Highlights
What are the main findings?
- We investigated whether changes in apelin levels in renal arterial perivascular adipose tissue (PVAT) are associated with the alteration of the favorable vasorelaxation response by the PVAT in the renal arteries of rats with metabolic syndrome (MetS). Furthermore, we examined whether apelin expression in PVAT was related to changes in kidney function in rats with MetS.
- This study demonstrated that apelin levels in the renal arterial PVAT were positively correlated with the PVAT-enhancing effects on relaxation in the renal arteries in rat models of human MetS. Furthermore, a high apelin level in the PVAT was related to indices of kidney function preservation, a high eGFR level, and a low urine protein level.
What is the implication of the main finding?
- High expression of apelin mRNA in PVAT is needed to maintain beneficial PVAT function (anti-contractile activity), resulting in the preservation of renal arterial tone and renal function;
- Angiotensin II-related AT1 receptor activity probably plays regulates apelin expression in PVAT.
Abstract
The perivascular adipose tissue (PVAT) regulates the arterial tone by releasing vasoactive molecules. PVAT dysfunction favoring the vasorelaxation response could contribute to the development of kidney disease in metabolic syndrome (MetS). Previously, we demonstrated that overactivation of angiotensin II signaling in the PVAT deteriorates the compensatory PVAT effects in rats with MetS (SHRSP.Z-Leprfa/IzmDmcr (SPZF) and SHR/NDmcr-cp (CP) rats). Apelin is an endogenous regulator of angiotensin II. Therefore, we investigated whether changes in apelin levels in the PVAT alter PVAT function and impair kidney function in MetS. Twenty-three-week-old male and female SPZF and CP rats were used. In the female CP rats, apelin mRNA levels in renal arterial PVAT, enhancing effects of the PVAT on acetylcholine-induced relaxation in renal arteries, and estimated glomerular filtration rate (eGFR) were the highest, and urine protein levels and homeostasis model assessment of insulin resistance (HOMA-IR) were the lowest. Apelin mRNA levels were positively correlated with the enhancing effects of the PVAT on vasorelaxation and eGFR but negatively correlated with urine protein levels and HOMA-IR. Moreover, apelin levels positively correlated with mRNA levels of angiotensin-converting enzyme 2 and angiotensin II type 1 receptor-associated protein, which are negative regulators of angiotensin II. This study suggests that a decline in apelin levels in the PVAT, probably owing to angiotensin II, is associated with PVAT dysfunction on vascular tone, resulting in impaired kidney function in MetS.
1. Introduction
Altered adipocyte functions, arising from hyperplasia and hypertrophy, contribute to the development of cardiovascular complications associated with metabolic syndrome (MetS) [1]. MetS is a significant risk factor for the development of chronic kidney disease (CKD) (odds ratio 2.08) in the general population [2]. Additionally, body mass index (BMI) is related to the development of kidney disease (odds ratio 1.23) in community-based populations [3]. Visceral obesity contributes to impaired renal function in Chinese adults of all ages and both sexes [4]. Furthermore, overactivation of the renin–angiotensin system (RAS) and insulin resistance are involved in the pathogenesis of obesity-related glomerulopathy, which leads to CKD [5,6,7]. Activation of the inflammatory process in obesity-related glomeruli has also been reported to play an important role in the development of CKD [8]. Para- and perirenal fat thicknesses are independent predictors of kidney dysfunction in type 2 diabetes [9]. Increased perirenal fat thickness and ectopic fat deposition in the kidneys are positively associated with albuminuria in patients with type 2 diabetes [10]. Perirenal fat had a higher predictive value for CKD than total, subcutaneous, or visceral fat in patients with type 2 diabetes [11].
The perivascular adipose tissue (PVAT), located around the blood vessels, regulates local blood vessel contractility by releasing vasoactive molecules [12,13,14]. The inhibitory role of the PVAT in the contractile response regulation of healthy rat aorta was first reported by Soltis et al. [15]. Human clinical data provide evidence that the PVAT surrounding the coronary arteries increases the risk of cardiovascular events [16,17]. Our previous work demonstrated that the mesenteric arterial PVAT provides compensatory effects for impaired acetylcholine (ACh)-induced nitric oxide (NO)-dependent vasorelaxation in SPZF rats (17 and 20 weeks of age), but this compensatory system becomes markedly attenuated with the progression of MetS (23 and 30 weeks of age) [18]. The dysfunction of the PVAT favoring the vasorelaxation response could be a reason for kidney disease development, as chronic ischemia resulting from reduced blood flow and hypoxic conditions contributes to the progression of CKD [19,20]. Perivascular renal sinus fat in individuals has been reported to play a role in microalbuminuria pathogenesis during the prediabetic stage [21]. Therefore, we proposed that the PVAT plays an important compensatory role when the vascular endothelial function is impaired in patients with MetS and previously demonstrated that renal arterial PVAT activity differs according to the severity of metabolic disorders and sex in rat models of human MetS, SHR/NDmcr-cp (CP), and SHRSP.Z-Leprfa/IzmDmcr (SPZF) rats [22]. We found that overactivation of angiotensin II (Ang II) type 1 receptor (AT1R) signaling, resulting from an increase in the AT1R to AT1R-associated protein (ATRAP) ratio in the renal arterial PVAT, may lead to a decline in compensatory PVAT effects [22].
Apelin is produced and released from adipocytes. Apelin acts as an endogenous negative regulator of Ang II through promoting the production of angiotensin-converting enzyme 2 (ACE2), which enhances the degradation of Ang II [23]. A recent review focused on apelin as a promising potential therapeutic target for kidney disease [24], and apelin analogs and receptor agonists have been discussed as therapeutic strategies [25,26]. In vivo and in vitro studies have demonstrated that apelin induces NO-dependent vasorelaxation in animals and humans [27,28,29]. We previously demonstrated that apelin increases ACh-induced vasorelaxation, and decreased apelin expression in the PVAT is involved in the deterioration of the enhancing effects of vasorelaxation in the mesenteric arteries of SPZF rats with MetS [30,31].
Therefore, in the present study, we investigated whether changes in apelin levels in the renal arterial PVAT are associated with the alteration of the favorable vasorelaxation response by the PVAT in the renal arteries of rats with MetS, similar to the findings in the mesenteric artery. Furthermore, we examined whether apelin expression in the PVAT was related to changes in kidney function in rats with MetS. This study was designed as an add-on to a previous study that focused on the relationship between PVAT function and Ang II signaling in the renal arteries of SPZF and CP rats [22], which are models for MetS.
2. Materials and Methods
2.1. Experimental Animals
Twenty-three-week-old male and female SPZF and CP rats (n = 16 of each line, n = 8 of each sex), established by the Disease Model Cooperative Research Association (Kyoto, Japan), were purchased from Japan SLC, Inc. (Hamamatsu, Japan). This study was designed as an add-on to our previous study published in Biomolecules [22], and additional rats were not used. They were housed under specific pathogen-free conditions on a 12 h day/night cycle with food (standard chow, CE-2, Clea Japan Inc., Tokyo, Japan) and water available ad libitum in an animal house. The age range of animals is based on published aging time-course studies, where the breakdown of the PVAT compensatory system was observed in the mesenteric arteries of male SPZF rates [18] but preserved in female SPZF rats [31]. The SPZF line was established by breeding stroke-prone spontaneously hypertensive rats with Zucker obese rats as a preclinical model of human hypertension with MetS [32]. The CP line is an inbred subline of SHR/N-corpulent rats with a corpulent (cp) mutation in the leptin receptor (Lepr), which is an autosomal recessive trait that causes Lepr deficiency.
2.2. Metabolic Parameters and Kidney Function
The data of body weight, waist circumference–body length ratio, systolic blood pressure (sBP), and serum glucose and insulin levels are reproduced from our previous publication in Biomolecules [22]. sBP was measured using the tail-cuff method (MK-2000; Muromachi, Tokyo, Japan). Serum glucose and insulin levels were measured using commercial kits (Rat Insulin ELISA Kit, Morinaga Biochemistry Lab., Tokyo, Japan, and Glucose C-II, FUJIFILM Wako Chemicals, Co., Osaka, Japan). In the present study, total cholesterol (T-Chol), triglyceride (TG), albumin, glycated albumin (GA), and creatinine levels were measured in serum collected in our previous study [22]. T-Chol and TG were measured using commercial kits (Triglyceride E and Cholesterol E, FUJIFILM Wako Chemicals, Co.). Serum albumin, GA, and creatinine were measured using enzymatic and Bromocresol Green methods at Oriental Yeast Co., Ltd. (Nagahama, Japan). GA was expressed as the percentage of GA in total albumin. The estimated glomerular filtration rate (eGFR) was calculated using serum creatinine levels after referring to the calculation formula by The Japanese Society of Nephrology and Pharmacotherapy [https://jsnp.org/egfr/] (accessed on 20 October 2024). The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated using serum insulin and glucose levels determined in a previous study [22].
The data on urine protein and glucose levels are reproduced from our previous publication in Biomolecules [22]. Protein and glucose levels in urine were recorded using UROPAPER III ‘Eiken’ (E-UR80, Eiken Chemical Co., Ltd., Tochigi, Japan) on a 6-point scale (minus [none detected] = 0; 15 mg/100 mL = 1; 30 mg/100 mL = 2; 100 mg/100 mL = 3; 300 mg/100 mL = 4; 1000 mg/100 mL = 5) and on a 5-point scale (minus [none detected] = 0; 50 mg/100 mL = 1; 100 mg/100 mL = 2; 250 mg/100 mL = 3; 500 mg/100 mL = 4), respectively, according to the manufacturer’s instructions [18].
2.3. Vasorelaxation in Arteries
Relaxation in response to ACh was determined using the organ bath method described in a previous study [22]. Briefly, renal arteries with or without intact PVAT were cut into 3 mm rings and mounted isometrically at an optimal resting tension (0.3 g) in 10 mL organ baths filled with Krebs buffer. After contraction was evoked by phenylephrine (3 µM), ACh was added cumulatively into the baths containing the arterial rings, and isometric tension changes were measured. In the present study, the relaxation-enhancing effects of PVAT were assessed based on the differences in the maximum response of renal arteries with or without PVAT to ACh.
2.4. Quantitative Real-Time PCR Assay
Renal arterial PVATs, corrected for in a previous study [22], were used. Total RNA was extracted from frozen tissues and then purified using an RNeasy fibrous kit following the manufacturer’s instructions (Qiagen K.K., Tokyo, Japan). To assess the purity of RNA, the ratio of absorbance at 260 nm and 280 nm was calculated, and a ratio of ~2.0 was considered pure. The mRNA transcript levels of apelin in renal arterial PVAT were measured by quantitative real-time PCR using a TaqMan RNA-to-CT 1-step kit and a LightCycler 1.5 (Roche Diagnostics Japan K.K., Tokyo, Japan). A triad housekeeping gene expression approach (ribosomal protein 18S, β-glucuronidase, and β-actin) was used to normalize the sample material, and the efficiency of each primer set was included in all calculations. Data were combined from three independent experiments.
We used the following commercially available gene-specific probes: Roche Applied Science, Universal ProbeLibrary product ID: apelin, 04686896001; ACE1, 433182_Ace1; ACE2, 433182_Ace2; AT1R, 04688503001; AT2R, 4331182_Agtr2; and ATRAP, 04684982001; ribosomal protein 18S, 04688937001; β-glucuronidase, 04688015001; β-actin, 04686900001; and gene-specific primers designed by the Assay Design Center (Roche Applied Science, San Francisco, CA, USA) were purchased from Life Technologies Japan, Ltd. (Tokyo, Japan).
2.5. Data Analyses
Data are expressed as mean ± standard error of the mean (SEM). The means between groups were analyzed using one-way analysis of variance (ANOVA), followed by the Bonferroni post hoc test using GraphPad Prism (GraphPad 10 for macOS, GraphPad, San Diego, CA, USA). Linear regression analysis was performed using GraphPad Prism software. Statistical significance was set at p < 0.05.
2.6. Drugs
Additional reagents and chemicals were purchased from suppliers, as follows: L-phenylephrine hydrochloride (Sigma-Aldrich Co., LLC., St. Louis, MO, USA) and acetylcholine chloride (Daiichi Pharmaceutical Co., Ltd., Tokyo, Japan). Other chemicals of analytical reagent grade were purchased from Nacalai Tesque, Inc. (Kyoto, Japan).
3. Results
3.1. Metabolic Parameters and Kidney Functions
Table 1 shows the characteristics of the rats. The CP rats had significantly heavier body weights than the SPZF rats, and the females of both strains had significantly lower weights than the males. There were no significant differences in waist circumference–body length ratio, which was calculated as an index of abdominal obesity, among the four groups. sBP levels in the male and female CP rats were significantly lower than those in the male and female SPZF rats, respectively.

Table 1.
Characteristics of male and female SHRSP.Z-Leprfa/IzmDmcr rats (SPZF) and SHR/NDmcr-cp rats (CP) at 23 weeks of age.
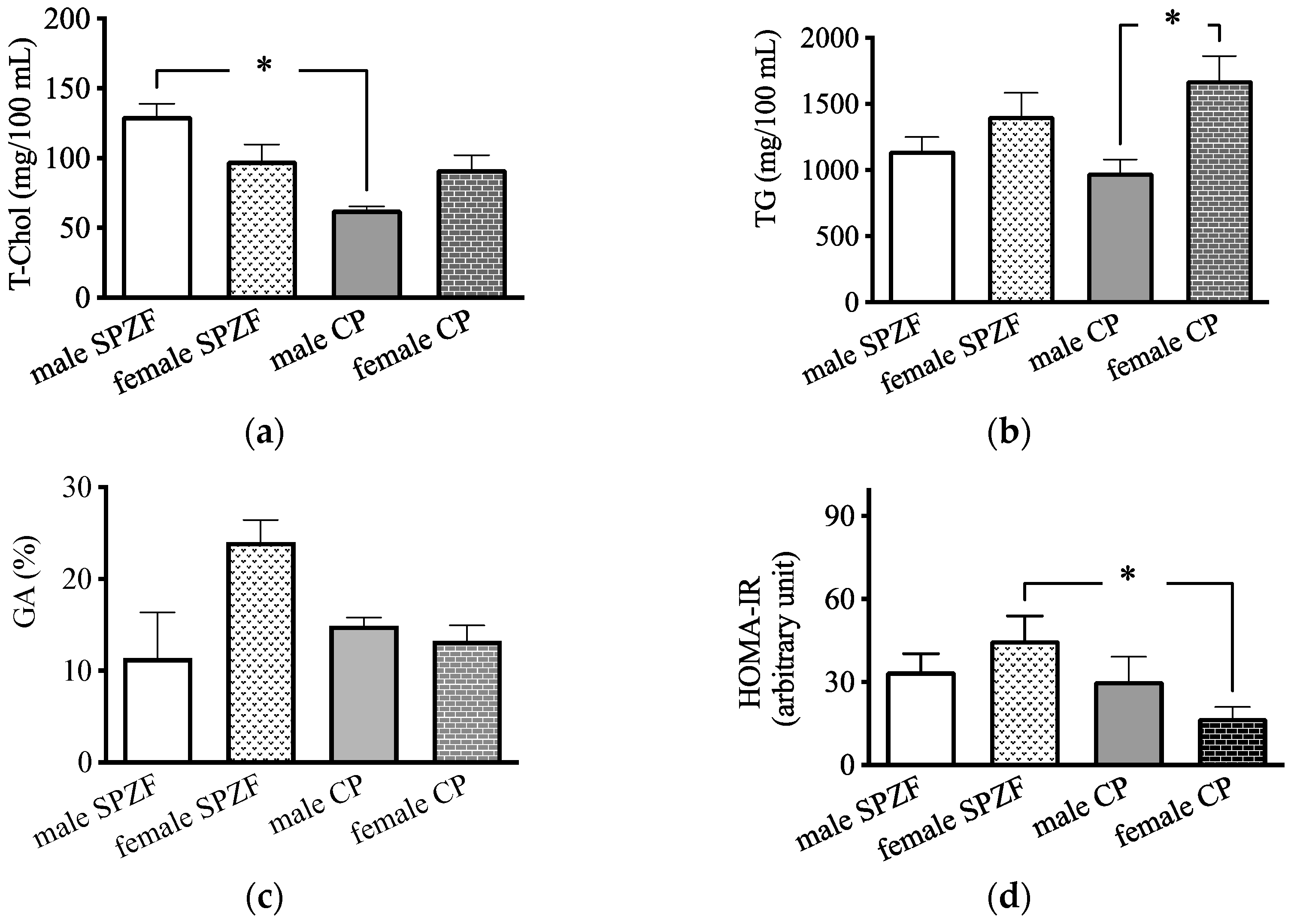
Serum T-Chol levels in the male CP group were significantly lower than those in the male SPZF group (Figure 1a), and serum TG levels in the female CP group were significantly higher than those in the male CP group (Figure 1b). There were no significant differences in the GA levels among the four groups (Figure 1c); however, the HOMA-IR in the female CP group was significantly lower than that in the other groups (Figure 1d).
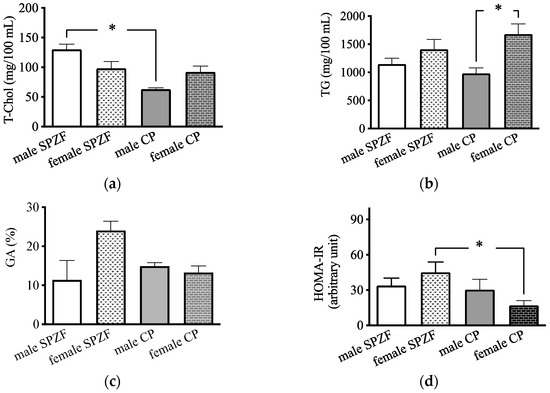
Figure 1.
Serum total cholesterol (T-Chol, (a)), serum triglyceride (TG, (b)), serum glycated albumin (GA, (c)), and homeostasis model assessment of insulin resistance (HOMA-IR, (d)) in male and female SHRSP.Z-Leprfa/IzmDmcr (SPZF) rats and SHR/NDmcr-cp (CP) rats at 23 weeks of age (rats/group, n = 8). Data are expressed as means ± SEM. * p < 0.05. Statistical comparisons of means between groups were performed using one-way ANOVA followed by Bonferroni post hoc test.
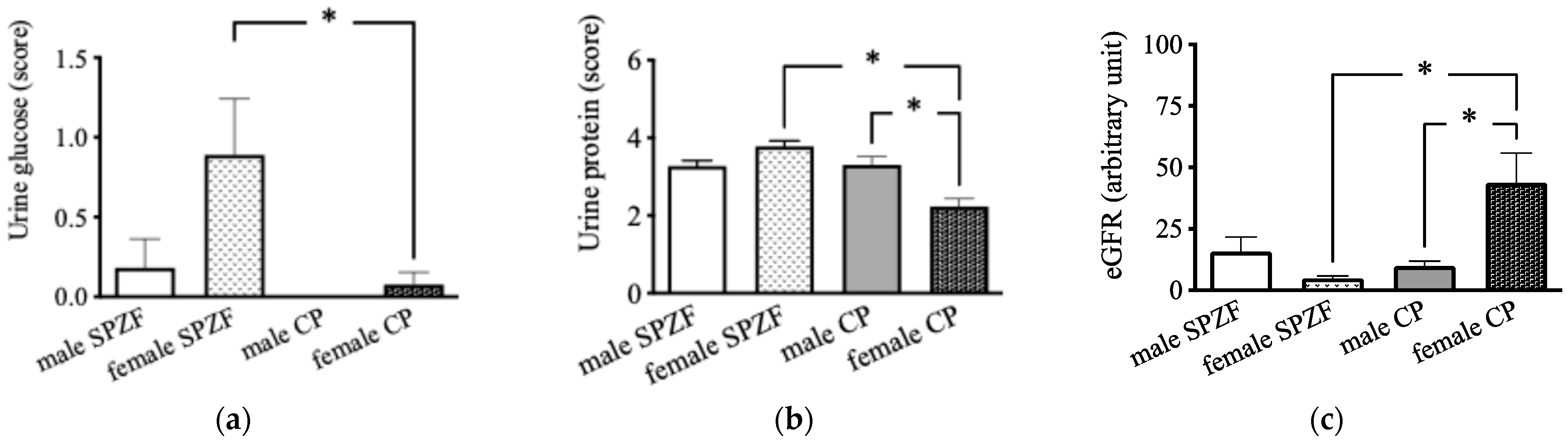
On contrast, urine glucose levels in the female SPZF rats were significantly higher than those in the male and female CP rats (Figure 2a). Urine protein levels were the lowest in the female CP group (Figure 2b). The eGFR in the female CP group was significantly higher than that in the other groups (Figure 2c).
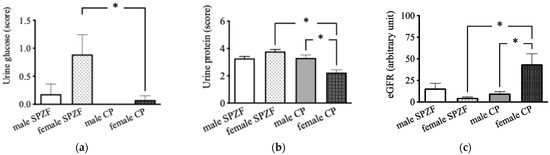
Figure 2.
Urine glucose (a), urine protein (b), and estimated glomerular filtration rate (eGFR, (c)) in male and female SHRSP.Z-Leprfa/IzmDmcr (SPZF) rats and SHR/NDmcr-cp (CP) rats at 23 weeks of age (rats/group, n = 8). Data are expressed as means ± SEM. * p < 0.05. Statistical comparisons of means between groups were performed using one-way ANOVA followed by Bonferroni post hoc test. Data on urine glucose and protein levels reported are reproduced from our previous publication in Biomolecules [22].
3.2. Changes in Apelin Expression in PVAT and Enhancing Effects of PVAT on Vasorelaxation
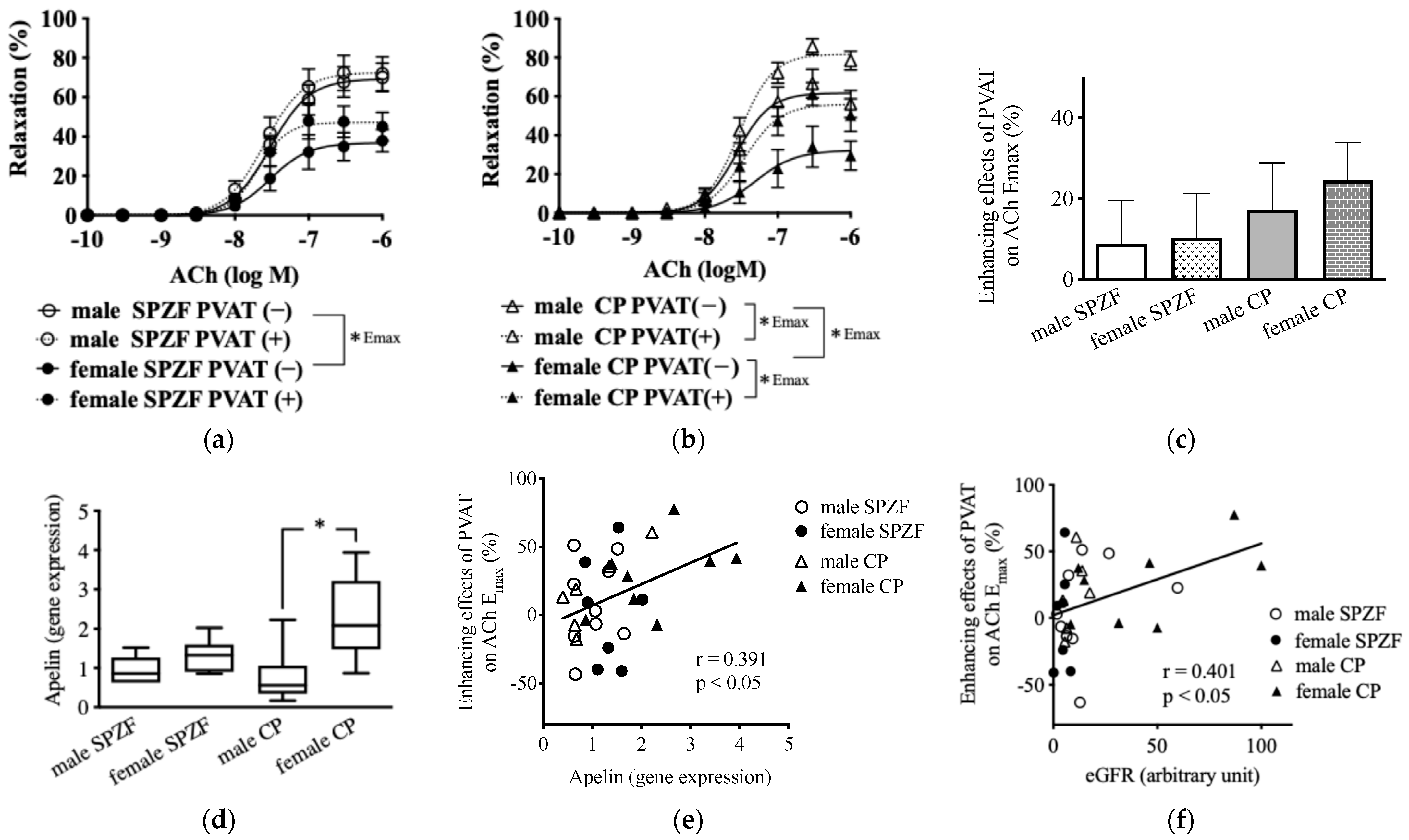
In renal arteries without PVAT, as indicated by the bold line, Ach-induced relaxation was significantly lower in females than in males in both strains. In contrast, Ach-induced relaxation in the presence of PVAT, as indicated by the dash line, was significantly increased in both sexes in CP rats but not in SPZF rats (Figure 1a,b). The enhancing effects of the PVAT on Ach-induced relaxation, assessed based on the differences in the maximum response of renal arteries with or without PVAT to Ach, tended to increase in the female CP group among all groups (Figure 3c). The mRNA levels of apelin in the renal arterial PVAT of the female CP group were significantly higher than those in the other groups (Figure 3d), and the enhancement of relaxation by the PVAT was positively correlated with apelin mRNA levels in the renal arterial PVAT (Figure 3e). The enhancement of relaxation by the PVAT was also positively correlated with eGFR (Figure 3f).
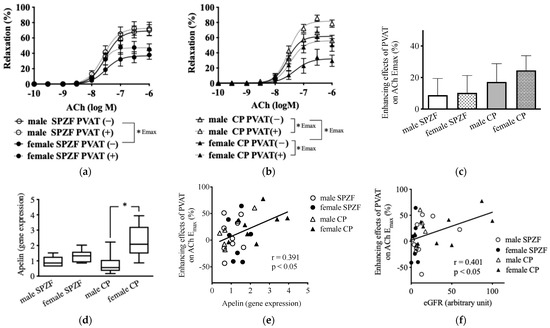
Figure 3.
Vascular relaxation in response to acetylcholine (ACh) in renal arteries with [PVAT(+)] or without [PVAT(−)] perivascular adipose tissue (PVAT) (a,b), PVAT-related enhancement of vasorelaxation in renal arteries (c), mRNA expression of apelin in renal arterial PVAT (d), and correlations between enhancement of vasorelaxation and apelin (e) or estimated glomerular filtration rate (eGFR, (f)) in male and female SHRSP.Z-Leprfa/IzmDmcr (SPZF) rats and SHR/NDmcr-cp (CP) rats at 23 weeks of age (rats/group, n = 8). Enhancing effects of PVAT were assessed by differences in maximum response to acetylcholine (ACh) in renal arteries with or without PVAT. * p < 0.05. Data are expressed as means ± SEM. * p < 0.05. Statistical comparisons of means between groups were performed using one-way ANOVA followed by Bonferroni post hoc test. Reported data of (a,b) are reproduced from our previous publication in Biomolecules [22].
3.3. Relationship Between Apelin Expression in PVAT and Kidney Function/Metabolic Parameter
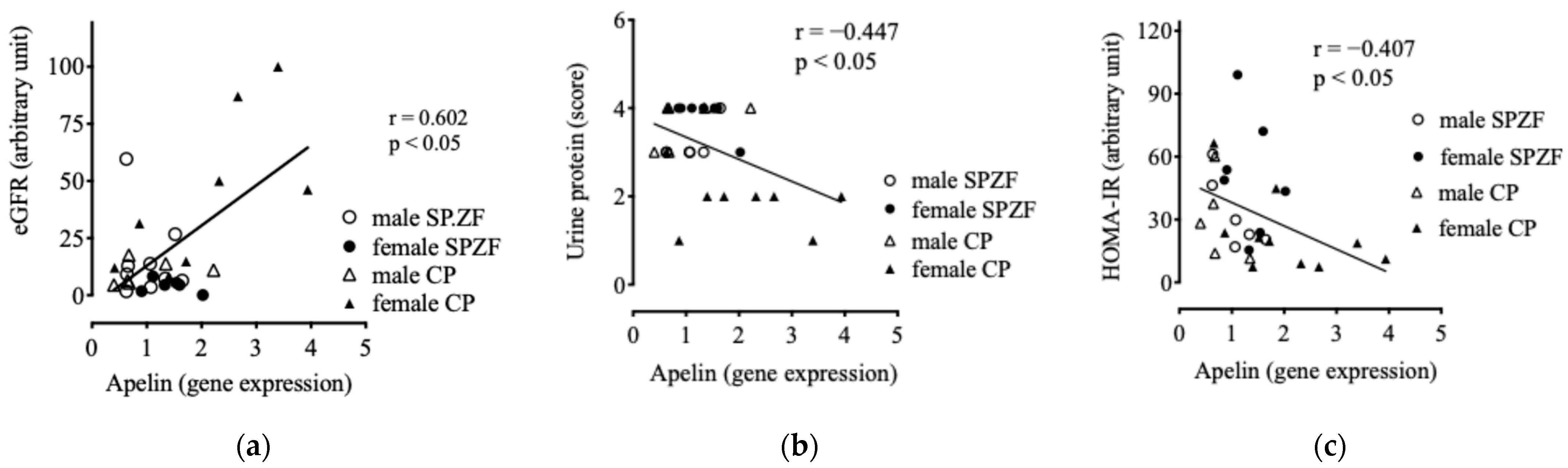
Apelin mRNA levels in the renal arterial PVAT positively correlated with eGFR (Figure 4a) and negatively correlated with urine protein levels (Figure 4b). Furthermore, apelin mRNA levels negatively correlated with HOMA-IR (Figure 4c), but did not correlate with sBP (p = 0.246), serum T-Chol levels (p = 0.551), and serum TG levels (p = 0.121).
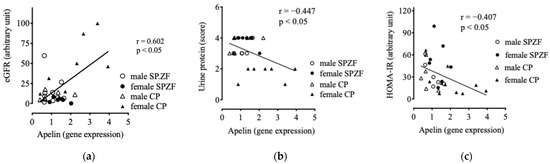
Figure 4.
Correlations between mRNA expression of apelin in renal arterial perivascular adipose tissues (PVATs) and estimated glomerular filtration rate (eGFR, (a)), urine protein (b), and homeostasis model assessment of insulin resistance (HOMA-IR, (c)) in male and female SHRSP.Z-Leprfa/IzmDmcr (SHRSP.ZF) rats and SHR/NDmcr-cp (CP) rats at 23 weeks of age (rats/group, n = 8).
3.4. Relationship Between Apelin and Ang II-Related Molecule Expression in PVAT
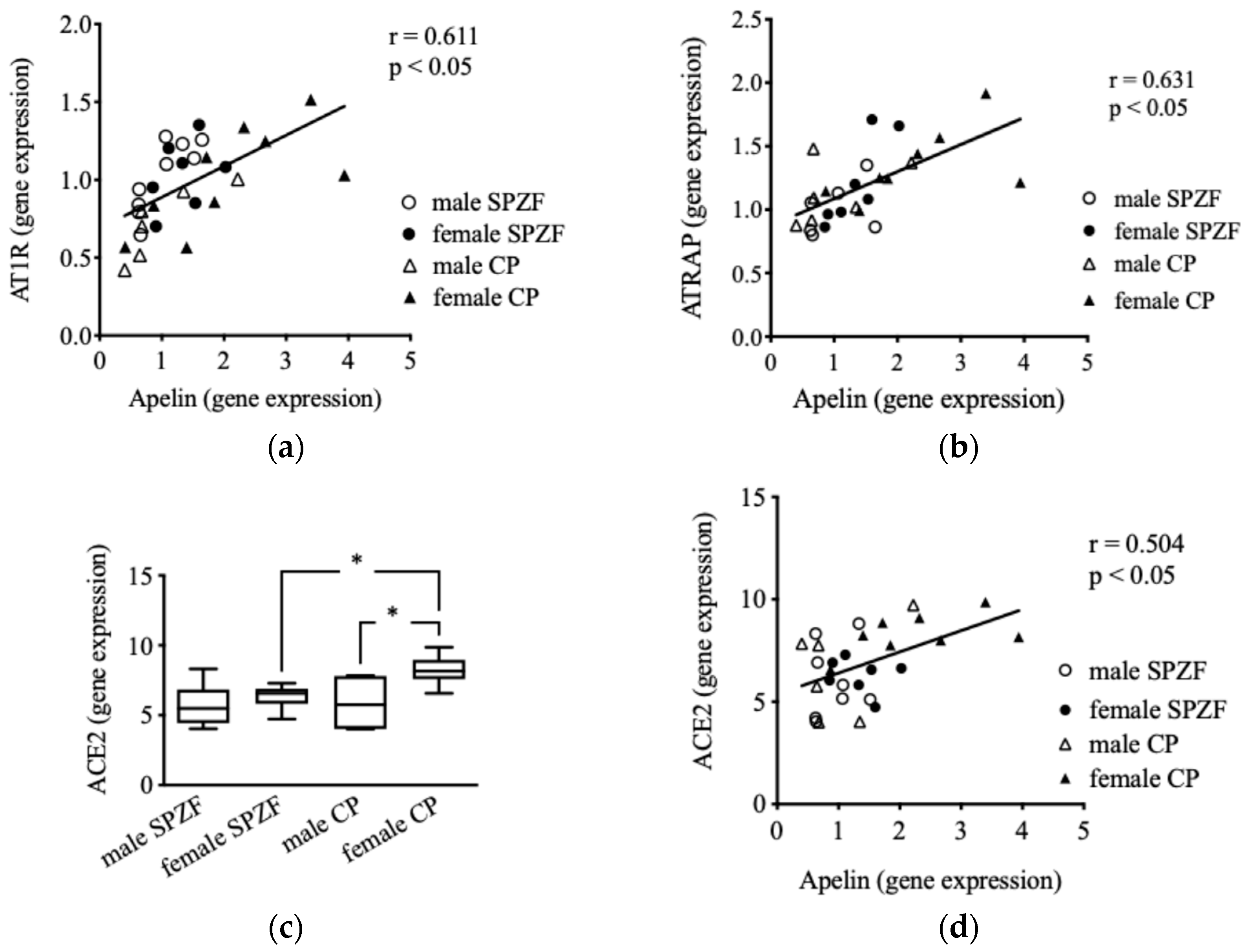
No significant difference was observed in mRNA levels concerning AT1R and ATRAP in the renal arterial PVAT among the groups [22]; however, AT1R (Figure 5a) and ATRAP (Figure 5b) mRNA levels positively correlated with apelin mRNA levels in the renal arterial PVAT. In contrast, no correlation was observed between AT2R mRNA and apelin levels (p = 0.531). Furthermore, no change in ACE1 mRNA levels was observed in the renal arterial PVAT (p = 0.132), but ACE2 mRNA levels in the female CP group were significantly higher than those in the other groups (Figure 5c). ACE1 mRNA levels did not correlate with apelin levels (p = 0.789), but a positive correlation was observed between apelin and ACE2 mRNA levels (Figure 5d). No correlation was observed between AT1R, AT2R, ATRAP, ACE1, or ACE2 mRNA levels in the renal arterial PVAT and eGFR or urine protein.
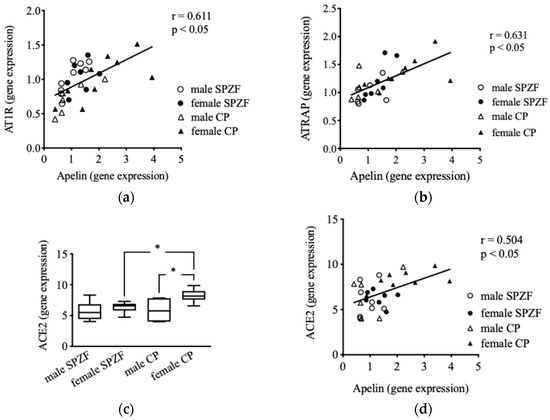
Figure 5.
Correlations between mRNA expression of apelin and angiotensin II type 1 receptor (AT1R) (a), AT1R-associated protein (ATRAP, (b)), and angiotensin-converting enzyme 2 (ACE2, (d)), as well as mRNA expression of ACE 2 (c) in renal arterial perivascular adipose tissues (PVAT) in male and female SHRSP.Z-Leprfa/IzmDmcr (SPZF) rats and SHR/NDmcr-cp (CP) rats at 23 weeks of age (rats/group, n = 8). * P < 0.05. Statistical comparisons of means between groups were performed using one-way ANOVA followed by Bonferroni post hoc test.
3.5. Relationship Between Metabolic Parameters and Kidney Function/Metabolic Parameter
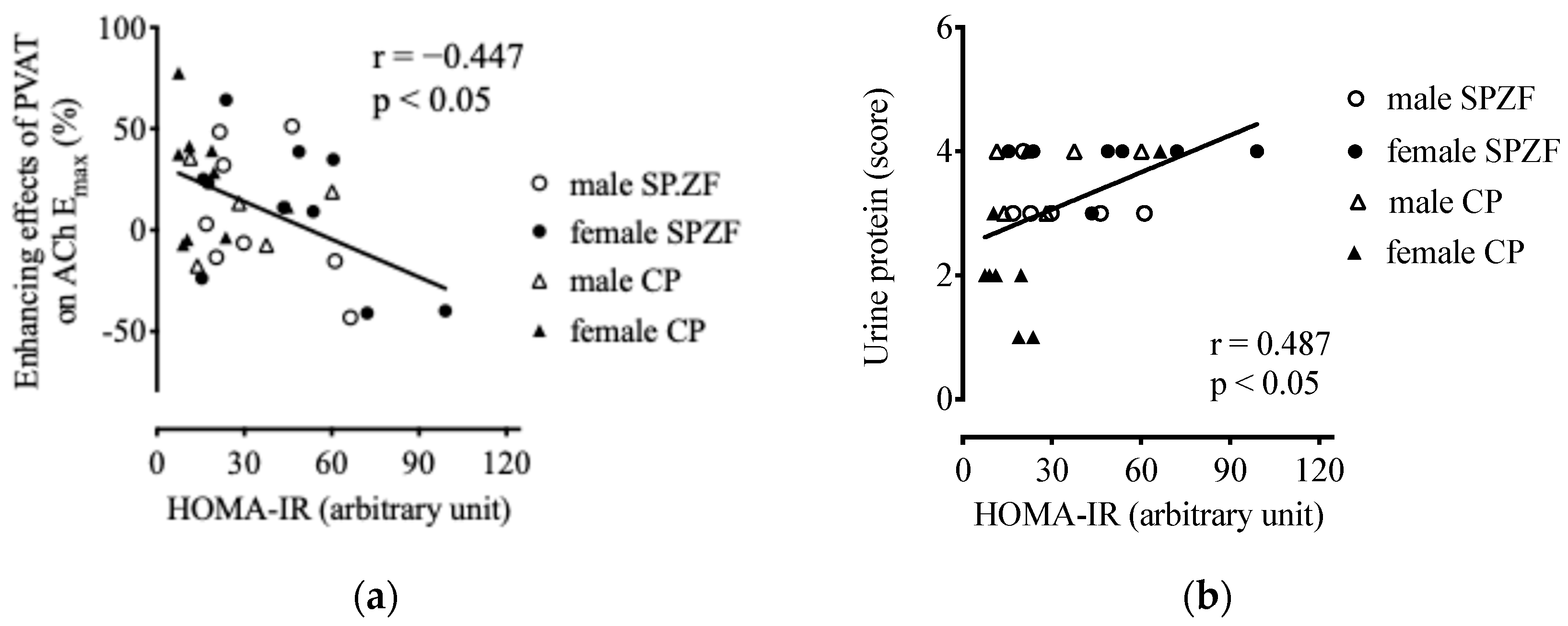
HOMA-IR negatively correlated with the enhancing effects of the PVAT on ACh-induced relaxation (Figure 6a). HOMA-IR was not related to eGFR (p = 0.07) but was positively related to urine protein levels (Figure 6b). Furthermore, there was no correlation between HOMA-IR and sBP (p = 0.263), serum T-Chol (p = 0.296), or serum TG levels (p = 0.532).
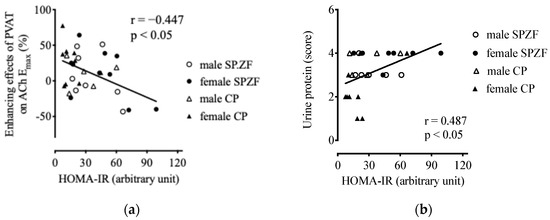
Figure 6.
Correlations between homeostasis model assessment of insulin resistance (HOMA-IR) and enhancement of vasorelaxation by PVAT (a) and urine protein (b) in male and female SHRSP.Z-Leprfa/IzmDmcr (SPZF) rats and SHR/NDmcr-cp (CP) rats at 23 weeks of age (rats/group, n = 8). Enhancing effects of PVAT were assessed by differences in maximum response to acetylcholine (ACh) in renal arteries with or without PVAT.
sBP was not significantly related to either eGFR (p = 0.070) or urine protein levels (p = 0.060). Furthermore, serum T-Chol level was not related to eGFR (p = 0.907) and urine protein levels (p = 0.732), but serum TG level was related to eGFR (r = 0.569, p < 0.05) and urine protein levels (r = −0.543, p < 0.05).
4. Discussion
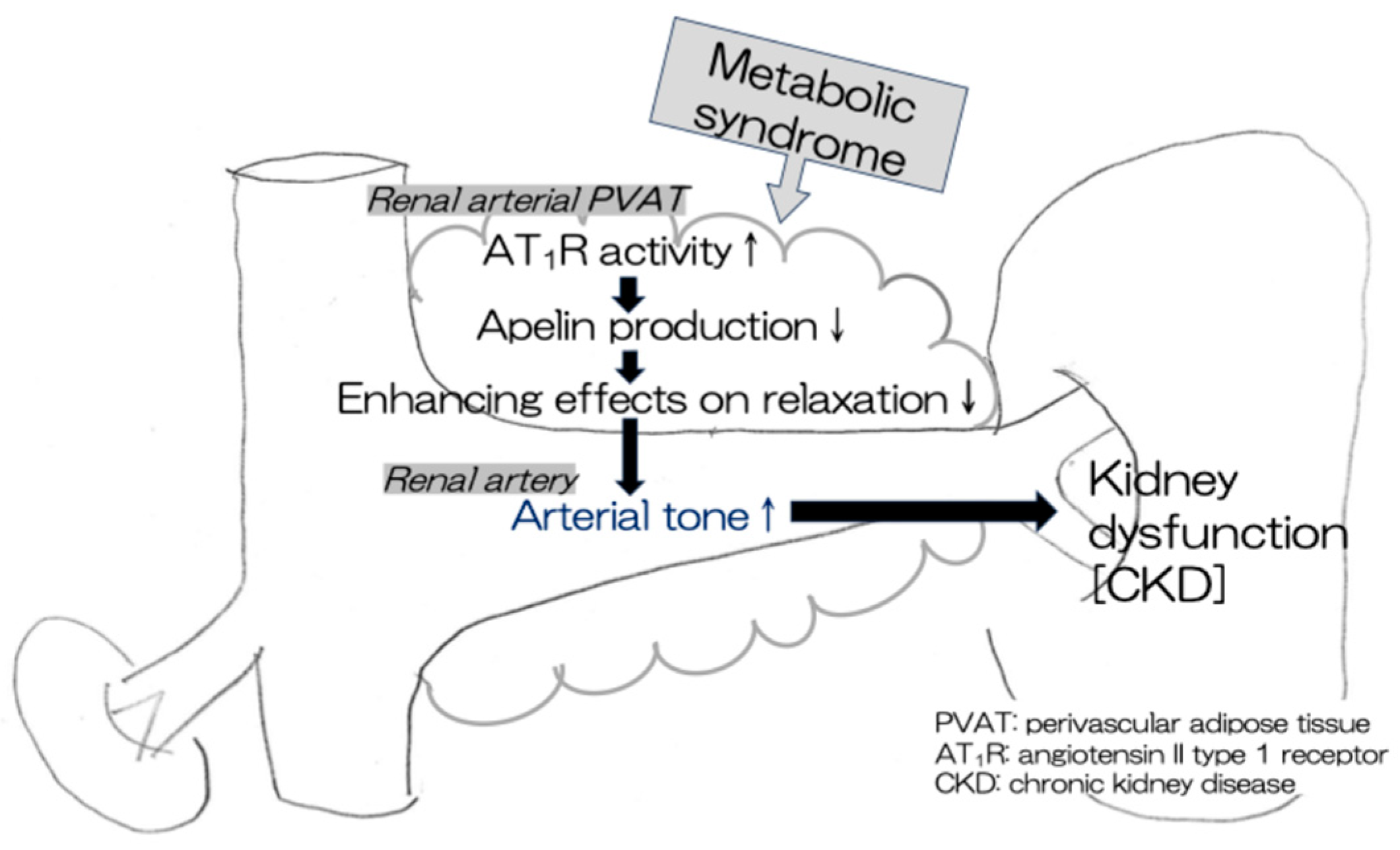
This study demonstrated that apelin levels in the renal arterial PVAT were positively correlated with the PVAT-enhancing effects on relaxation in renal arteries in rat models of human MetS (CP and SPZF rats). Furthermore, a high apelin level in the PVAT was related to a high eGFR level and a low urine protein level, which are indices of kidney function preservation. This study suggests that a high expression of apelin mRNA in the PVAT is needed to maintain the beneficial PVAT function (anti-contractile activity), resulting in the preservation of renal arterial tone and renal function. Apelin levels in the mesenteric arterial PVAT are associated with the alteration of the favorable vasorelaxation response by the PVAT in the mesenteric arteries of rats with MetS [25,27]. There may be no site-dependent differences in the mechanism by which the PVAT promotes the vasorelaxant response via apelin production/release. Additionally, we observed a positive correlation between apelin and either ACE2 or ATRAP levels, which are known as negative regulators of Ang II-related AT1R activity [23,26,33,34,35], in the renal arterial PVAT. Enhancement of Ang II-mediated AT1R activity in the PVAT probably involves a decrease in apelin expression, leading to the disappearance of the beneficial PVAT function induced by apelin in MetS (Figure 7).
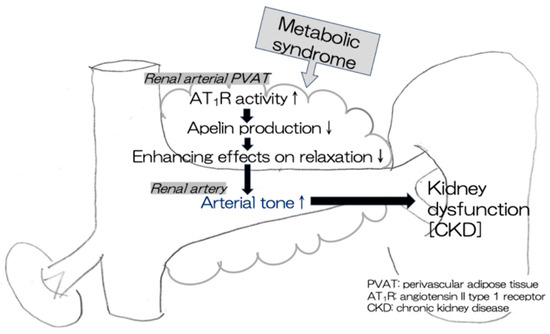
Figure 7.
A decline in compensatory PVAT effects leads to kidney dysfunction in MetS. ↑ increase, ↓ decrease.
Apelin, produced and released from adipocytes, acts as a vasodilator in the vasculature of both animals and humans. Apelin induces NO production via endothelial NO synthase activation, and apelin administration induces hypotension in mice in vivo experiments [36]. Similarly, apelin elicits concentration-dependent NO-mediated relaxation in isolated human arteries [28] and causes NO-dependent arterial vasodilation in vivo in humans [29]. Apelin expression in adipocytes is regulated by insulin [37], and its serum concentration correlates with increasing BMI in humans with obesity [37,38]. The present study shows that apelin mRNA levels were affected by the severity of insulin resistance in rats with MetS. Additionally, a high mRNA level of apelin in the renal arterial PVAT was positively related to the degree of compensatory PVAT vasorelaxation effects, coincident with non-reduced kidney function, which is defined as a high eGFR level and a low urine protein level. The enhancing effects of the PVAT on ACh-induced vasorelaxation were also positively correlated with eGFR. Together, these results suggest that the high expression of apelin in the renal arterial PVAT can maintain arterial tone, which may preserve kidney function. Apelin analogs and receptor agonists have recently been discussed as therapeutic strategies for cardiovascular diseases (CVDs), kidney diseases, and insulin resistance [25,26].
The apelin system plays the opposite role to the RAS in metabolic, kidney, and cardiovascular diseases [23,25,36,39]. It is well known that apelin and angiotensin II are vasodilatory and vasocontractile factors, respectively. Previous studies have discussed the crosstalk between the apelin system and RAS in organs and tissues, as apelin promotes the production of ACE2, which enhances the degradation of Ang II [23,26]. A positive correlation between apelin and ACE2 mRNA levels was observed in the renal arterial PVAT of MetS rats. Decreased ACE2 expression may be one reason for the activation of Ang II signaling in the PVAT. Conversely, one study reported that the cardiac apelin system is markedly downregulated in experimental heart failure and may be directly regulated by the AT1R system [40]. Overactivation of AT1R signaling, resulting from an increase in the AT1R-to-ATRAP ratio, has been observed in the renal arterial PVAT of CP and SPZF rats [22]. Since ATRAP suppresses the overactivation of adipose AT1R signaling [33,34,35], compensatory enhancement of AT1R activity resulted from a decrease in ATRAP expression, which probably led to a decline in apelin expression in the PVAT. The ATRAP mRNA and apelin levels positively correlated in the renal arterial PVAT of MetS. Furthermore, a decrease in the eGFR and an increase in urine protein, which indicates worsening renal function, correlated with the apelin level in the renal arterial PVAT but not with the AT1R, ATRAP, and ACE2 levels. Taken together, the activation of the AT1R system in the PVAT may cause the decrease in apelin expression and reduction in the vasodilatory effect of apelin by the PVAT, leading to worsening renal function in MetS.
MetS is a cluster of conditions, including excess abdominal fat, increased blood pressure, elevated blood sugar levels, and abnormal lipid levels. The potential effect of high blood pressure on kidney function may not be negligible, considering that CP and SPZF rats were used in the present study as animal models of MetS with hypertension. According to a recent clinical report, the comorbidities of hypertension and diabetes (hazard ratio 2.83) and hypertension only (hazard ratio 1.56) were significantly associated with the development of CKD in the general Japanese community, but diabetes only (hazard ratio 1.22) was not [41]. However, neither urine protein nor eGFR showed a significant relationship with sBP in rats with MetS. These results suggest that increased blood pressure is not an essential factor for the severity of renal dysfunction in MetS. Furthermore, serum T-Chol levels are not related to the impairment of kidney function in MetS rats. Thus, the present study shows that renal arterial PVAT-derived apelin is an important predictor of kidney dysfunction independent of blood pressure and lipid metabolism in MetS. Additionally, high serum GA level and HOMA-IR were coincident not only with high urine glucose level but also high urine protein level and low eGFR in female SPZF rats, which have low apelin mRNA levels in the PVAT. Contrastingly, even though of the same sex, female CP rats showed lower HOMA-IR, urine glucose, and protein levels, as well as high eGFR coincident with a high apelin mRNA level in the PVAT. These findings raise the possibility that female hormones do not have a critical role in the severity of metabolic parameters and apelin mRNA expression in the PVAT.
5. Limitations
The limitations of this study include the absence of protein level measurement of apelin in the PVAT. Further studies using apelin receptor antagonists are required to fully assess whether apelin contributes to the effect of the PVAT on vasorelaxation. Furthermore, clarity regarding the direct involvement of Ang II in decreasing apelin levels in the PVAT is warranted. The differences between adipocyte types, such as brown, white, or browning adipocytes, in the PVAT may possibly be associated with the release of apelin. Although no relationship was observed between eGFR and HOMA-IR, we cannot exclude the possibility that worsening insulin resistance directly induces renal dysfunction independent of apelin. At present, we cannot explain why serum TG levels are positively correlated with eGFR and negatively correlated with urine protein levels.
6. Conclusions
The findings of this study indicate that high apelin mRNA expression in the PVAT is required for beneficial PVAT function (anti-contractile activity), leading to renal arterial tone and renal function preservation in MetS. Apelin from the renal arterial PVAT may serve as an important predictor of renal function in patients with MetS. Ang II-related AT1R activity probably plays a role in regulating apelin expression in the PVAT.
Author Contributions
S.K. designed the experiments; S.K., K.M.-F. and R.F. performed the experiments and analyzed the data; S.K. and K.S. funded acquisition and supervised the study; S.K. interpreted the data and carried out writing—original draft preparation and writing—review and editing tasks. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partially supported by JSPS KAKENHI (grant number JP21K06777 to S.K., 1 April 2021).
Institutional Review Board Statement
All protocols involving the care and use of animals were approved by the Animal Ethics Committee and performed in accordance with the Guidelines for the Care and Use of Laboratory Animals at Mukogawa Women’s University (protocol numbers: P-12-2021-01-A, P-12-2022-01-A, and P-12-2023-01-A; 17 March 2021; 16 March 2022; 27 March 2023).
Informed Consent Statement
Not applicable as this study did not involve humans.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. The data are not publicly available due to specific ethical and privacy considerations.
Acknowledgments
We thank Mao Nishikawa, Miyu Nishimura, Aska Masazumi, Kaho Nojima, and Chihiro Suzuki at the Mukogawa Women’s University for their technical support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pérez-Torres, I.; Gutiérrez-Alvarez, Y.; Guarner-Lans, V.; Díaz-Díaz, E.; Manzano Pech, L.; Caballero-Chacón, S.D.C. Intra-abdominal fat adipocyte hypertrophy through a progressive alteration of lipolysis and lipogenesis in metabolic syndrome rats. Nutrients 2019, 11, 1529. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, T.; Kiyohara, Y.; Kubo, M.; Yonemoto, K.; Tanizaki, Y.; Doi, Y.; Hirakata, H.; Iida, M. Metabolic syndrome and CKD in a general Japanese population: The Hisayama Study. Am. J. Kidney Dis. 2006, 48, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.S.; Larson, M.G.; Leip, E.P.; Culleton, B.; Wilson, P.W.; Levy, D. Predictors of new-onset kidney disease in a community-based population. JAMA 2004, 291, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Tsao, Y.C.; Chen, J.Y.; Yeh, W.C.; Li, W.C. Gender- and Age-Specific Associations between Visceral Obesity and Renal Function Impairment. Obes. Facts 2019, 12, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Montoro, J.I.; Morales, E.; Cornejo-Pareja, I.; Tinahones, F.J.; Fernandez-Garcia, J.C. Obesity-related glomerulopathy: Current approaches and future perspectives. Obes. Rev. 2022, 23, e13450. [Google Scholar] [CrossRef]
- D’Agati, V.D.; Chagnac, A.; de Vries, A.P.; Levi, M.; Porrini, E.; Herman-Edelstein, M.; Praga, M. Obesity-related glomerulopathy: Clinical and pathologic characteristics and pathogenesis. Nat. Rev. Nephrol. 2016, 12, 453–471. [Google Scholar] [CrossRef]
- Wei, L.; Li, Y.; Yu, Y.; Xu, M.; Chen, H.; Li, L.; Peng, T.; Zhao, K.; Zhuang, Y. Obesity-Related Glomerulopathy: From Mechanism to Therapeutic Target. Diabetes Metab. Syndr. Obes. 2021, 14, 4371–4380. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Z.; Xiang, Z.; Zeng, C.; Chen, Z.; Ma, X.; Li, L. Obesity-related glomerulopathy: Insights from gene expression profiles of the glomeruli derived from renal biopsy samples. Endocrinology 2006, 147, 44–50. [Google Scholar] [CrossRef]
- Lamacchia, O.; Nicastro, V.; Camarchio, D.; Valente, U.; Grisorio, R.; Gesualdo, L.; Cignarelli, M. Para- and perirenal fat thickness is an independent predictor of chronic kidney disease, increased renal resistance index and hyperuricaemia in type-2 diabetic patients. Nephrol. Dial. Transplant. 2011, 26, 892–898. [Google Scholar] [CrossRef]
- Shen, F.C.; Cheng, B.C.; Chen, J.F. Peri-renal fat thickness is positively associated with the urine albumin excretion rate in patients with type 2 diabetes. Obes. Res. Clin. Pract. 2020, 14, 345–349. [Google Scholar] [CrossRef]
- Chen, X.; Mao, Y.; Hu, J.; Han, S.; Gong, L.; Luo, T.; Yang, S.; Qing, H.; Wang, Y.; Du, Z.; et al. Perirenal Fat Thickness Is Significantly Associated with the Risk for Development of Chronic Kidney Disease in Patients With Diabetes. Diabetes 2021, 70, 2322–2332. [Google Scholar] [CrossRef] [PubMed]
- Agabiti-Rosei, C.; Paini, A.; De Ciuceis, C.; Withers, S.; Greenstein, A.; Heagerty, A.M.; Rizzoni, D. Modulation of Vascular Reactivity by Perivascular Adipose Tissue (PVAT). Curr. Hypertens. Rep. 2018, 20, 44. [Google Scholar] [CrossRef] [PubMed]
- Sawicka, M.; Janowska, J.; Chudek, J. Potential beneficial effect of some adipokines positively correlated with the adipose tissue content on the cardiovascular system. Int. J. Cardiol. 2016, 222, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Bibi, A.; Valoti, M.; Fusi, F. Perivascular Adipose Tissue and Vascular Smooth Muscle Tone: Friends or Foes? Cells 2023, 12, 1196. [Google Scholar] [CrossRef] [PubMed]
- Soltis, E.E.; Cassis, L.A. Influence of perivascular adipose tissue on rat aortic smooth muscle responsiveness. Clin. Exp. Hypertens. A 1991, 13, 277–296. [Google Scholar] [CrossRef]
- Lin, A.; Dey, D.; Wong, D.T.L.; Nerlekar, N. Perivascular Adipose Tissue and Coronary Atherosclerosis: From Biology to Imaging Phenotyping. Curr. Atheroscler. Rep. 2019, 21, 47. [Google Scholar] [CrossRef]
- Ohyama, K.; Matsumoto, Y.; Takanami, K.; Ota, H.; Nishimiya, K.; Sugisawa, J.; Tsuchiya, S.; Amamizu, H.; Uzuka, H.; Suda, A.; et al. Coronary Adventitial and Perivascular Adipose Tissue Inflammation in Patients with Vasospastic Angina. J. Am. Coll. Cardiol. 2018, 71, 414–425. [Google Scholar] [CrossRef]
- Kagota, S.; Iwata, S.; Maruyama, K.; McGuire, J.J.; Shinozuka, K. Time-Dependent Differences in the Influence of Perivascular Adipose Tissue on Vasomotor Functions in Metabolic Syndrome. Metab. Syndr. Relat. Disord. 2017, 15, 233–239. [Google Scholar] [CrossRef]
- Heyman, S.N.; Khamaisi, M.; Rosen, S.; Rosenberger, C. Renal parenchymal hypoxia, hypoxia response and the progression of chronic kidney disease. Am. J. Nephrol. 2008, 28, 998–1006. [Google Scholar] [CrossRef]
- Spencer, S.; Wheeler-Jones, C.; Elliott, J. Hypoxia and chronic kidney disease: Possible mechanisms, therapeutic targets, and relevance to cats. Vet. J. 2021, 274, 105714. [Google Scholar] [CrossRef]
- Wagner, R.; Machann, J.; Lehmann, R.; Rittig, K.; Schick, F.; Lenhart, J.; Artunc, F.; Linder, K.; Claussen, C.D.; Schleicher, E.; et al. Exercise-induced albuminuria is associated with perivascular renal sinus fat in individuals at increased risk of type 2 diabetes. Diabetologia 2012, 55, 2054–2058. [Google Scholar] [CrossRef] [PubMed]
- Kagota, S.; Futokoro, R.; McGuire, J.J.; Maruyama-Fumoto, K.; Shinozuka, K. Modulation of Vasomotor Function by Perivascular Adipose Tissue of Renal Artery Depends on Severity of Arterial Dysfunction to Nitric Oxide and Severity of Metabolic Parameters. Biomolecules 2022, 12, 870. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Kuc, R.E.; Brame, A.L.; Dyson, A.; Singer, M.; Glen, R.C.; Cheriyan, J.; Wilkinson, I.B.; Davenport, A.P.; Maguire, J.J. [Pyr(1)]Apelin-13((1-12)) Is a Biologically Active ACE2 Metabolite of the Endogenous Cardiovascular Peptide [Pyr(1)]Apelin-13. Front. Neurosci. 2017, 11, 92. [Google Scholar] [CrossRef] [PubMed]
- Chapman, F.A.; Nyimanu, D.; Maguire, J.J.; Davenport, A.P.; Newby, D.E.; Dhaun, N. The therapeutic potential of apelin in kidney disease. Nat. Rev. Nephrol. 2021, 17, 840–853. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.A.; Vergara, A.; Wang, X.; Vederas, J.C.; Oudit, G.Y. Apelin pathway in cardiovascular, kidney, and metabolic diseases: Therapeutic role of apelin analogs and apelin receptor agonists. Peptides 2022, 147, 170697. [Google Scholar] [CrossRef] [PubMed]
- Chapman, F.A.; Maguire, J.J.; Newby, D.E.; Davenport, A.P.; Dhaun, N. Targeting the apelin system for the treatment of cardiovascular diseases. Cardiovasc. Res. 2023, 119, 2683–2696. [Google Scholar] [CrossRef]
- Tatemoto, K.; Takayama, K.; Zou, M.X.; Kumaki, I.; Zhang, W.; Kumano, K.; Fujimiya, M. The novel peptide apelin lowers blood pressure via a nitric oxide-dependent mechanism. Regul. Pept. 2001, 99, 87–92. [Google Scholar] [CrossRef]
- Salcedo, A.; Garijo, J.; Monge, L.; Fernandez, N.; Luis Garcia-Villalon, A.; Sanchez Turrion, V.; Cuervas-Mons, V.; Dieguez, G. Apelin effects in human splanchnic arteries. Role of nitric oxide and prostanoids. Regul. Pept. 2007, 144, 50–55. [Google Scholar] [CrossRef]
- Japp, A.G.; Cruden, N.L.; Amer, D.A.; Li, V.K.; Goudie, E.B.; Johnston, N.R.; Sharma, S.; Neilson, I.; Webb, D.J.; Megson, I.L.; et al. Vascular effects of apelin in vivo in man. J. Am. Coll. Cardiol. 2008, 52, 908–913. [Google Scholar] [CrossRef]
- Kagota, S.; Maruyama-Fumoto, K.; Iwata, S.; Shimari, M.; Koyanagi, S.; Shiokawa, Y.; McGuire, J.J.; Shinozuka, K. Perivascular Adipose Tissue-Enhanced Vasodilation in Metabolic Syndrome Rats by Apelin and N-Acetyl(-)l-Cysteine-Sensitive Factor(s). Int. J. Mol. Sci. 2018, 20, 106. [Google Scholar] [CrossRef]
- Kagota, S.; Futokoro, R.; Maruyama-Fumoto, K.; McGuire, J.J.; Shinozuka, K. Perivascular Adipose Tissue Compensation for Endothelial Dysfunction in the Superior Mesenteric Artery of Female SHRSP.Z-Leprfa/IzmDmcr Rats. J. Vasc. Res. 2022, 59, 209–220. [Google Scholar] [CrossRef]
- Hiraoka-Yamamoto, J.; Nara, Y.; Yasui, N.; Onobayashi, Y.; Tsuchikura, S.; Ikeda, K. Establishment of a new animal model of metabolic syndrome: SHRSP fatty (fa/fa) rats. Clin. Exp. Pharmacol. Physiol. 2004, 31, 107–109. [Google Scholar] [CrossRef] [PubMed]
- Ishida, J.; Hashimoto, T.; Hashimoto, Y.; Nishiwaki, S.; Iguchi, T.; Harada, S.; Sugaya, T.; Matsuzaki, H.; Yamamoto, R.; Shiota, N.; et al. Regulatory roles for APJ, a seven-transmembrane receptor related to angiotensin-type 1 receptor in blood pressure in vivo. J. Biol. Chem. 2004, 279, 26274–26279. [Google Scholar] [CrossRef] [PubMed]
- Boucher, J.; Masri, B.; Daviaud, D.; Gesta, S.; Guigne, C.; Mazzucotelli, A.; Castan-Laurell, I.; Tack, I.; Knibiehler, B.; Carpene, C.; et al. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology 2005, 146, 1764–1771. [Google Scholar] [CrossRef] [PubMed]
- Krist, J.; Wieder, K.; Kloting, N.; Oberbach, A.; Kralisch, S.; Wiesner, T.; Schon, M.R.; Gartner, D.; Dietrich, A.; Shang, E.; et al. Effects of weight loss and exercise on apelin serum concentrations and adipose tissue expression in human obesity. Obes. Facts 2013, 6, 57–69. [Google Scholar] [CrossRef]
- Fischer, C. A patent review of apelin receptor (APJR) modulators (2014–2019). Expert Opin. Ther. Pat. 2020, 30, 251–261. [Google Scholar] [CrossRef]
- Iwanaga, Y.; Kihara, Y.; Takenaka, H.; Kita, T. Down-regulation of cardiac apelin system in hypertrophied and failing hearts: Possible role of angiotensin II-angiotensin type 1 receptor system. J. Mol. Cell Cardiol. 2006, 41, 798–806. [Google Scholar] [CrossRef]
- Wakui, H.; Dejima, T.; Tamura, K.; Uneda, K.; Azuma, K.; Maeda, A.; Ohsawa, M.; Kanaoka, T.; Azushima, K.; Kobayashi, R.; et al. Activation of angiotensin II type 1 receptor-associated protein exerts an inhibitory effect on vascular hypertrophy and oxidative stress in angiotensin II-mediated hypertension. Cardiovasc. Res. 2013, 100, 511–519. [Google Scholar] [CrossRef]
- Maeda, A.; Tamura, K.; Wakui, H.; Dejima, T.; Ohsawa, M.; Azushima, K.; Kanaoka, T.; Uneda, K.; Matsuda, M.; Yamashita, A.; et al. Angiotensin receptor-binding protein ATRAP/Agtrap inhibits metabolic dysfunction with visceral obesity. J. Am. Heart Assoc. 2013, 2, e000312. [Google Scholar] [CrossRef]
- Azushima, K.; Ohki, K.; Wakui, H.; Uneda, K.; Haku, S.; Kobayashi, R.; Haruhara, K.; Kinguchi, S.; Matsuda, M.; Maeda, A.; et al. Adipocyte-specific enhancement of angiotensin II type 1 receptor-associated protein ameliorates diet-induced visceral obesity and insulin resistance. J. Am. Heart Assoc. 2017, 6, e004488. [Google Scholar] [CrossRef]
- Kaneyama, A.; Hirata, A.; Hirata, T.; Imai, Y.; Kuwabara, K.; Funamoto, M.; Sugiyama, D.; Okamura, T. Impact of hypertension and diabetes on the onset of chronic kidney disease in a general Japanese population. Hypertens. Res. 2023, 46, 311–320. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).