounds. Adv. Wound Care 2022, 11, 639–649. [Google Scholar] [CrossRef]
- Subramaniam, T.; Fauzi, M.B.; Lokanathan, Y.; Law, J.X. The Role of Calcium in Wound Healing. Int. J. Mol. Sci. 2021, 22, 6486. [Google Scholar] [CrossRef]
- Wang, J.; Cai, N.; Chan, V.; Zeng, H.; Shi, H.; Xue, Y.; Yu, F. Antimicrobial Hydroxyapatite Reinforced-Polyelectrolyte Complex Nanofibers with Long-Term Controlled Release Activity for Potential Wound Dressing Application. Colloids Surf. A Physicochem. Eng. Asp. 2021, 624, 126722. [Google Scholar] [CrossRef]
- Yousefian, F.; Hesari, R.; Jensen, T.; Obagi, S.; Rgeai, A.; Damiani, G.; Bunick, C.G.; Grada, A. Antimicrobial Wound Dressings: A Concise Review for Clinicians. Antibiotics 2023, 12, 1434. [Google Scholar] [CrossRef] [PubMed]
- Kalinov, K.; Ignatova, M.; Maximova, V.; Rashkov, I.; Manolova, N. Modification of Electrospun Poly(ε-Caprolactone) Mats by Formation of a Polyelectrolyte Complex between Poly(Acrylic Acid) and Quaternized Chitosan for Tuning of Their Antibacterial Properties. Eur. Polym. J. 2014, 50, 18–29. [Google Scholar] [CrossRef]
- Mat Amin, K.A.; Gilmore, K.J.; Matic, J.; Poon, S.; Walker, M.J.; Wilson, M.R.; in het Panhuis, M. Polyelectrolyte Complex Materials Consisting of Antibacterial and Cell-Supporting Layers. Macromol. Biosci. 2012, 12, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Thaler, M.; Khosravi, I.; Hirschmann, M.T.; Kort, N.P.; Zagra, L.; Epinette, J.A.; Liebensteiner, M.C. Disruption of Joint Arthroplasty Services in Europe during the COVID-19 Pandemic: An Online Survey within the European Hip Society (EHS) and the European Knee Associates (EKA). Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 1712–1719. [Google Scholar] [CrossRef]
- Torger, B.; Müller, M. In Situ-ATR–FTIR Analysis on the Uptake and Release of Streptomycin from Polyelectrolyte Complex Layers. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 104, 546–553. [Google Scholar] [CrossRef]
- Ajdnik, U.; Zemljič, L.F.; Plohl, O.; Pérez, L.; Trček, J.; Bračič, M.; Mohan, T. Bioactive Functional Nanolayers of Chitosan-Lysine Surfactant with Single- And Mixed-Protein-Repellent and Antibiofilm Properties for Medical Implants. ACS Appl. Mater. Interfaces 2021, 13, 23352–23368. [Google Scholar] [CrossRef]
- Debas, H.T.; Donkor, P.; Gawande, A.; T Jamison, D.; E Kruk, M.; Mock, C.N. (Eds.) Essential Surgery: Disease Control Priorities, 3rd ed.; The International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA, 2015; Volume 1. [Google Scholar] [PubMed]
- Shokry, M.M.; Khalil, I.A.; El-Kasapy, A.; Osman, A.; Mostafa, A.; Salah, M.; El-Sherbiny, I.M. Multifunctional Prosthetic Polyester-Based Hybrid Mesh for Repairing of Abdominal Wall Hernias and Defects. Carbohydr. Polym. 2019, 223, 115027. [Google Scholar] [CrossRef]
- David, N.; Nallaiyan, R. Biologically Anchored Chitosan/Gelatin-SrHAP Scaffold Fabricated on Titanium against Chronic Osteomyelitis Infection. Int. J. Biol. Macromol. 2018, 110, 206–214. [Google Scholar] [CrossRef]
- Puigmal, N.; Ramos, V.; Artzi, N.; Borrós, S. Poly(β-Amino Ester)s-Based Delivery Systems for Targeted Transdermal Vaccination. Pharmaceutics 2023, 15, 1262. [Google Scholar] [CrossRef]
- Gasmi, S.N.; Kirdi, R.; Khames, M. Antimicrobial Study and in Vitro Evaluation of Coated Contact Lenses with Polyelectrolyte Complex Based on Chitosan and Sodium Algina. Acta Microbiol. Hell. 2020, 65, 223–233. [Google Scholar]
- Kim, J.; Hwang, J.; Seo, Y.; Jo, Y.; Son, J.; Choi, J. Engineered Chitosan–Xanthan Gum Biopolymers Effectively Adhere to Cells and Readily Release Incorporated Antiseptic Molecules in a Sustained Manner. J. Ind. Eng. Chem. 2017, 46, 68–79. [Google Scholar] [CrossRef]
- Kumar, A.; Ahuja, M. Carboxymethyl Gum Kondagogu-Chitosan Polyelectrolyte Complex Nanoparticles: Preparation and Characterization. Int. J. Biol. Macromol. 2013, 62, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Racovita, S.; Vasiliu, A.-L.; Bele, A.; Schwarz, D.; Steinbach, C.; Boldt, R.; Schwarz, S.; Mihai, M. Complex Calcium Carbonate/Polymer Microparticles as Carriers for Aminoglycoside Antibiotics. RSC Adv. 2018, 8, 23274–23283. [Google Scholar] [CrossRef] [PubMed]
- Vodná, L.; Bubeníková, S.; Bakoš, D. Chitosan Based Hydrogel Microspheres as Drug Carriers. Macromol. Biosci. 2007, 7, 629–634. [Google Scholar] [CrossRef]
- Tadros, M.I. Controlled-Release Effervescent Floating Matrix Tablets of Ciprofloxacin Hydrochloride: Development, Optimization and in Vitro–in Vivo Evaluation in Healthy Human Volunteers. Eur. J. Pharm. Biopharm. 2010, 74, 332–339. [Google Scholar] [CrossRef]
- Jain, S.K.; Prajapati, N.; Rajpoot, K.; Kumar, A. A Novel Sustained Release Drug–Resin Complex-Based Microbeads of Ciprofloxacin HCl. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1891–1900. [Google Scholar] [CrossRef]
- Nugraheni, P.S.; Soeriyadi, A.H.; Sediawan, W.B.; Budhijanto, W. Comparison of Formulation Methods to Produce Nano-Chitosan as Inhibitor Agent for Bacterial Growth. J. Eng. Tech. Sci. 2019, 51, 431–442. [Google Scholar] [CrossRef]
- Rubini, D.; Banu, S.F.; Subramani, P.; Hari, B.N.V.; Gowrishankar, S.; Pandian, S.K.; Wilson, A.; Nithyanand, P. Extracted Chitosan Disrupts Quorum Sensing Mediated Virulence Factors in Urinary Tract Infection Causing Pathogens. Pathog. Dis. 2019, 77, ftz009. [Google Scholar] [CrossRef]
- Von Ohle, C.; Gieseke, A.; Nistico, L.; Decker, E.M.; deBeer, D.; Stoodley, P. Real-Time Microsensor Measurement of Local Metabolic Activities in Ex Vivo Dental Biofilms Exposed to Sucrose and Treated with Chlorhexidine. Appl. Environ. Microbiol. 2010, 76, 2326–2334. [Google Scholar] [CrossRef]
| Polyelectrolyte | Functional Group 1 | Properties | Source | Refs. |
|---|---|---|---|---|
| Dextran sulfate | -SO42⁻ | Highly soluble in water; forms gels with counterions |
| [82,83] |
| Polyacrylic acid | -COOH | Water solubility; high-water absorption; gel formation |
| [84,85] |
| Polymalic acid | -COOH | Soluble in water; forms gels in aqueous environments |
| [86,87] |
| Polyvinylsulfonic acid sodium salt | -SO3Na | High water solubility; complex formation with cations |
| [88,89] |
| Polystyrene carboxylic acid | -COOH | Complex formation; viscosity adjustment |
| [90,91] |
| Polyacrylamide acid | -CO2NH2 | Gel-forming; high-water retention |
| [92,93] |
| PEC | Tested Bacteria | Application | Refs. |
|---|---|---|---|
| CS/Alg | E. coli, S. aureus, K. pneumoniae, and P. aeruginosa |
| [69,109,110,111,112] |
| CS/ChS | B. subtilis and E. coli |
| [10,11,69] |
| CS/HA | P. gingivalis |
| [11,113] |
| CS/PCD | S. aureus and E. coli |
| [101,114,115,116] |
| CMC/HACC | E. coli and S. aureus |
| [98] |
| CS/Dex-P | S. aureus and E. coli |
| [101] |
| CS/γ-PGA | S. aureus and E. coli |
| [45,117] |
| Alg/QHEC-Et | M. luteus and P. aeruginosa |
| [118] |
| Xanthan/CS | S. aureus and E. coli |
| [119] |
| PPy/PA | S. aureus and E. coli |
| [120] |
| PEI/Alg | S. aureus and E. coli |
| [121] |
| PEI/DS | S. mutans, S. sanguinis, and P. gingivalis |
| [122] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsayyed, R.; Ribeiro, A.; Cabral-Marques, H. Polyelectrolytes and Polyelectrolyte Complexes as Future Antibacterial Agents. Bacteria 2024, 3, 452-475. https://doi.org/10.3390/bacteria3040031
Alsayyed R, Ribeiro A, Cabral-Marques H. Polyelectrolytes and Polyelectrolyte Complexes as Future Antibacterial Agents. Bacteria. 2024; 3(4):452-475. https://doi.org/10.3390/bacteria3040031
Chicago/Turabian StyleAlsayyed, Rahaf, Adriana Ribeiro, and Helena Cabral-Marques. 2024. "Polyelectrolytes and Polyelectrolyte Complexes as Future Antibacterial Agents" Bacteria 3, no. 4: 452-475. https://doi.org/10.3390/bacteria3040031
APA StyleAlsayyed, R., Ribeiro, A., & Cabral-Marques, H. (2024). Polyelectrolytes and Polyelectrolyte Complexes as Future Antibacterial Agents. Bacteria, 3(4), 452-475. https://doi.org/10.3390/bacteria3040031








