Detection of Trematodes from the Host Exotic Aquatic Snail Melanoides tuberculata in an Urban Stormwater System
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
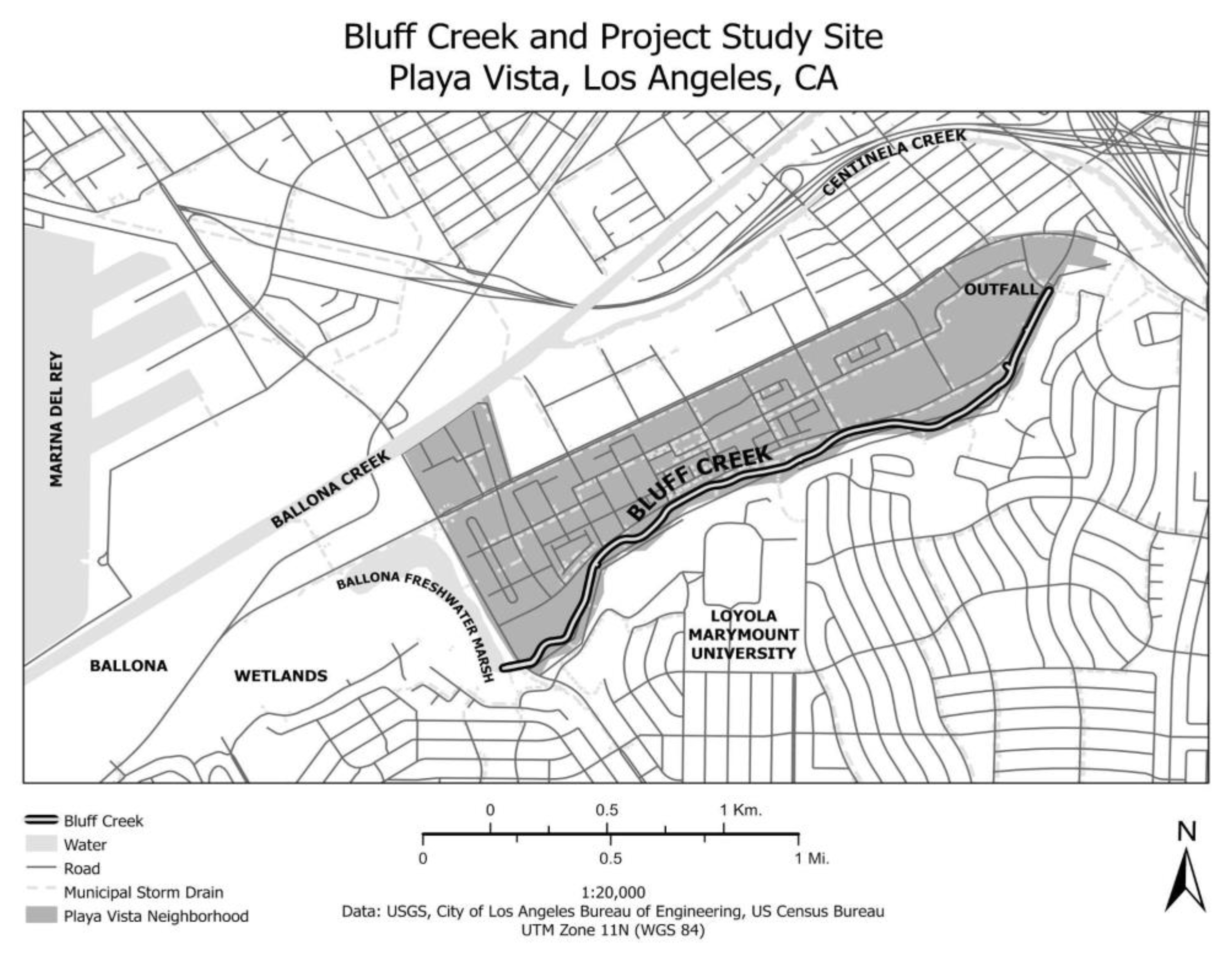
2.1. Sample Collection
2.2. Laboratory Testing
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krailas, D.; Namchote, S.; Koonchornboon, T.; Dechruksa, W.; Boonmekam, D. Trematodes obtained from the thiarid freshwater snail Melanoides tuberculata (Müller, 1774) as vector of human infections in Thailand. Zoosystematics Evol. 2014, 90, 57. [Google Scholar] [CrossRef] [Green Version]
- Facon, B.; Pointier, J.-P.; Glaubrecht, M.; Poux, C.; Jarne, P.; David, P. A molecular phylogeography approach to biological invasions of the New World by parthenogenetic Thiarid snails. Mol. Ecol. 2003, 12, 3027–3039. [Google Scholar] [CrossRef] [PubMed]
- Maciaszek, R.; Marcinek, D.; Sosnowski, W. Alien tropical snail, the red-rimmed melania Melanoides tuberculata Müller, 1774 (Thiaridae) from artificial lake in Warsaw, Central Poland. World Sci. News 2019, 122, 285–290. [Google Scholar]
- Maciaszek, R.; Sosnowski, W.; Wilk, S. Tropical snail Melanoides tuberculata Müller, 1774 (Thiaridae) found in thermally polluted canal in Central Poland. World Sci. News 2019, 122, 249–254. [Google Scholar]
- Gl, P. Malacological Review 1; Freshwater Snails of Taiwan: Taiwan, China, 1973. [Google Scholar]
- Piechocki, A.; Wawrzyniak-Wydrowska, B.; Zdanowski, B. Melanoides tuberculatus (O. F. Müller, 1774) (Orthogastropoda: Thiaridae), a gastropod species new for the fauna of Poland. Folia Malacol. 2003, 11, 39–41. [Google Scholar] [CrossRef]
- Peso, J.G.; Pérez, D.C.; Vogler, R.E. The invasive snail Melanoides tuberculata in Argentina and Paraguay. Limnologica 2011, 41, 281–284. [Google Scholar] [CrossRef] [Green Version]
- Murray, H.D. Tarebia granifera and Melanoides tuberculata in Texas. Annual Report to the American Malacological Union; American Malacological Union: TX, USA, 1964; pp. 15–16. Available online: https://www.biodiversitylibrary.org/ (accessed on 1 October 2022).
- Murray, H.D. The Introduction and Spread of Thiarids in the United States. Biologist 1971, 53, 133–135. [Google Scholar]
- Duggan, I.C. The freshwater aquarium trade as a vector for incidental invertebrate fauna. Biol. Invasions 2010, 12, 3757–3770. [Google Scholar] [CrossRef]
- Kwong, K.L.; Dudgeon, D.; Wong, P.K.; Qiu, J.W. Secondary production and diet of an invasive snail in freshwater wetlands: Implications for resource utilization and competition. Biol. Invasions 2010, 12, 1153–1164. [Google Scholar] [CrossRef] [Green Version]
- Karatayev, A.Y.; Burlakova, L.E.; Karatayev, V.A.; Padilla, D.K. Introduction, distribution, spread, and impacts of exotic freshwater gastropods in Texas. Hydrobiologia 2008, 619, 181–194. [Google Scholar] [CrossRef]
- McClure, M.R. Novel introduction for the invasive red-rim melania Melanoides tuberculate (müller) in southeastern texas. Southwest. Nat. 2021, 64, 232–235. [Google Scholar] [CrossRef]
- Facon, B.; Machline, E.; Pointier, J.; David, P. Variation in Desiccation Tolerance in Freshwater Snails and Its Consequences for Invasion Ability. Biol. Invasions 2004, 6, 283–293. [Google Scholar] [CrossRef]
- Pointier, J.P. Invading freshwater snails and biological control in Martinique Island, French West Indies. Mem. Inst. Oswaldo Cruz 2001, 96, 67–74. [Google Scholar] [CrossRef]
- Duggan, I.C. First record of a wild population of the tropical snail Melanoides tuberculata in New Zealand natural waters. N. Z. J. Mar. Freshw. Res. 2002, 36, 825–829. [Google Scholar] [CrossRef]
- Mitchell, A.J.; Brandt, T.M. Temperature Tolerance of Red-Rim Melania Melanoides tuberculatus, an Exotic Aquatic Snail Established in the United States. Trans. Am. Fish. Soc. 2005, 134, 126–131. [Google Scholar] [CrossRef]
- Pinto, H.A.; De Melo, A.L. A checklist of trematodes (Platyhelminthes) transmitted by Melanoides tuberculata (Mollusca: Thiaridae). Zootaxa 2011, 2799, 15. [Google Scholar] [CrossRef] [Green Version]
- Ben-Ami, F.; Heller, J. Spatial and temporal patterns of parthenogenesis and parasitism in the freshwater snail Melanoides tuberculata. J. Evol. Biol. 2005, 18, 138–146. [Google Scholar] [CrossRef]
- Dagan, Y.; Kosman, E.; Ben-Ami, F. Cost of resistance to trematodes in freshwater snail populations with low clonal diversity. BMC Ecol. 2017, 17, 40. [Google Scholar] [CrossRef] [Green Version]
- Madsen, H.; Frandsen, F. The spread of freshwater snails including those of medical and veterinary importance. Acta Trop. 1989, 46, 139–146. [Google Scholar] [CrossRef]
- Tang, Z.-L.; Huang, Y.; Yu, X.-B. Current status and perspectives of Clonorchis sinensis and clonorchiasis: Epidemiology, pathogenesis, omics, prevention and control. Infect. Dis. Poverty 2016, 5, 71. [Google Scholar] [CrossRef] [Green Version]
- Scholz, T.; Aguirre-Macedo, M.L.; Salgadomaldonado, G. Trematodes of the family Heterophyidae (Digenea) in Mexico: A review of species and new host and geographical records. J. Nat. Hist. 2001, 35, 1733–1772. [Google Scholar] [CrossRef]
- Tang, Z.Z.; Tang, C.; Chen, Q.; Lin, X.; Weng, Y.; He, Y. Studies of philophthalmosis of domestic-fowls in Fujian. Acta Zool. Sin. 1980, 26, 232–242. [Google Scholar]
- Tolley-Jordan, L.; Chadwick, M.; Triplett, J. New records of digenetic trematodes infecting Melanoides tuberculata (O.F. Müller, 1774) in Florida, USA. BioInvasions Rec. 2022, 11, 149–164. [Google Scholar] [CrossRef]
- Taylor, D.W. Freshwater Mollusks of California: A Distributional Checklist; California Fish and Game: Sacramento, CA, USA, 1981; pp. 140–163.
- Taxonomy, G.B.I.F.G.B. Melanoides tuberculata (O.F.Müller, 1774) in GBIF Secretariat. 2021. Available online: https://www.gbif.org/species/4362965 (accessed on 1 October 2022).
- Survey, U.S.G. Nonindigenous Aquatic Species Database. Gainsville, FL, USA; 2021. Available online: https://nas.er.usgs.gov/ (accessed on 1 October 2022).
- Wong, S.S.Y.; Poon, R.W.S.; To, K.K.W.; Chan, J.F.W.; Lu, G.; Xing, F.; Cheng, V.C.C.; Yuen, K.-Y. Improving the specific diagnosis of trematode, cestode and nematode infections by a multiplex single-tube real-time PCR assay. J. Clin. Pathol. 2019, 72, 487–492. [Google Scholar] [CrossRef]
- Díaz, M.T.; Hernandez, L.E.; Bashirullah, A.K. Studies on the life cycle of Haplorchis pumilio (Looss, 1896) (Trematoda: Heterophyidae) in Venezuela. Rev. Científica 2008, 18, 35–42. [Google Scholar]
- Kim, T.-H.; Kim, J.-H.; Seo, H.-G.; Kim, K.-I.; Min, E.-Y.; Cho, M.-Y.; Choi, H.-S.; Jung, S.-H.; Han, H.-J. The First Report of a Trematodes Infection in Purple Shell, Rapana venosa, in Korea. J. Fishries Mar. Sci. Educ. 2019, 31, 984–993. [Google Scholar] [CrossRef]
- Heneberg, P. Phylogenetic data suggest the reclassification of Fasciola jacksoni (Digenea: Fasciolidae) as Fascioloides jacksoni comb. nov. Parasitol Res. 2013, 112, 1679–1689. [Google Scholar] [CrossRef]
- Rees, W.J. The aerial dispersal of mollusca. J. Molluscan Stud. 1965, 36, 269–282. [Google Scholar] [CrossRef]
- Boag, D.A. Dispersal in pond snails: Potential role of waterfowl. Can. J. Zool. 1986, 64, 904–909. [Google Scholar] [CrossRef]
- Hall, C.W.J. Protecting the Ballona Wetlands in West Los Angeles: A Look Back at Three Decades of Urban Habitat Advocacy. Golden Gate U. Envtl. LJ 2012, 6, 25. [Google Scholar]
- Service, U.S.F.W. National Wetlands Inventory. 2018. Available online: https://fwsprimary.wim.usgs.gov/wetlands/apps/wetlands-mapper/ (accessed on 1 October 2022).
- Mitchell, A.J.; Overstreet, R.M.; Goodwin, A.E.; Brandt, T.M. Spread of an exotic fish-gill trematode. Fisheries 2005, 30, 11–16. [Google Scholar] [CrossRef]
- Wongsawad, C.; Wongsawad, P.; Sukontason, K.; Phalee, A.; Noikong-Phalee, W.; Chai, J.Y. Discrimination 28S ribosomal gene of trematode cercariae in snails from Chiang Mai Province, Thailand. Southeast Asian J. Trop. Med. Public Health 2016, 47, 199–206. [Google Scholar] [PubMed]
- Lotfy, W.M.; Perera, V.B.V.P.; Loker, E.S.; Brant, S.V.; Rajapakse, R.P.V.J.; Laursen, J.R.; Demiaszkiewicz, A.; DeJong, R.J.; LE, T.H. Evolutionary Origins, Diversification, and Biogeography of Liver Flukes (Digenea, Fasciolidae). Am. J. Trop. Med. Hyg. 2008, 79, 248–255. [Google Scholar] [CrossRef] [Green Version]
- Rajapakse, R.P.V.J.; Lawton, S.P.; Karunathilake, K.J.K.; Perera, B.V.P.; Nguyen, N.T.B.; Le, T.H. Molecular characterization of Fasciola jacksoni from wild elephants (Elephas maximus maximus) of Sri Lanka: A taxonomic evaluation. Parasitology 2019, 146, 1247–1255. [Google Scholar] [CrossRef] [Green Version]
- Radomyos, P.; Bunnag, D.; Harinasuta, T. Haplorchis pumilio (Looss) infection in man in northeastern Thailand. Southeast Asian J. Trop. Med. Public Health 1983, 14, 223–227. [Google Scholar] [PubMed]
- Chai, J.-Y.; Sohn, W.-M.; Jung, B.-K.; Yong, T.-S.; Eom, K.S.; Min, D.-Y.; Insisiengmay, B.; Insisiengmay, S.; Phommasack, B.; Rim, H.-J. Intestinal Helminths Recovered from Humans in Xieng Khouang Province, Lao PDR with a Particular Note on Haplorchis pumilio Infection. Korean J. Parasitol. 2015, 53, 439–445. [Google Scholar] [CrossRef]
- Sommerville, C. The life history of Haplorchis pumilio (Looss, 1896) from cultured tilapias. J. Fish Dis. 1982, 5, 233–241. [Google Scholar] [CrossRef]
- Sato, M.; Sanguankiat, S.; Pubampen, S.; Kusolsuk, T.; Maipanich, W.; Waikagul, J. Egg Laying Capacity of Haplorchis taichui (Digenea: Heterophyidae) in Humans. Korean J. Parasitol. 2009, 47, 315–318. [Google Scholar] [CrossRef] [Green Version]
- Chai, J.-Y.; Shin, E.-H.; Lee, S.-H.; Rim, H.-J. Foodborne Intestinal Flukes in Southeast Asia. Korean J. Parasitol. 2009, 47, S69–S102. [Google Scholar] [CrossRef]
- Chai, J.-Y.; Jung, B.-K. Foodborne intestinal flukes: A brief review of epidemiology and geographical distribution. Acta Trop. 2019, 201, 105210. [Google Scholar] [CrossRef]
- Pulido-Murillo, E.A.; Furtado, L.F.V.; Melo, A.L.; Rabelo, É.M.L.; Pinto, H.A. Fishborne Zoonotic Trematodes Transmitted by Melanoides tuberculata Snails, Peru. Emerg. Infect. Dis. 2018, 24, 606–608. [Google Scholar] [CrossRef] [Green Version]
- Authority, L.A.H.S. 2022 Greater Los Angeles Homeless County—Los Angeles County Data Summary. 2022. Available online: https://www.lahsa.org/news?article=893-2022-greater-los-angeles-homeless-count-data (accessed on 1 October 2022).
- Authority, L.A.H.S. Results from the 2019 Homeless Count for Los Angeles County by Census Tract Accessed via City of Los Angeles GIS Hub. 2019. Available online: https://www.lahsa.org/news?article=557-2019-greater-los-angeles-homeless-count-results (accessed on 1 October 2022).
- Ismail, N.S.; Arif, A.M.S. Population dynamics of Melanoides tuberculata (Thiaridae) snails in a desert spring, United Arab Emirates and infection with larval trematodes. Hydrobiologia 1993, 257, 57–64. [Google Scholar] [CrossRef]
- Rader, R.B.; Belk, M.C.; Keleher, M.J. The Introduction of an Invasive Snail (Melanoides tuberculata) to Spring Ecosystems of the Bonneville Basin, Utah. J. Freshw. Ecol. 2003, 18, 647–657. [Google Scholar] [CrossRef]
- Derraik, J. The potential significance to human health associated with the establishment of the snail Melanoides tuberculata in New Zealand. N. Z. Med. J. 2008, 121, 25–32. [Google Scholar]


| BLAST * Result | % Identity | NCBI+ Accession Number | M. tuberculata Specimens | Emitted From M. tubeculata Samples (into DI Water) | Previously Found in M. tuberculata |
|---|---|---|---|---|---|
| Fasciola jacksoni | 83.70% | MN970006.1 | X | ||
| Haplorchis pumilio | 100% | MG738252.1 | X | X [18] | |
| Haplorchis pumilio | 98.11% | MN745941.1 | X | X [18] | |
| Parorchis sp. TH-2019 | 100% | LC438643.1 | X | X | |
| Unclassified Trematoda sp. distomecercaria WN-2016 | 100% | KU820969.1 | X | X |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Post, J.M.; Reasch, R.J.; Bailey, E.S. Detection of Trematodes from the Host Exotic Aquatic Snail Melanoides tuberculata in an Urban Stormwater System. Zoonotic Dis. 2022, 2, 258-266. https://doi.org/10.3390/zoonoticdis2040021
Post JM, Reasch RJ, Bailey ES. Detection of Trematodes from the Host Exotic Aquatic Snail Melanoides tuberculata in an Urban Stormwater System. Zoonotic Diseases. 2022; 2(4):258-266. https://doi.org/10.3390/zoonoticdis2040021
Chicago/Turabian StylePost, Jason M., Rachael J. Reasch, and Emily S. Bailey. 2022. "Detection of Trematodes from the Host Exotic Aquatic Snail Melanoides tuberculata in an Urban Stormwater System" Zoonotic Diseases 2, no. 4: 258-266. https://doi.org/10.3390/zoonoticdis2040021
APA StylePost, J. M., Reasch, R. J., & Bailey, E. S. (2022). Detection of Trematodes from the Host Exotic Aquatic Snail Melanoides tuberculata in an Urban Stormwater System. Zoonotic Diseases, 2(4), 258-266. https://doi.org/10.3390/zoonoticdis2040021






