Neural Network Modulation of Ayahuasca: A Systematic Review of Human Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Extraction
Quality Evaluation of Selected Studies
3. Results
3.1. Article Screening and Inclusion
3.2. Results from Selected Studies
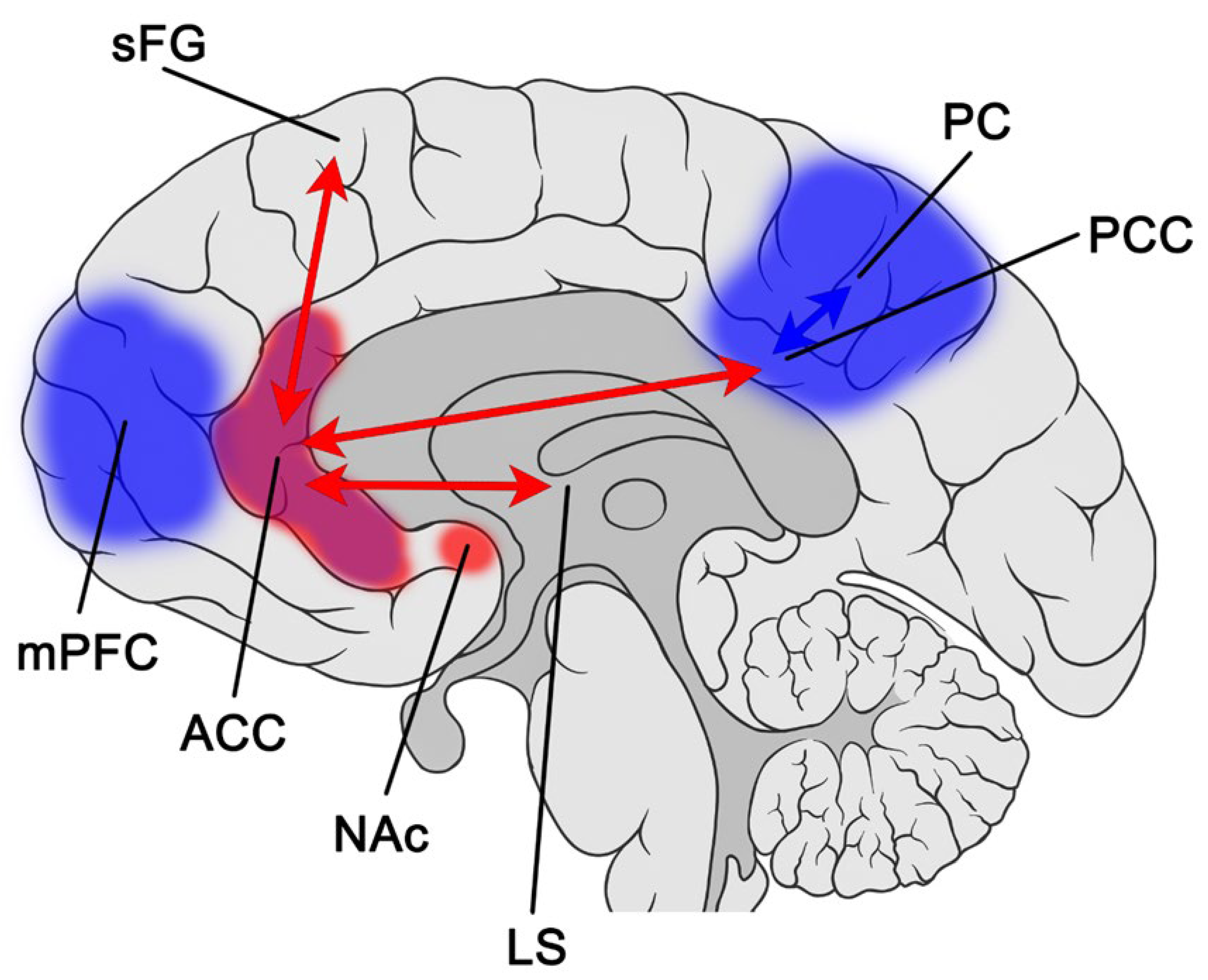
3.2.1. Single Photon Emission Computed Tomography (SPECT) Acute Effects (Molecular Imaging)
3.2.2. Magnetic Resonance Spectroscopy (MRS) and Functional Magnetic Resonance Imaging (fMRI) Subacute Effects
3.2.3. Structural Magnetic Resonance Imaging (MRI) Long-Term Effects
3.2.4. Functional Magnetic Resonance Imaging (fMRI) Acute Effects
3.2.5. Quality Assessment of Selected Studies
4. Discussion
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dos Santos, R.G.; Balthazar, F.M.; Bouso, J.C.; Hallak, J.E. The current state of research on ayahuasca: A systematic review of human studies assessing psychiatric symptoms, neuropsychological functioning, and neuroimaging. J. Psychopharmacol. 2016, 30, 1230–1247. [Google Scholar] [CrossRef]
- McKenna, D.J.; Towers, G.H.; Abbott, F. Monoamine oxidase inhibitors in South American hallucinogenic plants: Tryptamine and beta-carboline constituents of ayahuasca. J. Ethnopharmacol. 1984, 10, 195–223. [Google Scholar] [CrossRef]
- Chi, T.; Gold, J.A. A review of emerging therapeutic potential of psychedelic drugs in the treatment of psychiatric illnesses. J. Neurol. Sci. 2020, 411, 116715. [Google Scholar] [CrossRef]
- Buckner, R.L.; Andrews-Hanna, J.R.; Schacter, D.L. The brain’s default network: Anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008, 1124, 1–38. [Google Scholar] [CrossRef]
- Gattuso, J.J.; Perkins, D.; Ruffell, S.; Lawrence, A.J.; Hoyer, D.; Jacobson, L.H.; Timmermann, C.; Castle, D.; Rossell, S.L.; Downey, L.A.; et al. Default Mode Network Modulation by Psychedelics: A Systematic Review. Int. J. Neuropsychopharmacol. 2022. [Google Scholar] [CrossRef]
- Coutinho, J.; Fernandes, C.J.; Soares, J.M.; Maia, L.; Gonçalves, Ó.F.; Sampaio, A. Default mode network dissociation in depressive and anxiety states. Brain Imaging Behav. 2016, 10, 147–157. [Google Scholar] [CrossRef]
- Cumming, P.; Scheidegger, M.; Dornbierer, D.; Palner, M.; Quednow, B.B.; Martin-Soelch, C. Molecular and Functional Imaging Studies of Psychedelic Drug Action in Animals and Humans. Molecules 2021, 26, 2451. [Google Scholar] [CrossRef] [PubMed]
- Kadriu, B.; Greenwald, M.; Henter, I.D.; Gilbert, J.R.; Kraus, C.; Park, L.T.; Zarate, C.A. Ketamine and Serotonergic Psychedelics: Common Mechanisms Underlying the Effects of Rapid-Acting Antidepressants. Int. J. Neuropsychopharmacol. 2020, 24, 8–21. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- National Heart, Lung and Blood Institute. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 6 March 2023).
- Riba, J.; Romero, S.; Grasa, E.; Mena, E.; Carrió, I.; Barbanoj, M.J. Increased frontal and paralimbic activation following ayahuasca, the pan-amazonian inebriant. Psychopharmacology 2006, 186, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Prado, D.A.; Pinto, J.; Crippa, J.; Santos, A.; Ribeiro, S.; Araujo, D.; Zuardi, A.; Chaves, C.; Hallak, J. P.1.e.025 Effects of the Amazonian psychoactive plant beverage ayahuasca on prefrontal and limbic regions during a language task: A fMRI study. Eur. Neuropsychopharmacol. 2009, 19, S314–S315. [Google Scholar] [CrossRef]
- de Araujo, D.B.; Ribeiro, S.; Cecchi, G.A.; Carvalho, F.M.; Sanchez, T.A.; Pinto, J.P.; de Martinis, B.S.; Crippa, J.A.; Hallak, J.E.; Santos, A.C. Seeing with the eyes shut: Neural basis of enhanced imagery following ayahuasca ingestion. Hum. Brain Mapp. 2012, 33, 2550–2560. [Google Scholar] [CrossRef] [PubMed]
- Bouso, J.C.; Palhano-Fontes, F.; Rodríguez-Fornells, A.; Ribeiro, S.; Sanches, R.; Crippa, J.A.S.; Hallak, J.E.; de Araujo, D.B.; Riba, J. Long-term use of psychedelic drugs is associated with differences in brain structure and personality in humans. Eur. Neuropsychopharmacol. 2015, 25, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Palhano-Fontes, F.; Andrade, K.C.; Tófoli, L.F.; Santos, A.C.; Crippa, J.A.S.; Hallak, J.E.C.; Ribeiro, S.; De Araujo, D.B. The Psychedelic State Induced by Ayahuasca Modulates the Activity and Connectivity of the Default Mode Network. PLoS ONE 2015, 10, e0118143. [Google Scholar] [CrossRef]
- Sanches, R.F.; de Lima Osório, F.; Dos Santos, R.G.; Macedo, L.R.; Maia-de-Oliveira, J.P.; Wichert-Ana, L.; de Araujo, D.B.; Riba, J.; Crippa, J.A.; Hallak, J.E. Antidepressant Effects of a Single Dose of Ayahuasca in Patients with Recurrent Depression: A SPECT Study. J. Clin. Psychopharmacol. 2016, 36, 77–81. [Google Scholar] [CrossRef]
- Sampedro, F.; Revenga, M.D.L.F.; Valle, M.; Roberto, N.; Domínguez-Clavé, E.; Elices, M.; Luna, L.E.; Crippa, J.A.S.; Hallak, J.E.C.; de Araujo, D.B.; et al. Assessing the Psychedelic “After-Glow” in Ayahuasca Users: Post-Acute Neurometabolic and Functional Connectivity Changes Are Associated with Enhanced Mindfulness Capacities. Int. J. Neuropsychopharmacol. 2017, 20, 698–711. [Google Scholar] [CrossRef]
- Viol, A.; Palhano-Fontes, F.; Onias, H.; de Araujo, D.B.; Hövel, P.; Viswanathan, G.M. Characterizing Complex Networks Using Entropy-Degree Diagrams: Unveiling Changes in Functional Brain Connectivity Induced by Ayahuasca. Entropy 2019, 21, 128. [Google Scholar] [CrossRef]
- Viol, A.; Palhano-Fontes, F.; Onias, H.; de Araujo, D.B.; Viswanathan, G.M. Shannon entropy of brain functional complex networks under the influence of the psychedelic Ayahuasca. Sci. Rep. 2017, 7, 7388. [Google Scholar] [CrossRef]
- Pasquini, L.; Palhano-Fontes, F.; Araujo, D.B. Subacute effects of the psychedelic ayahuasca on the salience and default mode networks. J. Psychopharmacol. 2020, 34, 623–635. [Google Scholar] [CrossRef]
- Simonsson, O.; Bouso, J.C.; Kurth, F.; Araújo, D.B.; Gaser, C.; Riba, J.; Luders, E. Preliminary evidence of links between ayahuasca use and the corpus callosum. Front. Psychiatry 2022, 13, 1002455. [Google Scholar] [CrossRef]
- Castelhano, J.; Lima, G.; Teixeira, M.; Soares, C.; Pais, M.; Castelo-Branco, M. The Effects of Tryptamine Psychedelics in the Brain: A meta-Analysis of Functional and Review of Molecular Imaging Studies. Front. Pharmacol. 2021, 12, 739053. [Google Scholar] [CrossRef]
- Preller, K.H.; Razi, A.; Zeidman, P.; Stämpfli, P.; Friston, K.J.; Vollenweider, F.X. Effective connectivity changes in LSD-induced altered states of consciousness in humans. Proc. Natl. Acad. Sci. USA 2019, 116, 2743–2748. [Google Scholar] [CrossRef]
- Barrett, F.S.; Krimmel, S.R.; Griffiths, R.R.; Seminowicz, D.A.; Mathur, B.N. Psilocybin acutely alters the functional connectivity of the claustrum with brain networks that support perception, memory, and attention. Neuroimage 2020, 218, 116980. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Leech, R.; Hellyer, P.J.; Shanahan, M.; Feilding, A.; Tagliazucchi, E.; Chialvo, D.R.; Nutt, D. The entropic brain: A theory of conscious states informed by neuroimaging research with psychedelic drugs. Front. Hum. Neurosci. 2014, 8, 20. [Google Scholar] [CrossRef]
- Katchborian-Neto, A.; Santos, W.T.; Nicácio, K.J.; Corrêa, J.O.A.; Murgu, M.; Martins, T.M.M.; Gomes, D.A.; Goes, A.M.; Soares, M.G.; Dias, D.F.; et al. Neuroprotective potential of Ayahuasca and untargeted metabolomics analyses: Applicability to Parkinson’s disease. J. Ethnopharmacol. 2020, 255, 112743. [Google Scholar] [CrossRef]
- Saeger, H.N.; Olson, D.E. Psychedelic-inspired approaches for treating neurodegenerative disorders. J. Neurochem. 2021, 162, 109–127. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Erritzoe, D.; Williams, T.; Stone, J.M.; Reed, L.J.; Colasanti, A.; Tyacke, R.J.; Leech, R.; Malizia, A.L.; Murphy, K.; et al. Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc. Natl. Acad. Sci. USA 2012, 109, 2138–2143. [Google Scholar] [CrossRef]
- Drevets, W.C.; Savitz, J.; Trimble, M. The Subgenual Anterior Cingulate Cortex in Mood Disorders. CNS Spectrums 2008, 13, 663–681. [Google Scholar] [CrossRef]
- Pizzagalli, D.A.; Holmes, A.; Dillon, D.G.; Goetz, E.L.; Birk, J.; Bogdan, R.; Dougherty, D.D.; Iosifescu, D.V.; Rauch, S.L.; Fava, M. Reduced Caudate and Nucleus Accumbens Response to Rewards in Unmedicated Individuals with Major Depressive Disorder. Am. J. Psychiatry 2009, 166, 702–710. [Google Scholar] [CrossRef]
- Fitzgerald, P.B.; Laird, A.R.; Maller, J.; Daskalakis, Z.J. A meta-analytic study of changes in brain activation in depression. Hum. Brain Mapp. 2008, 29, 683–695. [Google Scholar] [CrossRef]
- Fuentes, J.J.; Fonseca, F.; Elices, M.; Farré, M.; Torrens, M. Therapeutic Use of LSD in Psychiatry: A Systematic Review of Randomized-Controlled Clinical Trials. Front. Psychiatry 2019, 10, 943. [Google Scholar] [CrossRef] [PubMed]
- Reiff, C.M.; Richman, E.E.; Nemeroff, C.B.; Carpenter, L.L.; Widge, A.S.; Rodriguez, C.I.; Kalin, N.H.; McDonald, W.M. Psychedelics and Psychedelic-Assisted Psychotherapy. Am. J. Psychiatry 2020, 177, 391–410. [Google Scholar] [CrossRef] [PubMed]
- Grob, C.S.; Danforth, A.L.; Chopra, G.S.; Hagerty, M.; McKay, C.R.; Halberstadt, A.L.; Greer, G.R. Pilot Study of Psilocybin Treatment for Anxiety in Patients with Advanced-Stage Cancer. Arch. Gen. Psychiatry 2011, 68, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Osório, F.D.L.; Sanches, R.F.; Macedo, L.R.; dos Santos, R.G.; Maia-De-Oliveira, J.P.; Wichert-Ana, L.; de Araujo, D.B.; Riba, J.; Crippa, J.A.; Hallak, J.E. Antidepressant effects of a single dose of ayahuasca in patients with recurrent depression: A preliminary report. Braz. J. Psychiatry 2015, 37, 13–20. [Google Scholar] [CrossRef]
- Griffiths, R.R.; Johnson, M.W.; Carducci, M.A.; Umbricht, A.; Richards, W.A.; Richards, B.D.; Cosimano, M.P.; Klinedinst, M.A. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J. Psychopharmacol. 2016, 30, 1181–1197. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Bolstridge, M.; Rucker, J.; Day, C.M.J.; Erritzoe, D.; Kaelen, M.; Bloomfield, M.; Rickard, J.A.; Forbes, B.; Feilding, A.; et al. Psilocybin with psychological support for treatment-resistant depression: An open-label feasibility study. Lancet Psychiatry 2016, 3, 619–627. [Google Scholar] [CrossRef]
- Ross, S.; Bossis, A.; Guss, J.; Agin-Liebes, G.; Malone, T.; Cohen, B.; Mennenga, S.E.; Belser, A.; Kalliontzi, K.; Babb, J.; et al. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: A randomized controlled trial. J. Psychopharmacol. 2016, 30, 1165–1180. [Google Scholar] [CrossRef]
- Goodwin, G.M.; Aaronson, S.T.; Alvarez, O.; Arden, P.C.; Baker, A.; Bennett, J.C.; Bird, C.; Blom, R.E.; Brennan, C.; Brusch, D.; et al. Single-Dose Psilocybin for a Treatment-Resistant Episode of Major Depression. N. Engl. J. Med. 2022, 387, 1637–1648. [Google Scholar] [CrossRef]
- Bogenschutz, M.P.; Forcehimes, A.E.; Pommy, J.A.; Wilcox, C.E.; Barbosa, P.C.R.; Strassman, R.J. Psilocybin-assisted treatment for alcohol dependence: A proof-of-concept study. J. Psychopharmacol. 2015, 29, 289–299. [Google Scholar] [CrossRef]
- Johnson, M.W.; Garcia-Romeu, A.; Cosimano, M.P.; Griffiths, R.R. Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. J. Psychopharmacol. 2014, 28, 983–992. [Google Scholar] [CrossRef]
- Moreno, F.A.; Wiegand, C.B.; Taitano, E.K.; Delgado, P.L. Safety, Tolerability, and Efficacy of Psilocybin in 9 Patients with Obsessive-Compulsive Disorder. J. Clin. Psychiatry 2006, 67, 1735–1740. [Google Scholar] [CrossRef]
- Rodrigues, L.S.; Rossi, G.N.; Rocha, J.M.; Osório, F.L.; Bouso, J.C.; Hallak, J.E.C.; dos Santos, R.G. Effects of ayahuasca and its alkaloids on substance use disorders: An updated (2016–2020) systematic review of preclinical and human studies. Eur. Arch. Psychiatry Clin. Neurosci. 2022, 272, 541–556. [Google Scholar] [CrossRef]
- Rossi, G.N.; Dias, I.C.D.S.; Baker, G.; Saiz, J.C.B.; Dursun, S.M.; Hallak, J.E.C.; Dos Santos, R.G. Ayahuasca, a potentially rapid acting antidepressant: Focus on safety and tolerability. Expert Opin. Drug Saf. 2022, 21, 789–801. [Google Scholar] [CrossRef]
- Rossi, G.N.; Hallak, J.E.C.; Saiz, J.C.B.; Dos Santos, R.G. Safety issues of psilocybin and LSD as potential rapid acting antidepressants and potential challenges. Expert Opin. Drug Saf. 2022, 21, 761–776. [Google Scholar] [CrossRef]
- Bramer, W.M.; Rethlefsen, M.L.; Kleijnen, J.; Franco, O.H. Optimal database combinations for literature searches in systematic reviews: A prospective exploratory study. Syst. Rev. 2017, 6, 245. [Google Scholar] [CrossRef]
- Dos Santos, R.G.; Bouso, J.C. Translational evidence for ayahuasca as an antidepressant: What’s next? Braz. J. Psychiatry 2019, 41, 275–276. [Google Scholar] [CrossRef]
- McCulloch, D.E.-W.; Knudsen, G.M.; Barrett, F.S.; Doss, M.K.; Carhart-Harris, R.L.; Rosas, F.E.; Deco, G.; Kringelbach, M.L.; Preller, K.H.; Ramaekers, J.G.; et al. Psychedelic resting-state neuroimaging: A review and perspective on balancing replication and novel analyses. Neurosci. Biobehav. Rev. 2022, 138, 104689. [Google Scholar] [CrossRef]

| Authors | Study Design and Quality Rating (QR, 0 to 1) | Sample | Drug and Dose | Neuroimaging Technique | Main Results |
|---|---|---|---|---|---|
| Riba et al., 2006 [11] | Randomized, double-blind, placebo-controlled. QR: 0.71 | 15 healthy male volunteers with previous experience of hallucinogen use and not diagnosed with psychiatric disorders (DSM-IV). | Lyophilized, encapsulated, and orally administered ayahuasca in concentrations equivalent to 1.0 mg DMT/kg or 0.75 g lactose capsules as placebo. | Single photon emission tomography (SPECT). | Activation of frontal and paralimbic brain regions (p < 0.002). Increased blood perfusion bilaterally in the regions of the anterior insula, with great intensity in the right hemisphere, and in the anterior cingulate cortex/medial front of the right hemisphere. Activity increases in the left amygdala and parahippocampal gyrus (p < 0.002). |
| Almeida Prado et al., 2009 [12] | Double-blind. QR: 0.71 | 10 healthy volunteers, 5 women, with experiences of chronic use of ayahuasca from the UDV church. | Ayahuasca in an average dose of 150 mL, with a concentration of 0.65 mg/kg of DMT. | Functional magnetic resonance imaging (fMRI). | Elevation in the scores of the scales: BPRS (p < 0.001), YMRS (p < 0.001), and CADSS (p = 0.001 and p < 0.001). Bilateral activation of the cingulate, superior, medial and frontomedial gyrus regions, medial and superior temporal gyrus, and the precuneus. |
| De Araujo et al., 2012 [13] | Open-label. QR: 0.75 | 9 healthy volunteers with regular use of ayahuasca recruited from Igreja do Santo Daime, compared with a control group composed of 26 individuals. * | Ayahuasca in a single dose of 2.2 mL/kg at concentrations of 0.8 mg/mL of DMT and 0.21 mg/mL of harmine. | Functional magnetic resonance imaging (fMRI). | An increase in BOLD signal was reported in the bilateral precuneus, cuneus, and lingual, fusiform, middle occipital, parahippocampal, posterior cingulate, superior temporal, superior and middle frontal, and inferior frontal gyri when comparing before and after ayahuasca intake (all p values < 0.05). By increasing the intensity of the retrieved images to the same level as the natural image, ayahuasca gives inner experiences a reality status. |
| Bouso et al., 2015 [14] | Cross-sectional case-control. QR: 0.41 | 22 participants with previous ayahuasca experience recruited from the Santo Daime church compared with a control of 22 matched participants. † | No dose was administered. | Magnetic resonance imaging (MRI). | Ayahuasca users showed significant differences in cortical thickness (CT) (p < 0.002), in the midline structures of the brain, with thinning in the posterior cingulate cortex (PCC). PCC CT values were inversely correlated with the intensity and duration of previous ayahuasca use and scores of self-transcendence. Significantly elevated scores of self-transcendence (p = 0.001), self-forgetfulness (p = 0.001), transpersonal identification (p = 0.001), and spiritual acceptance (p = 0.001) were reported in ayahuasca users when compared to controls. |
| Palhano-Fontes et al., 2015 [15] | Cross-sectional case-control. QR: 0.41 | 9 healthy volunteers with regular use of ayahuasca recruited from Igreja do Santo Daime, compared with a control group composed of 26 individuals. * | Ayahuasca in a single dose of 2.2 mL/kg at concentrations of 0.8 mg/mL of DMT and 0.21 mg/mL of harmine. | Functional magnetic resonance imaging (fMRI) | Within 9 analyzed DMN regions of interest, there was a significant reduction in connectivity in 6 of them (p < 0.001). In 2 related to speech, there was an increase in connectivity, which may be related to the requested task. |
| Sanches et al., 2016 [16] | Open-label. QR: 0.75 | 17 volunteers diagnosed with major depressive disorder. 3 of the volunteers were in mild depressive episodes, 13 in moderate depressive episodes, and 1 in severe depressive episode. | Ayahuasca in a single dose of 2.2 mL/kg at concentrations of 0.8 mg/mL of DMT and 0.21 mg/mL of harmine. | Single photon emission tomography (SPECT) | Significant decreases in HAM-D and MADRS depression scales from 80 to 180 min and on D21 (p < 0.001). As for the BPRS, effects were observed from the first day of administration to the 21st day. The Anxiety-Depression subscale (from 40 to 180 min, p < 0.01; and from D1 to D21, p < 0.001), Thought Disorder (180 min, p < 0.05; and on D1, D14, and D21). There was an increase in the CADSS index (p < 0.01) between 40 and 80 min. Furthermore, a significant increase (p < 0.01) in blood perfusion in the subungual area, nucleus accumbens, and insula was also noted. |
| Sampedro et al., 2017 [17] | Open-label. QR: 0.58 | 16 healthy volunteers, with previous experience of using ayahuasca. | Ayahuasca in a single 148 mL dose containing 0.3 mg/mL of DMT, 0.86 mg/mL of harmine, 0.17 mg/mL of tetrahydroharmine and 0.04 mg/mL of harmaline. | Magnetic resonance spectroscopy (MRS) | Involvement of glutamatergic neurotransmitters in psychedelic effects. Neurometabolic changes in posterior cingulate cortex and increased connectivity between the anterior cingulate cortex and medial temporal lobe. |
| Viol et al., 2017 [18] | Open-label. QR: 0.50 | 9 healthy volunteers with regular use of ayahuasca recruited from Igreja do Santo Daime, compared with a control group composed of 26 individuals. * | Ayahuasca in a single dose of 2.2 mL/kg in concentrations of 0.8 mg/mL of DMT and 0.21 mg/mL of harmine. | Functional magnetic resonance imaging (fMRI) | The neural networks became locally enlarged after using ayahuasca. On the other hand, the brain’s functional network, globally, became less connected. |
| Viol et al., 2019 [19] | Open-label. QR: 0.50 | 9 healthy volunteers with regular use of ayahuasca recruited from Igreja do Santo Daime, compared with a control group composed of 26 individuals.* | Ayahuasca in a single dose of 2.2 mL/kg in concentrations of 0.8 mg/mL of DMT and 0.21 mg/mL of harmine. | Functional magnetic resonance imaging (fMRI) | Ayahuasca ingestion tends to lead to higher geodesic entropy compared to the ordinary state. Geodesic distance becomes less constrained after substance use, making the network wider, which leads to greater diversity within the network of brain nodes. |
| Pasquini et al., 2020 [20] | Randomized, placebo-controlled study. QR: 0.57 | 50 healthy participants with no previous ayahuasca experience. | Ayahuasca in a single dose of 1 mL/kg containing 0.36 mg/mL of DMT, 1.86 mg/mL of harmine, 0.24 mg/mL 0.03 mg/mL of harmaline, and 1.20 mg/mL of tetrahydroharmine or placebo. | Functional magnetic resonance imaging (fMRI) | Significant increases in the HRS after ayahuasca administration (p < 0.009). Functional connectivity increased in the area of interest located in the anterior cingulate cortex and in the superior frontal gyrus (p < 0.05), with a tendency to the left hemisphere. On the other hand, there was a decrease in DMN in the ayahuasca group, predominantly affecting the posterior cingulate cortex (p < 0.1). |
| Simonsson et al., 2022 [21] | Cross-sectional, case-control study. QR: 0.66 | 22 participants with previous ayahuasca experience recruited from the Santo Daime church compared with a control of 22 matched participants. † | No dose was administered. | Magnetic resonance imaging (MRI). | The corpus callosum was thicker in the ayahuasca group than in the control group (p = 0.006). Additionally, the ayahuasca group reported 123 past ayahuasca sessions on average (range: 30–352) and was observed a significant positive correlation between callosal thickness and the number of sessions (p = 0.026), although statistical significance was not maintained after multiple comparisons. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, G.H.d.M.; Rodrigues, L.S.; Rocha, J.M.; Rossi, G.N.; Ona, G.; Bouso, J.C.; Hallak, J.E.C.; dos Santos, R.G. Neural Network Modulation of Ayahuasca: A Systematic Review of Human Studies. Psychoactives 2023, 2, 76-91. https://doi.org/10.3390/psychoactives2010006
Santos GHdM, Rodrigues LS, Rocha JM, Rossi GN, Ona G, Bouso JC, Hallak JEC, dos Santos RG. Neural Network Modulation of Ayahuasca: A Systematic Review of Human Studies. Psychoactives. 2023; 2(1):76-91. https://doi.org/10.3390/psychoactives2010006
Chicago/Turabian StyleSantos, Guilherme Henrique de Morais, Lucas Silva Rodrigues, Juliana Mendes Rocha, Giordano Novak Rossi, Genís Ona, José Carlos Bouso, Jaime Eduardo Cecilio Hallak, and Rafael Guimarães dos Santos. 2023. "Neural Network Modulation of Ayahuasca: A Systematic Review of Human Studies" Psychoactives 2, no. 1: 76-91. https://doi.org/10.3390/psychoactives2010006
APA StyleSantos, G. H. d. M., Rodrigues, L. S., Rocha, J. M., Rossi, G. N., Ona, G., Bouso, J. C., Hallak, J. E. C., & dos Santos, R. G. (2023). Neural Network Modulation of Ayahuasca: A Systematic Review of Human Studies. Psychoactives, 2(1), 76-91. https://doi.org/10.3390/psychoactives2010006







