Morphological and Immunohistochemical Characterization of Bone Structure and Cell–Cell Communication in a Rat Osteoporosis Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Tissue Collection
2.3. Undecalcified Technovit 9100 Embedding
2.4. Masson–Goldner Trichrome Staining
2.5. Decalcification and Paraffin Embedding
2.6. Hematoxylin–Eosin (HE) Staining of the Paraffin Sections
2.7. Picrosirrus Red (PSR) Collagen Staining
2.8. ALP Staining
2.9. TRAP Enzymatic Staining
2.10. Connexin 43 Immunohistochemistry
2.11. Imaging and Figure Reconstruction
2.12. Histomorphometric Analyses
2.13. Statistical Analysis
3. Results
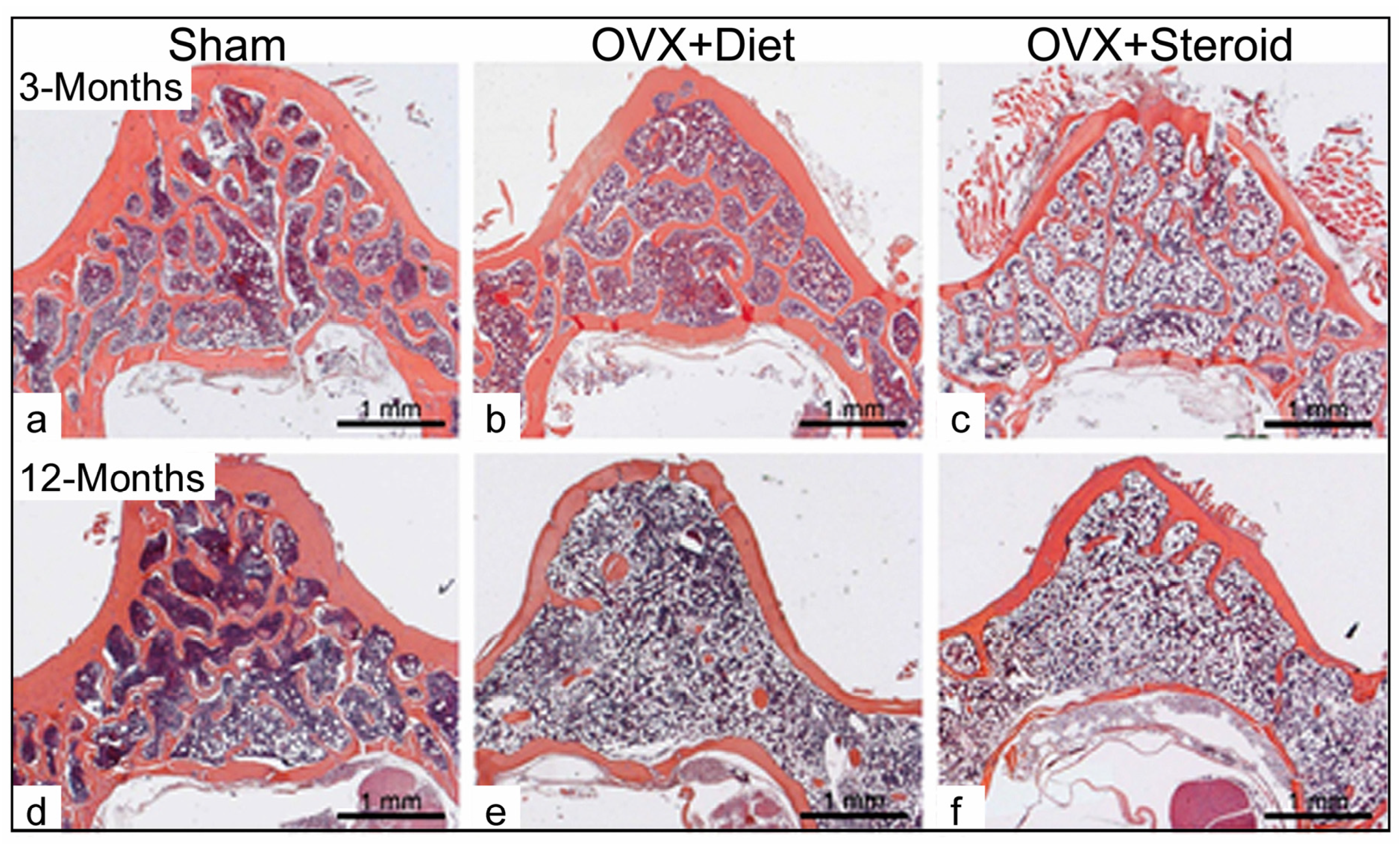
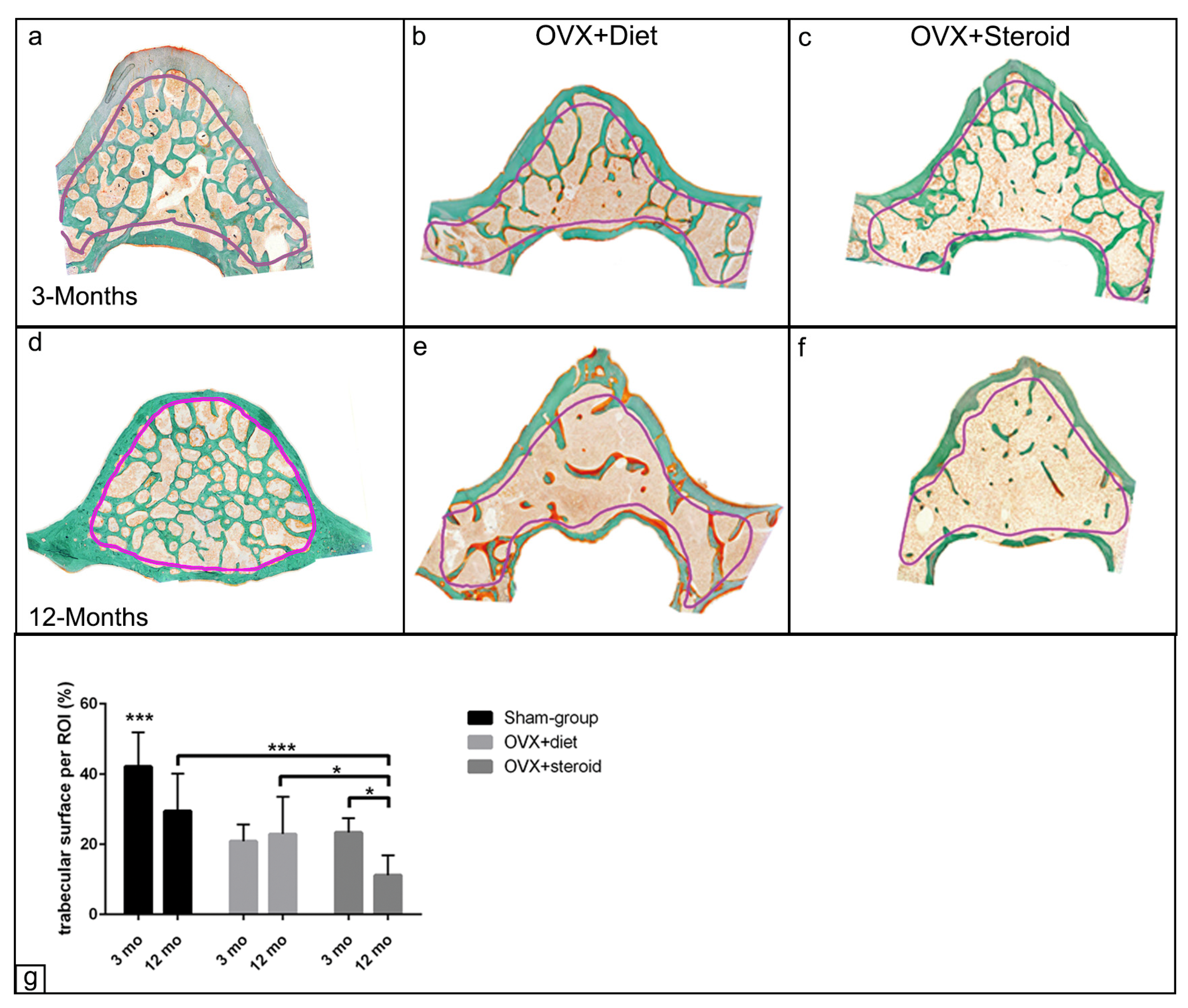
3.1. Alterations in Bone Architecture in the Osteoporotic Rat
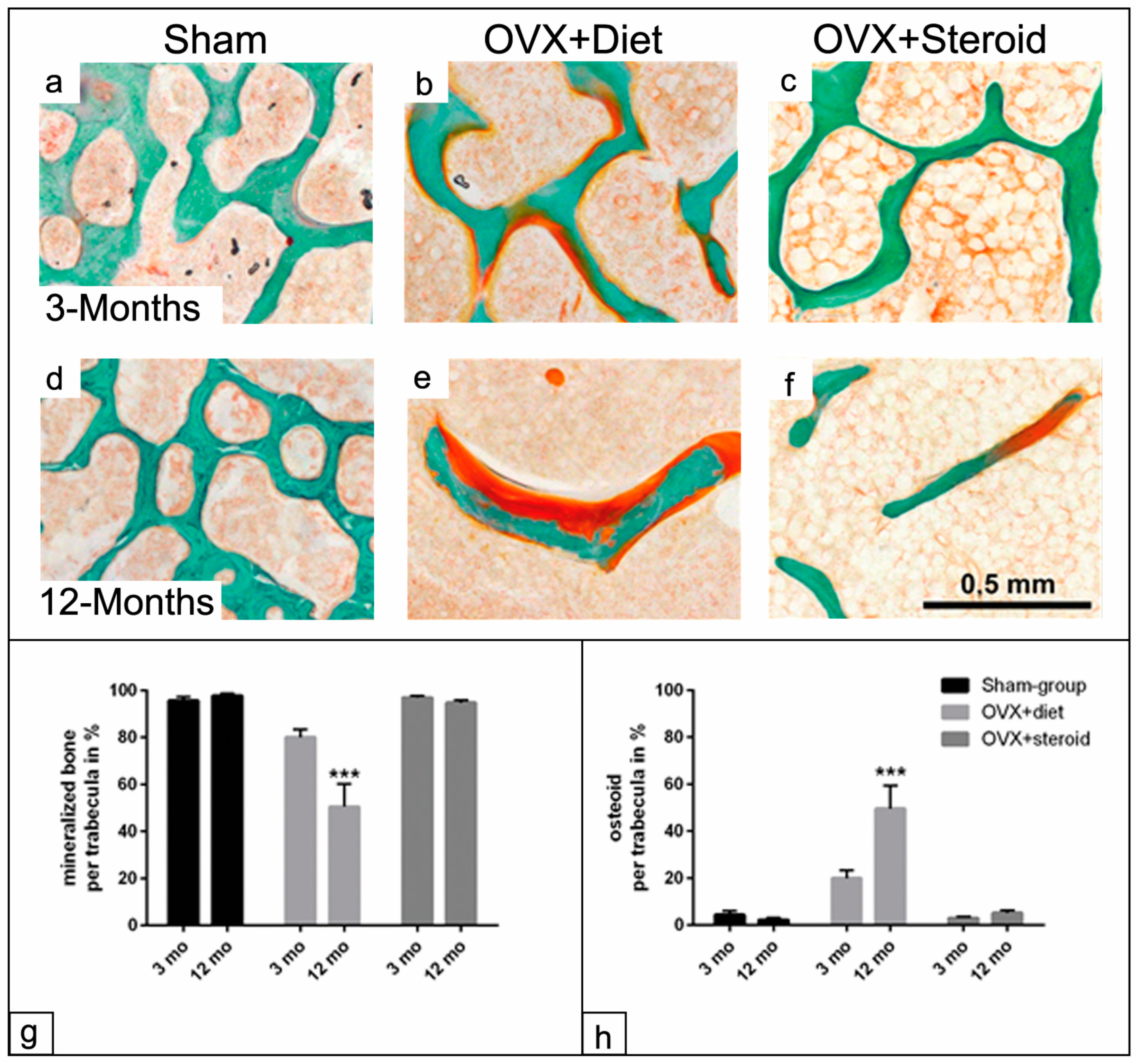
3.2. Mineralization Capacity of the Trabecular Surface in the Osteoporotic Rat
3.3. ALP Enzyme Histochemistry of Osteoblasts
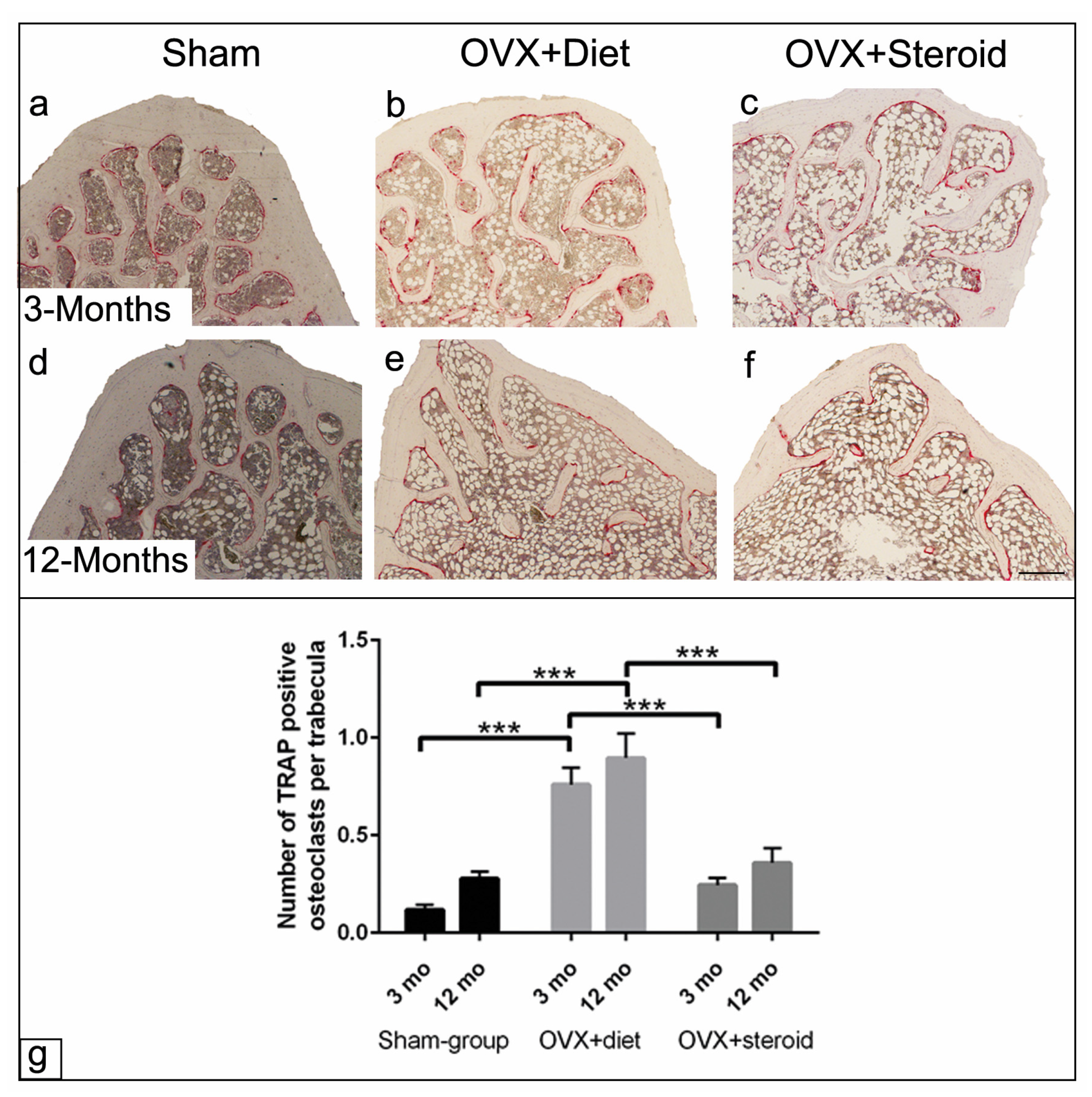
3.4. TRAP Activity of Osteoclasts by Enzyme Histochemistry
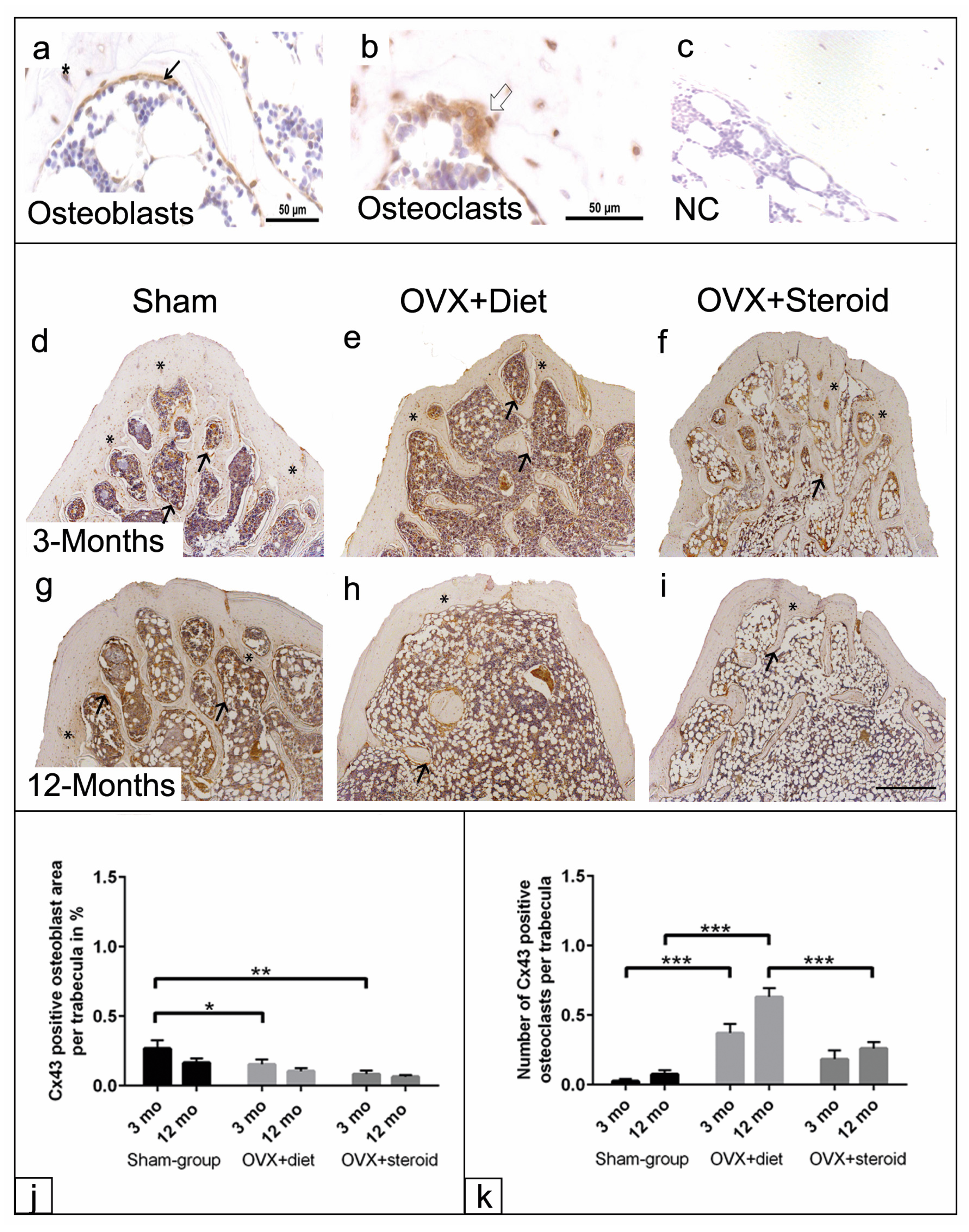
3.5. Cx43 Immunohistochemistry of Osteoblasts and Osteoclasts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zappitelli, T.; Aubin, J.E. The “connexin” between bone cells and skeletal functions. J. Cell. Biochem. 2014, 115, 1646–1658. [Google Scholar] [CrossRef] [PubMed]
- Stains, J.P.; Civitelli, R. Gap junctions in skeletal development and function. Biochim. Biophys. Acta 2005, 1719, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, P. Physiology of Bone. Endocr. Dev. 2015, 28, 33–55. [Google Scholar] [CrossRef]
- Brandi, M.L. Microarchitecture, the key to bone quality. Rheumatology 2009, 48 (Suppl. S4), iv3–iv8. [Google Scholar] [CrossRef] [PubMed]
- Bonucci, E.; Ballanti, P. Osteoporosis-bone remodeling and animal models. Toxicol. Pathol. 2014, 42, 957–969. [Google Scholar] [CrossRef] [PubMed]
- Johnell, O.; Kanis, J.A. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos. Int. 2006, 17, 1726–1733. [Google Scholar] [CrossRef]
- Sanches, D.S.; Pires, C.G.; Fukumasu, H.; Cogliati, B.; Matsuzaki, P.; Chaible, L.M.; Torres, L.N.; Ferrigno, C.R.A.; Dagli, M.L.Z. Expression of connexins in normal and neoplastic canine bone tissue. Vet. Pathol. 2009, 46, 846–859. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, L.I. Connexin 43 and bone: Not just a gap junction protein. Actual. Osteol. 2011, 7, 79–90. [Google Scholar]
- Hu, Z.; Riquelme, M.A.; Gu, S.; Jiang, J.X. Regulation of Connexin Gap Junctions and Hemichannels by Calcium and Calcium Binding Protein Calmodulin. Int. J. Mol. Sci. 2020, 21, 8194. [Google Scholar] [CrossRef]
- Plotkin, L.I. Connexin 43 hemichannels and intracellular signaling in bone cells. Front. Physiol. 2014, 5, 131. [Google Scholar] [CrossRef]
- Riquelme, M.A.; Cardenas, E.R.; Xu, H.; Jiang, J.X. The Role of Connexin Channels in the Response of Mechanical Loading and Unloading of Bone. Int. J. Mol. Sci. 2020, 21, 1146. [Google Scholar] [CrossRef] [PubMed]
- Buo, A.M.; Stains, J.P. Gap junctional regulation of signal transduction in bone cells. FEBS Lett. 2014, 588, 1315–1321. [Google Scholar] [CrossRef]
- Wagner, A.-S.; Glenske, K.; Wolf, V.; Fietz, D.; Mazurek, S.; Hanke, T.; Moritz, A.; Arnhold, S.; Wenisch, S. Osteogenic differentiation capacity of human mesenchymal stromal cells in response to extracellular calcium with special regard to connexin 43. Ann. Anat. 2017, 209, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.-S.; Glenske, K.; Henß, A.; Kruppke, B.; Rößler, S.; Hanke, T.; Moritz, A.; Rohnke, M.; Kressin, M.; Arnhold, S.; et al. Cell behavior of human mesenchymal stromal cells in response to silica/collagen based xerogels and calcium deficient culture conditions. Biomed. Mater. 2017, 12, 45003. [Google Scholar] [CrossRef]
- Lecanda, F.; Warlow, P.M.; Sheikh, S.; Furlan, F.; Steinberg, T.H.; Civitelli, R. Connexin 43 deficiency causes delayed ossification, craniofacial abnormalities, and osteoblast dysfunction. J. Cell Biol. 2000, 151, 931–944. [Google Scholar] [CrossRef] [PubMed]
- Watkins, M.; Grimston, S.K.; Norris, J.Y.; Guillotin, B.; Shaw, A.; Beniash, E.; Civitelli, R. Osteoblast connexin43 modulates skeletal architecture by regulating both arms of bone remodeling. Mol. Biol. Cell 2011, 22, 1240–1251. [Google Scholar] [CrossRef] [PubMed]
- Matemba, S.F.; Lie, A.; Ransjö, M. Regulation of osteoclastogenesis by gap junction communication. J. Cell. Biochem. 2006, 99, 528–537. [Google Scholar] [CrossRef]
- Glenske, K.; Wagner, A.-S.; Hanke, T.; Cavalcanti-Adam, E.A.; Heinemann, S.; Heinemann, C.; Kruppke, B.; Arnhold, S.; Moritz, A.; Schwab, E.H.; et al. Bioactivity of xerogels as modulators of osteoclastogenesis mediated by connexin 43. Biomaterials 2014, 35, 1487–1495. [Google Scholar] [CrossRef]
- Wenisch, S.; Cavalcanti-Adam, E.A.; Tryankowski, E.; Raabe, O.; Kilian, O.; Heiss, C.; Alt, V.; Arnhold, S.; Schnettler, R. Light- and transmission-electron-microscopic investigations on distribution of CD44, connexin 43 and actin cytoskeleton during the foreign body reaction to a nanoparticular hydroxyapatite in mini-pigs. Acta biomaterialia 2012, 8, 2807–2814. [Google Scholar] [CrossRef]
- Herde, K.; Hartmann, S.; Brehm, R.; Kilian, O.; Heiss, C.; Hild, A.; Alt, V.; Bergmann, M.; Schnettler, R.; Wenisch, S. Connexin 43 expression of foreign body giant cells after implantation of nanoparticulate hydroxyapatite. Biomaterials 2007, 28, 4912–4921. [Google Scholar] [CrossRef]
- Batra, N.; Kar, R.; Jiang, J.X. Gap junctions and hemichannels in signal transmission, function and development of bone. Biochim. Biophys. Acta 2012, 1818, 1909–1918. [Google Scholar] [CrossRef] [PubMed]
- Lelovas, P.P.; Xanthos, T.T.; Thoma, S.E.; Lyritis, G.P.; Dontas, I.A. The laboratory rat as an animal model for osteoporosis research. Comp. Med. 2008, 58, 424–430. [Google Scholar]
- Yousefzadeh, N.; Kashfi, K.; Jeddi, S.; Ghasemi, A. Ovariectomized rat model of osteoporosis: A practical guide. EXCLI J. 2020, 19, 89–107. [Google Scholar] [CrossRef] [PubMed]
- El Khassawna, T.; Böcker, W.; Brodsky, K.; Weisweiler, D.; Govindarajan, P.; Kampschulte, M.; Thormann, U.; Henss, A.; Rohnke, M.; Bauer, N.; et al. Impaired extracellular matrix structure resulting from malnutrition in ovariectomized mature rats. Histochem. Cell Biol. 2015, 144, 491–507. [Google Scholar] [CrossRef] [PubMed]
- El Khassawna, T.; Böcker, W.; Govindarajan, P.; Schliefke, N.; Hürter, B.; Kampschulte, M.; Schlewitz, G.; Alt, V.; Lips, K.S.; Faulenbach, M.; et al. Effects of multi-deficiencies-diet on bone parameters of peripheral bone in ovariectomized mature rat. PLoS ONE 2013, 8, e71665. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, P.; Böcker, W.; El Khassawna, T.; Kampschulte, M.; Schlewitz, G.; Huerter, B.; Sommer, U.; Dürselen, L.; Ignatius, A.; Bauer, N.; et al. Bone matrix, cellularity, and structural changes in a rat model with high-turnover osteoporosis induced by combined ovariectomy and a multiple-deficient diet. Am. J. Pathol. 2014, 184, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, P.; Khassawna, T.; Kampschulte, M.; Böcker, W.; Huerter, B.; Dürselen, L.; Faulenbach, M.; Heiss, C. Implications of combined ovariectomy and glucocorticoid (dexamethasone) treatment on mineral, microarchitectural, biomechanical and matrix properties of rat bone. Int. J. Exp. Pathol. 2013, 94, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Goergen, J.; Wenisch, S.; Raabe, O.; Moritz, A.; Schlewitz, G.; Schnettler, R.; Hempel, U.; Heiss, C.; Arnhold, S. Characterization of Bone-Marrow-Derived Stem Cells in Osteoporotic Models of the Rat. ISRN Stem Cells 2013, 2013, 262451. [Google Scholar] [CrossRef]
- Heiss, C.; Govindarajan, P.; Schlewitz, G.; Hemdan, N.Y.A.; Schliefke, N.; Alt, V.; Thormann, U.; Lips, K.S.; Wenisch, S.; Langheinrich, A.C.; et al. Induction of osteoporosis with its influence on osteoporotic determinants and their interrelationships in rats by DEXA. Med. Sci. Monit. 2012, 18, BR199–BR207. [Google Scholar] [CrossRef]
- Donath, K.; Breuner, G. A method for the study of undecalcified bones and teeth with attached soft tissues. The Säge-Schliff (sawing and grinding) technique. J. Oral Pathol. 1982, 11, 318–326. [Google Scholar] [CrossRef]
- Schwartz, A.V. Marrow fat and bone: Review of clinical findings. Front. Endocrinol. 2015, 6, 40. [Google Scholar] [CrossRef]
- Curtis, E.M.; Dennison, E.M.; Cooper, C.; Harvey, N.C. Osteoporosis in 2022: Care gaps to screening and personalised medicine. Best Pract. Res. Clin. Rheumatol. 2022, 36, 101754. [Google Scholar] [CrossRef] [PubMed]
- Gkastaris, K.; Goulis, D.G.; Potoupnis, M.; Anastasilakis, A.D.; Kapetanos, G. Obesity, osteoporosis and bone metabolism. J. Musculoskelet. Neuronal Interact. 2020, 20, 372–381. [Google Scholar] [PubMed]
- Egermann, M.; Goldhahn, J.; Schneider, E. Animal models for fracture treatment in osteoporosis. Osteoporos. Int. 2005, 16 (Suppl. S2), S129–S138. [Google Scholar] [CrossRef]
- Abdelfattah Abulfadle, K.; Refaat Abdelkader Atia, R.; Osama Mohammed, H.; Saad Ramadan, R.; Mohammed, N.A. The potential anti-osteoporotic effect of exercise-induced increased preptin level in ovariectomized rats. Anat. Sci. Int. 2023, 98, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Kalu, D.N. The ovariectomized rat model of postmenopausal bone loss. Bone Miner. 1991, 15, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Takano-Yamamoto, T.; Rodan, G.A. Direct effects of 17 beta-estradiol on trabecular bone in ovariectomized rats. Proc. Natl. Acad. Sci. USA 1990, 87, 2172–2176. [Google Scholar] [CrossRef] [PubMed]
- Wronski, T.J.; Lowry, P.L.; Walsh, C.C.; Ignaszewski, L.A. Skeletal alterations in ovariectomized rats. Calcif. Tissue Int. 1985, 37, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Green, C.R.; Bowles, L.; Crawley, A.; Tickle, C. Expression of the connexin43 gap junctional protein in tissues at the tip of the chick limb bud is related to the epithelial-mesenchymal interactions that mediate morphogenesis. Dev. Biol. 1994, 161, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Lecanda, F.; Towler, D.A.; Ziambaras, K.; Cheng, S.L.; Koval, M.; Steinberg, T.H.; Civitelli, R. Gap junctional communication modulates gene expression in osteoblastic cells. Mol. Biol. Cell 1998, 9, 2249–2258. [Google Scholar] [CrossRef]
- Schiller, P.C.; D’Ippolito, G.; Balkan, W.; Roos, B.A.; Howard, G.A. Gap-junctional communication is required for the maturation process of osteoblastic cells in culture. Bone 2001, 28, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Loiselle, A.E.; Jiang, J.X.; Donahue, H.J. Gap junction and hemichannel functions in osteocytes. Bone 2013, 54, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Grimston, S.; Civitelli, R.; Thomopoulos, S. Deletion of connexin43 in osteoblasts/osteocytes leads to impaired muscle formation in mice. J. Bone Miner. Res. 2015, 30, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Gramsch, B.; Gabriel, H.D.; Wiemann, M.; Grümmer, R.; Winterhager, E.; Bingmann, D.; Schirrmacher, K. Enhancement of connexin 43 expression increases proliferation and differentiation of an osteoblast-like cell line. Exp. Cell Res. 2001, 264, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhou, Z.; Saunders, M.M.; Donahue, H.J. Modulation of connexin43 alters expression of osteoblastic differentiation markers. Am. J. Physiol. Cell Physiol. 2006, 290, C1248-55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Paul, E.M.; Sathyendra, V.; Davison, A.; Sharkey, N.; Bronson, S.; Srinivasan, S.; Gross, T.S.; Donahue, H.J. Enhanced osteoclastic resorption and responsiveness to mechanical load in gap junction deficient bone. PLoS ONE 2011, 6, e23516. [Google Scholar] [CrossRef] [PubMed]
- Bivi, N.; Condon, K.W.; Allen, M.R.; Farlow, N.; Passeri, G.; Brun, L.R.; Rhee, Y.; Bellido, T.; Plotkin, L.I. Cell autonomous requirement of connexin 43 for osteocyte survival: Consequences for endocortical resorption and periosteal bone formation. J. Bone Miner. Res. 2012, 27, 374–389. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, L.I.; Speacht, T.L.; Donahue, H.J. Cx43 and mechanotransduction in bone. Curr. Osteoporos. Rep. 2015, 13, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Kim, M.R.; Noh, J.M.; Kim, S.J.; Ka, S.-O.; Kim, J.H.; Park, B.-H.; Park, J.H. Glucocorticoid Suppresses Connexin 43 Expression by Inhibiting the Akt/mTOR Signaling Pathway in Osteoblasts. Calcif. Tissue Int. 2016, 99, 88–97. [Google Scholar] [CrossRef]
- Gao, J.; Cheng, T.S.; Qin, A.; Pavlos, N.J.; Wang, T.; Song, K.; Wang, Y.; Chen, L.; Zhou, L.; Jiang, Q.; et al. Glucocorticoid impairs cell-cell communication by autophagy-mediated degradation of connexin 43 in osteocytes. Oncotarget 2016, 7, 26966–26978. [Google Scholar] [CrossRef]
- Weinstein, R.S.; Jilka, R.L.; Parfitt, A.M.; Manolagas, S.C. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J. Clin. Investig. 1998, 102, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Henneicke, H.; Gasparini, S.J.; Brennan-Speranza, T.C.; Zhou, H.; Seibel, M.J. Glucocorticoids and bone: Local effects and systemic implications. Trends Endocrinol. Metab. 2014, 25, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.E.; Dai, A.; Tiffee, J.C.; Li, H.H.; Mundy, G.R.; Boyce, B.F. Estrogen promotes apoptosis of murine osteoclasts mediated by TGF-beta. Nat. Med. 1996, 2, 1132–1136. [Google Scholar] [CrossRef] [PubMed]
- Angel, N.Z.; Walsh, N.; Forwood, M.R.; Ostrowski, M.C.; Cassady, A.I.; Hume, D.A. Transgenic mice overexpressing tartrate-resistant acid phosphatase exhibit an increased rate of bone turnover. J. Bone Miner. Res. 2000, 15, 103–110. [Google Scholar] [CrossRef]
- Goodenough, D.A.; Paul, D.L. Beyond the gap: Functions of unpaired connexon channels. Nat. Rev. Mol. Cell Biol. 2003, 4, 285–294. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glenske, K.; Eldaey, A.; Schaalo, S.; Arnhold, S.; Heiss, C.; Schnettler, R.; Wenisch, S.; Elashry, M.I. Morphological and Immunohistochemical Characterization of Bone Structure and Cell–Cell Communication in a Rat Osteoporosis Model. Anatomia 2024, 3, 93-109. https://doi.org/10.3390/anatomia3020008
Glenske K, Eldaey A, Schaalo S, Arnhold S, Heiss C, Schnettler R, Wenisch S, Elashry MI. Morphological and Immunohistochemical Characterization of Bone Structure and Cell–Cell Communication in a Rat Osteoporosis Model. Anatomia. 2024; 3(2):93-109. https://doi.org/10.3390/anatomia3020008
Chicago/Turabian StyleGlenske, Kristina, Asmaa Eldaey, Stephanie Schaalo, Stefan Arnhold, Christian Heiss, Reiner Schnettler, Sabine Wenisch, and Mohamed I. Elashry. 2024. "Morphological and Immunohistochemical Characterization of Bone Structure and Cell–Cell Communication in a Rat Osteoporosis Model" Anatomia 3, no. 2: 93-109. https://doi.org/10.3390/anatomia3020008
APA StyleGlenske, K., Eldaey, A., Schaalo, S., Arnhold, S., Heiss, C., Schnettler, R., Wenisch, S., & Elashry, M. I. (2024). Morphological and Immunohistochemical Characterization of Bone Structure and Cell–Cell Communication in a Rat Osteoporosis Model. Anatomia, 3(2), 93-109. https://doi.org/10.3390/anatomia3020008






