Waste-Derived Chars: A Comprehensive Review
Abstract
1. Introduction
2. Feedstock for Waste-Derived Char Production
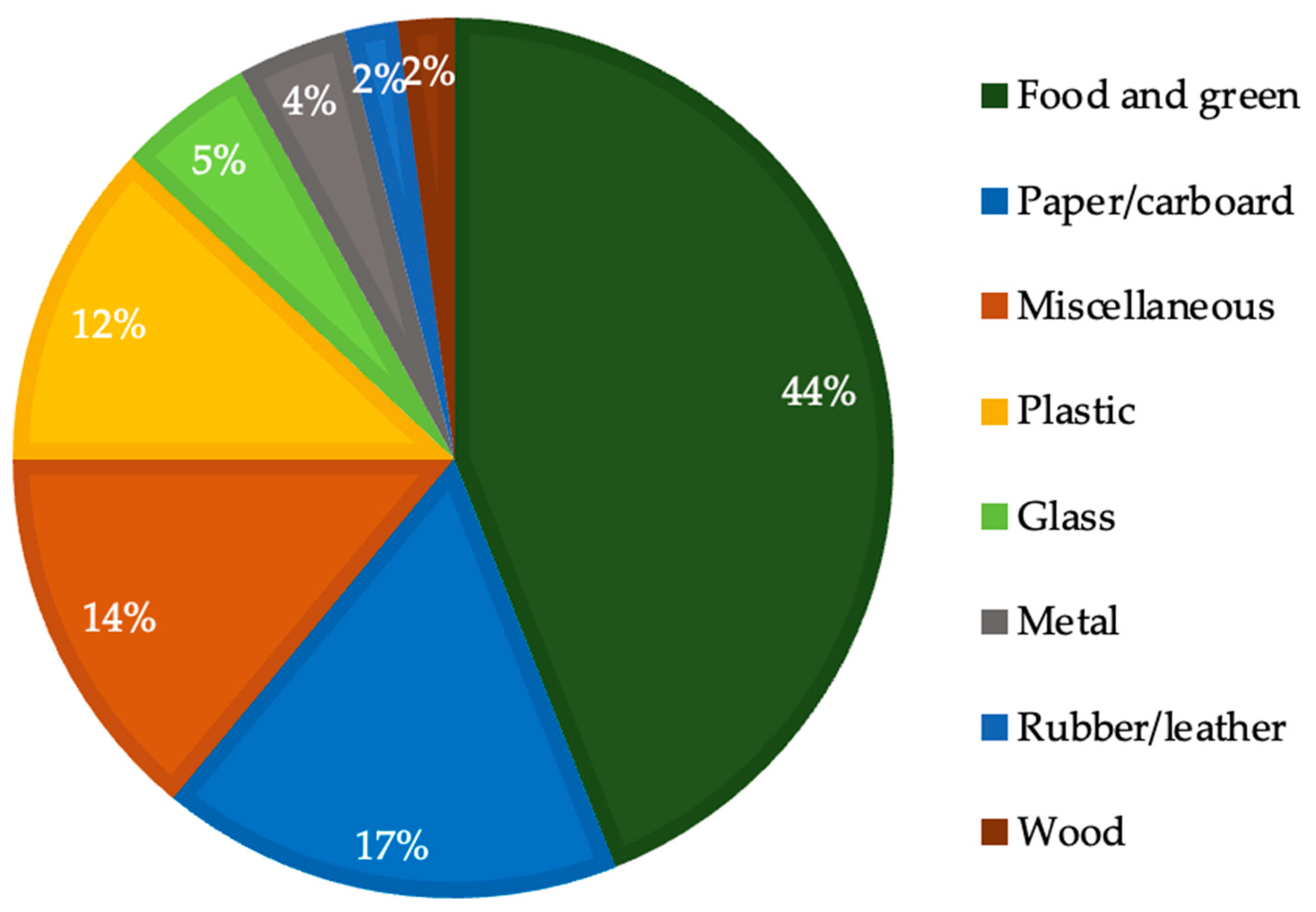
2.1. Municipal Solid Waste
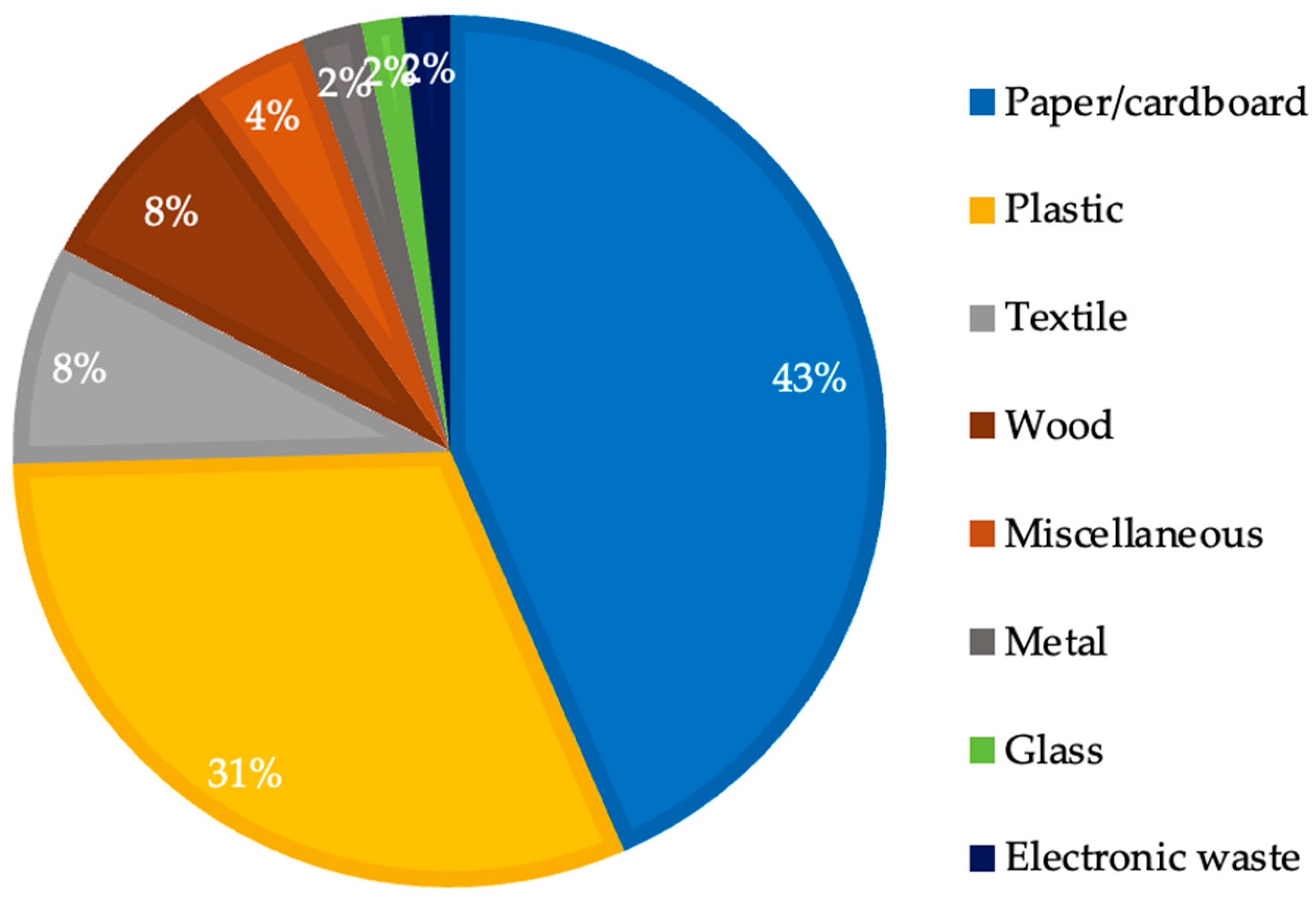
2.2. Industrial Solid Waste
2.3. Construction and Demolition Waste
3. Waste-Derived Char Production Technologies
3.1. Torrefaction
3.2. Pyrolysis
3.2.1. Slow Pyrolysis
3.2.2. Fast Pyrolysis
3.2.3. Flash Pyrolysis
3.3. Gasification
3.4. Hydrothermal Carbonization
3.5. Summary of Thermochemical Process Conditions for Producing Waste-Derived Char
4. Properties of Waste-Derived Chars
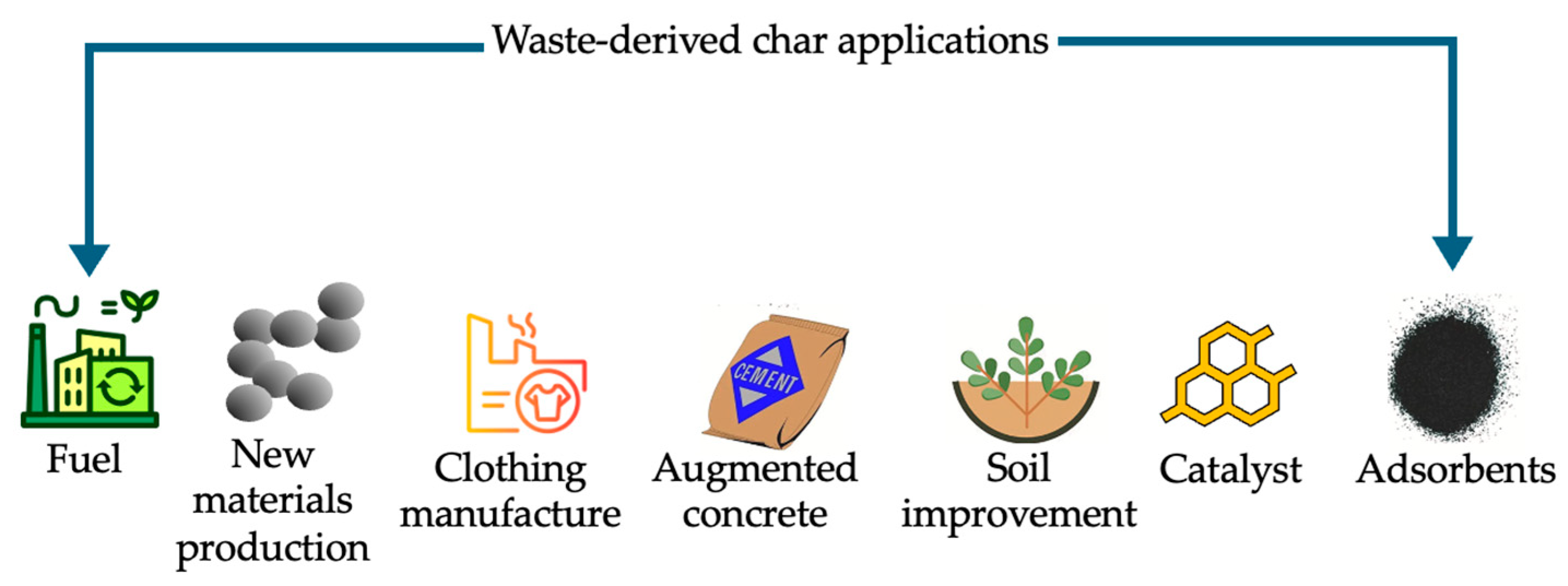
5. Different Applications for Waste-Derived Char
5.1. Material Applications
5.1.1. Adsorbents
5.1.2. Catalyst
5.1.3. Soil Improvement
5.1.4. Other Material Applications
5.2. Energy Applications
6. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ganesapillai, M.; Mehta, R.; Tiwari, A.; Sinha, A.; Bakshi, H.S.; Chellappa, V.; Drewnowski, J. Waste to Energy: A Review of Biochar Production with Emphasis on Mathematical Modelling and Its Applications. Heliyon 2023, 9, e14873. [Google Scholar] [CrossRef]
- United Nations Environment Programme Solid Waste Management. Available online: https://www.unep.org/explore-topics/resource-efficiency/what-we-do/cities/solid-waste-management (accessed on 5 February 2024).
- Co-Energy Waste to Biochar. Available online: https://co-energy.energy/waste-to-energy-solutions/waste-to-biochar/ (accessed on 5 February 2024).
- International Biochar Initiative. Standardized Product Definition and Product Testing Guidelines for Biochar That Is Used in Soil. 2015, IBI-STD-2.1. Available online: https://biochar-international.org/wp-content/uploads/2018/04/IBI_Biochar_Standards_V2.1_Final.pdf (accessed on 5 February 2024).
- Infurna, G.; Caruso, G.; Dintcheva, N.T. Sustainable Materials Containing Biochar Particles: A Review. Polymers 2023, 15, 343. [Google Scholar] [CrossRef]
- Çay, A.; Yanık, J.; Akduman, Ç.; Duman, G.; Ertaş, H. Application of Textile Waste Derived Biochars onto Cotton Fabric for Improved Performance and Functional Properties. J. Clean. Prod. 2020, 251, 119664. [Google Scholar] [CrossRef]
- Li, S.; Skelly, S. Physicochemical Properties and Applications of Biochars Derived from Municipal Solid Waste: A Review. Environ. Adv. 2023, 13, 100395. [Google Scholar] [CrossRef]
- Longo, A.; Nobre, C.; Sen, A.; Panizio, R.; Brito, P.; Gonçalves, M. Torrefaction Upgrading of Heterogenous Wastes Containing Cork and Chlorinated Polymers. Environments 2022, 9, 99. [Google Scholar] [CrossRef]
- Mei, Z.; Chen, D.; Qian, K.; Yin, L.; Hong, L. Producing Methane from Dry Municipal Solid Wastes: A Complete Roadmap and the Influence of Char Catalyst. Energy 2024, 290, 130180. [Google Scholar] [CrossRef]
- Guo, M.; Wang, K.; Bing, X.; Cheng, J.; Zhang, Y.; Sun, X.; Guan, B.; Yu, J. Pyrolysis of Plastics-Free Refuse Derived Fuel Derived from Municipal Solid Waste and Combustion of the Char Products in Lab and Pilot Scales: A Comparative Study. Fuel 2024, 359, 130335. [Google Scholar] [CrossRef]
- Nobre, C.; Alves, O.; Longo, A.; Vilarinho, C.; Gonçalves, M. Torrefaction and Carbonization of Refuse Derived Fuel: Char Characterization and Evaluation of Gaseous and Liquid Emissions. Bioresour. Technol. 2019, 285, 121325. [Google Scholar] [CrossRef]
- Hwang, I.H.; Matsuto, T.; Tanaka, N.; Sasaki, Y.; Tanaami, K. Characterization of Char Derived from Various Types of Solid Wastes from the Standpoint of Fuel Recovery and Pretreatment before Landfilling. Waste Manag. 2007, 27, 1155–1166. [Google Scholar] [CrossRef]
- Assima, G.P.; Marie-Rose, S.; Lavoie, J.M. Role of Fixed Carbon and Metal Oxides in Char during the Catalytic Conversion of Tar from RDF Gasification. Fuel 2018, 218, 406–416. [Google Scholar] [CrossRef]
- Wang, W.; Huang, J.; Wu, T.; Ren, X.; Zhao, X. Research on the Preparation of Biochar from Waste and Its Application in Environmental Remediation. Water 2023, 15, 3387. [Google Scholar] [CrossRef]
- Hu, Q.; Jung, J.; Chen, D.; Leong, K.; Song, S.; Li, F.; Mohan, B.C.; Yao, Z.; Prabhakar, A.K.; Lin, X.H.; et al. Biochar Industry to Circular Economy. Sci. Total Environ. 2021, 757, 43820. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Waste Incineration and Public Health (2000); National Academies Press: Washington, DC, USA, 2000. [Google Scholar]
- Kaza, S.; Yao, L.; Bhada-Tata, P.; Woerden, V.F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050. Available online: http://hdl.handle.net/10986/30317 (accessed on 16 February 2023).
- Statista. Average Annual per Capita Municipal Waste Generated by OECD Countries as of 2022. Available online: https://www.statista.com/statistics/478928/leading-countries-by-per-capita-generated-municipal-waste/ (accessed on 7 February 2024).
- Statista. Global Waste Generation—Statistics & Facts. Available online: https://www.statista.com/topics/4983/waste-generation-worldwide/#editorsPicks (accessed on 25 February 2024).
- Purchase, C.K.; Al Zulayq, D.M.; O’brien, B.T.; Kowalewski, M.J.; Berenjian, A.; Tarighaleslami, A.H.; Seifan, M. Circular Economy of Construction and Demolition Waste: A Literature Review on Lessons, Challenges, and Benefits. Materials 2022, 15, 76. [Google Scholar] [CrossRef] [PubMed]
- Nanda, S.; Berruti, F. Municipal Solid Waste Management and Landfilling Technologies: A Review. Environ. Chem. Lett. 2021, 19, 1433–1456. [Google Scholar] [CrossRef]
- Saikia, S.; Kalamdhad, A.S. Assessment of Pyrolysis Potential of Indian Municipal Solid Waste and Legacy Waste via Physicochemical and Thermochemical Characterization. Bioresour. Technol. 2024, 394, 130289. [Google Scholar] [CrossRef] [PubMed]
- Rudra, S.; Tesfagaber, Y.K. Future District Heating Plant Integrated with Municipal Solid Waste (MSW) Gasification for Hydrogen Production. Energy 2019, 180, 881–892. [Google Scholar] [CrossRef]
- Jamro, I.A.; Chen, G.; Baloch, H.A.; Wenga, T.; Ma, W. Optimization of Municipal Solid Waste Air Gasification for Higher H2 Production along with the Validation via Kinetics and Statistical Approaches. Fuel 2022, 322, 124137. [Google Scholar] [CrossRef]
- Castillo, K.J.T.; Maguyon-Detras, M.C.; Migo, V.P.; Alfafara, C.G. Parametric and Optimization Studies for Biochar Production from Municipal Solid Wastes (MSW) via Pyrolysis. IOP Conf. Ser. Mater. Sci. Eng. 2020, 778, 012078. [Google Scholar] [CrossRef]
- Białowiec, A.; Pulka, J.; Stępień, P.; Manczarski, P.; Gołaszewski, J. The RDF/SRF Torrefaction: An Effect of Temperature on Characterization of the Product—Carbonized Refuse Derived Fuel. Waste Manag. 2017, 70, 91–100. [Google Scholar] [CrossRef]
- García, R.; González-Vázquez, M.P.; Rubiera, F.; Pevida, C.; Gil, M.V. Co-Pelletization of Pine Sawdust and Refused Derived Fuel (RDF) to High-Quality Waste-Derived Pellets. J. Clean Prod. 2021, 328, 129635. [Google Scholar] [CrossRef]
- CEWEP. Latest Eurostat Figures: Municipal Waste Treatment 2021. Available online: https://www.cewep.eu/municipal-waste-treatment-2020-2/ (accessed on 5 April 2024).
- Directive (UE) 2018/850; Directive (EU) 2018/850 of the European Parliament and of the Council of 30 May 2018 Amending Directive 1999/31/EC on the Landfill of Waste. European Union: Maastricht, The Netherlands, 2018; pp. 100–108.
- CEN/TC 343; Solid Recovered Fuels. CEN — European Committee for Standardization: Brussels, Belgium, 2021. Available online: https://standards.iteh.ai/catalog/tc/cen/ea946eb8-b158-4ab3-87b3-83415b1f48a9/cen-tc-343 (accessed on 5 April 2024).
- Robinson, T.; Bronson, B.; Gogolek, P.; Mehrani, P. Sample Preparation for Thermo-Gravimetric Determination and Thermo-Gravimetric Characterization of Refuse Derived Fuel. Waste Manag. 2016, 48, 265–274. [Google Scholar] [CrossRef]
- de Azevedo, A.R.G.; Costa, A.M.; Cecchin, D.; Pereira, C.R.; Marvila, M.T.; Adesina, A. Economic Potential Comparative of Reusing Different Industrial Solid Wastes in Cementitious Composites: A Case Study in Brazil. Environ. Dev. Sustain. 2022, 24, 5938–5961. [Google Scholar] [CrossRef]
- Khoshsepehr, Z.; Alinejad, S.; Alimohammadlou, M. Exploring Industrial Waste Management Challenges and Smart Solutions: An Integrated Hesitant Fuzzy Multi-Criteria Decision-Making Approach. J. Clean. Prod. 2023, 420, 138327. [Google Scholar] [CrossRef]
- Sharma, P.; Gaur, V.K.; Gupta, S.; Varjani, S.; Pandey, A.; Gnansounou, E.; You, S.; Ngo, H.H.; Wong, J.W.C. Trends in Mitigation of Industrial Waste: Global Health Hazards, Environmental Implications and Waste Derived Economy for Environmental Sustainability. Sci. Total Environ. 2022, 811, 152357. [Google Scholar] [CrossRef]
- Yang, C.K.; Ma, H.W.; Liu, K.H.; Yuan, M.H. Measuring Circular Economy Transition Potential for Industrial Wastes. Sustain. Prod. Consum. 2023, 40, 376–388. [Google Scholar] [CrossRef]
- Quan, Z.; Xu, X.; Wang, W.; Jiang, J.; Gao, S. Do Industrial Solid Waste Recycling and Technological Innovation Promote Low-Carbon Development in China? New Insights from NARDL Approach. Sci. Total Environ. 2024, 916, 170446. [Google Scholar] [CrossRef]
- EUROSTAT. Waste Statistics. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Waste_statistics#Total_waste_generation (accessed on 28 February 2024).
- Nasrullah, M.; Vainikka, P.; Hannula, J.; Hurme, M. Elemental Balance of SRF Production Process: Solid Recovered Fuel Produced from Commercial and Industrial Waste. Fuel 2015, 145, 1–11. [Google Scholar] [CrossRef]
- Edirisinghe, L.G.L.M.; de Alwis, A.A.P.; Prakash, S.; Wijayasundara, M.; Hemali, N.A.A. A Volume-Based Analysis Method to Determine the Economic Value of Mixed Industrial Waste. Clean. Environ. Syst. 2023, 11, 100142. [Google Scholar] [CrossRef]
- Our World in Data. Waste Management. Available online: https://ourworldindata.org/waste-management (accessed on 5 March 2024).
- European Parliament. The Impact of Textile Production and Waste on the Environment (Infographics). Available online: https://www.europarl.europa.eu/topics/en/article/20201208STO93327/the-impact-of-textile-production-and-waste-on-the-environment-infographics (accessed on 5 March 2024).
- European Parliament Environmental. Impact of the Textile and Clothing Industry: What Consumers Need to Know. Available online: https://www.europarl.europa.eu/thinktank/en/document/EPRS_BRI(2019)633143 (accessed on 5 March 2024).
- Ellen MacArthur Foundation. A New Textiles Economy: Redesigning Fashion’s Future. Available online: https://www.ellenmacarthurfoundation.org/a-new-textiles-economy (accessed on 6 March 2024).
- Jiang, H.; Lu, P.; Xue, Z.; Wu, H. Torrefaction of Combustible Construction and Demolition Waste: Evolution of Fuel Performance, Heavy Metal Migration and Combustion Characteristics. J. Energy Inst. 2024, 113, 101510. [Google Scholar] [CrossRef]
- Directive 2008/98/EC; Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on Waste and Repealing Certain Directives (Text with EEA Relevance). European Union: Maastricht, The Netherlands, 2008; pp. 3–30.
- Wu, H.; Zuo, J.; Zillante, G.; Wang, J.; Yuan, H. Status Quo and Future Directions of Construction and Demolition Waste Research: A Critical Review. J. Clean. Prod. 2019, 240, 118163. [Google Scholar] [CrossRef]
- Gálvez-Martos, J.L.; Styles, D.; Schoenberger, H.; Zeschmar-Lahl, B. Construction and Demolition Waste Best Management Practice in Europe. Resour. Conserv. Recycl. 2018, 136, 166–178. [Google Scholar] [CrossRef]
- European Commission. Construction and Demolition Waste. Available online: https://environment.ec.europa.eu/topics/waste-and-recycling/construction-and-demolition-waste_en (accessed on 7 March 2024).
- Giorgi, S.; Lavagna, M.; Campioli, A. Guidelines for Effective and Sustainable Recycling of Construction and Demolition Waste. In Designing Sustainable Technologies, Products and Policies; Springer: Cham, Switzerland, 2018; pp. 211–221. [Google Scholar] [CrossRef]
- IDEA Consult. Analysis of Certain Waste Streams and the Potential of Industrial Symbiosis to Promote Waste as a Resource for EU Industry-Final Report Dirk Nelen Flemish Institute for Technological Research; European Commission: Brussels, Belgium, 2015. [Google Scholar]
- Jiang, H.; Lu, P.; Xue, Z.; Gong, R. Co-Combustion Characteristics of Paper Sludge and Combustible Construction and Demolition Waste. J. Energy Inst. 2023, 110, 101352. [Google Scholar] [CrossRef]
- Alves, O.; Calado, L.; Panizio, R.M.; Nobre, C.; Monteiro, E.; Brito, P.; Gonçalves, M. Gasification of Solid Recovered Fuels with Variable Fractions of Polymeric Materials. Energies 2022, 15, 8139. [Google Scholar] [CrossRef]
- Lokmit, C.; Nakason, K.; Kuboon, S.; Jiratanachotikul, A.; Panyapinyopol, B. A Comparison of Char Fuel Properties Derived from Dry and Wet Torrefaction of Oil Palm Leaf and Its Techno-Economic Feasibility. Mater. Sci. Energy Technol. 2023, 6, 192–204. [Google Scholar] [CrossRef]
- Haykiri-Acma, H.; Kurt, G.; Yaman, S. Properties of Biochars Obtained from RDF by Carbonization: Influences of Devolatilization Severity. Waste Biomass Valorization 2017, 8, 539–547. [Google Scholar] [CrossRef]
- Gao, N.; Wang, F.; Quan, C.; Santamaria, L.; Lopez, G.; Williams, P.T. Tire Pyrolysis Char: Processes, Properties, Upgrading and Applications. Prog. Energy Combust. Sci. 2022, 93, 101022. [Google Scholar] [CrossRef]
- Biakhmetov, B.; Dostiyarov, A.; Ok, Y.S.; You, S. A Review on Catalytic Pyrolysis of Municipal Plastic Waste. Wiley Interdiscip. Rev. Energy Environ. 2023, 12, e495. [Google Scholar] [CrossRef]
- Eze, W.U.; Umunakwe, R.; Obasi, H.C.; Ugbaja, M.I.; Uche, C.C.; Madufor, I.C. Plastics Waste Management: A Review of Pyrolysis Technology. Clean Technol. Recycl. 2021, 1, 50–69. [Google Scholar] [CrossRef]
- Chen, G.; Wang, J.; Yu, F.; Wang, X.; Xiao, H.; Yan, B.; Cui, X. A Review on the Production of P-Enriched Hydro/Bio-Char from Solid Waste: Transformation of P and Applications of Hydro/Bio-Char. Chemosphere 2022, 301, 134646. [Google Scholar] [CrossRef]
- Veses, A.; Sanahuja-Parejo, O.; Callén, M.S.; Murillo, R.; García, T. A Combined Two-Stage Process of Pyrolysis and Catalytic Cracking of Municipal Solid Waste for the Production of Syngas and Solid Refuse-Derived Fuels. Waste Manag. 2020, 101, 171–179. [Google Scholar] [CrossRef]
- Adeniyi, A.G.; Iwuozor, K.O.; Emenike, E.C.; Ajala, O.J.; Ogunniyi, S.; Muritala, K.B. Thermochemical Co-Conversion of Biomass-Plastic Waste to Biochar: A Review. Green Chem. Eng. 2024, 5, 31–49. [Google Scholar] [CrossRef]
- Ruiz, B.; Fuente, E.; Pérez, A.; Taboada-Ruiz, L.; Sanz, J.M.; Calvo, L.F.; Paniagua, S. Employment of Conventional and Flash Pyrolysis for Biomass Wastes from the Textile Industry with Sustainable Prospects. J. Anal. Appl. Pyrolysis 2023, 169, 105864. [Google Scholar] [CrossRef]
- Lu, J.S.; Chang, Y.; Poon, C.S.; Lee, D.J. Slow Pyrolysis of Municipal Solid Waste (MSW): A Review. Bioresour. Technol. 2020, 312, 123615. [Google Scholar] [CrossRef]
- Brown, L.J.; Collard, F.X.; Görgens, J. Fast Pyrolysis of Fibre Waste Contaminated with Plastic for Use as Fuel Products. J. Anal. Appl. Pyrolysis 2019, 138, 261–269. [Google Scholar] [CrossRef]
- Rauch, R.; Kiros, Y.; Engvall, K.; Kantarelis, E.; Brito, P.; Nobre, C.; Santos, S.M.; Graefe, P.A. Hydrogen from Waste Gasification. Hydrogen 2024, 5, 70–101. [Google Scholar] [CrossRef]
- Dogu, O.; Pelucchi, M.; Van de Vijver, R.; Van Steenberge, P.H.M.; D’hooge, D.R.; Cuoci, A.; Mehl, M.; Frassoldati, A.; Faravelli, T.; Van Geem, K.M. The Chemistry of Chemical Recycling of Solid Plastic Waste via Pyrolysis and Gasification: State-of-the-Art, Challenges, and Future Directions. Prog. Energy Combust. Sci. 2021, 84, 100901. [Google Scholar] [CrossRef]
- Abdelaal, A.; Antolini, D.; Piazzi, S.; Patuzzi, F.; Villot, A.; Gerente, C.; Baratieri, M. Steam Reforming of Tar Using Biomass Gasification Char in a Pilot-Scale Gasifier. Fuel 2023, 351, 128898. [Google Scholar] [CrossRef]
- James, A.M.R.; Yuan, W.; Wang, D.; Wang, D.; Kumar, A. The Effect of Gasification Conditions on the Surface Properties of Biochar Produced in a Top-Lit Updraft Gasifier. Appl. Sci. 2020, 10, 688. [Google Scholar] [CrossRef]
- Nobre, C.; Alves, O.; Durão, L.; Şen, A.; Vilarinho, C.; Gonçalves, M. Characterization of Hydrochar and Process Water from the Hydrothermal Carbonization of Refuse Derived Fuel. Waste Manag. 2021, 120, 303–313. [Google Scholar] [CrossRef]
- Alves, O.; Nobre, C.; Durão, L.; Monteiro, E.; Brito, P.; Gonçalves, M. Effects of Dry and Hydrothermal Carbonisation on the Properties of Solid Recovered Fuels from Construction and Municipal Solid Wastes. Energy Convers. Manag. 2021, 237, 114101. [Google Scholar] [CrossRef]
- Picone, A.; Volpe, M.; Malik, W.; Volpe, R.; Messineo, A. Role of Reaction Parameters in Hydrothermal Carbonization with Process Water Recirculation: Hydrochar Recovery Enhancement and Energy Balance. Biomass Bioenergy 2024, 181, 107061. [Google Scholar] [CrossRef]
- Picone, A.; Volpe, M.; Codignole Lùz, F.; Malik, W.; Volpe, R.; Messineo, A. Co-Hydrothermal Carbonization with Process Water Recirculation as a Valuable Strategy to Enhance Hydrochar Recovery with High Energy Efficiency. Waste Manag. 2024, 175, 101–109. [Google Scholar] [CrossRef]
- Bridgwater, A. V Review of Fast Pyrolysis of Biomass and Product Upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- You, S.; Ok, Y.S.; Chen, S.S.; Tsang, D.C.W.; Kwon, E.E.; Lee, J.; Wang, C.H. A Critical Review on Sustainable Biochar System through Gasification: Energy and Environmental Applications. Bioresour. Technol. 2017, 246, 242–253. [Google Scholar] [CrossRef]
- Kara, M. Environmental and Economic Advantages Associated with the Use of RDF in Cement Kilns. Resour. Conserv. Recycl. 2012, 68, 21–28. [Google Scholar] [CrossRef]
- Han, S.W.; Lee, J.J.; Tokmurzin, D.; Lee, S.H.; Nam, J.Y.; Park, S.J.; Ra, H.W.; Mun, T.Y.; Yoon, S.J.; Yoon, S.M.; et al. Gasification Characteristics of Waste Plastics (SRF) in a Bubbling Fluidized Bed: Effects of Temperature and Equivalence Ratio. Energy 2022, 238 Pt C, 121944. [Google Scholar] [CrossRef]
- Galvagno, S.; Casciaro, G.; Casu, S.; Martino, M.; Mingazzini, C.; Russo, A.; Portofino, S. Steam Gasification of Tyre Waste, Poplar, and Refuse-Derived Fuel: A Comparative Analysis. Waste Manag. 2009, 29, 678–689. [Google Scholar] [CrossRef]
- Arena, U. Process and Technological Aspects of Municipal Solid Waste Gasification: A Review. Waste Manag. 2012, 32, 625–639. [Google Scholar] [CrossRef]
- Flamme, S.; Geiping, J. Quality Standards and Requirements for Solid Recovered Fuels: A Review. Waste Manag. Res. 2012, 30, 335–353. [Google Scholar] [CrossRef]
- Escudero-Curiel, S.; Giráldez, A.; Pazos, M.; Sanromán, Á. From Waste to Resource: Valorization of Lignocellulosic Agri-Food Residues through Engineered Hydrochar and Biochar for Environmental and Clean Energy Applications—A Comprehensive Review. Foods 2023, 12, 3646. [Google Scholar] [CrossRef]
- Bhatt, M.; Wagh, S.; Chakinala, A.G.; Pant, K.K.; Sharma, T.; Joshi, J.B.; Shah, K.; Sharma, A. Conversion of Refuse Derived Fuel from Municipal Solid Waste into Valuable Chemicals Using Advanced Thermo-Chemical Process. J. Clean. Prod. 2021, 329, 129653. [Google Scholar] [CrossRef]
- Taherymoosavi, S.; Verheyen, V.; Munroe, P.; Joseph, S.; Reynolds, A. Characterization of Organic Compounds in Biochars Derived from Municipal Solid Waste. Waste Manag. 2017, 67, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Sewu, D.D.; Ohemeng-Boahen, G.; Lee, D.S.; Woo, S.H. Characterization and Adsorption Performance Evaluation of Waste Char By-Product from Industrial Gasification of Solid Refuse Fuel from Municipal Solid Waste. Waste Manag. 2019, 91, 33–41. [Google Scholar] [CrossRef]
- Lin, Y.; Ma, X.; Peng, X.; Yu, Z. A Mechanism Study on Hydrothermal Carbonization of Waste Textile. Energy Fuels 2016, 30, 7746–7754. [Google Scholar] [CrossRef]
- Rangappa, H.S.; Herath, I.; Lin, C.; Ch, S. Industrial Waste-Based Adsorbents as a New Trend for Removal of Water-Borne Emerging Contaminants. Environ. Pollut. 2024, 343, 123140. [Google Scholar] [CrossRef] [PubMed]
- Abbas, Z.; Ali, S.; Rizwan, M.; Zaheer, I.E.; Malik, A.; Riaz, M.A.; Shahid, M.R.; Rehman, M.Z.u.; Al-Wabel, M.I. A Critical Review of Mechanisms Involved in the Adsorption of Organic and Inorganic Contaminants through Biochar. Arab. J. Geosci. 2018, 11, 448. [Google Scholar] [CrossRef]
- Hoslett, J.; Ghazal, H.; Ahmad, D.; Jouhara, H. Removal of Copper Ions from Aqueous Solution Using Low Temperature Biochar Derived from the Pyrolysis of Municipal Solid Waste. Sci. Total Environ. 2019, 673, 777–789. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Shen, B.; Li, F.; Tian, L.; Singh, S.; Wang, F. Elemental Mercury Removal Using Biochar Pyrolyzed from Municipal Solid Waste. Fuel Process. Technol. 2015, 133, 43–50. [Google Scholar] [CrossRef]
- Čespiva, J.; Jadlovec, M.; Výtisk, J.; Serenčíšová, J.; Tadeáš, O.; Honus, S. Softwood and Solid Recovered Fuel Gasification Residual Chars as Sorbents for Flue Gas Mercury Capture. Environ. Technol. Innov. 2023, 29, 102970. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.H.; Kwon, E.E. Biochar as a Catalyst. Renew. Sustain. Energy Rev. 2017, 77, 70–79. [Google Scholar] [CrossRef]
- Assima, G.P.; Paquet, A.; Lavoie, J.M. Utilization of MSW-Derived Char for Catalytic Reforming of Tars and Light Hydrocarbons in the Primary Syngas Produced During Wood Chips and MSW-RDF Air Gasification. Waste Biomass Valorization 2019, 10, 1203–1222. [Google Scholar] [CrossRef]
- Gunarathne, V.; Ashiq, A.; Ramanayaka, S.; Wijekoon, P.; Vithanage, M. Biochar from Municipal Solid Waste for Resource Recovery and Pollution Remediation. Environ. Chem. Lett. 2019, 17, 1225–1235. [Google Scholar] [CrossRef]
- Jia, Y.; Li, H.; He, X.; Li, P.; Wang, Z. Effect of Biochar from Municipal Solid Waste on Mechanical and Freeze–Thaw Properties of Concrete. Constr. Build. Mater. 2023, 368, 130374. [Google Scholar] [CrossRef]
- Lee, S.; Kim, Y.T.; Lin, K.Y.A.; Lee, J. Plastic-Waste-Derived Char as an Additive for Epoxy Composite. Materials 2023, 16, 2602. [Google Scholar] [CrossRef]
- Lin, Y.; Ma, X.; Peng, X.; Yu, Z.; Fang, S.; Lin, Y.; Fan, Y. Combustion, Pyrolysis and Char CO2-Gasification Characteristics of Hydrothermal Carbonization Solid Fuel from Municipal Solid Wastes. Fuel 2016, 181, 905–915. [Google Scholar] [CrossRef]
- Nobre, C.; Longo, A.; Vilarinho, C.; Gonçalves, M. Gasification of Pellets Produced from Blends of Biomass Wastes and Refuse Derived Fuel Chars. Renew. Energy 2020, 154, 1294–1303. [Google Scholar] [CrossRef]
- Castro, C.; Gonçalves, M.; Longo, A.; Vilarinho, C.; Ferreira, M.; Ribeiro, A.; Pacheco, N.; Teixeira, J.C. Influence of Temperature in the Thermo-Chemical Decomposition of Below-Stoichiometric RDF Char—A Macro TGA Study. Energies 2023, 16, 3064. [Google Scholar] [CrossRef]


| Characteristics | Units | MSW | RDF/SRF | RDF | |||
|---|---|---|---|---|---|---|---|
| Moisture | wt.%, wb | 8.3 | 6.3 | 5.5 | 8.5 | 22.9 | 8.5 |
| Volatile matter | wt.%, db | 64.6 | 78.6 | 64.8 | 57.4 | 76.0 | 70.4 |
| Fixed carbon | 20.2 | 9.0 | 6.4 | 16.2 | n.d | 3.6 | |
| Ash | 6.8 | 12.4 | 23.3 | 26.4 | 14.3 | 26.0 | |
| C | 70.6 | 51.6 | 61.1 | 36.9 | 57.0 | 46.8 | |
| H | 11.9 | 6.3 | 6.8 | 4.2 | 7.2 | 5.4 | |
| N | 0.2 | 0.8 | 0.9 | 0.8 | n.d | 1.1 | |
| O | 10.8 | 28.7 | 30.3 | 31.7 | n.d | 20.4 | |
| S | 4.5 | 0.2 | 0.9 | n.d | 0.3 | 0.3 | |
| HHV | MJ/kg, db | 35.2 | 21.2 | n.d | 14.4 | 26.9 | 11.4 |
| Ref. | [22] | [23] | [24] | [25] | [26] | [27] | |
| Characteristics | Units | ISW | ||
|---|---|---|---|---|
| Moisture | wt.%, wb | 10.5 | 26.5 | 4.8 |
| Volatile matter | wt.%, db | 74.9 | n.d | 68.3 |
| Fixed carbon | 8.2 | n.d | 7.6 | |
| Ash | 16.9 | 16.6 | 24.1 | |
| C | wt.%, db | 41.1 a | 48.8 | 42.6 |
| H | 5.6 a | 7.0 | 5.6 | |
| N | 2.5 a | 0.6 | 0.6 | |
| O | 50.4 a | 18.0 | 27.1 | |
| S | 0.4 a | 0.2 | 0.0 | |
| HHV | MJ/kg, db | 16.4 | 19.8 | 17.2 |
| Ref. | [11] | [38] | [8] | |
| Characteristics | Units | CDW | |||
|---|---|---|---|---|---|
| Moisture | wt.%, wb | n.d | 11.9 | 9.4 | 6.0 |
| Volatile matter | wt.%, db | 78.1 | 92.4 | 93.9 | 85.5 |
| Fixed carbon | 11.6 | 1.6 | 0.0 | 9.5 | |
| Ash | 10.4 | 6.0 | 6.1 | 5.0 | |
| C | wt.%, daf | 52.3 | 51.7 | 52.5 | 52.7 |
| H | 6.8 | 6.3 | 6.3 | 6.6 | |
| N | 2.8 | 1.5 | 1.4 | 1.3 | |
| O | 27.5 | 40.4 | 39.8 | 39.3 | |
| S | 0.2 | 0.1 | 0.1 | 0.1 | |
| HHV | MJ/kg, db | 26.9 | 18.5 | 21.0 | 24.7 |
| Ref. | [51] | [52] | |||
| Process Conditions | Process | |||||
|---|---|---|---|---|---|---|
| Torrefaction | Pyrolysis | Gasification | HTC | |||
| Slow | Fast | Flash | ||||
| T (°C) | 200–300 | <700 | <800 | <1200 | <1200 | <350 |
| Residence time | Minutes–hours | Hours | Seconds | Few seconds | Seconds–minutes | Minutes–hours |
| Heating rate (°C/s) | 0.07–0.18 | 0.02–1.0 | 10–200 | >1000 | Moderate–very fast | 1–12 |
| Primary product | Char | Char | Bio-oil | Bio-oil or gas | Syngas | Hydrochar |
| Waste-derived char yield (%) | 60–80 | 35–89 | 12 | 25 | 10–39 | 35–80 |
| Torrefaction | Pyrolysis | Gasification | HTC | |||||
|---|---|---|---|---|---|---|---|---|
| Feedstock | MSW | RDF from ISW | RDF from MSW | CDW | SRF from MSW | RDF from MSW | RDF from ISW | SRF from CDW and MSW |
| Process conditions | 300 °C 1 h | 300 °C 30 min | 400 °C 30 min | 500 °C 1 h | 700 °C | 850 °C | 250 °C 30 min | 300 °C 30 min |
| Sample composition | 59% polymers, 17% lignocellulosic material | 22.9% plastic, 17.9% paper/cardboard, 7.9% textiles, 5% wood, 0.5% aluminum, 0.2% glass, 42.5% miscellaneous components | 66% textiles, 17.1% paper, 16.9% plastics | 39.1% paper, 32.2% wood, 11.8% plastics, 9.2% glass, 7.6% miscellaneous components | 80% plastics, 10% HDPE, 10% paper and chopstick wood | n.d | 22.9% plastic, 17.9% paper/cardboard, 7.9% textiles, 5% wood, 0.5% aluminum, 0.2% glass, 42.5% miscellaneous components | 65.87% wood, 17.27% plastics, 16.85% paper/cardboard |
| Ash wt.%, db | 28.7 | 22.8 | 20.1 | 41.9 | 88.5 b | 66.8 | 4.8 | 3.0 |
| Volatile matter wt.%, db | 58.3 | 56.3 | 69.8 | 26.5 | 4.9 b | n.d | 81.8 | 48.7 |
| Fixed carbon wt.%, db | 13.0 | 20.9 | 10.1 | 31.6 | 2.5 b | n.d | 13.4 | 51.4 |
| HHV (MJ/kg, db) | 16.0 | 19.9 | 28.1 | 16.28 | 2.6 | n.d | 26.1 | 28.4 |
| C wt.%, daf | 61.3 | 61.0 | 77.2 | 40.5 a | 8.1 a | 18.5 a | 61.9 | 73.0 |
| H wt.%, daf | 5.8 | 6.2 | 8.9 | 2.3 a | n.d | 0.7 a | 7.9 | 4.6 |
| N wt.%, daf | 0.5 | 1.3 | 2.1 | 0.6 a | n.d | 0.2 a | 1.8 | 0.8 |
| S wt.%, daf | 0.0 | 0.3 | 0.6 | 1.9 a | 0.003 a | 0.0 a | 0.0 | 0.0 |
| O wt.%, daf | 32.4 | 31.2 | 11.2 | n.d | n.d | 13.8 a | 28.4 | 21.6 |
| Cl wt.%, db | 8.5 | 1.8 | n.d | 4.9 | 0.2 | n.d | 1.27 | 2.4 |
| K wt.%, db | 0.02 | 4.0 | n.d | 0.1 | n.d | n.d | n.d | 0.6 |
| Zn wt.%, db | 0.05 | 6.8 | n.d | 0.2 | n.d | n.d | n.d | n.d |
| Ca wt.%, db | 13.2 | 34.1 | n.d | 9.1 | n.d | n.d | n.d | 7.6 |
| BET surface area (m2/g) | n.d | n.d | 0.7 | n.d | 10.4 | 12.0 | n.d | n.d |
| Ref. | [8] | [11] | [54,74] | [12] | [75] | [76] | [11,68] | [69] |
| Sample | Functionality | Wavenumber (cm−1) | Ref. |
|---|---|---|---|
| Pyrolysis RDF (from MSW) char (500 °C for 90 min) | O-H stretch (phenol and alcohols) | 3700–3500 | [80] |
| Aromatic C–H functional group | 2365 | ||
| N–H functional group | 2319 | ||
| C–H stretching vibration and conjugated aromatic carbonyl | 1690 | ||
| Inorganic compounds (lumino-silicate, calcium oxide, or metal oxide) | 1500–1300 | ||
| Pyrolysis MSW char (550 °C for 30 min) | Aliphatic O-H bond | 3200–3500 | [81] |
| Saturated symmetric and asymmetric C-H stretching vibration (aliphatic C-H) | 2875, 1935 | ||
| C=C or/and saturated C-H bending vibration | 1430 | ||
| C-O/Si-O bond (inorganic oxide compounds, such as phosphorus and sulfur) | 1101–1160 | ||
| P-O-P bond | 878 | ||
| C=O or C-O bond (carbonate functional groups) | 820 | ||
| O-Si-O (calcite and sílica) | 700 | ||
| Gasification SRF (from MSW) char (835 °C) | Hydrogen bonded O-H stretch | 3405 | [82] |
| C-H asymmetric/symmetric stretch | 2917, 2848 | ||
| Alkenyl C=C stretch overlapped by an open chain imino (C=N) | 1635 | ||
| Methyl C-H asymmetric bend | 1429 | ||
| Aliphatic-phosphate stretch (P-O-C) stretch | 1022 | ||
| Polysulphide (S-S) stretch | 536 | ||
| HTC waste textile (from MSW) char (230 and 280 °C for 30, 60, 90 min) | OH-stretching vibration bands of hydroxyl and carboxyl groups | 3200–3600 | [83] |
| Aromatic benzene ring =C–H stretching | 3000–3100 | ||
| C=O functional group | 1600–1800 | ||
| Aromatic C-C stretching | 1460–1600 | ||
| Esters C-O-C stretching | 1200–1360 | ||
| Aliphatic ether C-O or alcohol C-O stretching | 1100–1160 | ||
| Aldehydes -CHO stretching | 900–980 | ||
| Aromatic out-of-plane C-H bending | 750–875 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, S.M.; Gonçalves, M.; Brito, P.; Nobre, C. Waste-Derived Chars: A Comprehensive Review. Waste 2024, 2, 218-239. https://doi.org/10.3390/waste2030013
Santos SM, Gonçalves M, Brito P, Nobre C. Waste-Derived Chars: A Comprehensive Review. Waste. 2024; 2(3):218-239. https://doi.org/10.3390/waste2030013
Chicago/Turabian StyleSantos, Santa Margarida, Margarida Gonçalves, Paulo Brito, and Catarina Nobre. 2024. "Waste-Derived Chars: A Comprehensive Review" Waste 2, no. 3: 218-239. https://doi.org/10.3390/waste2030013
APA StyleSantos, S. M., Gonçalves, M., Brito, P., & Nobre, C. (2024). Waste-Derived Chars: A Comprehensive Review. Waste, 2(3), 218-239. https://doi.org/10.3390/waste2030013










