Coronary In-Stent Restenosis Predictors following Drug-Eluting Stent Implantation: A Meta-Analysis Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Eligibility Criteria
2.3. Study Quality Assessment
2.4. Data Extraction
2.5. Statistical Analysis
3. Results
3.1. Study Selection Process
3.2. In-Stent Restenosis Predictors
3.3. Subgroup Analysis
3.4. Heterogeneity among Studies and Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buccheri, D.; Piraino, D.; Andolina, G.; Cortese, B. Understanding and Managing In-Stent Restenosis: A Review of Clinical Data, from Pathogenesis to Treatment. J. Thorac. Dis. 2016, 8, E1150–E1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dangas, G.D.; Claessen, B.E.; Caixeta, A.; Sanidas, E.A.; Mintz, G.S.; Mehran, R. In-Stent Restenosis in the Drug-Eluting Stent Era. J. Am. Coll. Cardiol. 2010, 56, 1897–1907. [Google Scholar] [CrossRef] [Green Version]
- Tan, L.; Wang, X.; Yuan, K.; Yin, T.; Du, R.; Shen, L.; Zhu, Z.; Yu, S.; Zhang, H.; Wang, G. Structural and Temporal Dynamics Analysis on Drug-Eluting Stents: History, Research Hotspots and Emerging Trends. Bioact. Mater. 2023, 23, 170–186. [Google Scholar] [CrossRef]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on Myocardial Revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef] [Green Version]
- Cassese, S.; Byrne, R.A.; Tada, T.; Pinieck, S.; Joner, M.; Ibrahim, T.; King, L.A.; Fusaro, M.; Laugwitz, K.L.; Kastrati, A. Incidence and Predictors of Restenosis after Coronary Stenting in 10,004 Patients with Surveillance Angiography. Heart 2014, 100, 153–159. [Google Scholar] [CrossRef]
- Farooq, V.; Gogas, B.D.; Serruys, P.W. Restenosis Delineating the Numerous Causes of Drug-Eluting Stent Restenosis. Circ. Cardiovasc. Interv. 2011, 4, 195–205. [Google Scholar] [CrossRef] [Green Version]
- Zheng, C.; Kang, J.; Park, K.W.; Han, J.K.; Yang, H.M.; Kang, H.J.; Koo, B.K.; Kim, H.S. The Predictors of Target Lesion Revascularization and Rate of In-Stent Restenosis in the Second-Generation Drug-Eluting Stent Era. J. Interv. Cardiol. 2019, 2019, 3270132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kastrati, A.; Dibra, A.; Mehilli, J.; Mayer, S.; Pinieck, S.; Pache, J.; Dirschinger, J.; Schömig, A. Predictive Factors of Restenosis after Coronary Implantation of Sirolimus- or Paclitaxel-Eluting Stents. Circulation 2006, 113, 2293–2300. [Google Scholar] [CrossRef]
- Takasawa, Y.; Iijima, R.; Shiba, M.; Nakamura, M.; Sugi, K. Predictor of Subsequent Target Lesion Revascularization in Patients with Drug-Eluting Stent Restenosis Undergoing Percutaneous Coronary Intervention. J. Cardiol. 2010, 55, 391–396. [Google Scholar] [CrossRef] [Green Version]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.A.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-Analyses of Health Care Interventions: Checklist and Explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef] [Green Version]
- Rohman, M.S.; Waranugraha, Y.; Masbuchin, A.N.; Baskoro, S.S.; Sishartami, L.W.; Pratiwi, B.B. Coronary In-Stent Restenosis Predictors Following Drug-Eluting Stent Implantation: A Meta-Analysis Study. Inplasy Protoc. 2023. [Google Scholar] [CrossRef]
- Waranugraha, Y.; Rizal, A.; Firdaus, A.J.; Sihotang, F.A.; Akbar, A.R.; Lestari, D.D.; Firdaus, M.; Nurudinulloh, A.I. The Superiority of High-power Short-duration Radiofrequency Catheter Ablation Strategy for Atrial Fibrillation Treatment: A Systematic Review and Meta-analysis Study. J. Arrhythmia 2021, 37, 975–989. [Google Scholar] [CrossRef] [PubMed]
- Quintana, D.S. From Pre-Registration to Publication: A Non-Technical Primer for Conducting a Meta-Analysis to Synthesize Correlational Data. Front. Psychol. 2015, 6, 1549. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Zhou, S.; Jin, J.; Tian, F.; Han, Y.; Wang, J.; Liu, J.; Chen, Y. Chronic Treatment with Trimetazidine after Discharge Reduces the Incidence of Restenosis in Patients Who Received Coronary Stent Implantation: A 1-Year Prospective Follow-up Study. Int. J. Cardiol. 2014, 174, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Chang, F.J.; Wang, Y.; You, P.H.; Chen, H.C.; Han, W.Q.; Wang, J.W.; Zhong, N.E.; Min, Z.Q. Factors Influencing Stent Restenosis after Percutaneous Coronary Intervention in Patients with Coronary Heart Disease: A Clinical Trial Based on 1-Year Follow-Up. Med. Sci. Monit. 2019, 25, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Doi, H.; Maehara, A.; Mintz, G.S.; Weissman, N.J.; Yu, A.; Wang, H.; Mandinov, L.; Popma, J.J.; Ellis, S.G.; Grube, E.; et al. Impact of In-Stent Minimal Lumen Area at 9 Months Poststent Implantation on 3-Year Target Lesion Revascularization-Free Survival: A Serial Intravascular Ultrasound Analysis from the TAXUS IV, V, and VI Trials. Circ. Cardiovasc. Interv. 2008, 1, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Du, J.B.; Zhang, W.; Li, N.; Jiang, H.; Liu, Y.; Gao, J.; Chen, S.T.; Cong, H.L.; Wei, Y.L. Association Study of Matrix Metalloproteinase 3 5A/6A Polymorphism with in-Stent Restenosis after Percutaneous Coronary Interventions in a Han Chinese Population. J. Int. Med. Res. 2020, 48, 0300060519827145. [Google Scholar] [CrossRef] [Green Version]
- Gil, R.J.; Bil, J.; Kern, A.; Iñigo-Garcia, L.A.; Formuszewicz, R.; Dobrzycki, S.; Vassilev, D.; Mehran, R. Angiographic Restenosis in Coronary Bifurcations Treatment with Regular Drug Eluting Stents and Dedicated Bifurcation Drug-Eluting BiOSS Stents: Analysis Based on Randomized POLBOS i and POLBOS II Studies. Cardiovasc. Ther. 2020, 2020, 6760205. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Lara, J.; Heo, J.H.; Brugaletta, S.; Garg, S.; Garcia-Garcia, H.M.; Van Geuns, R.J.; Silber, S.; Windecker, S.; Serruys, P.W. Risk of Target Lesion Failure in Relationship to Vessel Angiographic Geometry and Stent Conformability Using the Second Generation of Drug-Eluting Stents. Am. Heart J. 2011, 162, 1069–1079.e2. [Google Scholar] [CrossRef]
- Hong, S.J.; Kim, M.H.; Ahn, T.H.; Ahn, Y.K.; Bae, J.H.; Shim, W.J.; Ro, Y.M.; Lim, D.S. Multiple Predictors of Coronary Restenosis after Drug-Eluting Stent Implantation in Patients with Diabetes. Heart 2006, 92, 1119–1124. [Google Scholar] [CrossRef] [Green Version]
- Hoppmann, P.; Erl, A.; Türk, S.; Tiroch, K.; Mehilli, J.; Schömig, A.; Kastrati, A.; Koch, W. No Association of Chromosome 9p21.3 Variation With Clinical and Angiographic Outcomes After Placement of Drug-Eluting Stents. JACC Cardiovasc. Interv. 2009, 2, 1149–1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ino, Y.; Kubo, T.; Kitabata, H.; Shimamura, K.; Shiono, Y.; Orii, M.; Okochi, K.; Sougawa, H.; Tanimoto, T.; Komukai, K.; et al. Impact of Hinge Motion on In-Stent Restenosis after Sirolimus-Eluting Stent Implantation. Circ. J. 2011, 75, 1878–1884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, L.O.; Thayssen, P.; Junker, A.; Maeng, M.; Tilsted, H.H.; Kaltoft, A.; Hansen, K.N.; Christiansen, E.H.; Kristensen, S.D.; Ravkilde, J.; et al. Comparison of Outcomes in Patients with versus without Diabetes Mellitus after Revascularization with Everolimus- and Sirolimus-Eluting Stents (from the SORT OUT IV Trial). Am. J. Cardiol. 2012, 110, 1585–1591. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.J.; Mintz, G.S.; Park, D.W.; Lee, S.W.; Kim, Y.H.; Lee, C.W.; Han, K.H.; Kim, J.J.; Park, S.W.; Park, S.J. Mechanisms of In-Stent Restenosis after Drug-Eluting Stent Implantation Intravascular Ultrasound Analysis. Circ. Cardiovasc. Interv. 2011, 4, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.W.; Park, D.W.; Lee, B.K.; Kim, Y.H.; Hong, M.K.; Kim, J.J.; Park, S.W.; Park, S.J. Predictors of Restenosis after Placement of Drug-Eluting Stents in One or More Coronary Arteries. Am. J. Cardiol. 2006, 97, 506–511. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, D.W.; Kim, Y.H.; Yun, S.C.; Kim, W.J.; Kang, S.J.; Lee, S.W.; Lee, C.W.; Park, S.W.; Park, S.J. Incidence, Predictors, Treatment, and Long-Term Prognosis of Patients with Restenosis after Drug-Eluting Stent Implantation for Unprotected Left Main Coronary Artery Disease. J. Am. Coll. Cardiol. 2011, 57, 1349–1358. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Liu, Y.; Jiang, H.; Ding, X.; Zhu, R.; Li, B.; Zhao, Y. Plasma Levels of Interleukin 18, Interleukin 10, and Matrix Metalloproteinase-9 and -137G/C Polymorphism of Interleukin 18 Are Associated with Incidence of in-Stent Restenosis after Percutaneous Coronary Intervention. Inflammation 2013, 36, 1129–1135. [Google Scholar] [CrossRef]
- Mehta, R.H.; Leon, M.B.; Sketch, M.H. The Relation Between Clinical Features, Angiographic Findings, and the Target Lesion Revascularization Rate in Patients Receiving the Endeavor Zotarolimus-Eluting Stent for Treatment of Native Coronary Artery Disease: An Analysis of ENDEAVOR I, ENDEAVOR II, ENDEAVOR II Continued Access Registry, and ENDEAVOR III. Am. J. Cardiol. 2007, 100, S62–S70. [Google Scholar] [CrossRef]
- Qin, Z.; Zheng, F.W.; Zeng, C.; Zhou, K.; Geng, Y.; Wang, J.L.; Li, Y.P.; Ji, Q.W.; Zhou, Y.J. Elevated Levels of Very Low-Density Lipoprotein Cholesterol Independently Associated with in-Stent Restenosis in Diabetic Patients after Drug-Eluting Stent Implantation. Chin. Med. J. 2017, 130, 2326–2332. [Google Scholar] [CrossRef]
- Rathore, S.; Terashima, M.; Katoh, O.; Matsuo, H.; Tanaka, N.; Kinoshita, Y.; Kimura, M.; Tuschikane, E.; Nasu, K.; Ehara, M.; et al. Predictors of Angiographic Restenosis after Drug Eluting Stents in the Coronary Arteries: Contemporary Practice in Real World Patients. EuroIntervention 2009, 5, 349–354. [Google Scholar] [CrossRef]
- Sun, J.; Yu, H.; Liu, H.; Pu, D.; Gao, J.; Jin, X.; Liu, X.; Yan, A. Correlation of Pre-Operative Circulating Inflammatory Cytokines with Restenosis and Rapid Angiographic Stenotic Progression Risk in Coronary Artery Disease Patients Underwent Percutaneous Coronary Intervention with Drug-Eluting Stents. J. Clin. Lab. Anal. 2020, 34, e23108. [Google Scholar] [CrossRef]
- Yin, J.; Shen, L.; Ji, M.; Wu, Y.; Cai, S.; Chen, J.; Yao, Z.; Ge, J. Inverse Relationship between Serum VEGF Levels and Late In-Stent Restenosis of Drug-Eluting Stents. BioMed Res. Int. 2017, 2017, 8730271. [Google Scholar] [CrossRef]
- Zeng, W.P.; Zhang, R.; Li, R.; Luo, J.F.; Hu, X.F. Association of the Endothelial Nitric Oxide Synthase Gene T786C Polymorphism with In-Stent Restenosis in Chinese Han Patients with Coronary Artery Disease Treated with Drug-Eluting Stent. PLoS ONE 2017, 12, e0170964. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.; Yang, M.; Lin, J.; Zhu, H.; Lu, Y.; Wang, B.; Xue, Y.; Fang, C.; Tang, L.; Xu, B.; et al. Association of Seven Renin Angiotensin System Gene Polymorphisms with Restenosis in Patients Following Coronary Stenting. J. Renin-Angiotensin-Aldosterone Syst. 2017, 18, 147032031668877. [Google Scholar] [CrossRef] [Green Version]
- Stettler, C.; Allemann, S.; Egger, M.; Windecker, S.; Meier, B.; Diem, P. Efficacy of Drug Eluting Stents in Patients with and without Diabetes Mellitus: Indirect Comparison of Controlled Trials. Heart 2006, 92, 650–657. [Google Scholar] [CrossRef]
- Gilbert, J.; Raboud, J.; Zinman, B. Meta-Analysis of the Effect of Diabetes on Restenosis Rates among Patients Receiving Coronary Angioplasty Stenting. Diabetes Care 2004, 27, 990–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheuner, M.T.; Setodji, C.M.; Pankow, J.S.; Blumenthal, R.S.; Keeler, E. Relation of Familial Patterns of Coronary Heart Disease, Stroke, and Diabetes to Subclinical Atherosclerosis: The Multi-Ethnic Study of Atherosclerosis. Genet. Med. 2008, 10, 879–887. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.C.; Allen, J.; Blair, S.N.; Bonow, R.O.; Brass, L.M.; Fonarow, G.C.; Grundy, S.M.; Hiratzka, L.; Jones, D.; Krumholz, H.M.; et al. AHA/ACC Guidelines for Secondary Prevention for Patients With Coronary and Other Atherosclerotic Vascular Disease: 2006 Update. Endorsed by the National Heart, Lung, and Blood Institute. J. Am. Coll. Cardiol. 2006, 47, 2130–2139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, S.; Mu, G.; Wei, S.; Liu, Z.; Wang, Z.; Xiang, Q.; Cui, Y. Associations Between Polymorphisms of Endothelial Nitric Oxide Synthase, Matrix Metalloproteinase 3, Angiotensinogen, and Angiotensin II Type 1 Receptor and Risk of Restenosis after Percutaneous Coronary Intervention: A Meta-Analysis. Clin. Ther. 2020, 42, 458–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lloyd-Jones, D.M.; Nam, B.-H.; D’Agostino, R.; Levy, D.; Wang, T.J.; Wilson, P.W.F.; Donnell, C.J.O. Parental Cardiovascular Disease as a Risk Factor for Cardiovascular Disease in Middle-Aged Adults A Prospective Study of Parents and Offspring. JAMA 2004, 291, 2204–2211. [Google Scholar] [CrossRef] [Green Version]
- Goff, D.C.; Lloyd-Jones, D.M.; Bennett, G.; Coady, S.; D’Agostino, R.B.; Gibbons, R.; Greenland, P.; Lackland, D.T.; Levy, D.; O’Donnell, C.J.; et al. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 63, 2935–2959. [Google Scholar] [CrossRef] [Green Version]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 74, e177–e232. [Google Scholar] [CrossRef]
- Venkatason, P.; Salleh, N.M.; Zubairi, Y.; Hafidz, I.; Ahmad, W.A.W.; Han, S.K.; Zuhdi, A.S.M. The Bizzare Phenomenon of Smokers’ Paradox in the Immediate Outcome Post Acute Myocardial Infarction: An Insight into the Malaysian National Cardiovascular Database-Acute Coronary Syndrome (NCVD-ACS) Registry Year 2006–2013. Springerplus 2016, 5, 534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cutlip, D.E.; Chauhan, M.S.; Baim, D.S.; Ho, K.K.L.; Popma, J.J.; Carrozza, J.P.; Cohen, D.J.; Kuntz, R.E. Clinical Restenosis after Coronary Stenting: Perspectives from Multicenter Clinical Trials. J. Am. Coll. Cardiol. 2002, 40, 2082–2089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, R.T.; Liu, J.; Zhou, Y.; Hu, B.L. Association of Smoking with Restenosis and Major Adverse Cardiac Events after Coronary Stenting: A Meta-Analysis. Pak. J. Med. Sci. 2015, 31, 1002–1008. [Google Scholar] [CrossRef]
- Moussa, I.D.; Mohananey, D.; Saucedo, J.; Stone, G.W.; Yeh, R.W.; Kennedy, K.F.; Waksman, R.; Teirstein, P.; Moses, J.W.; Simonton, C. Trends and Outcomes of Restenosis After Coronary Stent Implantation in the United States. J. Am. Coll. Cardiol. 2020, 76, 1521–1531. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Balachander, J. Predictor of In-Stent Restenosis in Patients with Drug-Eluting Stent (PRIDE)- a Retrospective Cohort Study. Clín. Investig. Arterioscler. 2021, 33, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Kitahara, H.; Okada, K.; Kimura, T.; Yock, P.G.; Lansky, A.J.; Popma, J.J.; Yeung, A.C.; Fitzgerald, P.J.; Honda, Y. Impact of Stent Size Selection on Acute and Long-Term Outcomes after Drug-Eluting Stent Implantation in de Novo Coronary Lesions. Circ. Cardiovasc. Interv. 2017, 10, e004795. [Google Scholar] [CrossRef]
- Applegate, R.J.; Sacrinty, M.T.; Kutcher, M.A.; Santos, R.M.; Gandhi, S.K.; Little, W.C. Effect of Length and Diameter of Drug-Eluting Stents versus Bare-Metal Stents on Late Outcomes. Circ. Cardiovasc. Interv. 2009, 2, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Moses, J.W.; Leon, M.B.; Popma, J.J.; Fitzgerald, P.J.; Holmes, D.R.; O’Shaughnessy, C.; Caputo, R.P.; Kereiakes, D.J.; Williams, D.O.; Teirstein, P.S.; et al. Sirolimus-Eluting Stents versus Standard Stents in Patients with Stenosis in a Native Coronary Artery. N. Engl. J. Med. 2003, 349, 1315–1323. [Google Scholar] [CrossRef] [Green Version]
- Holmes, D.R.; Leon, M.B.; Moses, J.W.; Popma, J.J.; Cutlip, D.; Fitzgerald, P.J.; Brown, C.; Fischell, T.; Wong, S.C.; Midei, M.; et al. Analysis of 1-Year Clinical Outcomes in the SIRIUS Trial. Circulation 2004, 109, 634–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, I.J.; Koh, Y.S.; Lim, S.; Kim, J.J.; Chang, M.; Kang, M.; Hwang, B.H.; Kim, C.J.; Kim, T.H.; Seo, S.M.; et al. Impact of the Stent Length on Long-Term Clinical Outcomes Following Newer-Generation Drug-Eluting Stent Implantation. Am. J. Cardiol. 2014, 113, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Song, H.G.; Kang, S.J.; Ahn, J.M.; Kim, W.J.; Lee, J.Y.; Park, D.W.; Lee, S.W.; Kim, Y.H.; Lee, C.W.; Park, S.W.; et al. Intravascular Ultrasound Assessment of Optimal Stent Area to Prevent In-Stent Restenosis after Zotarolimus-, Everolimus-, and Sirolimus-Eluting Stent Implantation. Catheter. Cardiovasc. Interv. 2014, 83, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, L.; Song, Y.; Chen, L.; Xue, Q.; Tian, J.; Wang, Y.; Chen, Y. Efficacy and Safety of Limus-Eluting versus Paclitaxel-Eluting Coronary Artery Stents in Patients with Diabetes Mellitus: A Meta-Analysis. Int. J. Cardiol. 2015, 184, 680–691. [Google Scholar] [CrossRef]
- Nakatsuma, K.; Shiomi, H.; Natsuaki, M.; Morimoto, T.; Igarashi, K.; Kadota, K.; Muramatsu, T.; Tanabe, K.; Morino, Y.; Akasaka, T.; et al. Second-Generation versus First-Generation Drug-Eluting Stents in Patients with and without Diabetes Mellitus: Pooled Analysis from the RESET and NEXT Trials. Cardiovasc. Interv. Ther. 2018, 33, 125–134. [Google Scholar] [CrossRef] [PubMed]
- De Innocentiis, C.; Zimarino, M.; De Caterina, R. Is Complete Revascularisation Mandated for All Patients with Multivessel Coronary Artery Disease? Interv. Cardiol. Rev. 2018, 13, 45–50. [Google Scholar] [CrossRef]
- Mikhail, G.W.; Airoldi, F.; Tavano, D.; Chieffo, A.; Rogacka, R.; Carlino, M.; Montorfano, M.; Sangiorgi, G.; Corvaja, N.; Michev, I.; et al. The Use of Drug Eluting Stents in Single and Multivessel Disease: Results from a Single Centre Experience. Heart 2004, 90, 990–994. [Google Scholar] [CrossRef]
- TsiGkas, G.G.G.; Karantalis, V.; Hahalis, G.; Alexopoulos, D. Stent Restenosis, Pathophysiology and Treatment Options: A 2010 Update. Hell. J. Cardiol. 2011, 52, 149–157. [Google Scholar]
- Kastrati, A.; Schoemig, A.; Elezi, S.; Dirschinger, J.; Mehilli, J.; Schuhlen, H.; Blasini, R.; Neumann, F.J. Prognostic Value of the Modified American College of Cardiology/American Heart Association Stenosis Morphology Classification for Long-Term Angiographic and Clinical Outcome after Coronary Stent Placement. Circulation 1999, 100, 1285–1290. [Google Scholar] [CrossRef] [Green Version]
- Alraies, M.; Darmoch, F.; Tummala, R.; Waksman, R. Diagnosis and Management Challenges of In-Stent Restenosis in Coronary Arteries. World J. Cardiol. 2017, 9, 640–647. [Google Scholar] [CrossRef]


| Study | Year of Publication | Design | Center Involved | Country | Sample | DES Type | Follow-Up Duration | ISR Definition | Modified Jadad Scale | NOS |
|---|---|---|---|---|---|---|---|---|---|---|
| Chen et al. [14] | 2014 | RCT | Single-center | China | 768 | Sirolimus, paclitaxel | 12 months | Stenosis >50% of the luminal diameter of the target lesion at follow up | 6 | NA |
| Cheng et al. [15] | 2019 | Cohort | Single-center | China | 1132 | NA | 1 year | Stenosis >50% of the luminal diameter of the target lesion at follow up | NA | 8 |
| Doi et al. [16] | 2008 | RCT | Multicenter | United States | 331 | NA | 3 years | If the lesion diameter stenosis was ≥70%, no additional clinical evidence of ischemia was needed. If the lesion diameter stenosis was >50% but <70%, one of the following pieces of evidence was needed: (1) positive functional study corresponding to the area served by the target lesion; (2) ischemic ECG changes at rest in a distribution consistent with the target vessel; or (3) ischemic symptoms referable to the target lesion. If the lesion diameter stenosis was ≤50%, a markedly positive functional study or ECG changes corresponding to the area served by the target vessel was needed. | 6 | NA |
| Du et al. [17] | 2019 | Cohort | Single-center | China | 818 | Sirolimus, paclitaxel, everolimus | 6 months | Stenosis >50% of the luminal diameter of the target lesion at follow up | NA | 7 |
| Gil et al. [18] | 2020 | RCT | Multicenter | Poland | 445 | Paclitaxel | 12 months | Stenosis >50% of the luminal diameter of the target lesion at follow up | 5 | NA |
| Gomez-Lara et al. [19] | 2011 | Cohort | Multicenter | Multinational | 2292 | Everolimus, zotarolimus | 5 years | Stenosis ≥50% of the luminal diameter of the target lesion at follow up | NA | 7 |
| Hong et al. [20] | 2006 | Cohort | Multicenter | Korea | 211 | Sirolimus, paclitaxel | 6 months | Stenosis >50% of the luminal diameter of the target lesion at follow up | NA | 7 |
| Hoppmann et al. [21] | 2009 | Cohort | Multicenter | Germany | 1683 | Sirolimus, paclitaxel | 6, 8, and 36 months | Stenosis >50% of the luminal diameter of the target lesion at follow up | NA | 8 |
| Ino et al. [22] | 2011 | Cohort | Single-center | Japan | 469 | Sirolimus | 6–9 months | Stenosis >50% of the luminal diameter of the target lesion at follow up | NA | 7 |
| Jensen et al. [23] | 2012 | RCT | Multicenter | Denmark | 2774 | Everolimus, sirolimus | 18 months | Stenosis ≥50% of the luminal diameter of the target lesion at follow up | 6 | NA |
| Kang et al. [24] | 2011 | Cohort | Multicenter | Republic of Korea | 366 | Paclitaxel, sirolimus, zotarolimus | 13–13.5 months | Stenosis >50% of the luminal diameter of the target lesion at follow up | NA | 7 |
| Kastrati et al. [8] | 2006 | Cohort | multicenter | Germany | 1845 | Sirolimus, paclitaxel | 6–8 months | Stenosis >50% of the luminal diameter of the target lesion at follow up | NA | 7 |
| Lee et al. [25] | 2006 | Cohort | Single center | Republic of Korea | 1228 | Sirolimus, paclitaxel | 6 months | Stenosis >50% of the luminal diameter of the target lesion at follow up | NA | 8 |
| Lee et al. [26] | 2011 | Cohort | Single-center | Republic of Korea | 402 | Sirolimus, paclitaxel, zotarolimus | 1 years | Stenosis ≥50% of the luminal diameter of the target lesion at follow up | NA | 7 |
| Liu et al. [27] | 2013 | Cohort | Single center | China | 687 | NA | 9–24 months | Stenosis >50% of the luminal diameter of the target lesion at follow up | NA | 7 |
| Mehta et al. [28] | 2007 | RCT | Multicenter | United States | 1306 | Zotarolimus | 9 months | Stenosis >50% of the luminal diameter of the target lesion at follow up | 6.5 | NA |
| Qin et al. [29] | 2017 | Cohort | Single-center | China | 1206 | NA | 2 years | Stenosis ≥50% of the luminal diameter of the target lesion at follow up | NA | 7 |
| Rathore et al. [30] | 2009 | Cohort | Single-center | Japan | 1885 | Sirolimus, paclitaxel | 9 months | Stenosis >50% of the luminal diameter of the target lesion at follow up | NA | 8 |
| Sun et al. [31] | 2020 | Cohort | Single-center | China | 2010 | Sirolimus | 12 months | Stenosis ≥50% of the luminal diameter of the target lesion at follow up | NA | 7 |
| Yin et al. [32] | 2017 | Cohort | Single-center | China | 158 | NA | 5 years | Stenosis ≥50% of the luminal diameter of the target lesion at follow up | NA | 7 |
| Zeng et al. [33] | 2017 | Cohort | Single-center | China | 425 | NA | 10–12 months | Stenosis >50% of the luminal diameter of the target lesion at follow up | NA | 9 |
| Zhu et al. [34] | 2016 | Cohort | Single-center | China | 352 | NA | 18 months | Stenosis ≥50% of the luminal diameter of the target lesion at follow up. | NA | 8 |
| Variables | Number of Studies | In-Stent Restenosis | No In-Stent Restenosis | p-Value of Heterogeneity | I2 (%) | Odds Ratio | Model | 95% CI | p-Value of Eggers’s Test | p-Value | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | Total | Yes | Total | |||||||||
| Alcohol consumption | 6 | 169 (24) | 705 | 874 (23) | 3799 | 0.10 | 46 | 1.00 | Fixed | 0.82 to 1.23 | 0.57 | 0.97 |
| Bifurcation lesion | 10 | 252 (23) | 1084 | 1754 (21) | 8183 | <0.01 | 82 | 1.31 | Random | 0.84 to 2.03 | 0.62 | 0.24 |
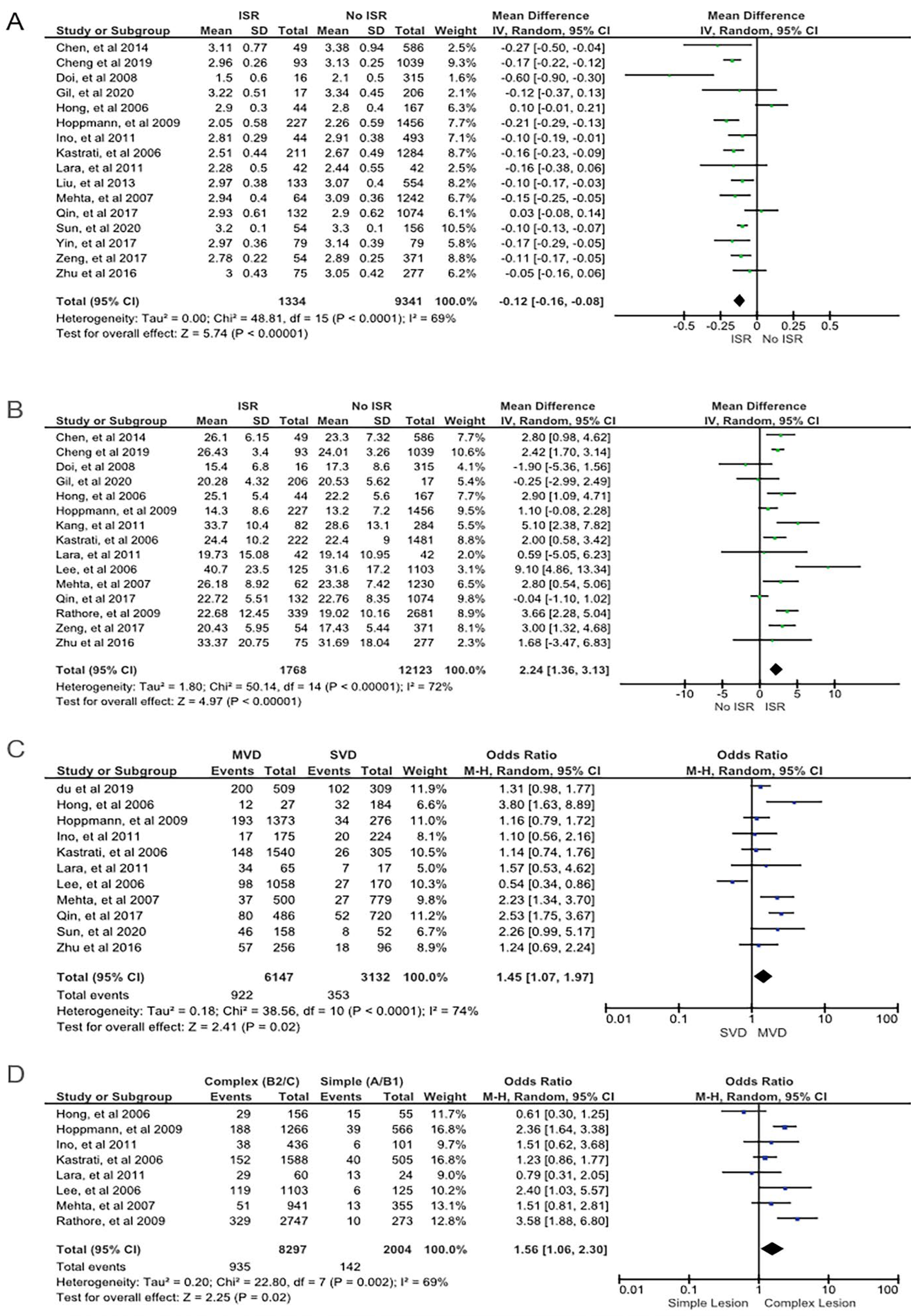
| Complex lesion (type B2/C) | 8 | 935 (87) | 1077 | 7362 (80) | 9224 | <0.01 | 69 | 1.56 | Random | 1.06 to 2.30 | 0.44 | 0.02 |
| Complex lesion (type C) | 5 | 230 (47) | 485 | 1437 (41) | 3518 | 0.85 | 0 | 1.33 | Fixed | 1.09 to 1.62 | 0.06 | <0.01 |
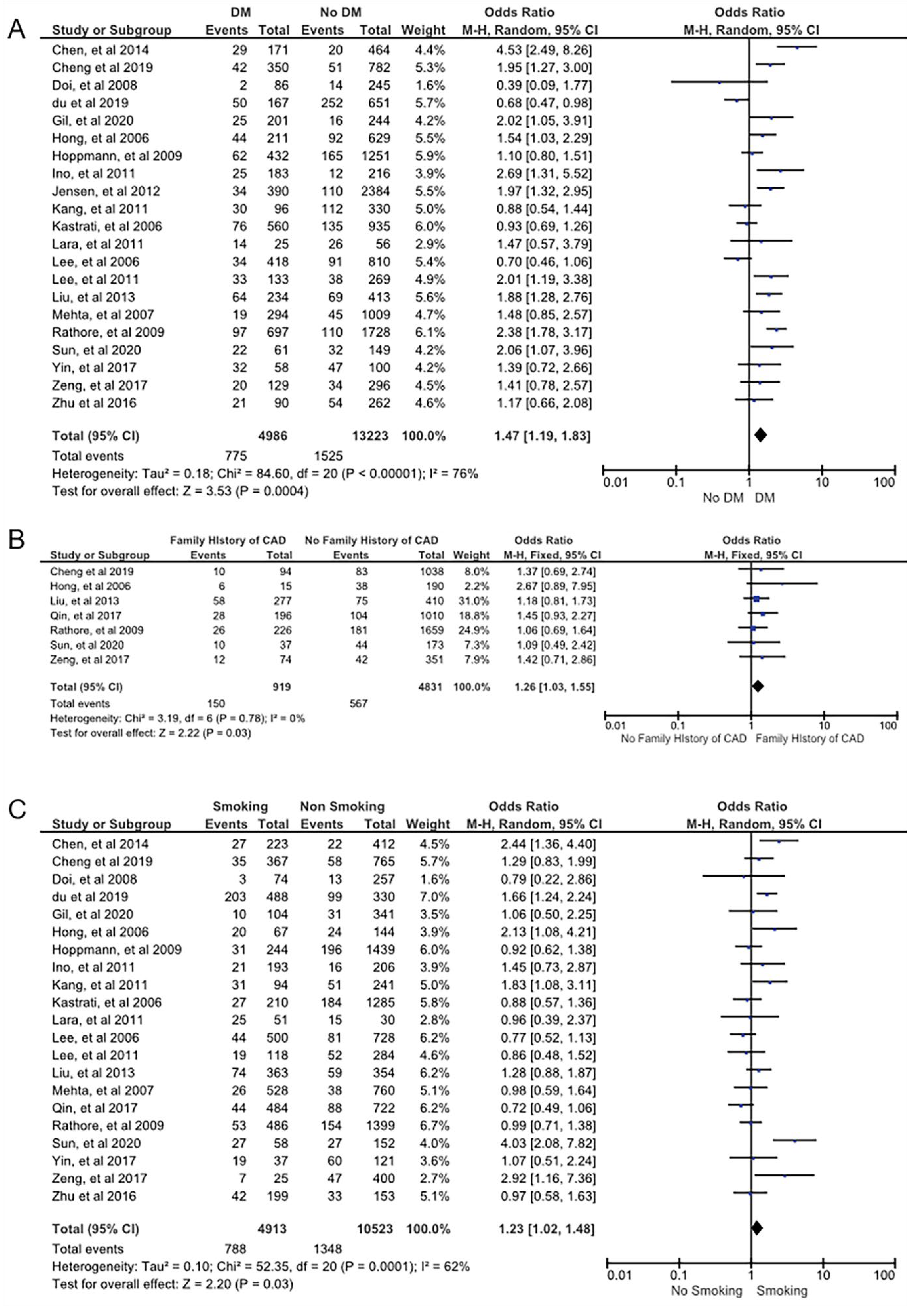
| DM | 21 | 775 (34) | 2300 | 4211 (26) | 15,909 | <0.01 | 76 | 1.47 | Random | 1.19 to 1.83 | 0.42 | <0.01 |
| Dyslipidemia | 16 | 874 (50) | 1742 | 6017 (53) | 11,412 | 0.03 | 45 | 1.04 | Random | 0.88 to 1.23 | 0.22 | 0.62 |
| Family history of CAD | 7 | 150 (21) | 717 | 769 (15) | 5033 | 0.79 | 0 | 1.26 | Fixed | 1.03 to 1.55 | 0.17 | 0.03 |
| Hypertension | 19 | 1294 (61) | 2116 | 8944 (66) | 13,505 | <0.01 | 86 | 1.10 | Random | 0.81 to 1.48 | 0.59 | 0.55 |
| Male gender | 21 | 1526 (75) | 2047 | 9308 (73) | 12,693 | <0.01 | 80 | 0.99 | Random | 0.75 to 1.30 | 0.55 | 0.94 |
| MVD | 11 | 922 (72) | 1275 | 5225 (65) | 8004 | <0.01 | 74 | 1.45 | Random | 1.07 to 1.97 | 0.42 | 0.02 |
| Smoking | 21 | 788 (37) | 2136 | 4125 (31) | 13,300 | <0.01 | 62 | 1.23 | Random | 1.02 to 1.48 | 0.32 | 0.03 |
| Stent type (limus-eluting stent) | 8 | 858 (70) | 1233 | 6755 (74) | 9137 | <0.01 | 80 | 0.83 | Random | 0.59 to 1.17 | 0.42 | 0.29 |
| Target vessel (LAD) | 15 | 738 (47) | 1558 | 5117 (46) | 11,080 | 0.24 | 19 | 1.02 | Fixed | 0.91 to 1.13 | 0.11 | 0.79 |
| Baseline Characteristics | Number of Studies | In-Stent Restenosis | No In-Stent Restenosis | p-Value of Heterogeneity | I2 (%) | Model | MD | 95% CI | p-Value of Eggers’s Test | p-Value | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | n | Mean ± SD | n | |||||||||
| Age | 21 | 64.19 ± 11.89 | 2112 | 63.49 ± 12.94 | 13,121 | <0.01 | 87 | Random | 0.52 | −0.90 to 1.95 | 3.13 | 0.47 |
| BMI | 7 | 25.86 ± 3.15 | 675 | 25.46 ± 4.56 | 2911 | <0.01 | 80 | Random | 0.54 | −0.15 to 1.23 | 0.20 | 0.12 |
| Stent diameter | 16 | 2.81 ± 0.39 | 1334 | 2.91 ± 0.42 | 9341 | <0.01 | 69 | Random | −0.12 | −0.16 to −0.08 | 0.18 | <0.01 |
| Stent length | 15 | 25.30 ± 10.92 | 1768 | 22.66 ± 9.84 | 12,123 | <0.01 | 72 | Random | 2.24 | 1.36 to 3.13 | 0.20 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rohman, M.S.; Waranugraha, Y.; Masbuchin, A.N.; Baskoro, S.S.; Sishartami, L.W.; Pratiwi, B.B. Coronary In-Stent Restenosis Predictors following Drug-Eluting Stent Implantation: A Meta-Analysis Study. J. Vasc. Dis. 2023, 2, 266-281. https://doi.org/10.3390/jvd2030020
Rohman MS, Waranugraha Y, Masbuchin AN, Baskoro SS, Sishartami LW, Pratiwi BB. Coronary In-Stent Restenosis Predictors following Drug-Eluting Stent Implantation: A Meta-Analysis Study. Journal of Vascular Diseases. 2023; 2(3):266-281. https://doi.org/10.3390/jvd2030020
Chicago/Turabian StyleRohman, Mohammad Saifur, Yoga Waranugraha, Ainun Nizar Masbuchin, Shalahuddin Suryo Baskoro, Lintang Widya Sishartami, and Bunga Bella Pratiwi. 2023. "Coronary In-Stent Restenosis Predictors following Drug-Eluting Stent Implantation: A Meta-Analysis Study" Journal of Vascular Diseases 2, no. 3: 266-281. https://doi.org/10.3390/jvd2030020





