Abstract
With increasing space exploration, there is a rising need to evaluate the impact of spaceflight on astronauts’ health, including the effects of space-associated hazards such as microgravity. Astronauts’ reports of experienced symptoms upon spaceflight include a notable prevalence of dry eye disease (DED). Hence, there is a pressing need to understand the pathogenesis and mechanism behind space-associated DED onset, which will subsequently guide the development of necessary therapies to reduce dry eye symptoms among astronauts. One critical effect of spaceflight includes alterations to the gut microbiome. On Earth, the prior literature has established the presence of an ocular surface–gut axis and the potential role of gut dysbiosis in DED onset. Meanwhile, the literature about astronauts’ health underscores the presence of space-associated gut microbiome composition alterations and the presence of DED separately. Therefore, in this opinion article, we review and present the current literature regarding the ocular surface–gut axis on Earth and regarding potential translations to spaceflight. We present the view that, based on the existing literature, the ocular surface–gut axis may be a critical mechanism for the pathogenesis of DED in space, and this axis needs to be further explored in the context of identifying ways to reduce astronauts’ experiences of DED during spaceflight.
1. Introduction
Dry eye disease (DED) is common in human beings on Earth and may occur in up to 30% of astronauts on the International Space Station (ISS) [1]. In post-mission surveys, 15.7% of astronauts flying on both ISS and space shuttle missions reported symptoms of DED [2]. Commonly reported symptoms among astronauts include eye irritation, foreign body sensation, conjunctival injection, eye burning, eye pain, blurry vision, tear film insufficiency, and eye strain [1,2]. Terrestrial DED is a multifactorial, inflammatory, and cyclical condition that results in symptoms (e.g., itching, burning, stinging, and foreign body sensations) and signs (e.g., corneal epithelial erosions, staining with vital dyes, increased tear break up time, and tear film changes) [2,3]. Similar to the terrestrial impacts, DED can be uncomfortable for astronauts and may negatively impact their quality of life, productivity, performance, and mental state [2,3]. During space travel, astronauts are exposed to a vast number of potential hazards (Figure 1), such as radiation, microgravity, closed environments, confinement, isolation, and loneliness [4]. Additionally, vestibular alterations and space motion sickness may occur from fluid shifts in microgravity [4]. One notable space-associated outcome includes inflight alterations to the gut microbiome composition [5]. In this article, we review the ocular surface–gut axis on Earth and in spaceflight as well as the potential implications of intestinal alterations in microgravity on the tear film and DED in space. We opine that the ocular surface–gut axis is a salient mechanism that may be involved in the pathogenesis of space-associated DED onset and that needs to be further explored in light of continuously increasing space travel.
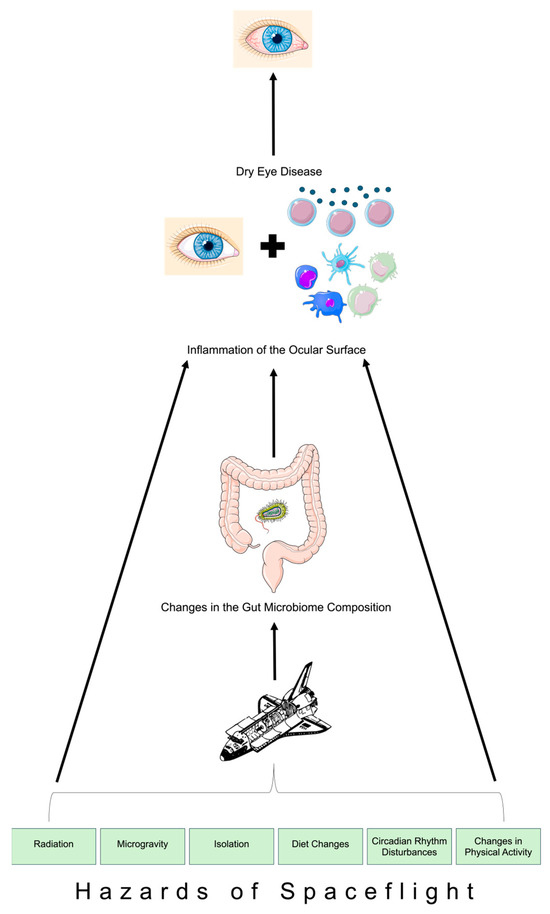
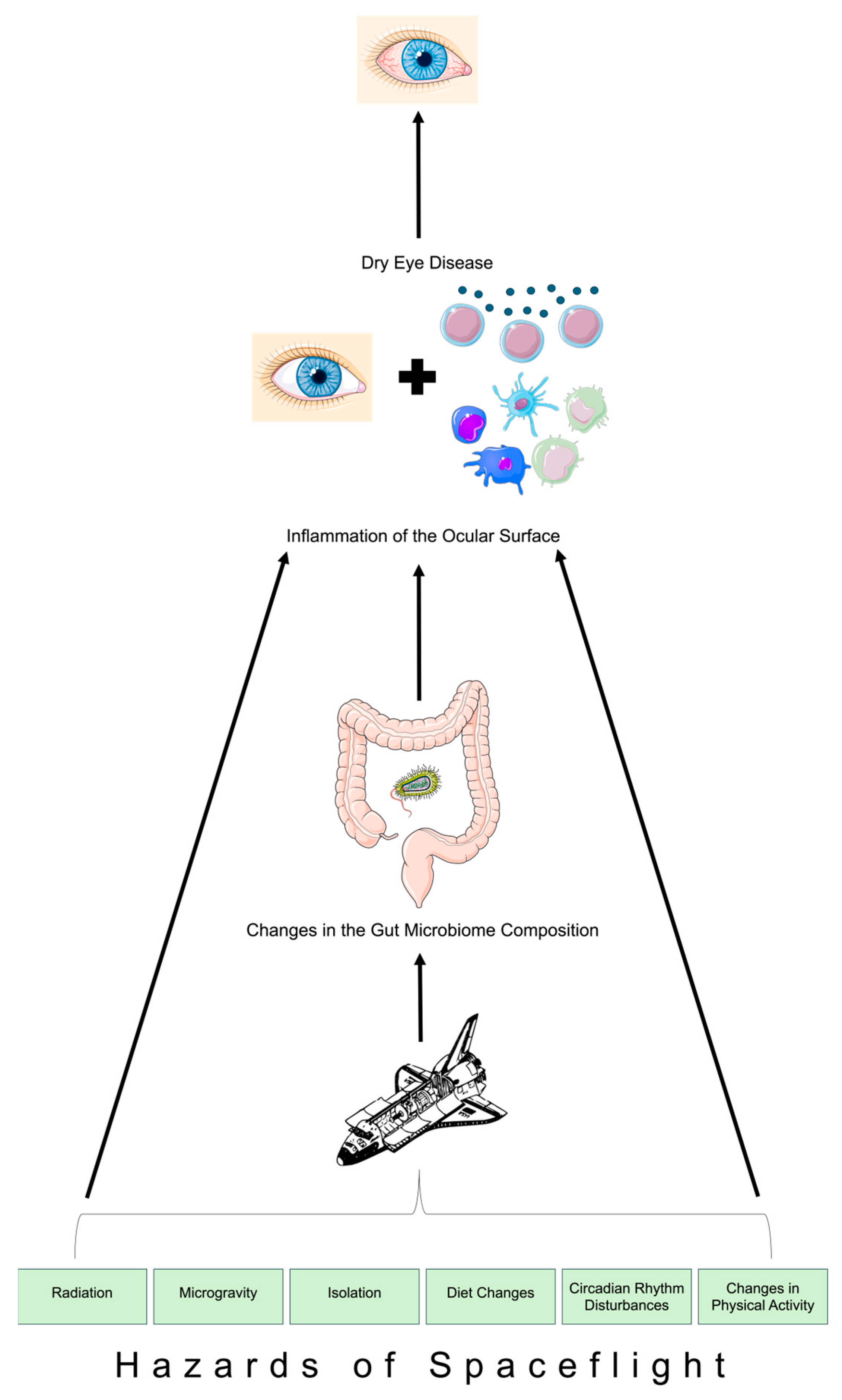
Figure 1.
Schematic depiction of the ocular surface–gut axis in spaceflight. Parts of Figure 1 were created using unmodified art from Servier Medical Art under a Creative Commons Attribution 4.0 License (https://creativecommons.org/licenses/by/4.0/) and Clkr [6] (accessed on 3 March 2024).
2. Methods
In this article, we queried two databases (PubMed and Google Scholar) with the following keywords separately and in various combinations: Gut Microbiome, Gut Dysbiosis, Ocular Surface, Ocular Surface–Gut Axis Dry Eye Disease, Cornea, Microgravity, Spaceflight, Astronaut, and Space Ophthalmology. From the search results, peer-reviewed sources in the English language were eligible for inclusion in this article. The search was not excluded based on year of publication; however, the publication dates of the included sources in this paper range from 2013 to 2024. We reviewed 30 sources that appeared in both PubMed and Google Scholar as well as one additional source that was found in Google Scholar alone. Thus, a total of 31 sources were reviewed, which are included in this paper.
3. Gut Microbiome
The gut microbiome includes about 100 trillion bacterial cells and over 1000 bacterial species, and crosstalk between the host and the microbes of the gut microbiome is essential for the development and maturation of the host’s immune responses [7,8]. The mutualistic relationship between the human host and inhabiting gut bacterial cells arises from other host-serving bacterial functions as well, such as involvement in nutrition and protection from colonization of pathogenic organisms [7]. In light of the mutualistic relationship between the host and gut microbiome, gut dysbiosis, or a disruption to the gut microbiome composition, is a critical inciting factor for subsequent disease development [7]. The reduction in microbiome diversity, loss of commensal bacteria, and increase in pathogenic bacteria contribute to an imbalance between protective bacteria and harmful pathogens residing in the gut [7,8]. Subsequently, alterations to the gut microbiome composition may negatively impact normal metabolic processes, thus giving rise to disease [8]. As a result, it is essential to identify causative factors for gut dysbiosis, both on Earth and in space.
4. Dry Eye Disease (DED)
DED may be categorized into multiple subtypes, including aqueous-deficient, evaporative, and mixed forms of dry eye [9]. Aqueous-deficient DED stems from reduced or absent tear production, which may be attributed to lacrimal gland insult or dysfunction (e.g., Sjögren syndrome) [9]. Meanwhile, evaporative DED arises from disproportionately greater tear evaporation rates (e.g., meibomian gland dysfunction, blinking rate reduction, contact lens use, or allergies) with normal lacrimal gland function [9]. Additionally, there is a mixed form of dry eye that commonly presents as a combination of both the aqueous-deficient and evaporative forms of DED [9,10].
Regardless of the type of DED, the pathophysiology of DED often begins with hyperosmolarity of tears from either a reduced volume of produced tears or higher evaporation rates, which then activates inflammatory cascades, leading to corneal tear film and corneal epithelium disruption [9]. Additional outcomes of ocular surface inflammation include apoptosis of goblet cells and lacrimal gland dysfunction [11]. Subsequently, inflammatory processes (e.g., activation of MAP kinase and NF-kB pathways) can result in secondary inflammatory cytokine production (e.g., MMP-9, TNF-α, IL-1α, and IL-1β) and thus secondary DED [9]. When inflammation of the lacrimal gland is a causative factor, inflammatory factors from lacrimal glands may enter tears and reach the ocular surface, leading to increased inflammation of the ocular surface and subsequent associated DED [9].
The evaporative form of DED is the most prevalent type of DED [9]. Notably, one causative factor for evaporative DED is meibomian gland dysfunction, which includes hypersecretory, hyposecretory, and obstructive meibomian gland dysfunction [12]. Being the most common type of meibomian gland dysfunction, the obstructive form is attributed to hyperkeratinization and subsequent blockage of the meibomian gland duct with keratinized cells [12]. In fact, hyperkeratinization of the meibomian gland duct may be stimulated by inflammation [12]. This manifests as dilation of the meibomian gland duct, followed by acinar atrophy and meibomian gland destruction [12]. The loss of meibomian gland function reduces lipid levels in the tear film, leading to greater tear film evaporation [12].
5. Dry Eye Disease (DED) and the Ocular Surface Microbiome
DED is a multifactorial condition that is driven by inflammation of the ocular surface. In fact, a notable elucidating factor for the inflammatory nature of DED is the ocular surface microbiome [13]. Numerous studies examining the ocular surface show how altered compositions of the ocular surface microbiome are associated with inflammation of the ocular surface, thus contributing to DED onset. More specifically, antibiotic-induced dysbiosis of the ocular surface microbiome is associated with greater generation of inflammatory cytokines and reduced regulatory T-cell differentiation as well as ensuing ocular surface inflammation [14]. Schlegel and colleagues showed that, among patients with DED, Propionibacteriaceae were more common in lids and Corynebacterium tuberculostearicum, which are commensal and non-pathogenic bacteria, were more prevalent in the conjunctiva when compared to healthy controls [14,15]. Furthermore, Willis et al. noted that individuals with DED experience shifts in the levels of Staphylococcus spp. and Pseudomonas spp. in the closed-eye ocular surface microbiome [13]. Additionally, Gupta et al. conducted 16S ribosomal RNA sequencing of samples from the conjunctiva of patients with DED, showing that there are greater levels of microbial DNA and bacteria alpha-diversity in comparison to samples from healthy controls [15]. However, the alterations to the ocular surface microbiome may be dependent on the subtype of DED, for Andersson et al. noted that individuals with aqueous tear-deficient dry eye have lower microbiota diversity than healthy controls [16]. In addition, the presence of an autoimmune condition with DED leads to varied ocular surface microbiome compositions compared to the presence of DED alone [17]. These studies collectively display the terrestrial relationship between DED and ocular surface microbiome alterations; however, there is an absence of sufficient evidence about ocular surface microbiome composition shifts among astronauts with versus without DED upon space travel. In light of the current data regarding the prevalence of astronauts with DED upon spaceflight, there is a need to explore the impact of space travel, including space-associated hazards, on ocular surface microbiome dysregulation and DED onset.
6. Terrestrial Ocular Surface–Gut Axis
Although the ocular surface has notably fewer bacteria than the gut microbiome, both the ocular surface and gut have distinct microbiomes that are dysregulated among individuals with DED [18]. Recent evidence has begun to elucidate the presence of an ocular surface–gut axis on Earth. For instance, when mice are treated with oral tributyrin, they experience less ocular surface inflammation upon exposure to a desiccating environment with low humidity levels compared to mice that did not receive oral tributyrin treatment [11]. Tributyrin is a precursor compound of butyrate, which is a short-chain fatty acid that is made by commensal bacteria in the gut upon fermentation of consumed fiber [11]. These findings show that compounds that are produced by the gut microbiome, such as butyrate, may lead to alterations in inflammatory pathways on the ocular surface, further confirming the ocular surface–gut axis [11]. Similarly, oral lactoferrin treatment protects against DED by maintaining tear quantities among mice exposed to desiccating stress, which may be attributed to the ability of lactoferrin to reduce inflammatory cytokine production and to modulate the gut microbiome by inducing protective short-chain fatty acid production [19]. On the other hand, gut microbiome dysbiosis, through vancomycin antibiotic treatment, opposes the protective effects of lactoferrin by reducing the gut’s short-chain fatty acid levels [19]. In a similar way, murine models of Sjögren syndrome that were exposed to dysbiosis-inducing antibiotics and desiccating environments displayed less mucin-producing goblet cells and more severe DED compared to controls that were not treated with antibiotics, further suggesting a role for the ocular surface–gut axis in DED development [20].
An additional crucial component of the ocular surface–gut axis includes the role of the lacrimal gland. Terrestrially, data from mice models show that alterations to the gut microbiome composition are associated with increased inflammation and fat accumulation in the lacrimal glands [21]. In light of the lacrimal gland’s salient role in producing tears that protect the ocular surface, such findings show the potential link between gut dysbiosis and an increased risk of DED onset [21]. Additionally, C57BL/6 mice treated with antibiotics to induce gut dysbiosis are found to have greater levels of inflammation and lymphocytes in the lacrimal gland [22]. These terrestrial findings about the ocular surface–gut–lacrimal gland axis provide a potential foundation for the pathophysiology of DED onset among astronauts during space travel.
Additionally, clinical studies among human subjects showed that patients with clinical Sjögren syndrome and patients with DED both displayed gut dysbiosis compared to control samples [19]. Gut dysbiosis levels were highest among patients with Sjögren syndrome, followed by patients with DED, when compared to controls [23]. Furthermore, a nonrandomized clinical trial was conducted to evaluate the safety of fecal microbial transplants for individuals with immune-mediated dry eye [24]. Here, results showed that after fecal microbial transplant, 80% of individuals’ gut microbiome composition transitioned closer towards their donors’ microbiome composition upon transplant, and 50% of individuals subjectively experienced improvement in DED symptoms post-transplant [24]. These findings collectively underscore how gut dysbiosis may be related to the development and progression of DED. While the prior literature evaluates the role of terrestrial gut dysbiosis in DED development, there is a need to explore how microgravity-induced gut dysbiosis and spaceflight hazard-mediated gut dysbiosis contribute to astronauts’ DED onset and progression.
7. Spaceflight and the Ocular Surface–Gut Axis
Space travel and its associated hazards (Figure 1) pose critical impacts on astronauts’ overall health and physiology, including the ocular surface [1,25,26]. This also includes dysregulation of the immune system, which gives rise to infection susceptibility [11]. For instance, simulated microgravity environments have been associated with increased rates of biofilm generation by Klebsiella pneumoniae [5]. The gut microbiome communicates with the host immune system, so dysregulation of the immune system upon exposure to microgravity during spaceflight is associated with dysregulation of the gut microbiome as well [5]. During short-duration spaceflight, astronauts experience altered compositions of their gut microbiomes, including an increase in Firmicutes levels and a decrease in Bacteroides levels [5]. However, a notable finding is that such changes to the gut microbiome were nearly not evident four weeks after spaceflight since the gut microbiome closely matched preflight compositions [5]. Long-duration spaceflight similarly impacts the gut microbiome composition through a significant elevation in the Firmicutes-to-Bacteroides ratio while in flight [27]. Such a transition in the gut microbiome during long-duration spaceflight was reported based on the NASA Twin Study, which compared one male twin in space on a 1-year mission and his terrestrial-bound twin [27]. The twin who was aboard experienced greater variations in gut microbiome composition during the time period of the flight compared to the Earth-bound twin during the same time period [27]. Similar to short-duration spaceflight, the changes to the gut microbiome upon long-duration spaceflight were also transient, for the twin’s space-associated elevations in the Firmicutes-to-Bacteroides ratio returned to preflight levels weeks after returning to Earth [27]. While the terrestrial literature elucidates evidence of the ocular surface–gut axis, the role of this axis in the pathogenesis of DED in space remains unknown. Considering that up to 30% of astronauts on the International Space Station report experiences of DED-associated symptoms and considering that space-associated gut dysbiosis is transient [1], there is a need to evaluate whether astronauts’ symptoms of DED disappear upon returning to Earth. Currently, there is an absence of adequate literature studies regarding astronauts’ progression of DED after returning to Earth. However, comparing the progression of astronauts’ DED with the composition of the gut microbiome after spaceflight would allow for a better understanding of the ocular surface–gut axis’s involvement in astronauts’ ocular surface health.
Regarding inflammation, human fibroblast cells that have been flown in space display the activation of NF-κB, which consequently leads to the generation of proinflammatory cytokines [28]. Also, during long-duration spaceflight, plasma concentrations of proinflammatory cytokines were significantly elevated compared to prior baseline concentrations [29]. Proinflammatory cytokines are a significant mediator of tear film instability in DED [9], so the activation of inflammatory cascades during space travel is a notable contributor to DED onset among astronauts. Additionally, murine models showed that age-dependent alterations in the gut microbiome were significantly associated with the DED phenotype and DED severity on Earth [30]. Similarly, crew members experience both inflammation as a part of accelerated aging [31] and alterations in the gut microbiome upon spaceflight [5,27], which together point towards the ocular surface–gut axis as a potential mediator for astronauts’ DED development during spaceflight.
In short, the prior literature regarding astronauts’ health elucidates alterations to the gut microbiome composition while in flight, an increase in inflammatory cascades, and an increase in proinflammatory cytokines. On Earth, these factors are related to the onset, development, and progression of DED. Therefore, we emphasize the need for an evaluation of the role of the ocular surface–gut axis as the underlying mechanism for astronauts’ DED upon spaceflight.
8. Limitations
While the presented literature underscores and supports the strength of the presented model regarding the ocular surface–gut axis serving as a mechanism for the pathogenesis of space-associated DED onset, we recognize that there are weaknesses inherent in this topic as well. For instance, terrestrially, meibomian gland dysfunction is a significant cause of evaporative DED, which is the most common form of DED; however, there is an absence of adequate literature studies regarding the terrestrial relationship between gut dysbiosis and meibomian gland dysfunction, thus limiting commentary about the role of the meibomian gland in the space-associated ocular surface–gut axis. Primarily, to our knowledge, there is an absence of a unifying study that explores the space-associated alterations to the gut microbiome and to the ocular surface within the same sample. Our opinion regarding the importance of further exploring the role of the ocular surface–gut axis in the development of DED among astronauts stems from our review of the prior literature that individually elucidates the presence of inflammation, alterations to the gut microbiome composition, aberrations to ocular surface health, and DED risk among astronauts. However, without a directly unifying study that explores the relationship between both variables (i.e., gut health and ocular health) within the same sample of astronauts, there is a risk of potential confounding variables being at play or the risk of a coincidental correlation when evaluating the ocular surface–gut axis in spaceflight. However, this is unlikely to be the case since terrestrial research has provided evidence for the presence of an ocular surface–gut axis within the same sample and within the same study on Earth [11,19,23,24], and the literature on astronaut health has also confirmed, albeit individually, the presence of disturbances to the gut microbiome [5,27], the ocular surface [1,2], and inflammatory pathways [28,29,31] due to hazards that are associated with space travel.
9. Conclusions
With increasingly expanding efforts towards space exploration, there is a growing concern regarding the impact of space travel on astronauts’ health and physiology. The current literature, thus far, has described that space-associated hazards affect the gut microbiome compositions during both short- and long-duration spaceflight. Similarly, in studies that are unrelated to gut microbiome analyses, astronauts have reported a striking prevalence of dry eye symptoms and DED upon space travel. Although terrestrial research has highlighted the presence of an ocular surface–gut axis and has described the relationship between gut microbiome dysbiosis and DED onset, there is a need for space medicine research to similarly explore the role of the ocular surface–gut axis in DED onset among astronauts specifically.
Alterations to the gut microbiome and activation of inflammatory pathways during space travel may play a role in space-associated changes in the ocular surface–gut axis and subsequent DED development (Figure 1). The mechanistic cascade begins with changes in the composition of the gut microbiome, which are incited by numerous hazards associated with space travel, including microgravity. Such changes are subsequently associated with accompanying inflammation of the tear film and corneal epithelium, ultimately leading to DED onset among astronauts. Future work should aim to further explore the pathogenesis of DED during spaceflight with particular attention towards the role of the ocular surface–gut axis. Such evidence may pave the way for therapies to be identified in order to ameliorate astronauts’ DED onset (e.g., administering short-chain fatty acid stimulators such as omega-3 fatty acid supplements prior to spaceflight or increasing fiber quantities in astronauts’ diet during space travel) [11,32].
Author Contributions
Conceptualization, R.S. and J.O.; methodology, R.S., J.O., E.W., J.B. and A.G.L.; investigation, R.S., J.O., E.W., J.B. and A.G.L.; resources, R.S., J.O., E.W., J.B. and A.G.L.; data curation, R.S., J.O., E.W., J.B. and A.G.L.; writing—original draft preparation, R.S.; writing—review and editing, R.S., J.O., E.W., J.B. and A.G.L.; visualization, R.S., J.O., E.W., J.B. and A.G.L.; supervision, A.G.L.; project administration, R.S., J.O., E.W., J.B. and A.G.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
Andrew G. Lee is a consultant for the National Aeronautics and Space Administration (NASA). John Berdahl is an employee of Vance Thompson Vision. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Ax, T.; Ganse, B.; Fries, F.N.; Szentmáry, N.; de Paiva, C.S.; March de Ribot, F.; Jensen, S.O.; Seitz, B.; Millar, T.J. Dry eye disease in astronauts: A narrative review. Front. Physiol. 2023, 14, 1281327. [Google Scholar] [CrossRef] [PubMed]
- Meer, E.; Grob, S.R.; Lehnhardt, K.; Sawyer, A. Ocular complaints and diagnoses in spaceflight. NPJ Microgravity 2024, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Barabino, S.; Labetoulle, M.; Rolando, M.; Messmer, E.M. Understanding Symptoms and Quality of Life in Patients with Dry Eye Syndrome. Ocul. Surf. 2016, 14, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Pal, N.; Goswami, S.; Singh, R.; Yadav, T.; Singh, R.P. Precautions & Possible Therapeutic Approaches of Health Hazards of Astronauts in Microgravity. Int. J. Aerosp. Psychol. 2021, 31, 149–161. [Google Scholar] [CrossRef]
- Liu, Z.; Luo, G.; Du, R.; Sun, W.; Li, J.; Lan, H.; Chen, P.; Yuan, X.; Cao, D.; Li, Y.; et al. Effects of spaceflight on the composition and function of the human gut microbiota. Gut Microbes 2020, 11, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Ocal. Space Shuttle 1 [Clip art]. Clker: 2007. Available online: https://www.clker.com/clipart-15271.html (accessed on 3 March 2024).
- Wu, G.D.; Lewis, J.D. Analysis of the human gut microbiome and association with disease. Clin. Gastroenterol. Hepatol. 2013, 11, 774–777. [Google Scholar] [CrossRef] [PubMed]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.-K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Messmer, E.M. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch. Ärzteblatt Int. 2015, 112, 71–81; quiz 82. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, L.; Hernandez, H.; Coats, R.A.; Yu, Z.; Pflugfelder, S.C.; Britton, R.A.; de Paiva, C.S. Gut-derived butyrate suppresses ocular surface inflammation. Sci. Rep. 2022, 12, 4512. [Google Scholar] [CrossRef]
- Jester, J.V.; Parfitt, G.J.; Brown, D.J. Meibomian gland dysfunction: Hyperkeratinization or atrophy? BMC Ophthalmol. 2015, 15 (Suppl. S1), 156. [Google Scholar] [CrossRef] [PubMed]
- Willis, K.A.; Postnikoff, C.K.; Freeman, A.; Rezonzew, G.; Nichols, K.; Gaggar, A.; Lal, C.V. The closed eye harbours a unique microbiome in dry eye disease. Sci. Rep. 2020, 10, 12035. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, I.; De Goüyon Matignon de Pontourade, C.M.F.; Lincke, J.-B.; Keller, I.; Zinkernagel, M.S.; Zysset-Burri, D.C. The Human Ocular Surface Microbiome and Its Associations with the Tear Proteome in Dry Eye Disease. Int. J. Mol. Sci. 2023, 24, 14091. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Chhibber-Goel, J.; Gupta, Y.; Mukherjee, S.; Maitra, A.; Sharma, A.; Tandon, R. Ocular conjunctival microbiome profiling in dry eye disease: A case control pilot study. Indian J. Ophthalmol. 2023, 71, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Andersson, J.; Vogt, J.K.; Dalgaard, M.D.; Pedersen, O.; Holmgaard, K.; Heegaard, S. Ocular surface microbiota in patients with aqueous tear-deficient dry eye. Ocul. Surf. 2021, 19, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Wan, Y.; Li, T.; Zhang, M.; Song, Y.; Hu, Y.; Sun, Y.; Li, L. Comparison of the Ocular Microbiomes of Dry Eye Patients with and without Autoimmune Disease. Front. Cell. Infect. Microbiol. 2021, 11, 716867. [Google Scholar] [CrossRef] [PubMed]
- Watane, A.; Raolji, S.; Cavuoto, K.; Galor, A. Microbiome and immune-mediated dry eye: A review. BMJ Open Ophthalmol. 2022, 7, e000956. [Google Scholar] [CrossRef] [PubMed]
- Connell, S.; Kawashima, M.; Nakamura, S.; Imada, T.; Yamamoto, H.; Tsubota, K.; Fukuda, S. Lactoferrin Ameliorates Dry Eye Disease Potentially through Enhancement of Short-Chain Fatty Acid Production by Gut Microbiota in Mice. Int. J. Mol. Sci. 2021, 22, 12384. [Google Scholar] [CrossRef] [PubMed]
- de Paiva, C.S.; Jones, D.B.; Stern, M.E.; Bian, F.; Moore, Q.L.; Corbiere, S.; Streckfus, C.F.; Hutchinson, D.S.; Ajami, N.J.; Petrosino, J.F.; et al. Altered Mucosal Microbiome Diversity and Disease Severity in Sjögren Syndrome. Sci. Rep. 2016, 6, 23561. [Google Scholar] [CrossRef]
- Qi, D.; Zou, S.; Lu, D.; Pei, X.; Huang, S.; Huang, D.-L.; Liu, J.; Si, H.; Li, Z. Long-term high fructose intake promotes lacrimal gland dysfunction by inducing gut dysbiosis in mice. Exp. Eye Res. 2023, 234, 109573. [Google Scholar] [CrossRef]
- Trujillo-Vargas, C.M.; Schaefer, L.; Alam, J.; Pflugfelder, S.C.; Britton, R.A.; de Paiva, C.S. The gut-eye-lacrimal gland-microbiome axis in Sjögren Syndrome. Ocul. Surf. 2020, 18, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Choi, S.H.; Yoon, C.H.; Kim, M.K. Gut dysbiosis is prevailing in Sjögren’s syndrome and is related to dry eye severity. PLoS ONE 2020, 15, e0229029. [Google Scholar] [CrossRef] [PubMed]
- Watane, A.; Cavuoto, K.M.; Rojas, M.; Dermer, H.; Day, J.O.; Banerjee, S.; Galor, A. Fecal Microbial Transplant in Individuals with Immune-Mediated Dry Eye. Am. J. Ophthalmol. 2022, 233, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Masalkhi, M.; Ong, J.; Waisberg, E.; Lee, A.G. Ocular immunology and inflammation under microgravity conditions and the pathogenesis of spaceflight associated neuro-ocular syndrome (SANS). Eye 2024, 38, 1799–1801. [Google Scholar] [CrossRef] [PubMed]
- Waisberg, E.; Ong, J.; Masalkhi, M.; Zaman, N.; Kamran, S.A.; Sarker, P.; Tavakkoli, A.; Lee, A.G. The Case for Expanding Visual Assessments during Spaceflight. Prehospital Disaster Med. 2023, 38, 518–521. [Google Scholar] [CrossRef]
- Garrett-Bakelman, F.E.; Darshi, M.; Green, S.J.; Gur, R.C.; Lin, L.; Macias, B.R.; McKenna, M.J.; Meydan, C.; Mishra, T.; Nasrini, J.; et al. The NASA Twins Study: A multidimensional analysis of a year-long human spaceflight. Science 2019, 364, eaau8650. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lu, T.; Wong, M.; Wang, X.; Stodieck, L.; Karouia, F.; Story, M.; Wu, H. Transient gene and microRNA expression profile changes of confluent human fibroblast cells in spaceflight. FASEB J. 2016, 30, 2211–2224. [Google Scholar] [CrossRef]
- Krieger, S.S.; Zwart, S.R.; Mehta, S.; Wu, H.; Simpson, R.J.; Smith, S.M.; Crucian, B. Alterations in Saliva and Plasma Cytokine Concentrations during Long-Duration Spaceflight. Front. Immunol. 2021, 12, 725748. [Google Scholar] [CrossRef]
- Yoon, C.H.; Ryu, J.S.; Moon, J.; Kim, M.K. Association between aging-dependent gut microbiome dysbiosis and dry eye severity in C57BL/6 male mouse model: A pilot study. BMC Microbiol. 2021, 21, 106. [Google Scholar] [CrossRef]
- Capri, M.; Conte, M.; Ciurca, E.; Pirazzini, C.; Garagnani, P.; Santoro, A.; Longo, F.; Salvioli, S.; Lau, P.; Moeller, R.; et al. Long-term human spaceflight and inflammaging: Does it promote aging? Ageing Res. Rev. 2023, 87, 101909. [Google Scholar] [CrossRef]
- Meer, E.; Grob, S.; Antonsen, E.L.; Sawyer, A. Ocular conditions and injuries, detection and management in spaceflight. NPJ Microgravity 2023, 9, 37. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).