Biochar Extracts Can Modulate the Toxicity of Persistent Free Radicals in the Nematode Caenorhabditis elegans
Abstract
1. Introduction
2. Material and Method
2.1. Strains and Maintenance
2.2. Preparation and Characterization of Biochars
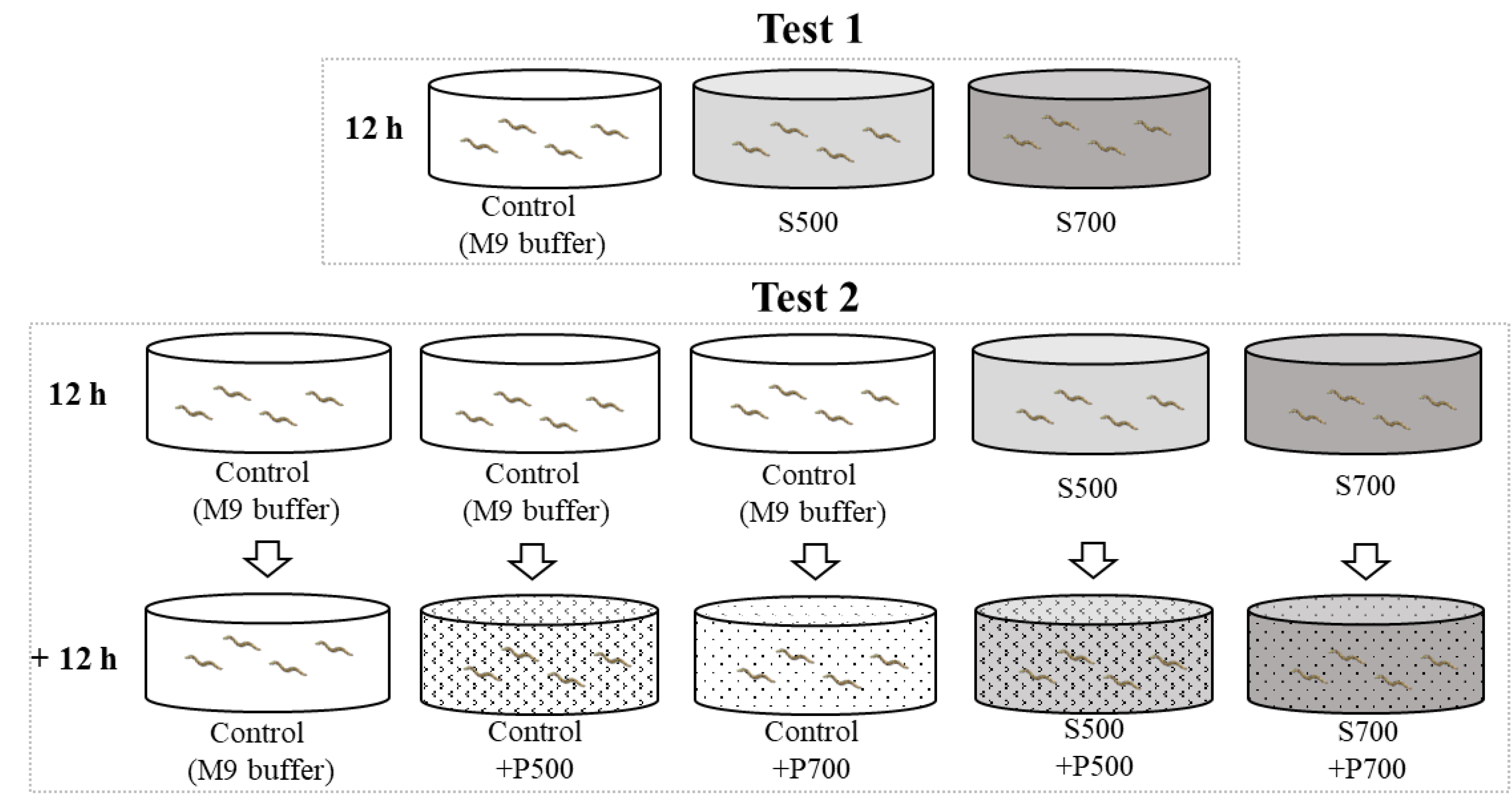
2.3. Exposure Condition
2.4. Neurotoxic Assays
2.5. The Autonomic Behavior Assay of Selected Supernatant
2.6. Statistical Analysis
3. Results and Discussion
3.1. Only Washed Particles from 700 °C Pyrolysis Caused Neurobehavioral Changes
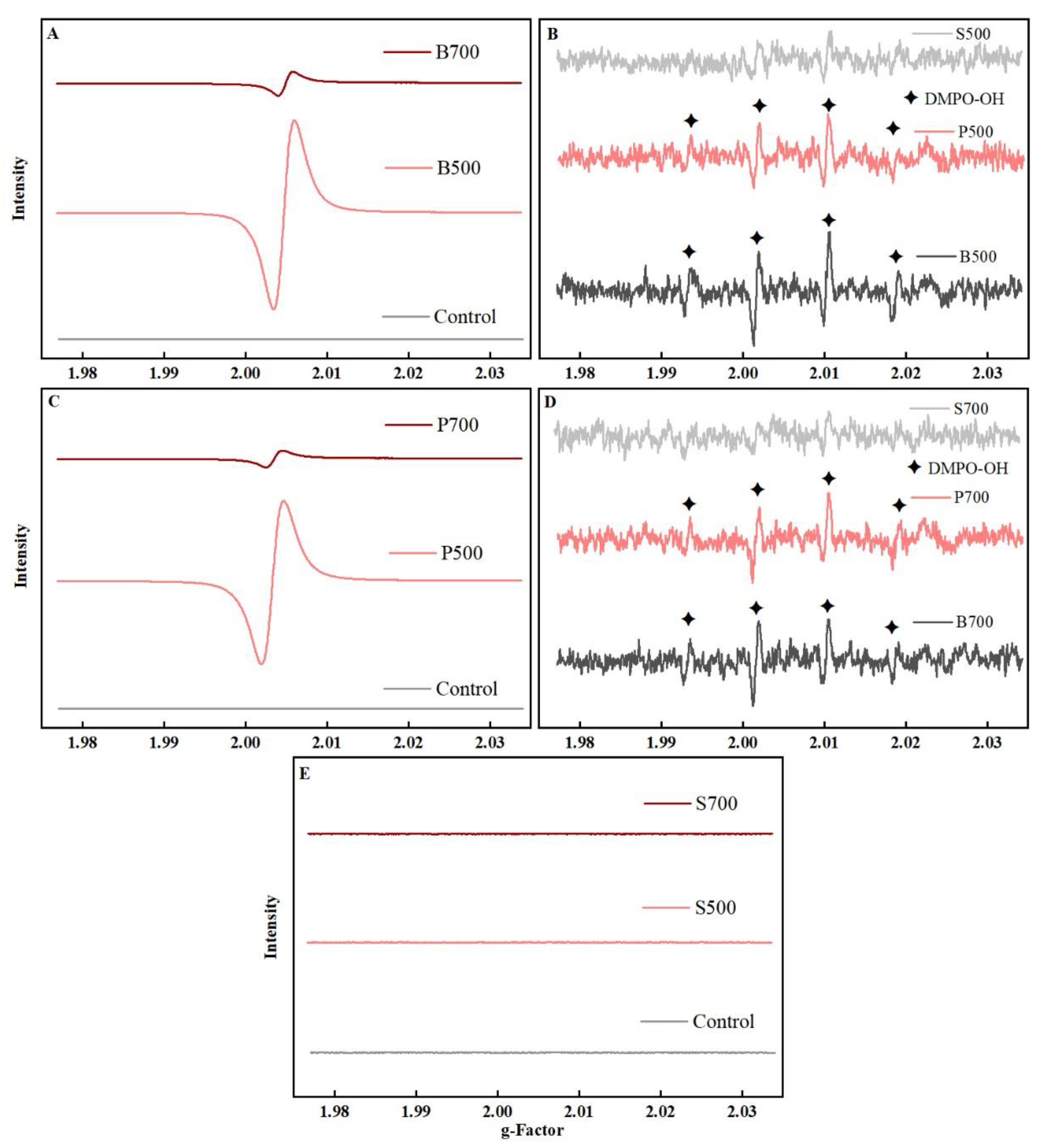
3.2. EPFRs Reactivity Might Play a Crucial Role in Neurotoxicity
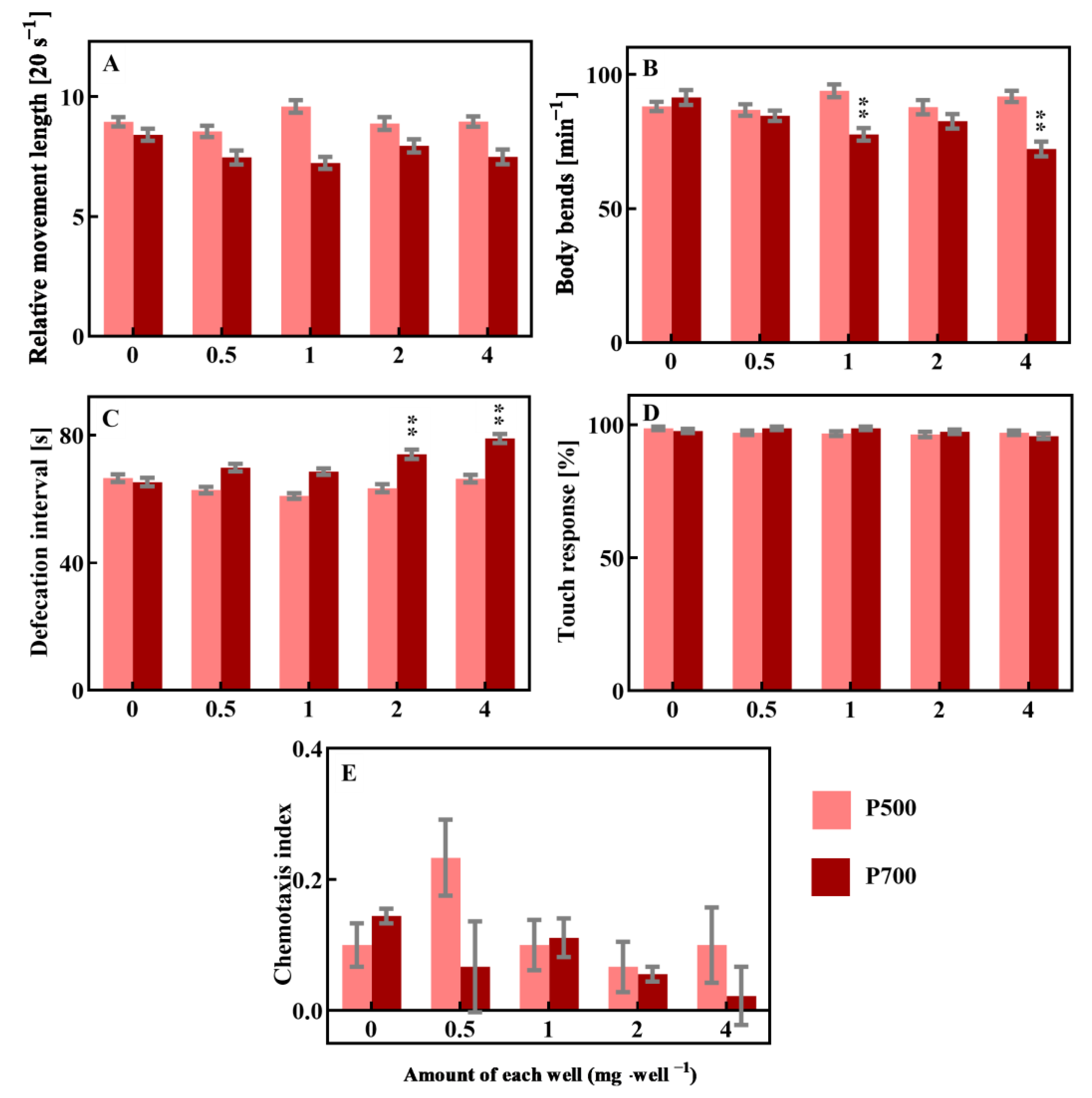
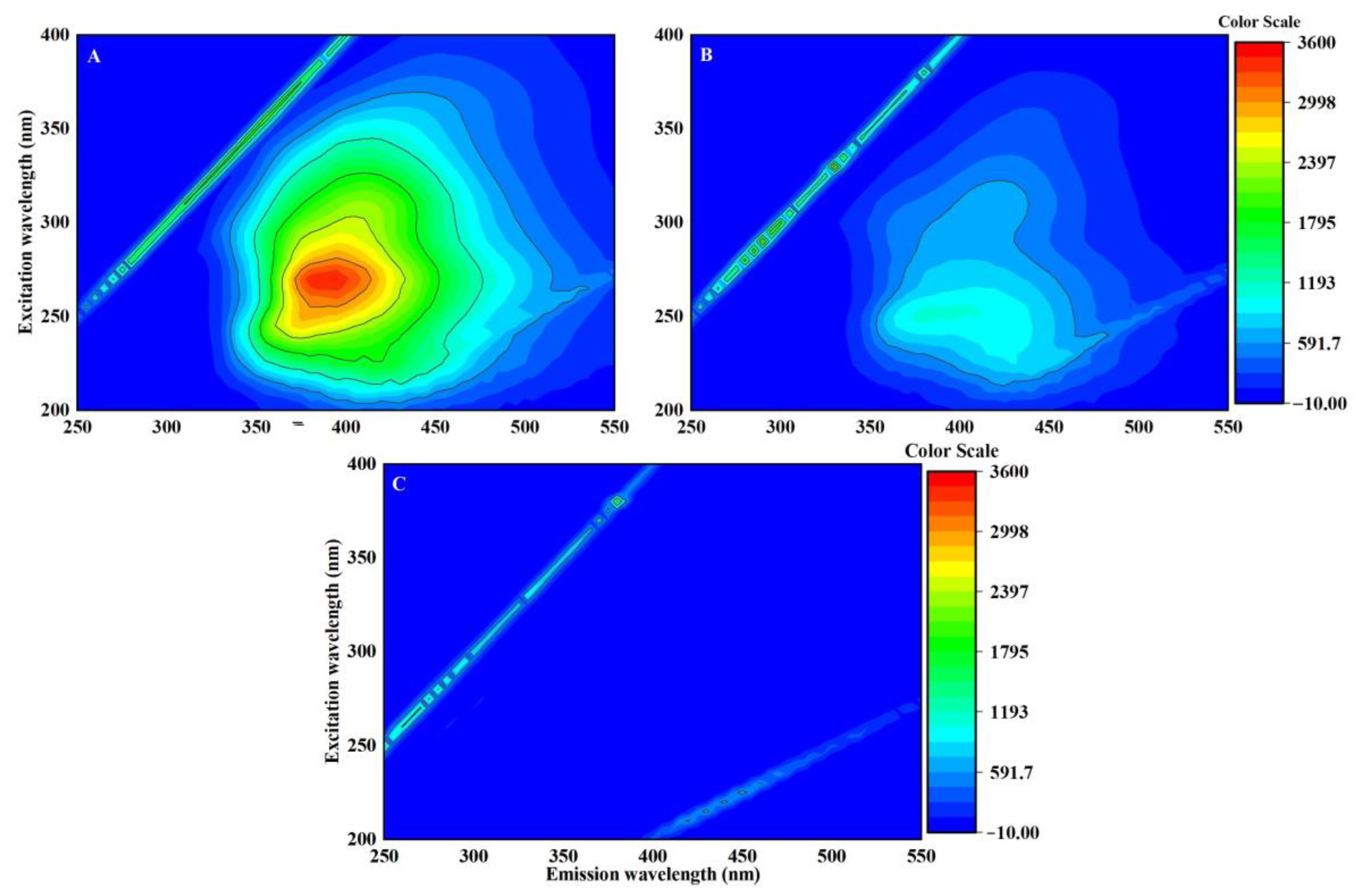
3.3. DOM Can Modulate the Toxicity of EPFRs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Naggar, A.; Lee, S.S.; Rinklebe, J.; Farooq, M.; Song, H.; Sarmah, A.K.; Zimmerman, A.R.; Ahmad, M.; Shaheen, S.M.; Ok, Y.S. Biochar application to low fertility soils: A review of current status, and future prospects. Geoderma 2019, 337, 536–554. [Google Scholar] [CrossRef]
- Yu, S.; Liu, J.; Yin, Y.; Shen, M. Interactions between engineered nanoparticles and dissolved organic matter: A review on mechanisms and environmental effects. J. Environ. Sci. (China) 2018, 63, 198–217. [Google Scholar] [CrossRef]
- Spokas, K.A.; Cantrell, K.B.; Novak, J.M.; Archer, D.W.; Ippolito, J.A.; Collins, H.P.; Boateng, A.A.; Lima, I.M.; Lamb, M.C.; McAloon, A.J.; et al. Biochar: A synthesis of its agronomic impact beyond carbon sequestration. J. Environ. Qual. 2012, 41, 973–989. [Google Scholar] [CrossRef] [PubMed]
- Beesley, L.; Moreno-Jimenez, E.; Gomez-Eyles, J.L.; Harris, E.; Robinson, B.; Sizmur, T. A review of biochars' potential role in the remediation, revegetation and restoration of contaminated soils. Environ. Pollut. 2011, 159, 3269–3282. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Sarswat, A.; Ok, Y.S.; Pittman, C.U., Jr. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent--a critical review. Bioresour. Technol. 2014, 160, 191–202. [Google Scholar] [CrossRef]
- Hussain, M.; Farooq, M.; Nawaz, A.; Al-Sadi, A.M.; Solaiman, Z.M.; Alghamdi, S.S.; Ammara, U.; Ok, Y.S.; Siddique, K.H.M. Biochar for crop production: Potential benefits and risks. J. Soils Sediments 2017, 17, 685–716. [Google Scholar] [CrossRef]
- Hale, S.E.; Lehmann, J.; Rutherford, D.; Zimmerman, A.R.; Bachmann, R.T.; Shitumbanuma, V.; O'Toole, A.; Sundqvist, K.L.; Arp, H.P.; Cornelissen, G. Quantifying the total and bioavailable polycyclic aromatic hydrocarbons and dioxins in biochars. Environ. Sci. Technol. 2012, 46, 2830–2838. [Google Scholar] [CrossRef]
- Liao, S.; Pan, B.; Li, H.; Zhang, D.; Xing, B. Detecting free radicals in biochars and determining their ability to inhibit the germination and growth of corn, wheat and rice seedlings. Environ. Sci. Technol. 2014, 48, 8581–8587. [Google Scholar] [CrossRef]
- Malev, O.; Contin, M.; Licen, S.; Barbieri, P.; De Nobili, M. Bioaccumulation of polycyclic aromatic hydrocarbons and survival of earthworms (Eisenia andrei) exposed to biochar amended soils. Environ. Sci. Pollut. Res. 2016, 23, 3491–3502. [Google Scholar] [CrossRef]
- Wang, P.; Pan, B.; Li, H.; Huang, Y.; Dong, X.; Ai, F.; Liu, L.; Wu, M.; Xing, B. The overlooked occurrence of environmentally persistent free radicals in an area with low-rank coal burning, Xuanwei, China. Environ. Sci. Technol. 2018, 52, 1054–1061. [Google Scholar] [CrossRef]
- Lian, F.; Xing, B. Black carbon (biochar) in water/soil environments: Molecular structure, sorption, stability, and potential risk. Environ. Sci. Technol. 2017, 51, 13517–13532. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zeng, G.; Huang, D.; Lai, C.; Chen, M.; Cheng, M.; Tang, W.; Tang, L.; Dong, H.; Huang, B.; et al. Biochar for environmental management: Mitigating greenhouse gas emissions, contaminant treatment, and potential negative impacts. Chem. Eng. J. 2019, 373, 902–922. [Google Scholar] [CrossRef]
- Pan, B.; Li, H.; Lang, D.; Xing, B. Environmentally persistent free radicals: Occurrence, formation mechanisms and implications. Environ. Pollut. 2019, 248, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dai, Q.; Jin, X.; Dong, X.; Peng, J.; Wu, M.; Liang, N.; Pan, B.; Xing, B. Negative impacts of biochars on urease activity: High pH, heavy metals, polycyclic aromatic hydrocarbons, or free radicals? Environ. Sci. Technol. 2018, 52, 12740–12747. [Google Scholar] [CrossRef] [PubMed]
- Lieke, T.; Zhang, X.; Steinberg, C.E.W.; Pan, B. Overlooked risks of biochars: Persistent free radicals trigger neurotoxicity in Caenorhabditis elegans. Environ. Sci. Technol. 2018, 52, 7981–7987. [Google Scholar] [CrossRef]
- Steinberg, C.E.W.; Sturm, A.; Kelbel, J.; Lee, S.K.; Hertkorn, N.; Freitag, D.; Kettrup, A.A. Changes of Acute Toxicity of Organic Chemicals to Daphnia magnain the Presence of Dissolved Humic Material (DHM). Acta Hydrochim. Hydrobiol. 1992, 20, 326–332. [Google Scholar] [CrossRef]
- Steinberg, C.E.; Saul, N.; Pietsch, K.; Meinelt, T.; Rienau, S.; Menzel, R. Dissolved humic substances facilitate fish life in extreme aquatic environments and have the potential to extend the lifespan of Caenorhabditis elegans. Ann. Environ. Sci. 2007, 1, 81–90. [Google Scholar]
- Yang, X.; Jiang, C.; Hsu-Kim, H.; Badireddy, A.R.; Dykstra, M.; Wiesner, M.; Hinton, D.E.; Meyer, J.N. Silver nanoparticle behavior, uptake, and toxicity in Caenorhabditis elegans: Effects of natural organic matter. Environ. Sci. Technol. 2014, 48, 3486–3495. [Google Scholar] [CrossRef]
- Lutz, I.; Jie, Z.; Opitz, R.; Kloas, W.; Ying, X.; Menzel, R.; Steinberg, C.E. Environmental signals: Synthetic humic substances act as xeno-estrogen and affect the thyroid system of Xenopus laevis. Chemosphere 2005, 61, 1183–1188. [Google Scholar] [CrossRef]
- Menzel, R.; Rodel, M.; Kulas, J.; Steinberg, C.E. CYP35: Xenobiotically induced gene expression in the nematode Caenorhabditis elegans. Arch. Biochem. Biophys. 2005, 438, 93–102. [Google Scholar] [CrossRef]
- Menzel, R.; Sturzenbaum, S.; Barenwaldt, A.; Kulas, J.; Steinberg, C.E. Humic material induces behavioral and global transcriptional responses in the nematode Caenorhabditis elegans. Environ. Sci. Technol. 2005, 39, 8324–8332. [Google Scholar] [CrossRef] [PubMed]
- Menzel, R.; Menzel, S.; Tiedt, S.; Kubsch, G.; Stosser, R.; Bahrs, H.; Putschew, A.; Saul, N.; Steinberg, C.E. Enrichment of humic material with hydroxybenzene moieties intensifies its physiological effects on the nematode Caenorhabditis elegans. Environ. Sci. Technol. 2011, 45, 8707–8715. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Oleszczuk, P.; Pan, B.; Xing, B. Environmental behavior of engineered biochars and their aging processes in soil. Biochar 2019, 1, 339–351. [Google Scholar] [CrossRef]
- Rajapaksha, A.U.; Ok, Y.S.; El-Naggar, A.; Kim, H.; Song, F.; Kang, S.; Tsang, Y.F. Dissolved organic matter characterization of biochars produced from different feedstock materials. J. Environ. Manag. 2019, 233, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Rombola, A.G.; Torri, C.; Vassura, I.; Venturini, E.; Reggiani, R.; Fabbri, D. Effect of biochar amendment on organic matter and dissolved organic matter composition of agricultural soils from a two-year field experiment. Sci. Total Environ. 2022, 812, 151422. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xiong, X.; He, M.; Xu, Z.; Hou, D.; Zhang, W.; Ok, Y.S.; Rinklebe, J.; Wang, L.; Tsang, D.C.W. Roles of biochar-derived dissolved organic matter in soil amendment and environmental remediation: A critical review. Chem. Eng. J. 2021, 424, 130387. [Google Scholar] [CrossRef]
- Ju, J.; Saul, N.; Kochan, C.; Putschew, A.; Pu, Y.; Yin, L.; Steinberg, C.E. Cyanobacterial xenobiotics as evaluated by a Caenorhabditis elegans neurotoxicity screening test. Int. J. Environ. Res. Public Health 2014, 11, 4589–4606. [Google Scholar] [CrossRef]
- Li, D.; Hockaday, W.C.; Masiello, C.A.; Alvarez, P.J.J. Earthworm avoidance of biochar can be mitigated by wetting. Soil Biol. Biochem. 2011, 43, 1732–1737. [Google Scholar] [CrossRef]
- Intani, K.; Latif, S.; Islam, M.; Müller, J. Phytotoxicity of Corncob Biochar before and after Heat Treatment and Washing. Sustainability 2018, 11, 30. [Google Scholar] [CrossRef]
- Lieke, T.; Steinberg, C.E.; Ju, J.; Saul, N. Natural Marine and Synthetic Xenobiotics Get on Nematode's Nerves: Neuro-Stimulating and Neurotoxic Findings in Caenorhabditis elegans. Mar. Drugs 2015, 13, 2785–2812. [Google Scholar] [CrossRef]
- Dellinger, B.; Lomnicki, S.; Khachatryan, L.; Maskos, Z.; Hall, R.W.; Adounkpe, J.; McFerrin, C.; Truong, H. Formation and stabilization of persistent free radicals. Proc. Combust. Inst. 2007, 31, 521–528. [Google Scholar] [CrossRef]
- Yang, J.; Pan, B.; Li, H.; Liao, S.; Zhang, D.; Wu, M.; Xing, B. Degradation of p-nitrophenol on biochars: Role of persistent free radicals. Environ. Sci. Technol. 2016, 50, 694–700. [Google Scholar] [CrossRef]
- Khachatryan, L.; Dellinger, B. Environmentally persistent free radicals (EPFRs)-2. Are free hydroxyl radicals generated in aqueous solutions? Environ. Sci. Technol. 2011, 45, 9232–9239. [Google Scholar] [CrossRef] [PubMed]
- Khachatryan, L.; Vejerano, E.; Lomnicki, S.; Dellinger, B. Environmentally persistent free radicals (EPFRs). 1. Generation of reactive oxygen species in aqueous solutions. Environ. Sci. Technol. 2011, 45, 8559–8566. [Google Scholar] [CrossRef] [PubMed]
- Wen, G.; Wang, S.J.; Ma, J.; Huang, T.L.; Liu, Z.Q.; Zhao, L.; Xu, J.L. Oxidative degradation of organic pollutants in aqueous solution using zero valent copper under aerobic atmosphere condition. J. Hazard. Mater. 2014, 275, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Hackbarth, S.; Vogt, R.D.; Roder, B.; Burnison, B.K.; Steinberg, C.E. Photogeneration of singlet oxygen by humic substances: Comparison of humic substances of aquatic and terrestrial origin. Photochem. Photobiol. Sci. 2004, 3, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Luo, H.; Zhang, C.; Zeng, G.; Lai, C.; Cheng, M.; Wang, R.; Deng, R.; Xue, W.; Gong, X.; et al. Nonnegligible role of biomass types and its compositions on the formation of persistent free radicals in biochar: Insight into the influences on Fenton-like process. Chem. Eng. J. 2019, 361, 353–363. [Google Scholar] [CrossRef]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence excitation-emission matrix regional integration to quantify spectra for dissolved organic matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef]
- Helms, J.R.; Stubbins, A.; Perdue, E.M.; Green, N.W.; Chen, H.; Mopper, K. Photochemical bleaching of oceanic dissolved organic matter and its effect on absorption spectral slope and fluorescence. Mar. Chem. 2013, 155, 81–91. [Google Scholar] [CrossRef]
- Liu, C.-H.; Chu, W.; Li, H.; Boyd, S.A.; Teppen, B.J.; Mao, J.; Lehmann, J.; Zhang, W. Quantification and characterization of dissolved organic carbon from biochars. Geoderma 2019, 335, 161–169. [Google Scholar] [CrossRef]
- Gui, X.; Liu, C.; Li, F.; Wang, J. Effect of pyrolysis temperature on the composition of DOM in manure-derived biochar. Ecotoxicol. Environ. Saf. 2020, 197, 110597. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhao, B.; Zhang, X.; Li, L.; Zhao, Y.; Li, Y.; Duan, K. Investigating Biochar-Derived Dissolved Organic Carbon (DOC) Components Extracted Using a Sequential Extraction Protocol. Materials 2022, 15. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhang, H.; Ni, J.; Xiang, Y.; Wei, R.; Qian, W.; Chen, W. Spectral characteristics coupled with self-organizing maps analysis on different molecular size-fractionated water-soluble organic carbon from biochar. Sci. Total Environ. 2023, 857, 159424. [Google Scholar] [CrossRef]
- Zhang, H.; Qian, W.; Wu, L.; Yu, S.; Wei, R.; Chen, W.; Ni, J. Spectral characteristics of dissolved organic carbon (DOC) derived from biomass pyrolysis: Biochar-derived DOC versus smoke-derived DOC, and their differences from natural DOC. Chemosphere 2022, 302, 134869. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Huang, P.; Xu, X.; Sun, H.; Jiang, B.; Liao, Y. Spectroscopic and molecular characterization of biochar-derived dissolved organic matter and the associations with soil microbial responses. Sci. Total Environ. 2020, 708, 134619. [Google Scholar] [CrossRef] [PubMed]
- Tomczyk, A.; Kubaczynski, A.; Szewczuk-Karpisz, K. Assessment of agricultural waste biochars for remediation of degraded water-soil environment: Dissolved organic carbon release and immobilization of impurities in one- or two-adsorbate systems. Waste Manag. 2023, 155, 87–98. [Google Scholar] [CrossRef]
- Reemtsma, T.; These, A.; Linscheid, M.; Leenheer, J.; Spitzy, A. Molecular and structural characterization of dissolved organic matter from the deep ocean by FTICR-MS, including hydrophilic nitrogenous organic molecules. Environ. Sci. Technol. 2008, 42, 1430–1437. [Google Scholar] [CrossRef]
- Collin, B.; Tsyusko, O.V.; Starnes, D.L.; Unrine, J.M. Effect of natural organic matter on dissolution and toxicity of sulfidized silver nanoparticles to Caenorhabditis elegans. Environ. Sci. Nano 2016, 3, 728–736. [Google Scholar] [CrossRef]
- Haitzer, M.; Abbt-Braun, G.; Traunspurger, W.; Steinberg, C.E.W. Effects of humic substances on the bioconcentration of polycyclic aromatic hydrocarbons: Correlations with spectroscopic and chemical properties of humic substances. Environ. Toxicol. Chem. 1999, 18, 2782–2788. [Google Scholar] [CrossRef]
- Lieke, T.; Steinberg, C.E.W.; Pan, B.; Perminova, I.V.; Meinelt, T.; Knopf, K.; Kloas, W. Phenol-rich fulvic acid as a water additive enhances growth, reduces stress, and stimulates the immune system of fish in aquaculture. Sci. Rep. 2021, 11, 174. [Google Scholar] [CrossRef]
- Timofeyev, M.A.; Shatilina, Z.M.; Bedulina, D.S.; Menzel, R.; Steinberg, C.E. Natural organic matter (NOM) has the potential to modify the multixenobiotic resistance (MXR) activity in freshwater amphipods Eulimnogammarus cyaneus and E. verrucosus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2007, 146, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Haitzer, M.; Hoss, S.; Traunspurger, W.; Steinberg, C. Effects of dissolved organic matter (DOM) on the bioconcentration of organic chemicals in aquatic organisms--a review. Chemosphere 1998, 37, 1335–1362. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, C.E.W.; Brüggemann, R. Ambiguous Ecological Control by Dissolved Humic Matter (DHM) and Natural Organic Matter (NOM): Trade-offs between Specific and Non-specific EffectsWe dedicate this paper to Prof. Dr. Fritz H. Frimmel on the occasion of his 60th birthday anniversary. Acta Hydrochim. Et Hydrobiol. 2001, 29, 399. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Saul, N.; Lieke, T.; Chen, Y.; Wu, M.; Pan, B.; Steinberg, C.E.W. Biochar Extracts Can Modulate the Toxicity of Persistent Free Radicals in the Nematode Caenorhabditis elegans. Appl. Biosci. 2023, 2, 71-83. https://doi.org/10.3390/applbiosci2010007
Zhang X, Saul N, Lieke T, Chen Y, Wu M, Pan B, Steinberg CEW. Biochar Extracts Can Modulate the Toxicity of Persistent Free Radicals in the Nematode Caenorhabditis elegans. Applied Biosciences. 2023; 2(1):71-83. https://doi.org/10.3390/applbiosci2010007
Chicago/Turabian StyleZhang, Xuchao, Nadine Saul, Thora Lieke, Yi Chen, Min Wu, Bo Pan, and Christian E. W. Steinberg. 2023. "Biochar Extracts Can Modulate the Toxicity of Persistent Free Radicals in the Nematode Caenorhabditis elegans" Applied Biosciences 2, no. 1: 71-83. https://doi.org/10.3390/applbiosci2010007
APA StyleZhang, X., Saul, N., Lieke, T., Chen, Y., Wu, M., Pan, B., & Steinberg, C. E. W. (2023). Biochar Extracts Can Modulate the Toxicity of Persistent Free Radicals in the Nematode Caenorhabditis elegans. Applied Biosciences, 2(1), 71-83. https://doi.org/10.3390/applbiosci2010007





