Abstract
Median Arcuate Ligament Syndrome, also known as Dunbar’s syndrome, is a rare condition caused by stenosis of the celiac artery (CAS) through the fibrous arch connecting the diaphragmatic branches. It manifests as postprandial abdominal pain, nausea, vomiting, weight loss and increased epigastric tenderness. The condition most commonly affects young females without coexisting vascular comorbidities. Diagnosis is difficult due to the non-specific symptoms, often overlapping with other gastrointestinal diseases. Standard investigations include duplex ultrasound, computed tomography angiography (CTA) and contrast-enhanced magnetic resonance imaging (CE-MRA). Treatment mainly consists of surgical release of the arch ligament, which can be performed by open, laparoscopic or robotic methods. Surgery is often supported by celiac truncal stenting for residual stenosis, which significantly improves vascular flow. Alternative approaches include visceral plexus blocks and novel hybrid techniques, such as a combination of ligament release and endovascular treatment of the celiac trunk. In severe cases, vascular by-passes are recommended. The aim of this paper is to discuss the clinical manifestations, diagnostic possibilities, therapeutic options and directions for further research on MALS from the perspective of a vascular surgeon. It emphasizes the need for a multidisciplinary approach, including collaboration between the surgeon, radiologist, gastroenterologist and psychologist, which enables comprehensive disease management and improved quality of life for patients. In addition, the need for further development of diagnostic and therapeutic methods for early diagnosis and effective treatment was pointed out.
1. Introduction
Median Arcuate Ligament Syndrome (MALS) also known as Dunbar’s syndrome is one of the main factors leading to Celiac Artery Stenosis (CAS) [1]. The celiac trunk (CT) is an unpaired, branching artery located immediately below the Median Arcuate Ligament (MAL), originating from the abdominal aorta at the Th11-L1 level [2]. Causes of CAS can be internal such as atherosclerosis, or external such as pancreatitis, malignant tumors, or MALS [3]. MALS is defined as external compression of the celiac trunk and celiac plexus by the fibrous arch connecting the diaphragmatic crura, or MAL [4,5,6]. Individuals with a higher position of celiac artery or a lower position of median arcuate ligament are predisposed to this condition [7]. This very rare syndrome affecting 0.002% of the population, and its diagnostic and surgical management are not well defined [8]. The characteristic presentation of a patient with MALS is that of a young female, without comorbidities of atherosclerotic phenotype. MALS may remain asymptomatic in up to 25% of affected patients, while, according to different article, between 15% and 50% of asymptomatic cases can still be identified [9,10]. The most common associated symptoms include postprandial abdominal pain, nausea and vomiting, weight loss, bloating, abdominal bruit and increased epigastric tenderness [2,11,12]. The presentation of clinical symptoms is due to hemodynamic disturbances in the CA, leading to malperfusion of visceral organs supplied by this vessel such as the spleen, pancreas, liver, stomach and proximal part of the duodenum [4]. Symptoms are caused by vascular and neuropathic components. The vascular component is responsible for a decrease in blood flow due to compression of the CT. The neuropathic component, on the contrary, results in irritation of the celiac plexus and visceral pain [13]. In addition, chronic compression of the CA by the MAL may promote the formation of aneurysms within the visceral arteries branching from the CA, such as aneurysms in the pancreatoduodenal artery arch [14]. They develop due to increased flow in the collateral circulation from the superior mesenteric artery (SMA) to the visceral arteries [15]. It is important to point out that in both paediatric and adult patients affected by MALS, psychiatric symptoms such as anxiety and depression are common. In the adult group, it is estimated that more than one third of patients are likely due to the prolonged diagnostic process and chronic pain [16,17]. However, depression or anxiety may not disappear despite the resolution of symptoms in the post-operative period, so psychological care and the use of behavioral-cognitive therapy in such patients is extremely important [18].
The aim of this review is to discuss the clinical and imaging features, therapeutic management and potential future research directions that may be useful in the early diagnosis and treatment of MALS from a vascular surgeon’s perspective. In addition, the review emphasizes, the need for a multidisciplinary approach involving the vascular surgeon, radiologist, gastroenterologist and psychologist to optimally manage patients with MALS.
For this review, we extensively searched the PubMed, Embase and ClinicalTrials.gov databases up until February 2025. The subject researched was “Dunbar syndrome” or “Median Arcuate Ligament Syndrome”, along with the following terms: celiac artery stenosis, endovascular and surgery treatment. Articles in a language other than English were excluded.
2. Clinical Presentation
MALS is classified as a condition that is challenging to diagnose based on clinical symptoms alone. Many of them are frequently associated with other gastrointestinal disorders, significantly complicating effective diagnosis [19]. The primary clinical manifestation triad includes postprandial or exercise-induced abdominal pain, primarily localized in the epigastric region, unintentional, marked weight loss and abdominal bruise, which is clearly audible during exhalation [20]. Abdominal pain is present in more than 90% of patients, epigastric localization in about 70%, the incidence of postprandial pain varies from 48% to 85%, epigastric bruits are about 30%, non-intentional weight loss from 40 to 67%, vomiting in about 30%, while the incidence of postexertional pain is about 10% [21,22]. During physical exercise, the demand for blood increases in skeletal muscles, which may lead to a redistribution of blood flow from the splanchnic region to the muscles. In the case of MALS, the already restricted blood flow in the celiac artery may be further reduced during physical exertion, leading to ischemia of the abdominal organs and the onset of abdominal pain [16,20]. It is noteworthy that patients with postexertional pain had a higher success rate with surgery, whereas patients in whom vomiting was predominant did not see significant improvement after surgery [21]. Therefore, physical activity may cause additional gastrointestinal ischaemia, which expands the spectrum of symptoms in MALS. Furthermore, the case descriptions characterize post-exercise pain in MALS as epigastric tension progressing to lower abdominal pain and the need to defecate. Interestingly, postprandial abdominal pain was not reported in the described cases. The abdominal pain in patients with MALS may be due to a complicated mechanism of perfusion redistribution as well as sympathetic nerve irritation [23,24]. The characteristic feature of abdominal pain is its worsening after food intake. This pain is acute and recurrent. The literature most commonly describes cases of patients who experienced pain for months before seeking medical assistance [10,25,26]. Other common clinical symptoms correlated with gastrointestinal disorders include vomiting, nausea, diarrhea, bloating. It is worth noting that some patients with MALS suffer from behavioral disorders, such as anxiety disorder [20]. Furthermore, some of these patients experience an extreme fear of food [9,16].
The more severe and rare complications associated with MALS include stenosis and occlusion of the celiac trunk. CT occlusion occurred in only 5% of patients with MALS [27]. Furthermore, CT compression by MAL leads to CT stenosis chronic organ ischemia and microcirculatory disruption. Therefore, there was a significant improvement in microcirculation, mainly in the stomach and duodenum after MALR. An increase in hemoglobin concentration in the gastric and duodenal walls and an increase blood flow velocity in the duodenum. Interestingly, a decrease in gastric oxygen saturation was observed, which may have been due to increased demand from previously hypoxic tissues [28]. Studies show that thrombosis of the SMA during MALS causes acute intestinal ischemia, leading to necrosis and requiring partial intestinal resection [29]. Notable is the occurrence of MALS in conjunction with autoimmune diseases—a case involving a young female with MALS, secondary APS, and SLE resulted in thrombosis of the celiac trunk, splenic artery, and common hepatic artery [25]. However, this is one case currently reported in the literature. Despite well-developed collateral circulation, the patient experienced a splenic infarction.
3. Diagnostics
MALS symptoms such as postprandial epigastric pain or nausea are non-specific and therefore this diagnosis should be considered only after excluding other, more common diseases. Firstly, the patient must undergo meticulous physical examination, lab tests and abdominal ultrasonography. Moreover, examinations such as gastroduodenoscopy, abdominal/pelvic ultrasonography, abdominal tomography are usually performed [30]. European guidelines on management of chronic mesenteric ischemia (CMI) recommend use of contrast-enhanced magnetic resonance angiography (CE-MRA), computed tomography angiography (CTA) or expiratory/inspiratory duplex ultrasound (DUS) in the diagnosis of celiac trunk compression (4). In MALS diagnostics DUS is used to measure parameters such as end-expiratory upturn-angle of the celiac trunk and inspiratory/expiratory peak systolic velocity. Several ultrasonographic criteria of MALS including mentioned parameters have been proposed in a few of the research articles published so far, however there is no consensus [31,32,33]. In the expiratory phase, there is greater compression of the CT by the MAL, leading to an increase in the systolic velocity of blood flow in the CT. During the inspiratory phase, the MAL retracts, the compression is reduced and leads to normalization of blood flow. In some patients with MALS, vasodilation behind the site of stenosis, namely post-stenotic dilation [16]. By using Doppler ultrasonography, one can detect the discontinuation of blood flow and increased blood flow in the proximal part of the CT Promising for the diagnosis of MALS expiratory peak velocity (PV) higher than 350 cm/s and the bending of the celiac trunk greater than 50° had a sensitivity of 83% and a specificity of 100% [10]. Other article reports peak systolic velocity of greater than 249 cm/sec in the expiratory phase with normalization during inspiration as pathognomonic for celiac artery compression [34]. MAL assessment should be performed on inspiration and expiration during Angio-CT and DSA, as celiac trunk stenosis is often not visible on inspiration, which may contribute to misinterpretation of the results [35]. Endoscopic DUS (E-DUS) could be a potential alternative for transabdominal DUS (TA-DUS). In a study by Safi et al. E-DUS was demonstrated to be more sensitive and specific than TA-DUS in diagnosing CMI [13,36].
Another diagnostic modality used to evaluate patients with suspected MALS is CTA, which allows for meticulous assessment of celiac trunk and MAL anatomy. Some of the MALS characteristics in CTA scan include inferior displacement of the celiac trunk, proximal compression of celiac artery, MAL thickening, Angio CT on inspiration and expiration, celiac artery stenosis and “hook sign” (Figure 1) [37]. To date, MAL thickening has been considered significant when thickness is greater than 4 mm. However, recent studies indicate a mean MAL thickness at CTA in men and women of 8.4 ± 2.52 mm and 6.9 ± 2.41 mm, respectively. In addition, the mean distance of the MAL from the CT was 1.32 ± 2.04 mm, and the most common angle of celiac trunk exit was the obtuse angle, which is consistent with adaptation to compression [38,39]. Interestingly, in a study by Ganapathy et al. there was no significant correlation between anatomic measurements such as CA angle, CA diameter, thickness of MAL and MALS symptoms such as pain, weight loss, nausea, vomiting [38]. Therefore, diagnosis should be based not only on the assessment of the thickness of the MAL, but also the distance of the CT from the MAL, the determination of the angle of departure of the CT, the presence of post-stenotic CT dilatation and the assessment of the dynamics of flow through the celiac trunk on inspiration and expiration [39]. “Hook sign” is defined as focal narrowing in the proximal celiac artery and is useful in differentiating MALS from other pathologies [40]. Chan et al. characterized “hook sign” in their study as a fold angle of CA lower than 135°. MALS patients in comparison with non-MALS patients had significantly more severe fold angle of CA in CTA scan (p=0,002) [41]. “Hook sign” in CTA scan had the sensitivity of 71% and specificity of 59%. CE-MRA can be considered as a potential alternative to CTA in patients who should avoid exposure to radiation or have allergy for contrast used in CTA [4].
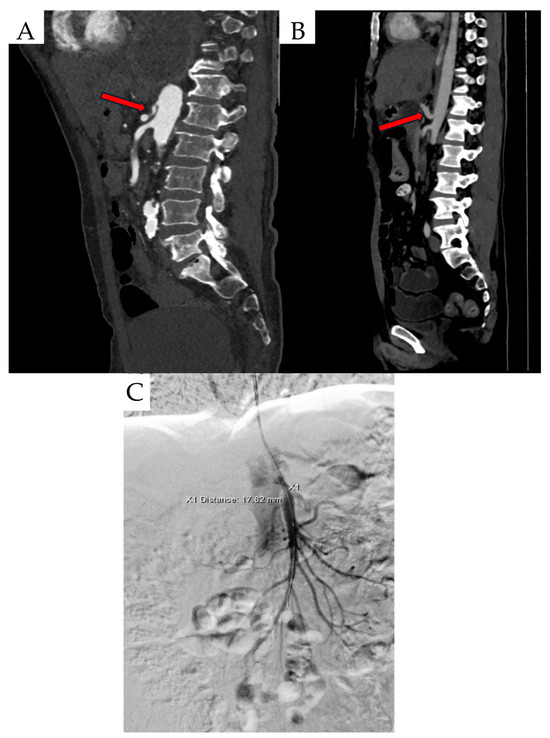
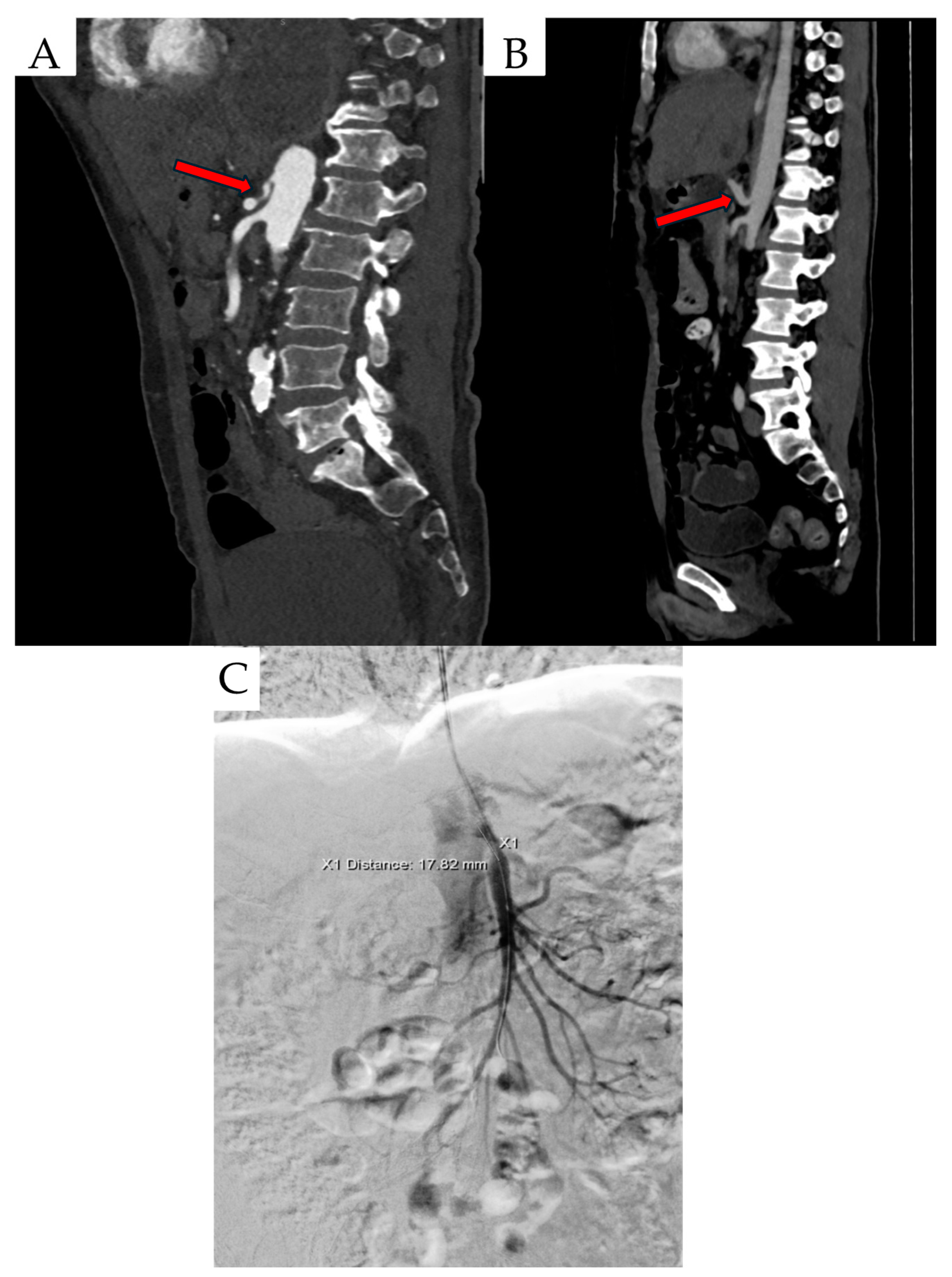
Figure 1.
Created based on unpublished clinical data from Department of Vascular Surgery and Angiology, Pomeranian Medical University in Szczecin. (A) CTA findings include inferior displacement of the CT by proximal celiac artery compression. (B) Celiac artery stenosis and ‘hook’ sign (red arrows). (C) Digital subtractive angiography (DSA) scan in a patient with MALS.
In recent years, thanks to better understanding of MALS pathophysiology, the celiac plexus block (CPB) has been gaining popularity. It can be used as an additional examination to qualify patients for median arcuate ligament release (MALR) surgery. In one retrospective descriptive study 96% of CPB-responders benefited from MALR [42]. Moreover DeCarlo et al. demonstrated that absence of CPB-response is a factor of increased hazard of treatment failure [8]. The use of standardized diagnostic protocol could improve patient outcomes (Figure 2). Lanka et al. evaluated the efficacy of a protocol that included, among other more usual steps, referral to a pain rehabilitation clinic and a provocative mesenteric angiogram and/or CPB. Patients diagnosed using of standardized protocol in comparison with the control group had greater relief from abdominal pain (65.9% vs. 50.0%, p < 0.04) [43]. Several less common methods for MALS diagnostics have been evaluated so far. For example, mesenteric ischemia in MALS can be diagnosed with the use of gastric tonometry. In tonometry a catheter is used to measure the partial pressure of carbon dioxide (PCO2) in the intestines. Next PCO2 value is used to calculate intestinal wall pH which can be used to assess intestinal perfusion [44]. Another unusual procedure which can help diagnose MALS is fractional flow reserve (FFR). FFR can provide the information if the celiac trunk stenosis is hemodynamically significant [45]. Intravascular ultrasound is another interesting diagnostic modality which can be used to assess the exact stenosis rate of the celiac artery [46]. 4D-Flow MRI can be used in MALS to visualize abnormal arterial flow dynamics [47].
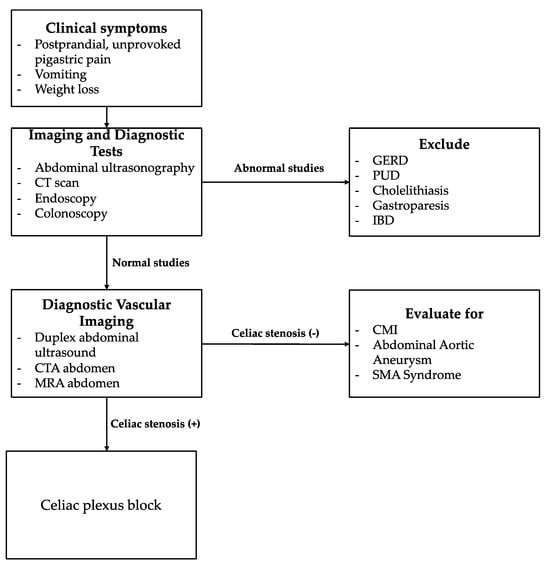
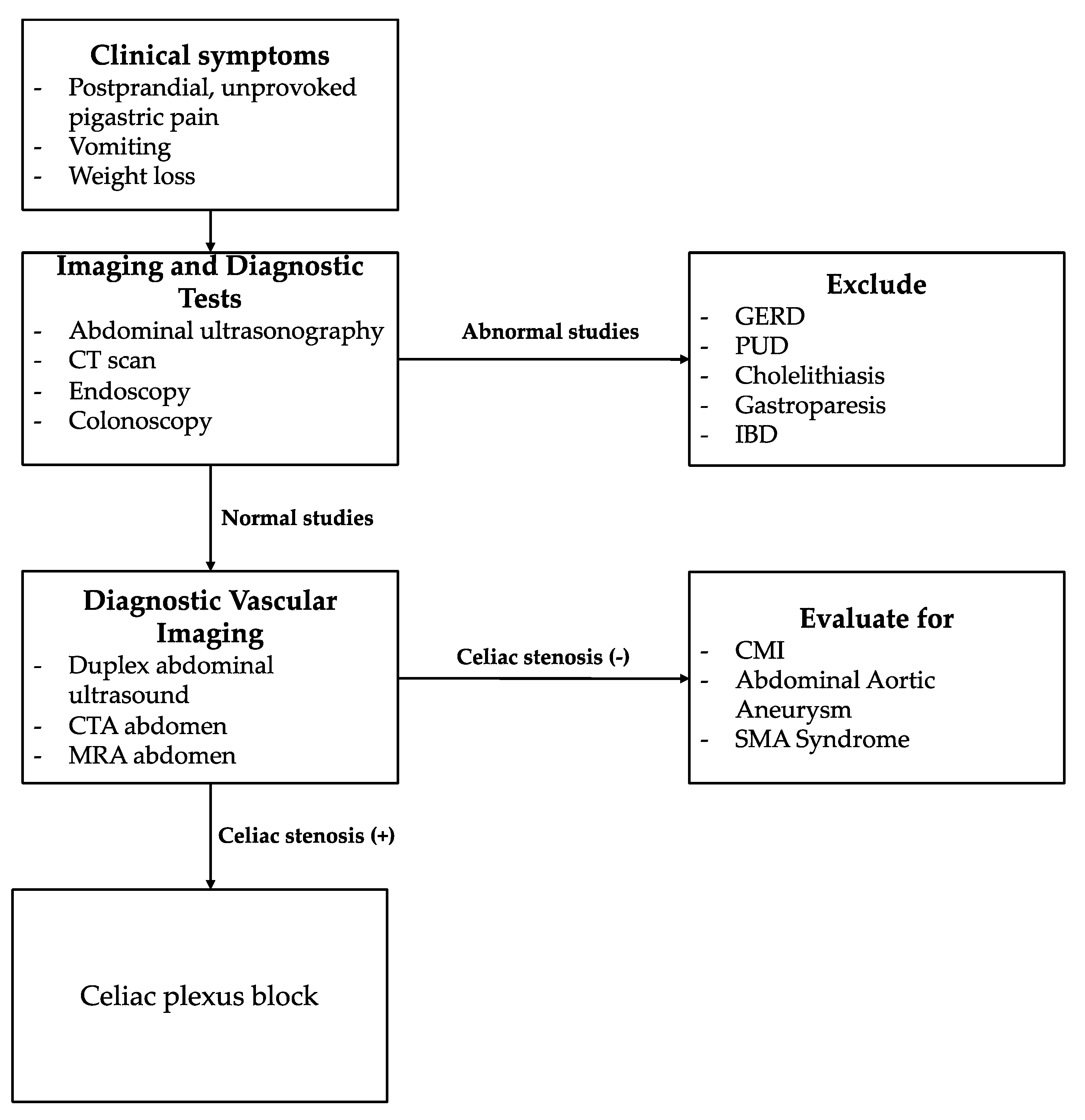
Figure 2.
Diagnostic Algorithm for Unexplained Epigastric Pain. Focus on Celiac Artery Stenosis. The algorithm was developed based on personal experience and a review of the literature.
4. Therapeutic Options
In the treatment of MALS, various therapeutic options are offered. Currently the most popular surgical treatment of Dunbar Syndrome is MALR. After surgery, there is symptom relief in 96% of patients [48]. Long-term follow-up confirms symptom relief after MALR in more than 50% of patients [49,50]. Relief of symptoms in only half of patients may result from residual visceral artery stenosis, incomplete transection of the MAL or secondary fibrosis. Chronic compression may also result in irreversible damage to the celiac trunk. This operation can be performed open, laparoscopic (LMALR) or robotic (RMALR). The Laparoscopic approach, which is probably the most widely used technique, has several advantages over open surgery. In a large cross-sectional study LMALR was associated with less major complications (0.5% vs. 4.0%, p = 0.02) and percentage of patients who were reoperated (0% vs. 2.6%, p = 0.03) [48]. Moreover, in two retrospective reviews of large patient databases laparoscopic approach in comparison with open was characterized by fewer perioperative complications, especially ileus. In addition, LMALR was associated with shorter length of hospitalization and smaller hospital charges than open surgery [8,51]. Recently robotic approach is gaining in popularity. Several studies compared this novel technique with LMALR. In the study by DeCarlo et al. the use of robotic surgery was demonstrated to be a factor of increased hazard of treatment failure (HR = 1.73; 95% CI, 1.16–2.59; p = 0.007) [8]. In contrast with this finding, Shin et al. reported larger reduction of postoperative abdominal pain in RMALR cohort comparison with LMALR group (76.9% vs. 50%; p = 0.0487). However, there was no significant difference in reduction of other symptoms [52]. Furthermore, in another study by Khrucharoen et al. the overall clinical improvement after RMALR and LMALR was similar [53]. All these studies had some limitations including a small number of RMALR cases and the so far provided evidence is contradictory. Although it is recommended to further evaluate the results of RMALR, it may become the standard in the future. However, there is no doubt that RMALR allows for better anatomical visualization, which enables greater precision in the preparation of vessels and celiac plexus [54]. MALR carries risks for the patient. The reported factors potentially associated with treatment failure include hypertension, gastroparesis, dysphagia or odynophagia, GERD, no relief from CPB, larger number of pain locations [8,22]. Intriguingly, in the study by DeCarlo et al. patients who underwent more gastrointestinal examinations were more likely to benefit from MALR [8]. This underlines the necessity of thorough diagnostic workup.
Based on the previous articles, the preferred treatment strategy is ligament release and stabilization of the celiac trunk flow. Ligament release results in almost 90% symptom relief [26,55]. As reported by Schneider et al. CT stenting was the preferred treatment strategy in >70% CT stenosis after MAL release. In addition, 92% of patients were treated with the covered balloon expandable stent achieving a high success rate. At 6-month follow-up, the percentage of primary patency was 82%, while secondary patency due to reintervention was 100% [56]. Moreover, there were no deaths within a month after surgery. An interesting treatment approach was performed in a patient with an anatomical abnormality, namely celiacomesenteric trunk (CMT) and SMA aneurysm. Only MALR with visceral plexus resection was performed. At six-month follow-up, satisfactory treatment effects were obtained [57].
The ACR Appropriateness Criteria guidelines report that after MAL release, stenting is recommended in patients with residual CT stenosis, i.e., >30%, because it reduces the risk of recurrent symptoms by improving hemodynamics. In addition, they point out that the use of endovascular methods alone may not be sufficient due to chronic changes in the vessel and external compression [55].
The use of only PTA and stenting without MAL release is possible in asymptomatic patients. It is possible to implant only an uncovered balloon-expandable stent such as Express Vascular SD (Boston Scientific, Marlborough, MA, USA) with a diameter of 6.0–14 mms. At 15-month follow-up, improvements in visceral artery hemodynamics and stent patency were observed, confirming the high efficacy of this method [58]. Stenting before MAL release is also performed in patients with celiac trunk dissection and aneurysm of the pancreatic duodenal artery. The self-expanding E-LUMINEXX® stent (Bard Peripheral Vascular, Tempe, AZ, USA) is implanted into the true lumen of the celiac trunk. After intraoperative effective blood flow improvement, aneurysm embolization was performed. In the next stage, the MAL was released. The effectiveness of this strategy is confirmed by the absence of restenosis symptoms and the patency of the stent in the patient at 6- and 12-month follow-up [52].
The similar strategy based on CT stenting in the first stage is recommended before MAL resection during pancreatoduodenectomy in patients with MALS. This provides patency prior to surgical CT by preventing retrograde flow [59]. One invasive alternative with high efficacy for complex stenosis is to perform an aorto-hepatic by-pass after MALS release using a polytetrafluoroethylene (ePTFE) prosthesis, resulting in successful revascularization [60]. The proposed approach can be extremely effective, as MALS can be a complication of pancreatoduodenectomy due to removal of the pancreatic artery arch and displacement of the fat-rich intestinal mesentery, leading to excessive flexion of the visceral trunk. In such a situation, implantation of a non-covered metal stent into the CT is necessary [61].
Complex treatment strategy is described for aneurysms secondary to MALS which is an extremely rare pathology accounting for 2% of all abdominal aneurysms. However, MALS aneurysms can account for almost 70% of pancreatoduodenal artery (PDA) aneurysms [62]. These arise due to hemodynamic overload and increased flow in the collateral circulation mainly through the pancreaticoduodenal arteries and the gastroduodenal circulation. Insufficient collateral circulation can result in bowel resection due to ischemia [63].
The recommended treatment strategy for MAL is transcatheter PDA embolization with PTA and celiac trunk stenting in the first stage and MALR in the second stage. It is extremely important to recanalize the CA because in long-term follow-up, failure to recanalize the CA results in recurrent visceral artery aneurysms [56,57,64]. It is also possible to perform an aorto-hepatic by-pass in the first stage, reducing the hyperkinetic circulation. This treatment strategy may be considered in cases of celiac trunk closure and critical SMA stenosis. However, by-pass has numerous limitations as it carries a high risk of complications such as bleeding, thrombosis, or obstruction [58]. Long-term imaging follow-up of patients should be based on Angio CT [57].
Patients with advanced CT compression by MAL should be monitored long-term after F/B-EVAR. Increased risk and complexity of these procedures have been observed in patients with MAL resulting from longer operating times and significantly higher blood loss. Moreover, at follow-up, 30-day mortality was almost 3% higher in the MALS group [65,66].
In addition to the preferred therapeutic strategies, sensitization of the celiac plexus plays an important role in the pathophysiology of MALS. Resulting from excessive stimulation of the celiac plexus caused by mechanical compression of the MAL, release of the ligament reduces stimulation. In addition, ischemia is one of the triggers of celiac plexus nerve stimulation, so improving blood flow through the CT can relieve pain [67]. Celiac plexus block (CPB), which periodically interrupt neuropathic pain transmission, may also be of key clinical importance in reducing pain, allowing the clinician to assess whether abdominal pain is due to nerve fiber irritation. In addition, improvement of gastroparesis after CPB has been reported, suggesting a correlation of visceral nerve compression with gastrointestinal motility [68]. However, this requires further study. In some extreme clinical situations, visceral plexus ganglionectomy may be considered. But such resection is aimed only at patients with severe visceral pain. It is worth emphasizing that non-invasive MAL release in asymptomatic patients is extremely important, as preservation of the visceral plexus reduces the risk of perioperative complications [69].
5. MALS: Associations and Future Perspectives
The thrombotic incident in a young individual with autoimmune diseases should prompt consideration of MALS in the diagnostic process. It is important to emphasize that this condition may act as a triggering factor for thrombotic events in patients with APS. However, there is insufficient research on this subject to draw definitive conclusions [25].
Studies confirm that MALS can coexist with other conditions involving arterial compression. Among a group of 18 patients, as many as 13 were found to have Nutcracker syndrome [70]. The coexistence of these two pathologies may be explained by the presence of excessive lumbar lordosis in such patients. On the other hand, a kyphotic posture in these individuals reduced visceral pain due to decreased tension in the median arcuate ligament. The overlap of compression syndromes presents a significant diagnostic challenge due to additional symptoms that differ from the classic clinical presentation of MALS [71]. Many studies describe the presence of aneurysms and pseudoaneurysms of the pancreaticoduodenal artery in the majority of patients with MALS [72,73]. These result from elevated intravascular pressure in the pancreaticoduodenal arcade caused by celiac trunk compression. Additionally, the pressure gradient in the pancreaticoduodenal arteries is reversed, leading to hemodynamic disturbances and, consequently, aneurysm formation in these vessels. Such aneurysms may compress the common bile duct, resulting in jaundice.
Various diagnostic and therapeutic strategies have been discussed in the previous sections. Importantly, MALS is currently the subject of two clinical trials (Table 1). One study is a randomized clinical trial that focuses on evaluating the efficacy of endoscopic release in CT comparing this with sham surgery. In another study, Kazmi et al. report that patients with MALS have significantly elevated plasma α-glutathione-S-transferase (α-GST) levels compared to healthy individuals (p < 0.001). Furthermore, this potential marker has been shown to be closely associated with intestinal ischemia and not associated with disorders of other organs such as the pancreas or liver [74].

Table 1.
A list of selected clinical trials including MALS patients with estimated completion date in the future with statuses “Recruiting” and “Active, not recruiting” registered at the https://www.clinicaltrials.gov (accessed on 28 January 2025).
6. Conclusions
In conclusion, clinical cases of patients with MALS accompanied by vascular diseases require close collaboration between a gastroenterologist, radiologist, and vascular surgeon. This involves a lengthy diagnostic process, where more common conditions must first be ruled out. Radiological diagnostics of MALS often do not correlate with patients’ clinical symptoms, which is important in understanding this complex clinical problem—only 6.7% of the global population has a narrowed celiac trunk, and in most cases, it is asymptomatic [50]. Therefore, future research should focus on creating validated diagnostic criteria, which combine radiological features with clinical symptoms. Moreover, inclusion of CPB in such a protocol to address neurogenic MALS seems especially promising. Furthermore, investigation of potential MALS biomarkers is another interesting research direction.
Author Contributions
Conceptualization, P.S., P.R.; writing—original draft preparation, P.S., J.S., K.S., A.S., P.G., M.W. and P.R.; writing—review and editing, P.S., J.S., K.S., A.S., P.G., M.W. and P.R; supervision, P.R., P.G., P.S. and M.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available in this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dembinski, J.; Robert, B.; Sevestre, M.-A.; Freyermuth, M.; Yzet, T.; Dokmak, S.; Regimbeau, J.-M. Celiac axis stenosis and digestive disease: Diagnosis, consequences and management. J. Visc. Surg. 2020, 158, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.N.; Lamb, K.; Relles, D.; Moudgill, N.; DiMuzio, P.J.; Eisenberg, J.A. Median Arcuate Ligament Syndrome—Review of This Rare Disease. JAMA Surg. 2016, 151, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Takemura, N.; Inagaki, F.; Mihara, F.; Shida, Y.; Tajima, T.; Kokudo, N. Diagnosis of celiac artery stenosis using multidetector computed tomography and evaluation of the collateral arteries within the mesopancreas of patients undergoing pancreaticoduodenectomy. Clin. Anat. 2020, 34, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Terlouw, L.G.; Moelker, A.; Abrahamsen, J.; Acosta, S.; Bakker, O.J.; Baumgartner, I.; Boyer, L.; Corcos, O.; van Dijk, L.J.; Duran, M.; et al. European guidelines on chronic mesenteric ischaemia–joint United European Gastroenterology, European Association for Gastroenterology, Endoscopy and Nutrition, European Society of Gastrointestinal and Abdominal Radiology, Netherlands Association of Hepatogastroenterologists, Hellenic Society of Gastroenterology, Cardiovascular and Interventional Radiological Society of Europe, and Dutch Mesenteric Ischemia Study group clinical guidelines on the diagnosis and treatment of patients with chronic mesenteric ischaemia. United Eur. Gastroenterol. J. 2020, 8, 371–395. [Google Scholar] [CrossRef]
- Iqbal, S.; Chaudhary, M. Median arcuate ligament syndrome (Dunbar syndrome). Cardiovasc. Diagn. Ther. 2021, 11, 1172–1176. [Google Scholar] [CrossRef]
- Dunbar, J.D.; Molnar, W.; Beman, F.F.; Marable, S.A. Compression of the celiac trunk and abdominal angina. Am. J. Roentgenol. 1965, 95, 731–744. [Google Scholar] [CrossRef]
- Ito, K.; Takemura, N.; Oikawa, R.; Inagaki, F.; Mihara, F.; Kokudo, N. Detailed anatomy and procedure of celiac artery decompression in median arcuate ligament syndrome. Langenbeck’s Arch. Surg. 2021, 406, 1717–1722. [Google Scholar] [CrossRef]
- DeCarlo, C.; Woo, K.; van Petersen, A.S.; Geelkerken, R.H.; Chen, A.J.; Yeh, S.L.; Kim, G.Y.; Henke, P.K.; Tracci, M.C.; Schneck, M.B.; et al. Factors associated with successful median arcuate ligament release in an international, multi-institutional cohort. J. Vasc. Surg. 2022, 77, 567–577.e2. [Google Scholar] [CrossRef]
- Maddox, K.; Farrell, T.M.; Pascarella, L. Median Arcuate Ligament Syndrome: Where Are We Today? Am. Surg. 2024, 91, 284–291. [Google Scholar] [CrossRef]
- Nikolova, D.; Antovic, S.; Karagjozov, P.; Kitevski, V.; Trajkovska, M.; Dimitrova, M.G.; Deriban, G.; Trpcevska, E.N.; Nikolovska, A.V. Chronic Abdominal Pain, an Overlooked Diagnosis of Median Arcuate Ligament Syndrome (MALS). Prilozi 2022, 43, 95–99. [Google Scholar] [CrossRef]
- Kozhimala, M.; Chan, S.M.B.; Weininger, G.B.; Sumpio, B.J.; Levine, L.J.; Harris, S.; Zheng, S.B.; E Longo, W.; Chaar, C.O.M.; Guzman, R.J.M.; et al. Prevalence and Characteristics of Patients with Median Arcuate Ligament Syndrome in a Cohort Diagnosed with Celiac Artery Compression. J. Am. Coll. Surg. 2022, 236, 1085–1091. [Google Scholar] [CrossRef]
- Metz, F.M.; Blauw, J.T.; Brusse-Keizer, M.; Kolkman, J.J.; Bruno, M.J.; Geelkerken, R.H. Systematic Review of the Efficacy of Treatment for Median Arcuate Ligament Syndrome. Eur. J. Vasc. Endovasc. Surg. 2022, 64, 720–732. [Google Scholar] [CrossRef] [PubMed]
- Sghaier, A.; Jelassi, M.A.; Fradi, K.; Mraidha, M.H.; Hamila, F.; Youssef, S. Median arcuate ligament syndrome (Dunbar syndrome): A diagnosis not to be underestimated. Radiol. Case Rep. 2023, 19, 636–641. [Google Scholar] [CrossRef]
- Sarad, N.; Basilious, M.; Nag, U.; Jethmalani, N.; Agrusa, C.; Ellozy, S.; DeRubertis, B.; Connolly, P. Presentation and management of true aneurysms of the pancreaticoduodenal arcade with concomitant celiac artery stenosis using the endovascular approach. J. Vasc. Surg. Cases Innov. Tech 2024, 10, 101499. [Google Scholar] [CrossRef] [PubMed]
- Yamana, F.; Ohata, T.; Kitahara, M.; Nakamura, M.; Yakushiji, H.; Nakahira, S. Blood flow modification might prevent secondary rupture of multiple pancreaticoduodenal artery arcade aneurysms associated with celiac axis stenosis. J. Vasc. Surg. Cases Innov. Tech. 2020, 6, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Zbinden, S.; Forgo, G.; Kucher, N.; Barco, S. Practice-Based Management Data of Consecutive Subjects Assessed for the Median Arcuate Ligament Syndrome at a Single Tertiary Institution. Clin. Pract. 2024, 14, 1911–1920. [Google Scholar] [CrossRef]
- Stiles-Shields, C.; Osos, S.; Sunnquist, M.L.; Mak, G.Z.; Skelly, C.L.; Drossos, T. Patient-Reported Experiences With Median Arcuate Ligament Syndrome: Resilience and Resources Required. Clin. Pract. Pediatr. Psychol. 2022, 10, 44–53. [Google Scholar] [CrossRef]
- Skelly, C.L.; Stiles-Shields, C.; Goldenthal, H.; Bohr, N.; Feldman, E.; Mak, G.Z.; Drossos, T. Median arcuate ligament syndrome: A cost analysis to determine the economic burden of a rarely diagnosed disease. Front. Psychol. 2024, 14, 1166744. [Google Scholar] [CrossRef]
- Chen, A.J.; Yeh, S.; Dhindsa, Y.; Lawrence, P.F.; Woo, K. Outcomes of Median Arcuate Ligament Release: A Single Institution Retrospective Review. Ann. Vasc. Surg. 2023, 94, 296–300. [Google Scholar] [CrossRef]
- Goodall, R.; Langridge, B.; Onida, S.; Ellis, M.; Lane, T.; Davies, A.H. Median arcuate ligament syndrome. J Vasc Surg. 2020, 71, 2170–2176. [Google Scholar] [CrossRef]
- Ho, K.K.F.; Walker, P.; Smithers, B.M.; Foster, W.; Nathanson, L.; O’Rourke, N.; Shaw, I.; McGahan, T. Outcome predictors in median arcuate ligament syndrome. J. Vasc. Surg. 2017, 65, 1745–1752. [Google Scholar] [CrossRef]
- Pather, K.; Kärkkäinen, J.M.; Tenorio, E.R.; Bower, T.C.; Kalra, M.; DeMartino, R.; Colglazier, J.; Oderich, G.S. Long-term symptom improvement and health-related quality of life after operative management of median arcuate ligament syndrome. J. Vasc. Surg. 2020, 73, 2050–2058.e4. [Google Scholar] [CrossRef] [PubMed]
- Desmond, C.P.; Roberts, S.K. Exercise-related abdominal pain as a manifestation of the median arcuate ligament syndrome. Scand. J. Gastroenterol. 2004, 39, 1310–1313. [Google Scholar] [CrossRef]
- Harr, J.N.; Haskins, I.N.; Brody, F. Median arcuate ligament syndrome in athletes. Surg. Endosc. 2016, 31, 476. [Google Scholar] [CrossRef] [PubMed]
- Janiak, P.; Smoleńska, Ż.; Skotarczak, M.; Zdrojewski, Z. Celiac trunk thrombosis in a patient with antiphospholipid syndrome induced by median arcuate ligament compression: A case presentation and literature review. Rheumatol. Int. 2023, 44, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Diab, J.; Diab, V.; Berney, C.R. A diagnostic workup and laparoscopic approach for median arcuate ligament syndrome. ANZ J. Surg. 2022, 92, 1742–1747. [Google Scholar] [CrossRef]
- Weber, J.M.; Boules, M.; Fong, K.; Abraham, B.; Bena, J.; El-Hayek, K.; Kroh, M.; Park, W.M. Median Arcuate Ligament Syndrome Is Not a Vascular Disease. Ann. Vasc. Surg. 2015, 30, 22–27. [Google Scholar] [CrossRef]
- Edwin, G.v.G.; Skraastad, B.K.B.; Hisdal, J.; Wisløff, T.; Sundhagen, J.O.; Kazmi, S.S.H. Assessment of Transserosal Microcirculation with Visible Light Spectroscopy and Laser Doppler Flowmetry in Patients with Median Arcuate Ligament Syndrome and Chronic Mesenteric Ischemia. Vasc. Heal. Risk Manag. 2025, 21, 61–69. [Google Scholar] [CrossRef]
- Cassim, N.; Diljohn, J.; Rampersad, F.S.; Chan, A. Superior Mesenteric Artery Thrombosis in a Patient With Median Arcuate Ligament Syndrome. Cureus 2023, 15, e39351. [Google Scholar] [CrossRef]
- Mak, G.Z.; Speaker, C.; Anderson, K.; Stiles-Shields, C.; Lorenz, J.; Drossos, T.; Liu, D.C.; Skelly, C.L. Median arcuate ligament syndrome in the pediatric population. J. Pediatr. Surg. 2013, 48, 2261–2270. [Google Scholar] [CrossRef]
- Gruber, H.; Loizides, A.; Peer, S.; Gruber, I. Ultrasound of the median arcuate ligament syndrome: A new approach to diagnosis. Med. Ultrason. 2012, 14, 5–9. [Google Scholar]
- Miura, D.; Hiwatashi, R.; Sakita, M.; Sakata, T. A new comprehensive ultrasonic diagnostic method for celiac artery compression syndrome that hybridizes “arterial compression hook sign” and peak systolic velocity. J. Ultrasound 2021, 24, 289–295. [Google Scholar] [CrossRef]
- Römer, C.; Fischer, T.; Haase, O.; Möckel, M.; Hamm, B.; Lerchbaumer, M.H. Assessment of celiac artery compression using color-coded duplex sonography. Clin. Hemorheol. Microcirc. 2020, 76, 413–423. [Google Scholar] [CrossRef]
- Rodriguez, J.H. Median arcuate ligament syndrome: A clinical dilemma. Clevel. Clin. J. Med. 2021, 88, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Accogli, S.; Napoli, V.; Gabelloni, M.; Cioni, R.; Caramella, D. Complications of Vascular Impingement in the Median Arcuate Ligament Syndrome. ECR 20182018. Available online: https://epos.myesr.org/poster/esr/ecr2018/C-2499 (accessed on 28 January 2025).
- Safi, N.; Ånonsen, K.V.; Berge, S.T.; Medhus, A.W.; Sundhagen, J.O.; Hisdal, J.; Kazmi, S.S.H. Early Identification of Chronic Mesenteric Ischemia with Endoscopic Duplex Ultrasound. Vasc. Heal. Risk Manag. 2022, ume 18, 233–243. [Google Scholar] [CrossRef]
- Koc, M.; Artas, H.; Serhatlioglu, S. The investigation of incidence and multidetector computed tomography findings of median arcuate ligament syndrome. Turk. J. Med. Sci. 2018, 48, 1214–1218. [Google Scholar] [CrossRef] [PubMed]
- Ganapathy, A.; Mohakud, S.; Rout, S.; Joy, P.; Alagappan, A.; Manokaran, A. A radio-anatomical study of median arcuate ligament syndrome: Unveiling the morphology and morphometry of median arcuate ligament, celiac trunk, and superior mesenteric artery. Abdom. Imaging. 2024, 49, 3297–3308. [Google Scholar] [CrossRef]
- Dyches, R.P.; Eaton, K.J.; Smith, H.F. The Roles of Celiac Trunk Angle and Vertebral Origin in Median Arcuate Ligament Syndrome. Diagnostics 2020, 10, 76. [Google Scholar] [CrossRef] [PubMed]
- Horton, K.M.; Talamini, M.A.; Fishman, E.K. Median Arcuate Ligament Syndrome: Evaluation with CT Angiography. RadioGraphics 2005, 25, 1177–1182. [Google Scholar] [CrossRef]
- Chan, S.M.; Weininger, G.; Kozhimala, M.; Sumpio, B.J.; Levine, L.J.; Harris, S.; Zheng, S.; Chaar, C.I.O.; Guzman, R.J.; Sumpio, B.E. Utility of Hook Sign in the Diagnosis of Median Arcuate Ligament Syndrome. Ann. Vasc. Surg. 2023, 94, 165–171. [Google Scholar] [CrossRef]
- Shnayder-Adams, M.M.; Masotti, M.; Sanogo, M.L. Clinical Outcomes after Median Arcuate Ligament Release in Patients Responsive to Celiac Plexus Block. J. Vasc. Interv. Radiol. 2024, 35, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Lanka, S.P.; Hakaim, A.; Bowers, S.; Erben, Y.; Bruce, B.; Cangemi, D.; Stone, W.; Paz-Fumagalli, R.; Ritchie, C.; Gloviczki, P.; et al. Institutionalized Adoption of a Protocol for the Management of Median Arcuate Ligament Syndrome Correlates with Improved Surgical Outcomes. Ann. Vasc. Surg. 2024, 110, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Faries, P.L.; Narula, A.; Veith, F.J.; Pomposelli, F.B.; Marsan, B.U.; LoGerfo, F.W. The Use of Gastric Tonometry in the Assessment of Celiac Artery Compression Syndrome. Ann. Vasc. Surg. 2000, 14, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, I.R.; Abdulbaki, A.; Azemi, T. Median arcuate ligament syndrome: Use of fractional flow reserve in documentation of chronic mesenteric ischemia. Vasc. Med. 2014, 19, 317–321. [Google Scholar] [CrossRef]
- de Lara, F.V.; Higgins, C.; Hernandez-Vila, E.A. Median Arcuate Ligament Syndrome Confirmed with the Use of Intravascular Ultrasound. Tex. Hear. Inst. J. 2014, 41, 57–60. [Google Scholar] [CrossRef]
- Roberts, G.S.; François, C.J.; Starekova, J.; Roldán-Alzate, A.; Wieben, O. Non-invasive assessment of mesenteric hemodynamics in patients with suspected chronic mesenteric ischemia using 4D flow MRI. Abdom. Imaging 2021, 47, 1684–1698. [Google Scholar] [CrossRef]
- Romero-Velez, G.; Barajas-Gamboa, J.S.; Pantoja, J.P.; Corcelles, R.; Rodriguez, J.; Navarrete, S.; Park, W.M.; Kroh, M. A nationwide analysis of median arcuate ligament release between 2010 and 2020: A NSQIP Study. Surg. Endosc. 2022, 37, 140–147. [Google Scholar] [CrossRef]
- Antony, A.; Ravindran, S.K.; Jayan, N.P.; Yadukrishna, S.; Sebastian, R.; Kumar, A.; Shyamkumar, S. Performing median arcuate ligament release surgery in celiac artery compression syndrome: Insights from a tertiary care hospital. J. Minimal Access Surg. 2024, 20, 318–325. [Google Scholar] [CrossRef]
- Woestemeier, A.; Semaan, A.; Block, A.; Arensmeyer, J.; Dohmen, J.; Kania, A.; Verrel, F.; Mücke, M.; Kalff, J.C.; Lingohr, P. Prognostic factors for the long term outcome after surgical celiac artery decompression in MALS. Orphanet J. Rare Dis. 2023, 18, 334. [Google Scholar] [CrossRef]
- Alnahhal, K.I.; Tedesco, A.; Khan, Z.Z.; Irshad, A.; Salehi, P. Median Arcuate Ligament Syndrome: Comparing the Safety of Open and Laparoscopic Management in a Large Cohort. Ann. Vasc. Surg. 2023, 96, 215–222. [Google Scholar] [CrossRef]
- Shin, T.H.; Rosinski, B.; Strong, A.; Fayazzadeh, H.; Fathalizadeh, A.; Rodriguez, J.; El-Hayek, K. Robotic versus laparoscopic median arcuate ligament (MAL) release: A retrospective comparative study. Surg. Endosc. 2021, 36, 5416–5423. [Google Scholar] [CrossRef] [PubMed]
- Khrucharoen, U.; Juo, Y.-Y.; Chen, Y.; Jimenez, J.C.; Dutson, E.P. Short- and intermediate-term clinical outcome comparison between laparoscopic and robotic-assisted median arcuate ligament release. J. Robot. Surg. 2019, 14, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Barron-Cervantes, N.M.; Martinez-Esteban, A.; Gardner-Hilbert, E.F.; Villegas-Tovar, E.; Faes-Petersen, R.; Gidi, A.D.G. A Novel Minimally Invasive Robotic-Assisted Surgery Technique for Treatment of Median Arcuate Ligament Syndrome: A Case Report. Cureus 2024, 16, e66933. [Google Scholar] [CrossRef] [PubMed]
- Lam, A.; Kim, Y.-J.; Fidelman, N.; Higgins, M.; Cash, B.D.; Charalel, R.A.; Guimaraes, M.S.; Kwan, S.W.; Patel, P.J.; Plett, S.; et al. ACR Appropriateness Criteria® Radiologic Management of Mesenteric Ischemia: 2022 Update. J. Am. Coll. Radiol. 2022, 19, S433–S444. [Google Scholar] [CrossRef]
- Schneider, M.; Longchamp, J.; Uldry, E.; Corpataux, J.-M.; Kefleyesus, A.; Halkic, N. Systematic hybrid laparoscopic and endovascular treatment of median arcuate ligament syndrome: A single-center experience. Front. Surg. 2023, 10. [Google Scholar] [CrossRef] [PubMed]
- Braet, D.J.; Pourak, K.; Davis, F.M.; Eliason, J.L.; Vemuri, C. Superior mesenteric aneurysm associated with median arcuate ligament syndrome and a single celiacomesenteric trunk. J. Vasc. Surg. Cases Innov. Tech. 2023, 9, 101348. [Google Scholar] [CrossRef]
- Sunohara, D.; Miura, T.; Nomoto, F.; Itagaki, T.; Komatsu, T.; Mochidome, T.; Kasai, T.; Ikeda, U. Effectiveness of endovascular therapy using stents in a patient with celiac artery compression syndrome due to the median arcuate ligament: A case report. J. Cardiol. Cases 2023, 28, 128–131. [Google Scholar] [CrossRef]
- Shintakuya, R.; Kohashi, T.; Nakashima, A.; Oishi, K.; Honmyo, N.; Hihara, J.; Kagawa, E.; Mukaida, H. How to do it: Endovascular stent and ligament resection during pancreaticoduodenectomy for coeliac axis stenosis in median arcuate ligament syndrome. ANZ J Surg. 2021, 91, 2824–2826. [Google Scholar] [CrossRef]
- Choi, K.W.; Min, S.-K. Renovascular Hypertension due to Median Arcuate Ligament Syndrome Treated by Open Bypass after Failed Endovascular Treatment. Vasc. Spéc. Int. 2022, 38, 42. [Google Scholar] [CrossRef]
- Hanaki, T.; Sakamoto, T.; Yata, S.; Murakami, Y.; Fujiwara, Y. Successful Interventional Radiology for Acute Median Arcuate Ligament Syndrome After Pancreaticoduodenectomy. Cureus 2021, 13. [Google Scholar] [CrossRef]
- Arino, H.; Wada, M.; Kobayashi, H.; Yoshida, A.; Oka, S.; Inokuma, T. Metachronous Rupture of Pancreatoduodenal Artery Aneurysm with Median Arcuate Ligament Syndrome: A Case Report and Review of 11 Cases. Intern. Med. 2025, 64, 665–671. [Google Scholar] [CrossRef]
- Sugaya, T.; Suzuki, T.; Wada, J.; Shimizu, H.; Uchihara, D.; Yokogawa, Y.; Ichii, O.; Tai, M.; Ejiri, Y.; Ohira, H. Transarterial embolization for ruptured pancreaticoduodenal artery aneurysm due to segmental arterial mediolysis combined with median arcuate ligament syndrome: A case report. Clin. J. Gastroenterol. 2023, 16, 859–863. [Google Scholar] [CrossRef] [PubMed]
- Saiga, A.; Koizumi, J.; Osumi, K.; Ota, J.; Kubota, Y.; Wada, T.; Akutsu, A.; Watanabe, M.; Murai, S.; Masuda, M.; et al. Celiac Artery Dissection and Retroperitoneal Hemorrhage in Median Arcuate Ligament Syndrome Treated With a Stent and Transcatheter Arterial Embolization: Preprocedural 4-Dimensional Computed Tomography Angiography Assessment. Vasc. Endovasc. Surg. 2021, 56, 75–79. [Google Scholar] [CrossRef]
- Manunga, J.; Cravero, E.; Goldman, J.; Stanberry, L.I.; Stephenson, E.; Harris, K.M.; Skeik, N. Examining the impact of median arcuate ligament-induced celiac artery compression on target vessel patency, long-term survival and device integrity in fenestrated and branched endovascular repairs. J. Vasc. Surg. 2024, 80, 996–1005.e1. [Google Scholar] [CrossRef] [PubMed]
- Squizzato, F.; Oderich, G.S.; Tenorio, E.R.; Mendes, B.C.; DeMartino, R.R. Effect of celiac axis compression on target vessel-related outcomes during fenestrated-branched endovascular aortic repair. J. Vasc. Surg. 2021, 73, 1167–1177.e1. [Google Scholar] [CrossRef]
- Matsumoto, K.; Shinozaki, H.; Shinozaki, S.; Yukisawa, S.; Kimata, M.; Terauchi, T.; Sata, N. Clinical outcomes after surgical decompression of median arcuate ligament syndrome—An observational study. Indian J. Gastroenterol. 2024, 43, 638–644. [Google Scholar] [CrossRef]
- Bower, K.S.; McCarthy, C.C.; Vyasa, P.; Nagarsheth, K.; Desai, M.J. Celiac plexus block: A diagnostic tool for neurogenic median arcuate ligament syndrome. Pain Pr. 2024, 25. [Google Scholar] [CrossRef] [PubMed]
- Kayashima, H.; Minagawa, R.; Inokuchi, S.; Koga, T.; Miura, N.; Kajiyama, K. Laparoscopic treatment of median arcuate ligament syndrome without ganglionectomy of the celiac plexus in the hybrid operating room: Report of a case. Int. J. Surg. Case Rep. 2021, 81, 105840. [Google Scholar] [CrossRef]
- Sahm, M.; Otto, R.; Pross, M.; Scholbach, T.; Mantke, R. Laparoscopic therapy of the coeliac artery compression syndrome: A critical analysis of the current standard procedure. Ind. Mark. Manag. 2020, 102, 104–109. [Google Scholar] [CrossRef]
- Donnelly, L.; Turner, B.; Davies, A.H. Atypical case of coexistent vascular compression syndromes: Median arcuate ligament syndrome and nutcracker syndrome. BMJ Case Rep. 2023, 16, e257754. [Google Scholar] [CrossRef]
- Matsumoto, K.; Shinozaki, H.; Shinozaki, S.; Terauchi, T.; Lefor, A.K.; Sata, N. Normalization of Flow in the Common Hepatic Artery after Decompression of Median Arcuate Ligament Syndrome with Diminution of a Pancreatoduodenal Arcade Aneurysm. Case Rep. Gastroenterol. 2022, 16, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Jalili, J.; Javadrashid, R.; Alvandfar, D.; Falahatian, M.; Jafarizadeh, A.; Alihosseini, S.; Hashemizadeh, S.E. Obstructive jaundice as a rare complication of multiple pancreaticoduodenal artery aneurysms due to median arcuate ligament syndrome: A case report and review of the literature. J. Med Case Rep. 2023, 17, 385. [Google Scholar] [CrossRef] [PubMed]
- Kazmi, S.S.H.; Safi, N.; Berge, S.T.; Kazmi, M.; Sundhagen, J.O.; Julien, K.; Thorsby, P.M.; Ånonsen, K.V.; Medhus, A.W.; Hisdal, J. Plasma α-Glutathione S-Transferase in Patients with Chronic Mesenteric Ischemia and Median Arcuate Ligament Syndrome. Vasc. Heal. Risk Manag. 2022, 18, 567–574. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).