Abstract
Chicory (Cichorium intybus L.) is a low-height perennial or biennial herb from the family of Asteraceae. Investigation of different in vitro regeneration strategies of Cichorium intybus and increasing the number of secondary metabolites in vitro regenerated plant samples were the aims of the research. Callus and plant regenerations were achieved in basal plant growth media supplemented with plant growth regulators (PGRs). Whole plant regeneration was carried out by direct organogenesis from leaf explant in Murashige and Skoog (MS) and B5 media supplemented with naphthalene acetic (NAA) acid and indole-3-butyric acid (IBA). The highest callus quantity was produced in MS medium supplemented with indole-3-acetic acid (IAA) and benzyl amino purine (BAP). The combination and concentrations of PGRs used in MS and B5 media not only provided root and shoot formation with callus, but also caused a change in the amounts of phenolic components. In addition, some PGRs used caused an increase in the number of phenolic compounds in callus and shoots developed from the leaf explant. When plants that grow in vitro and in vivo are compared with each other, it has been determined that plants grown in vivo contain higher amounts of some phenolic compounds. In vivo and in vitro samples were extracted in ethanol/water (80:20 v/v). The analysis of phenolic compounds (caftaric, chicoric, and chlorogenic acids and esculin) were performed in high-performance liquid chromatography (HPLC) and inulin was in UV spectrophotometry. The caftaric and chlorogenic acids and inulin concentrations were higher in vivo samples than that in vitro. Contrarily, esculin, and chicoric acid concentrations were higher in the in vitro regenerated samples. The higher concentration of valuable compounds in the in vitro regenerated samples, especially in callus tissue, gives hope for large-scale production of secondary metabolites under laboratory conditions.
1. Introduction
Chicory (Cichorium intybus L.) is a small aromatic biennial or perennial herb belonging to the family of Asteraceae. Chicory is known also as cornflower, blue weed, ragged sailors, blue dandelion, and horseweed. The first recordings of the cultivation of chicory were found in papyruses of ancient Egyptians as a coffee substitute, vegetable, medicinal plant, and occasionally for animal foraging [1]. The recent habitat of the plant ranges in temperate and semi-arid regions of the world such as the Mediterranean region, Mid-Asia, and Northern Africa [2].
The medical aspects of Cichorium intybus L. are an example of the oldest herb approach [3]. Chicory was used for the treatment of diseases such as diarrhea, cough, cancer, and hangover, for purification of the biliary tract, liver disease, as spasmolytic, to the relief of ailments related to mild digestive disorders (such as a feeling of abdominal fullness, flatulence, and slow digestion) and temporary loss of appetite [4,5]. Extended healing properties of chicory were for tuberculosis, haemorrhoids, sore throat, rashes, abdominal cramps, deafness, melancholy, and laxative for children. For thousands of years, the study of herbs was bequeathed from generation to generation throughout civilization [6].
Chicory contains many medicinally relevant compounds such as esculin, inulin, volatile compounds (monoterpenes and sesquiterpenes), flavonoids, coumarins, and vitamins [7]. Inulin is a reserve carbohydrate, feasible for diabetics to replace fat or sugar and reduce the calories of food and in inulin clearance tests to measure glomerular filtration rates. The root of chicory due to the high content of inulin that is easily extracted is considered one of the potential sources for inulin production on an industrial scale [8]. Chicory features have high potential in clonal propagation in in vitro culture and cultivators enhance production for medicinal purposes [9]. Hence, plant tissue culture offers an alternative source for the controlled production of these products [10]. Secondary plant metabolites are organic compounds that are indirectly involved in normal growth, development, or organism reproduction. They play a significant role in pollination, defence against pathogens, and abiotic stress. Despite significant increase in the demand for secondary metabolites and functioning in industrial medicine and agriculture, the habitat of plants has shrunk due to economic development, diseases, and climate change. Chicory phytocompounds have appeared as a cutting-edge topic due to their widespread usage [11]. Efficient isolations of chicory secondary metabolites and other materials required the development of extraction technique approaches [12].
The present study aimed to monitor in vitro regeneration and increase the strategy of secondary metabolite (caftaric, chlorogenic, chicoric acids, inulin, and esculin) of chicory plant tissue culture. The results of the study provided a wider perspective on the successful approaches to cultivating the plant in vitro and focusing on increasing the concentration of chicoric, caftaric, chlorogenic acids, esculin, and inulin of the plant in controlled conditions. In this study, quantitative determination and increase in the strategy of phenolic compounds in chicory produced in vitro conditions were carried out by using PGRs at different concentrations and combinations for the first time.
2. Material and Methods
2.1. Plant Sample
Cichorium intybus L. was collected from the field of the Campus of Van Yuzuncu Yil University, Van-Türkiye on October 20, 2016, when the plant was in the flowering stages. The description of the plant species was made according to the Flora of Turkey, Davis. The samples were then dried in dark conditions in the laboratory of the Science Faculty at Van Yuzuncu Yil University. The collected sample of the plant was separated into roots, stems, leaves, and flowers. After drying, the samples were ground into powder and placed in a fridge at a temperature −20 °C until analysis.
2.2. Plant Tissue Culture Studies
The experimental setup was sterilized in an autoclave at 121 °C and 1.5 atmosphere pressure: media for 25 min, while metal and glass equipment for 1 h. Three types of media; B5 (Gamborg B5 medium G0209), Murashige and Skoog (MS) [13], and White (WO227) were used [14], supplemented with agarose of concentrations from 3 g L−1 to 6 g L−1 and 30 g L−1 sucrose. pH was adjusted between 5.7 and 5.8, using 1 M HCL and 1 M of NaOH [15].
The explants were soaked in tap water for about 30 min. After that, their surface was sterilized with 70% ethanol for 30 s and washed with distilled water 3–4 times. Then, the explants were put in sodium hypochlorite 5% commercial bleach for 3 min. Finally, the explants were washed with distilled water 4–5 times. After sterilization, explants were incubated in the media supplemented with plant growth regulators.
Explants were cultivated in ready-made media in Petri dishes (covered by parafilm) and incubated in growth chamber. The growth chamber was characterized as the manufacturer mentioned; Phytotron, Sanyo, Gellenkamp PLC, UK, at a temperature of 25 ± 2 °C under 16 h light/8 h dark, provided by cool white fluorescent lamps. The young leaves of the plant field samples with young leaves of the plant were surface-sterilized and incubated in MS, B5 medium [16] (Bajaj et al., 2021) and were used to regenerate the whole plant via indirect organogenesis in basal plant media supplemented with PGRs in different concentrations and combinations. Since positive results are unobtainable in White medium in plant regeneration, MS and B5 mediums were mostly used in the study.
2.3. Extraction and Analysis of Phenolic Composition and Polysaccharide (Inulin) of Cichorium intybus L.
Extraction processes were carried out in different parts of the plant both through in vivo and in vitro [4].
- Roots in vivo
- Leaves in vivo
- Stems in vivo
- Flowers in vivo
- Leaves in vitro
- Callus in vitro
Extractions were performed in Eppendorf tubes with 100 mg of pulverized plant material applying 1 mL of aqueous ethanol (80% ethanol diluted in water). Then, samples were sonicated for 15 min, centrifuged for 10 min, 10,000 rpm, (FA-45-24- 11 rotor, Eppendorf, Germany), and then for pellets extractions were repeated. The supernatant was stored at temperature −20 °C until analysis, as determined as proper by [17].
An aliquot (3 μL) of every ethanolic extract was injected in a chromatographic column at 30 °C (150 × 2.1 mm i.d., 5 μm Luna Synergy Hydro, Phenomenex). Samples were separated using 0.5% formic acid in purified water (A) and 0.5% formic acid in acetonitrile (B) at a flow rate of 200 μL/min. The gradient approached was 0% B for 2 min, then 40% B for 6 min, following 60% of B for 8 min, and 100% B for 4 min. Then, the gradient was held for 4 min at the rate of 100% of B. The range of the photodiode array detector was from 190 nm to 520 nm. Ions for mass spectrophotometer were generated using an electrospray source in either the positive or negative mode (depending on analytics) under conditions set following optimization using chlorogenic acid (negative) or quercetin-3-glucoside (positive). MS experiments in the full scan (parent and product-specific) and the selected reaction monitoring (SRM) mode were conducted. The composition of phenolic compounds was characterized based on their UV spectrum, retention time, co-chromatography with commercial standards, when available, and MS fragmentation patterns.
The HPLC system consisted of a SPD-M10ADvp diode array detector, two LC-10ADvp pumps, SIL-10ADvp auto-injector, CTO-1- ADvp column oven, DGU-12A degasser, and SCL-10A system controller (Shimadzu Corporation, Kyoto, Japan) equipped with an Atlantis column (dC18, 4.6 mm i.d x 100 mm length, 5 μm particle size, Waters Associates, Chippendale, NSW, Australia). Analytical HPLC was run at 30 °C and monitored at 280 nm, 326 nm, and 370 nm with an injection volume of 10 μL. The following solvents in purified water with a flow rate of 1.0 mL/min were used: A, 0.5% trifluoroacetic acid (TFA), and B, 0.5% TFA + 95% acetonitrile. The elution profile was a linear gradient elution for B of 0% to 20% for 3 min, to 40% for 5 min, to 60% for 5 min, to 80% for 5 min, and 100% for 5 min. The gradient elution was held at the rate of 100% B for 5 min. The identity of compounds was confirmed by co-chromatography and comparison of their spectral characteristics with those of authentic standards of esculin, chlorogenic acid, and chicoric acid.
100 mg of dried material was extracted separately in 10 mL of warm water (50 °C) to 1 mL of sample. An equal volume of concentration of HCl and 0.1 mL resorcinol were added and made up to 10 mL with distilled water. The mixture was warmed in a water bath for 10 min and the absorbance was read at 490 nm in a spectrophotometer. The results were expressed as mg of inulin content (inulin equivalent) per gram of dry weight of the lyophilized powder (mg inulin equivalent/g DW), based on the UV inulin standard curve and against a blank control. The analyses were conducted in triplicate.
2.4. Statistical Analysis
Mean values were calculated based on the results of 3 replicates for each sample. (n = 3). The experimental data were subjected to one-way analysis of variance and post-hoc Tukey’s b test using IBM SPSS version 20 and were performed to assess differences between the samples at the level of p < 0.05.
3. Results
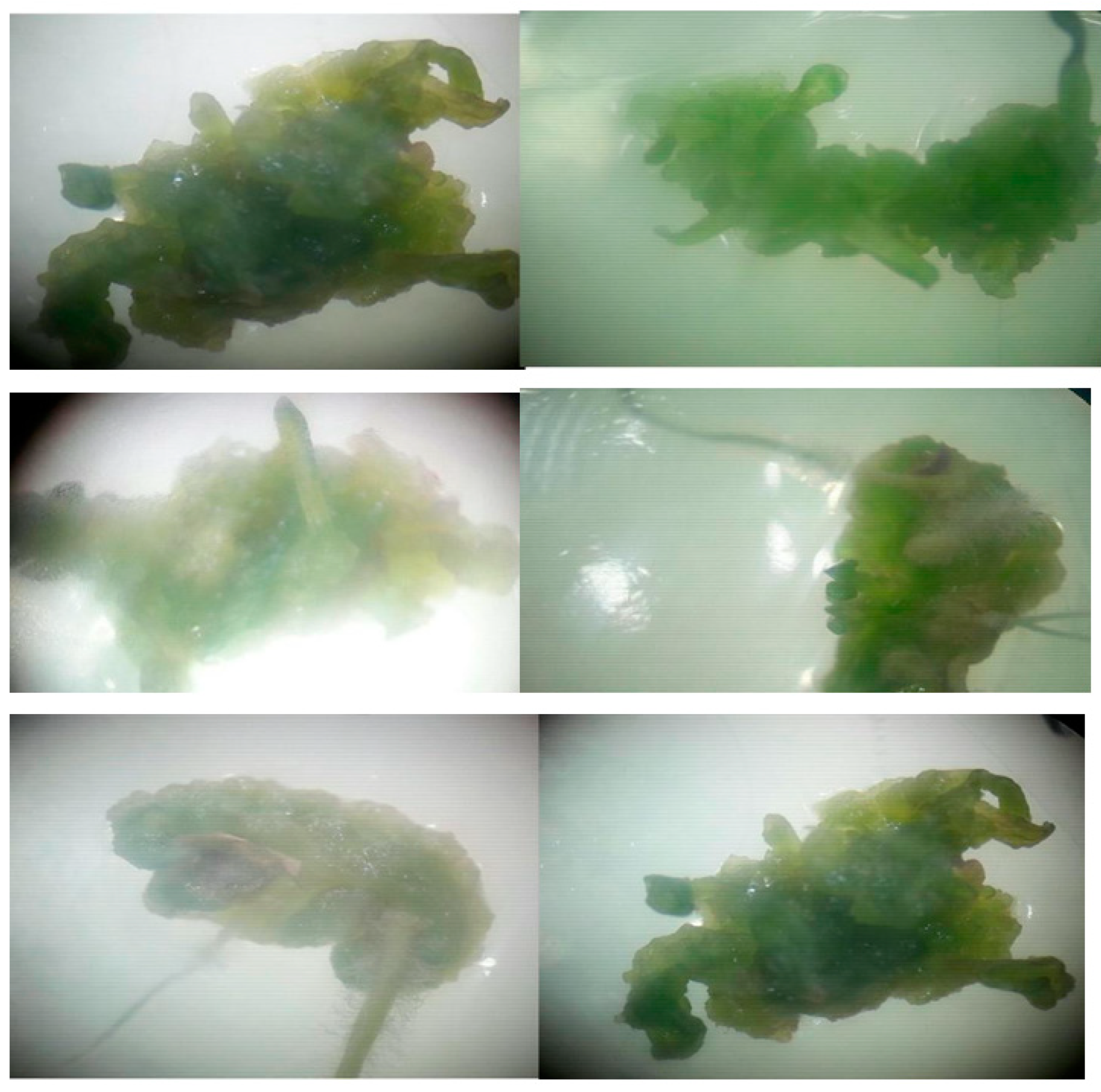
Young leaves of the plant collected from the field were surface-sterilized and incubated in MS and B5 medium supplemented with different concentrations and combinations of plant growth regulators (IAA, IBA, NAA, BAP, GA3). In this study, in which the leaf was used as an explant source, the use of IBA together with NAA in MS medium caused the development of shoots with callus from the explants, the use of BAP together with IAA caused the development of calli, and the use of GA3 together with NAA and IBA led to the development of shoots with callus. The use of IBA and BAP together provided root development with calli. IAA, IBA and BAP PGRs were used in B5 medium. The use of BAP together with IAA caused the development of shoots together with calli. In the same way, the use of IAA together with IBA provided the development of shoots together with calli. Shoot and root regeneration occurred after two weeks (Figure 1).
Figure 1.
Calli regeneration from explants after two weeks.
Maturation of plantlets occurred at the bottles, where they had been transferred to PGRs-free B5 and MS medium. Then seedlings were planted in soil and kept in a natural condition in the laboratory. Plant regeneration was stimulated more efficiently in B5 medium supplemented with 0.125 g L−1 IBA + 0.1 g L−1 NAA (Table 1).

Table 1.
In vitro callus production from Cichorium intybus L.
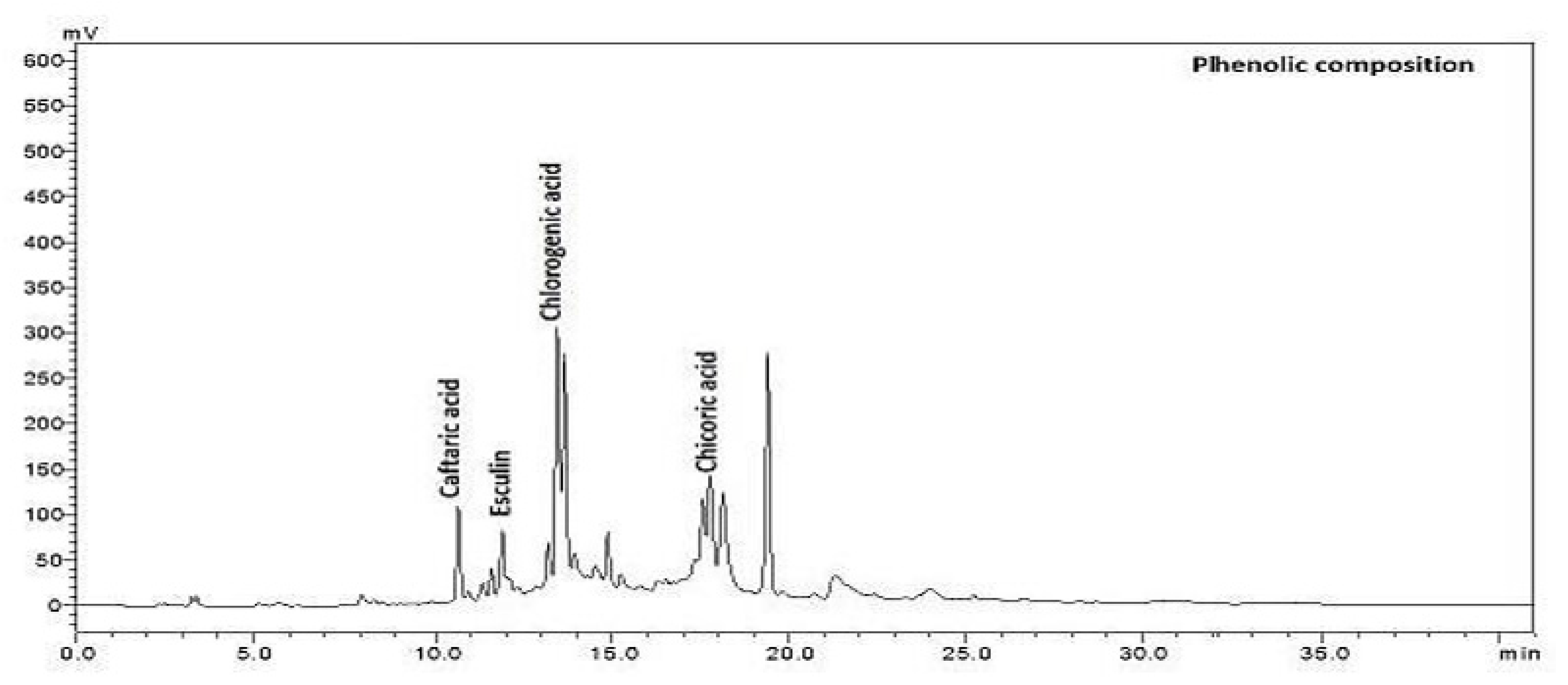
The main phenolic compositions, caftaric, chlorogenic, chicoric acids, and esculin of Cichorium intybus L., were extracted from the sample of in vivo and in vitro regenerated plants and analysed in HPLC (Table 2).

Table 2.
Phenolic compositions of Cichorium intybus L.
Among the phenolic compounds, the amount of chicoric acid was highest in flowers growing in vivo and lowest in roots growing in vivo. The caftaric acid amount was highest in leaves growing in vitro and lowest in roots and flowers growing in vivo. The esculin amount was highest in roots growing in vivo (Figure 2). It was determined that the amount of chlorogenic acid was the highest in the leaves growing in vitro and the lowest in the callus developed in vitro in flowers growing in vivo (Table 2).
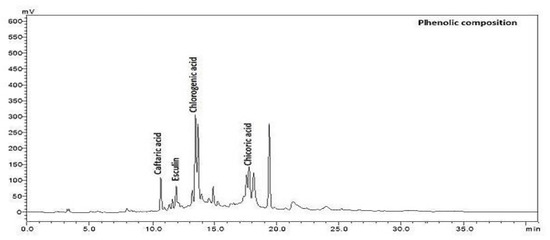
Figure 2.
The chromatogram of phenolic composition of Cichorium intybus L.
Inulin as a major polysaccharide in Cichorium intybus L. was extracted and analysed in a spectrophotometer. In the root of the in vivo sample, inulin concentration was the highest (37.9 ± 0.5 a mg/g-dw), while the lowest was found in the callus (14.3 ± 0.5 e mg/g-dw) (Table 3).

Table 3.
Concentration of inulin of Cichorium intybus L. using a spectrophotometer ([4]).
4. Discussion
Chicory is a relevant plant in pharmacy, due to the special ratio of compounds such as esculin, inulin, vitamins, coumarins, flavonoids, monoterpenes, and sesquiterpenes [7]. Chicory is prescribed as a digestive, diuretic, vomiting medicine, and anti-inflammatory. The plant is applied for the treatment of hangovers, haemorrhoids, diarrhoea, tuberculosis, cough, cancer, and purification of the biliary tract. Other healing advantages of chicory are used for liver complaints for relief of symptoms related to mild digestive disorders such as a feeling of abdominal fullness, flatulence, digestion inhibition, temporary loss of appetite, spasmolytic, sore throat, abdominal cramps, deafness [18], and laxative for children [5].
Callus production from shoots, leaves, and roots of Cichorium intybus L. was studied and increased based on PGRs. It was reported that MS and Linsmaier & Skoog (LS) basal media, containing different concentrations of 2, 4-D, caused a high amount of callus proliferation [19]. In the present study, leaves were used as an explant and incubated in three various media supplemented with different concentrations and combinations of PGRs. The prolific result was provided from MS medium supplemented with IAA and BAP. The results are two times higher than in the research cited [20]. It was determined that the IAA, BAP, IBA, and NAA used in MS medium increased the amount of chlorogenic acid in leaf explants, and the same situation caused an increase in the amount of inulin. However, no such relationship was found between the other explants used and the PGR. However, different metabolites may be synthesized depending on the type of PGRs added to the medium.
The proposed method is safer since the IBA and NAA are utilized contrary to amalgamate addition to Petunia hybrida Vilm. Cv. ‘‘Bravo” [21] or used in Cichorium intybus [6]. The application of the proposed method would not increase contamination with hazardous mercury [22].
Leaf was incubated in three different media supplemented with IBA and NAA. Shoot and root regeneration was observed in two weeks. Plantlets were isolated from Petri dishes and incubated in PGRs-free MS medium in jars. Seedlings were maturated after 3 to 4 weeks and planted in the soil and acclimated to natural environmental conditions. The result was supported by the study that reported that leaf explant incubated in MS medium supplemented with auxin and cytokinin initiates shoot and root development. Auxins are reported to initiate root and cytokinins initiate shoot. The combination of the two PGRs simultaneously initiated both shoots and roots [7]. IAA is an auxin with significant plant growth properties and can be used for full-scale plant propagation [23]. Plant growth regulators such as IBA function in the rooting stage of tissue culture [24]. In the present study, combining cytokinin and auxin provided a useful effect on direct organogenesis. Direct roots and shoots regeneration was stimulated by a combination of IBA with NAA.
In the present study, secondary metabolites extracted from in vivo- and in vitro-regenerated plant samples were quantitatively analyzed. The least secondary metabolite production was provided from callus with some minor exceptions from in vivo specimens. Although callus tissue has advantages for the first step for the suspension culture and bioreactor, long-term culture continuity with subcultures causes insufficient production of secondary metabolites. This can be explained by the fact that genes responsible for the synthesis of secondary metabolites in some non-differentiating tissues are not expressed, the metabolite is disintegrated immediately, the substrate is shifted to another pathway, and the produced metabolite is not at the storage site
In the research, the highest total phenolic contents were compared in leaves, flowers, stems, and roots. The results are also parallel to the report of Dalar and Konczak, (2014) [25], which concluded that a high concentration of total phenolic was found in leaves and flowers. Innocenti et al., (2005) [26] determined the same content of the phenolic compounds in red chicory, witloof chicory (Belgian endive), and green chicory,
Nandagopal and Kumari (2007) [7] achieved the concentration of esculin higher in the callus than in vivo roots. They also determined the maximum concentration of esculin in in vitro leaves and roots, while the minimum concentration of esculin was detected in callus and in in vivo leaf. In the present study, the maximum esculin concentration was determined to be in in vitro leaves while the minimum concentration of esculin was measured in in vivo leaves and roots. This is an important result for the present study through increasing secondary metabolite production in in vitro cultures. Producing secondary metabolites in controlled tissue culture was achieved. All of the samples regenerated in tissue culture produced secondary metabolites; in some cases, more than that of in vivo-regenerated specimens. In tissue culture, substances can be produced with a certain standard. Mass production of valuable chemicals in economic and medicinal terms can be achieved. Thus, the destruction of wild plants in nature can be reduced to a minimum and less land use can be achieved.
In the current study, the highest concentration of caftaric acids was found in flowers in vivo, and the lowest concentration was found in roots in vivo. Although the optimal results were provided from in vivo specimens, producing the compound with in vitro applications is also enough efficient for agriculture development. The amount of the compound in tissue culture depends on some modifications and applications. Caftaric acid brings a yellowish-gold colour to some white drinks. The acid is commonly known as cinnamates (hydroxycinnamic acid) [27]. Chlorogenic acid is an important phenylpropanoid with antioxidant properties. In the research, the concentration of chlorogenic acid was measured higher in in vivo samples than that of in vitro, including leaves. In in vivo samples, the lowest concentration of chlorogenic acid was found in calli, while the highest was in flowers. Phenylpropanoids function fundamentally in plants as protection against biotic and abiotic stresses, signaling molecules and building elements and pigments [28]. Phenylpropanoid production increases in response to stress conditions such as temperature, injury, UV irradiation, light density, and nutrient deficiency pathogen. The volumes of chlorogenic acid were increased by UV irradiation in Hypericum perforatum [29]. In this study, due to the lack of abiotic and biotic stress factors, the concentration of chlorogenic acid was insignificant in in vitro samples. Cichorium intybus L. plants growing in field conditions produced more chlorogenic acid because of exposing hard environmental conditions as a protective agent against stress factors [4]. Therefore, sunlight-exposed parts, such as leaves and flowers, featured the highest chlorogenic acid level.
In vitro samples obtained more chicoric acid than in vivo assays, with the highest level in the leaves. Chicoric acid production was accomplished in the samples that originated through tissue culture with higher concentrations than in the specimens provided from conventional field agriculture. Media compositions and PGRs in different combinations and concentrations may trigger secondary metabolite production. Callus also produced the compound. Chicoric acid protects plants from bacteria, viruses, fungi, nematodes, and insects that cause wounds in plants whose presence leads to higher ratios of production of chicoric acid [30].
In the current study, the highest inulin concentration was found in vivo in roots and leaves (where inulin is stored) while the lowest was in calli and flowers in vivo. A similar finding was obtained by Nandagopal and Kumari, (2007) [7] who reported the maximum concentration of the compound in the root of in vivo- and in vitro-regenerated plants. Inulin is fructan and is mostly found in the Asteraceae family as chicory. Recent pharmacological investigation of the root extract of the cornflower revealed immunomodulatory, antitumor, and anticancer properties [7]. In this study, inulin content was measured differently in various parts of the plant, and the most remarkable content was in the root in vivo and higher than in the leaves of plants grown in vitro and significantly less than that of flower and stem in vivo. The result is relevant for the industrial production of inulin, which becomes an important medical carbohydrate.
5. Conclusions
In tissue culture, including callus, the production of secondary metabolites due to supplementation of indole-3-acetic acid IAA and BAP was remarkable and allowed for restoration of the Cichorium intybus L. plant. The method can be developed for external application in a controlled environment in tissue culture. Application of abiotic stress factors on in vitro samples would shift plantations for the production of desired compounds, including those relevant to pharmacy. The production of valuable compounds in in vitro conditions, especially in callus tissue, gives hope for the manipulation of production at an industrial scale under laboratory conditions. Stress applied in in vitro conditions causes an increase in some phenolics in the plant, while it decreases some phenolic compounds. For this reason, which phenolic component is desired to be increased, the stress factor and PGR causing the increase in this compound should be determined and stress should be applied by creating appropriate conditions. Otherwise, all stress does not increase phenolic compounds together with any PGR used in vitro.
Author Contributions
Conceptualization, Y.A.A. and A.D.; methodology, A.E.; software, Y.A.A., F.A.O. and G.S.; validation, G.S., F.A.O. and M.T.; formal analysis, Y.A.A.; investigation, M.T.; resources, G.S.; data curation, Y.A.A., G.S. and F.A.O.; writing—original draft preparation, G.S., F.A.O.; writing—review and editing, M.T.; visualization, F.A.O., G.S. and M.T.; supervision, A.D.; project administration, A.D.; funding acquisition, M.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Van Yuzuncu Yıl University, Scientific Research Projects Management Unit (FYL-2017-5391), which supported this study.
Institutional Review Board Statement
Ethics committee approval is not required for this study.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are put in the manuscript.
Conflicts of Interest
The authors have declared that they do not have any conflict of interest in the publication of this article.
Abbreviations
| ANOVA | Analysis of Variance |
| BAP | Benzyl Amino Purine |
| HPLC | High-Pressure Liquid Chromatography |
| IAA | Indole 3 -Acetic Acid |
| IBA | Indole Butyric Acid |
| LC | Liquid Chromatography |
| LS | Linsmaier & Skoog |
| MS media | Murashige-Skoog media |
| NAA | Naphthalene Acetic acid |
| WO227 | White medium |
| G0209 | Gamborg B5 medium |
| PGR | Plant Growth Regulators |
| UV | Ultraviolet |
References
- Wang, Q.; Cui, J. Perspectives and utilization technologies of chicory (Cichorium intybus L.): A review. Afr. J. Biotechnol. 2011, 10, 1966–1977. [Google Scholar] [CrossRef]
- Liang, X.Y.; Zhang, X.Q.; Bai, S.Q.; Huang, L.K.; Luo, X.M.; Ji, Y.; Jiang, L.F. Genetic diversity and relationship of chicory (Cichorium intybus L.) using sequence-related amplified polymorphism markers. Genet. Mol. Res. 2014, 13, 7736–7746. [Google Scholar] [CrossRef] [PubMed]
- Puhlmann, M.L.; de Vos, W.M. Back to the roots: Revisiting the use of the fiber-rich Cichorium intybus L. Taproots. Adv. Nutr. 2020, 11, 878–889. [Google Scholar] [CrossRef] [PubMed]
- Abas, Y.A. Regeneration of Cichorium intybus L. in Tissue Vulture and Secondary Metabolite Analysis. Master’s Thesis, Van Yuzuncu Yil University, Van, Turkey, May 2018. [Google Scholar]
- Al-Snafi, A.E. Medical importance of Cichorium intybus—A review. IOSR J. Pharmasy 2016, 6, 41–56. [Google Scholar]
- Nandagopal, S.; Kumari, B.D.R. Adenine sulphate induced high frequency shoot organogenesis in callus and in vitro flowering of Cichorium intybus L. cv. Focus-a potent medicinal plant. Acta Agric. Slov. 2006, 87, 415–425. [Google Scholar]
- Kumari, B.D.R.; Ranjitha Kumari, B.D.; Nandagopal, S. Phytochemical and Antibacterial Studies of Chicory (Cichorium intybus L.)—A Multipurpose Medicinal Plant. Adv. Biol. Res. 2007, 1, 17–21. [Google Scholar]
- Maroufi, A.; van Bockstaele, E.; de Loose, M. Differential expression of fructan 1-exohydrolase genes involved in inulin biodegradation in chicory (Cichorium intybus) cultivars. Aust. J. Crop Sci. 2012, 6, 1362–1368. [Google Scholar]
- Doliński, R.; Olek, A. Micropropagation of wild chicory (Cichorium intybus L. var. silvestre Bisch.) from leaf explants. Acta Sci. Pol. Cultus 2013, 12, 33–44. [Google Scholar]
- Zahid, A. Transformation and Agrotechnology Strategies for Improved Levels of Secondary Metabolite in Cichorium intybus L.; Jamia Hamdard University: Jamia Hambdar, India, 2012. [Google Scholar]
- Meena, A.; Renu, S. Phytochemical screening of sterols extracted from Cichorium intybus in vivo and in vitro. World J. Pharm. Pharm. Sci. 2014, 3, 1349–1360. [Google Scholar]
- Starmans, D.A.J.; Nijhuis, H.H. Extraction of secondary metabolites from plant material: A review. Trends Food Sci. Technol. 1996, 7, 191–197. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Ibrahim, M.; El-Bahr, M.; Rady, M. In-vitro adventitious root production of Cichorium endivia L. and antioxidants, total phenolic, and total flavonoids assessments. Egypt. Pharm. J. 2019, 18, 216. [Google Scholar] [CrossRef]
- Dreschke, G.; Papirio, S.; Sisinni, D.M.G.; Lens, P.N.L.; Esposito, G. Effect of feed glucose and acetic acid on continuous biohydrogen production by Thermotoga neapolitana. Bioresour. Technol. 2019, 273, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, R.; Irvin, L.M.; Vaidya, B.N.; Dhekney, S.A.; Joshee, N. Optimizing plant regeneration and genetic transformation of Paulownia elongata. Biocatal. Agric. Biotechnol. 2021, 33, 101970. [Google Scholar] [CrossRef]
- Barreiro, K.; Dwivedi, O.P.; Valkonen, S.; Groop, P.H.; Tuomi, T.; Holthofer, H.; Rannikko, A.; Yliperttula, M.; Siljander, P.; Laitinen, S.; et al. Urinary extracellular vesicles: Assessment of pre-analytical variables and development of a quality control with focus on transcriptomic biomarker research. J. Extracell. Vesicles 2021, 10, e12158. [Google Scholar] [CrossRef]
- Eray, N.; Dalar, A.; Turker, M. The effects of abiotic stressors and signal molecules on phenolic composition and antioxidant activities of in vitro regenerated Hypericum perforatum (St. John’s Wort). South African J. Bot. 2020, 133, 253–263. [Google Scholar] [CrossRef]
- Gharari, Z.; Hanachi, P.; Sadeghinia, H.; Walker, T.R. Cichorium intybus bio-callus synthesized silver nanoparticles: A promising antioxidant, antibacterial and anticancer compound. Int. J. Pharm. 2022, 625, 122062. [Google Scholar] [CrossRef]
- Othman, M.; Helmi, L.M.; Hosni, A.M. Effect of growth regulator NAA and IBA applications on total phenolic and flavonoid compounds extracted from in vitro produced callus of chicory plant (Cichorium intybus L.). Arab. Univ. J. Agric. Sci. 2019, 27, 1929–1936. [Google Scholar] [CrossRef]
- Farooq, I.; Qadri, Z.A.; Rather, Z.A.; Nazki, I.T.; Banday, N.; Rafiq, S.; Masoodi, K.Z.; Noureldeen, A.; Mansoor, S. Optimization of an improved, efficient and rapid in vitro micropropagation protocol for Petunia hybrida Vilm. Cv. “Bravo.” Saudi J. Biol. Sci. 2021, 28, 3701–3709. [Google Scholar] [CrossRef]
- Lee, N.W.; Wang, H.Y.; Du, C.L.; Yuan, T.H.; Chen, C.Y.; Yu, C.J.; Chan, C.C. Air-polluted environmental heavy metal exposure increase lung cancer incidence and mortality: A population-based longitudinal cohort study. Sci. Total Environ. 2022, 810, 152186. [Google Scholar] [CrossRef]
- Chavda, J.; Dwsai, B.; Hha, S.; Tandel, M.; Patel, D. Effect of PGR on clonal propagation of tion of madhunashini (Gymnema Sylvestre R. BR) through rooted cutting. An. Int. Q. J. Life Sci. 2015, 10, 1645–1648. [Google Scholar]
- Gâteblé, G.; Pastor, M. Ontogenic stage, auxin type and concentration influence rooting of Oxera sulfurea stem cuttings. In Proceedings of the I International Symposium on the Labiatae: Advances in Production, Biotechnology and Utilisation 723, Sanremo, Italy, 22–25 February 2006; pp. 269–272. [Google Scholar]
- Dalar, A.; Konczak, I. Cichorium intybus from Eastern Anatolia: Phenolic composition, antioxidant and enzyme inhibitory activities. Ind. Crops Prod. 2014, 60, 79–85. [Google Scholar] [CrossRef]
- Innocenti, M.; Gallori, S.; Giaccherini, C.; Ieri, F.; Vincieri, F.F.; Mulinacci, N. Evaluation of the Phenolic Content in the Aerial Parts of Different Varieties of Cichorium intybus L. J. Agric. Food Chem. 2005, 53, 6497–6502. [Google Scholar] [CrossRef] [PubMed]
- Vanzo, A.; Cecotti, R.; Vrhovsek, U.; Torres, A.M.; Mattivi, F.; Passamonti, S. The Fate of trans-Caftaric Acid Administered into the Rat Stomach. J. Agric. Food Chem. 2007, 55, 1604–1611. [Google Scholar] [CrossRef] [PubMed]
- Weisshaar, B.; Jenkins, G.I. Phenylpropanoid biosynthesis and its regulation. Curr. Opin. Plant Biol. 1998, 1, 251–257. [Google Scholar] [CrossRef]
- Dixon’, R.A.; Paiva, N.L. Stress-lnduced Phenylpropanoid Metabolism. Plant Cell. 1995, 7, 1085–1097. [Google Scholar] [CrossRef]
- Lee, J.; Scagel, C.F. Chicoric acid: Chemistry, distribution, and production. Front. Chem. 2013, 1. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).