Abstract
Microwaves in the presence of enzymes can contribute to the preparation of a variety of medicinally active compounds. Microwave-induced enzymatic reactions are influenced by variables such as frequency, field strength, waveform, duration, and modulation. The activation of enzymes under microwave irradiation allows the study of simple and complex reactions that have never before been reported under these conditions. By combining enzyme catalysis with microwave technology and solvent-free chemical reactions, it is possible to prepare drug-related molecules. This review presents the most interesting microwave reactions performed by enzymes toward medicinally active molecules.
1. Introduction
A microwave is a low-energy electromagnetic wave that has a wavelength in the range of 1 mm to 1 m and a frequency in the range of 300 MHz to 300 GHz. In laboratories (and in households as well), microwave instrumentation almost exclusively uses microwaves operating at a frequency of 2.45 GHz (or 12.2 cm wavelength), since this frequency has the right penetration depth for laboratory reaction conditions [1]. Microwaves, like all other electromagnetic waves, comprise two perpendicular oscillating fields: a magnetic field and an electric field. In microwaves, the photon energy is relatively low, affecting only kinetic molecular excitation. The electric field is primarily responsible for generating heat in microwaves, interacting with molecules via dipolar rotation and ionic conduction. During the process of dipolar rotation, molecules oscillate continuously back and forth, trying to align their dipoles with the ever-oscillating electric field; the friction between each rotating molecule results in the generation of heat. The process of ionic conduction involves free ions traveling translationally through space in an attempt to align with a changing electric field. It is the friction between these moving species that results in the generation of heat, and the higher the temperature of the reaction mixture, the more effective the transfer of energy is, just like in dipolar rotation. As microwaves interact directly with the contents of a reaction mixture, energy is transferred more efficiently than with conventional heating techniques.
Compounds of medicinal significance have been synthesized through organic reactions induced by microwaves [1]. There has been a significant increase in the rate of reactions. As a result, researchers have hypothesized that rapid increases in temperature caused by automated or commercial microwaves could be responsible for the rate of accelerations. There are those, however, who believe that microwave radiation is responsible for accelerating reactions. Even though the main focus has remained on synthesizing important molecules and drug candidates, the debate has become very intense. The use of microwave-induced processes as a means of synthesizing medicinally important substrates has become increasingly popular worldwide. As a result, many chiral and achiral molecules that are effective against a wide variety of medical conditions can be prepared using this method.
A substrate or catalyst that is optically active is necessary for the preparation of chiral compounds. Asymmetric induction may not be sufficient in many instances, and the chiral compounds may not be optically pure. As a result, some products are less effective as medicines. The use of enzymes in asymmetric synthesis has gained popularity in organic and medicinal chemistry in response to high demand [2,3,4]. A systematic investigation of microwave-induced reactions for the preparation of chiral or achiral molecules using enzymatic pathways has yet to be conducted. In recent years, however, scientists have begun to show interest in this field. Each enzyme has its own characteristics. The enzyme is a highly active and specific biological catalyst made up of proteins with a high molecular weight [5]. The use of enzymes in microwave-induced reactions, however, seems to be prohibited by several theories or speculations. For instance, enzymes are believed to be intolerant of temperature and pH alterations [6]. In other words, enzymes are unable to function outside of their temperature and pH ranges. It is possible to break the weak forces that hold the enzyme’s structure together at high temperatures, causing the enzyme to change its shape as a result. The structural change may affect the rate of an enzymatic reaction, and may even be able to completely prevent that reaction from occurring in the first place.
In many instances, enzymes are also considered to be better at catalyzing reactions than chemicals [7]. The actual role of microwaves is not known even though the benefits of using microwaves have been demonstrated in thousands of publications.
A careful literature review revealed that many enzyme-catalyzed microwave reactions occur even at relatively high temperatures and under radiation. There is a profound difference between the rate acceleration of enzyme-mediated reactions being induced by microwaves and the rate acceleration observed in chemical reactions. By using chemical methods, non-chiral compounds are produced when chiral influences are absent. Additionally, if the study is conducted using enzymes in a microwave oven, even mild chemical reactions seem drastic. Microwave-induced enzyme reactions can even be conducted at very low power without requiring rapid temperature rises, which is normally required in microwave-assisted chemical reactions. This is quite an interesting observation to keep in mind. There may be a greater sensitivity to radiation than temperature in enzymatic reactions in a microwave oven. On the other hand, scientists believe that chemical reactions may take place as a result of a combination of both. These types of reactions may be controlled more by the thermal situation, according to most published papers. The precise control of radiation and temperature of microwave in various research projects has been investigated by our group [8,9]. The synergistic effect of microwave irradiation and enzyme catalysis significantly accelerates the reaction [10]. Biodegradation of toxic organic pollutants is explained in various studies by using enzymes from fungi, bacteria, and plants [11,12]. Microbial enzymes control bioremediation, which is an inexpensive, environmentally friendly biotechnology. As a result of these analyses, the toxicity of pollutants can be reduced and useful new products can be developed. Microwave technology and enzyme catalysis are thus nature-friendly methods that produce high yields of products with little solvent wastage [13,14]. With microwave irradiation, enzymes from diverse microorganisms aid a broad range of biochemical reactions. As of yet, microwave-induced reactions have not been explored for the application of biochemical methods.
This review describes the most interesting enzyme reactions occurring under microwave irradiation, based on examples related to medicinally active molecules. The following sections discuss how microwaves can be used to promote enzymatic reactions in order to prepare compounds that are medicinally active. We focused only on a few compounds since this is not a comprehensive review.
2. Microwave-Assisted Lipase-Catalyzed Reactions
An enzyme called lipase catalyzes the hydrolysis of lipids. There are several reasons why it is widely used as a biocatalyst, including its biodegradability, non-toxicity, specificity, high catalytic efficiency, pH-dependent activity, activity in organic solvents, low product inhibition, low reaction times, reusability, high yield in non-aqueous media, resistance to temperature, pH, and alcohol. Additionally, lipase plays a vital role in a wide range of industrial applications, including detergents, food, leather, textiles, pharmaceuticals, cosmetics, and paper. Furthermore, lipase is involved in the synthesis of organic compounds, polymer synthesis, functionalization reactions, transformations, oxidations, hydrolysis, and epoxidations, as well as the esterification and transesterification of polymers and organic compounds. The following sections discuss the synthesis of medicinally active compounds using lipases.
Due to the polar nature of lipase, it can absorb microwave energy very rapidly. Microwave energy in the form of heat and radiation can activate lipase significantly and help the enzyme to participate in chemical reactions. Interestingly, it appears that both the heat and radiation of the microwave are responsible for the success of lipase-catalyzed reactions. An exposure of the reactants with lipase at room temperature (at about 25–30 °C) in a domestic microwave oven for 10 min oven did not produce any products regardless of the nature of the reactions. This means that radiation by the microwave alone failed. Similarly, the same reaction failed to produce products if heated alone at a specific temperature outside a microwave. A combination of microwave heating and radiation is necessary to perform enzyme-catalyzed reactions successfully. All enzyme-mediated reactions described here follow this general principle.
Importantly, the microwave-induced enzyme-mediated reactions produced either desired products or the starting compounds remained intact. No side products were isolated from these reactions.
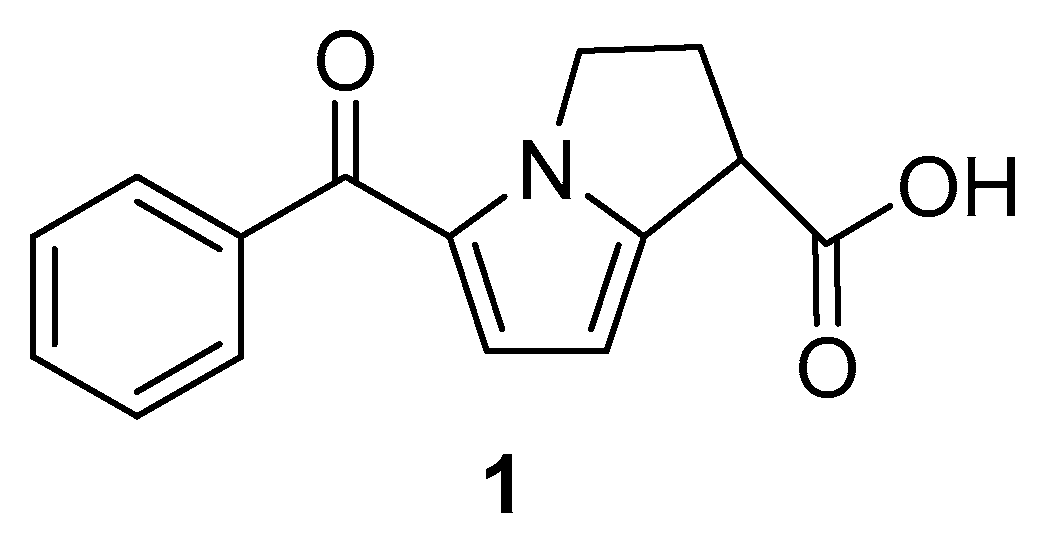
An anti-inflammatory and analgesic nonsteroidal drug, ketorolac has one stereogenic carbon center [15]. The structure of the molecule can be seen in Figure 1. The efficacy of ketorolac has been demonstrated to be higher than that of indomethacin, aspirin, naproxen, meperidine, ibuprofen, pentazocine, and morphine [16]. Ketorolac has achieved considerable therapeutic value in this way. Among the ketorolac enantiomers, the (R)-isomer is inactive and exhibits adverse effects, whereas the (S)-isomer exhibits very high therapeutic activity [17]. The preparation of the (S)-isomer is therefore crucial. There are several methods available for the stereoselective synthesis of (S)-isomer, including esterification in organic media catalyzed by microorganisms or enzymes, and hydrolysis in aqueous media [18]. In order to obtain the desired enantiomeric excess form of ketorolac, enzymatic biotransformation requires a longer reaction time.
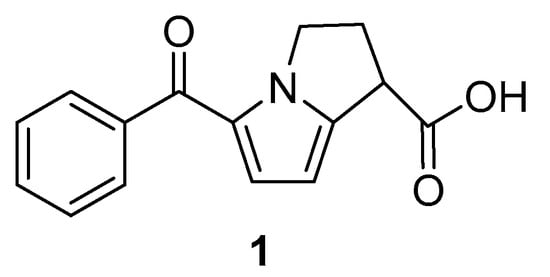
Figure 1.
Molecular structure of ketorolac.
Microwave-induced biocatalytic transformation is an important green method for chiral drug resolution. Our research group has been conducting green chemistry research using diverse methods [19,20].
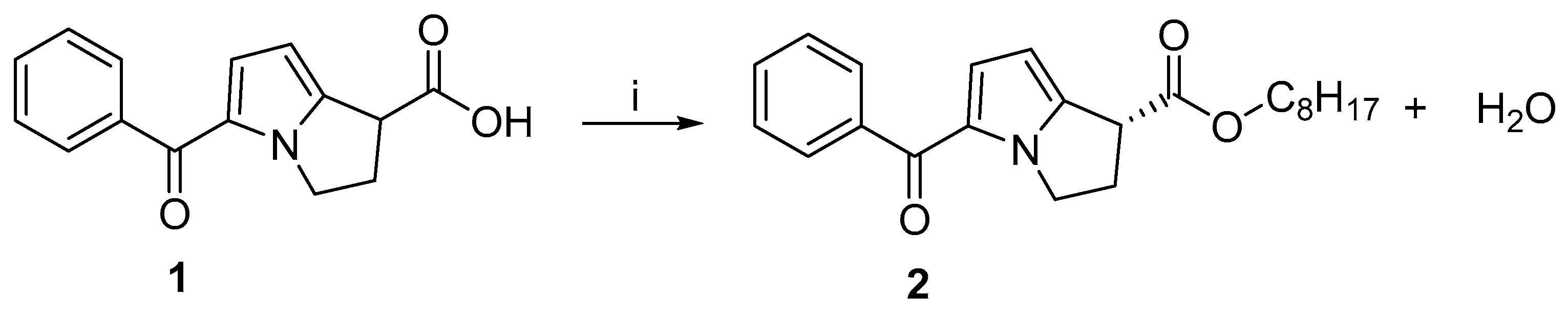
Through an esterification reaction using a lipase enzyme, the synergistic effect of microwave irradiation was investigated in the kinetic resolution of RS-(±)-ketorolac [21]. But the s-factor was not mentioned [21]. A variety of immobilized enzymes (Lipozyme TL IM, Novozym 435, Lipozyme RM IM, Lipase AYS amino, and Lipase Amano AS) have been evaluated for the kinetic resolution of RS-(±)-ketorolac under microwave conditions. Additionally, various growth parameters such as agitation speed, different solvents, temperature, catalyst concentration, and substrate concentration were examined. There was an increase in initial rates under microwave conditions compared to conventional methods. In comparison with other lipases, Novozym 435 lipase efficiently catalyzed the enantioselective esterification of RS-(±)-ketorolac. Figure 2 shows a schematic of the reaction. At 50 °C in 3 h, an enantiomeric excess of >99% and a conversion of 50% was achieved. It was demonstrated that the Ping-Pong bi–bi mechanism was followed by this reaction, inhibited by n-octanol.
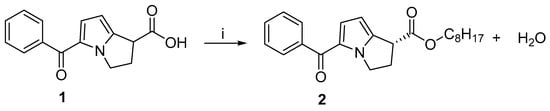
Figure 2.
The kinetic resolution of ketorolac induced by microwave irradiation and catalyzed by lipase. Reagents and conditions: (i) n-Octanol, Novozym 435, MWI.
By comparing the enantioselective resolution of ketorolac under conventional and microwave heating, the effect of temperature was investigated in the range of 30 to 60 °C. With an increase in temperature in the range of 30–60 °C under microwave irradiation, the initial rate increased from 4.67 × 10−1 to 21.33 × 10−1mol L−1 min−1 kg−1 of enzymes and the conversion increased from 23 to 53%. Due to the momentum provided by microwave energy, the reaction was completed faster than it would be with conventional heating methods due to the energy barrier being surpassed quickly. Enantioselectivity is not affected much by temperature change either in microwave irradiation or conventional heating.
On the Arrhenius plot, conventional and microwave activation energies were calculated to be approximately 9.02 and 10.29 kcal mol−1. It is likely that the microwave irradiation improved the collision frequency since the values were very close. Microwave irradiation increased the reaction rate, indicating that the effect may not be restricted to thermal effects. The microwave-absorbing nature of the reactants contributed to the faster reaction rate. The RS-(±)-ketorolac can be seen as an example of a microwave-absorbing material that features a high level of performance. The dipole of the RS-(±)-ketorolac may reorient rapidly, so that the functional unit becomes highly energetic at the interface between the RS-(±)-ketorolac and n-octanol, under microwave irradiation. Under microwave irradiation, the enzyme may act differently and receive more activity. Due to conformational changes in the enzyme, this occurs. In comparison to conventional heating, it facilitates substrate access to the enzyme’s active site.
Different growth parameters were investigated, such as the biocatalyst, temperature, and solvent. For the analysis, Novozym 435 (Candida antarctica lipase B), Lipozyme RM IM (Rhizomucor miehei), and Lipozyme TL IM (Thermomyces lanuginosus) lipases were tested. Lipase Novozym 435 showed the highest conversion rate of the three enzymes considered. A conversion rate of 83% was achieved with Novozym 435, 8% with Lipozyme RM IM, and a very minor conversion with Lipozyme TL IM. As compared to other enzymes studied, Novozym 435 showed the highest conversion, which can be attributed to its higher conformational flexibility towards R-isomer than S-isomer. Both conventional and microwave heating enhanced the initial rate at 60 °C. Compared to conventional methods, microwave irradiation increased initial rates by about fourfold.
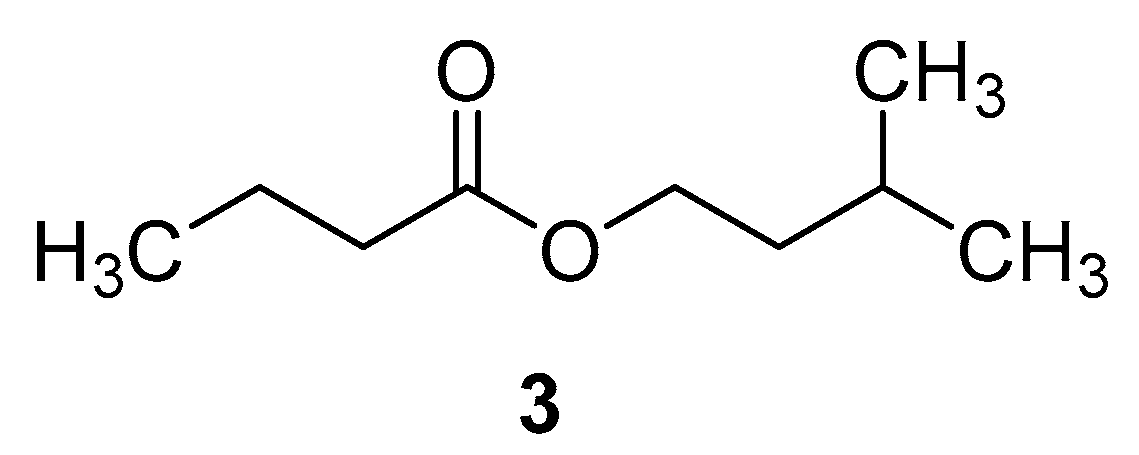
In Figure 3, isopentyl butanoate represents the butanoate ester of isoamylol; isopentyl butanoate is also called isoamyl butyrate. As a metabolite, this compound belongs to the class of fatty acid esters. It is also used in the preparation of fruit juice flavors as a flavoring agent. Additionally, it is widely used as a fragrance and flavor compound in the cosmetic, pharmaceutical, beverage, and food industries. Isoamyl alcohol and butyrate are normally esterified using lipase as a catalyst to produce this compound. It is also possible to synthesize isoamyl butyrate enzymatically under microwave conditions.
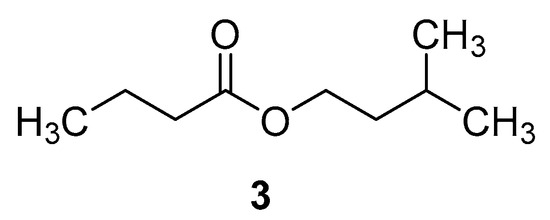
Figure 3.
Molecular structure of isoamyl butyrate.
A study investigating the effect of microwave irradiation on the enzyme-mediated synthesis of isoamyl butyrate ester in a solvent-free system was conducted by Bansode et al. [22]. Different parameters were analyzed to optimize the reaction conditions under the solvent-free condition to maximize the yield. At 60 °C and 700 W microwave power, about 95% conversion was achieved in 120 min. Data obtained from conventional and ultrasonic methods were compared with the thermodynamic data of the reaction. In the microwave method, the reaction rate increased due to the lower activation energy associated with the system. Thus, microwave energy enhanced lipase-catalyzed solvent-free esterification 1.5- to 5-fold compared to ultrasonic and conventional methods. Due to thermal denaturation of the enzyme, conversion decreased from 95% to 83% when the temperature was raised from 60 °C to 70 °C. In this study, the importance of temperature had been emphasized.
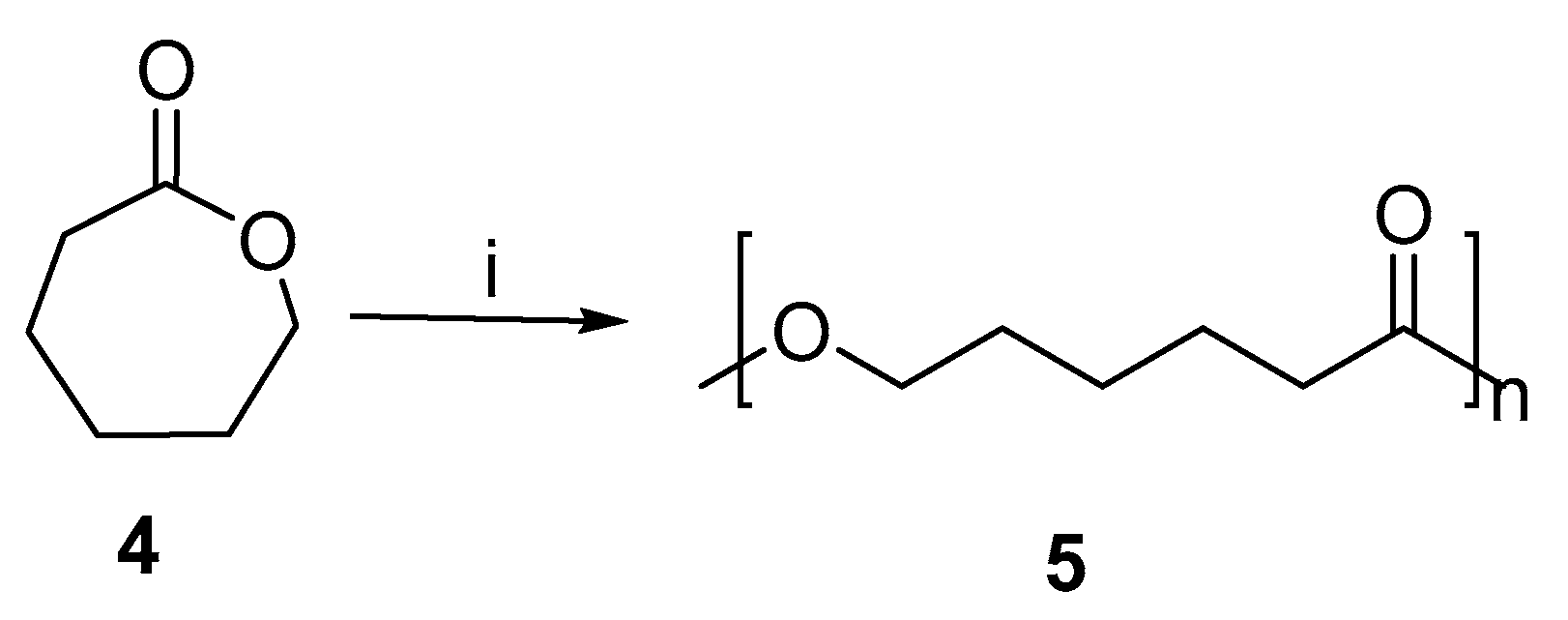
PCL (Poly-ε-caprolactone) is a biodegradable polyester. Moreover, it is used for modeling, splinting, prototyping systems, and the manufacture of special polyurethanes. As well as being used as an implantable biomaterial, as a device for drug delivery, and as a barrier or adhesion suture. Numerous studies have been conducted on PCL synthesis [23]. PCL was also synthesized using microwave-induced enzymes without solvent [24]. Figure 4 shows the ring-opening polymerization of ε-caprolactone.
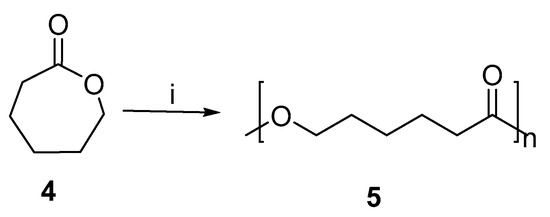
Figure 4.
Ring-opening polymerization of ε-caprolactone assisted by microwave. Reagents and conditions: (i) MWI, lipase.
Various growth parameters were tested, including microwave intensity, reaction time, and temperature. A polymerization reaction under optimal conditions resulted in PCL with a 1.2 polydispersity index and Mn of 20,624. Temperature also affected PCL’s properties positively, while microwave irradiation of high levels was ineffective. There is a close relationship between the stability of the catalytically active conformation of an enzyme and its interaction with the microenvironment in which it is located. In an organic medium, the hydrophobic interactions are more pronounced and the enzyme’s structural rigidity increases, resulting in a greater degree of thermal stability. It is possible that Caprolactone, being in a liquid form, could provide some thermal stability as well. Microwave energy is very efficiently absorbed by caprolactone. As a result of the increase in power, the instantaneous microenvironment temperature in the chemical reaction increased due to the increase in bulk temperature. It affected enzymes and their microenvironments, resulting in a loss of enzyme activity [25].
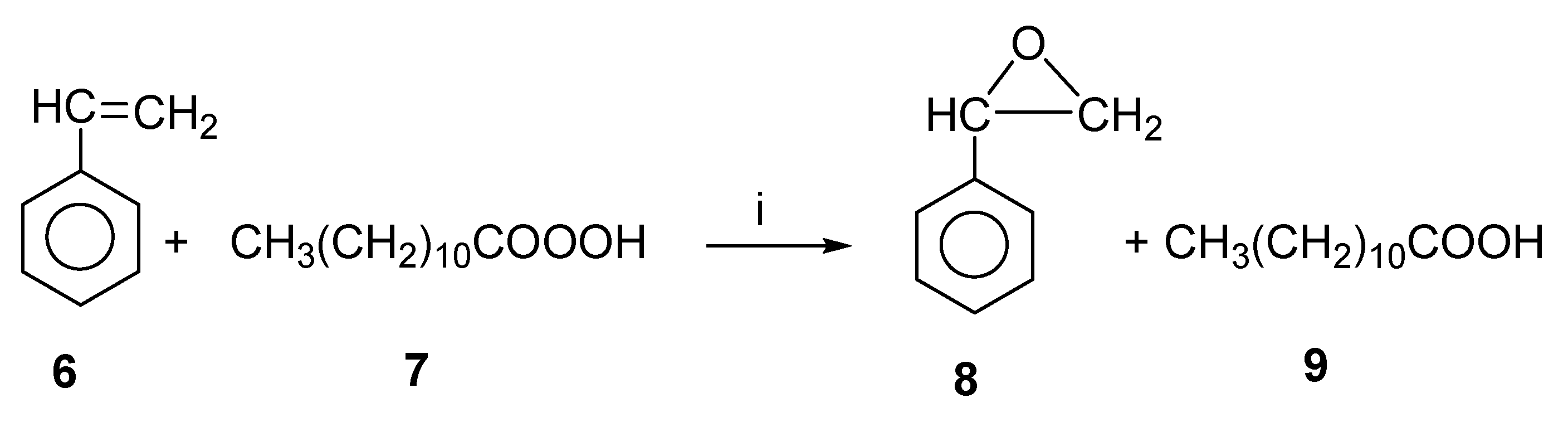
In the preparation of organic compounds of medicinal significance, styrene oxide is an efficient and important intermediate. Styrene-to-styrene oxide epoxidation catalyzed by lipases was investigated both in situ and ex situ using perlauric acid as the oxidizing agent, both by microwave as well as conventional heating [26]. Figure 5 shows the chemoenzymatic epoxidation of styrene oxide. As a first step, hydrogen peroxide and lauric acid were converted to perlauric acid by lipase (Candida antarctica). The synthesized perlauric acid was then reacted with styrene at the same time to produce styrene oxide. The enzyme was shown to be deactivated by hydrogen peroxide [27]. In order to enhance the enzyme’s activity and stability, microwave irradiation was considered.
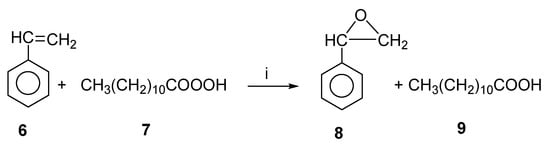
Figure 5.
Lipase-catalyzed styrene oxide synthesis. Reagents and conditions: (i) Lipase.
For the reaction, various lipases were considered, including PS-C “Amano”, Lipozyme RM IM, Novozym 435, and Lipozyme TL IM. It was found that Novozym 435 lipase was the most effective catalyst among those tested, achieving a conversion rate of 48% in three hours. In three hours, Lipase PS-C “Amano”, Lipozyme TL IM, and Lipozyme RM IM produced only conversions of 0, 7, and 11%.
With microwave heating, a temperature increase of 30 to 60 °C increased the reaction rate from 0.02 to 0.06 mol/(L-min-g-enz). By contrast, when the temperature was increased to 70 °C, the rate of reaction decreased to 0.045 mol/(L-min-g-enz). In conventional heating, however, the reaction rate increased from 0.02 to 0.05 mol/(L-min-g-enz) with an increase in temperature (30 to 60 °C). In the same way as microwave heating, an increase in temperature (70 °C) reduced the reaction rate to 0.045 mol/(L-min-g-enz). The microwave method resulted in a 33% increase in reaction rate over the conventional method at 60 °C. The microwave power of 35 W was sufficient to increase the reaction rate by such a large amount. At 35 W power, the reaction rate increased with temperature in accordance with the Arrhenius model. At higher temperatures, this model collapses due to irreversible lipase denaturation.
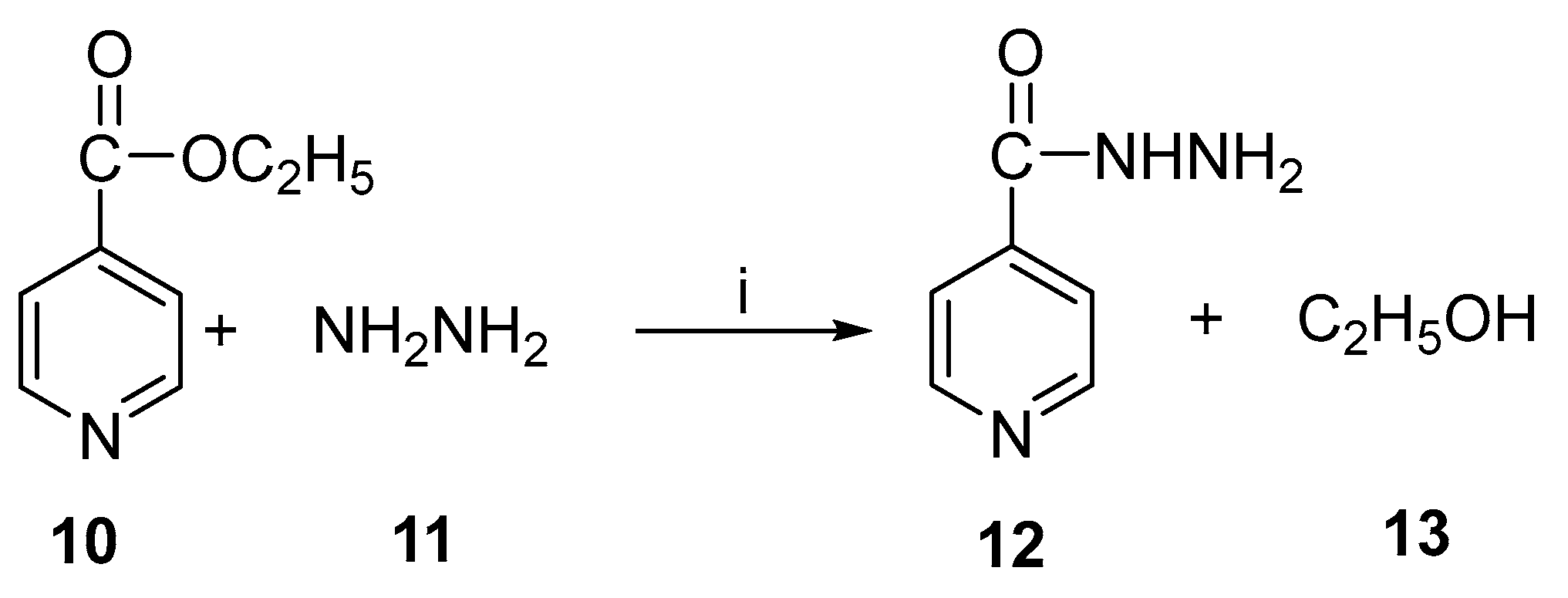
Isoniazid (isonicotinic acid hydrazide) is an antibacterial drug that is used primarily to treat tuberculosis. This compound was first prepared in 1912, and its anti-tuberculosis activity was discovered in 1952. There is a wide use of this drug in combination with other drugs such as ethambutol and rifampicin. Under reflux conditions for 7 h at 100 °C, isoniazid is chemically prepared from hydrazine hydrate and 4-cyanopyridine in the presence of sodium hydroxide [28]. There is also a method using hydrazine hydrate and ethyl isonicotinate. The use of immobilized lipase enzyme in the enzymatic preparation of isoniazid was also reported [29]. A study was conducted to determine whether it is possible to synthesize isoniazid enzymatically from ethyl isonicotinate and hydrazine hydrate in non-aqueous media with microwaves or conventional heating methods [30]. Figure 6 illustrates the reaction schematically.
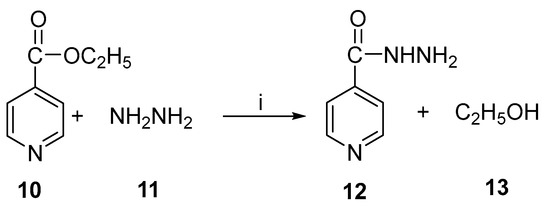
Figure 6.
Enzymatic synthesis of isoniazid. Reagents and conditions: (i) lipase, MW.
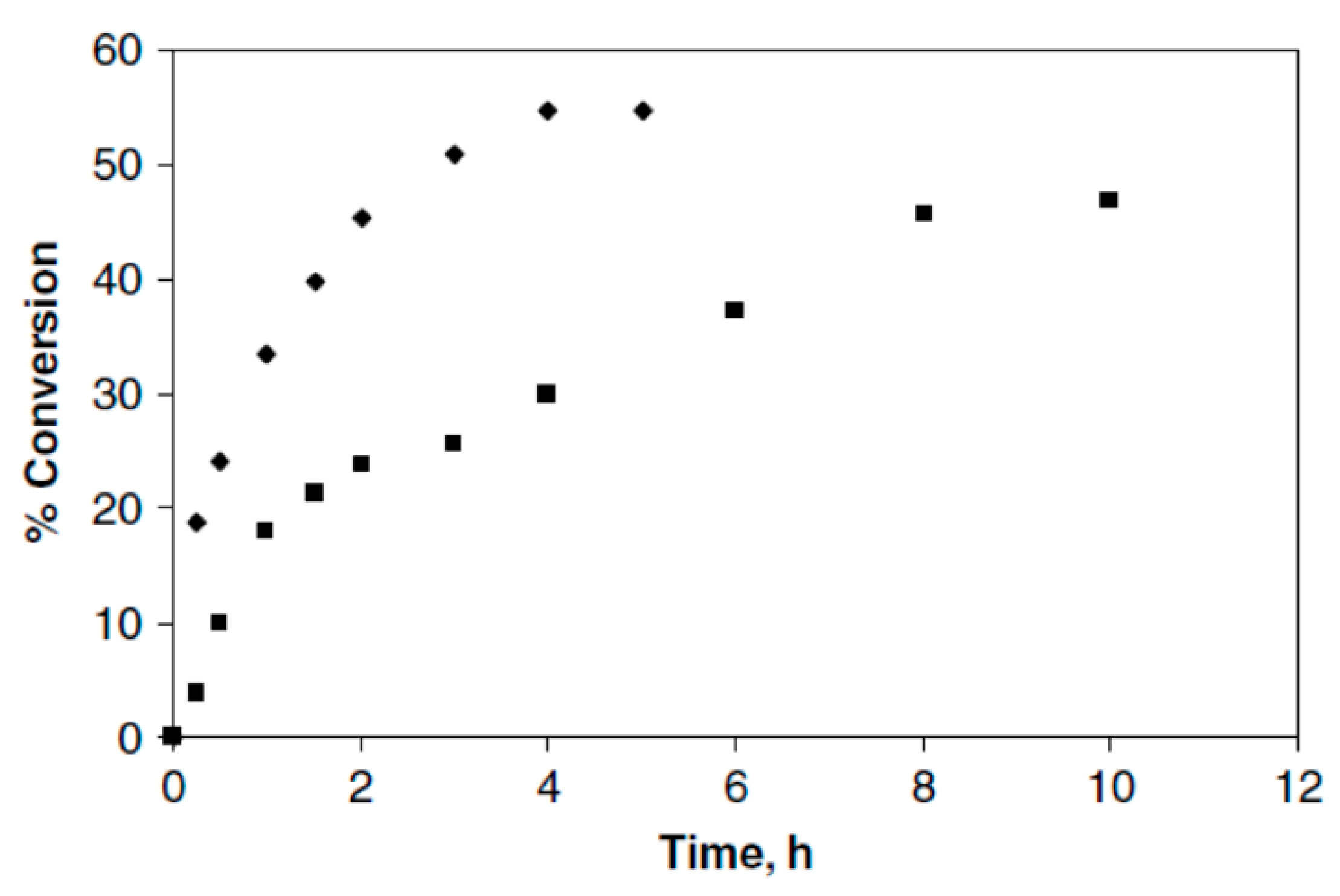
Lipozyme TL IM, Lipozyme RM IM, and Novozym 435 were all considered in this study. It was observed that Novozym 435 was the most active lipase among them. In comparison with conventional methods, microwave irradiation increased the final conversion and rate of reaction synergistically (Figure 7).
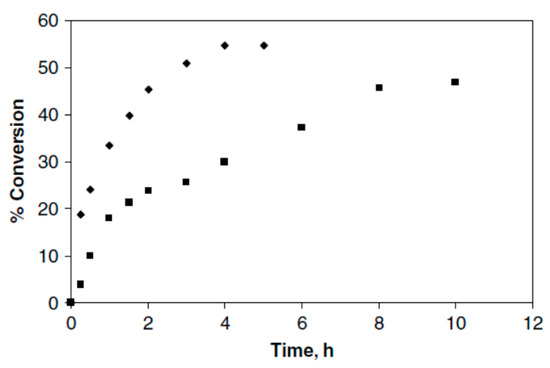
Figure 7.
Reaction rate and conversion. (■), conventional; (♦), microwave. Adapted from [30].
As a result of microwave irradiation, the enzyme activity was increased in the transesterification reaction. As compared to the conventional method, the rate of reaction improved 1.5-fold in the first 4 h. Microwave-induced transesterification achieved an equivalent product yield within a shorter period of time than conventional transesterification.
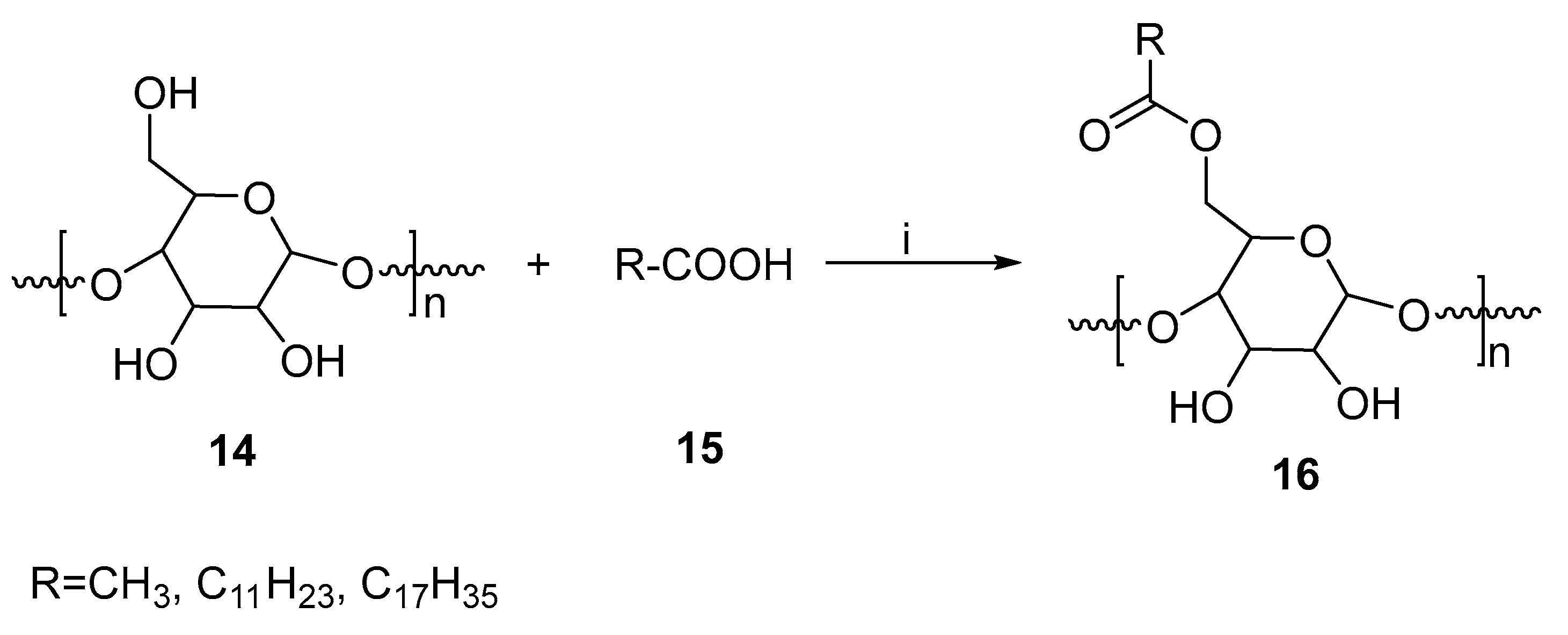
One of the most widely used pharmaceutical excipients is starch. With minimal processing, it meets most of the requirements for excipients. In addition, it is non-toxic, inexpensive, odorless, widely available, and biocompatible. There are a number of applications for modified starch. It can be used in a variety of applications, such as hot melt adhesives, tablet binders, cigarette filters, coatings, pharmaceutical aspects, and packaging materials [31]. It has been reported that various esterification methods such as chemical, enzymatic, and physical methods can be used to supply altered starches of high quality [32].
A method for esterifying starch using microwaves and biocatalysis has been described [33]. By employing microwave-induced enzyme transformations of starch, starch esters could be produced in abundance. In this method, hog pancreas lipase served as a biocatalyst. Dimethylformamide (DMF) and dimethyl sulfoxide (DMSO) were used as reaction media (Figure 8). An experiment was performed in which low-power microwave radiation was applied in the reaction mixture and the results compared to those obtained with conventional heating methods. The final temperature of the DMF process is much lower, and does not reach more than 80 °C when using a microwave power of 160 milli Watts per gram (mW/g). It was found that the internal temperature of the mixture could not be greater than 65 °C when only a low level of microwave power was applied (80 mW/g). In the case of DMSO, similar temperature profiles can also be observed; however, the maximum temperature is much higher. A total of 80 °C for 80 mW/g and about 100 °C for 160 mW/g, respectively, are the maximum temperatures. Based on the results of the study, microwave-assisted esterification in DMF with lauric acid as an acyl donor produced the highest substitution degree (around 0.51). Furthermore, the microwave method reduced the reaction time by 2.5 times. Using the method, starch ester was produced in a more efficient manner.
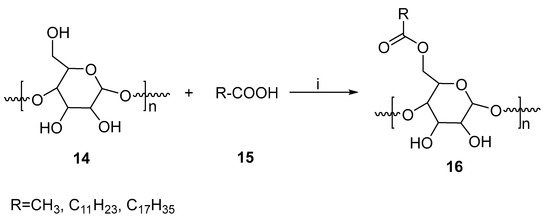
Figure 8.
Microwave-assisted esterification of starch. Reagents and conditions: (i) MWI, lipase, DMSO/DMF.
3. Microwave-Assisted Cellulase-Catalyzed Reactions
The decomposition of cellulose and related polysaccharides is known as cellulolysis. Enzymes that contain cellulase are usually used to catalyze cellulolysis, which hydrolyzes 1,4-glucoside linkages within the cellulose chain. Bacteria, protozoa, and fungi are the main producers of this enzyme. There are various types of cellulases, most of which differ in their structure and mechanism. There are many industries that use cellulases, including the food processing industry, textile industry, brewery and wine industry, laundry detergents, pulp and paper industry, livestock feed industry, agriculture, and pharmaceutical industry. Following is a discussion of how microwave irradiation affects cellulose activity.
In addition to being a water-soluble heteropolysaccharide, Astragalus polysaccharide also has bioactive properties (Figure 9). Astragalus polysaccharide can be extracted from Astragali Radix (the dry root of Astragalus membranaceus). There are a number of medicinal properties of this polysaccharide, including anti-aging, antitumor, immunomodulation, antioxidant, antiviral, cardiovascular protection, and anti-inflammatory properties [34]. Astragalus polysaccharides are composed of galactose, glucose, rhamnose, xylose, arabinose, mannose, galacturonic acid, and glucuronic acid. The biological activities of Astragalus polysaccharides depend on their structure and chemical composition, making its extraction crucial. In order to prepare astragalus polysaccharide, several extraction methods have been described [35].
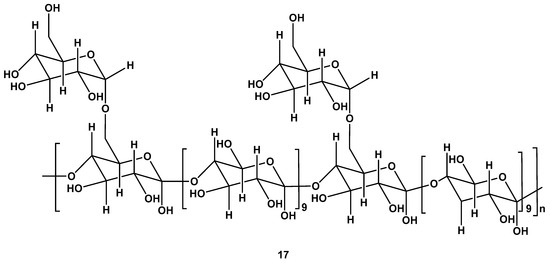
Figure 9.
The repeating unit of Astragalus polysaccharide.
Microwave-assisted cellulose hydrolysis was also studied to extract Astragalus polysaccharides [36]. It was found that enzymatic-microwave conditions could achieve a maximum extraction rate of about 16% and a maximum purity of about 88%, which was higher than that achieved using other methods. There were several conditions that seemed to be optimal for enzyme-microwave extraction, the most important being that the liquid-to-solid ratio was 10:1; the enzyme ratio was 57.6 U/g; and the cellulase reaction time was 60 min before 8 min of microwave irradiation (480 W). It is more efficient and faster to use microwave-assisted enzyme-catalyzed extraction to extract Astragalus polysaccharides from Astragali Radix.
4. Microwave-Assisted Glycosidases-Catalyzed Reactions
Glycosidases play a crucial role in carbohydrate metabolism as a part of perfect health. These enzymes are primarily responsible for the degradation of oligo- and polysaccharides in vivo. Glycosidases catalyze glycosidic bond hydrolysis in carbohydrates and other related compounds. Besides degrading biomass, they are also involved in the digestion of intestinal contents, immune defense strategies, post-translational modification of glycoproteins, pathogenesis mechanisms, lysosomal catabolism of glycoconjugates, and normal cell function.
In regard to biological activity, glycosides are a class of compounds that are biologically active. As an example, glycosides derived from alkaloid glycosides, vitamins, polyphenolic glycosides (flavonoids), glycosides derived from antibiotics, cardiac glycosides, glycopeptides, terpenoid glycosides, and steroid glycosides are a few of the many types of glycosides. There is also evidence that glycosides derived from different medicinal plants exhibit anticancer activity against a variety of cancer types [37]. It is possible to synthesize glycosidic bonds chemically in a number of different ways.
In an investigation that was carried out in dry media, glycosidase-catalyzed reversed hydrolysis and transglycosidation were performed using microwave assistance (Figure 10) [38]. As part of this study, some (trans)glycosidations were performed using almond-β-glucosidase. The preparation by reversed hydrolysis was carried out with hexane-1,6-diol and glucose, while transglycosidation was performed with propane-1,2-diol as acceptor and cellobiose and phenyl- β-D-glucoside as donors. A dry reaction medium was created by the co-immobilization of enzyme, acceptor, and donor from acetonitrile–water solution or water. Almond-β-glucosidase-catalyzed reversed hydrolysis was investigated utilizing type C aluminum oxide as the support whose pH is close to that of the enzyme’s optimal activity. The maximum conversion was 16% after 6 h under classical heating or after 1 h under microwave irradiation, while it was 60% after 5 days at 50 °C using 4 equiv. of acceptor in acetonitrile–water (9:1) mixture. However, due to the instability of this glucosidase, in the experiments at 80 °C, the reaction time could not be prolonged. Transglycosidation with almond-β-glucosidase was not successful due to hydrolysis of the phenyl-β-D-glucoside, donor 2, during the impregnation step.
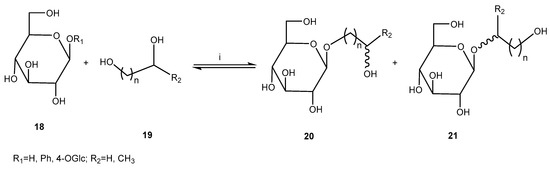
Figure 10.
Microwave-assisted transglycosylations catalyzed by glycosidases. Reagents and conditions: (i) MWI, glycosidase support.
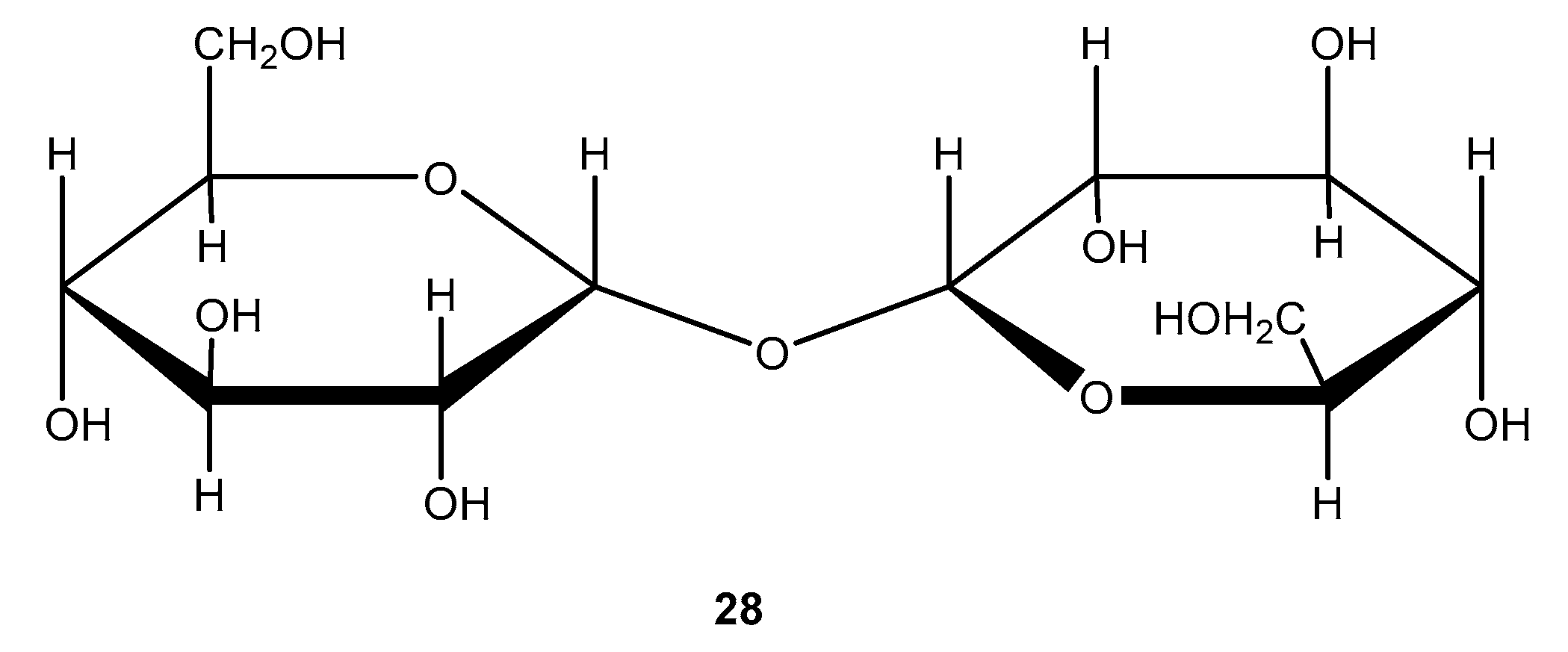
Oligosaccharides and their derivatives play a crucial role in many biochemical reactions. It has been demonstrated that oligosaccharides and their derivatives are widely used in a variety of applications, including therapeutics, cosmetics, the food industry, and diagnostic tools [39]. A derivative of an oligosaccharide called galacto-oligosaccharides stimulates the growth of Bifidobacteria in the human body [40].
Galacto-oligosaccharides are also considered growth factors for Bifidus. Intestinal Bifidobacteria are essential for maintaining intestinal balance, lactose tolerance, antitumorigenic activity, lowering serum cholesterol levels, milk digestibility, increasing calcium absorption from food, and synthesizing B-complex vitamins in the human body [41]. This makes galacto-oligosaccharide synthesis essential. There are several methods for synthesizing oligosaccharides [42]. Chemical synthesis, however, requires long deprotection steps to control selectivity, produces unwanted enantiomers, produces low yields, and generally involves very complicated procedures [43]. The enzymatic synthesis method offers a promising alternative.
With the use of beta-galactosidase from Kluyveromyceslactis and microwave irradiation, galacto-oligosaccharides can be prepared from lactose [44]. The synthesis was performed under microwave irradiation in a phosphate buffer supplemented with MgCl2, lactose, and immobilized beta-galactosidase (Figure 11). After 10 min of 100 °C heating, the reaction was stopped when lactose was depleted. Increased lactose concentrations, lower water activity in the media, and the addition of co-solvents enhanced the selectivity of galacto-oligosaccharides. With co-solvents (such as hexanol) and microwave irradiation exposed to the immobilized enzyme, the galacto-oligosaccharides selectivity was raised 217-fold compared with the conventional method.
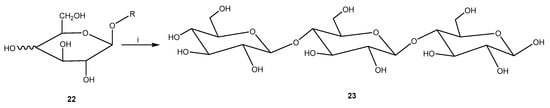
Figure 11.
Microwave-assisted transglycosylation of lactose using galactosidase. Reagents and conditions: (i) MWI, β-galactosidase.
Microwave-assisted transglycosylation of nucleotide-activated oligosaccharides was studied using Bacillus circulans galactosidase [45]. Figure 12 illustrates the pathway for synthesis.
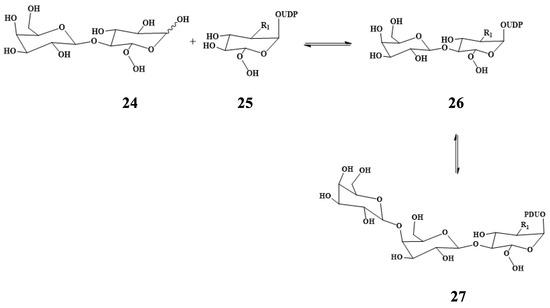
Figure 12.
Transglycosylation for the synthesis of UDP-Lac(NAc) using β-galactosidase.
Glycosylation involves covalently attaching saccharides to lipids, proteins, or other molecules. A glycosylation reaction takes place at a specific location under the guidance of an enzyme. Protein stabilization, protein folding, protein trafficking, quaternary structure, and protease protection are all dependent on glycosylation and deglycosylation [46]. Inflammation, receptor binding, and disease recovery are also affected by glycosylation [47].
Standard deglycosylation methods involve overnight digestion with the appropriate deglycosylating chemicals or enzymes. This method has been improved using several techniques. Deglycosylation using hydrophilic and hydrophobic chip technology [48], the optimization of reaction conditions using anhydrous trifluoromethane sulfonic acid [49], the PVDF-immobilization of glycosylated proteins followed by deglycosylating enzyme incubation [50], the development of hybrid deglycosylation enzymes [51], and the incubation of glycoproteins in the presence of surfactants with the peptide N-glycosidase F enzyme [52] are among the techniques.
Using peptide N-glycosidase F enzyme, the microwave-assisted removal of N-linked oligosaccharides was investigated [53]. The majority of protein samples were completely deglycosylated within 30 min without compromising their integrity. Compared to conventional deglycosylation methods, microwave-assisted enzymatic methods were significantly faster. This method was tested on a variety of glycoproteins. Deamidation, poor recovery, or cleavage of the protein backbone was not reported as damaging effects.
5. Microwave-Assisted Yeast-Catalyzed Reactions
The yeast family contains hundreds of species. Saccharomyces cerevisiae is one of the most well-known yeast species, commonly called baker’s or brewer’s yeast. Baker’s yeast is widely used in several biotechnological and medicinal applications. Various forms of Baker’s yeast are available depending on the moisture content. Additionally, organic compounds can be synthesized using it. Several reactions use it as a biocatalyst, for example. Among the reactions are condensation, oxidation, reduction, hydrogenation, cyclization, and hydrolysis—all are useful in drug synthesis. Baker’s yeast can be used in free form or immobilized form during different chemical reactions. Ethanol can also be synthesized using Baker’s yeast via fermentation. The following sections describe the variations in baker’s yeast activity due to microwave effects.
Disaccharides such as trehalose are non-reducing. As shown in Figure 13, trehalose contains two alpha, alpha-connected glucose moieties. It is considered to be the storehouse of glucose. In plants, fungi, bacteria, insects, and yeast, it can be found but not in vertebrates.
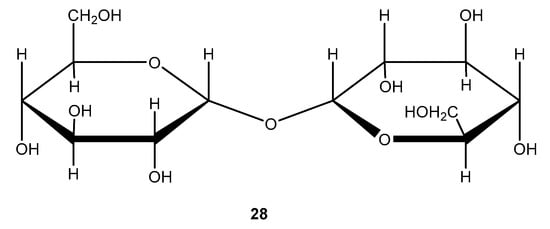
Figure 13.
Molecular structure of trehalose.
In addition to reserving glucose, trehalose also protects membranes and proteins from osmolarity, desiccation, heat, and frost as well as acting as a crucial component of mycobacterial cell walls and a mycolic acid donor, as well as serving as a transcriptional regulator and an allosteric inhibitor of glucose metabolism. As a practical preservative, this molecule is used to preserve foods, enzymes, cosmetics, and pharmaceuticals that are unstable [54]. There are five biosynthesis pathways reported for the synthesis of this molecule, but only three are commonly used [55]. There are three pathways in Corynebacteria and mycobacteria, but the majority of organisms possess only one pathway.
In large-scale production, trehalose is primarily synthesized from yeast (Saccharomyces cerevisiae). Yeast contains trehalase, an enzyme that hydrolyzes trehalose. The activity of trehalase was reported to control the extraction of trehalose from baker’s yeast [56]. Therefore, trehalase must be inactivated before extraction. Trehalase can be inactivated by adjusting pH, extraction temperature, and ethanol concentration. A high extraction ratio of trehalose can be obtained from thermally addressed yeast. It is possible to inactivate trehalase by irradiating fresh Saccharomyces cerevisiae with microwaves [57]. Microwave treatment disrupted the yeast cell and rendered the trehalase enzyme inactive after 60 s. Using microwave-treated yeast at room temperature, trehalose was easily extracted in 10 min by using water since the yeast cell was lysed and trehalase was inactive. Microwave-assisted pre-treatment is an efficient method for preparing trehalose from yeast. Different materials can also be prepared using microwave-assisted methods for intracellular products.
There is biological activity associated with beta-lactams, clinically active molecules. Among their medicinal properties are antibacterial, antifungal, anti-inflammatory, cholesterol absorption inhibitors, antihepatitis, antihyperglycemic, analgesic, and anticancer. As an example, hydroxy-beta-lactams can be used to make Taxol and Taxotere. As a part of developing numerous anticancer agents with their mechanism of actions, we have reported a number of medicinal aspects on this subject [58]. Figure 14 shows the molecular structure of Taxol and Taxotere, two clinically active anticancer drugs.
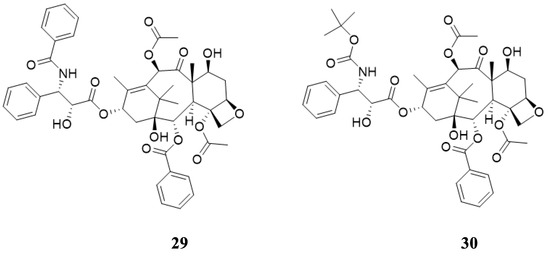
Figure 14.
Molecular structure of Taxol and Taxotere.
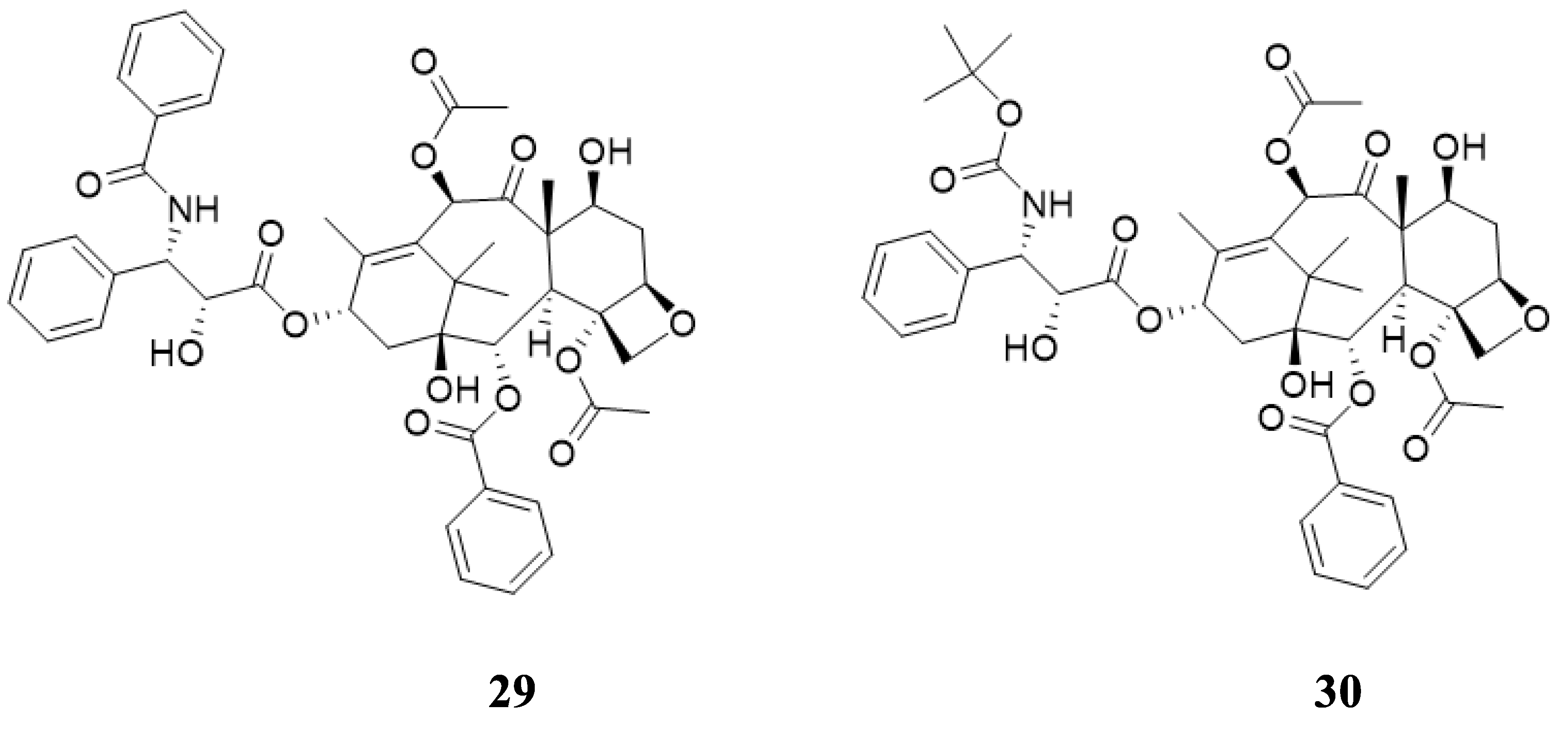
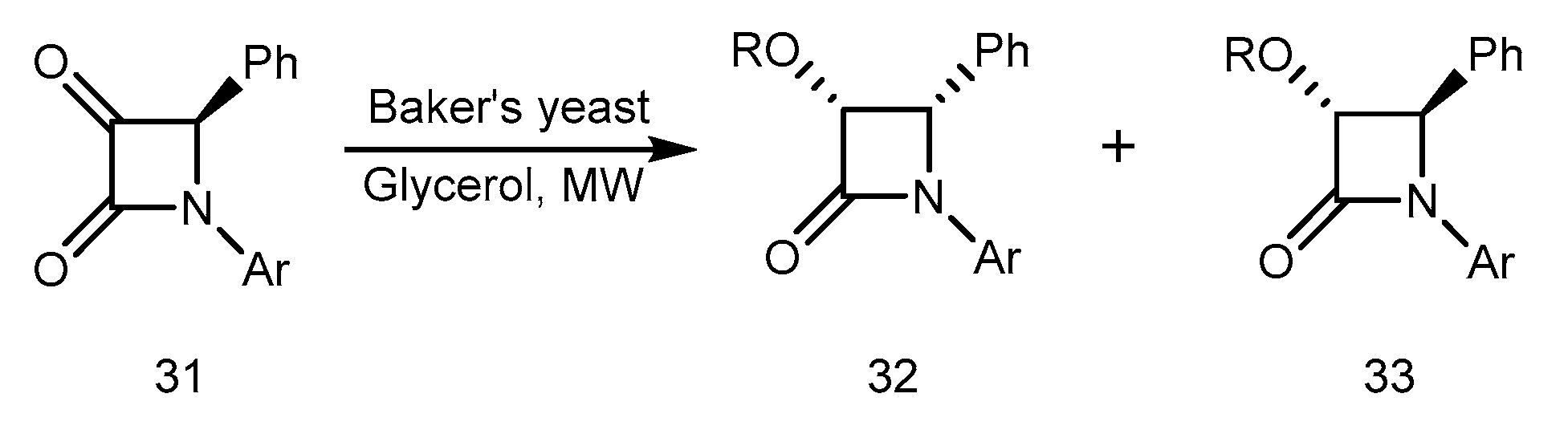
Microwave-induced Baker’s yeast-mediated reduction of 3-keto beta-lactams to chiral 3-hydroxy derivatives was reported by Banik et al. [59]. The keto functionality of alpha-keto-beta-lactam 31 in glycerol was reduced by Baker’s yeast (Saccharomyces Cerevisiae, type 3) using microwave irradiation. The hydroxyl compounds 32 and 33 were produced at a ratio of 3:1 and with a yield of 55%. Figure 15 illustrates the reaction schematically. A cis and trans configuration was indicated by NMR data for products 32 and 33. Beta-lactams were not studied in terms of enzymatic reactions by microwave. Beta-lactams have a variety of clinical applications, so microwave enzymatic reactions on them should be highly significant. The synthesis, properties, and reactivity of beta lactams constitutes the central part of our research endeavor [60].
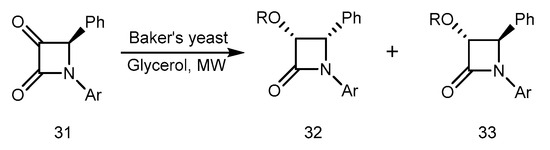
Figure 15.
Reduction of 3-keto β-lactams to chiral 3-hydroxy derivatives.
6. Conclusions
These examples demonstrate an increase in the initial rate under microwave irradiation compared with conventional heating. As a result, the reaction time for obtaining a similar conversion was reduced. Microwave radiation can be absorbed more efficiently by polar reactants than by less polar reactants, resulting in super molecular heating. As temperature increases, molecules can act faster and engage in more energetic interactions. This process occurs more quickly with microwave energy since the instantaneous temperature of the substances is higher than the normal bulk temperature. The detected rate enhancements may be due to this elemental factor. Microwave irradiation can cause enzymes to behave differently and become more efficient to some extent. Microwave irradiation and enzymatic catalysis work synergistically, as demonstrated by the examples given. Depending on the strength of the field, waveform, frequency, duration of exposure, and modulation, microwaves make various biological effects on enzymes.
The activity, selectivity, and stability of enzymes can be improved by microwave heating, according to studies. Since enzymes are very temperature-sensitive molecules, the application of microwave irradiation in the enzymatic synthesis of medicinally active compounds is constrained by the high temperatures associated with microwave heating. Various investigations are being conducted using new technologies while maintaining accurate power inputs and maintaining temperatures as low as 40 °C.
Several reports have shown that when microwaves are used in enzymatic reactions, the reaction time is drastically reduced and even superior selectivity is achieved. In spite of this, there are still many controversies and unexplained effects in this area that still need to be studied. There are still several limitations to the application of microwave heating technology, including non-homogeneous electromagnetic field distribution, thermally unstable rising temperatures, and an insufficient depth of microwave penetration, which reduces its efficiency. The limited penetration depth of microwaves causes a lack of uniformity in temperature distributions. In addition, other studies have shown that local overheat problems and hot spots may also cause thermal leakage, such as sudden spikes in system temperature. Microwave technology has other limitations, such as limited application, scalability, and health hazards. Combining microwave heating with other technologies is one way to overcome some of these limitations. In addition to improving product yields and energy efficiency, the combined technologies can reduce costs.
In spite of the fact that microwave-induced enzymatic reactions are not as widely used and popular as chemical synthesis, they have been successfully applied to a wide range of processes. This review discussed a wide range of topics including hydrolysis, transesterification, esterification, transglycosylation, ring-opening polymerization, deglycosylation, oxidation, optical resolution, condensation, and reduction, among others. There is no doubt that many more enzyme-catalyzed microwave-assisted reactions toward drugs and drug candidates will be discovered in the near future. As far as we are concerned, this is just the beginning of what we believe will be an incredibly exciting field of study.
Author Contributions
The authors have contributed equally to the preparation of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors are grateful to Prince Mohammad Bin Fahd University for its support.
Conflicts of Interest
Authors state no conflicts of interest. Kertz Ltd. had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Das, A.; Banik, B.K. Microwaves in Chemistry Applications: Fundamentals, Methods and Future Trends; Elsevier Science: Amsterdam, The Netherlands, 2021; p. 410. [Google Scholar]
- Hall, M. Enzymatic strategies for asymmetric synthesis. RSC Chem. Biol. 2021, 2, 958–989. [Google Scholar] [CrossRef] [PubMed]
- de Gonzalo, G.; Alcántara, A.R. Enzyme-Catalyzed Asymmetric Synthesis. In Catalytic Asymmetric Synthesis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2022; pp. 531–558. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/9781119736424.ch14 (accessed on 3 September 2024).
- Long, C.J.; He, Y.H.; Guan, Z. Emerging Strategies for Asymmetric Synthesis: Combining Enzyme Promiscuity and Photo-/Electro-redox Catalysis. Asian J. Org. Chem. 2023, 12, e202200685. [Google Scholar] [CrossRef]
- Ainsworth, S. Enzymes as Biological Catalysts. In Steady-State Enzyme Kinetics; Ainsworth, S., Ed.; Macmillan Education: London, UK, 1977; pp. 1–28. [Google Scholar] [CrossRef]
- Kishore, D.; Kundu, S.; Kayastha, A.M. Thermal, chemical and pH induced denaturation of a multimeric β-galactosidase reveals multiple unfolding pathways. PLoS ONE 2012, 7, e50380. [Google Scholar] [CrossRef] [PubMed]
- Mu, R.; Wang, Z.; Wamsley, M.C.; Duke, C.N.; Lii, P.H.; Epley, S.E.; Todd, L.C.; Roberts, P.J. Application of Enzymes in Regioselective and Stereoselective Organic Reactions. Catalysts 2020, 10, 832. [Google Scholar] [CrossRef]
- Das, A.; Yadav, R.N.; Banik, B.K. Microwave-induced ferrier rearrangement of hyroxy beta-lactams with glycals. Appl. Chem. Eng. 2024, 7, 1870. [Google Scholar] [CrossRef]
- Das, A.; Banik, B.K. Microwave-induced biocatalytic reactions toward medicinally important compounds. Phys. Sci. Rev. 2022, 7, 507–538. [Google Scholar]
- Kamble, M.P.; Chaudhari, S.A.; Singhal, R.S.; Yadav, G.D. Synergism of microwave irradiation and enzyme catalysis in kinetic resolution of (R,S)-1-phenylethanol by cutinase from novel isolate Fusarium ICT SAC1. Biochem. Eng. J. 2017, 117, 121–128. [Google Scholar] [CrossRef]
- Karigar, C.S.; Rao, S.S. Role of Microbial Enzymes in the Bioremediation of Pollutants: A Review. Enzym. Res. 2011, 2011, e805187. [Google Scholar] [CrossRef]
- Bhandari, S.; Poudel, D.K.; Marahatha, R.; Dawadi, S.; Khadayat, K.; Phuyal, S.; Shrestha, S.; Gaire, S.; Basnet, K.; Khadka, U.; et al. Microbial Enzymes Used in Bioremediation. J. Chem. 2021, 2021, e8849512. [Google Scholar] [CrossRef]
- Bashari, M.; Jin, Z.; Wang, J.; Zhan, X. A novel technique to improve the biodegradation efficiency of dextranase enzyme using the synergistic effects of ultrasound combined with microwave shock. Innov. Food Sci. Emerg. Technol. 2016, 35, 125–132. [Google Scholar] [CrossRef]
- Yu, D.; Wang, C.; Yin, Y.; Zhang, A.; Gao, G.; Fang, X. A synergistic effect of microwave irradiation and ionic liquids on enzyme-catalyzed biodiesel production. Green Chem. 2011, 13, 1869–1875. [Google Scholar] [CrossRef]
- Kim, Y.H.; Cheong, C.S.; Lee, S.H.; Kim, K.S. Enzymatic kinetic resolution of ketorolac. Tetrahedron Asymmetry 2001, 12, 1865–1869. [Google Scholar] [CrossRef]
- Kim, B.Y.; Doh, H.J.; Le, T.N.; Cho, W.J.; Yong, C.S.; Choi, H.G.; Kim, J.S.; Lee, C.-H.; Kim, D.-D. Ketorolac amide prodrugs for transdermal delivery: Stability and in vitro rat skin permeation studies. Int. J. Pharm. 2005, 293, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhuang, Y.; Chen, L.; Liu, J.; Li, D.; Ye, N. Process optimization for microwave-assisted direct liquefaction of Sargassum polycystum C.Agardh using response surface methodology. Bioresour. Technol. 2012, 120, 19–25. [Google Scholar]
- Palomer, A.; Cabré, M.; Ginesta, J.; Mauleón, D.; Carganico, G. Resolution of rac-ketoprofen esters by enzymatic reactions in organic media. Chirality 1993, 5, 320–328. [Google Scholar] [CrossRef]
- Das, A.; Banik, B.K. 15-Versatile thiosugars in medicinal chemistry. In Green Approaches in Medicinal Chemistry for Sustainable Drug Design; Banik, B.K., Ed.; Advances in Green and Sustainable Chemistry; Elsevier: Amsterdam, The Netherlands, 2020; pp. 549–574. [Google Scholar] [CrossRef]
- Das, A.; Banik, B.K. 26-Dipole moment in medicinal research: Green and sustainable approach. In Green Approaches in Medicinal Chemistry for Sustainable Drug Design; Banik, B.K., Ed.; Advances in Green and Sustainable Chemistry; Elsevier: Amsterdam, The Netherlands, 2020; pp. 921–964. [Google Scholar] [CrossRef]
- Shinde, S.D.; Yadav, G.D. Insight into microwave assisted immobilized Candida antarctica lipase B catalyzed kinetic resolution of RS-(±)-ketorolac. Process Biochem. 2015, 50, 230–236. [Google Scholar] [CrossRef]
- Bansode, S.R.; Rathod, V.K. Enzymatic sythesis of Isoamyl butyrate under microwave irradiation. Chem. Eng. Process.—Process Intensif. 2018, 129, 71–76. [Google Scholar] [CrossRef]
- Hoogenboom, R.; Schubert, U.S. Microwave-Assisted Polymer Synthesis: Recent Developments in a Rapidly Expanding Field of Research. Macromol. Rapid Commun. 2007, 28, 368–386. [Google Scholar] [CrossRef]
- Matos, T.D.; King, N.; Simmons, L.; Walker, C.; McClain, A.R.; Mahapatro, A.; Rispoli, F.J.; McDonnell, K.T.; Shah, V. Microwave assisted lipase catalyzed solvent-free poly-ε-caprolactone synthesis. Green Chem. Lett. Rev. 2011, 4, 73–79. [Google Scholar] [CrossRef]
- Réjasse, B.; Besson, T.; Legoy, M.D.; Lamare, S. Influence of microwave radiation on free Candida antarctica lipase B activity and stability. Org. Biomol. Chem. 2006, 4, 3703–3707. [Google Scholar] [CrossRef]
- Yadav, G.D.; Borkar, I.V. Kinetic modeling of microwave-assisted chemoenzymatic epoxidation of styrene. AIChE J. 2006, 52, 1235–1247. [Google Scholar] [CrossRef]
- Yadav, G.D.; Devi, K.M. Enzymatic synthesis of perlauric acid using Novozym 435. Biochem. Eng. J. 2002, 10, 93–101. [Google Scholar] [CrossRef]
- Sycheva, T.P.; Pavlova, T.N.; Shchukina, M.N. Synthesis of isoniazid from 4-cyanopyridine. Pharm. Chem. J. 1972, 6, 696–698. [Google Scholar] [CrossRef]
- Yadav, G.D.; Joshi, S.S.; Lathi, P.S. Enzymatic synthesis of isoniazid in non-aqueous medium. Enzym. Microb. Technol. 2005, 36, 217–222. [Google Scholar] [CrossRef]
- Yadav, G.D.; Sajgure, A.D. Synergism of microwave irradiation and enzyme catalysis in synthesis of isoniazid. J. Chem. Technol. Biotechnol. 2007, 82, 964–970. [Google Scholar] [CrossRef]
- Shogren, R.L. Rapid preparation of starch esters by high temperature/pressure reaction. Carbohydr. Polym. 2003, 52, 319–326. [Google Scholar] [CrossRef]
- Bao, J.; Xing, J.; Phillips, D.L.; Corke, H. Physical Properties of Octenyl Succinic Anhydride Modified Rice, Wheat, and Potato Starches. J. Agric. Food Chem. 2003, 51, 2283–2287. [Google Scholar] [CrossRef]
- Lukasiewicz, M.; Kowalski, S. Low power microwave-assisted enzymatic esterification of starch. Starch—Stärke 2012, 64, 188–197. [Google Scholar] [CrossRef]
- Wang, X.Y.; Wang, R.C.; Qu, Z.Y.; Zhu, Y.Z.; Li, Y.L. Advances on immunoregulation effect of astragalus polysaccharides. Front. Nat. Prod. 2022, 1, 971679. [Google Scholar] [CrossRef]
- Wang, J.; Jia, J.; Song, L.; Gong, X.; Xu, J.; Yang, M.; Li, M. Extraction, Structure, and Pharmacological Activities of Astragalus Polysaccharides. Appl. Sci. 2018, 9, 122. [Google Scholar] [CrossRef]
- Dong, L.L.; Huang, X.; Qi, Y.G.; Feng, H. Study on the enzymatic-microwave extraction of Astragalus polysaccharides. J. Zhejiang Univ. Technol. 2011, 3, 220–232. [Google Scholar]
- Khan, H.; Saeedi, M.; Nabavi, S.M.; Mubarak, M.S.; Bishayee, A. Glycosides from Medicinal Plants as Potential Anticancer Agents: Emerging Trends Towards Future Drugs. Curr. Med. Chem. 2019, 26, 2389–2406. [Google Scholar] [CrossRef] [PubMed]
- Gelo-Pujic, M.; Guibé-Jampel, E.; Loupy, A.; Trincone, A. Enzymatic glycosidation in dry media under microwaveirradiation. J. Chem. Soc. Perkin. Trans. 1 1997, 7, 1001–1002. [Google Scholar] [CrossRef]
- Monsan, P.; Paul, F. Enzymatic synthesis of oligosaccharides. FEMS Microbiol. Rev. 1995, 16, 187–192. [Google Scholar] [CrossRef]
- Kullen, M.J.; Khil, J.; Busta, F.F.; Gallaher, D.D.; Brady, L.J. Carbohydrate source and bifidobacteria influence the growth of Clostridium perfringens in vivo and in vitro. Nutr. Res. 1998, 18, 1889–1897. [Google Scholar] [CrossRef]
- Hughes, D.B.; Hoover, D.G. Viability and enzymatic activity of bifidobacteria in milk. J. Dairy Sci. 1995, 78, 268–276. [Google Scholar] [CrossRef]
- Öhrlein, R. Glycosyltransferase-Catalyzed Synthesis of Non-Natural Oligosaccharides. In Biocatalysis—From Discovery to Application; Fessner, W.-D., Archelas, A., Demirjian, D.C., Furstoss, R., Griengl, H., Jaeger, K.-E., Morís-Varas, E., Öhrlein, R., Reetz, M.T., Reymond, J.-L., et al., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; Volume 200, pp. 227–254. [Google Scholar] [CrossRef]
- Flowers, H.M. Chemical synthesis of oligosaccharides. Methods Enzymol. 1987, 138, 359–404. [Google Scholar]
- Maugard, T.; Gaunt, D.; Legoy, M.D.; Besson, T. Microwave-assisted synthesis of galacto-oligosaccharides from lactose with immobilized beta-galactosidase from Kluyveromyces lactis. Biotechnol. Lett. 2003, 25, 623–629. [Google Scholar] [CrossRef]
- Kamerke, C.; Pattky, M.; Huhn, C.; Elling, L. Synthesis of UDP-activated oligosaccharides with commercial β-galactosidase from Bacillus circulans under microwave irradiation. J. Mol. Catal. B Enzym. 2012, 79, 27–34. [Google Scholar] [CrossRef]
- Hare, J.F. Intracellular pathways of folded and misfolded amyloid precursor protein degradation. Arch. Biochem. Biophys. 2006, 451, 79–90. [Google Scholar] [CrossRef]
- Dwek, R.A. Biological importance of glycosylation. Dev. Biol. Stand. 1998, 96, 43–47. [Google Scholar] [PubMed]
- Ge, Y.; Gibbs, B.F.; Masse, R. Complete chemical and enzymatic treatment of phosphorylated and glycosylated proteins on ProteinChip arrays. Anal. Chem. 2005, 77, 3644–3650. [Google Scholar] [CrossRef] [PubMed]
- Raju, T.S.; Davidson, E.A. New approach towards deglycosylation of sialoglycoproteins and mucins. Biochem. Mol. Biol. Int. 1994, 34, 943–954. [Google Scholar] [PubMed]
- Papac, D.I.; Briggs, J.B.; Chin, E.T.; Jones, A.J. A high-throughput microscale method to release N-linked oligosaccharides from glycoproteins for matrix-assisted laser desorption/ionization time-of-flight mass spectrometric analysis. Glycobiology 1998, 8, 445–454. [Google Scholar] [CrossRef]
- Kwan, E.M.; Boraston, A.B.; McLean, B.W.; Kilburn, D.G.; Warren, R.A.J. N-Glycosidase—Carbohydrate-binding module fusion proteins as immobilized enzymes for protein deglycosylation. Protein Eng. Des. Sel. 2005, 18, 497–501. [Google Scholar] [CrossRef][Green Version]
- Yu, Y.Q.; Gilar, M.; Kaska, J.; Gebler, J.C. A rapid sample preparation method for mass spectrometric characterization of N-linked glycans. Rapid Commun. Mass Spectrom. 2005, 19, 2331–2336. [Google Scholar] [CrossRef]
- Sandoval, W.N.; Arellano, F.; Arnott, D.; Raab, H.; Vandlen, R.; Lill, J.R. Rapid removal of N-linked oligosaccharides using microwave assisted enzyme catalyzed deglycosylation. Int. J. Mass Spectrom. 2007, 259, 117–123. [Google Scholar] [CrossRef]
- Newman, Y.M.; Ring, S.G.; Colaco, C. The role of trehalose and other carbohydrates in biopreservation. Biotechnol. Genet. Eng. Rev. 1993, 11, 263–294. [Google Scholar] [CrossRef]
- Elbein, A.D.; Pan, Y.T.; Pastuszak, I.; Carroll, D. New insights on trehalose: A multifunctional molecule. Glycobiology 2003, 13, 17R–27R. [Google Scholar] [CrossRef]
- Yoshikawa, Y.; Matsumoto, K.; Nagata, K.; Sato, T. Extraction of Trehalose from Thermally-treated Bakers’ Yeast. Biosci. Biotechnol. Biochem. 1994, 58, 1226–1230. [Google Scholar] [CrossRef]
- Chuanbin, L.; Jian, X.; Fengwu, B.; Zhiguo, S. Trehalose extraction from Saccharomyces cerevisiae after microwave treatment. Biotechnol. Tech. 1998, 12, 941–943. [Google Scholar] [CrossRef]
- Banik, B.K.; Das, A. Natural Products as Anticancer Agents; Elsevier: Amsterdam, The Netherlands, 2023; p. 444. [Google Scholar]
- Das, A.; Yadav, R.N.; Banik, B.K. A Novel Baker’s Yeast-Mediated Microwave-Induced Reduction of Racemic 3-Keto-2-Azetidinones: Facile Entry to Optically Active Hydroxy β-Lactam Derivatives. Curr. Organocatal. 2022, 9, 195–198. [Google Scholar]
- Das, A.; Yadav, R.N.; Banik, B.K. Conceptual design and cost-efficient environmentally Benign synthesis of beta-lactams. Phys. Sci. Rev. 2023, 8, 4053–4084. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).