Abstract
Background: Metritis, a common postpartum uterine infection in bovines, poses substantial challenges in livestock management, including compromised fertility and economic losses. Poor uterine drug penetration and systemic side effects, necessitating innovative localised delivery systems and limiting current systemic antibiotic treatments. Aim: This study aimed to develop and evaluate the potential effect of the ofloxacin-loaded hydrogel as a localised drug delivery system to treat metritis in bovine. The focus was on achieving sustained drug release, enhanced antibacterial efficacy and reduced inflammation in the endometrium. Materials and Methods: The CS/PVA hydrogel was synthesised using a freeze–thaw method and further optimised for drug encapsulation efficiency (96.7 ± 2.1%), stability and biocompatibility. Physicochemical characterisation included swelling behaviour, mechanical properties and rheological analysis. In vitro drug release profiles in the simulated uterine fluid were assessed over 72 h and antibacterial activity was tested against common uterine pathogens such as Escherichia coli and S. aureus. In vivo studies were conducted on bovines diagnosed with endometritis to evaluate clinical recovery. Results: The SEM image of the ofloxacin-loaded CS/PVA hydrogel resulted in a smooth and porous structure demonstrating larger pore size than the blank. The rheological study suggested higher stability and elastic behaviour. Antibacterial assays on E. coli and S. aureus revealed significant inhibition zones, respectively, indicating potent efficacy. In vivo, evaluated on treated bovine, reduced bacterial loads were exhibited (2.86 × 105A CFU/mL → 6.37 × 102B CFU/mL), clinical improvement was marked and uterine inflammation was resolved. Conclusions: Ofloxacin-loaded hydrogels represent a promising localised treatment for bovine metritis, offering sustained antibacterial action and improved clinical outcomes. This approach addresses the limitations of systemic antibiotic therapies and provides a practical solution for enhanced veterinary care. Further studies are recommended to validate these findings in more extensive field trials and explore commercialisation potential.
1. Introduction
Metritis is a major reproductive health issue in dairy cattle, contributing to reduced fertility, prolonged calving intervals, decreased milk production and significant economic losses [1]. This condition is characterised by inflammation of the uterine lining (endometritis) and deeper layers of the uterus. It is primarily caused by bacterial pathogens such as Trueperella pyogenes, Escherichia coli, Fusobacterium necrophorum and Staphylococcus aureus and other pathogenic bacteria accompanied by systemic signs such as fever, reduced food intake and decreased milk production. The persistent risk factors are dystocia, retained placenta, poor hygiene during calving and immunosuppression, further exacerbating the condition [2]. If not treated promptly, uterine infections can progress to chronic endometritis, pyometra or infertility, severely impacting herd productivity. Globally, the prevalence of uterine infections ranges from 10% to 40%, varying with management practices and environmental factors [3]. Poor hygiene during calving, improper handling of postpartum complications and a lack of effective treatment options contribute to this high prevalence. In addition to compromising reproductive performance, these infections also increase the culling rates of affected animals, further impacting farm profitability [4].
Systemic or intrauterine antibiotic therapies, including cephalosporins, oxytetracycline and penicillin, are the most common treatments for uterine infections. While these therapies can help manage infections, they have significant limitations [5]. However, these approaches need to be revised. Systemic antibiotics often fail to achieve sufficient drug concentrations at the infectious sites due to poor vascularisation in inflamed uterine tissue. Furthermore, systemic treatments can lead to antibiotic residues in milk and meat, necessitating withdrawal periods and posing public health concerns. Overuse of antibiotics also accelerates the development of antimicrobial resistance, reducing the long-term efficacy of these treatments [6]. These challenges highlight the need for localised drug delivery systems to ensure targeted treatment, reduce systemic exposure and improve therapeutic outcomes [7].
Ofloxacin, a second-generation fluoroquinolone, has broad-spectrum antimicrobial actions towards gram-positive and gram-negative bacteria, making it a prospective therapeutic agent for treating metritis. It has bactericidal effects because it suppresses bacterial DNA gyrase and topoisomerase IV, two essential enzymes required for DNA replication and transcription. This dual-target mechanism enhances its efficacy and reduces the risk of resistance [8]. However, conventional delivery methods of ofloxacin, such as systemic or intrauterine administration, often fail to maintain sustained therapeutic concentrations at the infectious site. Additionally, ofloxacin demonstrates favourable pharmacokinetics, including excellent tissue penetration and prolonged activity, making it an ideal candidate for localised delivery to the uterine environment [9].
Nanotechnology enhances the therapeutic potential of any active moiety by addressing limitations associated with conventional drug delivery methods. Incorporating bioactive molecules in nanostructures facilitates controlled and sustained drug release, ensuring consistent therapeutic concentrations at the infection site while minimising systemic exposure and drug wastage. Furthermore, the nanoscale size of these carriers allows for better interaction with bacterial cells and biofilms, enhancing antimicrobial efficacy. By integrating nanotechnology, the delivery of ofloxacin can be optimised to achieve more effective pathogen eradication in metritis [10].
Hydrogels are multifaceted cross-linked matrices made up of hydrophilic composites that are critical in transporting bioactive compounds. High retention of water, effective mass transfer, similarity to natural tissues, softness that reduces surrounding tissue friction and responsiveness to physiological alterations are some of the unique characteristics of the hydrogel [11]. Several nonsynthetic or synthetic polymers were used to fabricate it: poly (vinyl alcohol), chitosan, alginate, etc. Selecting appropriate polymers and cross-linking techniques is essential to achieve the desired feature of the hydrogel [12]. PVA (medical-grade) is widely used due to its high safety, low toxicity, biocompatibility and stable chemical properties. It is extensively researched for various industrial and medical applications, including wound dressings, cartilage repair and drug delivery [13]. PVA’s large hydroxyl groups form hydrogels with good mechanical character and physicochemical qualities without compromising its biological properties, which can be improved by blending it with other polymers to create a safer physical cross-linking network (via freeze–thaw cycles), which eliminates the need for hazardous chemical cross-linking agents [14]. Additionally, chitosan (CS) is biologically active, safer, compatible, mucoadhesive and biodegradable, making it a choice of copolymer. It can be used to create antibacterial hydrogels with strong mechanical qualities because of its many free amino groups and capacity to cationise in acidic environments, which inhibits the growth of bacteria [15]. Pinto et al. tested the effectiveness of PVA/CS/alginate hydrogel loaded with antibiotic meropenem. They found that the hydrogel inhibits bacterial growth in S. aureus and E. coli due to the presence of CS in the hydrogel structure. The binding of CS to negatively charged bacterial cell walls caused cell rupture and disrupted membrane permeability, followed by binding to DNA, inhibiting DNA replication and cell death [16]. Similarly, Khan et al. performed an antibacterial, degradable and pH-responsive study of CS/Guar gum/PVA hydrogel for wound dressing. At pH 7, 98% of paracetamol was released in 140 min, followed by the Peppas model [17].
Minimal research has been conducted in the field of delivering ofloxacin antibiotics via hydrogel for the treatment of endometritis in large animals. This study aims to evaluate the effect of PVA and CS hydrogel on bovine endometrium. Hydrogel, composed of hydrophilic polymers, has excellent adhesion and retention properties, making it easy to apply and prevent microbes from spreading. It is injectable, self-healing and easy to use, with chitosan having antimicrobial activity. The hydrogel works with various uterine fluids, improving drug delivery and absorbing inflammatory exudates. It can also absorb purulent discharge from dead bacteria and leukocytes, allowing for more decisive antimicrobial actions. The hydrogel can also take in postpartum uterine fluids, like lochia, to keep the area clean and promote healing. Increased vascular permeability during infection may lead to plasma-like fluid uptake, allowing for more favourable retention and treatment of the hydrogel with uterine secretions. The formulation is designed for direct application to the uterine lining, allowing localised and sustained release of ofloxacin, reducing systemic exposure and enhancing antimicrobial efficacy. The formulation’s drug loading efficiency, release kinetics, mechanical properties and biocompatibility were characterised. Preliminary in vivo studies were conducted to evaluate their therapeutic potential. These formulations offer a sustainable approach to managing uterine infections, addressing the limitations of existing therapies and improving livestock health and productivity.
2. Results and Discussion
2.1. Organoleptic Assay
The organoleptic responses of CS/PVA hydrogels disclose the significant distinctions in the tactile and tangible characteristics of formulations with varying PVA:CS ratios. Hydrogels with a higher PVA content (9:1 and 8:2) had a firmer texture and a slightly more transparent appearance than those with a higher chitosan content (7:3, 6:4 and 5:5), which were softer and more opaque, as shown in Table 1 and Figure 1. The increased chitosan ratio resulted in a more slippery and moisturised hydrogel, which improves the mucoadhesive characteristics. Hydrogels with more chitosan have a slightly higher gel consistency, most likely due to variations in polymer interactions. Furthermore, formulations with a higher chitosan content exhibited a somewhat less uniform appearance, with occasional microscopic abnormalities detected. Despite these differences, all formulations were odourless and free of apparent phase separation, indicating their suitability for intrauterine use.

Table 1.
Organoleptic evaluation of the blank CS/PVA hydrogels.

Figure 1.
Visual observation of blank hydrogel with different PVA:CS ratios.
2.2. Gel Fraction
To objectively assess the level of cross-linking, the gel content in hydrogels (different PVA:CS ratio) was examined. The effect of CS on the gel content indicates a reduction in the percentage of gel content with an elevation in the CS concentration. The H1 sample (90.7 ± 0.9%) with a higher PVA content of gel suggests a higher level of cross-linking due to PVA, resulting in a stable cross-linking network. Contrarily, H5 (72.7 ± 2.0%) and H4 (73.8 ± 1.1%), with a higher CS content, revealed a low gel fraction due to the reduced availability of PVA chains and weaker cross-linking interactions. This aligns with chitosan’s inherent properties, which increase hydrophilicity and sol fraction [18]. The strength of cross-linking via the physical method, i.e., the freeze–thaw method, indicates lower cross-linking achieved by CS than PVA. Thus, chitosan can enhance the flexibility and strength of hydrogels by inhibiting the cross-linking reaction between polymers, thereby adjusting the strength and gel fraction [19]. Hydrogel H3 (80.8 ± 1.2%) evoked moderate gel fraction, well-balanced cross-linking density, adequate structural integrity and enhanced flexibility due to the increased chitosan content.
2.3. Porosity
H1 hydrogel (9:1) showed high cross-linking density due to the PVA predominance, resulting in densely packed networks with the lowest porosity. The porosity elevates when the ratio is elevated to 8:2 and 7:3, suggesting a more open network topology and a decrease in cross-linking density. An increase in the content of CS (6:4 and 5:5) results in a more hydrophilic and loosely packed polymer network, which in turn produces the highest porosity. Nevertheless, the hydrogel’s structural integrity may be endangered by the high porosity of the 5:5 ratio, making it a less appropriate drug delivery system required for prolonged drug release [20]. To preserve mechanical stability and give enough space for drug accommodation, the 7:3 and 6:4 ratios produced a balanced porosity.
2.4. Swelling Ratio
Three sequential processes are involved in the complex phenomena of hydrogel swelling behaviour: network expansion, chain relaxation within the hydrated gels and solvent migration into the network [21]. Functional groups cause electrostatic repulsion inside the network, which causes the gel to expand and eventually reach equilibrium. Stronger electrostatic repulsion among polymer chains and a quicker swelling rate are caused by the network’s increased number of free amino groups [22]. The increase in the swelling ratio with an increase in the CS concentration may be due to the low cross-linking of the hydrogel. A higher concentration of PVA is highly cross-linked due to more hydroxyl groups, resulting in less water absorption [23,24]. The swelling ratio is an essential parameter for optimising PVA:CS hydrogels, as it directly influences drug loading and release efficiency. At a 9:1 ratio, the hydrogel exhibits a low swelling ratio due to the dominance of PVA, limiting water absorption and drug diffusion. As the chitosan content increases, the swelling ratio improves moderately, allowing for better hydration and potential drug release. At a 7:3 ratio, the hydrogel balances structural integrity and hydrophilicity, ensuring sufficient water uptake for effective drug release while maintaining mechanical strength for localised application. At a 6:4 ratio, the swelling ratio significantly increases, enhancing water absorption and drug release but reducing mechanical stability. At a 5:5 ratio, the hydrogel achieves maximum swelling due to the hydrophilic nature of chitosan, which is ideal for drug release in a moist uterine environment. However, compromised structural integrity may limit its retention at the application site. The swelling ratio was also performed in PBS (pH 7). The observed result was slightly lower, as in the case of the PVA:CS hydrogel (7:3) retaining more water than PBS [25]. The salting-out action of the buffer’s ions, which prevent polymeric chains from hydrating, may cause the formulations’ poorer retention capacity in PBS than water.
2.5. Morphological Analysis of Hydrogel
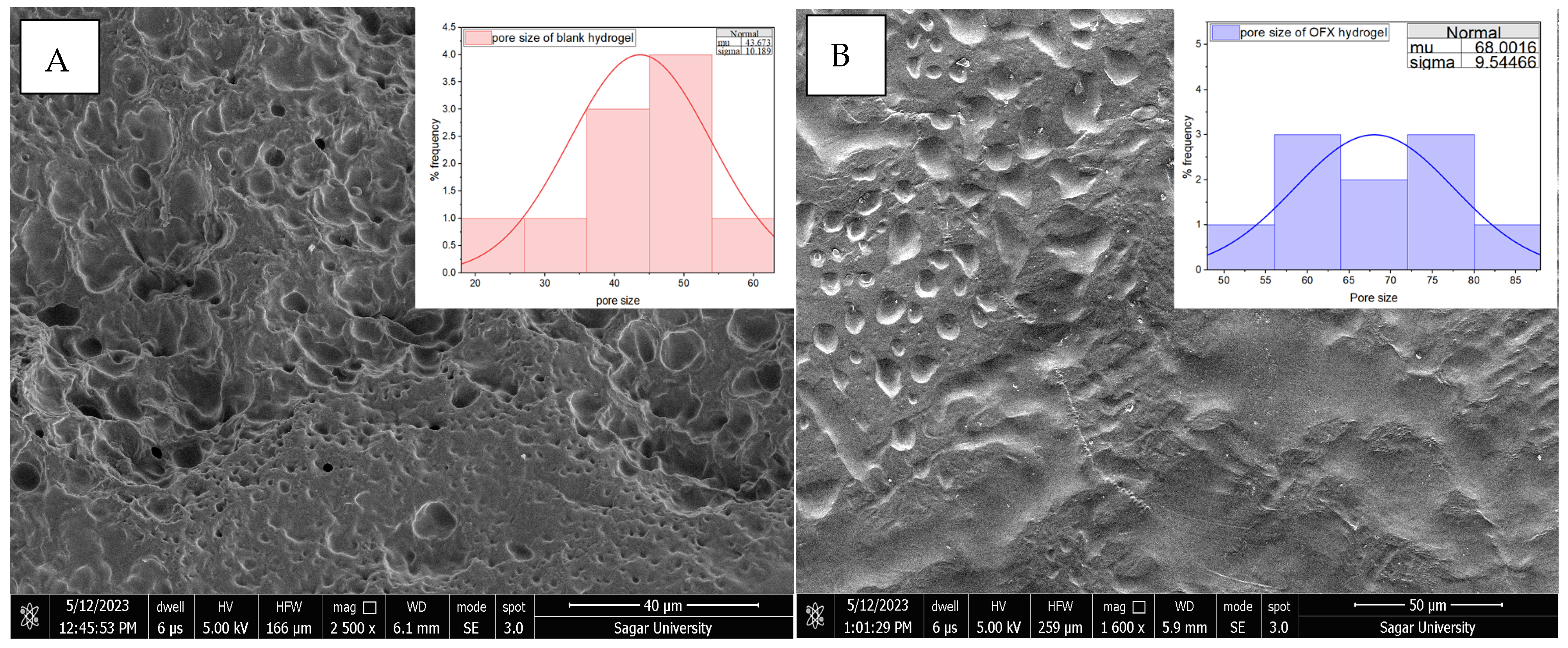
The PVA/CS (7:3) hydrogel was selected for further analysis by considering the aforementioned parameters. H3 showed moderate polymeric behaviour and regulated the swelling ratio, emerging as the best formulation, combining significant absorption with mechanical integrity for successful intrauterine drug delivery. The ofloxacin-loaded hydrogel and the blank hydrogel’s surface topology were examined using SEM, as shown in Figure 2, which offers essential information about their possible functional and structural variations. Since its microstructural characteristics, such as porosity, surface roughness and pore size, directly impact hydrogels’ water absorption, swelling behaviour and drug loading capability, SEM is essential for describing them. The SEM investigation of blank hydrogel (39.02 µm) revealed a highly porous and uneven structure, suggesting the possibility of high water retention and drug encapsulation. On the other hand, OFX-H3 (44.31 µm) had a smoother and more compact shape, with slightly increased pore size and prominent depressions, indicating that the drug had been well integrated into the polymeric matrix.
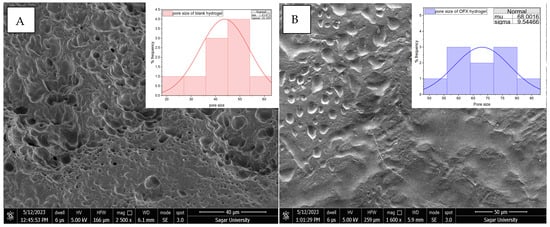
Figure 2.
SEM image of (A) CS/PVA hydrogel and (B) OFX-CS/PVA hydrogel.
2.6. Structural Analysis by FTIR, DSC and XRD
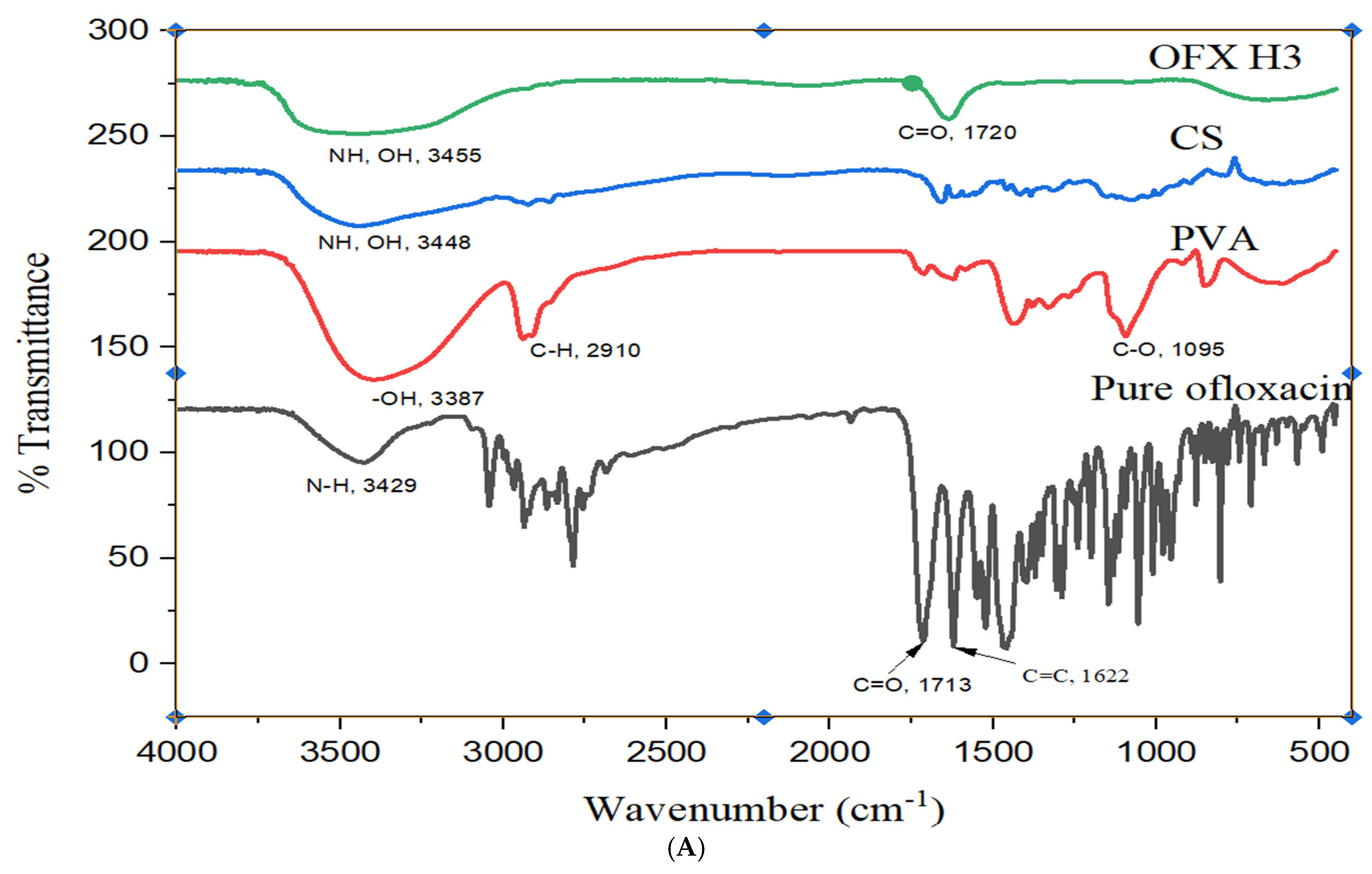
The FTIR analysis provides detailed insight into the OFX, PVA, CS and their composite hydrogel (OFX-CS/PVA Hydrogel) structural features in Figure 3A. The spectrum of OFX displayed characteristic peaks at approximately 1720 cm−1 corresponding to C=O stretching of the carbonyl group, 1620 cm−1 attributed to C=C stretching in the aromatic ring and a broad band around 3300–3500 cm−1 due to N-H stretching of the secondary amine group. These peaks confirm the molecular structure of the drug [26]. For PVA, the spectrum exhibited a broad O-H stretching band around 3200–3500 cm−1, indicative of hydroxyl groups, alongside C-H stretching at ~2900 cm−1 and C-O stretching at ~1090 cm−1, which are consistent with the polymer backbone of poly (vinyl alcohol). Similarly, the CS spectrum showed prominent peaks at ~3400 cm−1 for O-H and N-H stretching, 1650 cm−1 (Amide I) for C=O stretching, 1590 cm−1 (Amide II) for N-H bending and ~1070 cm−1 for C-O stretching, all of which align with the polysaccharide structure of chitosan [27]. The OFX-CS/PVA Hydrogel spectrum demonstrated notable shifts and intensity changes, indicating interactions among the components. The broad O-H and N-H stretching band (~3300–3500 cm−1) in the hydrogel was slightly shifted and exhibited reduced intensity, suggesting hydrogen bonding between PVA, CS and OFX. The C=O stretching peak at ~1720 cm−1 from OFX showed diminished intensity, further supporting the formation of interactions between OFX and the hydrogel matrix. Additionally, the C-O stretching peaks (~1090–1070 cm−1) were retained but displayed minor shifts, reflecting the integration of OFX into the CS/PVA network. These spectral features confirm the hydrogel’s successful formation and the OFX encapsulation, with evidence of molecular interactions enhancing the structural integrity of the composite material.
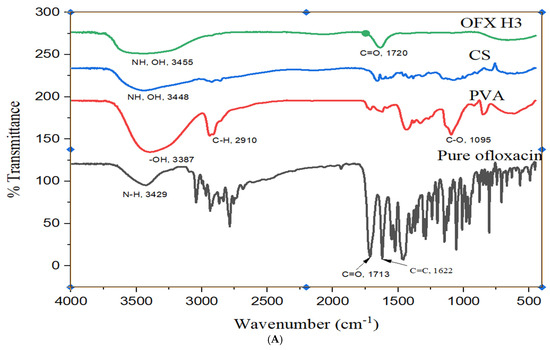
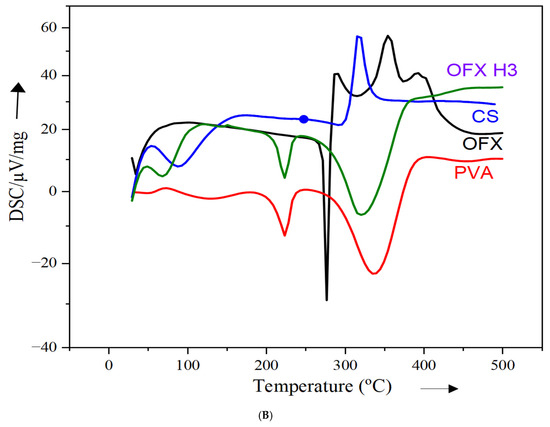
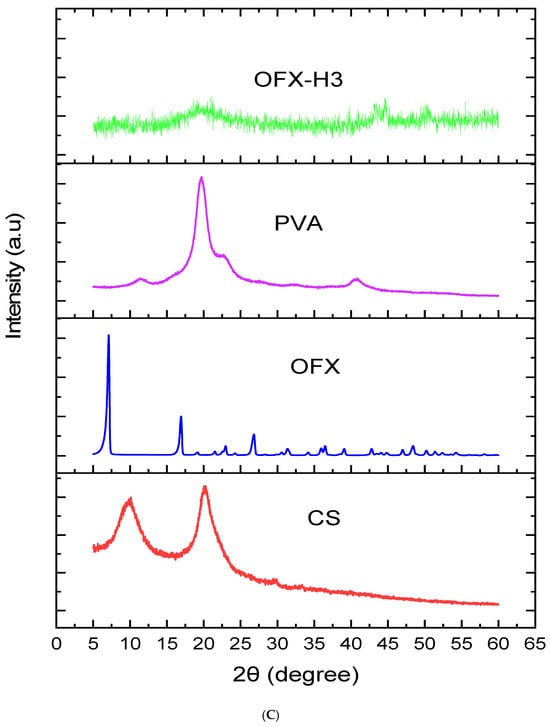
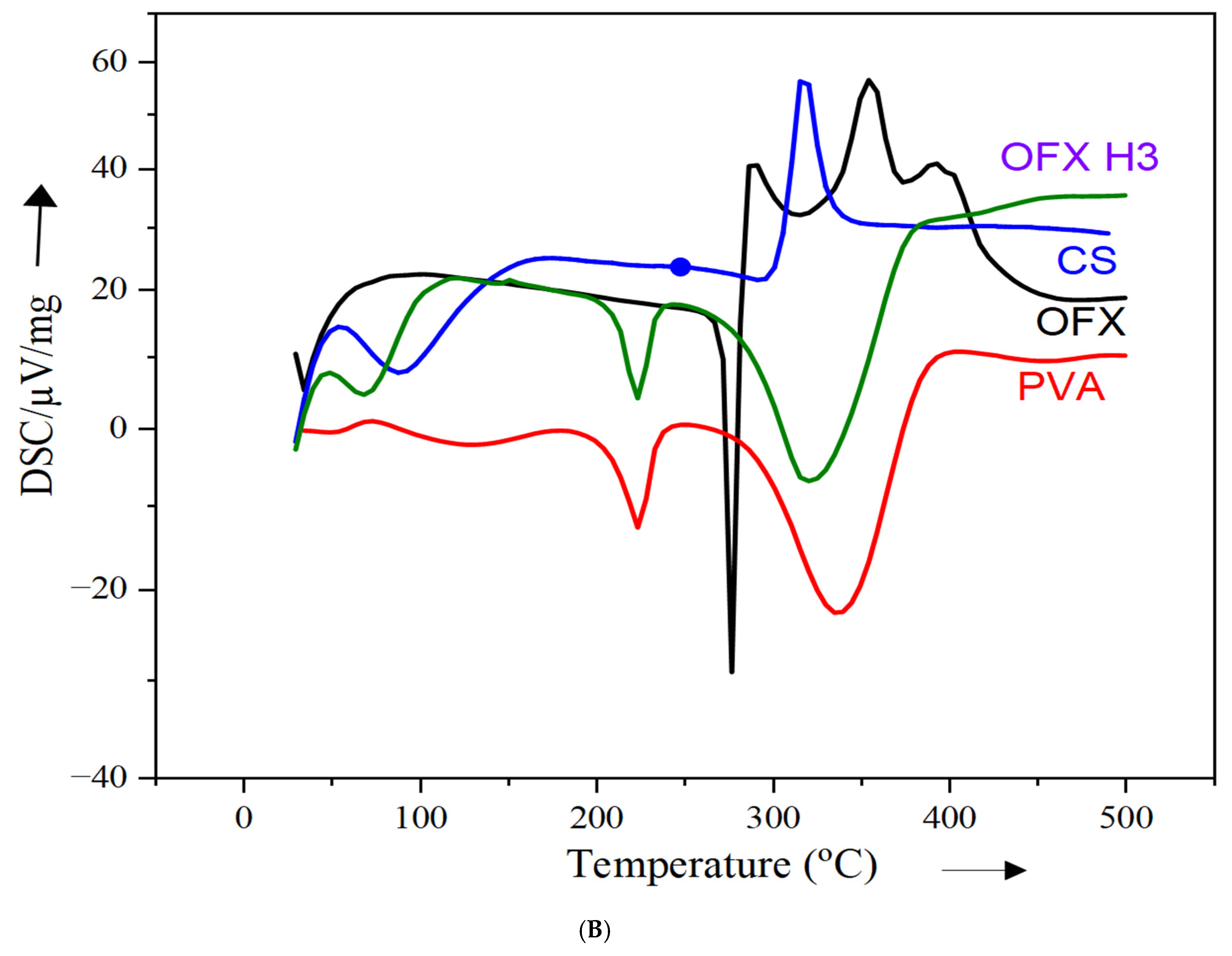
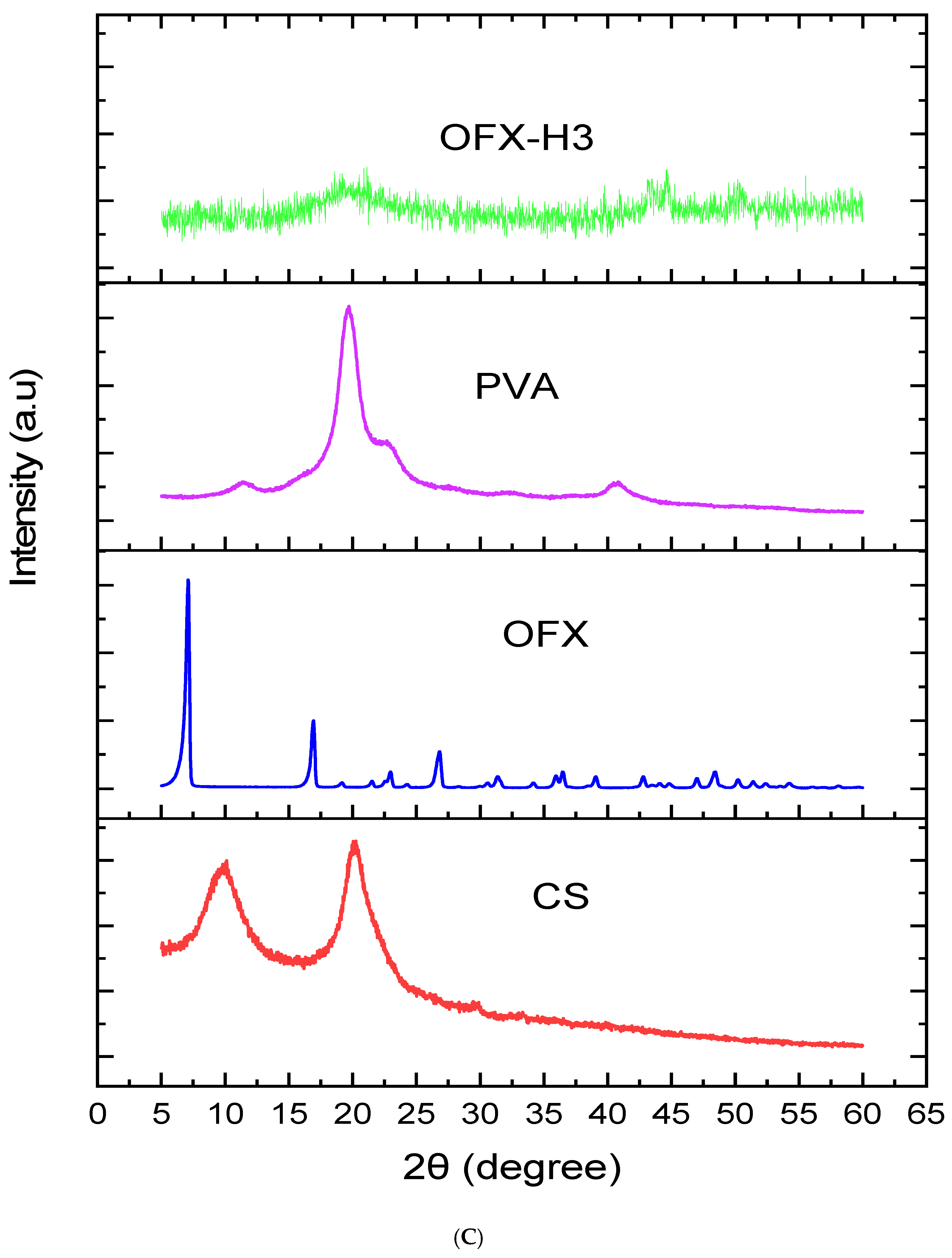
Figure 3.
Structural analysis of OFX, polymers and OFX H3 by (A) FTIR, (B) DSC and (C) XRD.
The DSC thermogram provides insights into the thermal properties of individual components (PVA, ofloxacin and chitosan) and the ofloxacin-loaded composite (OFX H3). In Figure 3B, the red curve, representing PVA, exhibits an endothermic peak at approximately 220 °C, corresponding to its melting or dehydration process, which reflects its crystalline nature [27]. The green curve for pure ofloxacin shows a sharp melting peak at around 275 °C, confirming its crystalline structure [26]. The blue curve for chitosan reveals a broad endothermic peak at approximately 100–150 °C, indicative of its amorphous nature and associated with water loss or relaxation transitions in the polymer [27]. In contrast, the black curve for the OFX H3 composite shows significant thermal changes. The sharp melting peak of ofloxacin observed at 275 °C is no longer visible, suggesting a reduction in crystallinity due to the incorporation of the drug into the polymeric matrix. The broad thermal transitions observed indicate strong interactions within the composite between PVA, chitosan and ofloxacin. These changes confirm the successful molecular dispersion or encapsulation of ofloxacin, ensuring compatibility of the components. The observed thermal shifts and reduced crystallinity highlight the potential of this composite formulation to improve drug release and bioavailability in drug delivery applications.
Figure 3C illustrates the X-ray diffraction pattern and provides insights into the crystalline and amorphous nature of the samples. The blue curve of pure ofloxacin (OFX) exhibits sharp, intense peaks at specific 2θ values, indicating its highly crystalline structure. Key diffraction peaks for OFX are observed around 10°, 20°, 25° and 30° 2θ. In contrast, the PVA shows a sharp peak centred around 20° 2θ, reflecting its semicrystalline nature, while the chitosan (CS) demonstrated distinct peaks around 10° and 20° 2θ, reflecting its partially crystalline structure. Nevertheless, the XRD pattern of the OFX-PVA/CS hydrogel shows either diminished or absent sharp crystalline peaks of OFX, which is accompanied by the emergence of a broad, amorphous peak. This implies that the drug is molecularly dispersed within the hydrogel matrix, and strong polymer-drug interactions have significantly decreased the crystallinity. Amorphisation increases the solubility and bioavailability of the drug, thus making the hydrogel formulation more advantageous for controlled drug release [28,29,30].
2.7. Mechanical Analysis by Viscosity Test
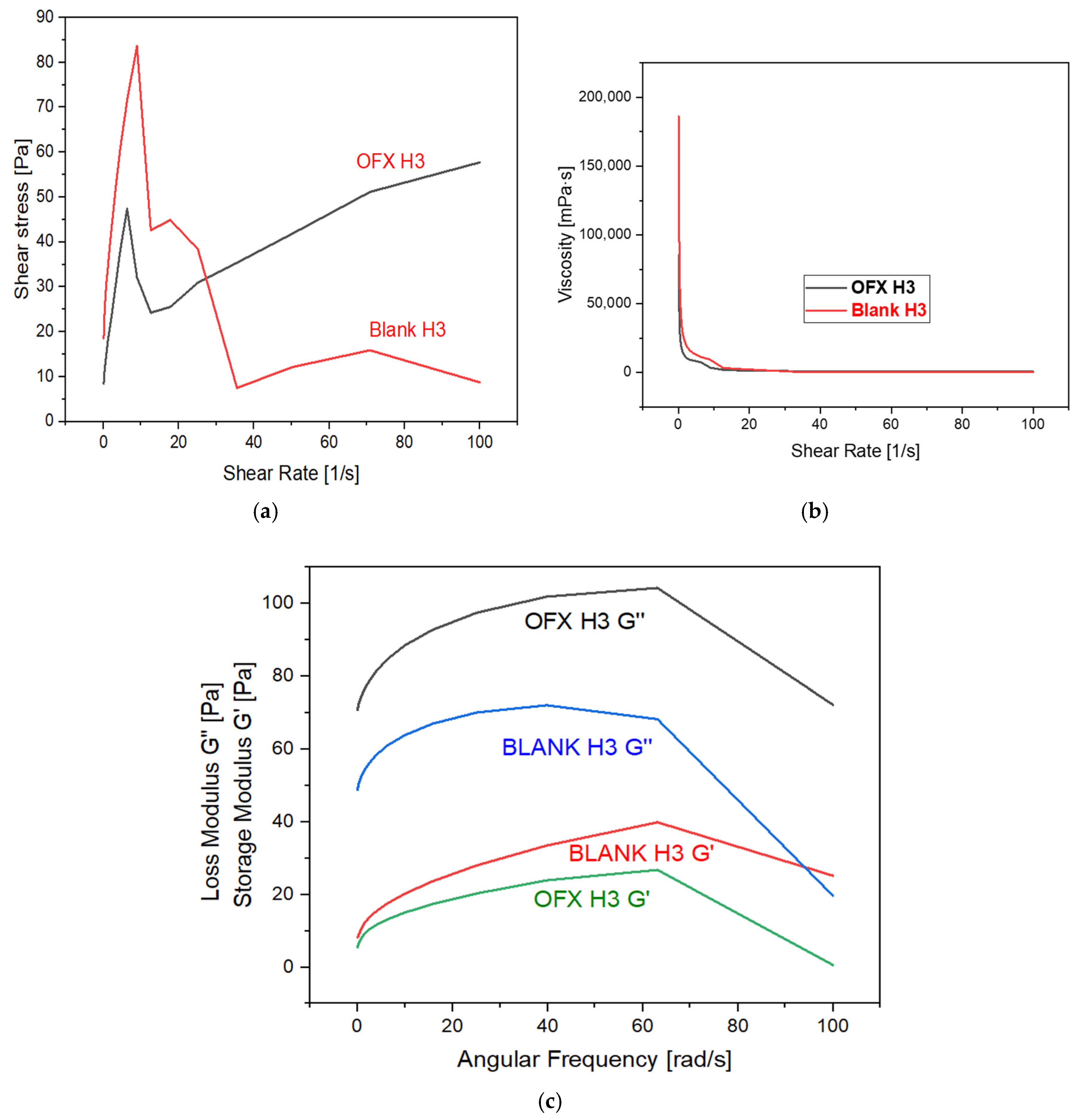
The rheological properties of the samples, OFX H3 and Blank H3, were analysed to understand their mechanical behaviour under the shear and dynamic conditions mentioned in Figure 4. The shear stress versus the shear rate plot shows that OFX H3 exhibits a steady increase in shear stress with an increasing shear rate, indicating stable and predictable behaviour. In contrast, blank H3 displays an initial peak followed by a decline and levelling-off at higher shear rates, which suggests shear-thinning and irregular stress behaviour. Similarly, the viscosity versus shear rate graph reveals that both samples exhibit shear-thinning properties, as the viscosity decreases significantly with an increasing shear rate. However, OFX H3 maintains slightly higher viscosity at comparable shear rates, suggesting more excellent stability under shear. The dynamic moduli analysis highlights key differences in the elastic and viscous components of the two samples. For OFX H3, the loss modulus (G”) dominates over the storage modulus (G’) across the frequency range, indicating a predominantly viscous nature. While both G’ and G” increase with frequency, the viscous component remains the primary contributor. Conversely, blank H3 exhibits relatively higher elastic contributions, as indicated by its higher G’ values compared to OFX H3, suggesting enhanced structural integrity under dynamic conditions. Both samples show frequency-dependent strengthening, with G’ and G” increasing with angular frequency. Thus, OFX H3 demonstrates stable shear-stress growth and more excellent viscosity stability under shear, making it ideal for applications requiring predictable flow properties. Blank H3, with its higher elastic contributions, could be more suitable for applications that require improved structural integrity under deformation [31].
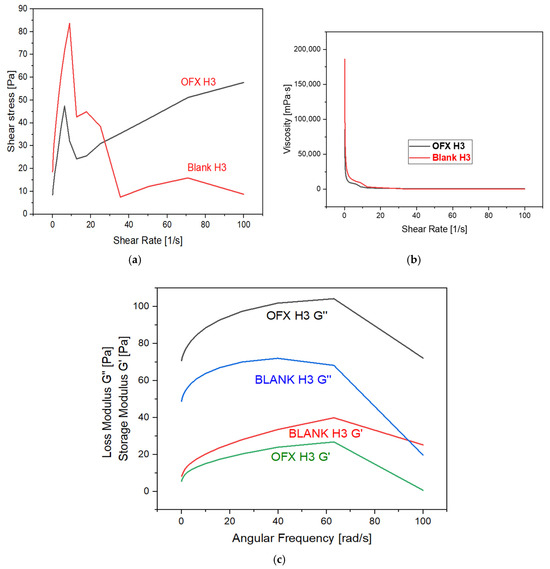
Figure 4.
Graph represents: (a) shear stress vs. shear rate; (b) viscosity vs. shear rate; and (c) storage and loss modulus vs. angular frequency.
2.8. Entrapment Efficiency
The entrapment efficiency of ofloxacin-loaded CS/PVA hydrogel prepared in a 7:3 ratio was 96.7 ± 2.1%. This indicates that the hydrogel matrix effectively encapsulates a significant portion of the drug; unencapsulated drugs might be due to wastage/leftovers in the beaker during formulation preparation.
2.9. In-Vitro Drug Release and Kinetics
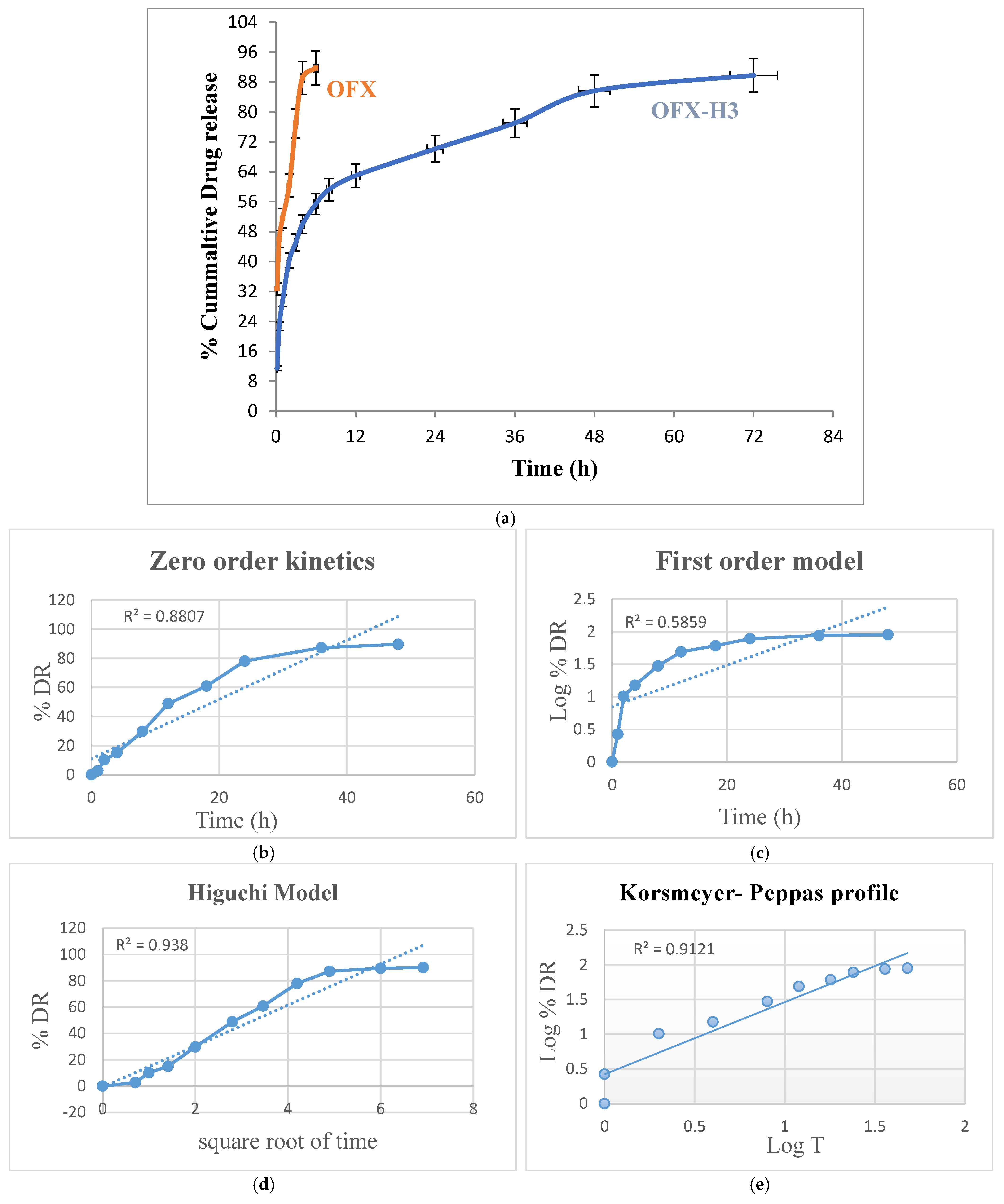
The in vitro drug release study of pure ofloxacin and ofloxacin-loaded CS/PVA hydrogel was conducted over 72 h to evaluate and compare their release profiles. The pure ofloxacin exhibited a rapid release, with approximately 95–100% of the drug released within the first hours. This immediate release can be attributed to the absence of a controlled-release mechanism, as the drug directly dissolved into the release medium [32]. In contrast, the ofloxacin-loaded hydrogel demonstrated a sustained release profile. An initial burst release of approximately 15–25% was observed within the first 1–2 h, likely due to the release of surface-bound or loosely entrapped drug molecules. Following this, the release rate slowed, governed by the diffusion of the drug through the hydrogel matrix and possibly by the swelling of the hydrogel.
By the end of the 72 h study, the hydrogel released approximately 95% of the encapsulated drug in a controlled manner. The burst-release phase ensures an immediate therapeutic effect, while the sustained-release phase maintains drug availability over an extended period, reducing the frequency of drug administration (Figure 5a). The two key features of a delivery system crucial for characterising the drug disintegration or dispersion profile are drug release mechanisms and kinetics. It is now widely recognised that modifications to formulation and processing circumstances, quantitative and qualitative, can affect pharmaceutical systems’ drug release and in vivo performance [33]. One of the logical ways to evaluate and forecast the in vivo bioperformance of novel delivery systems is by using mathematical models. Accordingly, the drug release data of formulations of OFX-H3 were fitted into various models, including Korsmeyer-Peppas, zero order, first order and Higuchi equation [34] Kinetic Modeling to Explain the Release of Medicine from Drug Delivery Systems Kinetic Modeling to Explain the Release of Medicine from Drug Delivery Systems. The best-fitting equation for the developed OFX-loaded hydrogel formulations was determined using the correlation coefficient value (R2). The Higuchi and Korsmeyer Peppas model best described the drug release mechanism of OFX-H3 in Figure 5e, indicating that the drug release is proportionate to time and independent of the amount of drug still in the delivery system. The n value of the Korsmeyer-Peppas plot was 1.048, indicating that the drug release did not follow the Fickian and non-Fickian diffusion-controlled mechanisms. The linear regression in Higuchi’s plot implies that diffusion is one of the mechanisms for drug release. The model proposes super case II transport, which denotes a drastic alteration in the formulation of the polymeric matrix that is appropriate for a diffusion matrix formulation [19]. The findings from investigations on release kinetics are shown in Figure 5b–e.
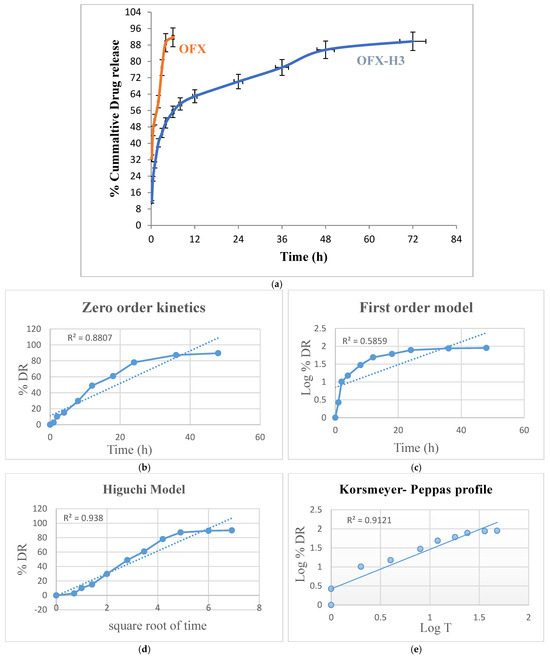
Figure 5.
(a) In vitro drug release of ofloxacin solution and OFX-H3, graph of various release kinetic models of OFX-H3; (b) zero order kinetics; (c) first order model; (d) Higuchi model; (e) Korsmeyer-Peppas profile.
2.10. In Vitro Antimicrobial Study
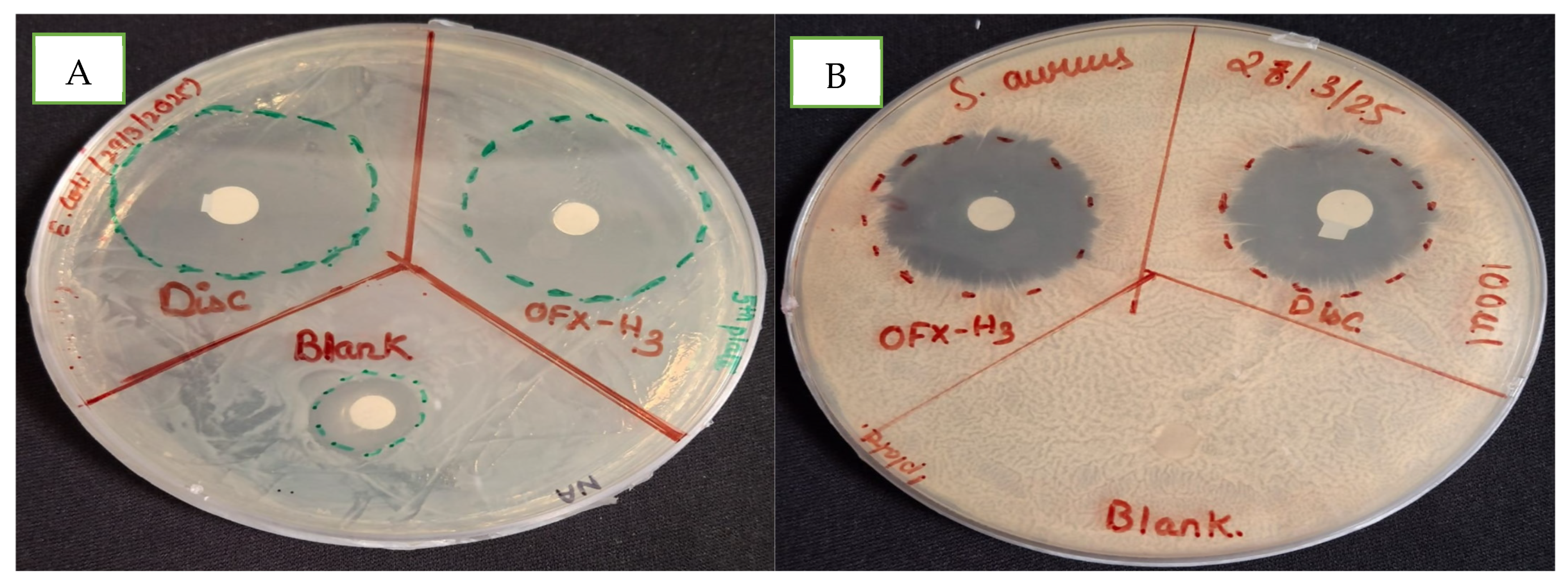
The efficacy of hydrogel formulations was tested against representative gram-negative and gram-positive bacteria (i.e., E. coli and S. aureus) using the agar diffusion method. The antimicrobial performance of the hydrogel was compared with the blank hydrogel and ofloxacin-loaded hydrogel in Figure 6. In Figure 6A, the blank hydrogel denoted by Blank and OFX-H3 showed a 15 mm and 31 mm zone of inhibition (ZOI) in E. coli, confirming the presence of inherent antimicrobial activity in the hydrogel matrix. In Figure 6B, the culture plate of S. aureus had no ZOI in the blank hydrogel denoted by Blank, whereas OFX -H3 had 26 mm compared to the control OFX antibiotic disc (22 mm) resprented as disc. The drug-loaded hydrogel exhibited the largest zone of inhibition (ZOI), reflecting its potent antibacterial activity due to the immediate availability of the drug in the medium. A slightly smaller zone of inhibition in the blank hydrogel in E. coli acts as a synergistic system for OFX-H3. This might be due to the presence of chitosan with antibacterial activity attributable to the controlled release of the drug from the hydrogel matrix. This controlled-release mechanism allows the hydrogel to be used in effective drug concentrations over an extended period [35], making it suitable for the localised treatment of infections.
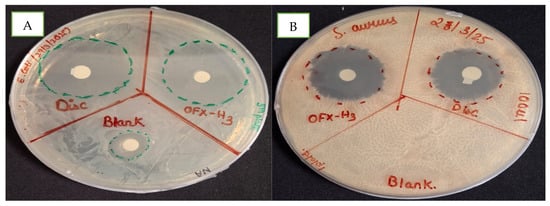
Figure 6.
In vitro antimicrobial study of blank and ofloxacin-loaded hydrogel in (A) E. coli and (B) S. aureus.
2.11. In Vivo Study
An in vivo study was performed to study the effect of formulations on the bacterial load of cervicovaginal mucus and polymorphonuclear cell count after endometrial cytology.
2.11.1. Effect of Treatment on Bacterial Load of Cervicovaginal Mucus
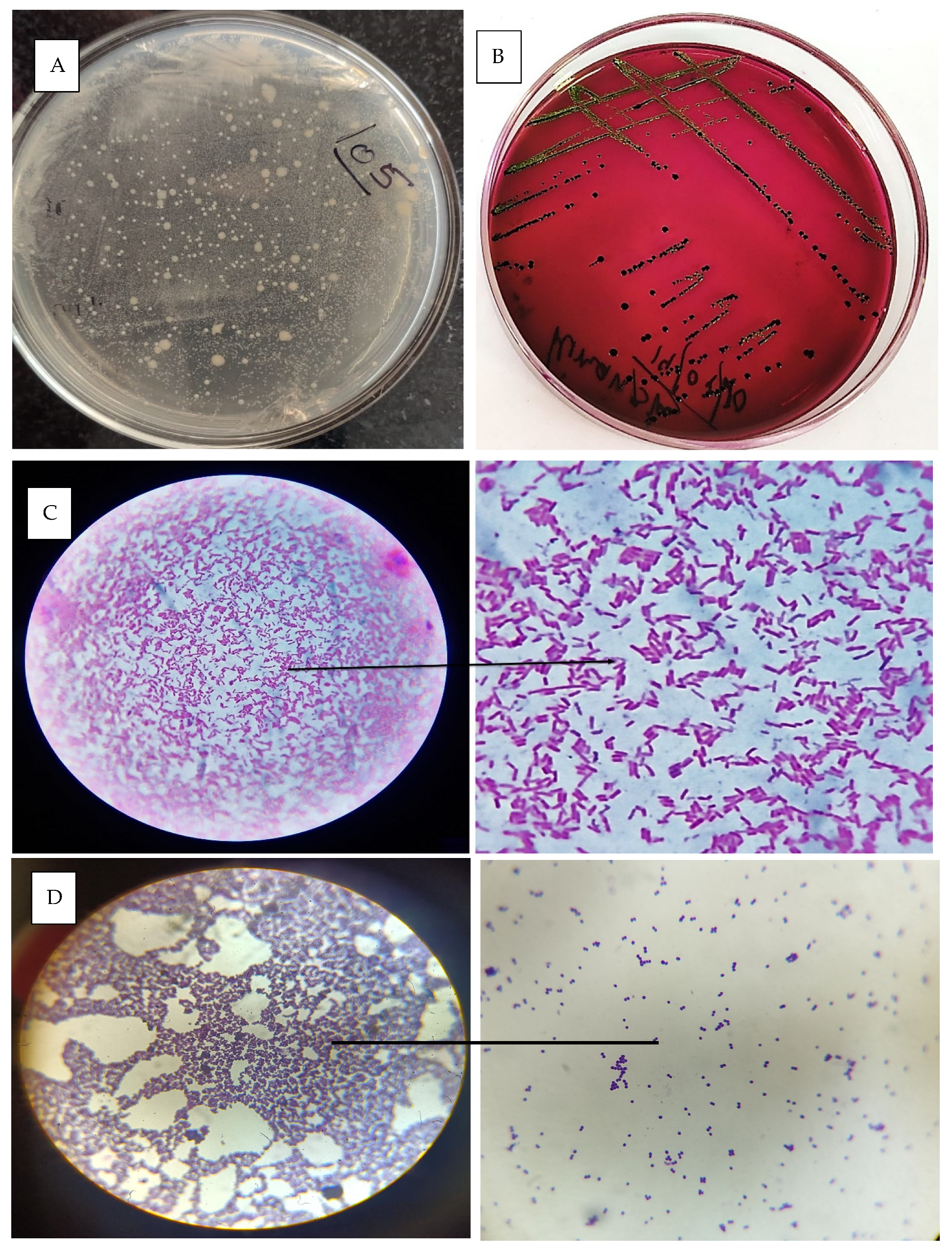
The cervicovaginal mucus samples were collected during oestrus immediately before AI. The effect of treatment on the bacterial load of cervicovaginal mucus discharge during oestrus was analysed. A standard plate of the colony-forming unit was prepared, as shown in Figure 7A, and further experiments were carried out in the control and treated groups. In the control group (Group 1), the bacterial load was slightly reduced, decreasing from 2.53 × 105A CFU/mL pretreatment to 1.98 × 105A CFU/mL posttreatment. However, this reduction was not statistically significant, as indicated by the same superscript letter “A” in both measurements. Conversely, in the hydrogel-treated group (Group 2), there was a substantial and statistically significant reduction in bacterial load, indicated by superscript “B”. The pretreatment load of 2.86 × 105A CFU/mL decreased drastically to 6.37 × 102B CFU/mL posttreatment. These findings suggest that the hydrogel treatment reduced the bacterial load in cervicovaginal mucus compared to the control group.
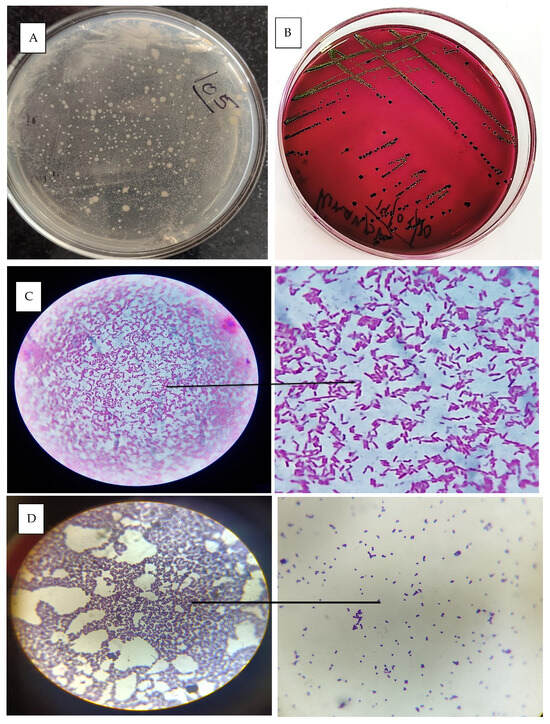
Figure 7.
(A) Standard plate for CFU counts; (B) E. coli colony grown in EMB agar; (C) microscopic image of gram-stained E. coli bacteria; (D) microscopic image of staphylococcus sps. Bacteria.
2.11.2. Bacterial Identification and Isolation
The identification and isolation of bacteria from the cervicovaginal mucus (CVM) of a cow with metritis involve collecting a sterile swab sample from the vaginal canal or cervix and processing it in the laboratory. The sample is inoculated onto selective and nonselective media, such as MLA, BHI and SDA, and incubated under appropriate conditions [36]. Figure 7B represents the growth of E. coli in EMB agar media. A critical step in identification is gram staining, which differentiates bacteria based on their cell wall structure. Gram-positive bacteria, such as Staphylococcus spp., retain the crystal violet stain and appear purple under the microscope (Figure 7D), while gram-negative bacteria, such as Escherichia coli, do not retain the crystal violet but take up the counterstain (safranin) and appear pink (Figure 7C). The most commonly isolated bacterium was E. coli, followed by Staphylococcus spp., mixed infection (gram-positive rods and gram-negative rods) and Candida spp. Brucella spp. was not detected in any CVM samples.
Various approaches were used, and the micro-organisms were isolated using differential media and selective media and identified using the IMViC test. Confirmatory identification was carried out using the molecular identification method. The correlation with the CFU count was established to understand the dominance of the micro-organism [37]. The results were statistically studied, and it was found that E. coli was the most dominant bacteria (42%) present in cattle CVM. However, the prevalence of Staphylococcus sp. (27%) and mixed infection (23%) was also recorded. It was observed that among fungal species, only Candida spp. was present in mucus samples, with a prevalence of only 08%.
2.11.3. Rectum Temperature and pH of Vaginal Mucus
During oestrous, the pH of cervicovaginal mucus and the rectal temperature (°F) were measured in the control and hydrogel treatment groups. There was no discernible change in the cows’ rectal temperatures between the control and treatment groups at both the pretreatment and posttreatment phases. Similarly, there was no discernible change (p < 0.05) between the control and treatment groups’ mean cervicovaginal mucus pH values at the pretreatment stage [38]. In the posttreatment phase, however, the treatment group’s mean cervicovaginal mucus pH value was substantially higher (p < 0.05) than the control group’s. In particular, the control group’s rectal temperature was 101.5 ± 0.1 °F before treatment and 101.3 ± 0.1 °F after treatment, whereas the treatment group’s was 102.06 ± 0.06 °F before treatment and 102.8 ± 0.1 °F after treatment. The control group’s cervicovaginal mucus had a pH of 7.3 ± 0.2 before treatment and 7.4 ± 0.2 after. On the other hand, the pH in the treatment group was 7.6 ± 0.3 before treatment and considerably raised to 8.9 ± 0.2 following therapy. Significant differences (p < 0.05) are indicated by different superscripts (alphabet) within a row.
2.11.4. Cytology for Diagnosis of Subclinical Metritis
The percentage of PMN cells, an indicator of inflammation or infection, was evaluated in the control group (Group 1) and the hydrogel-treated group (Group 2) before and after treatment. In Group 1, the pretreatment PMN percentage was 7.3 ± 0.3, significantly reducing to 2.7 ± 0.4 posttreatment. Similarly, in Group 2, the pretreatment PMN percentage was slightly higher at 7.8 ± 1.0, which was reduced to 2.5 ± 0.2 posttreatment. These results demonstrate a significant decrease in PMN cells in both groups posttreatment, with the hydrogel group showing comparable effectiveness to the control, highlighting its potential to reduce inflammation or infection. A proportion of more than 5% PMNs was observed as threshold volume, which results in a positive diagnosis of subclinical endometritis [39]. Statistical analysis indicated significant differences (p < 0.05) between pretreatment and posttreatment values within each group.
2.11.5. Statistical Analysis
The study’s mean and prevalence were calculated using triplicate experiments and the results were subsequently examined via ANOVA one-way classification using IBM SPSS Statistics 26.0. A significance level of (p < 0.05) was established for the results.
3. Materials and Methods
3.1. Materials
The chitosan (shrimp shells, deacetylated ± 75%) and PVA (60,000–12,500, Dalton, GA, USA) were obtained from HIMEDIA. Ofloxacin was purchased from Sigma Aldrich (St. Louis, MA, USA). The glacial acetic acid and other compounds were graded for analysis. The dairy cows were kept at Sardar Vallabhbhai Patel University of Agriculture and Technology in Meerut (IAEC Protocol: IAEC/SVPUAT/2022/109, Vallabh Vidyanagar, India).
3.2. Methods
3.2.1. Preparation of Hydrogel
The preparation of blank and drug-loaded CS/PVA hydrogels was carried out using the freeze–thaw method. This simple and eco-friendly approach avoids the use of chemical cross-linkers. Initially, 2 g of PVA was dissolved in 20 mL of distilled water, stirred constantly and heated to 80 °C to create a clear and homogeneous PVA solution (approx. 3 h). Separately, a chitosan solution was prepared by soaking 0.2 g chitosan in a 10 mL aqueous acetic acid solution and stirred until complete dissolution. The PVA and chitosan solutions were blended in appropriate ratios to form the hydrogel precursor. For drug-loaded hydrogels, ofloxacin was added to the mixture during the blending process, ensuring uniform dispersion of the drug throughout the matrix. The resulting blank and drug-loaded mixtures were poured into moulds and subjected to a repeated freeze–thaw process. This involved freezing the mixtures at −20 °C for 18 h and thawing at room temperature for another 6 h. This cycle was repeated multiple times, typically 3–5, to induce physical cross-linking between the polymer chains, forming a stable hydrogel network. The freeze–thaw process facilitates hydrogen bonding and crystalline domain formation within the hydrogel, enhancing its mechanical strength and stability [40]. The prepared hydrogels were then carefully removed from the moulds, washed with distilled water to remove unbound drugs or residual solvents and stored for further characterisation and evaluation.
3.2.2. Organoleptic Study
The organoleptic properties of the CS/PVA hydrogels were evaluated to assess their physical and sensory characteristics, ensuring suitability for intrauterine drug delivery. The evaluation included appearance, colour, texture, odour, clarity, homogeneity and hydration. The hydrogels were visually inspected under natural light for uniformity, the presence of bubbles and colour consistency [41].
3.2.3. Gel Fraction
Three sequential repetitions of the freeze–thaw process generated the intertwined hydrogels made of PVA and chitosan. A hydrophobic and entangled polymer network was created by cyclically freezing and thawing using distinct CS/PVA ratios in aqueous solutions. Following F–T cycles, all the samples were vacuum-dried for six h at 50 °C (W0). To remove the soluble components, they were submerged in distilled water for 24 h until their weight remained constant. After that, the gels were vacuum-dried at 50 °C (We). The gel fraction % was calculated using the steps that follow Equation (1).
3.2.4. Porosity
The porosity of the CS/PVA hydrogel was evaluated using a gravimetric method based on the displacement of a liquid in the hydrogel structure. Each ratio of hydrogel was initially weighed (Ww) and after 24 h of drying at 50 °C, the hydrogel was weighed again (Wd). After 48 h of ethanol immersion to reach adsorption saturation, the hydrogels were reweighed (Wl). Equation (2) was used to predict the hydrogel porosity [13].
3.2.5. Swelling Behaviour
The swelling behaviour of the CS/PVA hydrogel was assessed to evaluate its ability to absorb and retain fluid in an in vitro environment. For this, a controlled volume of simulated uterine fluid (or an equivalent fluid mimicking the composition of uterine secretions) was prepared and used in the experiment. A known quantity of the hydrogel, typically in the form of a cylindrical or spherical shape, was immersed in the fluid for a specific period, generally ranging from 1 to 24 h. During this time, the hydrogel was allowed to absorb the fluid under conditions that simulated the uterine environment using pH and temperature. After incubation, the hydrogel was gently removed, excess fluid was carefully blotted from its surface and its weight was measured again to determine the amount of fluid absorbed. The absorption capacity was calculated by comparing the weight of the swollen hydrogel to its initial dry weight. The % of fluid absorption was determined using the following formula.
Wswollen is the weight of the hydrogel after absorbing the uterine fluid and Wdry is the dry weight of the hydrogel. This method is crucial for evaluating the hydrogel’s suitability for localised drug delivery as it simulates the fluid dynamics of the uterine environment and provides insight into the hydrogel’s ability to maintain its structure while absorbing fluid.
Additionally, 50 mg of hydrogel was taken and heated in an oven at 50 °C for 24 h to assess water absorption. The samples were then allowed to come to room temperature in a desiccator. Subsequently, the samples were weighed and incubated for 48 h at 37 °C in 25 mL of PBS with a neutral pH of 7 and water. The samples were then carefully taken out of the buffer solution and weighed and any extra buffer on the sample surfaces was dried with filter paper. The formula (Equation (4)) was used to determine the % of swelling ratio:
where the weights of the dry samples and swollen samples were Wd and Ws, respectively [42].
3.2.6. Morphological Analysis
The surface morphology of the optimised CS/PVA hydrogel and ofloxacin-loaded CS/PVA hydrogel were characterised using scanning electron microscopy (SEM) (ThermoFisher Scientific, Waltham, MA, USA, CURAJ). The hydrogel samples were prepared and carefully dehydrated to prevent structural distortion during imaging. For this, the hydrogels were freeze-dried to the critical drying point. A small layer of gold was applied to the samples after they had dried and been mounted on aluminium stubs, employing dual-sided conductive adhesive to enhance the conductivity and prevent charging effects during SEM imaging.
3.2.7. Physiochemical Analysis
The physicochemical properties of the blank and drug-loaded hydrogel were evaluated to understand its structural integrity, chemical composition and functional attributes. This included the following:
Fourier Transform Infrared Spectroscopy
One milligram of Pure OFX, PVA, CS and OFX-loaded hydrogel (OFX-H3) was mixed with 100 mg of FTIR grade KBr and triturated individually, and the mixture was pressed in the hydraulic pump to make pellets. Then, the samples were analysed in the 400–4000 cm−1 range using Perkin Elmer (Springfield, IL, USA, CURAJ).
DSC
The PVA, CS, OFX and OFX-H3 thermal properties were analysed using DSC (NETZSCH GERATEBAU GmbH, Wittelsbacherstraße, Germany). Approximately 5–10 mg of the dried hydrogel sample was sealed in an aluminium pan and subjected to a heating rate of 10 °C/min under a nitrogen atmosphere. The DSC thermogram was recorded over a temperature range of 25–500 °C.
X-Ray Diffraction
XRD analysis was conducted to determine the crystalline or amorphous nature of the hydrogel. PVA, CS, OFX and OFX-H3 were analysed using an X-ray diffractometer [43].
3.2.8. Mechanical Analysis
Rheology (Viscosity)
A rheometer with a 50 mm diameter was used to analyse the rheological behaviour of the hydrogels at 25 °C. Before estimation, the freshly formed formulations were made and calibrated for a full day. The shear viscosity of the hydrogels was tested over a shear rate range of 0.01−100 1/s to investigate the shear-thinning behaviour. Using the shear strain (γ) range of 0.01–100% at an angular frequency (ω) = 10 rad/s and the ω-range of 0.1−100 rad/s at γ = 5%, respectively, the storage and loss modulus (G′ and G”) values were determined. For every hydrogel, the experiment was conducted three times [44].
3.2.9. Entrapment Efficiency
To determine the content of the drug present within the prepared formulation, preweighed formulations were dissolved in PBS (pH 7) at 37 °C to extract the excess drug. After the entire system was left for 24 h, the solution was filtered through a membrane filter with a pore size of 0.45 μm to remove any debris that might have been present. The clear liquid supernatant was then taken for spectrophotometric drug content determination using a UV–Vis spectrophotometer (UV-1800, Shimadzu, Kyoto, Japan) with a λmax of 294 nm for ofloxacin. Three duplicates of each experiment were conducted. The following formula was used to determine the hydrogels’ % drug entrapment efficiency:
3.2.10. Drug Release and Kinetics
The in vitro release profile of ofloxacin from the CS/PVA hydrogel was determined using a dialysis bag diffusion method. Ofloxacin-loaded hydrogel samples (equivalent to 5 mg of ofloxacin) were placed in dialysis bags (molecular weight cutoff: 12,000–14,000 Da) and immersed in 50 mL of PBS (pH 7) at 37 °C, simulating physiological conditions. The setup was agitated at 100 rpm using a magnetic stirrer to ensure uniform mixing. At certain intervals (1, 2, 4, 8, 12, 24, 36 and 48 h), 2 mL of the release medium was removed and swapped with new PBS to preserve sink conditions.
The samples were analysed for ofloxacin concentration using a UV–Vis spectrophotometer at 294 nm. A standard calibration curve (R2 = 0.9995) was prepared to determine the drug concentration.
We looked at the kinetics of drug release from hydrogel networks using the following mathematical models:
where , , and are the release rate constants; n is the release exponent that identifies the release mechanism; indicates the amount of drug released at time t; and specifies the starting concentration of the drug in the hydrogel [45].
3.2.11. In Vitro Microbial Study
The agar disc diffusion technique was used to conduct antibacterial tests of OFX-loaded hydrogels to combat gram-negative and gram-positive bacterial strains S. aureus and E. coli. This method involved filling petri plates with 30 mL of nutritional agar, followed by the even distribution of 100 μL of bacterial suspension. After that, 50 mg of OFX-H3, the blank hydrogel and the control (CA) were placed into the nutritional agar petri dishes; later, they were kept in an incubator at 37 °C. Following a 24 h incubation period, each drug concentration’s inhibitory zone diameter was evaluated. Three duplicates of the experiment were conducted [46].
3.2.12. In-Vivo Study
Animal Management
The Institutional Animal Ethics Committee approved all animal procedures (IAEC Protocol: IAEC/SVPUAT/2022/109). Every dairy cow in this experiment was kept at the Sardar Vallabhbhai Patel University of Agriculture and Technology in Meerut (U.P.). Dairy cows weighing 500 and 600 kg were given a mixed military ration twice daily to fulfil or surpass their nutritional needs.
Treatment and Sampling
Dairy cows with metritis (n = 12) were randomly assigned to two groups. The control group received a 3 mg/kg drug solution in PBS, as ofloxacin has pH-dependent solubility. The other group (experiment group) also received hydrogel based on their body weight. Treatments were conducted daily for 21 days once the formulation was administered to both groups. Five to thirty minutes before artificial insemination, a cervical mucous discharge (CMD) swab was obtained from each animal. The perineal area was scrubbed three or four times with soap and dried after the animal was restrained and its tail was secured. A sterile double-guarded swab instrument (Hi-media, Thane, India) was used to gather swabs for CMD. Under the guidance of palpation per rectum, a sterile swab was placed through the vagina into the cervical canal lumen. The traditional artificial insemination method was used to withdraw the swab under rectal guidance after being spun three or four times on the mucosa while moving forwards and backwards. Within 3 h of sampling, all the swabs were put in a transport medium and brought to the lab for microbiological analysis at room temperature.
Isolation and Identification of Bacteria
MacConkey lactose agar, brain heart infusion agar and Sabouraud dextrose agar plates were stained with swabs (Hi-media, India). The MacConkey agar and brain heart infusion agar plates were incubated in an aerobic incubator for 24 h at 37 °C. Incubation temperatures for the Sabouraud dextrose agar plate plated to isolate yeast sp. and mould sp. were 25 °C and 37 °C, respectively. The bacteria were identified using the isolates’ growth, biochemistry, colony morphology and microscopic morphology. Before being used for molecular and resistance testing, the identified colonies were kept in glycerol stock at −20 °C. After that, they were cultivated for 24 h on blood agar at 37 °C so that pure colonies could be subcultured for a further 24 h [47].
Rectum Temperature and pH of Vaginal Mucus
Rectal temperature and vaginal pH readings were taken every day at the same time from day 0 to day 21 and evaluated by a veterinarian [48].
Cytology for Diagnosis of Subclinical Endometritis
In this technique, cytobrush (Marfair Surgical Corporation, Ludhiana, India) was screwed on to the piston of the cytobrush gun and was inserted into the uterine cavity aseptically. The cytobrush was pulled back in the cytobrush cannon after being gently rolled against the uterine endometrium. The brush was rolled onto a sterile glass microscopic slide to prepare the cytologic slides and allowed to dry. In a lab, slides were fixed and stained using HiMedia. The percentage of polymorphonuclear cells was ascertained by counting cells under an oil immersion microscope (Olympus CX21; Olympus Corporation, Tokyo, Japan). The threshold volume for a positive diagnosis of subclinical endometritis was set at 5% PMN or higher [39,49].
4. Conclusions
Developing ofloxacin-loaded CS/PVA hydrogel offers a promising solution for treating bovine metritis. The hydrogel demonstrated efficient drug entrapment of more than 95% and a controlled, sustained release profile for up to 72 h, ensuring prolonged therapeutic action at the infection site while reducing the need for frequent administrations. Optimised hydrogel’s stable and elastic behaviour and enhanced swelling properties maintain structural integrity, provide moisture to the infected area and improve drug diffusion. Antibacterial assays showed mm inhibition zones against E. coli and S. aureus, confirming strong antimicrobial efficacy. In vivo studies revealed a 99.78% reduction in bacterial load and an improved clinical recovery rate, with no observed adverse effects. The localised drug delivery approach ensures a high concentration of the therapeutic agent in the uterus while minimising systemic side effects, making it a safer and more efficient alternative to conventional treatments. Furthermore, the hydrogel is cost-effective, easy to apply and reduces handling stress in animals, making it a practical solution for veterinary medicine. Overall, the ofloxacin-loaded CS/PVA hydrogel represents a significant advancement in managing bovine metritis, with the potential for commercialisation and adoption in clinical settings to improve treatment outcomes and animal welfare.
Author Contributions
Writing—original draft preparation, conceptualization, methodology, software, validation: P.K.; investigation, resources, data curation: M.K.S.; A.T.; J.P.; supervision, writing—review and editing, funding acquisition: A.K.G. All authors have read and agreed to the published version of the manuscript.
Funding
Department of Biotechnology (BT/PR46598/AAQ/1/938/2022.) and Indian Council of Medical Research (RBMH/FW/2021/9).
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Animal Ethics Committee of Sardar Vallabhbhai Patel University of Agriculture and Technology, Meerut (India) vide protocol code no. IAEC/SVPUAT/2023/125, dated 6 June 2023 for studies involving animals.
Informed Consent Statement
Not Applicable.
Data Availability Statement
No new data were created due to privacy or ethical restrictions.
Acknowledgments
We thank the Department of Biotechnology, ICMR, Central University of Rajasthan, Sardar Vallabhbhai Patel University of Agriculture and Technology.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| PVA | Poly(vinyl alcohol) |
| CS | Chitosan |
| OFX | Ofloxacin |
| DNA | Deoxyribonucleic acid |
| F-T cycles | Freeze–thaw cycles |
| DSC | Differential Scanning Calorimetry |
| FTIR | Fourier Transform Infrared Spectroscopy |
| XRD | X-Ray Diffraction |
| PBS | Phosphate buffer saline |
| S. aureus | Staphylococcus aureus |
| E. coli | Escherichia coli |
| CA | Control group |
| CMD | Cervical mucous discharge |
| MLA | MacConkey lactose agar |
| BHI | Brain heart infusion agar |
| SDA | Sabouraud dextrose agar |
| AI | Artificial insemination |
| CFU | Colony forming unit |
| CVM | Cervicovaginal mucus |
| °F | Degree Fahrenheit |
| SEM | Scanning electron microscopy |
References
- Negasee, K.A. Clinical Metritis and Endometritis in Diary Cattle: A Review. Vet. Med.-Open J. 2020, 5, 51–56. [Google Scholar] [CrossRef]
- Thulasiraman, S.; Thulasiraman, M.S.; Gunasekar, M.; Narayansamy, A.; Sampathkumar, K.U.; Kumar, R.; Alam, K. Uterine Infections/Metritis. In Periparturient Diseases of Cattle; Wiley: Hoboken, NJ, USA, 2024; pp. 135–151. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Molinari, P.C.C.; Ormsby, T.J.R.; Bromfield, J.J. Preventing postpartum uterine disease in dairy cattle depends on avoiding, tolerating and resisting pathogenic bacteria. Theriogenology 2020, 150, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, I.M.; Owens, S.E. Postpartum uterine infection and endometritis in dairy cattle. Anim. Reprod. 2017, 14, 622–629. [Google Scholar] [CrossRef]
- Haimerl, P.; Heuwieser, W. Invited review: Antibiotic treatment of metritis in dairy cows: A systematic approach. J. Dairy Sci. 2014, 97, 6649–6661. [Google Scholar] [CrossRef]
- Adegbeye, M.J.; Adetuyi, B.O.; Igirigi, A.I.; Adisa, A.; Palangi, V.; Aiyedun, S.; Alvarado-Ramírez, E.R.; Elghandour, M.M.M.Y.; Molina, O.M.; Oladipo, A.A.; et al. Comprehensive insights into antibiotic residues in livestock products: Distribution, factors, challenges, opportunities, and implications for food safety and public health. Food Control. 2024, 163, 110545. [Google Scholar] [CrossRef]
- Kumari, P.; Goyal, A.K. Challenges and opportunities in intravesical drug delivery approaches for the treatment of lower urinary diseases. J. Drug Deliv. Sci. Technol. 2024, 100, 106110. [Google Scholar] [CrossRef]
- Giguère, S.; Dowling, P.M. Fluoroquinolones. In Antimicrobial Therapy in Veterinary Medicine; Wiley: Hoboken, NJ, USA, 2013; pp. 295–314. [Google Scholar] [CrossRef]
- Kumar, A.; Jain, S.K.; Mishra, D.K.; Gautam, R. Influence of Drug Properties and Routes of Drug Administration on Design of Sustained and Controlled Release Systems. In Novel Carrier Systems for Targeted and Controlled Drug Delivery; Springer Nature: Singapore, 2024; pp. 1–46. [Google Scholar] [CrossRef]
- van Staden, D.; Gerber, M.; Lemmer, H.J.R. The Application of Nano Drug Delivery Systems in Female Upper Genital Tract Disorders. Pharmaceutics 2024, 16, 1475. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation; characterization; applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Oh, G.W.; Kim, S.C.; Cho, K.J.; Ko, S.C.; Lee, J.M.; Yim, M.J.; Kim, K.W.; Kim, H.S.; Kim, J.Y.; Lee, D.S.; et al. Poly(vinyl alcohol)/chitosan hydrogel incorporating chitooligosaccharide-gentisic acid conjugate with antioxidant and antibacterial properties as a potential wound dressing. Int. J. Biol. Macromol. 2024, 255, 128047. [Google Scholar] [CrossRef]
- Suflet, D.M.; Popescu, I.; Pelin, I.M.; Ichim, D.L.; Daraba, O.M.; Constantin, M.; Fundueanu, G. Dual Cross-Linked Chitosan/PVA Hydrogels Containing Silver Nanoparticles with Antimicrobial Properties. Pharmaceutics 2021, 13, 1461. [Google Scholar] [CrossRef]
- Liang, X.; Zhong, H.-J.; Ding, H.; Yu, B.; Ma, X.; Liu, X.; Chong, C.-M.; He, J. Polyvinyl Alcohol (PVA)-Based Hydrogels: Recent Progress in Fabrication, Properties, and Multifunctional Applications. Polymers 2024, 16, 2755. [Google Scholar] [CrossRef] [PubMed]
- Eivazzadeh-Keihan, R.; Noruzi, E.B.; Mehrban, S.F.; Aliabadi, H.A.M.; Karimi, M.; Mohammadi, A.; Maleki, A.; Mahdavi, M.; Larijani, B.; Shalan, A.E. Review: The latest advances in biomedical applications of chitosan hydrogel as a powerful natural structure with eye-catching biological properties. J. Mater. Sci. 2022, 57, 3855–3891. [Google Scholar] [CrossRef]
- Pinto, C.; Méndez, L.; Camacho-Rodríguez, B.; Silva-Cote, I. Antibacterial PVA/Chitosan/alginate/Meropenem-based hydrogel as a potential therapeutic strategy for chronic ulcers infections. Int. J. Polym. Mater. Polym. Biomater. 2024, 73, 1021–1033. [Google Scholar] [CrossRef]
- Khan, A.; Andleeb, A.; Azam, M.; Tehseen, S.; Mehmood, A.; Yar, M. Aloe vera and ofloxacin incorporated chitosan hydrogels show antibacterial activity, stimulate angiogenesis and accelerate wound healing in full thickness rat model. J. Biomed. Mater. Res. Part B Appl. Biomater. 2023, 111, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Zitouni, M.A.; Slimane, S.B.K. Preparation and Characterization of Hydrogels Based on Chitsoan/Polyvinyl Alcohol Blends. Adv. Mater. Res. 2015, 1105, 203–207. [Google Scholar] [CrossRef]
- Khairan, K.; Hasan, M.; Idroes, R.; Diah, M. Fabrication and Evaluation of Polyvinyl Alcohol/Corn Starch/Patchouli Oil Hydrogel Films Loaded with Silver Nanoparticles Biosynthesized in Pogostemon cablin Benth Leaves’ Extract. Molecules 2023, 28, 2020. [Google Scholar] [CrossRef]
- Khorasani, M.T.; Joorabloo, A.; Adeli, H.; Mansoori-Moghadam, Z.; Moghaddam, A. Design and optimization of process parameters of polyvinyl (alcohol)/chitosan/nano zinc oxide hydrogels as wound healing materials. Carbohydr. Polym. 2019, 207, 542–554. [Google Scholar] [CrossRef]
- Farazin, A.; Mohammadimehr, M.; Ghasemi, A.H.; Naeimi, H. Design; preparation, and characterization of CS/PVA/SA hydrogels modified with mesoporous Ag2O/SiO2 and curcumin nanoparticles for green, biocompatible, and antibacterial biopolymer film. RSC Adv. 2021, 11, 32775–32791. [Google Scholar] [CrossRef]
- Lin, S.-P.; Lo, K.-Y.; Tseng, T.-N.; Liu, J.-M.; Shih, T.-Y.; Cheng, K.-C. Evaluation of PVA/dextran/chitosan hydrogel for wound dressing. Cell. Polym. 2019, 38, 15–30. [Google Scholar] [CrossRef]
- Long, J.; Etxeberria, A.E.; Kornelsen, C.; Nand, A.V.; Ray, S.; Bunt, C.R.; Seyfoddin, A. Development of a Long-Term Drug Delivery System with Levonorgestrel-Loaded Chitosan Microspheres Embedded in Poly(vinyl alcohol) Hydrogel. ACS Appl. Bio Mater. 2019, 2, 2766–2779. [Google Scholar] [CrossRef]
- Shahzadi, U.; Zeeshan, R.; Tabassum, S.; Khadim, H.; Arshad, M.; Ansari, A.A.; Safi, S.Z.; Haq, R.I.U.; Asif, A. Physico-chemical properties and in-vitro biocompatibility of thermo-sensitive hydrogel developed with enhanced antimicrobial activity for soft tissue engineering. Polym. Adv. Technol. 2023, 34, 3870–3884. [Google Scholar] [CrossRef]
- Dolezel, R.; Dolezel, T.R.; Palenik, T.; Cech, S.; Kohoutova, L.; Vyskocil, M. Bacterial contamination of the uterus in cows with various clinical types of metritis and endometritis and use of hydrogen peroxide for intrauterine treatment. Vet. Med. 2010, 55, 504–511. [Google Scholar] [CrossRef]
- Vickers, L.A.; Burfeind, O.; von Keyserlingk, M.A.G.; Veira, D.M.; Weary, D.M.; Heuwieser, W. Technical note: Comparison of rectal and vaginal temperatures in lactating dairy cows. J. Dairy Sci. 2010, 93, 5246–5251. [Google Scholar] [CrossRef]
- Jeon, S.J.; Ma, Z.; Kang, M.; Galvão, K.N.; Jeong, K.C. Application of chitosan microparticles for treatment of metritis and in vivo evaluation of broad spectrum antimicrobial activity in cow uteri. Biomaterials 2016, 110, 71–80. [Google Scholar] [CrossRef]
- Kasimanickam, R.; Duffield, T.; Foster, R.; Gartley, C.; Leslie, K.; Walton, J.; Johnson, W. Endometrial cytology and ultrasonography for the detection of subclinical endometritis in postpartum dairy cows. Theriogenology 2004, 62, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Garnica-Palafox, I.M.; Estrella-Monroy, H.O.; Benítez-Martínez, J.A.; Bizarro, M.; Sánchez-Arévalo, F.M. Influence of Genipin and Multi-walled Carbon Nanotubes on the Dye Capture Response of CS/PVA Hybrid Hydrogels. J. Polym. Environ. 2022, 30, 4690–4709. [Google Scholar] [CrossRef]
- Figueroa-Pizano, M.D.; Vélaz, I.; Peñas, F.J.; Zavala-Rivera, P.; Rosas-Durazo, A.J.; Maldonado-Arce, A.D.; Martínez-Barbosa, M.E. Effect of freeze-thawing conditions for preparation of chitosan-poly (vinyl alcohol) hydrogels and drug release studies. Carbohydr. Polym. 2018, 195, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Rynkowska, E.; Fatyeyeva, K.; Kujawa, J. Chemically and Thermally Crosslinked PVA-Based Membranes: Effect on Swelling and Transport Behavior. Polymers 2019, 11, 1799. [Google Scholar] [CrossRef]
- Vo, T.M.; Piroonpan, T.; Preuksarattanawut, C.; Kobayashi, T.; Potiyaraj, P. Characterization of pH-responsive high molecular-weight chitosan/poly (vinyl alcohol) hydrogel prepared by gamma irradiation for localizing drug release. Bioresour. Bioprocess. 2022, 9, 89. [Google Scholar] [CrossRef]
- Rizwan, M.; Rizwan, R.M.; Yahya, R.; Hassan, A.; Yar, M.; Azzahari, A.D.; Selvanathan, V.; Sonsudin, F.; Abouloula, C.N. pH sensitive hydrogels in drug delivery: Brief history, properties, swelling, and release mechanism, material selection and applications. Polymers 2017, 9, 137. [Google Scholar] [CrossRef]
- Farid, O.; Farid, F.O.; Mansour, F.; Habib, M.; Robinson, J.; Tarleton, S. Investigating the sorption influence of poly(vinyl alcohol) (PVA) at different crosslinking content. J. Environ. Chem. Eng. 2016, 4, 293–298. [Google Scholar] [CrossRef]
- Bi, S.; Wang, P.; Hu, S.; Li, S.; Pang, J.; Zhou, Z.; Sun, G.; Huang, L.; Cheng, X.; Xing, S.; et al. Construction of physical-crosslink chitosan/PVA double-network hydrogel with surface mineralization for bone repair. Carbohydr. Polym. 2019, 224, 115176. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Khan, N.M.S.; Ranjha, N.M. Effect of degree of cross-linking on swelling and on drug release of low viscous chitosan/poly(vinyl alcohol) hydrogels. Polym. Bull. 2014, 71, 2133–2158. [Google Scholar] [CrossRef]
- Rade, P.P.; Garnaik, B. Ofloxacin-Loaded PLLA Nanofibrous Mats for Wound Dressing Applications. ACS Appl. Bio Mater. 2020, 3, 6648–6660. [Google Scholar] [CrossRef]
- Hyder, M.N.; Chen, P. Pervaporation dehydration of ethylene glycol with chitosan-poly(vinyl alcohol) blend membranes: Effect of CS-PVA blending ratios. J. Memb. Sci. 2009, 340, 171–180. [Google Scholar] [CrossRef]
- Sahoo, S.; Sahoo, C.K.S.; Chakraborti, C.K.; Mishra, S.C.; Nanda, U.N.; Naik, S. FTIR and XRD investigations of some fluoroquinolones. Int. J. Pharm. Pharm. Sci. 2011, 3, 165–170. [Google Scholar]
- Elashmawi, I.S.; Ismail, A.M.; Abdelghany, A.M. The incorporation of polypyrrole (PPy) in CS/PVA composite films to enhance the structural, optical, and the electrical conductivity. Polym. Bull. 2023, 80, 11379–11399. [Google Scholar] [CrossRef]
- Peng, L.; Zhou, Y.; Lu, W.; Zhu, W.; Li, Y.; Chen, K.; Zhang, G.; Xu, J.; Deng, Z.; Wang, D. Characterization of a novel polyvinyl alcohol/chitosan porous hydrogel combined with bone marrow mesenchymal stem cells and its application in articular cartilage repair. BMC Musculoskelet. Disord. 2019, 20, 257. [Google Scholar] [CrossRef]
- Tang, Y.F.; Du, Y.M.; Hu, X.W.; Shi, X.W.; Kennedy, J.F. Rheological characterisation of a novel thermosensitive chitosan/poly(vinyl alcohol) blend hydrogel. Carbohydr. Polym. 2007, 67, 491–499. [Google Scholar] [CrossRef]
- Varma, M.V.S.; Kaushal, A.M.; Garg, A.; Garg, S. Factors Affecting Mechanism and Kinetics of Drug Release from Matrix-Based Oral Controlled Drug Delivery Systems. Am. J. Drug Deliv. 2004, 2, 43–57. [Google Scholar] [CrossRef]
- Bowen, W.E.; Wang, Q.; Wuelfing, W.P.; Thomas, D.L.; Nelson, E.D.; Mao, Y.; Hill, B.; Thompson, M.; Gallagher, K.; Reed, R.A. A Biopharmaceutical Classification System Approach to Dissolution: Mechanisms and Strategies. In Biopharmaceutics Applications in Drug Development; Springer: Boston, MA, USA, 2008; pp. 290–316. [Google Scholar] [CrossRef]
- Askarizadeh, M.; Askarizadeh, N.M.; Esfandiari, N.; Honarvar, B.; Sajadian, S.A.; Azdarpour, A. Kinetic Modeling to Explain the Release of Medicine from Drug Delivery Systems. ChemBioEng Rev. 2023, 10, 1006–1049. [Google Scholar] [CrossRef]
- Sokker, H.H.; Ghaffar, A.M.A.; Gad, Y.H.; Aly, A.S. Synthesis and characterization of hydrogels based on grafted chitosan for the controlled drug release. Carbohydr. Polym. 2009, 75, 222–229. [Google Scholar] [CrossRef]
- Mekibib, B.; Belachew, M.; Asrade, B.; Abebe, R. Isolation; identification, and antibiogram profiles of bacteria from dairy cows with postpartum uterine infection in southern Ethiopia. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Basak, S.; Shetty, P.H. Conventional Microbial Counting and Identification Techniques. In Techniques to Measure Food Safety and Quality; Springer International Publishing: Cham, Switzerland, 2021; pp. 69–89. [Google Scholar] [CrossRef]
- Scott, H.M.; Atkins, G.; Willows, B.; McGregor, R. Effects of 2 commercially-available 9-way killed vaccines on milk production and rectal temperature in Holstein-Friesian dairy cows. Can. Vet. J. 2001, 42, 793–798. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).