Abstract
This review article explores the fundamental role of receptor targeting in overcoming drug resistance in cancer therapy, an area of critical concern given the persistently high rates of cancer morbidity and mortality globally. We highlight how receptor biology intersects with the development of therapeutic resistance with a specific focus on anti-angiogenic agents, immune checkpoint inhibitors, and monoclonal antibodies, which directly or indirectly influence receptor pathways. We also explore how other receptor tyrosine kinases can initially suppress tumor growth, yet often lead to resistance, underscoring the need for novel combinatorial approaches that incorporate advanced receptor modulation techniques. Further, the review delves into the mechanisms by which modulation of the tumor microenvironment and immune system via receptor pathways can overcome resistance to traditional immunotherapies. Additionally, emerging technologies in receptor-targeted nanomedicine are also highlighted, showcasing their potential to revolutionize drug delivery and improve therapeutic outcomes by targeting specific receptor interactions. Ultimately, this review calls for a deeper understanding of receptor dynamics to develop more precise interventions, including insights from various healthcare settings that can prevent or circumvent drug resistance, thus enhancing patient outcomes in oncology.
1. Introduction
Cancer therapeutics has witnessed significant advancements over the past few decades. However, cancer remains a major cause of death globally. In 2024, in the United States alone, there is a staggering projection of over two million new cancer cases and approximately 611,720 deaths from this disease. These numbers are a sobering reminder that there is an urgent need to address the challenges associated with cancer therapy and the eventual development of drug resistance to current therapeutic strategies [1]. Drug resistance in cancer results when cancer becomes tolerant to the treatment. This insensitivity to drugs arises from a variety of factors that can reform tumors, which can lead to recurrence and relapse. The development of resistance against treatment can occur within weeks of treatment or slowly over months or years. The tumor growth kinetics, tumor microenvironment (TME), therapeutic pressure, physical barriers, irreversible genetic mutations, non-compliance [2], and immune system are the multi-dimensional challenges that collectively contribute to the failure of treatments in cancer [3,4]. Such drug resistance adaptations can manifest either inherently or as an acquired trait, post-treatment. Uncontrollable drug resistance has profound clinical implications that can attenuate the efficacy of treatment regimens and increase the probability of recurrent, aggressive tumor phenotypes [5]. A high mortality rate is associated with the development of drug resistance, with over 90% of deaths being attributed to drug resistance [6]. This rise in cancer resistance mirrors the antibiotic resistance crisis, threatening our therapeutic arsenal against various cancers due to stagnation in new drug discoveries. To prevent a potential therapeutic deadlock, there is a need for innovative approaches to combat the growing challenge of cancer drug resistance [7]. The Cancer Moonshot Initiative was launched in 2016 by the National Cancer Institute (NCI), a collaborative effort aimed at accelerating scientific discovery and research to foster greater collaboration, advancing research, and sharing cancer data. The goals of this initiative are to identify novel candidates [8] for cancer treatment, explore preventive measures, and enhance cancer detection methods. The research initiatives of the Cancer Moonshot, launched initially in 2016 have created important insights into cancer mechanisms, potential treatments, and prevention strategies. The Adult Immunotherapy Network is a specific aim of the Cancer Moonshot initiative focused on developing immune-based approaches for cancer treatment and prevention in adult patients. The Cancer Moonshot Biobank is another specific aim that voluntarily collects blood and tissue samples from people with cancer and sends them out to researchers to advance our understanding of cancer [9,10,11].
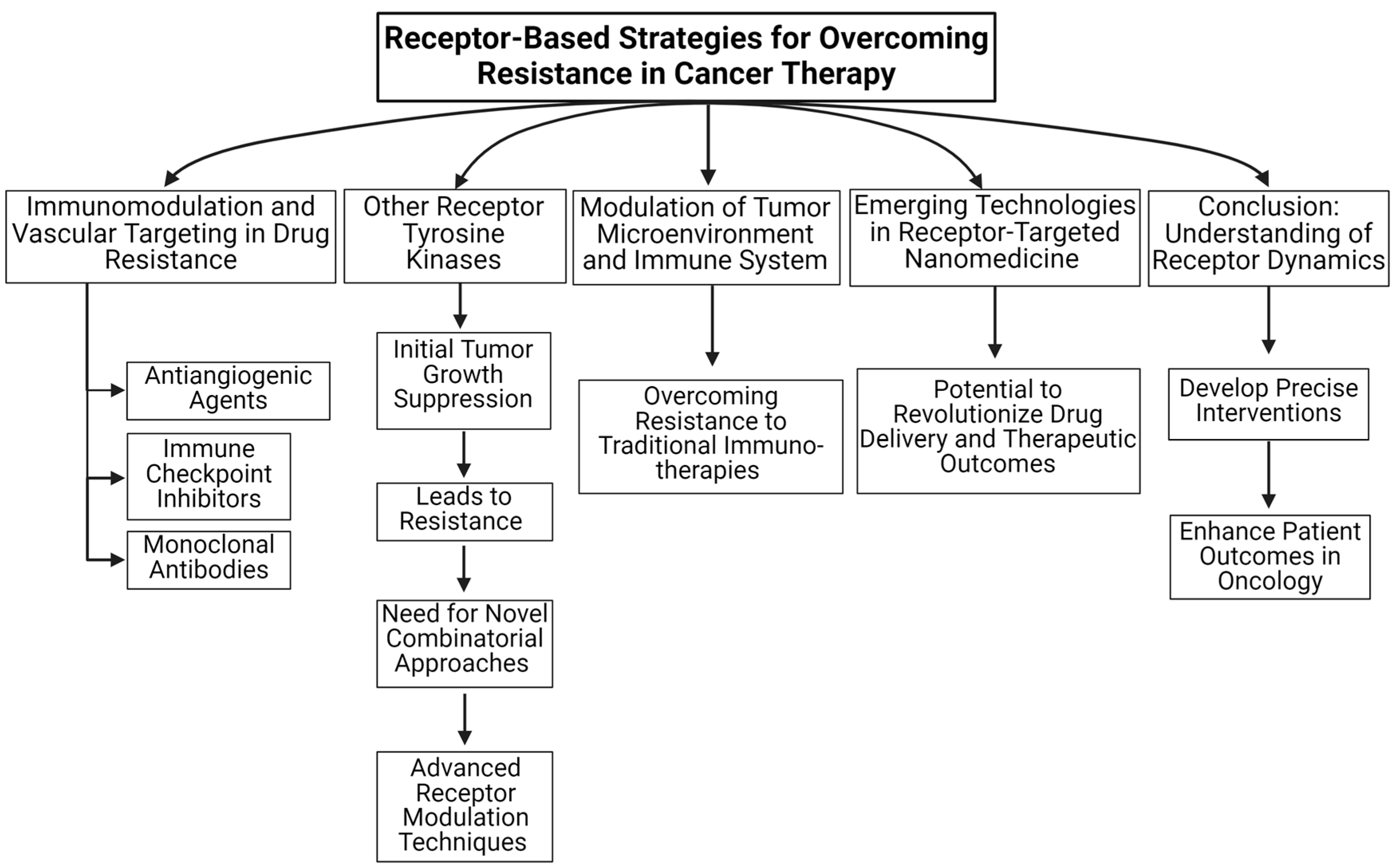
Given the urgent threat of drug resistance, especially considering the expected cancer deaths in the future, this review article delves into the multifaceted strategies to overcome drug resistance in cancer, focusing on the roles of vascular targeting, immunomodulation, monoclonal antibodies, emerging technologies, and natural approaches. Our review highlights how understanding and countering drug resistance in various cancer forms requires an integrated approach, combining traditional methods with novel therapies (Figure 1). We explore anti-angiogenic strategies and their role in overcoming cancer drug resistance, emphasizing the necessity of combination therapies. Such vascular targeting therapies focus on disrupting the blood supply to tumors. By inhibiting the formation of new blood vessels, we can limit tumor growth and enhance drug delivery. Vascular therapy seems to hold the potential to improve overall outcomes when combined with other therapies. Another promising avenue to tackle drug resistance is to harness the immune system to recognize and attack cancer cells. Immune checkpoint inhibitors seem to enhance the body’s natural defenses against tumors. Targeting specific immune pathways that are impacted by the TME to overcome drug resistance is discussed in detail. Monoclonal antibodies (mAbs) are engineered to recognize specific proteins in cancer cells and block growth signals to enhance immune responses and directly attack cancer cells. Here, we have discussed the role of monoclonal antibodies in addressing therapeutic resistance, which is continuously evolving in cancer treatment [12]. Other emerging technologies in nanomedicine, like nano-engagers, nanoparticles, and nanobodies, have been used to co-deliver cancer therapies to overcome resistance. Such drug delivery approaches have been shown to alter the tumor microenvironment offering promising avenues for developing more effective cancer therapies. We will also detail specific drug mechanisms that can be targeted to overcome drug resistance at the end of this review.
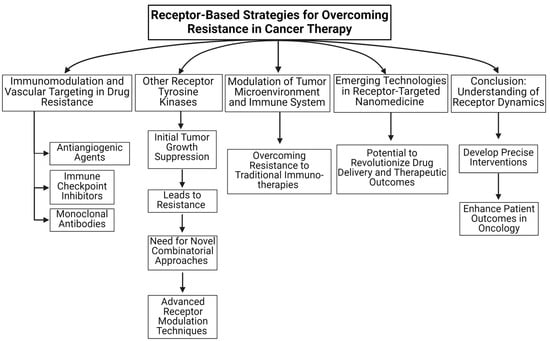
Figure 1.
An overview of receptor-based strategies for overcoming resistance in cancer therapy is summarized in this review article.
2. Strategies to Overcome Drug Resistance
2.1. Immunomodulation and Vascular Targeting in Drug Resistance
Harnessing the immune system to recognize and attack cancer cells is a promising avenue. In this section, we will explore the use of immunotherapy and vascular targeting in mitigating cancer drug resistance. We will address anti-angiogenic strategies, highlighting the importance of understanding and counteracting resistance in various cancer forms through combination therapies. Next, we will discuss resistance to immune checkpoint inhibitors (ICIs), which have improved long-term survival outcomes [13,14]. Unfortunately, ICIs are only effective in patients with immune-responsive tumors. Many cancer patients have a mixed response to the ICI therapy; they either do not respond to the ICIs or experience disease progression after an initial period of response. Resistance to ICI treatment is a significant challenge in oncology. Here, we will focus on resistance mechanisms to PD1/PDL1 blockade therapies. The use of PD1/PDL1 in combination therapies has been at the center stage in almost 80% of 4400 clinical trials of the current clinical trial landscape trying to evaluate the efficacy of PD1/PDL1 monoclonal antibodies (mAbs) [15].
Immunomodulation is widely used in multiple myeloma (MM) therapy. Immunomodulatory drugs, like thalidomide (THAL), seem to be beneficial for MM therapy. When used in conjunction with the proteasome inhibitors bortezomib and dexamethasone, thalidomide exhibits high clinical efficacy in patients eligible for autologous stem cell transplantation. Though effective, THAL causes adverse drug reactions, which may necessitate careful patient monitoring and management strategies [16]. The development of new immunomodulatory agents based on THAL—like lenalidomide and pomalidomide—could be incorporated into multiple treatment regimens, such as monotherapy or combination therapy [17].
2.1.1. Antiangiogenic Strategies to Overcome Cancer Drug Resistance
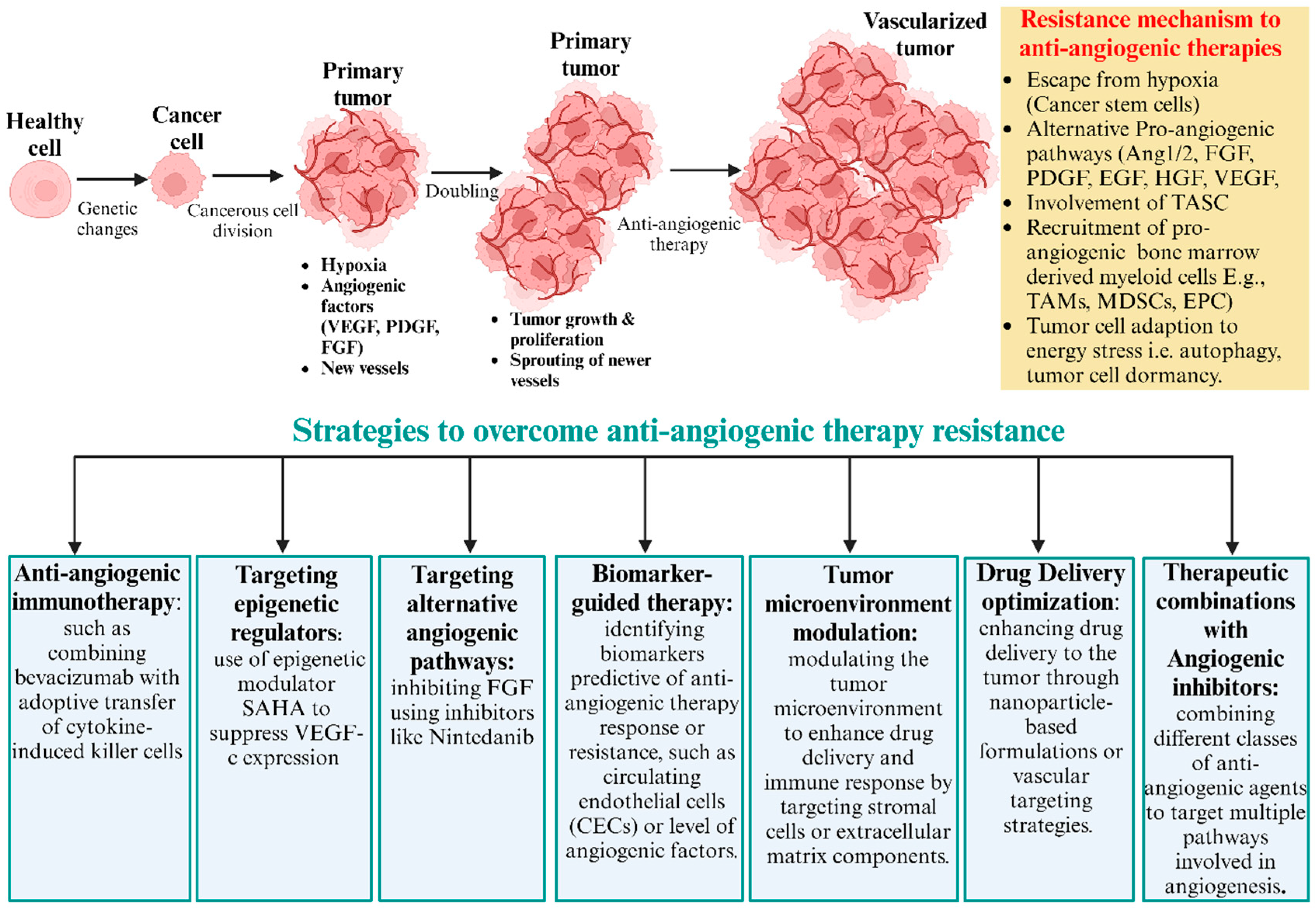
The process of forming a new blood vessel from pre-existing ones, angiogenesis, is crucial for cancer progression. Antiangiogenic therapy, which targets the vascular supply to tumors, has emerged as a potent strategy in cancer treatment, particularly those targeting vascular endothelial growth factor (VEGF) and its receptors. This section explores the mechanisms behind resistance to anti-angiogenic strategies and potential receptor-based methods to overcome resistance (Figure 2).
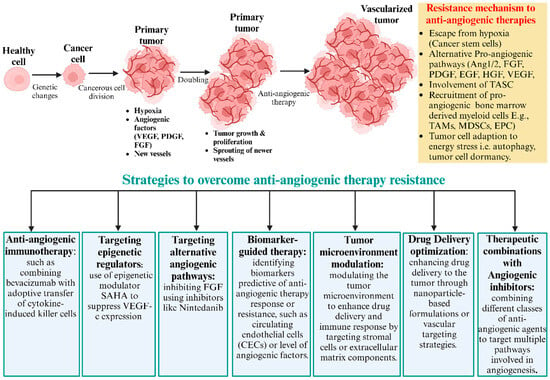
Figure 2.
Common scenario of anti-angiogenesis treatments. Anti-angiogenesis treatments initially inhibit primary tumor growth but often lead to eventual relapse due to the development of therapy resistance. Tumor cells resist through hypoxia and the upregulation of alternative pro-angiogenic factors, like Ang1/2, FGF, PDGF, EGF, HGF, and VEGF. Additionally, recruited vascular modulator cells, like TAMs, MDSCs, and EPCs, promote new blood vessel formation. Tumor cells adapt to energy stress and survival mechanisms, like autophagy, dormancy, and cancer stem cells. Resistance to anti-angiogenic drugs could be overcome by various approaches. This includes targeting alternative angiogenic pathways, anti-angiogenic immunotherapy, tumor microenvironment modulation, drug delivery optimization, and therapeutic combinations with angiogenic inhibitors. Combination therapies aim to enhance treatment efficacy and prevent tumor relapse.
The following are examples of agents used to hinder tumor blood vessel formation: bevacizumab (monoclonal antibody against VEGF), sorafenib (a small-molecule tyrosine kinase inhibitor (TKI)), aflibercept (decoy receptor or VEGF trap), ramucirumab (VEGFR2 inhibitor). The efficacy of these drugs has been demonstrated across cancer types. Yet, resistance to these drugs is unavoidable and complex. Thus, there is a need to comprehensively understand the mechanisms of action, plausible mechanisms of resistance, and novel approaches to combat it.
Acquired drug resistance to anti-angiogenic therapy results from various factors, such as the following: (a) upregulation of compensatory pathways in tumors, such as the alternative pro-angiogenic signaling pathways PDGF, FGF, G-CSF, PGF, EGF, SDF-1, HGF, TGF, VEGF-C, angiopoietins, and DII-4, to compensate for the inhibited VEGF pathway; [18,19] (b) cellular recruitment of bone marrow-derived endothelial progenitor cells [20], pericyte progenitor cells [21], and tumor-associated macrophages [22] to sustain angiogenesis; (c) perivascular cells to help stabilize new blood cells, aiding in the continued supply of nutrients to the tumor [23]. A primary mechanism driving resistance to anti-angiogenic therapies in hepatocellular carcinoma (HCC) has been associated with YAP1 (Yes-Associated Protein 1), a transcriptional regulator that plays a crucial role in various cellular processes, including organ size control, cell proliferation, and apoptosis. In HCC, YAP1 is associated with the formation of tumor-associated blood vessel formation. The increased expression of YAP1 around tumor-associated blood vessels indicates its crucial role in angiogenesis, making it a potential target for therapeutic intervention. The activation of YAP1 leads to the transient expansion of stem-like cancer cells that circumvents the inhibitory effects of anti-angiogenic agents. Such insights may help formulate therapies, where YAP1 is targeted alongside conventional anti-angiogenic agents [24].
Cancers, such as melanoma, pancreatic cancer, breast cancer, and prostate cancer, which depend on angiogenic factors other than VEGF, show reduced responsiveness to anti-VEGF agents. Various preclinical mouse models and clinical trials indicate that inhibiting a specific growth factor can lead to the upregulation of others [25]. For example, a preclinical study has shown an increase in SDF1 and P1GF in mice treated with anti-VEGF2 compounds [26]. In a pancreatic cancer mouse model, the prolonged use of anti-VEGF antibodies elevated the expression of several pro-angiogenic growth factors, including Ang-1, Ephrin-A1/A2, and FGF2 [27]. In a clinical study by Willet et al., rectal cancer patients treated with BVZ exhibited significantly increased plasma levels of PIGF.
Targeting angiopoietin offers a promising strategy to overcome resistance to anti-vascular endothelial growth factor (VEGF) treatments. Angiopoietins, particularly Ang-1 and Ang-2, are crucial for the maturation and stabilization of blood vessels [28,29]. In preclinical studies, the simultaneous inhibition of both VEGF and Ang-2 has led to significant reductions in tumor growth and vascularization compared to VEGF inhibition alone [30]. Similarly, several preclinical cancer models have shown improved outcomes when both VEGF and FGF pathways are targeted [31,32]. Transforming growth factor-β (TGF-β) is a multifunctional cytokine involved in the modulation of angiogenesis through its effects on endothelial cells and the extracellular matrix (ECM) [33]. The upregulation of TGF-β expression has been noted in various cancer models that are resistant to anti-VEGF therapy [34].
In ovarian cancer, anti-angiogenic agents were the first approved targeted agents. Such therapies target the VEGF/VEGFR pathway, TK receptor, and non-VEGF/VEGFR targets and have demonstrated mixed efficacies either as monotherapy or when combined with chemotherapy [35]. In such situations, the benefits of using anti-angiogenic treatments must be carefully weighed, as such treatment paradigms have not increased cures but rather contributed to the development of anti-angiogenic resistance. A combination of poly (ADP-ribose) polymerase inhibitors (PARPi) or ICIs seems to be a promising strategy to overcome resistance and enhance the antitumor activity of anti-angiogenic agents.
Crizotinib, an anaplastic lymphoma kinase-tyrosine kinase inhibitor (ALK-TKI), when used in the treatment of non-small cell lung cancer (NSCLC), seems to develop resistance mediated by chemokine-driven angiogenesis. By using a combination therapy of crizotinib along with a multi-targeted TKI angiogenesis inhibitor like anlotinib, researchers have shown anlotinib’s effectiveness in reversing the chemokine-driven angiogenesis in echinoderm-microtubule-associate-protein-like-4 (EML4)-ALK-positive NSCLC [36].
The tumor microenvironment (TME) is indispensable for influencing drug resistance. Fibroblast activation protein-alpha (FAPα)-expressing hepatic stellate cells were implicated in causing resistance to anti-angiogenic drugs in colorectal cancer liver metastasis models. Targeting these stellate cells with the FAPα-activated prodrug Z-GP-DAVLBH can potentially re-sensitize tumors to angiogenic inhibitors, pointing toward a more integrative approach that considers the broader tumor environment [37]. Further elucidating resistance mechanisms, the role of endothelial p130cas (Crk-associated substrate) has been spotlighted in conferring resistance to anti-angiogenesis therapy. Targeting this molecular entity might be an additional avenue to boost the efficacy of existing therapeutic agents [38]. As we explore the complexities of the tumor microenvironment and its contribution to drug resistance, the identification of novel oncogenic factors also becomes crucial. For instance, one study demonstrates the oncogenic role of upregulated lymphoblastic leukemia-derived sequence-1 (LYL1) in the progression and metastasis of ovarian cancer [39]. Such insights are vital for developing targeted therapies that can intervene effectively in the signaling pathways mediated by these oncogenes, potentially circumventing the mechanisms that lead to resistance.
Myeloid-derived suppressor cells (MDSCs), neutrophils, monocytes, and macrophages are potentially involved in resistance to anti-angiogenic therapy by creating an immunosuppressive TME [40]. Notably, MDSCs have been shown to drive angiogenesis [41]. Various cytokines, including VEGF, chemokine C-C motif ligand 2 (CCL2), macrophage colony-stimulating factor (MCSF), and complement anaphylatoxin C5a, facilitate the recruitment of these cells to the TME [42,43,44]. Blocking the recruitment of these immunosuppressive cells to the TME could be a promising approach to overcoming resistance to anti-angiogenic therapy. A recent study by Ghouse et al. demonstrated that inhibiting C5aR1 in combination with an anti-angiogenic Listeria-based vaccine reduced or prevented metastasis by reducing vascular density and enhancing antitumor immunity compared to the approved anticancer drug sunitinib [45]. Consequently, combining angiogenic inhibitors with immune checkpoint inhibitors in HCC and RCC has shown greater promise than monotherapy [46,47].
Along with receptor tyrosine kinases (RTKs), integrins, immune checkpoint receptors, Toll-like receptors (TLRs), G-protein-coupled receptors (GPCRs), nuclear receptors, Notch receptors, and cytokine receptors all play critical roles in anti-angiogenic strategies for cancer therapy. Integrins, such as αvβ3 and αvβ5, facilitate cell–ECM adhesion and are targeted to inhibit new blood vessel formation, preventing tumor angiogenesis [48]. Immune checkpoint receptors (discussed in detail in Section 2.1.1, including PD-1 and CTLA-4) regulate immune responses and can amplify the antitumor immune response when combined with anti-angiogenic agents [49]. TLRs that recognize pathogen-associated (PAMPs) and damage-associated molecular patterns (DAMPs) can influence angiogenesis through the production of pro-inflammatory cytokines; targeting TLR4 can reduce tumor-associated inflammation and angiogenesis [42]. Angiogenesis and the migration of cancer cells are facilitated by GPCRs, like CXCR4 and CCR5, of which antagonists disrupt chemokine signaling pathways to reduce tumor vascularization [50,51]. Targeting nuclear receptors, which regulate gene expression influencing angiogenesis, can inhibit tumor growth [52]. Notch receptors are also essential in angiogenesis regulation; blocking Notch signaling can reduce dysfunctional blood vessel formation within tumors [53]. Finally, cytokine receptors, like IL-6R and TNFR, also regulate inflammation and angiogenesis in the TME; targeting these receptors can inhibit cytokine-driven angiogenic processes and enhance anti-cancer treatments [54]. Recent studies have demonstrated the effectiveness of these strategies in improving therapeutic outcomes and overcoming drug resistance in cancer treatment.
In summary, resistance remains a significant hurdle, while anti-angiogenic therapies have transformed the cancer treatment landscape. The convergence of studies underscores the importance of combination strategies, deeper mechanistic insights, and a holistic approach that considers the tumor microenvironment. As our understanding of resistance deepens, so does our arsenal against it, paving the way for more durable and effective cancer treatments.
2.1.2. Overcoming Immune Checkpoint Inhibitor Resistance in Cancer Treatment
The TME plays a critical role in shaping the therapeutic outcomes across diverse types of cancers. In the previous section, we saw how TME facilitates resistance to ant-angiogenic therapies. In this section, we will look at the influence of the TME on the efficacy of ICIs and its role in mitigating drug resistance.
Resistance to immune checkpoint inhibitors is not limited to a single cancer type; various malignancies have unique and shared resistance mechanisms and strategies to overcome such therapeutic interventions. Despite the transformative potential of ICIs in cancer treatment, many patients either do not respond or develop resistance [55,56]. To further improve the therapeutic efficacy of these drugs, it is essential to understand the fundamental mechanisms of resistance. Various studies have shown that resistance mechanisms to anti-PD1/PDL1 therapies include alterations in the tumor microenvironment, the upregulation of alternative immune checkpoints, and intrinsic tumor cell changes, like mutations and altered signaling pathways [57,58]. Most importantly, regulating PDL1 expression plays a crucial role in the success or failure of immuno-therapies [59,60]. The acquired resistance patterns, like neoantigen depletion, defects in antigen presentation machinery, aberrations of interferon signaling, tumor-induced exclusion/immunosuppression, tumor cell plasticity, and other immune checkpoint upregulation following ICI therapy, and the molecular underpinnings associated with these phenomena, have been examined under different treatment modalities [61].
To overcome resistance to PD1/PDL1 blockade in breast cancer, specific mechanisms of resistance, like reduced antigen presentation, an immunosuppressive tumor microenvironment, interactions with other immune checkpoints, and aberrant activation of oncological signaling, have been studied. Approaches like the combination of ICIs with other therapies, such as chemotherapy, radiotherapy, targeted therapy, oncologic virus, and neoantigen-based therapies, have been explored [62,63]. On a related note, a revolutionary approach utilizing mPEG-Masked Trispecific T-Cell Nano-engagers, combined with a Stimulator of Interferon Genes (STING) agonists, to successfully bypass immunotherapy resistance in triple-negative breast cancer has even been shown to have a vaccination effect [64].
The resistance mechanisms to anti-PD cancer immunotherapy highlight the intricate interplay among tumor cells, the immune micro-environment, and therapeutic agents [65]. Among the various factors regulating PDL1 expression in cancers, transcriptional, post-transcriptional, and post-translational modifications play a pivotal role. These regulations offer novel small-molecule therapeutics [57], which can be combined with other agents, such as mitogen-activated protein kinase (MAPK) inhibitors and cancer vaccines, for the direct modulation of PDL1 expression and to improve the anticancer efficacy of PD1/PDL1 blockade [55,58,66,67]. For instance, enhancing PDL1 degradation through ITCH (E3 ligase) during MAPK inhibitor therapy has shown potential in countering acquired resistance [59]. Similarly, in colorectal cancer, irreversible c-Jun N-Terminal Kinase (JNK) blockade or that in combination with a myeloid cell leukemia-1 (Mcl-1) antagonist has been proposed to counteract PDL1-mediated resistance to chemotherapy [68]. Also, combination therapies involving STING agonists have shown promise in addressing anti-PD-L1 resistance. For example, the combination of a STING agonist and CXC chemokine receptor 3 (CXCR3) antagonist disrupted immune tolerance to overcome anti-PDL1 resistance in lung adenocarcinomas undergoing oxidative stress [69,70,71].
As we explore the role of emerging technologies in overcoming drug resistance, nanomedicine presents a promising avenue. For instance, the nanoscale fluoropyrimidine polymer CF10 has demonstrated enhanced therapeutic efficacy in a rat model of colorectal cancer liver metastasis, highlighting the potential of nanotechnology to improve drug delivery and efficacy in complex cancer conditions [72].
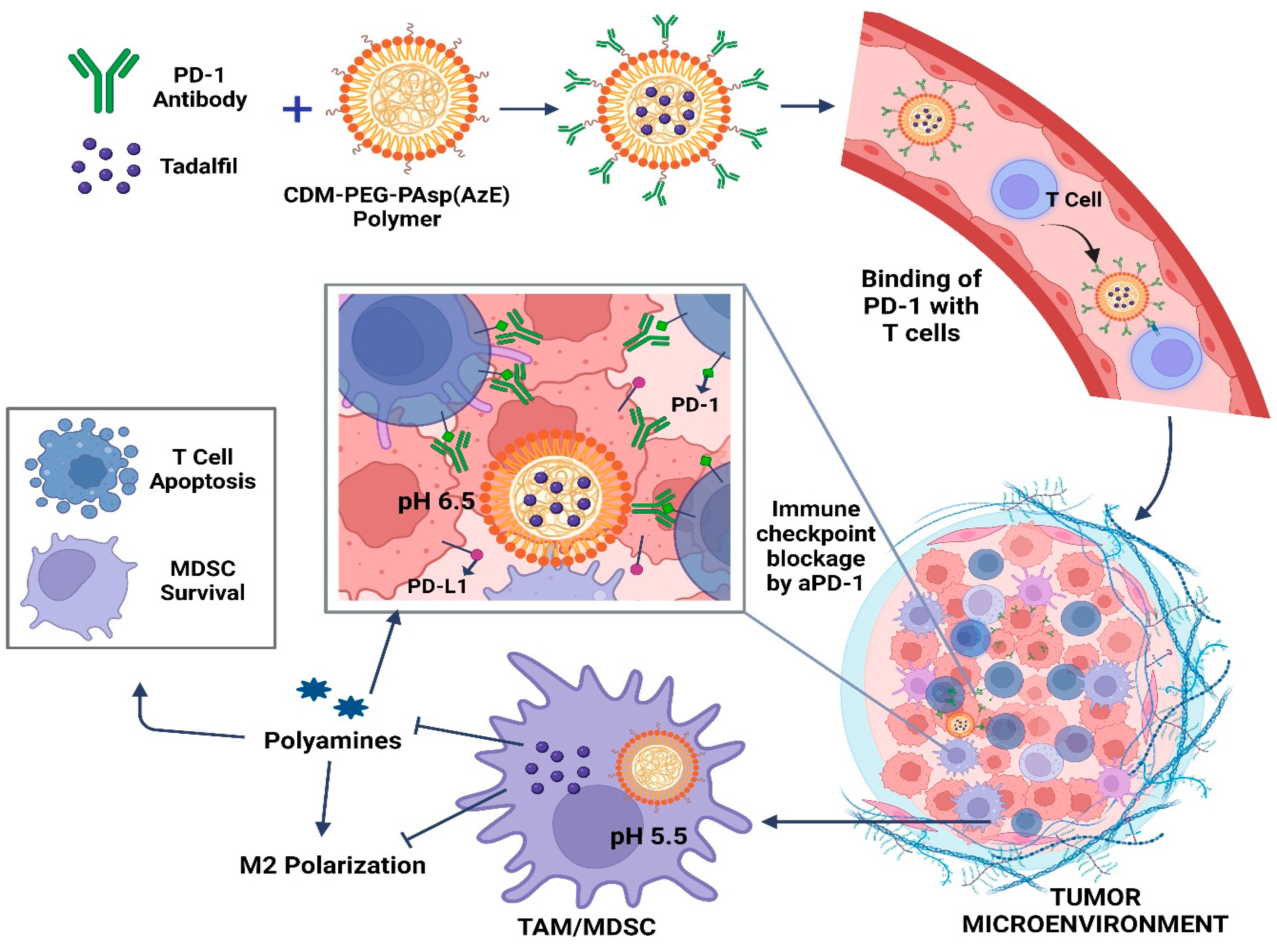
A novel dual pH-sensitive nanodrug has been developed, capable of carrying both Tadalafil (TA) and aPD-1 (PD-1 antibody). This innovative formulation achieves targeted drug delivery by binding to circulating programmed-cell-death-receptor-1-positive T cells, enabling their infiltration into the tumor. Moreover, within the acidic tumor microenvironment (TME), the nanodrug efficiently releases aPD-1 for immune checkpoint blockade (ICB), while TA remains encapsulated, effectively regulating tumor-associated macrophages (TAMs) and myeloid-derived suppressor cells (MDSCs). By leveraging the synergistic effects of TA and aPD-1, coupled with precise tumor targeting, this nanodrug combats M2 polarization and disrupts polyamine metabolism in TAMs and MDSCs, thereby overcoming the immunosuppressive TME. Consequently, this approach demonstrates remarkable therapeutic efficacy in hepatocellular carcinoma (HCC) through ICB, while minimizing adverse effects (Figure 3). CDM, 2-propionic acid-3-methylmaleic anhydride; PD-1, programmed cell death receptor 1; PD-L1, programmed death-ligand 1.
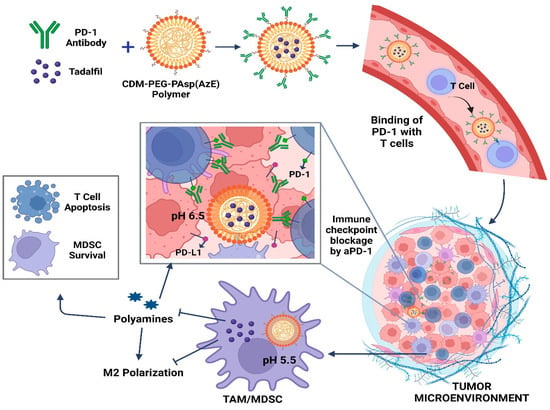
Figure 3.
Schematic depiction detailing how nanodrug delivery combats immune checkpoint blockade resistance in hepatocellular carcinoma (HCC) through their antitumor mechanisms.
The tumor infiltration of lymphocytes (TIL) against cancer plays an important role in eliminating cancer cells by attacking them. However, TIL’s effectiveness is hindered by some factors, such as immune system brakes or signals from the tumor that weaken the immune response. Depending on the type of immune infiltration, tumors can be divided into hot (high lymphocyte infiltrations), cold (few lymphocyte infiltrations), immune excluded (less lymphocyte infiltration), and immune desert (very low lymphocyte density). Tumor immune cell infiltration affects the immunotherapy receptivity and prognosis and is impacted by various immune cells, like M1/M2 macrophages, neutrophils, CD4+ T cells, and CD8+ T cells. Clinically, a high TIL density correlates with better outcomes in various cancers and indicates an active immune response. TILs predict responsiveness to immunotherapies like immune checkpoint inhibitors. Different cancers have different strategies to regulate immune infiltration. For example, in melanoma, a high mutational burden may lead to the production of neoantigens that attract immune cells, making these tumors more susceptible to immune checkpoint inhibitors. In colorectal cancer, the presence of a thick fibrotic stroma can physically prevent immune penetration to the tumor site, thereby decreasing the efficacy of immunotherapy. The degree and type of immune infiltration in breast cancer might vary depending on the subtype (e.g., triple-negative vs. hormone receptor-positive), with triple-negative breast cancer usually exhibiting higher levels of immune cell infiltration [73,74]. In hepatocellular carcinoma (HCC), dysregulated signaling pathways, such as TGF-β, JAK-STAT, and Wnt/β-Catenin, have a major impact on immune cell infiltration by influencing the activation and differentiation of T cells, NK cells, and macrophages [75]. Similarly, in ovarian cancer, the immune infiltration patterns differ between primary and metastatic sites. Primary tumors often exhibit immune-excluded or immune-desert patterns with low T-cell infiltration, whereas omental metastases may have higher numbers of T cells, though often in an exhausted or non-tumor-specific state [76].
Considering the various resistance mechanisms, a multi-pronged therapeutic approach, targeting different facets of the immune system and the tumor biology/TME, is essential. Multiple researchers have shown efficacy when targeting TANK-binding kinase 1 (TBK1), a key regulator of the innate immune response to counteract resistance in T-cell-cold solid tumors [77,78]. Similarly, novel targets and combinations, including the extracellular matrix, tumor-associated macrophages (TAMs), TAM receptors, and p38 MAPK inhibitors, have shown potential in preclinical and early clinical studies in modulating ICI resistance mechanisms and suggest avenues for potential combination therapies [79,80,81,82,83,84]. In hepatocellular carcinoma, the need to target tumor-associating myeloid cells and combine immune checkpoint blockades has been employed to evade sorafenib resistance [85]. This finding was supported by Wang et al. (2023), who outlined a strategic approach using the T-cell-mediated targeted delivery of tadalafil to mitigate immunosuppression through regulation of TAMs and myeloid-derived suppressor cells (MDSCs) by inhibiting M2 polarization and modify polyamine metabolism, thereby enhancing the efficacy of ICIs in liver cancer [86]. Focusing on the immune microenvironment, the potential of TLR5 agonists in combination with antibodies targeting CTLA-4 and PD1 as powerful tools to boost antitumor immunity and treat solid tumors refractory to ICIs has been underlined [87,88]. On another note, intra-tumoral heterogeneity and the microbial milieu can contribute to therapy resistance. A unique strategy targeting the interaction between PD-L2 and its binding partner, repulsive guidance molecule b (RGMb), can address microbiome-dependent resistance to PD-1 pathway inhibitors [66].
Furthermore, the unique role of chemokines and chemokine-regulated pathways in mediating therapy resistance and their utility as predictive biomarkers and prognostic factors in pancreatic cancer were thoroughly explored [89]. This work underscores the importance of the intricate tumor microenvironment and its key players. The potential of interleukins as targets has also been acknowledged. The targeted therapy against interleukin-34, which facilitates the differentiation of immunosuppressive cells within the TME, may offer a new horizon in overcoming therapy resistance across various cancer types [90]. Interestingly, it was discovered that a novel immune-cytokine called anti-PD-L1/IL-15, which combines anti-PD-L1 and IL-15 to deliver an immune-activating cytokine directly to the immunosuppressive TME and block the immune checkpoint at the same time, can overcome PDL1 blockade resistance and boost antitumor immunity [91]. Similarly, viral vectors expressing IL-2 in combination with anti-PD1 or anti-PDL1 have been shown to eradicate tumors in the intraperitoneal MC38-luc tumor model [92]. Even in sarcomas, an insightful role of the histologic subtype, tumor-infiltrating lymphocytes, and the tumor microenvironment challenges posed by immune checkpoint inhibitor resistance and potential avenues for therapeutic intervention have been discussed [56]. Furthermore, understanding the role of transforming growth factor-β (TGF-β) in non-inflamed cancers can provide insights into designing effective therapeutic strategies [93]. Innovative drug delivery systems, such as smart drug delivery platforms, can deliver high concentrations of drugs, modulate interferon signaling pathways, and reverse the immunosuppressive microenvironment to overcome small molecule and monoclonal antibody resistance in cancer immunotherapy [67].
The potential predictive factors for ICI immunotherapy and the strategies to counteract the resistance have also been addressed for esophageal cancer, another malignancy that presents significant resistance to PD1/PDL1 inhibitors [94]. This predictive factor provides the potential avenues for tailored treatments in immune therapies reflected by microsatellite-instability-high advanced colorectal cancers, known for their intricate resistance patterns [95,96]. A comprehensive overview of melanoma immunotherapy specifically spotlights neoantigens, antigen presentation, oncogenic pathways, DNA repair, T cell functionality, checkpoint regulators, chromatin modifiers, and copy-number alterations as predictive biomarkers for ICI responses and resistance [97]. Few recent studies have underscored the multifaceted nature of intra-tumoral immunotherapy mechanisms extending beyond mere checkpoint inhibition [60,80].
In conclusion, while resistance to cancer immunotherapies remains a significant challenge, ongoing research endeavors shed light on the intricate mechanisms at play. A multifaceted approach, integrating various therapeutic strategies, understanding the complex tumor–immune interface, innovative combination therapies, novel drug delivery systems, and targeted interventions are the way forward in overcoming resistance and improving patient outcomes [98,99,100,101,102].
3. Targeted Therapies and Specific Drug Resistance
This section delves into the details of resistance mechanisms specific to receptor-targeted cancer therapies. Highlighting agents, such as kinase inhibitors, anti-EGFR therapies, tyrosine kinase inhibitors (TKIs), and osimertinib, this section elucidates molecular resistance patterns and potential counterstrategies (Table 1). The focus extends to understanding resistance in particular cancers, like NSCLC, providing insights to refine therapeutic approaches for enhanced efficacy.

Table 1.
Summary of receptor-based strategies to overcome resistance in different cancers.
Overcoming Resistance to Kinase Inhibitors
The intricate interplay between targeted therapies and the development of specific drug resistance in cancer treatment underscores the critical role of receptor-mediated mechanisms. The advent of targeted therapies, specifically kinase inhibitors, anti-EGFR therapies, and tyrosine kinase inhibitors (TKIs), has revolutionized the management of various types of cancers, including non-small cell lung cancer (NSCLC), colorectal cancer, and hepatocellular carcinoma. These emerging therapies target specific receptors and signaling pathways, offering a more personalized approach to fighting against drug resistance, a major challenge in cancer therapy [119,120] and an obstacle to better patient outcomes.
EGFR inhibitors have shown promise in treating EGFR-mutant NSCLC and colorectal cancer. However, acquired resistance often limits their long-term effectiveness [119,121]. Studies suggest that inhibiting glutathione synthesis, a mechanism promoting cell survival, can overcome EGFR-targeted therapy resistance in lung cancer [122]. Similarly, the use of biparatopic METxMET antibody–drug conjugates, a novel antibody–drug conjugate targeting the mesenchymal epithelial transition (MET) receptor in MET-driven patient-derived models, has shown promise for overcoming resistance to EGFR TKIs in EGFR-mutant NSCLC [123]. The innovative use of biparatopic METxMET antibody–drug conjugates and novel compounds targeting specific EGFR mutations illustrates the potential of receptor-focused strategies to counter resistance. Other researchers have also highlighted the promise of antibody–drug conjugates in the landscape of EGFR-mutant NSCLC treatment [124].
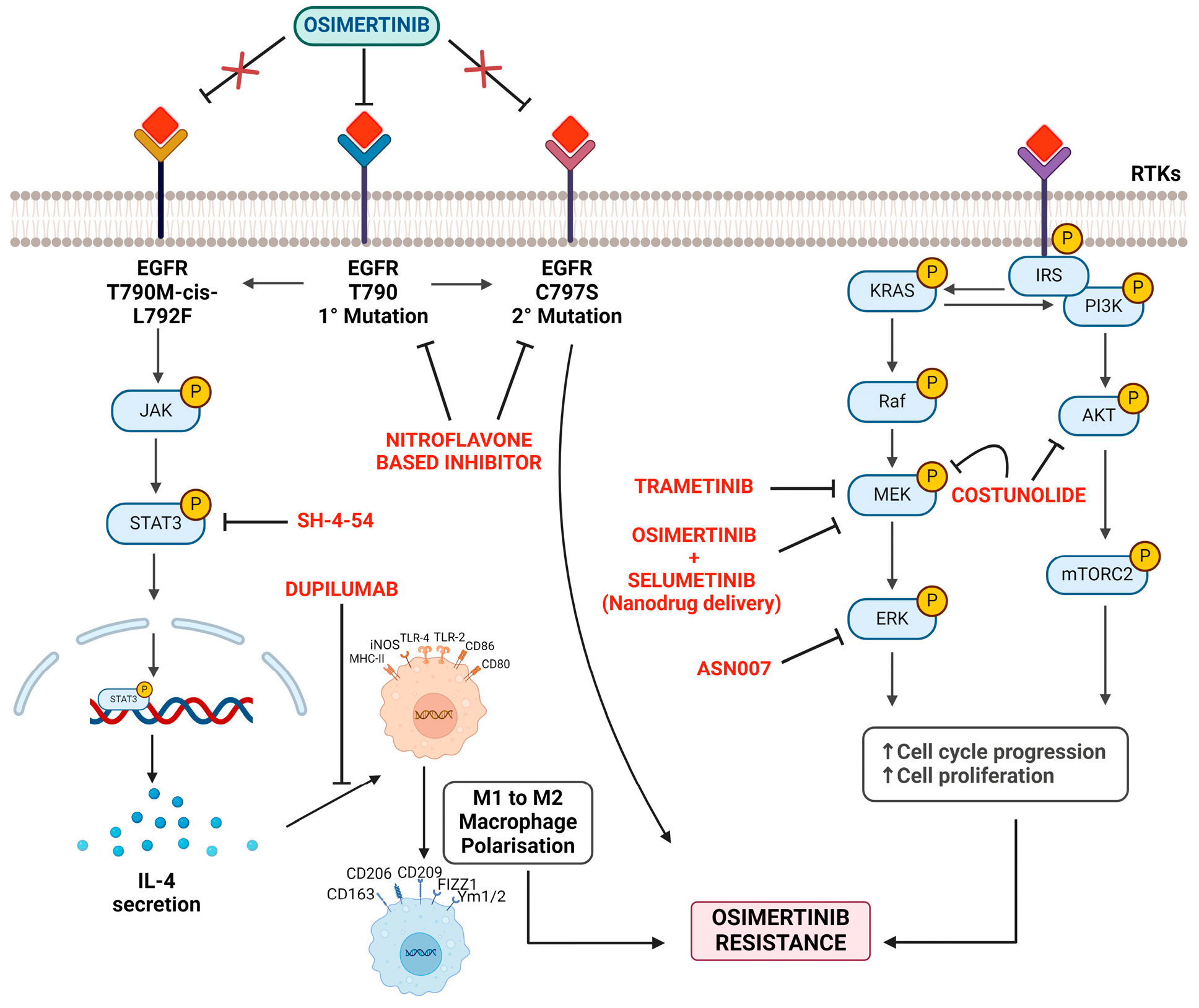
In the context of overcoming resistance to kinase inhibitors, the significance of the receptor structure and function becomes evident. For instance, osimertinib, a third-generation EGFR-TKI, is pivotal in treating EGFR-mutated NSCLC, particularly in tumors harboring the T790M mutation. However, the therapeutic landscape is increasingly confronted with acquired resistance, posing a significant challenge to clinicians [125,126]. Therefore, resistance to osimertinib, a key EGFR-TKI, further emphasizes the centrality of receptor interactions in therapeutic challenges. The development of secondary mutations within the EGFR receptor (such as C797S, that hinder drug binding) [126,127] itself or the activation of bypass signaling routes highlights the necessity of a deep understanding of receptor biology to devise effective counterstrategies (Figure 4). Among several new compounds to circumvent resistance issues, one such compound is a novel nitroflavone-based scaffold that may help design mutant-selective EGFR tyrosine kinase inhibitors targeting T790M and C797S resistance in advanced NSCLC [103]. Similarly, it has been demonstrated that combining D6, a synthetic derivative of a naturally occurring chemical from C. pilosula, with erlotinib or osimertinib can overcome EGFR-T790M-mediated resistance in NSCLC [128]. Another strategy of identifying the drugs targeting STAT3 to inhibit IL4 secretion, thereby preventing M2 macrophage polarization, might counter osimertinib resistance in NSCLC patients. This would address the EGFR p.T790M cis p.L792F mutation-induced resistance to osimertinib [129].
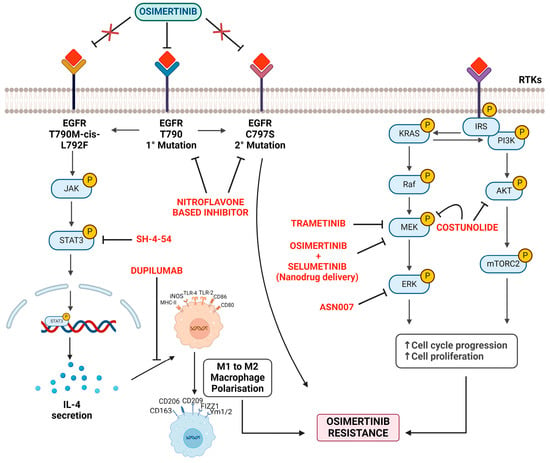
Figure 4.
Mechanisms and strategies to counter osimertinib resistance.
Processes of cell cycle progression and increased cell proliferation lead to osimertinib resistance. The strategies mentioned aim to counter or slow down this resistance by intervening at different stages. (a) EGFR mutations: this figure highlights the primary (EGFR T790) and secondary mutations (C797S) associated with osimertinib resistance. Another mutation is T790M-cis-L792F. Strategy: use the targeted therapy osimertinib to inhibit these mutations. However, resistance can eventually develop. (b) JAK-STAT3 pathway: the JAK enzyme’s activation leads to the phosphorylation and subsequent activation of the STAT3 protein. Strategy: implementing SH-4-54, which seems to target and inhibit the STAT3 pathway, reducing its activation. Furthermore, Dupilumab appears to counteract the pathway or its resulting IL-4 secretion. (c) Macrophage polarization: the diagram displays a phenotypic shift from M1 to M2 macrophages, influenced by factors like IL-4. Strategy: understanding and potentially controlling macrophage polarization may impact the tumor microenvironment and associated drug resistance. (d) KRAS signaling pathway: activation of the KRAS gene triggers a signaling cascade involving various proteins and kinases, such as PI3K, AKT, Raf, MEK, and ERK. RTKs (receptor tyrosine kinases) also influence this signaling pathway. Strategy: interventions include TRAMETINIB, which targets the MEK protein. Combined administration of OSIMERTINIB and SELUMETINIB (via nanodrug delivery) to target multiple points in the signal transduction pathway. COSTUNOLIDE, aiming at the AKT protein. ASN007 targeting ERK.
Outside the EGFR mutations, the activation of alternative pathways, including MAPK/ERK and PI3K/AKT, further confers osimertinib resistance [130,131]. Therefore, approaches, such as dual inhibitors of receptor signaling pathways and the co-delivery of pathway inhibitors via nanoparticles, underscore the evolving landscape of receptor-targeted interventions. Researchers have identified costunolide as a dual inhibitor of mitogen-activated protein kinase kinase (MEK1) and oncogene AKT1/2, presenting a potential solution against osimertinib resistance [107]. Nanoparticles’ potential to co-deliver osimertinib and selumetinib (MEK1/2 inhibitor) have also been investigated, effectively reversing osimertinib acquired resistance [108,132]. Furthermore, combined anti-EGF nanobodies with osimertinib enhance osimertinib’s antitumoral effect [133]. Trametinib (a MEK inhibitor) and ASN007 (an ERK inhibitor) have shown efficacy in overcoming resistance linked to the KRAS-G12V mutation and augmented ERK signaling, respectively [109,134]. Likewise, research indicates that addressing intrinsic apoptotic pathways may be instrumental in overcoming resistance to EGFR inhibitors. Targeting myeloid leukemia cell differentiation protein (Mcl-1), which inhibits cell death or promotes Bax, a pro-apoptotic protein, may reverse the acquired resistance to third-generation EGFR inhibitors [135]. Suggesting an alternate survival route, activation of the EGFR–STAT3–ABCB1 signaling axis has been implicated in acquired lenvatinib (inhibitor of multiple receptor tyrosine kinases) resistance in hepatocellular carcinoma. Inhibiting EGFR was shown to overcome this resistance, suggesting a potential cancer management strategy [110].
4. Receptor Degradation Strategies
Depleting the receptor tyrosine kinases (RTKs), such as EGFR and HER2, may be a viable strategy to overcome resistance to EGFR inhibitors in colorectal cancer [121,136,137]. This theme of receptor degradation is echoed by Hong et al., 2023, who emphasized the function of diarylheptanoid 35d in overcoming EGFR TKI resistance by promoting the HSP70-mediated lysosomal degradation of EGFR in EGFR-mutant lung cancer [138]. Others propose repurposing existing drugs like tamoxifen to counteract crosstalk between cytoplasmic estrogen receptor α and the EGFR/HER2 signaling pathway, effectively overcoming resistance to EGFR inhibitors [139].
In summary, while osimertinib offers a transformative therapeutic solution for NSCLC, resistance remains a pertinent challenge. Comprehensive reviews have reinforced the importance of understanding the plethora of resistance mechanisms and emphasized tailoring therapeutic strategies based on specific resistance origins [140,141]. Deciphering the molecular basis of resistance and tailoring treatments accordingly is imperative. Combining pathway inhibitors, nanotechnology, repurposing existing drugs, and understanding mutation-specific resistance opens the door for more effective interventions, promising to prolong patient survival and quality of life.
5. Insights from Clinical Settings
In the realm of receptor-based strategies to combat cancer drug resistance, the precision of diagnostic and prognostic tools also plays a pivotal role in tailoring and adjusting treatment protocols. Advanced imaging and molecular diagnostics guide the selection and effectiveness of receptor-targeted therapies, enabling a more personalized approach to cancer management. For example, a study by Huddleston et al. evaluates the prognostic efficacy of HPV-DNA testing compared to PET-CT imaging in cervical cancer, illustrating the potential of these technologies to refine treatment decisions based on receptor dynamics and therapeutic responses [142]. Such diagnostic advancements are crucial for monitoring resistance patterns and adjusting therapeutic strategies accordingly. Similarly, the strategies for overcoming cancer drug resistance and the context in which patients receive treatment—whether inpatient or outpatient—can significantly influence the efficacy of these targeted therapies. For example, the study by Varghese et al. investigates clinical evaluations in different healthcare settings, shedding light on how such environments can impact the administration and effectiveness of treatments, including receptor-targeted therapies [143]. This highlights the necessity of adapting our therapeutic approaches to fit the logistical and clinical realities faced by patients, ensuring that the potential of receptor-targeted strategies is fully realized in varying healthcare contexts.
In addition to biological and molecular challenges, logistical and administrative obstacles within healthcare settings also significantly impact the efficacy of receptor-targeted therapies in cancer treatment. For instance, a study on the transition from IV to oral highlights systemic barriers that can also affect cancer treatment protocols [144]. Understanding these obstacles is crucial for optimizing treatment regimens and enhancing the management of cancer drug resistance, ensuring that therapies are not only effective at a molecular level but also feasible and effective in real-world clinical settings.
6. Conclusions
This review underlines the importance of receptors in designing drugs and overcoming drug resistance in cancer therapy. By elucidating the mechanisms of resistance from a receptor-centric viewpoint, we underscore the potential of targeted therapies that manipulate the receptor structure, signaling, and interactions. The deployment of advanced computational modeling to predict receptor behavior and the application of physicochemical and biophysical properties to inform therapeutic design are poised to revolutionize cancer treatment. Moreover, integrating clinical insights from different healthcare settings, as demonstrated, highlights how systemic factors and real-world clinical environments impact the administration and effectiveness of receptor-targeted therapies. Such insights emphasize the necessity of adapting our therapeutic approaches to fit the logistical and clinical realities faced by patients, ensuring that the potential of receptor-targeted strategies is fully realized in varying healthcare contexts. Through a comprehensive analysis, an in-depth understanding of receptor biology, coupled with innovative therapeutic strategies, to target receptors, we can unveil new avenues to circumvent drug resistance, ultimately enhancing patient care in oncology.
Author Contributions
Conceptualization, N.S., S.K. and S.B.; Writing—original draft preparation, N.S., A.A.S., G.A. and M.D.; Writing—review and editing, A.S. and S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer Statistics, 2024. CA A Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Sah, N.; Ramaiah, B.; Abdulla; Gupta, A.K.; Thomas, S.M. Noncompliance with Prescription-Writing Guidelines in an Outpatient Department of a Tertiary Care Hospital: A Prospective, Observational Study. RGUHS J. Pharm. Sci. 2020, 10, 29–36. [Google Scholar] [CrossRef]
- Sharma, M.; Bakshi, A.K.; Mittapelly, N.; Gautam, S.; Marwaha, D.; Rai, N.; Singh, N.; Tiwari, P.; Agarwal, N.; Kumar, A.; et al. Recent Updates on Innovative Approaches to Overcome Drug Resistance for Better Outcomes in Cancer. J. Control. Release 2022, 346, 43–70. [Google Scholar] [CrossRef]
- Why Do Cancer Treatments Stop Working?—NCI. Available online: https://www.cancer.gov/about-cancer/treatment/research/drug-combo-resistance (accessed on 8 April 2024).
- Wang, X.; Zhang, H.; Chen, X. Drug Resistance and Combating Drug Resistance in Cancer. Cancer Drug Resist. 2019, 2, 141–160. [Google Scholar] [CrossRef]
- The Role and Mechanisms of Action of microRNAs in Cancer Drug Resistance|Clinical Epigenetics|Full Text. Available online: https://clinicalepigeneticsjournal.biomedcentral.com/articles/10.1186/s13148-018-0587-8 (accessed on 8 April 2024).
- Sah, N.; Ramaiah, B.; Koneri, R. The Pharmacist Role in Clinical Audit at an Indian Accredited Hospital: An Interventional Study. Indian J. Pharm. Pract. 2019, 12, 117–125. [Google Scholar] [CrossRef]
- Schumacher, T.J.; Sah, N.; Palle, K.; Rumbley, J.; Mereddy, V.R. Synthesis and Biological Evaluation of Benzofuran Piperazine Derivatives as Potential Anticancer Agents. Bioorg. Med. Chem. Lett. 2023, 93, 129425. [Google Scholar] [CrossRef]
- Adult Immunotherapy Network—Cancer Moonshot Recommendation—NCI. Available online: https://www.cancer.gov/research/key-initiatives/moonshot-cancer-initiative/implementation/adult-immunotherapy-network (accessed on 8 April 2024).
- Cancer Moonshot Biobank|National Cancer Institute. Available online: https://moonshotbiobank.cancer.gov (accessed on 8 April 2024).
- Cancer Moonshot Research Initiatives—NCI. Available online: https://www.cancer.gov/research/key-initiatives/moonshot-cancer-initiative/implementation (accessed on 8 April 2024).
- Tabatabaee, A.; Nafari, B.; Farhang, A.; Hariri, A.; Khosravi, A.; Zarrabi, A.; Mirian, M. Targeting Vimentin: A Multifaceted Approach to Combatting Cancer Metastasis and Drug Resistance. Cancer Metastasis Rev. 2024, 43, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Karasarides, M.; Cogdill, A.P.; Robbins, P.B.; Bowden, M.; Burton, E.M.; Butterfield, L.H.; Cesano, A.; Hammer, C.; Haymaker, C.L.; Horak, C.E.; et al. Hallmarks of Resistance to Immune-Checkpoint Inhibitors. Cancer Immunol. Res. 2022, 10, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Tian, W.; Wang, Z.; Zhang, J.; Zhou, R. Co-Inhibition of TIGIT and PD-1/PD-L1 in Cancer Immunotherapy: Mechanisms and Clinical Trials. Mol. Cancer 2023, 22, 93. [Google Scholar] [CrossRef]
- Upadhaya, S.; Neftelino, S.T.; Hodge, J.P.; Oliva, C.; Campbell, J.R.; Yu, J.X. Combinations Take Centre Stage in PD1/PDL1 Inhibitor Clinical Trials. Nat. Rev. Drug Discov. 2020, 20, 168–169. [Google Scholar] [CrossRef]
- Sah, N.; Ramaiah, B.; Koneri, R. Sulfasalazine-Induced Drug Rash with Eosinophilia and Systemic Symptoms Syndrome in a Seronegative Spondyloarthritis Patient: A Case Report. Indian J. Pharmacol. 2021, 53, 391–393. [Google Scholar] [CrossRef] [PubMed]
- Kulig, P.; Milczarek, S.; Bakinowska, E.; Szalewska, L.; Baumert, B.; Machaliński, B. Lenalidomide in Multiple Myeloma: Review of Resistance Mechanisms, Current Treatment Strategies and Future Perspectives. Cancers 2023, 15, 963. [Google Scholar] [CrossRef] [PubMed]
- Gacche, R.N.; Assaraf, Y.G. Redundant Angiogenic Signaling and Tumor Drug Resistance. Drug Resist. Updates 2018, 36, 47–76. [Google Scholar] [CrossRef]
- Melaccio, A.; Reale, A.; Saltarella, I.; Desantis, V.; Lamanuzzi, A.; Cicco, S.; Frassanito, M.A.; Vacca, A.; Ria, R. Pathways of Angiogenic and Inflammatory Cytokines in Multiple Myeloma: Role in Plasma Cell Clonal Expansion and Drug Resistance. J. Clin. Med. 2022, 11, 6491. [Google Scholar] [CrossRef]
- Shi, H.; Zhao, Z.; Jiang, W.; Zhu, P.; Zhou, N.; Huang, X. A Review Into the Insights of the Role of Endothelial Progenitor Cells on Bone Biology. Front. Cell Dev. Biol. 2022, 10, 878697. [Google Scholar] [CrossRef]
- Zhang, Z.-S.; Zhou, H.-N.; He, S.-S.; Xue, M.-Y.; Li, T.; Liu, L.-M. Research Advances in Pericyte Function and Their Roles in Diseases. Chin. J. Traumatol. 2020, 23, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, J.; Chen, Z.; Luo, J.; Guo, W.; Sun, L.; Lin, L. Targeting M2-like Tumor-Associated Macrophages Is a Potential Therapeutic Approach to Overcome Antitumor Drug Resistance. NPJ Precis. Oncol. 2024, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Nowosad, A.; Marine, J.-C.; Karras, P. Perivascular Niches: Critical Hubs in Cancer Evolution. Trends Cancer 2023, 9, 897–910. [Google Scholar] [CrossRef]
- Castven, D.; Czauderna, C.; Becker, D.; Pereira, S.; Schmitt, J.; Weinmann, A.; Shah, V.; Hajduk, J.; Keggenhoff, F.; Binder, H.; et al. Acquired Resistance to Antiangiogenic Therapies in Hepatocellular Carcinoma Is Mediated by Yes-Associated Protein 1 Activation and Transient Expansion of Stem-Like Cancer Cells. Hepatol. Commun. 2022, 6, 1140–1156. [Google Scholar] [CrossRef]
- Hida, K.; Maishi, N.; Matsuda, A.; Yu, L. Beyond Starving Cancer: Anti-Angiogenic Therapy. J. Med. Ultrason. 2023, 1–6. [Google Scholar] [CrossRef]
- Montemagno, C.; Pagès, G. Resistance to Anti-Angiogenic Therapies: A Mechanism Depending on the Time of Exposure to the Drugs. Front. Cell Dev. Biol. 2020, 8, 584. [Google Scholar] [CrossRef] [PubMed]
- Quan, L.; Ohgaki, R.; Hara, S.; Okuda, S.; Wei, L.; Okanishi, H.; Nagamori, S.; Endou, H.; Kanai, Y. Amino Acid Transporter LAT1 in Tumor-Associated Vascular Endothelium Promotes Angiogenesis by Regulating Cell Proliferation and VEGF-A-Dependent mTORC1 Activation. J. Exp. Clin. Cancer Res. 2020, 39, 266. [Google Scholar] [CrossRef] [PubMed]
- Kiss, E.A.; Saharinen, P. Anti-Angiogenic Targets: Angiopoietin and Angiopoietin Receptors. Tumor Angiogenesis 2019, 227–250. [Google Scholar] [CrossRef]
- Leong, A.; Kim, M. The Angiopoietin-2 and TIE Pathway as a Therapeutic Target for Enhancing Antiangiogenic Therapy and Immunotherapy in Patients with Advanced Cancer. Int. J. Mol. Sci. 2020, 21, 8689. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, I.; Baum, A.; Hofmann, M.H.; Trapani, F.; Reichel-Voda, C.; Ehrensperger, D.; Aichinger, M.; Ebner, F.; Budano, N.; Schweifer, N.; et al. Pharmacodynamic and Antitumor Activity of BI 836880, a Dual Vascular Endothelial Growth Factor and Angiopoietin 2 Inhibitor, Alone and Combined with Programmed Cell Death Protein-1 Inhibition. J. Pharmacol. Exp. Ther. 2023, 384, 331–342. [Google Scholar] [CrossRef]
- Szymczyk, J.; Sochacka, M.; Chudy, P.; Opalinski, L.; Otlewski, J.; Zakrzewska, M. FGF1 Protects FGFR1-Overexpressing Cancer Cells against Drugs Targeting Tubulin Polymerization by Activating AKT via Two Independent Mechanisms. Front. Oncol. 2022, 12, 1011762. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, Y.; Ji, H.; Sun, X.; Xie, S.; Chen, L.; Li, S.; Zeng, W.; Chen, R.; Tang, Q.; et al. Lenvatinib for Effectively Treating Antiangiogenic Drug-Resistant Nasopharyngeal Carcinoma. Cell Death Dis. 2022, 13, 724. [Google Scholar] [CrossRef]
- Chia, Z.-J.; Cao, Y.; Little, P.J.; Kamato, D. Transforming Growth Factor-β Receptors: Versatile Mechanisms of Ligand Activation. Acta Pharmacol. Sin. 2024, 45, 1337–1348. [Google Scholar] [CrossRef]
- Niu, M.; Yi, M.; Wu, Y.; Lyu, L.; He, Q.; Yang, R.; Zeng, L.; Shi, J.; Zhang, J.; Zhou, P.; et al. Synergistic Efficacy of Simultaneous Anti-TGF-β/VEGF Bispecific Antibody and PD-1 Blockade in Cancer Therapy. J. Hematol. Oncol. 2023, 16, 94. [Google Scholar] [CrossRef]
- Acharya, G.; Mani, C.; Sah, N.; Saamarthy, K.; Young, R.; Reedy, M.B.; Sobol, R.W.; Palle, K. CHK1 Inhibitor Induced PARylation by Targeting PARG Causes Excessive Replication and Metabolic Stress and Overcomes Chemoresistance in Ovarian Cancer. Cell Death Discov. 2024, 10, 278. [Google Scholar] [CrossRef]
- Role of Chemokine-Mediated Angiogenesis in Resistance towards Crizotinib and Its Reversal by Anlotinib in EML4-ALK Positive NSCLC—PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9153090/ (accessed on 9 April 2024).
- Qi, M.; Fan, S.; Huang, M.; Pan, J.; Li, Y.; Miao, Q.; Lyu, W.; Li, X.; Deng, L.; Qiu, S.; et al. Targeting FAPα-Expressing Hepatic Stellate Cells Overcomes Resistance to Antiangiogenics in Colorectal Cancer Liver Metastasis Models. J. Clin. Investig. 2023, 133, e157399. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Chelariu-Raicu, A.; Umamaheswaran, S.; Nick, A.M.; Stur, E.; Hanjra, P.; Jiang, D.; Jennings, N.B.; Chen, X.; Corvigno, S.; et al. Endothelial P130cas Confers Resistance to Anti-Angiogenesis Therapy. Cell Rep. 2022, 39, 110999. [Google Scholar] [CrossRef] [PubMed]
- Sah, N.; Peddibhotla, S.; Richardson, B.; Luna, P.; Bansal, N.A.; Mani, C.; Reedy, M.; Palle, K. Abstract A084: Oncogenic Role for Upregulated Lymphoblastic Leukemia Derived Sequence-1 in the Progression of Ovarian Cancer and Its Metastasis. Cancer Epidemiol. Biomark. Prev. 2023, 32, A084. [Google Scholar] [CrossRef]
- Lopes-Coelho, F.; Silva, F.; Gouveia-Fernandes, S.; Martins, C.; Lopes, N.; Domingues, G.; Brito, C.; Almeida, A.M.; Pereira, S.A.; Serpa, J. Monocytes as Endothelial Progenitor Cells (EPCs), Another Brick in the Wall to Disentangle Tumor Angiogenesis. Cells 2020, 9, 107. [Google Scholar] [CrossRef]
- Glover, A.; Zhang, Z.; Shannon-Lowe, C. Deciphering the Roles of Myeloid Derived Suppressor Cells in Viral Oncogenesis. Front. Immunol. 2023, 14, 1161848. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and Tumor Progression: Signaling Pathways and Targeted Intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar]
- Gadwa, J.; Bickett, T.E.; Darragh, L.B.; Knitz, M.W.; Bhatia, S.; Piper, M.; Court, B.V.; Bhuvane, S.; Nguyen, D.; Nangia, V.; et al. Complement C3a and C5a Receptor Blockade Modulates Regulatory T Cell Conversion in Head and Neck Cancer. J. Immunother. Cancer 2021, 9, e002585. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-B.; Wu, C.-Y.; Wang, X.-Y.; Deng, J.; Cao, W.-J.; Tang, Y.-Z.; Wan, C.-C.; Chen, Z.-T.; Zhan, W.-Y.; Shan, H.; et al. Targeting Inflammatory Macrophages Rebuilds Therapeutic Efficacy of DOT1L Inhibition in Hepatocellular Carcinoma. Mol. Ther. 2023, 31, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Ghouse, S.M.; Vadrevu, S.K.; Manne, S.; Reese, B.; Patel, J.; Patel, B.; Silwal, A.; Lodhi, N.; Paterson, Y.; Srivastava, S.K.; et al. Therapeutic Targeting of Vasculature in the Premetastatic and Metastatic Niches Reduces Lung Metastasis. J. Immunol. 2020, 204, 990–1000. [Google Scholar] [CrossRef]
- Tang, T.; Huang, X.; Zhang, G.; Hong, Z.; Bai, X.; Liang, T. Advantages of Targeting the Tumor Immune Microenvironment over Blocking Immune Checkpoint in Cancer Immunotherapy. Signal Transduct. Target. Ther. 2021, 6, 72. [Google Scholar] [CrossRef]
- Ciciola, P.; Cascetta, P.; Bianco, C.; Formisano, L.; Bianco, R. Combining Immune Checkpoint Inhibitors with Anti-Angiogenic Agents. J. Clin. Med. 2020, 9, 675. [Google Scholar] [CrossRef]
- Chen, J.-R.; Zhao, J.-T.; Xie, Z.-Z. Integrin-Mediated Cancer Progression as a Specific Target in Clinical Therapy. Biomed. Pharmacother. 2022, 155, 113745. [Google Scholar] [CrossRef]
- Yi, M.; Jiao, D.; Qin, S.; Chu, Q.; Wu, K.; Li, A. Synergistic Effect of Immune Checkpoint Blockade and Anti-Angiogenesis in Cancer Treatment. Mol. Cancer 2019, 18, 60. [Google Scholar] [CrossRef]
- Chaudhary, P.K.; Kim, S. An Insight into GPCR and G-Proteins as Cancer Drivers. Cells 2021, 10, 3288. [Google Scholar] [CrossRef]
- Kohli, K.; Pillarisetty, V.G.; Kim, T.S. Key Chemokines Direct Migration of Immune Cells in Solid Tumors. Cancer Gene Ther. 2022, 29, 10–21. [Google Scholar] [CrossRef]
- Crean, D.; Murphy, E.P. Targeting NR4A Nuclear Receptors to Control Stromal Cell Inflammation, Metabolism, Angiogenesis, and Tumorigenesis. Front. Cell Dev. Biol. 2021, 9, 589770. [Google Scholar] [CrossRef]
- Akil, A.; Gutiérrez-García, A.K.; Guenter, R.; Rose, J.B.; Beck, A.W.; Chen, H.; Ren, B. Notch Signaling in Vascular Endothelial Cells, Angiogenesis, and Tumor Progression: An Update and Prospective. Front. Cell Dev. Biol. 2021, 9, 642352. [Google Scholar] [CrossRef]
- Morris, R.M.; Mortimer, T.O.; O’Neill, K.L. Cytokines: Can Cancer Get the Message? Cancers 2022, 14, 2178. [Google Scholar] [CrossRef]
- Wang, Q.; Shen, X.; Chen, G.; Du, J. How to Overcome Resistance to Immune Checkpoint Inhibitors in Colorectal Cancer: From Mechanisms to Translation. Int. J. Cancer 2023, 153, 709–722. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, C.; Wang, H. Immune-Checkpoint Inhibitor Resistance in Cancer Treatment: Current Progress and Future Directions. Cancer Lett. 2023, 562, 216182. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Hsu, J.-M.; Yang, W.-H.; Hung, M.-C. Mechanisms Regulating PD-L1 Expression in Cancers and Associated Opportunities for Novel Small-Molecule Therapeutics. Nat. Rev. Clin. Oncol. 2022, 19, 287–305. [Google Scholar] [CrossRef]
- Improvement of the Anticancer Efficacy of PD-1/PD-L1 Blockade via Combination Therapy and PD-L1 Regulation—PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8917703/ (accessed on 10 April 2024).
- Yang, Z.; Wang, Y.; Liu, S.; Deng, W.; Lomeli, S.H.; Moriceau, G.; Wohlschlegel, J.; Piva, M.; Lo, R.S. Enhancing PD-L1 Degradation by ITCH during MAPK Inhibitor Therapy Suppresses Acquired Resistance. Cancer Discov. 2022, 12, 1942–1959. [Google Scholar] [CrossRef]
- Yuan, J.; Khilnani, A.; Brody, J.; Andtbacka, R.H.I.; Hu-Lieskovan, S.; Luke, J.J.; Diab, A.; Marabelle, A.; Snyder, A.; Cao, Z.A.; et al. Current Strategies for Intratumoural Immunotherapy—Beyond Immune Checkpoint Inhibition. Eur. J. Cancer 2021, 157, 493–510. [Google Scholar] [CrossRef]
- Zhou, B.; Gao, Y.; Zhang, P.; Chu, Q. Acquired Resistance to Immune Checkpoint Blockades: The Underlying Mechanisms and Potential Strategies. Front. Immunol. 2021, 12, 693609. [Google Scholar] [CrossRef]
- Nguyen, H.-M.; Sah, N.; Humphrey, M.R.M.; Rabkin, S.D.; Saha, D. Growth, Purification, and Titration of Oncolytic Herpes Simplex Virus. J. Vis. Exp. 2021, 171, 1–16. [Google Scholar] [CrossRef]
- Chen, X.; Feng, L.; Huang, Y.; Wu, Y.; Xie, N. Mechanisms and Strategies to Overcome PD-1/PD-L1 Blockade Resistance in Triple-Negative Breast Cancer. Cancers 2022, 15, 104. [Google Scholar] [CrossRef]
- Shen, M.; Chen, C.; Guo, Q.; Wang, Q.; Liao, J.; Wang, L.; Yu, J.; Xue, M.; Duan, Y.; Zhang, J. Systemic Delivery of mPEG-Masked Trispecific T-Cell Nanoengagers in Synergy with STING Agonists Overcomes Immunotherapy Resistance in TNBC and Generates a Vaccination Effect. Adv. Sci. 2022, 9, e2203523. [Google Scholar] [CrossRef]
- Vesely, M.D.; Zhang, T.; Chen, L. Resistance Mechanisms to Anti-PD Cancer Immunotherapy. Annu. Rev. Immunol. 2022, 40, 45–74. [Google Scholar] [CrossRef]
- Park, J.S.; Gazzaniga, F.S.; Wu, M.; Luthens, A.K.; Gillis, J.; Zheng, W.; LaFleur, M.W.; Johnson, S.B.; Morad, G.; Park, E.M.; et al. Targeting PD-L2-RGMb Overcomes Microbiome-Related Immunotherapy Resistance. Nature 2023, 617, 377–385. [Google Scholar] [CrossRef]
- Yi, W.; Yan, D.; Wang, D.; Li, Y. Smart Drug Delivery Systems to Overcome Drug Resistance in Cancer Immunotherapy. Cancer Biol. Med. 2023, 20, 248–267. [Google Scholar] [CrossRef]
- Irreversible JNK Blockade Overcomes PD-L1-Mediated Resistance to Chemotherapy in Colorectal Cancer|Oncogene. Available online: https://www.nature.com/articles/s41388-021-01910-6 (accessed on 10 April 2024).
- Combination of STING Agonist and CXCR3 Antagonist Disrupts Immune Tolerance to Overcome Anti-PD-L1 Resistance in Lung Adenocarcinoma under Oxidative Stress—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/abs/pii/S037811192200782X (accessed on 10 April 2024).
- Mani, C.; Acharya, G.; Kshirsagar, S.; Vijayan, M.; Khan, H.; Reddy, P.H.; Palle, K. A Novel Role for BRIP1/FANCJ in Neuronal Cells Health and in Resolving Oxidative Stress-Induced DNA Lesions. J. Alzheimers Dis. 2022, 85, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Luna, P.; Acharya, G.; Ochola, D.; Peddibhotla, S.; Mani, C.; Reedy, M.B.; Palle, K. Abstract 5496: Glutaminase Inhibition Induces Replication Stress in Ovarian Cancer Cells and Inhibition of Replication Checkpoint Causes Synthetic Lethality. Cancer Res. 2023, 83, 5496. [Google Scholar] [CrossRef]
- Okechukwu, C.C.; Ma, X.; Sah, N.; Mani, C.; Palle, K.; Gmeiner, W.H. Enhanced Therapeutic Efficacy of the Nanoscale Fluoropyrimidine Polymer CF10 in a Rat Colorectal Cancer Liver Metastasis Model. Cancers 2024, 16, 1360. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Liu, S.; Mao, M.; Gong, Y.; Li, X.; Lei, T.; Liu, C.; Wu, S.; Hu, Q. T-Cell Infiltration and Its Regulatory Mechanisms in Cancers: Insights at Single-Cell Resolution. J. Exp. Clin. Cancer Res. 2024, 43, 38. [Google Scholar] [CrossRef]
- Healey, N. Tumor Infiltrating Lymphocyte Approval Heralds New Era for Precision Cancer Immunotherapy. Nat. Med. 2024, 30, 1795–1796. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yuan, Z.; Li, Z.; He, X.; Zhang, Y.; Wang, X.; Su, J.; Wu, X.; Li, M.; Du, F.; et al. Key Oncogenic Signaling Pathways Affecting Tumor-Infiltrating Lymphocytes Infiltration in Hepatocellular Carcinoma: Basic Principles and Recent Advances. Front. Immunol. 2024, 15, 1354313. [Google Scholar] [CrossRef]
- Zhou, L.; Yi, M. Editorial: Harnessing Tumor Microenvironment for Gynecologic Cancer Therapy. Front. Immunol. 2024, 15, 1407128. [Google Scholar] [CrossRef]
- Targeting TBK1 to Overcome Resistance to Cancer Immunotherapy—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/36634707/ (accessed on 10 April 2024).
- Belmontes, B.; Sawant, D.V.; Zhong, W.; Tan, H.; Kaul, A.; Aeffner, F.; O’Brien, S.A.; Chun, M.; Noubade, R.; Eng, J.; et al. Immunotherapy Combinations Overcome Resistance to Bispecific T Cell Engager Treatment in T Cell-Cold Solid Tumors. Sci. Transl. Med. 2021, 13, eabd1524. [Google Scholar] [CrossRef]
- Dong, S.; Zhang, S.; Zhao, P.; Lin, G.; Ma, X.; Xu, J.; Zhang, H.; Hu, J.; Zou, C. A Combined Analysis of Bulk and Single-Cell Sequencing Data Reveals That Depleted Extracellular Matrix and Enhanced Immune Processes Co-Contribute to Fluorouracil Beneficial Responses in Gastric Cancer. Front. Immunol. 2022, 13, 999551. [Google Scholar] [CrossRef]
- Zhou, K.; Li, S.; Zhao, Y.; Cheng, K. Mechanisms of Drug Resistance to Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer. Front. Immunol. 2023, 14, 1127071. [Google Scholar] [CrossRef]
- Zhou, X.; Ni, Y.; Liang, X.; Lin, Y.; An, B.; He, X.; Zhao, X. Mechanisms of Tumor Resistance to Immune Checkpoint Blockade and Combination Strategies to Overcome Resistance. Front. Immunol. 2022, 13, 915094. [Google Scholar] [CrossRef] [PubMed]
- Addressing CPI Resistance in NSCLC: Targeting TAM Receptors to Modulate the Tumor Microenvironment and Future Prospects—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/35858709/ (accessed on 10 April 2024).
- Combination of P38 MAPK Inhibitor with PD-L1 Antibody Effectively Prolongs Survivals of Temozolomide-Resistant Glioma-Bearing Mice via Reduction of Infiltrating Glioma-Associated Macrophages and PD-L1 Expression on Resident Glioma-Associated Microglia|Brain Tumor Pathology. Available online: https://link.springer.com/article/10.1007/s10014-021-00404-3 (accessed on 10 April 2024).
- Zhao, J.; Dong, Y.; Zhang, Y.; Wang, J.; Wang, Z. Biophysical Heterogeneity of Myeloid-Derived Microenvironment to Regulate Resistance to Cancer Immunotherapy. Adv. Drug Deliv. Rev. 2022, 191, 114585. [Google Scholar] [CrossRef] [PubMed]
- Target Immune Components to Circumvent Sorafenib Resistance in Hepatocellular Carcinoma—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/37121146/ (accessed on 10 April 2024).
- T Cell-Mediated Targeted Delivery of Tadalafil Regulates Immunosuppression and Polyamine Metabolism to Overcome Immune Checkpoint Blockade Resistance in Hepatocellular Carcinoma—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/36813307/ (accessed on 10 April 2024).
- Gonzalez, C.; Williamson, S.; Gammon, S.T.; Glazer, S.; Rhee, J.H.; Piwnica-Worms, D. TLR5 Agonists Enhance Anti-Tumor Immunity and Overcome Resistance to Immune Checkpoint Therapy. Commun Biol 2023, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Immune Checkpoint Proteins: Signaling Mechanisms and Molecular Interactions in Cancer Immunotherapy—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/35341913/ (accessed on 10 April 2024).
- Gautam, S.K.; Basu, S.; Aithal, A.; Dwivedi, N.V.; Gulati, M.; Jain, M. Regulation of Pancreatic Cancer Therapy Resistance by Chemokines. Semin. Cancer Biol. 2022, 86, 69–80. [Google Scholar] [CrossRef]
- Monteleone, G.; Franzè, E.; Maresca, C.; Colella, M.; Pacifico, T.; Stolfi, C. Targeted Therapy of Interleukin-34 as a Promising Approach to Overcome Cancer Therapy Resistance. Cancers 2023, 15, 971. [Google Scholar] [CrossRef]
- Shi, W.; Lv, L.; Liu, N.; Wang, H.; Wang, Y.; Zhu, W.; Liu, Z.; Zhu, J.; Lu, H. A Novel Anti-PD-L1/IL-15 Immunocytokine Overcomes Resistance to PD-L1 Blockade and Elicits Potent Antitumor Immunity. Mol. Ther. 2023, 31, 66–77. [Google Scholar] [CrossRef]
- Wang, H.; Borlongan, M.; Hemminki, A.; Basnet, S.; Sah, N.; Kaufman, H.L.; Rabkin, S.D.; Saha, D. Viral Vectors Expressing Interleukin 2 for Cancer Immunotherapy. Hum. Gene Ther. 2023, 34, 878–895. [Google Scholar] [CrossRef]
- Yi, M.; Niu, M.; Zhang, J.; Li, S.; Zhu, S.; Yan, Y.; Li, N.; Zhou, P.; Chu, Q.; Wu, K. Combine and Conquer: Manganese Synergizing Anti-TGF-β/PD-L1 Bispecific Antibody YM101 to Overcome Immunotherapy Resistance in Non-Inflamed Cancers. J. Hematol. Oncol. 2021, 14, 146. [Google Scholar] [CrossRef]
- Cheng, C.; Zhuge, L.; Xiao, X.; Luan, S.; Yuan, Y. Overcoming Resistance to PD-1/PD-L1 Inhibitors in Esophageal Cancer. Front. Oncol. 2022, 12, 955163. [Google Scholar] [CrossRef]
- Advances in Immune Therapies for the Treatment of Microsatellite Instability-High/Deficient Mismatch Repair Metastatic Colorectal Cancer (Review)—PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8360619/ (accessed on 10 April 2024).
- Ding, P.R. Immunotherapy for microsatellite-instability-high advanced colorectal cancer. Zhonghua Wei Chang Wai Ke Za Zhi 2022, 25, 199–204. [Google Scholar] [CrossRef]
- Insights and Strategies of Melanoma Immunotherapy: Predictive Biomarkers of Response and Resistance and Strategies to Improve Response Rates—PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9820306/ (accessed on 10 April 2024).
- Choi, S.H.; Jung, D.; Kim, K.Y.; An, H.J.; Park, K.-S. Combined Use of Cisplatin plus Natural Killer Cells Overcomes Immunoresistance of Cisplatin Resistant Ovarian Cancer. Biochem. Biophys. Res. Commun. 2021, 563, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, P.; Barroso-Sousa, R.; Garrido-Castro, A.C.; McAllister, S.S.; Guerriero, J.L.; Mittendorf, E.; Curigliano, G.; Tolaney, S.M. Understanding Resistance to Immune Checkpoint Inhibitors in Advanced Breast Cancer. Expert Rev. Anticancer Ther. 2022, 22, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Liu, S.; Liu, J.; Hu, H.; Yang, L.; Zhao, Q.; Li, C.; Zhang, B.; Zhang, Y. Overcoming Resistance to Immunotherapy by Targeting GPR84 in Myeloid-Derived Suppressor Cells. Signal Transduct. Target. Ther. 2023, 8, 164. [Google Scholar] [CrossRef]
- Wei, C.-Y.; Zhu, M.-X.; Zhang, P.-F.; Huang, X.-Y.; Wan, J.-K.; Yao, X.-Z.; Hu, Z.-T.; Chai, X.-Q.; Peng, R.; Yang, X.; et al. PKCα/ZFP64/CSF1 Axis Resets the Tumor Microenvironment and Fuels Anti-PD1 Resistance in Hepatocellular Carcinoma. J. Hepatol. 2022, 77, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Strategies to Overcome Drug Resistance Using SHP2 Inhibitors—PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8727779/ (accessed on 10 April 2024).
- Cristina, M.; Emiliano, L.; Leonardo, S.; Giulia, S.; Roberta, G.; Adolfo, A.; Marta, S.S.; Paola, S.; Samuele, R.; Pierluigi, S.; et al. Identification of a Novel Nitroflavone-Based Scaffold for Designing Mutant-Selective EGFR Tyrosine Kinase Inhibitors Targeting T790M and C797S Resistance in Advanced NSCLC. Bioorg. Chem. 2022, 129, 106219. [Google Scholar] [CrossRef]
- Sun, Y.; Dong, Y.; Liu, X.; Zhang, Y.; Bai, H.; Duan, J.; Tian, Z.; Yan, X.; Wang, J.; Wang, Z. Blockade of STAT3/IL-4 Overcomes EGFR T790M-Cis-L792F-Induced Resistance to Osimertinib via Suppressing M2 Macrophages Polarization. EBioMedicine 2022, 83, 104200. [Google Scholar] [CrossRef]
- Yan, D.; Huelse, J.M.; Kireev, D.; Tan, Z.; Chen, L.; Goyal, S.; Wang, X.; Frye, S.V.; Behera, M.; Schneider, F.; et al. MERTK Activation Drives Osimertinib Resistance in EGFR-Mutant Non-Small Cell Lung Cancer. J. Clin. Investig. 2022, 132, e150517. [Google Scholar] [CrossRef]
- Fu, K.; Xie, F.; Wang, F.; Fu, L. Therapeutic Strategies for EGFR-Mutated Non-Small Cell Lung Cancer Patients with Osimertinib Resistance. J. Hematol. Oncol. 2022, 15, 173. [Google Scholar] [CrossRef]
- Tian, X.; Wang, R.; Gu, T.; Ma, F.; Laster, K.V.; Li, X.; Liu, K.; Lee, M.-H.; Dong, Z. Costunolide Is a Dual Inhibitor of MEK1 and AKT1/2 That Overcomes Osimertinib Resistance in Lung Cancer. Mol. Cancer 2022, 21, 193. [Google Scholar] [CrossRef]
- Chen, W.; Yu, D.; Sun, S.-Y.; Li, F. Nanoparticles for Co-Delivery of Osimertinib and Selumetinib to Overcome Osimertinib-Acquired Resistance in Non-Small Cell Lung Cancer. Acta Biomater. 2021, 129, 258–268. [Google Scholar] [CrossRef]
- Fukuda, K.; Otani, S.; Takeuchi, S.; Arai, S.; Nanjo, S.; Tanimoto, A.; Nishiyama, A.; Naoki, K.; Yano, S. Trametinib Overcomes KRAS-G12V-Induced Osimertinib Resistance in a Leptomeningeal Carcinomatosis Model of EGFR-Mutant Lung Cancer. Cancer Sci. 2021, 112, 3784–3795. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Zou, T.; Qin, W.; Shen, X.; Su, Y.; Li, J.; Chen, Y.; Zhang, Z.; Sun, H.; Zheng, Y.; et al. Inhibition of EGFR Overcomes Acquired Lenvatinib Resistance Driven by STAT3-ABCB1 Signaling in Hepatocellular Carcinoma. Cancer Res. 2022, 82, 3845–3857. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Long, L.; Liu, J.; Zhu, L.; Luo, F. Case Report: Anlotinib Reverses Nivolumab Resistance in Advanced Primary Pulmonary Lymphoepithelioma-Like Carcinoma with FGFR3 Gene Amplification. Front. Oncol. 2021, 11, 749682. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.-H.; Nam, A.-R.; Bang, J.-H.; Oh, K.-S.; Seo, H.-R.; Kim, J.-M.; Yoon, J.; Kim, T.-Y.; Oh, D.-Y. WEE1 Inhibition Reverses Trastuzumab Resistance in HER2-Positive Cancers. Gastric Cancer 2021, 24, 1003–1020. [Google Scholar] [CrossRef]
- Gomes, I.N.F.; da Silva-Oliveira, R.J.; da Silva, L.S.; Martinho, O.; Evangelista, A.F.; van Helvoort Lengert, A.; Leal, L.F.; Silva, V.A.O.; Dos Santos, S.P.; Nascimento, F.C.; et al. Comprehensive Molecular Landscape of Cetuximab Resistance in Head and Neck Cancer Cell Lines. Cells 2022, 11, 154. [Google Scholar] [CrossRef]
- Landi, N.; Ciaramella, V.; Ragucci, S.; Chambery, A.; Ciardiello, F.; Pedone, P.V.; Troiani, T.; Di Maro, A. A Novel EGFR Targeted Immunotoxin Based on Cetuximab and Type 1 RIP Quinoin Overcomes the Cetuximab Resistance in Colorectal Cancer Cells. Toxins 2023, 15, 57. [Google Scholar] [CrossRef]
- Kawasaki, N.; Yamashita-Kashima, Y.; Fujimura, T.; Yoshiura, S.; Harada, N.; Kondoh, O.; Yoshimura, Y. Resistance to Obinutuzumab-Induced Antibody-Dependent Cellular Cytotoxicity Caused by Abnormal Fas Signaling Is Overcome by Combination Therapies. Mol. Biol. Rep. 2022, 49, 4421–4433. [Google Scholar] [CrossRef]
- Tian, Y.; Chen, Z.-H.; Wu, P.; Zhang, D.; Ma, Y.; Liu, X.-F.; Wang, X.; Ding, D.; Cao, X.-C.; Yu, Y. MIR497HG-Derived miR-195 and miR-497 Mediate Tamoxifen Resistance via PI3K/AKT Signaling in Breast Cancer. Adv. Sci. (Weinh) 2023, 10, e2204819. [Google Scholar] [CrossRef]
- Park, S.-J.; Joo, S.H.; Lee, N.; Jang, W.-J.; Seo, J.H.; Jeong, C.-H. ACY-241, an HDAC6 Inhibitor, Overcomes Erlotinib Resistance in Human Pancreatic Cancer Cells by Inducing Autophagy. Arch. Pharm. Res. 2021, 44, 1062–1075. [Google Scholar] [CrossRef]
- You, Q.; Wang, J.; Yu, Y.; Li, F.; Meng, L.; Chen, M.; Yang, Q.; Xu, Z.; Sun, J.; Zhuo, W.; et al. The Histone Deacetylase SIRT6 Promotes Glycolysis through the HIF-1α/HK2 Signaling Axis and Induces Erlotinib Resistance in Non-Small Cell Lung Cancer. Apoptosis 2022, 27, 883–898. [Google Scholar] [CrossRef]
- Zhou, J.; Ji, Q.; Li, Q. Resistance to Anti-EGFR Therapies in Metastatic Colorectal Cancer: Underlying Mechanisms and Reversal Strategies. J. Exp. Clin. Cancer Res. 2021, 40, 328. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Huang, Z.; Han, L.; Gong, Y.; Xie, C. Mechanisms and Management of 3rd-generation EGFR-TKI Resistance in Advanced Non-small Cell Lung Cancer (Review). Int. J. Oncol. 2021, 59, 90. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Bhattacharya, A.; Li, Y.; Sexton, S.; Ling, X.; Li, F.; Zhang, Y. Depleting Receptor Tyrosine Kinases EGFR and HER2 Overcomes Resistance to EGFR Inhibitors in Colorectal Cancer. J. Exp. Clin. Cancer Res. 2022, 41, 184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.-R.; Zhang, Y.-F.; Lei, H.-M.; Tang, Y.-B.; Ma, C.-S.; Lv, Q.-M.; Wang, S.-Y.; Lu, L.-M.; Shen, Y.; Chen, H.-Z.; et al. Targeting AKR1B1 Inhibits Glutathione de Novo Synthesis to Overcome Acquired Resistance to EGFR-Targeted Therapy in Lung Cancer. Sci. Transl. Med. 2021, 13, eabg6428. [Google Scholar] [CrossRef]
- Oh, S.Y.; Lee, Y.W.; Lee, E.J.; Kim, J.H.; Park, Y.; Heo, S.G.; Yu, M.R.; Hong, M.H.; DaSilva, J.; Daly, C.; et al. Preclinical Study of a Biparatopic METxMET Antibody-Drug Conjugate, REGN5093-M114, Overcomes MET-Driven Acquired Resistance to EGFR TKIs in EGFR-Mutant NSCLC. Clin. Cancer Res. 2023, 29, 221–232. [Google Scholar] [CrossRef]
- Lim, S.M.; Kim, C.G.; Cho, B.C. Antibody-Drug Conjugates: A New Addition to the Treatment Landscape of EGFR-Mutant Non-Small Cell Lung Cancer. Cancer Res. 2022, 82, 18–20. [Google Scholar] [CrossRef]
- Zeng, Y.; Yu, D.; Tian, W.; Wu, F. Resistance Mechanisms to Osimertinib and Emerging Therapeutic Strategies in Non small Cell Lung Cancer. Curr. Opin. Oncol. 2022, 34, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Mao, T.; Wang, J.; Zheng, H.; Hu, Z.; Cao, P.; Yang, S.; Zhu, L.; Guo, S.; Zhao, X.; et al. Toward the Next Generation EGFR Inhibitors: An Overview of Osimertinib Resistance Mediated by EGFR Mutations in Non-Small Cell Lung Cancer. Cell Commun. Signal. 2023, 21, 71. [Google Scholar] [CrossRef]
- Tsubata, Y.; Tanino, R.; Isobe, T. Current Therapeutic Strategies and Prospects for EGFR Mutation-Positive Lung Cancer Based on the Mechanisms Underlying Drug Resistance. Cells 2021, 10, 3192. [Google Scholar] [CrossRef]
- Tang, X.; Cheng, L.; Li, G.; Yan, Y.-M.; Su, F.; Huang, D.-L.; Zhang, S.; Liu, Z.; Qian, M.; Li, J.; et al. A Small-Molecule Compound D6 Overcomes EGFR-T790M-Mediated Resistance in Non-Small Cell Lung Cancer. Commun. Biol. 2021, 4, 1391. [Google Scholar] [CrossRef]
- Malapelle, U.; Passiglia, F. A Strategy to Overcome EGFR p.T790M Cis p.L792F Induced Resistance to Osimertinib. EBioMedicine 2022, 83, 104213. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Zhou, Y.; Yu, W. A Review of Research Progress on Mechanisms and Overcoming Strategies of Acquired Osimertinib Resistance. Anticancer Drugs 2022, 33, e76–e83. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, M.; Shinde, Y.; Pawara, R.; Noolvi, M.; Surana, S.; Ahmad, I.; Patel, H. Emerging Approaches to Overcome Acquired Drug Resistance Obstacles to Osimertinib in Non-Small-Cell Lung Cancer. J. Med. Chem. 2022, 65, 1008–1046. [Google Scholar] [CrossRef] [PubMed]
- Markham, A.; Keam, S.J. Selumetinib: First Approval. Drugs 2020, 80, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Guardiola, S.; Sánchez-Navarro, M.; Rosell, R.; Giralt, E.; Codony-Servat, J. Anti-EGF Nanobodies Enhance the Antitumoral Effect of Osimertinib and Overcome Resistance in Non-Small Cell Lung Cancer (NSCLC) Cellular Models. Med. Oncol. 2022, 39, 195. [Google Scholar] [CrossRef]
- Ku, B.M.; Heo, J.Y.; Kim, J.; Sun, J.-M.; Lee, S.-H.; Ahn, J.S.; Park, K.; Ahn, M.-J. ERK Inhibitor ASN007 Effectively Overcomes Acquired Resistance to EGFR Inhibitor in Non-Small Cell Lung Cancer. Investig. New Drugs 2022, 40, 265–273. [Google Scholar] [CrossRef]
- Ma, G.; Deng, Y.; Qian, L.; Vallega, K.A.; Zhang, G.; Deng, X.; Owonikoko, T.K.; Ramalingam, S.S.; Fang, D.D.; Zhai, Y.; et al. Overcoming Acquired Resistance to Third-Generation EGFR Inhibitors by Targeting Activation of Intrinsic Apoptotic Pathway through Mcl-1 Inhibition, Bax Activation, or Both. Oncogene 2022, 41, 1691–1700. [Google Scholar] [CrossRef]
- Sah, N.; Luna, P.; Mani, C.; Gmeiner, W.; Palle, K. A Novel Fluoropyrimidine Drug to Treat Recalcitrant Colorectal Cancer. J. Pharmacol. Exp. Ther. 2023, 385, 441. [Google Scholar] [CrossRef]
- Sah, N.; Luna, P.; Mani, C.; Gmeiner, W.; Palle, K. Abstract 6178: A Novel Second-Generation Nano-Fluoropyrimidine to Treat Metastatic Colorectal Cancer and Overcome 5-Fluorouracil Resistance. Cancer Res. 2023, 83, 6178. [Google Scholar] [CrossRef]
- Hong, X.; Hsieh, M.-T.; Tseng, T.-Y.; Lin, H.-Y.; Chang, H.-C.; Yau, S.-T.; Cheng, W.-C.; Ke, B.; Liao, H.-H.; Wu, C.-Y.; et al. Diarylheptanoid 35d Overcomes EGFR TKI Resistance by Inducing Hsp70-Mediated Lysosomal Degradation of EGFR in EGFR-Mutant Lung Adenocarcinoma. J. Biol. Chem. 2023, 299, 104814. [Google Scholar] [CrossRef]
- Kwon, Y.-S.; Nam, K.-S.; Kim, S. Tamoxifen Overcomes the Trastuzumab-Resistance of SK-BR-3 Tumorspheres by Targeting Crosstalk between Cytoplasmic Estrogen Receptor α and the EGFR/HER2 Signaling Pathway. Biochem. Pharmacol. 2021, 190, 114635. [Google Scholar] [CrossRef] [PubMed]
- Zalaquett, Z.; Catherine Rita Hachem, M.; Kassis, Y.; Hachem, S.; Eid, R.; Raphael Kourie, H.; Planchard, D. Acquired Resistance Mechanisms to Osimertinib: The Constant Battle. Cancer Treat. Rev. 2023, 116, 102557. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Piotrowska, Z.; Soo, R.; Cho, B.C.; Lim, S.M. Combatting Acquired Resistance to Osimertinib in EGFR-Mutant Lung Cancer. Ther. Adv. Med. Oncol. 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Huddleston, C.; Fakhreddine, A.B.; Stroever, S.; Young, R.; Sah, N.; Palle, K.; Reedy, M. Abstract 1305: Comparison of HPV-DNA Testing to PET-CT Imaging as Prognostic Test Following Definitive Treatment for Cervical Cancer: A Retrospective Proof-of-Concept Study. Cancer Res. 2024, 84, 1305. [Google Scholar] [CrossRef]
- Varghese, J.; Sah, N.; Thomas, S.M.; Jose, J.C.; Ramaiah, B.; Koneri, R. PDG6 Clinical Evaluation of Skin and Soft Tissue Infections in Inpatient Versus Outpatient Setting at a Tertiary Care Hospital—A Prospective Study. Value Health 2020, 23, S521–S522. [Google Scholar] [CrossRef]
- Varghese, J.; Sah, N.; Ramaiah, B. PDG50 Obstacles in the IV to ORAL Antibiotic Shift for Eligible Patients at a Tertiary Care Hospital. Value Health 2020, 23, S527. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).