Preparation and In Vitro Testing of Brinzolamide-Loaded Poly Lactic-Co-Glycolic Acid (PLGA) Nanoparticles for Sustained Drug Delivery
Abstract
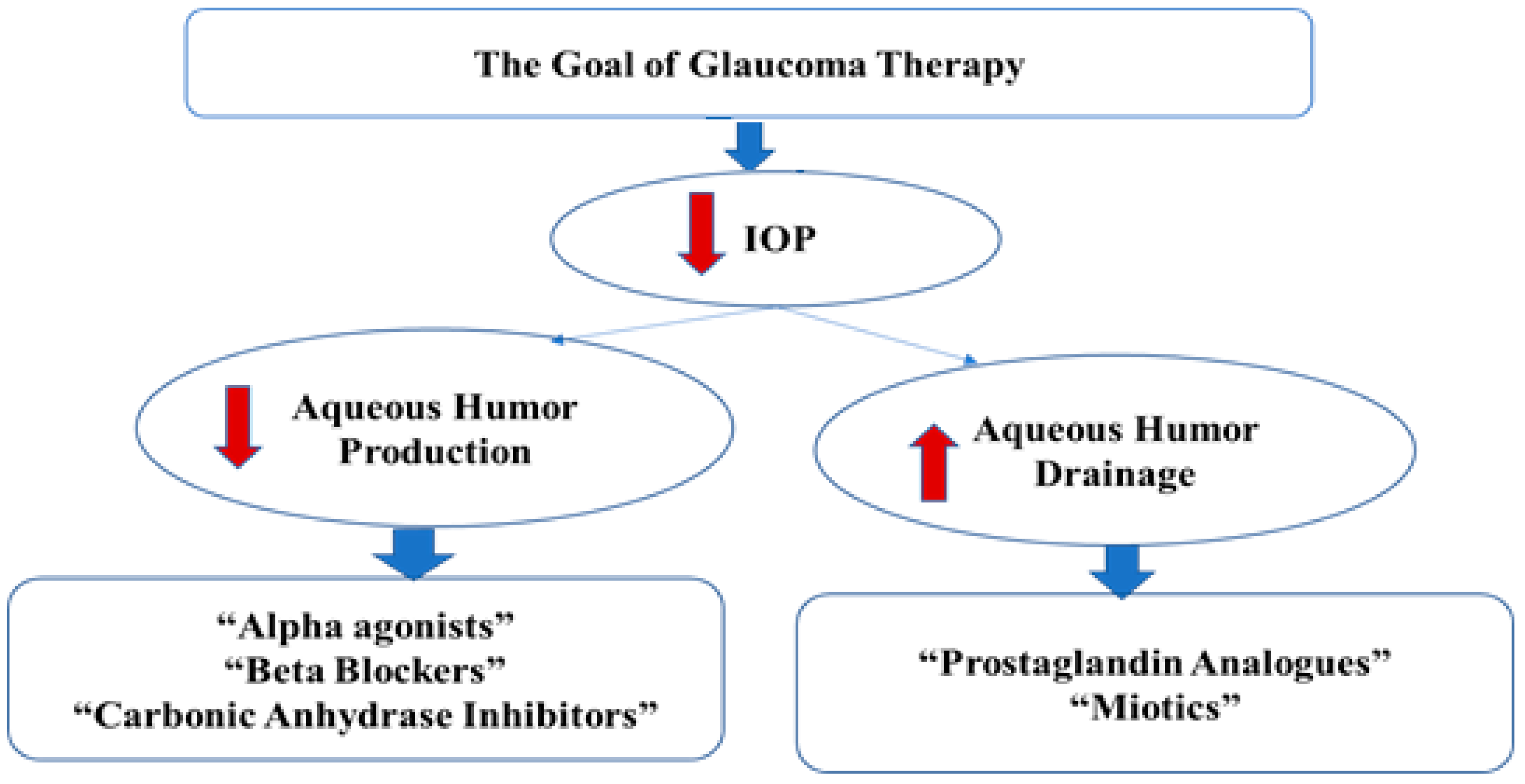
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Blank, Rhodamine-Loaded, and Brinzolamide-Loaded PLGA Nanoparticles
3. Characterization of the Prepared PLGA Nanoparticles
3.1. Particle Size Distribution and Zeta Potential
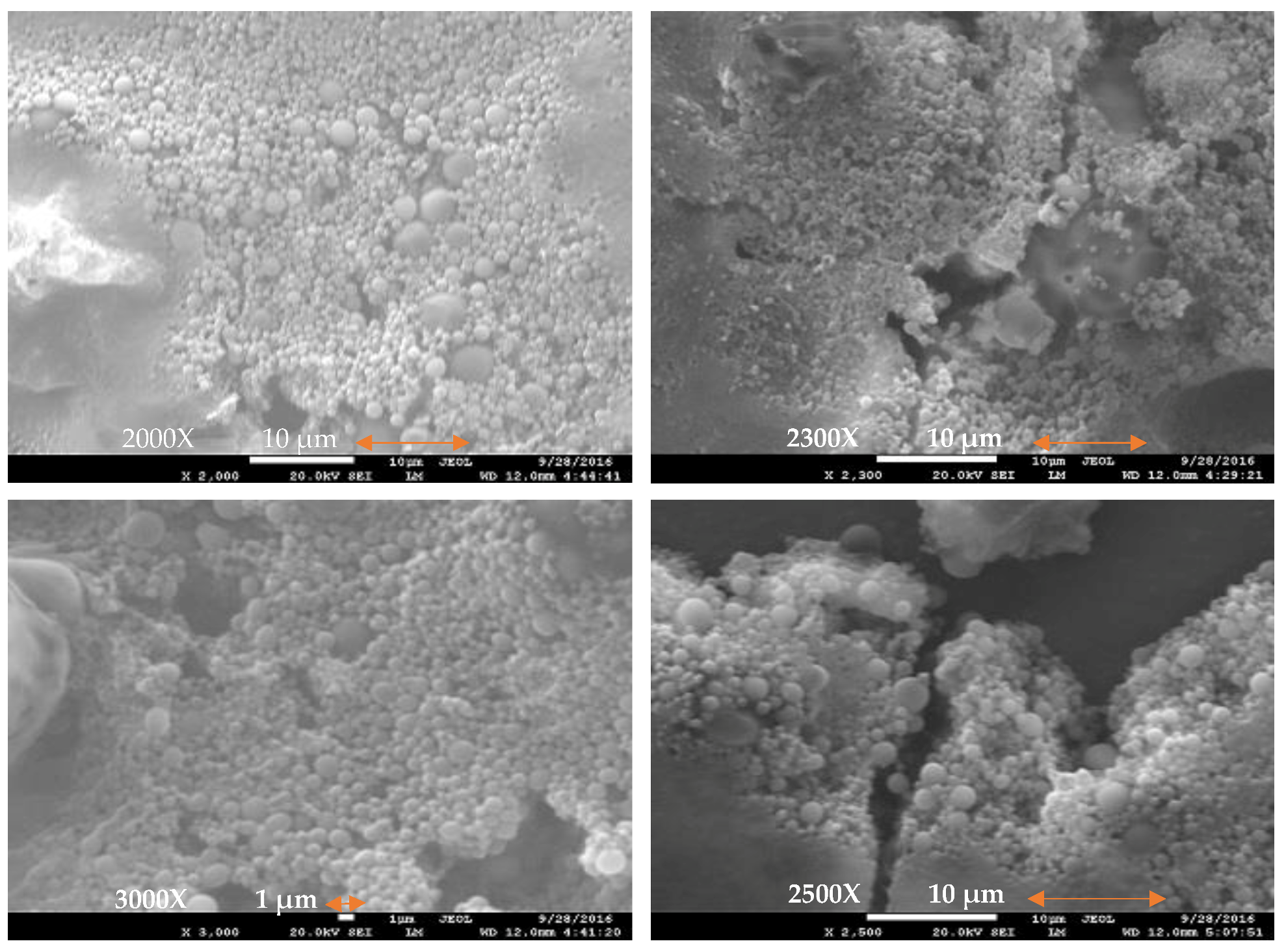
3.2. Scanning Electron Microscopy (SEM)
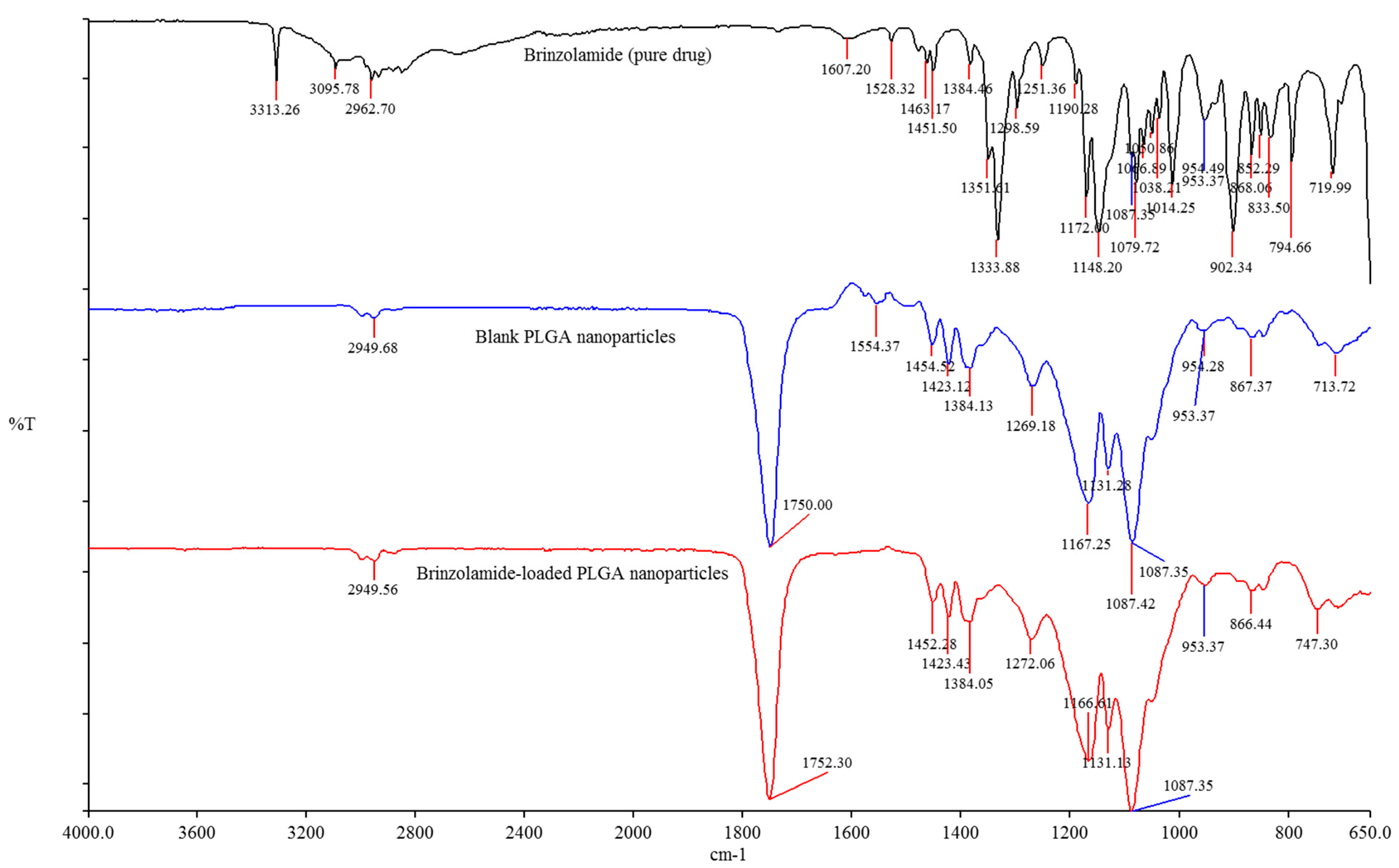
3.3. Fourier Transform Infrared (FTIR) Spectroscopy
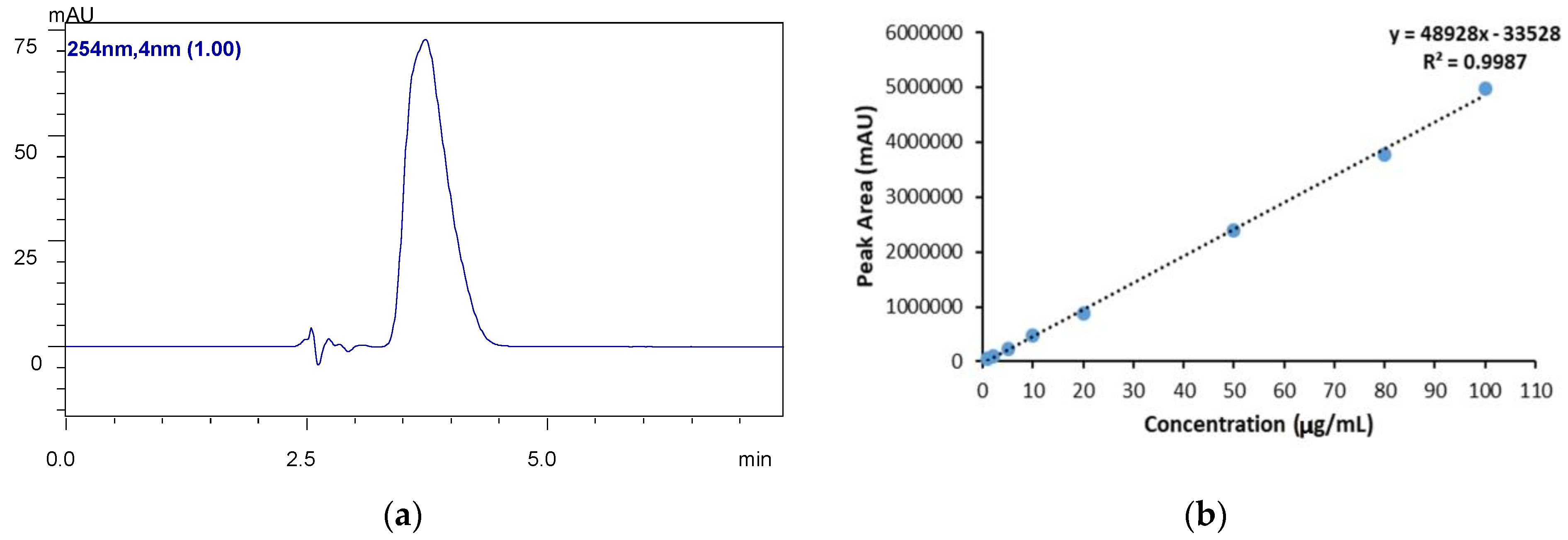
4. HPLC Analysis
5. Drug Loading (%DL) and Encapsulation Efficiency (%EE)
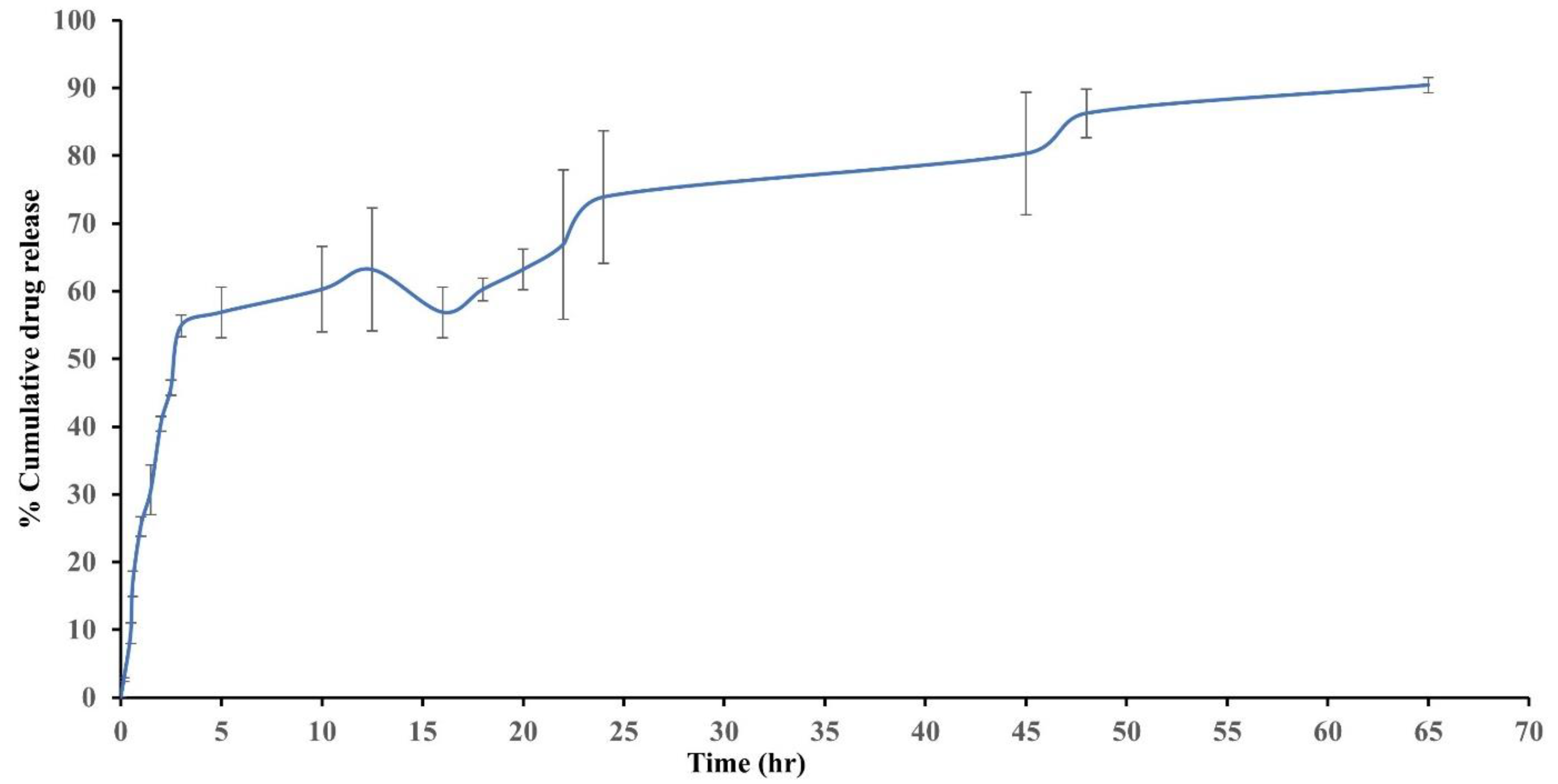
6. In Vitro Drug Release Studies
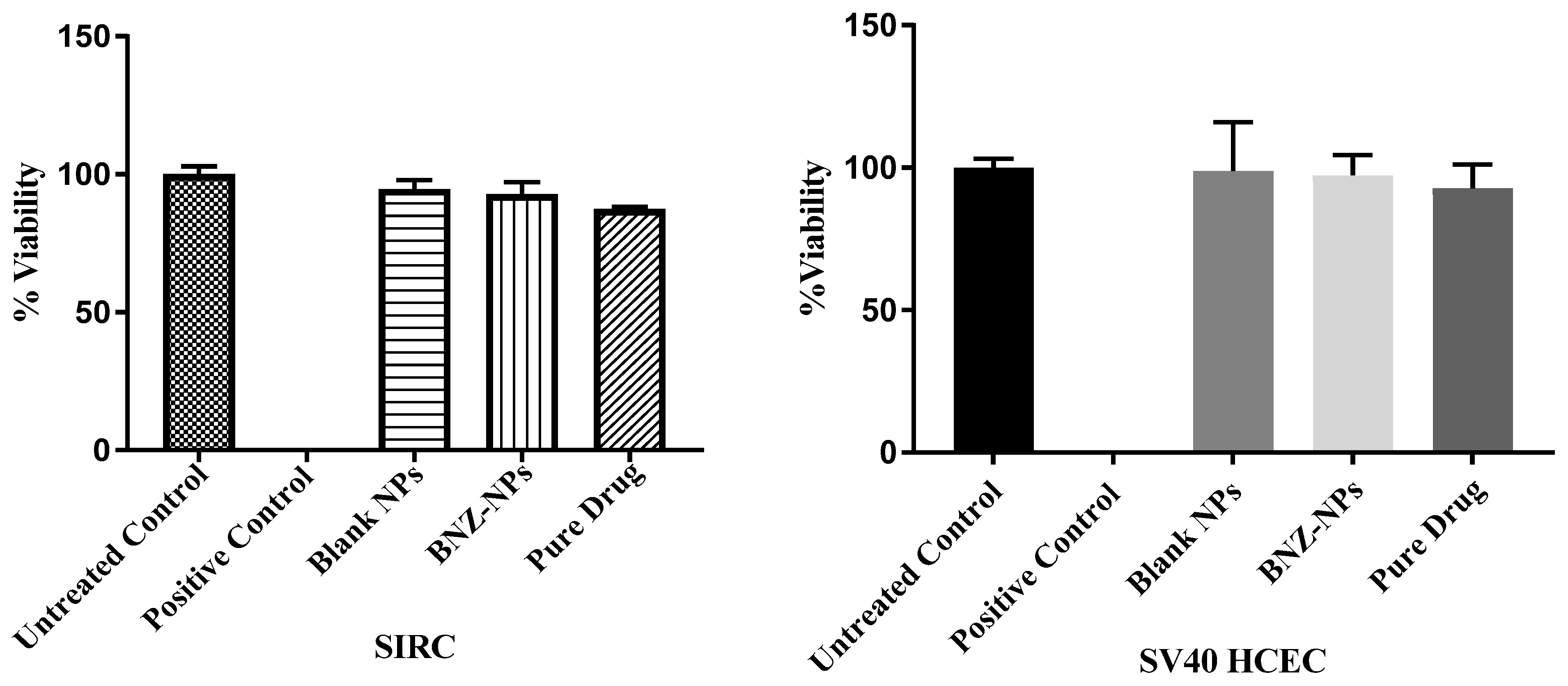
7. Cytotoxicity Studies of the Nanoparticle Formulation
7.1. Cell Cultures
7.2. In Vitro Cytotoxicity Assay
8. In Vitro Intracellular Localization of Nanoparticles
9. Results and Discussion
9.1. Physicochemical Characterization of BNZ-Loaded PLGA Nanoparticles: Particle Size and Zeta Potential Analysis
9.2. SEM and FTIR
9.3. In Vitro Release Study
9.4. Cytotoxicity Study
9.5. Cellular Uptake Study
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Resnikoff, S.; Pascolini, D.; Etya’ale, D.; Kocur, I.; Pararajasegaram, R.; Pokharel, G.P.; Mariotti, S.P. Global data on visual impairment in the year 2002. Bull. World Health Organ. 2004, 82, 844–851. [Google Scholar] [PubMed]
- Glaucoma Research Foundation. Glaucoma Facts and Stats; Glaucoma Research Foundation: San Francisco, CA, USA, 2016. [Google Scholar]
- Migdal, C. What therapy to use in glaucoma? In Ophthalmology, 2nd ed.; Yanoff, M., Duker, J.S., Eds.; Mosby: Boston, MA, USA, 2004; pp. 1539–1543. [Google Scholar]
- Dey, S.; Patel, J.; Anand, B.; Jain-Vakkalagadda, B.; Kaliki, P.; Pal, D.; Ganapathy, V.; Mitra, A. Molecular evidence and functional expression of P-glocoprotein (MDR1) in human and rabbit corneal epithelial cell lines. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2909–2918. [Google Scholar] [CrossRef] [PubMed]
- Lang, J. Ocular drug delivery conventional ocular formulations. Adv. Drug Deliv. Rev. 1995, 16, 39–43. [Google Scholar] [CrossRef]
- Bourlais, C.; Acar, L.; Zia, H.; Sado, P.; Needham, T.; Leverge, R. Ophthalmic drug delivery systems—Recent advances. Prog. Retin. Eye Res. 1998, 17, 33–58. [Google Scholar] [CrossRef] [PubMed]
- Negarsenker, M.; Londhe, V.; Nadkarni, G. Preparation and evaluation of liposomal formulations of tropicamide for ocular delivery. Int. J. Pharm. 1999, 190, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Sieg, J.; Robinson, J. Mechanistic studies on transcorneal penetration of pilocarpine. J. Pharm. Sci. 1976, 65, 1816–1822. [Google Scholar] [CrossRef]
- Gaudana, R.; Ananthula, H.; Parenky, A.; Mitra, A. Ocular drug delivery. AAPS J. 2010, 12, 348–360. [Google Scholar] [CrossRef]
- Urtti, A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1131–1135. [Google Scholar] [CrossRef]
- Lapalus, P.; Moulin, G.; Fredj, D. Kinetics of drugs in ophthalmology. Bull. Soc. D’ophtalmologie Fr. 1985, 1, 31–48. [Google Scholar]
- Schoenwald, R.D. Ocular pharmacokinetics. In Textbook of Ocular Pharmacology; Lippincott-Raven, Zimmerman, T.J., Kooner, K.S., Sharir, M., Fechtner, R.D., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1997; Volume 9, pp. 119–138. [Google Scholar]
- Labetoulle, M.; Frau, E.; Jeunne, C.L. Systemic adverse effects of topical ocular treatments. Presse Med. 2005, 34, 589–595. [Google Scholar] [CrossRef]
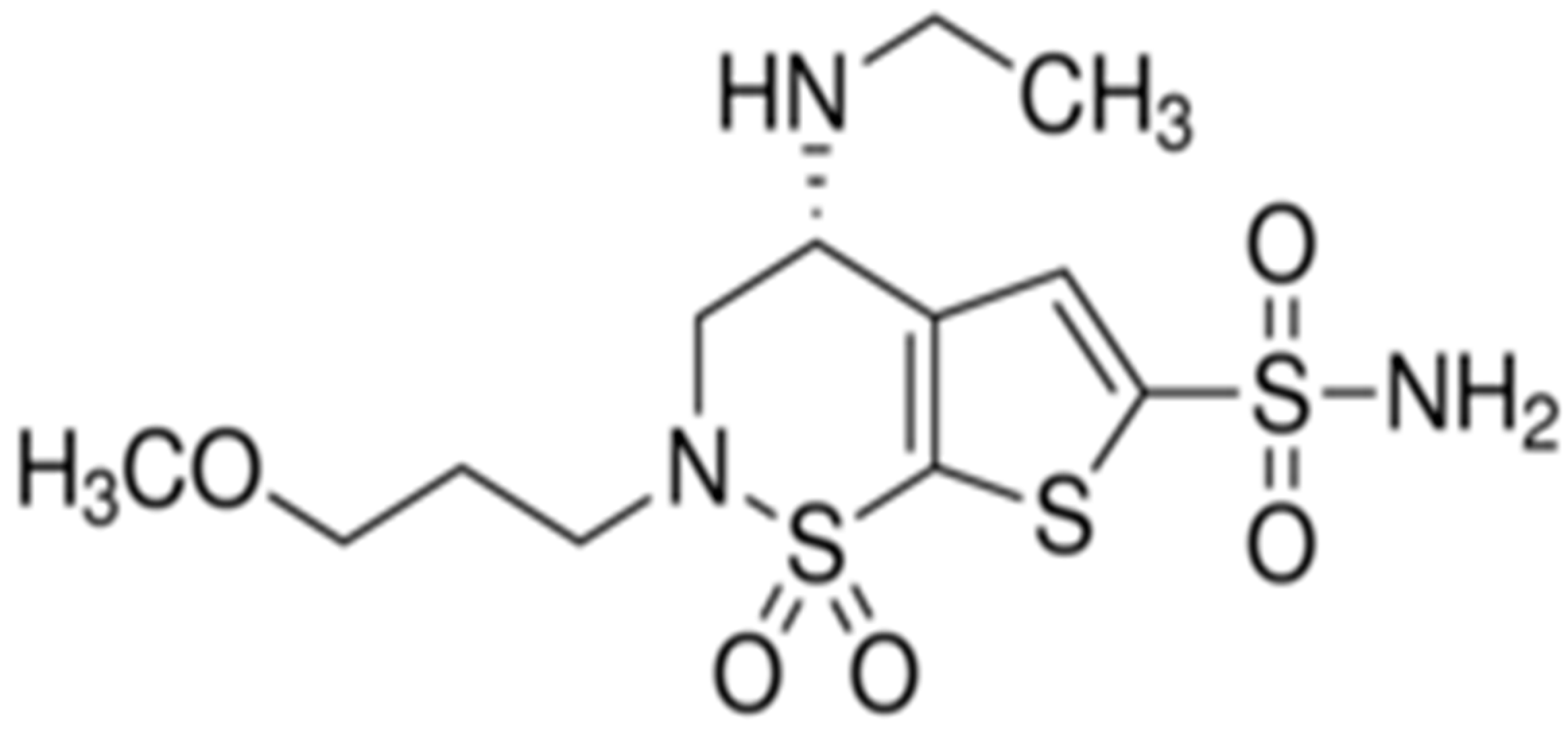
- Iester, M. Brinzolamide ophthalmic suspension: A review of its pharmacology and use in the treatment of open angle glaucoma and ocular hypertension. Clin. Ophthalmol. 2008, 2, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.A.; Higginbotham, E.J. Glaucoma and its treatment: A review. Am. J. Health-Syst. Pharm. 2005, 62, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.H.; Huang, J.Y.; Hung, P.T.; Shieh, J.W.; Chen, Y.F. Ocular hypotensive effect and safety of brinzolamide ophthalmic solution in open angle glaucoma patients. J. Formos. Med. Assoc. 2004, 103, 369–373. [Google Scholar] [PubMed]
- March, W.F.; Ochsner, K.I. The long-term safety and efficacy of brinzolamide 1.0% (azopt) in patients with primary open-angle glaucoma or ocular hypertension. The Brinzolamide Long-Term Therapy Study Group. Am. J. Ophthalmol. 2000, 129, 136–143. [Google Scholar] [CrossRef]
- Sharma, O.P.; Patel, V.; Mehta, T. Nanocrystal for ocular drug delivery: Hope or hype. Drug Deliv. Transl. Res. 2016, 6, 399–413. [Google Scholar] [CrossRef]
- Liu, S.; Jones, L.; Gu, F.X. Nanomaterials for ocular drug delivery. Macromol. Biosci. 2012, 12, 608–620. [Google Scholar] [CrossRef]
- USP29—NF24, Brinzolamide. Available online: http://www.pharmacopeia.cn/v29240/usp29nf24s0_m10206.html (accessed on 20 March 2016).
- Marques, M.; Loebenberg, R.; Almukainzi, M. Simulated Biological Fluids with Possible Application in Dissolution Testing. Dissolution Technol. 2011, 18, 15–28. [Google Scholar] [CrossRef]
- Toropainen, E.; Ranta, V.; Vellonen, K.; Palmgren, J.; Talvitie, A.; Laavola, M.; Suhonen, P.; Hamalainen, K.M.; Auriola, S.; Urtti, A. Paracellular and passive transcellular permeability in immortalized human corneal epithelial cell culture model. Eur. J. Pharm. Sci. 2003, 20, 99–106. [Google Scholar] [CrossRef]
- Nagai, N.; Ono, H.; Hashino, M.; Ito, Y.; Okamoto, N.; Shimomura, Y. Improved corneal toxicity and permeability of tranilast by the preparation of ophthalmic formulations containing its nanoparticles. J. Oleo Sci. 2014, 63, 177–186. [Google Scholar] [CrossRef]
- Gupta, H.; Aqil, M.; Khar, R.K.; Ali, A.; Bhatnagar, A.; Mittal, G. Sparfloxacin-loaded PLGA nanoparticles for sustained ocular drug delivery. Nanomedicine 2010, 6, 324–333. [Google Scholar] [CrossRef]
- Guinedi, A.S.; Mortada, N.D.; Mansour, S.; Hathout, R.M. Preparation and evaluation of reverse-phase evaporation and multilamellar niosomes as ophthalmic carriers of acetazolamide. Int. J. Pharm. 2005, 306, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Patravale, V.B.; Date, A.A.; Kulkarni, R.M. Nanosuspension: A promising drug delivery strategy. J. Pharm. Pharmacol. 2004, 56, 827–840. [Google Scholar] [CrossRef] [PubMed]
- Sahana, D.K.; Mittal, G.; Bhardwaj, V.; Kumar, M.N. PLGA nanoparticles for oral delivery of hydrophobic drugs: Influence of organic solvent on nanoparticle formation and release behavior in vitro and in vivo using estradiol as a model drug. J. Pharm. Sci. 2008, 97, 1530–1542. [Google Scholar] [CrossRef] [PubMed]
- Görner, T.; Gref, R.; Michenot, D.; Sommer, F.; Tran, M.N.; Dellacherie, E. Lidocaine-loaded biodegradable nanospheres. I. Optimization Of the drug incorporation into the polymer matrix. J. Control. Release 1999, 57, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Biswal, I.; Dinda, A. ’ Mohanty, S.; Dhara, M.; Das, D.; Chowdary, K.A.; Si, S. Influence of Drug/Polymer Ratio on the Encapsulation Efficiency of Highly Hydrophilic Drug. Asian J. Chem. 2011, 23, 1973–1978. [Google Scholar]
- Pavanveena, C.; Kavitha, K.; Anil, K.S.N. Formulation and evaluation of trimetazidine hydrochloride loaded chitosan microspheres. Int. J. Appl. Pharm. 2010, 2, 11–14. [Google Scholar]
- Park, J.H.; Jeong, H.; Hong, J.; Chang, M.; Kim, M.; Roy, S.; Chuck, R.S.; Lee, J.K.; Park, C.Y. The Effect of Silica Nanoparticles on Human Corneal Epithelial Cells. Sci. Rep. 2016, 6, 37762. [Google Scholar] [CrossRef]
- Heurtault, B.; Saulnier, P.; Pech, B.; Proust, J.E.; Benoit, J.P. Physico-chemical stability of colloidal lipid particles. Biomaterials 2003, 24, 4283–4300. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Zhang, Y.; Fang, D.; Xu, B.; Zhang, L.; Chen, T.; Ren, K.; Nie, Y.; Yao, S.; et al. Liposomes as a Novel Ocular Delivery System for Brinzolamide: In Vitro and In Vivo Studies. AAPS PharmSciTech 2016, 17, 710–717. [Google Scholar] [CrossRef]
- Marques, D.R.; Dos Santos, L.A. Analysis of Poly(Lactic-co-Glycolic Acid)/Poly(Isoprene) Polymeric Blend for Application as Biomaterial. Polímeros 2013, 23, 579–584. [Google Scholar] [CrossRef]
- Hines, D.J.; Kaplan, D.L. Poly (lactic-co-glycolic acid) controlled release systems: Experimental and modeling insights. Crit. Rev. Ther. Drug Carr. Syst. 2013, 30, 257–276. [Google Scholar] [CrossRef] [PubMed]
- Chiaraviglio, L.; Kirby, J.E. Evaluation of Impermeant, DNA-Binding Dye Fluorescence as a Real-Time Readout of Eukaryotic Cell Toxicity in a High Throughput Screening Format. ASSAY Drug Dev. Technol. 2014, 12, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Li, J.; Wu, L.; Wang, B.; Wang, Z.; Xu, Q.; Xin, H. Ophthalmic delivery of brinzolamide by liquid crystalline nanoparticles: In vitro and in vivo evaluation. AAPS PharmSciTech 2013, 14, 1063–1071. [Google Scholar] [CrossRef]
- Chakole, C.M.; Sahoo, P.K.; Pandey, J.; Chauhan, M.K. A green chemistry approach towards synthesizing hydrogel for sustained ocular delivery of brinzolamide: In vitro and ex vivo evaluation. J. Indian Chem. Soc. 2022, 99, 100323. [Google Scholar] [CrossRef]
- Anwar, M.; Muhammad, F.; Akhtar, B. Biodegradable nanoparticles as drug delivery devices. J. Drug Deliv. Sci. Technol. 2021, 64, 102638. [Google Scholar] [CrossRef]
- Rodrigo, M.; Garcia-Herrranz, D.; Aragon-Navas, A.; Subias, A.; Martinez-Rincon, T.; Mendez-Martinez, S.; Cardiel, M.J.; Garcia-Fejjoo, J.; Ruberte, J.; Herrero-Vanrell, R.; et al. Long-term corticosteroid-induced chronic glaucoma model produced by intracameral injection of dexamethasone-loaded PLGA microspheres. Drug Deliv. 2021, 28, 2427–2446. [Google Scholar] [CrossRef]
- Yang, F.; Cabe, M.; Nowak, H.A.; Langert, K.A. Chitosan/poly(lactic-co-glycolic)acid Nanoparticle Formulations with Finely-Tuned Size Distributions for Enhanced Mucoadhesion. Pharmaceutics 2022, 14, 95. [Google Scholar] [CrossRef]
- Shao, X.; Wei, Z.; Song, X.; Hao, L.; Cai, X.; Zhang, Z.; Peng, Q.; Lin, Y. Independent effect of polymeric nanoparticle zeta potential/surface charge, on their cytotoxicity and affinity to cells. Cell Prolif. 2015, 48, 465–474. [Google Scholar] [CrossRef]

| Formulation | PLGA 50:50 (mg) | Drug (mg) | Solvent (5 mL) | Surfactant | Particle Size (nm) | Polydispersity |
|---|---|---|---|---|---|---|
| F1 | 100 | - | Ethyl acetate | 0.8% PVA | 271.1 ± 4.3 ** | 0.112 ± 0.027 * |
| F2 | 100 | - | Ethyl acetate | 1.0% F-68 | 254.9 ± 2.9 ** | 0.107 ± 0.018 * |
| F3 | 100 | - | Ethyl acetate | 1.2% F-68 | 297.7 ± 12.8 ** | 0.117 ± 0.051 * |
| F4 | 100 | - | Ethyl acetate | 1.0% PVA | 327.8 ± 10.9 ** | 0.325 ± 0.012 ** |
| F5 | 100 | - | Dichloromethane | 2.0% PVA | 483.1 ± 27.9 * | 0.247 ± 0.043 ** |
| F6 | 100 | - | Ethyl acetate | 1.2% F-68/1.0% PVA | 271.7 ± 1.8 ** | 0.107 ± 0.012 * |
| F7 | 100 | - | Ethyl acetate | 1.0% PVA/1.2% F-68 | 276.1 ± 2.9 ** | 0.167 ± 0.021 ** |
| F8 | 100 | - | Ethyl acetate | 2.5% PVA | 310.3 ± 3.8 ** | 0.167 ± 0.024 ** |
| F9 | 100 | - | Ethyl acetate | 1.5% PVA | 284.9 ± 3.9 ** | 0.134 ± 0.018 ** |
| F10 | 100 | - | Ethyl acetate | 1.5% F-68 | 208.7 ± 3.4 | 0.075 ± 0.012 |
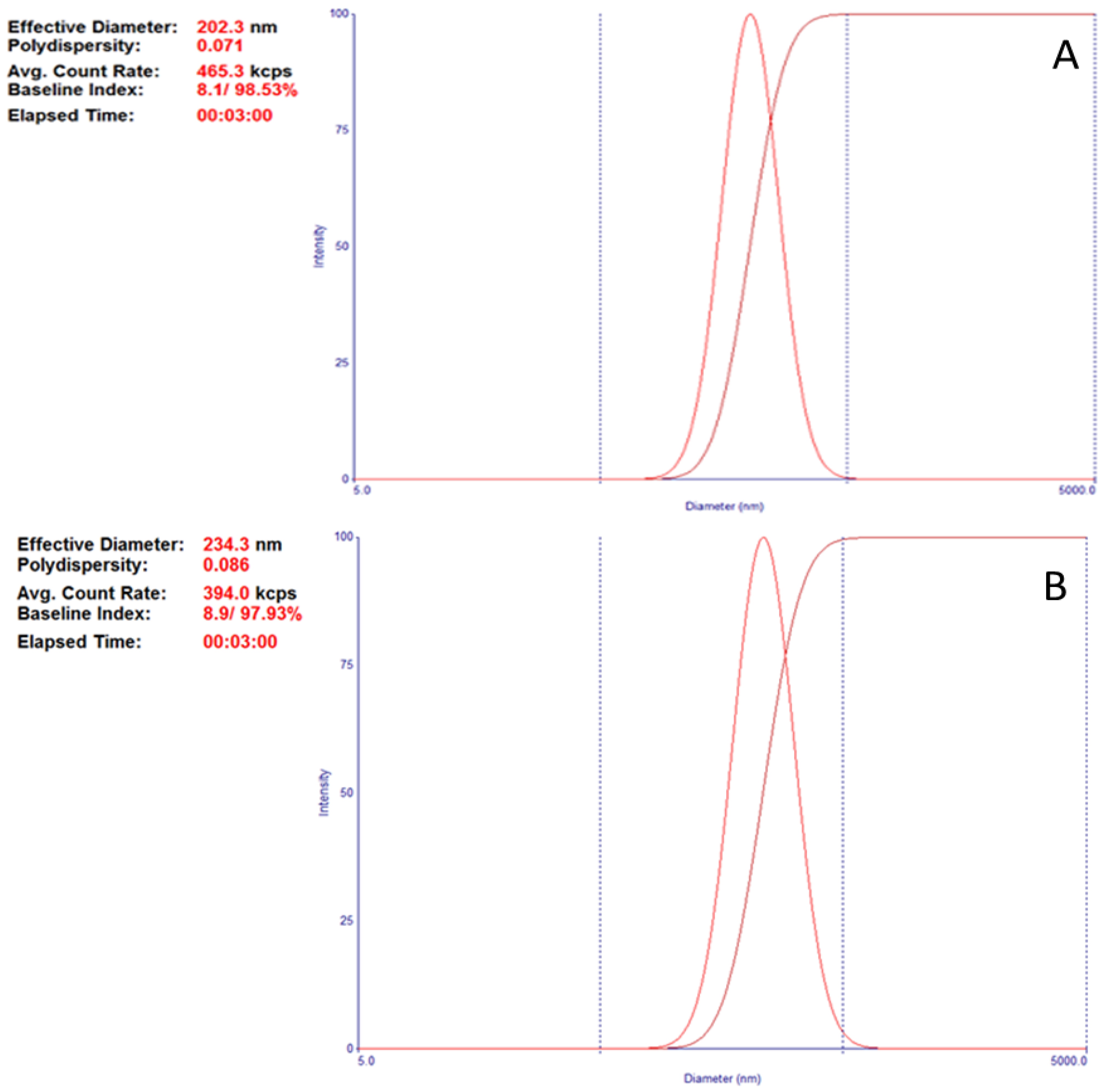
| F10B | 200 | - | Ethyl acetate | 1.5% F-68 | 202.3 ± 2.9 | 0.071 ± 0.032 |
| BNZ001 | 200 | 20 | Ethyl acetate | 1.5% F-68 | 234.3 ± 6.8 ** | 0.089 ± 0.028* |
| BNZ002 | 200 | 10 | Ethyl acetate | 1.5% F-68 | 129.6 ± 1.5 | 0.108 ± 0.047 |
| BNZ003 | 200 | 20 | Ethyl acetate | 1.5% PVA | 350.9 ± 3.4 ** | 0.113 ± 0.018 ** |
| BNZ004 | 200 | 30 | Dichloromethane | 1.5% F-68 | 303.3 ± 4.9 ** | 0.158 ± 0.004 ** |
| BNZ005 | 150 | 30 | Ethyl acetate | 1.5% F-68 | 130.7 ± 1.1 | 0.120 ± 0.012 |
| Rho123-NP1 | 200 | 1.5 mg Rho123 | Ethyl acetate | 1.5% F-68 | 195.2 ± 1.4 | 0.099 ± 0.017 |
| Formulation | Drug:Polymer | Surfactant | Zeta Potential (mV) | EE (%) | DL (%) |
|---|---|---|---|---|---|
| BNZ001 | 1:10 | 1.5% F-68 | −35.21 ± 1.52 ** | 64.65 | 12.93 |
| BNZ002 | 1:20 | 1.5% F-68 | −37.98 ± 1.39 ** | 74.18 | 7.42 |
| BNZ003 | 1:10 | 1.5% PVA | −33.12 ± 1.67 ** | 68.53 | 13.71 |
| BNZ004 | 1:7 | 1.5% F-68 | −40.73 ± 0.53 ** | 52.80 | 15.84 |
| BNZ005 | 1:5 | 1.5% F-68 | −38.57 ± 1.02 ** | 38.93 | 11.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ako-Adounvo, A.-M.; Karla, P.K. Preparation and In Vitro Testing of Brinzolamide-Loaded Poly Lactic-Co-Glycolic Acid (PLGA) Nanoparticles for Sustained Drug Delivery. J. Clin. Transl. Ophthalmol. 2024, 2, 1-14. https://doi.org/10.3390/jcto2010001
Ako-Adounvo A-M, Karla PK. Preparation and In Vitro Testing of Brinzolamide-Loaded Poly Lactic-Co-Glycolic Acid (PLGA) Nanoparticles for Sustained Drug Delivery. Journal of Clinical & Translational Ophthalmology. 2024; 2(1):1-14. https://doi.org/10.3390/jcto2010001
Chicago/Turabian StyleAko-Adounvo, Ann-Marie, and Pradeep K. Karla. 2024. "Preparation and In Vitro Testing of Brinzolamide-Loaded Poly Lactic-Co-Glycolic Acid (PLGA) Nanoparticles for Sustained Drug Delivery" Journal of Clinical & Translational Ophthalmology 2, no. 1: 1-14. https://doi.org/10.3390/jcto2010001
APA StyleAko-Adounvo, A.-M., & Karla, P. K. (2024). Preparation and In Vitro Testing of Brinzolamide-Loaded Poly Lactic-Co-Glycolic Acid (PLGA) Nanoparticles for Sustained Drug Delivery. Journal of Clinical & Translational Ophthalmology, 2(1), 1-14. https://doi.org/10.3390/jcto2010001






