Rickettsial Agents Associated with Ectoparasites in Attica, Greece
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
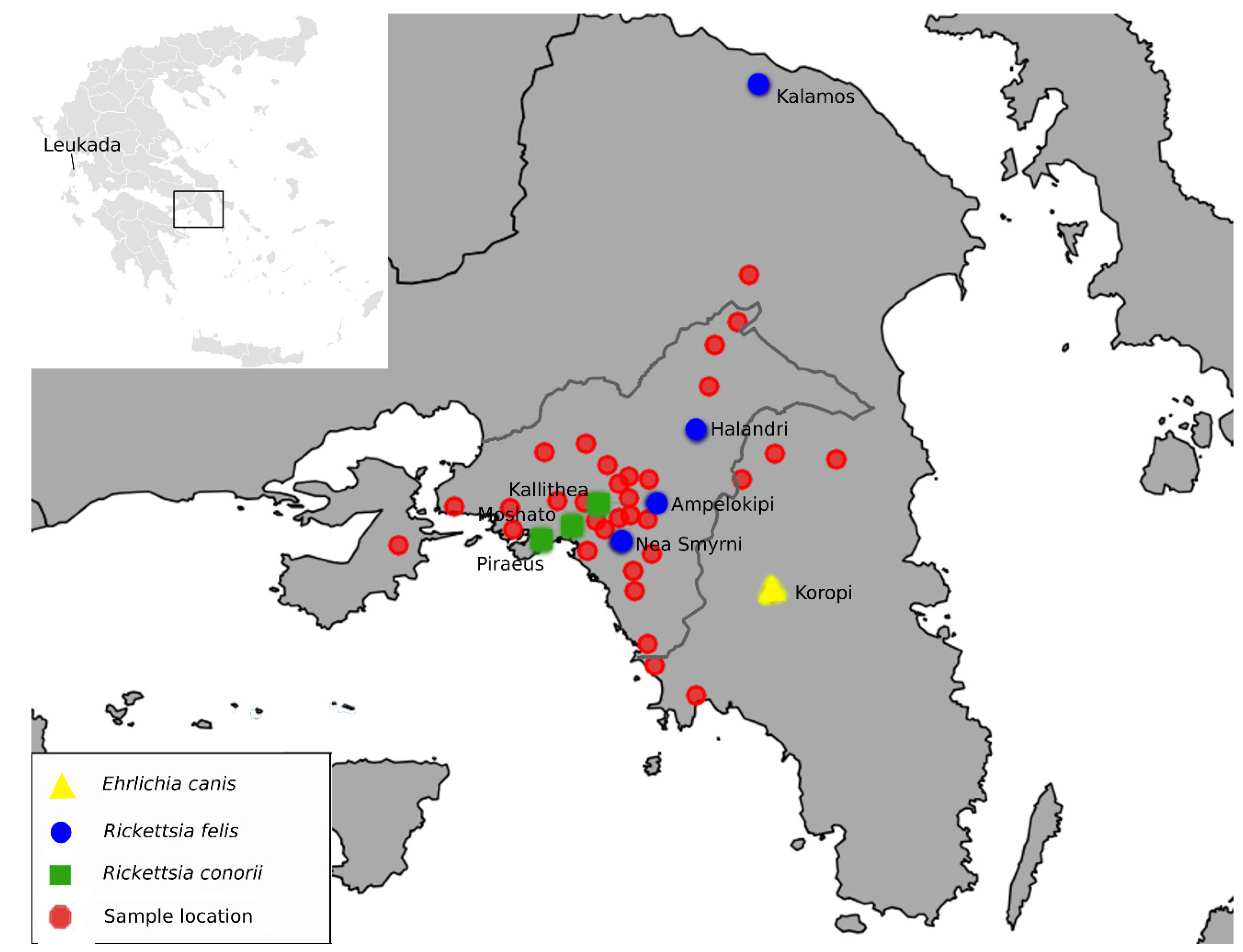
3. Results
4. Discussion
4.1. Rickettsia Felis
4.2. Rickettsia Conorii
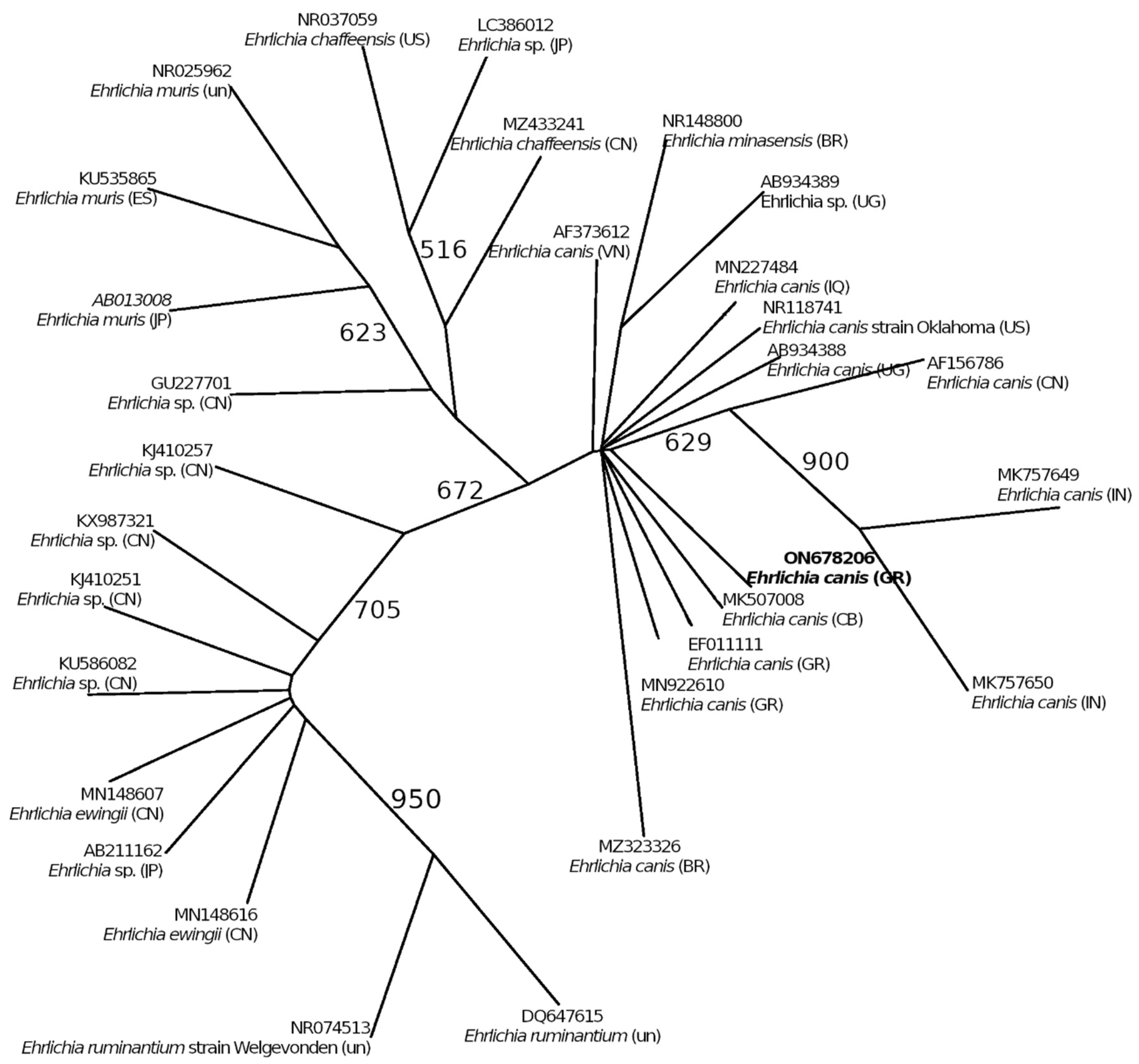
4.3. Ehrlichia canis
4.4. Wolbachia pipientis
4.5. Candidatus Midichloria mitochondrii
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Castelli, M.; Sassera, D.; Petroni, G. Biodiversity of “Non-Model” Rickettsiales and Their Association with Aquatic Organisms. In Rickettsiales: Biology, Molecular Biology, Epidemiology, and Vaccine Development; Thomas, S., Ed.; Springer International Publishing: Cham, Swizerland, 2016; pp. 59–91. [Google Scholar] [CrossRef]
- Angelakis, E.; Raoult, D. Rickettsia and Rickettsia-Like Organisms. In Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1666–1675.e1. [Google Scholar] [CrossRef]
- Tomassone, L.; Portillo, A.; Nováková, M.; de Sousa, R.; Oteo, J.A. Neglected Aspects of Tick-Borne Rickettsioses. Parasit. Vectors 2018, 11, 263. [Google Scholar] [CrossRef] [PubMed]
- Parola, P.; Davoust, B.; Raoult, D. Tick- and Flea-Borne Rickettsial Emerging Zoonoses. Vet. Res. 2005, 36, 469–492. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Alexander, W.; Gilligan, J.; Rikihisa, Y. The Importance of Rickettsiales Infections. In Rickettsiales: Biology, Molecular Biology, Epidemiology, and Vaccine Development; Springer International Publishing: Cham, Switzerland, 2017; pp. 3–21. [Google Scholar] [CrossRef]
- Makepeace, B.; Gill, A. Chapter 21: Wolbachia; Springer International Publishing: Cham, Switzerland, 2017; pp. 465–512. [Google Scholar] [CrossRef]
- Psaroulaki, A.; Spyridaki, I.; Ioannidis, A.; Babalis, T.; Gikas, A.; Tselentis, Y. First Isolation and Identification of Rickettsia conorii from Ticks Collected in the Region of Fokida in Central Greece. J. Clin. Microbiol. 2003, 41, 3317–3319. [Google Scholar] [CrossRef] [PubMed]
- Latrofa, M.S.; Angelou, A.; Giannelli, A.; Annoscia, G.; Ravagnan, S.; Dantas-Torres, F.; Capelli, G.; Halos, L.; Beugnet, F.; Papadopoulos, E.; et al. Ticks and Associated Pathogens in Dogs from Greece. Parasit. Vectors 2017, 10, 301. [Google Scholar] [CrossRef]
- Papa, A.; Xanthopoulou, K.; Kotriotsiou, T.; Papaioakim, M.; Sotiraki, S.; Chaligiannis, I.; Maltezos, E. Rickettsia Species in Human-Parasitizing Ticks in Greece. Trans. R. Soc. Trop. Med. Hyg. 2016, 110, 299–304. [Google Scholar] [CrossRef]
- Papa, A.; Tsioka, K.; Kontana, A.; Papadopoulos, C.; Giadinis, N. Bacterial Pathogens and Endosymbionts in Ticks. Ticks Tick-Borne Dis. 2017, 8, 31–35. [Google Scholar] [CrossRef]
- Moraga-Fernández, A.; Chaligiannis, Ι.; Cabezas-Cruz, A.; Papa, A.; Sotiraki, S.; de la Fuente, J.G.; Fernández de Mera, I. Molecular Identification of Spotted Fever Group Rickettsia in Ticks Collected from Dogs and Small Ruminants in Greece. Exp. Appl. Acarol. 2019, 78, 421–430. [Google Scholar] [CrossRef]
- Psaroulaki, A.; Ragiadakou, D.; Kouris, G.; Papadopoulos, B.; Chaniotis, B.; Tselentis, Y. Ticks, Tick-Borne Rickettsiae, and Coxiella burnetii in the Greek Island of Cephalonia. Ann. N. Y. Acad. Sci. 2006, 1078, 389–399. [Google Scholar] [CrossRef]
- Lane, R.P.; Crosskey, R.W. (Eds.) Medical Insects and Arachnids; Springer: Dordrecht, The Netherlands, 1993. [Google Scholar] [CrossRef]
- Walker, J.B.; Keirans, J.E.; Horak, I. The Genus Rhipicephalus (Acardi, Ixodidae): A Guide to the Brown Ticks of the World; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2000. [Google Scholar]
- Estrada-Peña, A.; Bouattour, A.; Camicas, J.-L.; Walker, A. Ticks of Domestic Animals in the Mediterranean Region. A Guide to Identification of Species; University of Zaragoza: Zaragoza, Spain, 2004. [Google Scholar]
- Duron, O.; Noël, V.; McCoy, K.D.; Bonazzi, M.; Sidi-Boumedine, K.; Morel, O.; Vavre, F.; Zenner, L.; Jourdain, E.; Durand, P.; et al. The Recent Evolution of a Maternally-Inherited Endosymbiont of Ticks Led to the Emergence of the Q Fever Pathogen, Coxiella burnetii. PLOS Pathog. 2015, 11, e1004892. [Google Scholar] [CrossRef]
- Maia, C.; Ferreira, A.; Nunes, M.; Vieira, M.L.; Campino, L.; Cardoso, L. Molecular Detection of Bacterial and Parasitic Pathogens in Hard Ticks from Portugal. Ticks Tick-Borne Dis. 2014, 5, 409–414. [Google Scholar] [CrossRef]
- Felsenstein, J. PHYLIP (Phylogeny Inference Package). Version 3.6 Distributed by the Author. Available online: https://evolution.genetics.washington.edu/phylip.html (accessed on 9 October 2022).
- Diarra, A.Z.; Kone, A.K.; Doumbo Niare, S.; Laroche, M.; Diatta, G.; Atteynine, S.A.; Coulibaly, M.; Sangare, A.K.; Kouriba, B.; Djimde, A.; et al. Molecular Detection of Microorganisms Associated with Small Mammals and Their Ectoparasites in Mali. Am. J. Trop. Med. Hyg. 2020, 103, 2542–2551. [Google Scholar] [CrossRef] [PubMed]
- Taillardat-Bisch, A.-V.; Raoult, D.; Drancourt, M. RNA Polymerase β-Subunit-Based Phylogeny of Ehrlichia Spp., Anaplasma Spp., Neorickettsia Spp. and Wolbachia pipientis. Int. J. Syst. Evol. Microbiol. 2003, 53, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Socolovschi, C.; Mediannikov, O.; Sokhna, C.; Tall, A.; Diatta, G.; Bassene, H.; Trape, J.-F.; Raoult, D. Rickettsia felis –Associated Uneruptive Fever, Senegal. Emerg. Infect. Dis. 2010, 16, 1140–1142. [Google Scholar] [CrossRef]
- Angelakis, E.; Mediannikov, O.; Parola, P.; Raoult, D. Rickettsia felis: The Complex Journey of an Emergent Human Pathogen. Trends Parasitol. 2016, 32, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Bechah, Y.; Socolovschi, C.; Raoult, D. Identification of Rickettsial Infections by Using Cutaneous Swab Specimens and PCR. Emerg. Infect. Dis. 2011, 17, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Hellenic Statistical Authority. Census Results of Population and Housing-ELSTAT. 2021. Available online: https://www.statistics.gr/2021-census-pop-hous-results (accessed on 9 November 2022).
- Giudice, E.; Pietro, S.D.; Alaimo, A.; Blanda, V.; Lelli, R.; Francaviglia, F.; Caracappa, S.; Torina, A. A Molecular Survey of Rickettsia felis in Fleas from Cats and Dogs in Sicily (Southern Italy). PLoS ONE 2014, 9, e106820. [Google Scholar] [CrossRef]
- Bitam, I.; Dittmar, K.; Parola, P.; Whiting, M.F.; Raoult, D. Fleas and Flea-Borne Diseases. Int. J. Infect. Dis. 2010, 14, e667–e676. [Google Scholar] [CrossRef]
- Feldman, S.H.; Easton, D.N. Chapter 17—Occupational Health and Safety. In The Laboratory Rat, 2nd ed.; Suckow, M.A., Weisbroth, S.H., Franklin, C.L., Eds.; American College of Laboratory Animal Medicine; Academic Press: Burlington, VT, USA, 2006; pp. 565–586. [Google Scholar] [CrossRef]
- Civen, R.; Ngo, V. Murine Typhus: An Unrecognized Suburban Vectorborne Disease. Clin. Infect. Dis. 2008, 46, 913–918. [Google Scholar] [CrossRef]
- Diakou, A.; Di Cesare, A.; Accettura, P.M.; Barros, L.; Iorio, R.; Paoletti, B.; Frangipane di Regalbono, A.; Halos, L.; Beugnet, F.; Traversa, D. Intestinal Parasites and Vector-Borne Pathogens in Stray and Free-Roaming Cats Living in Continental and Insular Greece. PLoS Negl. Trop. Dis. 2017, 11, e0005335. [Google Scholar] [CrossRef]
- Chochlakis, D.; Germanakis, A.; Chaliotis, G.; Kapetanaki, S.; Kalogeraki, L.; Gkika, E.; Partalis, N.; Polymili, G.; Tselentis, Y.; Psaroulaki, A. Potential Exposure of Humans to Rickettsia felis in Greece. Acta Trop. 2018, 178, 40–45. [Google Scholar] [CrossRef]
- Legendre, K.; Macaluso, K. Rickettsia Felis: A Review of Transmission Mechanisms of an Emerging Pathogen. Trop. Med. Infect. Dis. 2017, 2, 64. [Google Scholar] [CrossRef] [PubMed]
- Diakou, A.; Di Cesare, A.; Morelli, S.; Colombo, M.; Halos, L.; Simonato, G.; Tamvakis, A.; Beugnet, F.; Paoletti, B.; Traversa, D. Endoparasites and Vector-Borne Pathogens in Dogs from Greek Islands: Pathogen Distribution and Zoonotic Implications. PLoS Negl. Trop. Dis. 2019, 13, e0007003. [Google Scholar] [CrossRef] [PubMed]
- Daniel, S.A.; Manika, K.; Arvanmdou, M.; Antoniadis, A. Prevalence of Rickettsia conorii and Rickettsia typhi Infections in the Population of Northern Greece. Am. J. Trop. Med. Hyg. 2002, 66, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Psaroulaki, A.; Germanakis, A.; Gikas, A.; Scoulica, E.; Tselentis, Y. First Isolation and Genotypic Identification of Rickettsia conorii Malish 7 from a Patient in Greece. Eur. J. Clin. Microbiol. Infect. Dis. 2005, 24, 297–298. [Google Scholar] [CrossRef] [PubMed]
- Spernovasilis, N.; Markaki, I.; Papadakis, M.; Mazonakis, N.; Ierodiakonou, D. Mediterranean Spotted Fever: Current Knowledge and Recent Advances. Trop. Med. Infect. Dis. 2021, 6, 172. [Google Scholar] [CrossRef] [PubMed]
- Mylonakis, M.E.; Koutinas, A.F.; Leontides, L.S. Bone Marrow Mastocytosis in Dogs with Myelosuppressive Monocytic Ehrlichiosis (Ehrlichia Canis): A Retrospective Study. Vet. Clin. Pathol. 2006, 35, 311–314. [Google Scholar] [CrossRef]
- Komnenou, A.A.; Mylonakis, M.E.; Kouti, V.; Tendoma, L.; Leontides, L.; Skountzou, E.; Dessiris, A.; Koutinas, A.F.; Ofri, R. Ocular Manifestations of Natural Canine Monocytic Ehrlichiosis (Ehrlichia canis): A Retrospective Study of 90 Cases. Vet. Ophthalmol. 2007, 10, 137–142. [Google Scholar] [CrossRef]
- Theodorou, K.; Leontides, L.; Siarkou, V.I.; Petanides, T.; Tsafas, K.; Harrus, S.; Mylonakis, M.E. Synovial Fluid Cytology in Experimental Acute Canine Monocytic Ehrlichiosis (Ehrlichia canis). Vet. Microbiol. 2015, 177, 224–227. [Google Scholar] [CrossRef]
- Kostopoulou, D.; Gizzarelli, M.; Ligda, P.; Foglia Manzillo, V.; Saratsi, K.; Montagnaro, S.; Schunack, B.; Boegel, A.; Pollmeier, M.; Oliva, G.; et al. Mapping the Canine Vector-Borne Disease Risk in a Mediterranean Area. Parasit. Vectors 2020, 13, 282. [Google Scholar] [CrossRef]
- Angelou, A.; Gelasakis, A.I.; Verde, N.; Pantchev, N.; Schaper, R.; Chandrashekar, R.; Papadopoulos, E. Prevalence and Risk Factors for Selected Canine Vector-Borne Diseases in Greece. Parasit. Vectors 2019, 12, 283. [Google Scholar] [CrossRef]
- Bowman, D.; Little, S.E.; Lorentzen, L.; Shields, J.; Sullivan, M.P.; Carlin, E.P. Prevalence and Geographic Distribution of Dirofilaria immitis, Borrelia burgdorferi, Ehrlichia canis, and Anaplasma phagocytophilum in Dogs in the United States: Results of a National Clinic-Based Serologic Survey. Vet. Parasitol. 2009, 160, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.C.; Luz, M.F.; Granada, S.; Vilhena, H.; Nachum-Biala, Y.; Lopes, A.P.; Cardoso, L.; Baneth, G. Molecular Detection of Anaplasma bovis, Ehrlichia canis and Hepatozoon felis in Cats from Luanda, Angola. Parasit. Vectors 2018, 11, 167. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.; Rikihisa, Y.; Wen, B. Ehrlichia canis-like Agent Isolated from a Man in Venezuela: Antigenic and Genetic Characterization. J. Clin. Microbiol. 1996, 34, 2133–2139. [Google Scholar] [CrossRef] [PubMed]
- Unver, A.; Perez, M.; Orellana, N.; Huang, H.; Rikihisa, Y. Molecular and Antigenic Comparison of Ehrlichia canis Isolates from Dogs, Ticks, and a Human in Venezuela. J. Clin. Microbiol. 2001, 39, 2788–2793. [Google Scholar] [CrossRef]
- Perez, M.; Bodor, M.; Zhang, C.; Xiong, Q.; Rikihisa, Y. Human Infection with Ehrlichia canis Accompanied by Clinical Signs in Venezuela. Ann. N. Y. Acad. Sci. 2006, 1078, 110–117. [Google Scholar] [CrossRef]
- Werren, J.H.; Baldo, L.; Clark, M.E. Wolbachia: Master Manipulators of Invertebrate Biology. Nat. Rev. Microbiol. 2008, 6, 741–751. [Google Scholar] [CrossRef]
- Bordenstein, S.R.; Paraskevopoulos, C.; Dunning Hotopp, J.C.; Sapountzis, P.; Lo, N.; Bandi, C.; Tettelin, H.; Werren, J.H.; Bourtzis, K. Parasitism and Mutualism in Wolbachia: What the Phylogenomic Trees Can and Cannot Say. Mol. Biol. Evol. 2009, 26, 231–241. [Google Scholar] [CrossRef]
- Madhav, M.; Baker, D.; Morgan, J.A.T.; Asgari, S.; James, P. Wolbachia: A Tool for Livestock Ectoparasite Control. Vet. Parasitol. 2020, 288, 109297. [Google Scholar] [CrossRef]
- De Oliveira, C.D.; Gonçalves, D.S.; Baton, L.A.; Shimabukuro, P.H.F.; Carvalho, F.D.; Moreira, L.A. Broader Prevalence of Wolbachia in Insects Including Potential Human Disease Vectors. Bull. Entomol. Res. 2015, 105, 305–315. [Google Scholar] [CrossRef]
- Rolain, J.-M.; Franc, M.; Davoust, B.; Raoult, D. Molecular Detection of Bartonella quintana, B. koehlerae, B. henselae, B. clarridgeiae, Rickettsia felis and Wolbachia pipientis in Cat Fleas, France. Emerg. Infect. Dis. 2003, 9, 338–342. [Google Scholar] [CrossRef]
- Zurita, A.; Gutiérrez, S.G.; Cutillas, C. Infection Rates of Wolbachia sp. and Bartonella sp. in Different Populations of Fleas. Curr. Microbiol. 2016, 73, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Perveen, N.; Hussain, A.; Song, B.; Aziz, M.U.; Zeb, J.; Li, J.; George, D.; Cabezas-Cruz, A.; Sparagano, O. The Symbiotic Continuum Within Ticks: Opportunities for Disease Control. Front. Microbiol. 2022, 13, 854803. [Google Scholar] [CrossRef] [PubMed]
- Mariconti, M.; Epis, S.; Gaibani, P.; Dalla Valle, C.; Sassera, D.; Tomao, P.; Fabbi, M.; Castelli, F.; Marone, P.; Sambri, V.; et al. Humans Parasitized by the Hard Tick Ixodes ricinus Are Seropositive to Midichloria Mitochondrii: Is Midichloria a Novel Pathogen, or Just a Marker of Tick Bite? Pathog. Glob. Health 2012, 106, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Castelli, M.; McCarthy, U.; Petroni, G.; Bazzocchi, C. Transmission of Members of the “Candidatus Midichloriaceae” Family to Vertebrates and Possible Involvement in Disease Pathogenesis. In Rickettsiales: Biology, Molecular Biology, Epidemiology, and Vaccine Development; Thomas, S., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 283–292. [Google Scholar] [CrossRef]
- Bonnet, S.I.; Binetruy, F.; Hernández-Jarguín, A.M.; Duron, O. The Tick Microbiome: Why Non-Pathogenic Microorganisms Matter in Tick Biology and Pathogen Transmission. Front. Cell. Infect. Microbiol. 2017, 7. [Google Scholar] [CrossRef]
| Target Species | Target Gene | PCR Primer and Probe Sequences (5′–3′) | Product Size (bp) | Reference |
|---|---|---|---|---|
| SFG Rickettsia genus specific | gltA | F: GTG AAT GAA AGA TTA CAC TAT TTAT | 166 | [23] |
| R: GTA TCT TAG CAA TCA TTC TAA TAG C | ||||
| FAM-CTA TTA TGC TTG CGG CTG TCG GTT C-TAMRA | ||||
| Rickettsia conorii | Putative acetyltransferase | F: TTG GTA GGC AAG TAG CTA AGC AAA | 116 | [23] |
| R: GGA AGT ATA TGG GAA TGC TTT GAA | ||||
| FAM-GCG GTT ATT CCT GAA AAT AAG CCG GCA-TAMRA | ||||
| Rickettsia felis specific | Biotin synthase | F: ATG-TTC-GGG-CTTCCG-GTA-TG | 120 | [21] |
| R: CCG-ATT-CAG-CAGGTT-CTT-CAA | ||||
| 6-FAM- GCT-GCG-GCGGTA-TTT-TAG-GAA-TGGG-TAMRA | ||||
| Anaplasmataceae | 16S rRNA | EHR16SD: GGT ACC YAC AGA AGA AGTCC | 345 | [17] |
| EHR16SR: TAG CAC TCA TCG TTT ACAGC | ||||
| PanWolbachia | 23S rRNA | F: CCA AAA TTA CAG CTA AGT GG | 100 | [19] |
| R: AGT GAG CTG TTA CGC TTT CT 6FAM-TACAGCTAGGAGGTTGGCTT-TAMRA | ||||
| Wolbachia pipientis | rpoB | F: TCATGCGCGTTCAGTAGGAC | 103 | Unpublished |
| R: TGCCCAACATTCCATTTCAC 6FAM-AGGAAAGTCTCATTTTGGTGGTAGCG-TAMRA |
| Rickettsial Agent | Pathogen/ Endosymbiont | Prevalence (%) | Examined Ectoparasite | Method of Detection | Sequence Identity (%) | Query Cover (%) | GenBank ID |
|---|---|---|---|---|---|---|---|
| Anaplasmataceae | Ehrlichia canis | 2.4% | R. sanguineus s.l. | PCR | 100% (305/305 bp) | 100% | MN922610 |
| Ca. Midichloria mitochondrii | 2.4% | Ixodes sp. | PCR | 100% (305/305 bp) | 100% | KX359181 | |
| Wolbachia pipientis | 58.7% a | C. felis | PCR | 100% (305/305 bp) | 100% | CP051156 | |
| Wolbachia pipientis | 61.5% | C. felis | qPCR | na b | na b | ||
| SFG Rickettsiae | Rickettsia conorii | 7.3% | R. sanguineus s.l. | qPCR | na b | na b | |
| Rickettsia felis | 4.8% | C. felis | qPCR | na b | na b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liodaki, M.; Angelakis, E.; Spanakos, G.; Papadogiannaki, I.; Samarkos, M.; Daikos, G.L.; Christopoulou, B.; Piperaki, E.-T. Rickettsial Agents Associated with Ectoparasites in Attica, Greece. Zoonotic Dis. 2022, 2, 247-257. https://doi.org/10.3390/zoonoticdis2040020
Liodaki M, Angelakis E, Spanakos G, Papadogiannaki I, Samarkos M, Daikos GL, Christopoulou B, Piperaki E-T. Rickettsial Agents Associated with Ectoparasites in Attica, Greece. Zoonotic Diseases. 2022; 2(4):247-257. https://doi.org/10.3390/zoonoticdis2040020
Chicago/Turabian StyleLiodaki, Maria, Emmanouil Angelakis, Gregory Spanakos, Ioanna Papadogiannaki, Michael Samarkos, George L. Daikos, Barbara Christopoulou, and Evangelia-Theophano Piperaki. 2022. "Rickettsial Agents Associated with Ectoparasites in Attica, Greece" Zoonotic Diseases 2, no. 4: 247-257. https://doi.org/10.3390/zoonoticdis2040020
APA StyleLiodaki, M., Angelakis, E., Spanakos, G., Papadogiannaki, I., Samarkos, M., Daikos, G. L., Christopoulou, B., & Piperaki, E.-T. (2022). Rickettsial Agents Associated with Ectoparasites in Attica, Greece. Zoonotic Diseases, 2(4), 247-257. https://doi.org/10.3390/zoonoticdis2040020







