Synergistic Effect of Substance P with Insulin and Insulin-Like Growth Factor-I on Epithelial Migration of the Transformed Human Corneal Epithelial Cells (SV-40)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
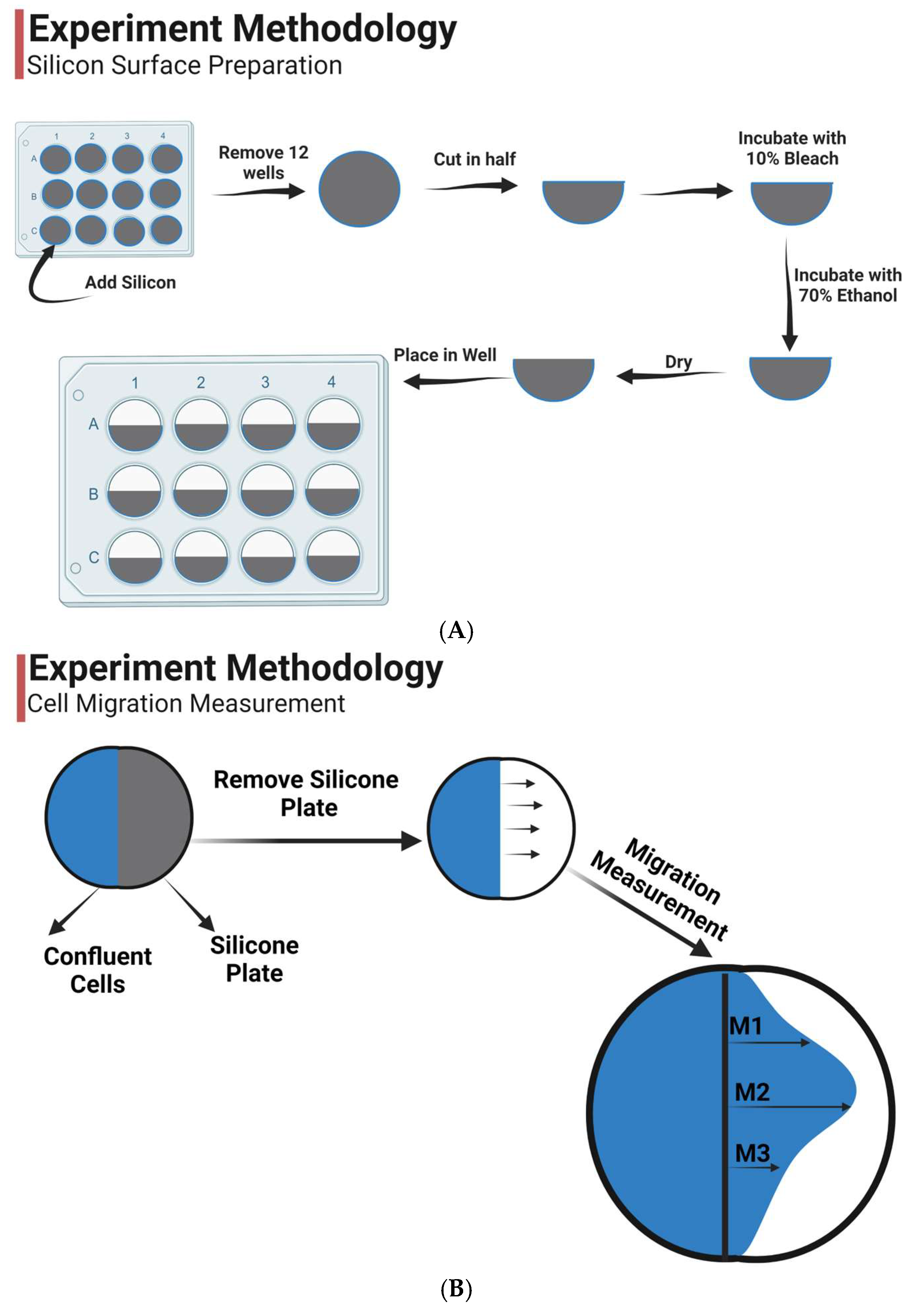
2.2. Preparation
2.3. Epithelial Migration
2.4. Statistical Analysis
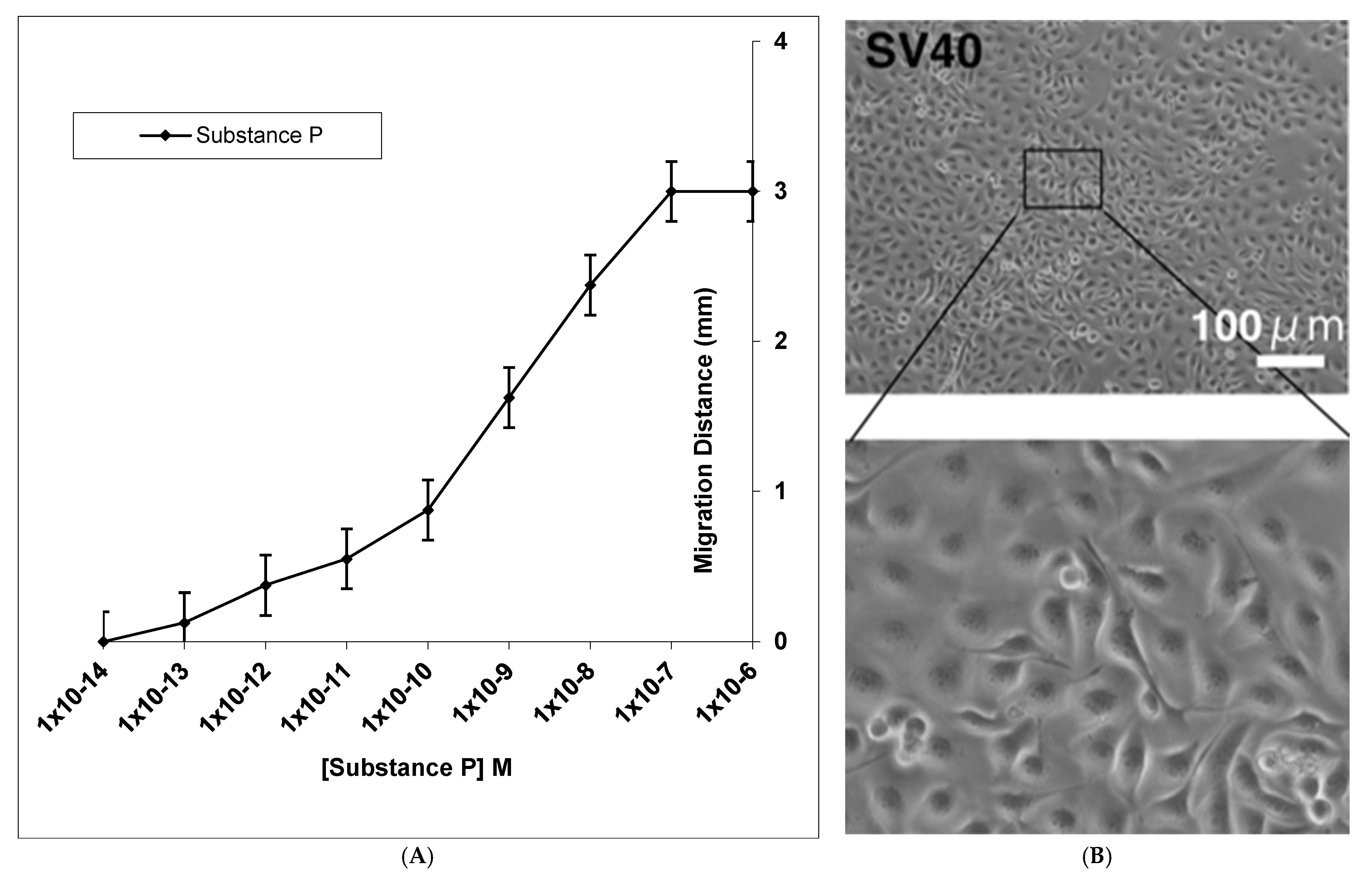
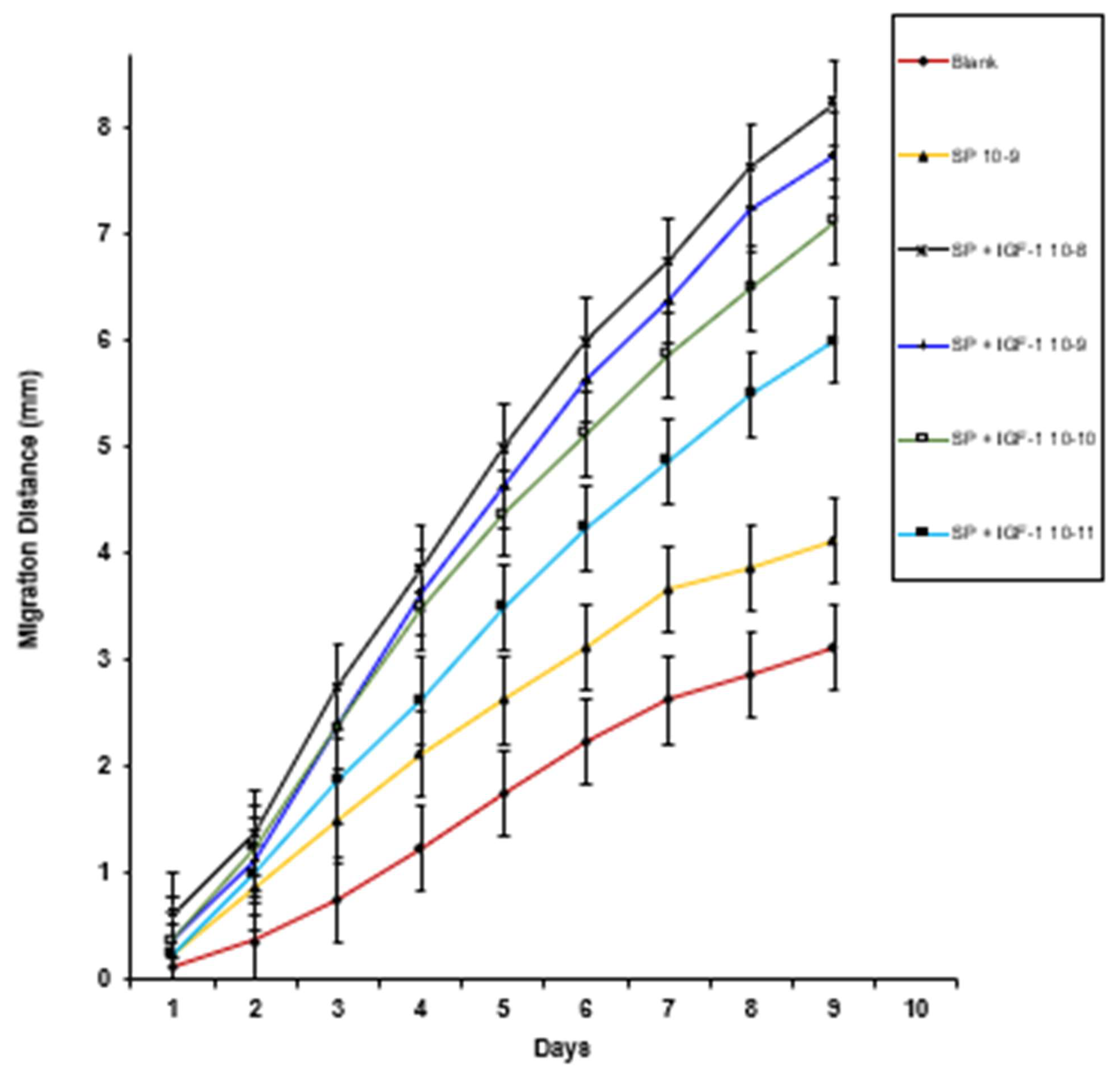
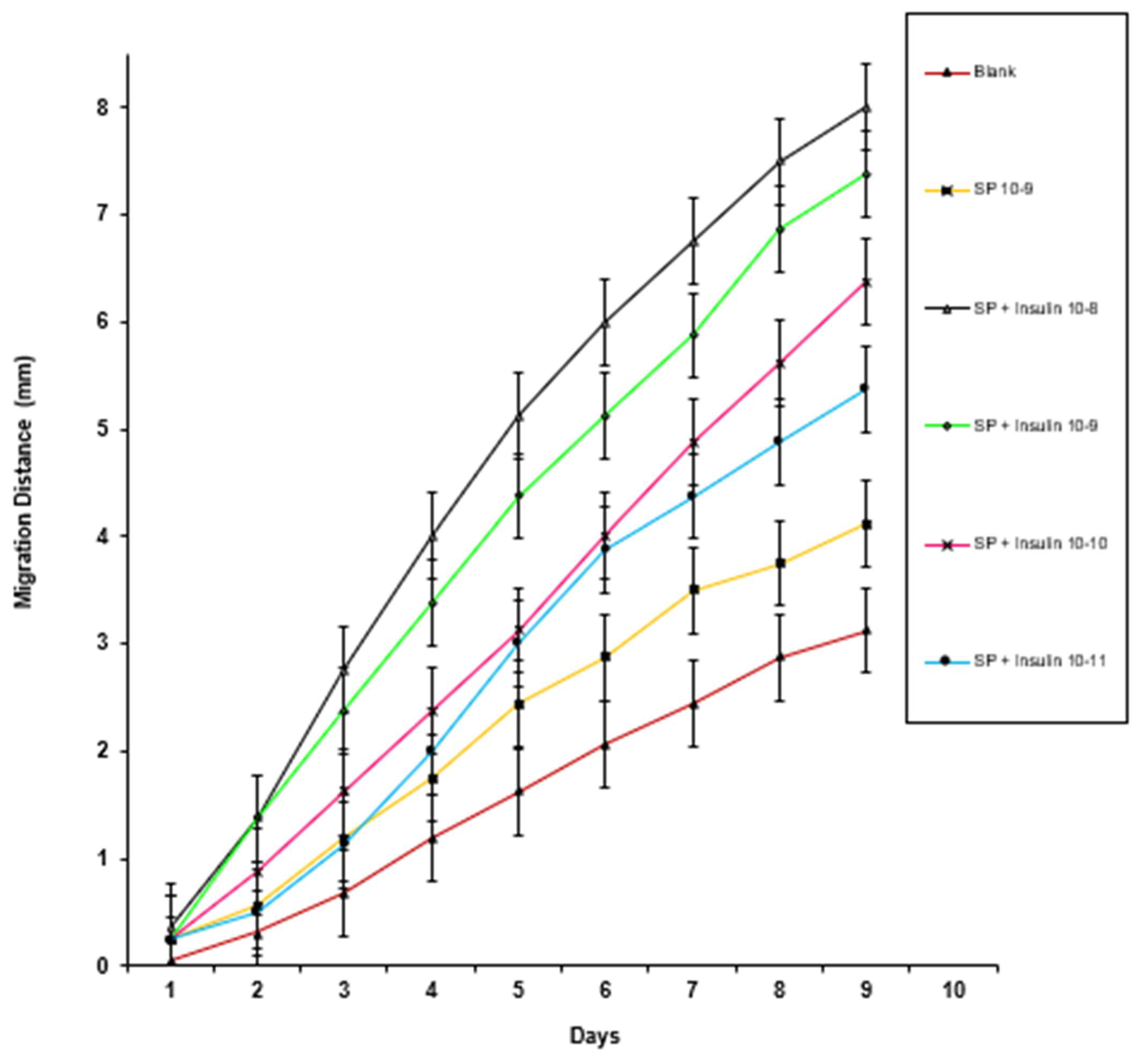
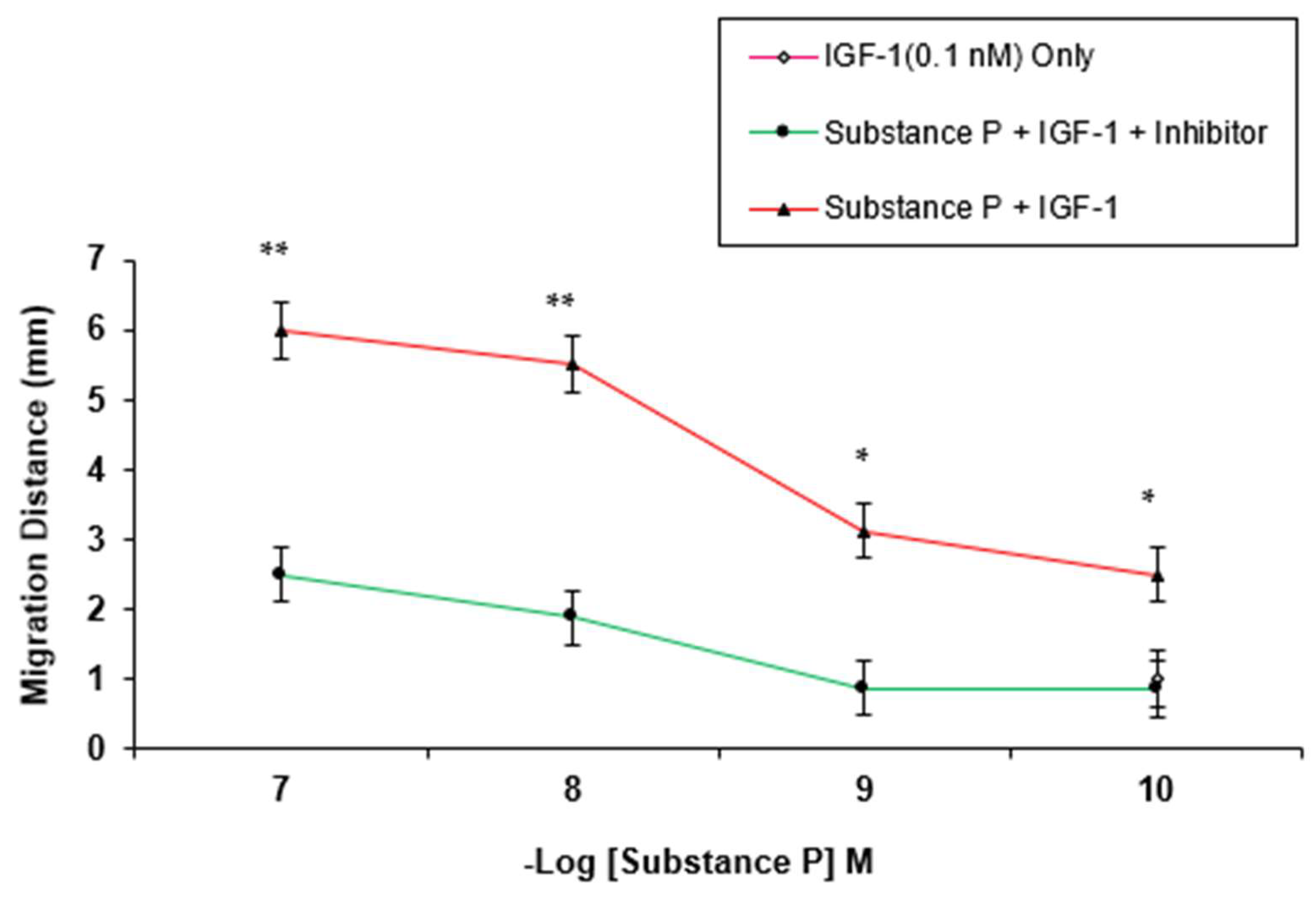
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, R.B.; Naderi, A.; Cho, W.; Ortiz, G.; Musayeva, A.; Dohlman, T.H.; Chen, Y.; Ferrari, G.; Dana, R. Modulating the tachykinin: Role of substance P and neurokinin receptor expression in ocular surface disorders. Ocul. Surf. 2022, 25, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.M.; Leeman, S.E. Isolation of a Sialogogic Peptide from Bovine Hypothalamic Tissue and Its Characterization as Substance P. J. Biol. Chem. 1970, 245, 4784–4790. [Google Scholar] [CrossRef] [PubMed]
- Magendie, F. Pris Entaire de Physiologie/par F. Magendie; Chez Muignon-Marvis: Paris, France, 1825. [Google Scholar]
- Lewis, R.A. Corneal Anesthesia After Percutaneous Radiofrequency Trigeminal Rhizotomy. Arch. Ophthal. 1982, 100, 301. [Google Scholar] [CrossRef]
- Davies, M.S. Corneal anaesthesia after alcohol injection of the trigeminal sensory root. Examination of 100 anaesthetic corneae. Br. J. Ophthal. 1970, 54, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Payne, F. Text-book of Ophthalmology: The Neurology of Vision, Motor and Optical Anomalies, Vol. IV. W. Stewart Duke-Elder. St. Louis, Mo.: C. V. Mosby, 1949. 3473- 4627 pp. $20.00. Science 1950, 111, 433–434. [Google Scholar] [CrossRef]
- Beuerman, R.W.; Schimmelpfennig, B. Sensory denervation of the rabbit cornea affects epithelial properties. Exp. Neurol. 1980, 69, 196–201. [Google Scholar] [CrossRef]
- Cintron, C.; Schneider, H.; Kublin, C. Corneal scar formation. Exp. Eye Res. 1973, 17, 251–259. [Google Scholar] [CrossRef]
- Fitzgerald, G.G.; Cooper, J.R. Acetylcholine as a possible sensory mediator in rabbit corneal epithelium. Biochem. Pharm. 1971, 20, 2741–2748. [Google Scholar] [CrossRef]
- Mackie, R.M.; Parratt, D.; Jenkins, W.M.M. The relationship between immunological parameters and response to therapy in resistant oral candidosis. Br. J. Dermatol. 1978, 98, 343–348. [Google Scholar] [CrossRef]
- Takeya, K.; Mlnamishima, Y.; Amako, K.; Ohnishi, Y. A small rod-shaped pyocin. Virology 1967, 31, 166–168. [Google Scholar] [CrossRef]
- Okada, Y.; Sumioka, T.; Reinach, P.S.; Miyajima, M.; Saika, S. Roles of Epithelial and Mesenchymal TRP Channels in Mediating Inflammatory Fibrosis. Front. Immunol. 2022, 12, 731674. [Google Scholar] [CrossRef]
- Reid, T.W.; Murphy, C.J.; Iwahashi, C.K.; Foster, B.A.; Mannis, M.J. Stimulation of epithelial cell growth by the neuropeptide substance P. J. Cell. Biochem. 1993, 52, 476–485. [Google Scholar] [CrossRef]
- Reid, T.W. Growth control of cornea and lens epithelial cells. Prog. Retin. Eye Res. 1994, 13, 507–554. [Google Scholar] [CrossRef]
- Ofuji, K.; Nakamura, M.; Nishida, T. Signaling regulation for synergistic effects of substance P and insulin-like growth factor-1 or epidermal growth factor on corneal epithelial migration. Jpn. J. Ophthalmol. 2000, 44, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.M.; Lamberts, D.; Reid, T.; Nishida, T.; Murphy, C. Neurotrophic and Anhidrotic Keratopathy Treated With Substance P and Insulinlike Growth Factor 1. Arch. Ophthal. 1997, 115, 926. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.J.; Marfurt, C.F.; McDermott, A.; Bentley, E.; Abrams, G.A.; Reid, T.W.; Campbell, S. Spontaneous chronic corneal epithelial defects (SCCED) in dogs: Clinical features, innervation, and effect of topical SP, with or without IGF-1. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2252–2261. [Google Scholar]
- Nakamura, M.; Ofuji, K.; Chikama, T.; Nishida, T. Combined effects of substance P and insulin-like growth factor-1 on corneal epithelial wound closure of rabbit in vivo. Curr. Eye Res. 1997, 16, 275–278. [Google Scholar] [CrossRef]
- Nakamura, M.; Ofuji, K.; Chikama, T.; Nishida, T. The NK1 receptor and its participation in the synergistic enhancement of corneal epithelial migration by substance P and insulin-like growth factor-1. Br. J. Pharmacol. 1997, 120, 547–552. [Google Scholar] [CrossRef]
- Nakamura, M.; Chikama, T.; Nishida, T. Synergistic effect with Phe-Gly-Leu-Met-NH2 of the C-terminal of substance P and insulin-like growth factor-1 on epithelial wound healing of rabbit cornea. Br. J. Pharmacol. 1999, 127, 489–497. [Google Scholar] [CrossRef]
- Nakamura, M.; Kawahara, M.; Nakata, K.; Nishida, T. Restoration of corneal epithelial barrier function and wound healing by substance P and IGF-1 in rats with capsaicin-induced neurotrophic keratopathy. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2937–2940. [Google Scholar] [CrossRef]
- Yamada, N.; Yanai, R.; Kawamoto, K.; Nagano, T.; Nakamura, M.; Inui, M.; Nishida, T. Promotion of corneal epithelial wound healing by a tetrapeptide (SSSR) derived from IGF-1. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3286–3292. [Google Scholar] [CrossRef] [PubMed]
- Yamada, N.; Yanai, R.; Nakamura, M.; Inui, M.; Nishida, T. Role of the C domain of IGFs in synergistic promotion, with a substance P-derived peptide, of rabbit corneal epithelial wound healing. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Furuya, K.; Wu, T.; Orimoto, A.; Sugano, E.; Tomita, H.; Kiyono, T.; Kurose, T.; Takai, Y.; Fukuda, T. The transcriptome of wild-type and immortalized corneal epithelial cells. Sci. Data 2021, 8, 126. [Google Scholar] [CrossRef] [PubMed]
- Bee, J.A.; Kuhl, U.; Edgar, D.; von der Mark, K. Avian corneal nerves: Co-distribution with collagen type IV and acquisition of substance P immunoreactivity. Investig. Ophthalmol. Vis. Sci. 1988, 29, 101–107. [Google Scholar]
- Miller, A.; Costa, M.; Furness, J.B.; Chubb, I.W. Substance P immunoreactive sensory nerves supply the rat iris and cornea. Neurosci. Lett. 1981, 23, 243–249. [Google Scholar] [CrossRef]
- Bentley, E.; Abrams, G.A.; Covitz, D.; Cook, C.S.; Fischer, C.A.; Hacker, D.; Stuhr, C.M.; Reid, T.W.; Murphy, C.J. Morphology and immunohistochemistry of spontaneous chronic corneal epithelial defects (SCCED) in dogs. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2262–2269. [Google Scholar]
- Kopel, J.; Keshvani, C.; Mitchell, K.; Reid, T. The Activity of Substance P (SP) on the Corneal Epithelium. J. Clin. Transl. Ophthalmol. 2023, 1, 35–51. [Google Scholar] [CrossRef]
- Lynch, D.R.; Snyder, S.H. Neuropeptides: Multiple Molecular Forms, Metabolic Pathways, and Receptors. Ann. Rev. Biochem. 1986, 55, 773–799. [Google Scholar] [CrossRef]
- Nilsson, J.; von Euler, A.M.; Dalsgaard, C.-J. Stimulation of connective tissue cell growth by substance P and substance K. Nature 1985, 315, 61–63. [Google Scholar] [CrossRef]
- Payan, D.G. Receptor-mediated mitogenic effects of substance P on cultured smooth muscle cells. Biochem. Biophys. Res. Commun. 1985, 130, 104–109. [Google Scholar] [CrossRef]
- Payan, P. Biographie de l’auteur. In À L’assaut du Palais; Éditions Universitaires d’Avignon: Avignon, France, 2021; p. 133. [Google Scholar] [CrossRef]
- Ziche, M.; Morbidelli, L.; Pacini, M.; Dolara, P.; Maggi, C.A. NK1-receptors mediate the proliferative response of human fibroblasts to tachykinins. Br. J. Pharm. 1990, 100, 11–14. [Google Scholar] [CrossRef]
- Ziche, M.; Morbidelli, L.; Pacini, M.; Geppetti, P.; Alessandri, G.; Maggi, C.A. Substance P stimulates neovascularization in vivo and proliferation of cultured endothelial cells. Microvasc. Res. 1990, 40, 264–278. [Google Scholar] [CrossRef] [PubMed]
- Lotz, M.; Carson, D.A.; Vaughan, J.H. Substance P Activation of Rheumatoid Synoviocytes: Neural Pathway in Pathogenesis of Arthritis. Science 1987, 235, 893–895. [Google Scholar] [CrossRef] [PubMed]
- Zachary, I.; Woll, P.J.; Rozengurt, E. A role for neuropeptides in the control of cell proliferation. Dev. Biol. 1987, 124, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Sakiyama, T.; Kuwayama, Y.; Ishimoto, I.; Sasaoka, A.; Shiosaka, S.; Tohyama, M.; Manabe, R.; Shiotani, Y. Ontogeny of substance p-containing structures in the ocular tissue of the rat: An immunohistochemical analysis. Dev. Brain Res. 1984, 13, 275–281. [Google Scholar] [CrossRef]
- Sasaoka, A.; Ishimoto, I.; Kuwayama, Y.; Sakiyama, T.; Manabe, R.; Shiosaka, S.; Inagaki, S.; Tohyama, M. Overall distribution of substance P nerves in the rat cornea and their three-dimensional profiles. Investig. Ophthalmol. Vis. Sci. 1984, 25, 351–356. [Google Scholar]
- Marfurt, C.F.; Murphy, C.J.; Florczak, J.L. Morphology and neurochemistry of canine corneal innervation. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2242–2251. [Google Scholar]
- Nakamura, M.; Nishida, T.; Ofuji, K.; Reid, T.W.; Mannis, M.J.; Murphy, C.J. Synergistic effect of substance P with epidermal growth factor on epithelial migration in rabbit cornea. Exp. Eye Res. 1997, 65, 321–329. [Google Scholar] [CrossRef]
- Nishida, T.; Nakamura, M.; Konma, T.; Ofuji, K.; Nagano, K.; Tanaka, T.; Enoki, M.; Reid, T.W.; Brown, S.M.; Murphy, C.J.; et al. Neurotrophic keratopathy—Studies on substance P and the clinical significance of corneal sensation. Nippon Ganka Gakkai Zasshi 1997, 101, 948–974. [Google Scholar]
- Ghiasi, Z.; Gray, T.; Tran, P.; Dubielzig, R.; Murphy, C.; McCartney, D.L.; Reid, T.W. The Effect of Topical Substance-P Plus Insulin-like Growth Factor-1 (IGF-1) on Epithelial Healing After Photorefractive Keratectomy in Rabbits. Trans. Vis. Sci. Technol. 2018, 7, 12. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, P.; Kopel, J.; Reid, T.W. Synergistic Effect of Substance P with Insulin and Insulin-Like Growth Factor-I on Epithelial Migration of the Transformed Human Corneal Epithelial Cells (SV-40). J. Clin. Transl. Ophthalmol. 2023, 1, 79-90. https://doi.org/10.3390/jcto1030010
Tran P, Kopel J, Reid TW. Synergistic Effect of Substance P with Insulin and Insulin-Like Growth Factor-I on Epithelial Migration of the Transformed Human Corneal Epithelial Cells (SV-40). Journal of Clinical & Translational Ophthalmology. 2023; 1(3):79-90. https://doi.org/10.3390/jcto1030010
Chicago/Turabian StyleTran, Phat, Jonathan Kopel, and Ted W. Reid. 2023. "Synergistic Effect of Substance P with Insulin and Insulin-Like Growth Factor-I on Epithelial Migration of the Transformed Human Corneal Epithelial Cells (SV-40)" Journal of Clinical & Translational Ophthalmology 1, no. 3: 79-90. https://doi.org/10.3390/jcto1030010
APA StyleTran, P., Kopel, J., & Reid, T. W. (2023). Synergistic Effect of Substance P with Insulin and Insulin-Like Growth Factor-I on Epithelial Migration of the Transformed Human Corneal Epithelial Cells (SV-40). Journal of Clinical & Translational Ophthalmology, 1(3), 79-90. https://doi.org/10.3390/jcto1030010






