Abstract
Background/Objectives: Bacterial infections remain among the top ten major public health concerns, contributing to a high number of incidences of disease and mortality worldwide, exacerbated by the rise of multidrug-resistant bacteria (MDRB). Consequently, it is crucial to develop novel antimicrobial strategies, including the use of functional nanoparticles. Gold nanoparticles (GNPs) have emerged as promising candidates due to their unique optical properties, particularly their ability to efficiently convert absorbed light into heat through the photothermal (PT) effect, which can be harnessed for bacteria eradication. Methods: Chitosan was modified with 3-mercaptopropionic acid to introduce sulfur groups, facilitating gold deposition onto chitosan nanoparticle (TCNPs) surface. The gold shell was subsequently formed via a seed-mediated method, wherein gold seeds were adsorbed onto TCNPs and further grown to form the shell. Photothermal effect on the bacterial viability was evaluated. Results: TCNPs with a size of 178 nm and spherical morphology were obtained. After the gold shell (TCNP@Au) exhibited a photothermal conversion efficiency of 31%, making them a promising photothermal agent for bacterial clearance. Notably, the viability of Escherichia coli was significantly reduced in the presence of TCNP@Au and was almost eradicated upon PT treatment. In contrast, TCNP@Aus were non-toxic for Staphylococcus aureus. Conclusions: TCNP@Au demonstrated favorable photothermal properties, presenting a novel nanoplatform for antibacterial applications, particularly against Gram-negative bacteria. However, further investigation is required to optimize the PT-based strategies against Gram-positive bacteria, such as S. aureus.
1. Introduction
Today, bacterial infections remain a serious public health concern, a challenge further exacerbated by the emergence of multidrug-resistant bacteria (MDRBs). The principal MDRBs include both Gram-positive and Gram-negative bacteria, such as S. aureus, S. pneumoniae, E. faecium, and E. faecalis (Gram-positive) and A. baumannii, E. coli, P. aeruginosa, and K. pneumoniae (Gram-negative) [1]. Patients infected with MDRBs face: (i) an increased risk of mortality. For instance, in 2019, approximately 1.3 million deaths globally were attributed to MDRBs, and this number could rise to 10 million by 2050 if no effective action are taken to combat these microorganisms [1,2]; and (ii) prolonged illness, leading to rising medical costs [3], due to the high expense associated with accessing next-generation antibiotics and extended hospitalization periods. In this regard, burden estimates from World Organization for Animal Health and World Bank Group indicate that MDRBs cost health systems approximately 66 billion dollars per year, with projections suggesting that these costs could continue to rise over the next 25 years [2,3]. Therefore, it is of paramount importance to seek alternative approaches to combat these bacteria. This effort includes the discovery of new antimicrobial drugs, either synthesized or isolated from natural sources, as well as the development of novel strategies for bacterial clearance, including the design and fabrication of functional nanoparticles (FNPs). From a broader perspective, the antimicrobial effect of the FNPs can be an intrinsic property, depending on their chemical composition and their ability to respond to external physical stimuli. Furthermore, the antimicrobial activity of previously ineffective or “obsolete” drugs can be enhanced by loading them into FNPs, enabling targeted delivery to bacteria.
In this regard, plasmonic nanoparticles have emerged as promising candidates for antimicrobial applications, with gold nanoparticles (GNPs) standing out among them due to their optical properties, stability, and biocompatibility [4,5]. Their unique optical property is characterized by a strong absorption band, known as the surface plasmon resonance (SPR), which arises from the collective oscillation of conduction band electrons at the surface of GNPs when they resonate with incident electromagnetic radiation of a specific energy. There are two remarkable characteristics of GNPs,(i) the SPR of GNPs can be tuned across a wide wavelength range, from visible to NIR region, by simply varying their size and shape [6]. For instance, spherical gold nanoparticles (SGNP) exhibit SPR at approximately 520 nm, with the absorption peak broadening and red shifting as particle size increases. In contrast, anisotropic gold nanostructures -such as nanorods, cubes, and core-shell architectures- display broader SPR bands in the NIR region, which can be further tunable by adjusting their aspect ratio; (ii) all these GNPs can efficiently convert the absorbed light into heat through nonradiative electron relaxation, dissipating the thermal energy into the surrounding medium [7]. This phenomenon, known as the photothermal effect (PT), is a critical property for alternative therapeutic applications, such as photothermal therapy (PTT), which has been explored for inactivation of viruses, fungi, and tumor cells and even MRDBs [6,8,9].
In the present report, gold nanoshell (GNSs) grown on a dielectric template based on chitosan nanoparticles are proposed for the clearance of Gram-positive and Gram-negative bacteria via photothermal effect. Several studies have reported the synthesis of GNSs on dielectric templates, such as AuS2 SiO2, PLGA and polystyrene NPs [9,10,11]. However, no reports were found, at least within the reviewed literature, where chitosan nanoparticles have been used as dielectric template for the growth of a gold shell.
2. Results
2.1. Chitosan Modification and Synthesis of Chitosan Nanoparticles (TCNP)
2.1.1. Chitosan Modification
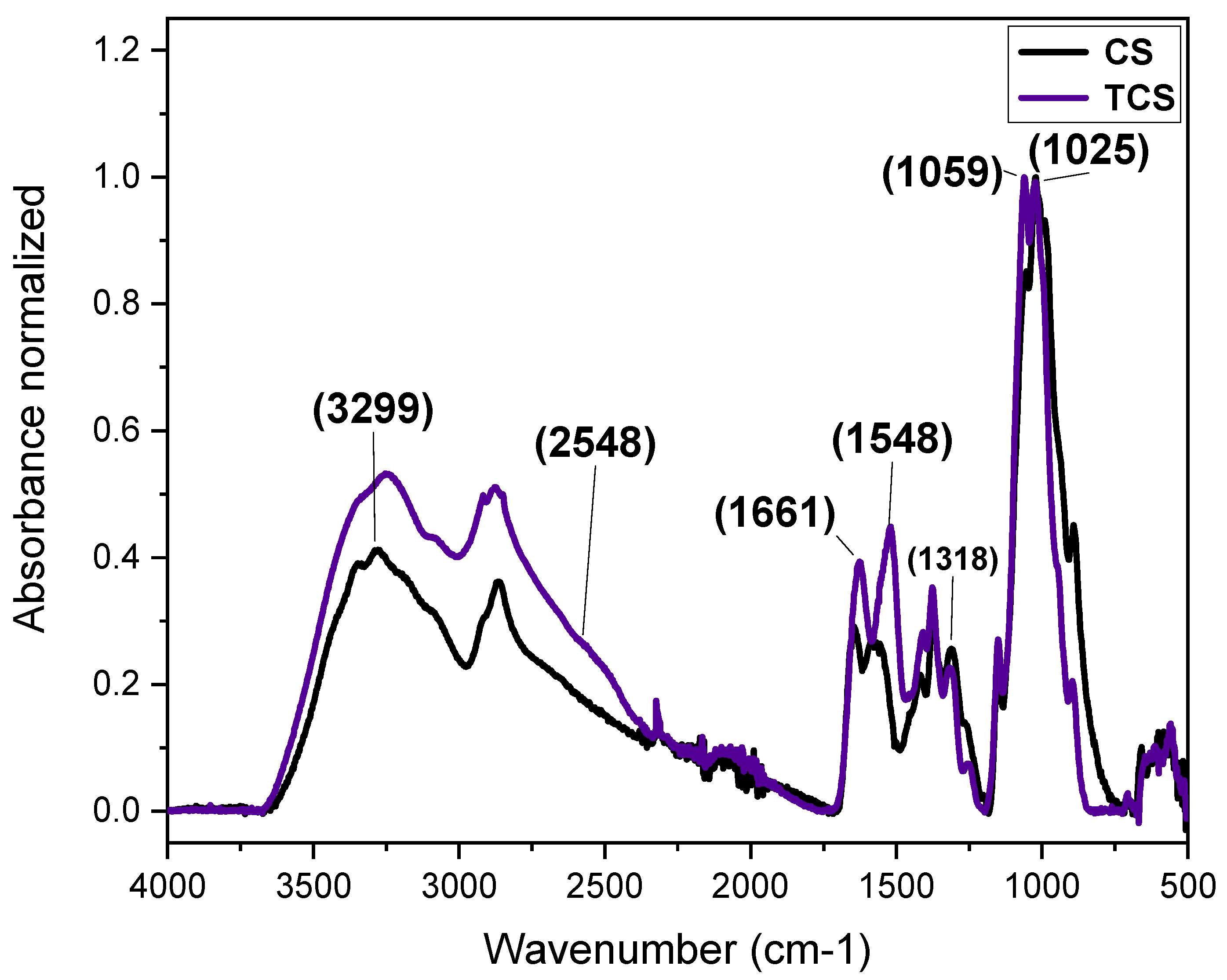
The chemical modification of chitosan is typically performed to alter its physicochemical properties in aqueous media, including its hydrophilic/hydrophobic balance and reactivity in water. Given the strong affinity of sulfur for gold [12,13], 3-mercaptopropionic acid (3-MPA) was conjugated to the amine groups of chitosan to facilitate the growth of a gold shell, achieved via a gold seed-mediated growth process, onto the surface of TCNP. The chemical attachment of 3-MPA to chitosan was confirmed by FTIR in ATR mode. The FTIR spectra of native CS and TCS are shown in Figure 1. The black line corresponds to the FTIR spectrum of native CS, which shows characteristic stretching bands centered at 3299 cm−1, attributed to –OH and -NH2 groups. The stretching vibrations of -CH2− o in the pyranose ring and –CH3 of acetyl groups of CS appeared at the range of 2950–2780 cm−1 [14]. The peak at 1661 cm−1 corresponds to C=O stretching vibration of amide I, while the peak at 1578 cm−1 result from the overlapping of -NH in amide II and -NH2 groups [14,15,16]. Additional bands at 1420 cm−1 and 1058 cm−1 are correspond to the asymmetric bending vibrations of -CH2− and stretching C–O–C of pyranose rings, respectively. Peaks at 1318 cm−1 and 1025 cm−1 are related to C–N stretching (amide III) and the symmetric C–O–C stretching [17,18]. In the spectrum of TCs (blue line), increased peak intensities for amide I and amide II, –CH2−, C=O, and amide III indicate successful chemical modification [19]. Additionally, a weak shoulder at 2548 cm−1 confirms the presence of thiol (-SH) group [12], corroborating the successful thiolation.
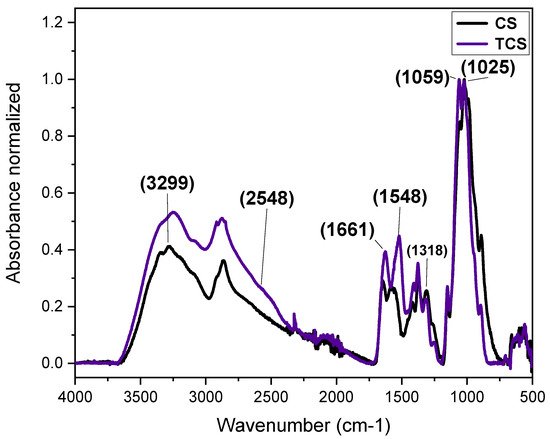
Figure 1.
FTIR-ATR spectra recorded for native CS (black) and TCS (blue).
2.1.2. Synthesis of TCNPs
TCNPs were synthesized using the ionic gelation method, with TPP as a crosslinking agent. To optimize the formation of spherical TCNPs with sizes below 200 nm, various synthesis conditions were evaluated, including pH values (4.5, 4.8, 5.0 and 5.2) and TPP:CS ratios (1.2:1, 1:1, 0.8:1 and 0.6:1 w:w). The results are summarized in Table 1. Based on the observed morphology and size of TCNPs, the optimal synthesis conditions were achieved at pH 4.8 with a TPP:CS ratio of 0.8:1. These conditions appear to favor TCNP formation due to several factors: (i) the amount of TPP is sufficient to promote effective ionic crosslinking with TCS, whereas both lower and higher TPP:CS ratios tend to produce larger nanoparticles; (ii) at pH 4.8, electrostatic interactions between TPP and thiolated chitosan are optimized, playing a key role in nanoparticle formation; and (iii) under these pH conditions, TCS chains adopt a partially coiled structure, allowing for availability of a sufficient number of protonated amino groups essential for crosslinking, thereby facilitating the formation of spherical TCNPs with sizes below 200 nm [20,21]. Interestingly, despite the relatively low zeta potential values (+14 mV) obtained for the TCNPs under optimal formulation, the nanosystem exhibited substantial colloidal stability. Literature indicates that colloidal stability can be inferred from its zeta potential: values between |30|−|20| mV suggests stability due sufficient electrostatic repulsion between nanoparticles; values between |20|−|10| mV indicate a metastable system; and values below |10| mV typically signify an unstable colloidal system prone to aggregation. In this context, the optimized formulation can be classified as a metastable suspension that nonetheless remained stable for at least 10 days. Although TCNPs with sizes under 200 nm were also obtained under other pH conditions and TPP:TCS ratios conditions (see Table 1), those formulation showed poor colloidal stability in the aqueous media and higher polydispersity index (PDI) values, indicating a non-uniform size distribution [22].

Table 1.
Effect on the mean size, polydispersity index and zeta potential of the different pH and TPP:Cs ratios in the synthesis of the TCNPs. Values are presented as mean ± SD (n = 3).
Figure 2 presents the AFM images of TCNPs synthesized at a TPP:TCS ratio of 0.8:1 under different pH conditions (4.5, 4.8, 5.0 and 5.2). It can be observed that TCNPs synthesized at pH 4.5 and 4.8 show a spherical morphology, with approximate size of 190 nm and 150 nm, respectively (Figure 2a,b). These sizes measurements are consistent with the hydrodynamic diameters determined by DLS (215 nm and 178 nm). Furthermore, TCNPs synthesized at pH 4.8 conditions demonstrate enhanced stability, whereas those obtained at pH 4.5 exhibited poor stability, consisting with previous reports [23]. At higher pH values, the synthesized TCNPs displayed increased polydispersity and polymorphic structures. Based on these findings, TCNPs synthesized at pH 4.8 were chosen as the dielectric template for gold shell growth.
2.2. Core-Shell Chitosan-Gold Nanoparticles
Gold shells were grown onto the surface of TCNPs using a seed-mediated method, which involves the growth of shell layer from gold seed previously adsorbed onto the surface of nanoparticle. This approach is similar to the synthesis of gold shell using silver and platinum NPs as templates [24,25,26], or their deposition onto dielectric nanoparticles as silicon oxide and polymer NPs [27,28,29]. For instance, when silver nanoparticles are used as growth templates, gold hollow-shell structures are spontaneously formed through a galvanic replacement process. In contrast, a dielectric core–gold shell structure is obtained when silicon oxide or polymer nanoparticles serve as the growth scaffold. This process consists of two consecutive stages: The adsorption of gold seeds onto the surface of the dielectric core, and the two-dimensional (2D) shell growth, which occurs after gold seed adsorption, thorough deposition of gold atom (typically Au+) in the presence of a moderate reductant. The adsorption of gold seeds can be further enhanced by modifying the surface of the dielectric nanoparticles thorough chemical attachment of functional groups with strong affinity to gold, such as thiol (-SH) groups. In this regard, the chitosan structure was modified by conjugating 3-MPA motifs, resulting in TCS, which was then used to synthesize TCNPs with surface-exposed -SH groups.
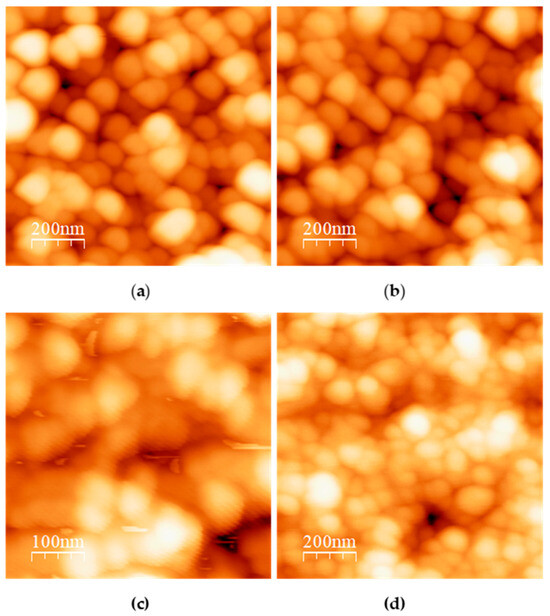
Figure 2.
AFM micrographs of TCNP synthesized using a TPP:Cs ratio 0.8:1 (w:w) at different pH values (a) 4.5, (b) 4.8, (c) 5.0, (d) 5.2. AFM images were analyzed with the free online WSxM software [30].
Figure 2.
AFM micrographs of TCNP synthesized using a TPP:Cs ratio 0.8:1 (w:w) at different pH values (a) 4.5, (b) 4.8, (c) 5.0, (d) 5.2. AFM images were analyzed with the free online WSxM software [30].
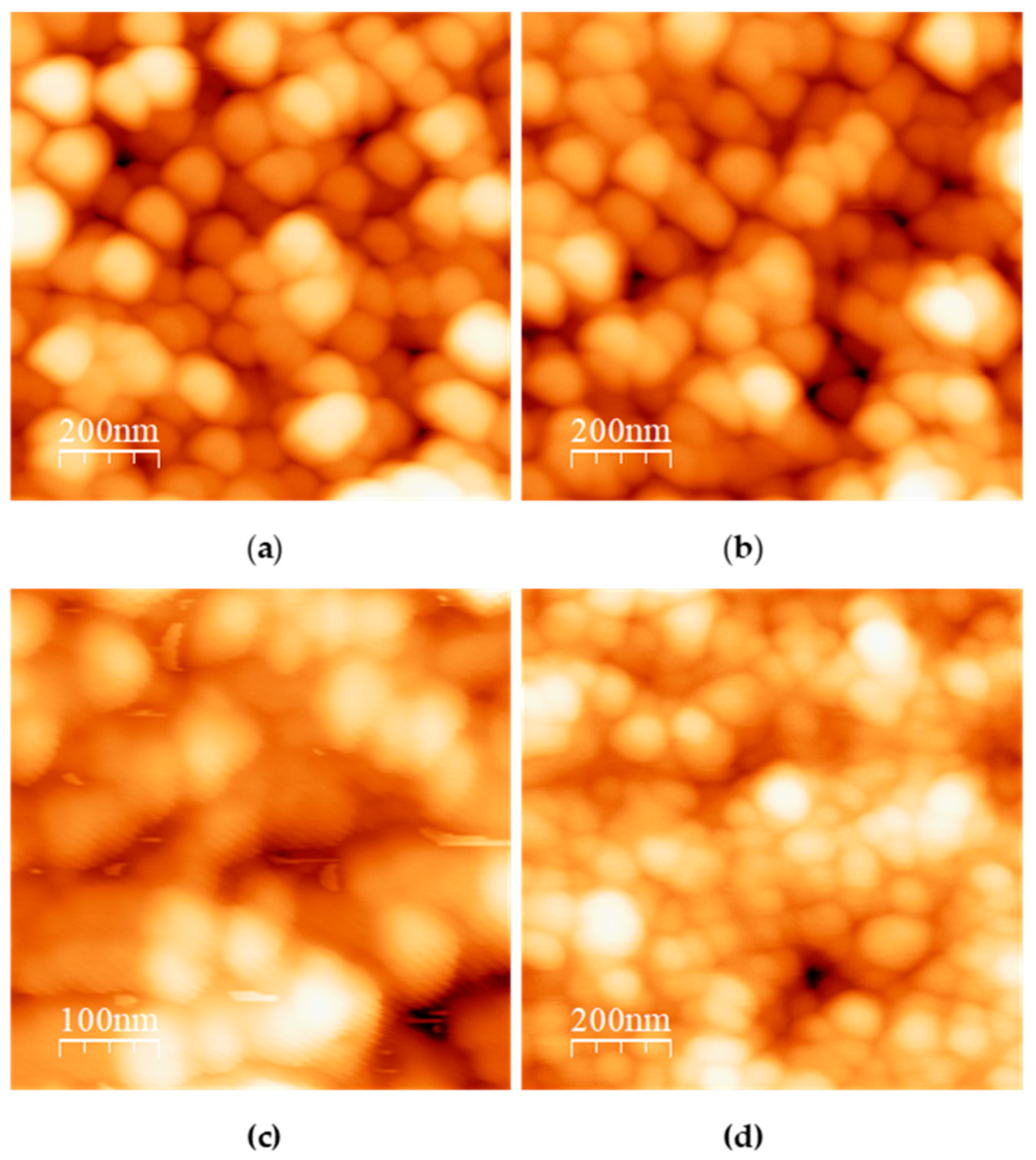
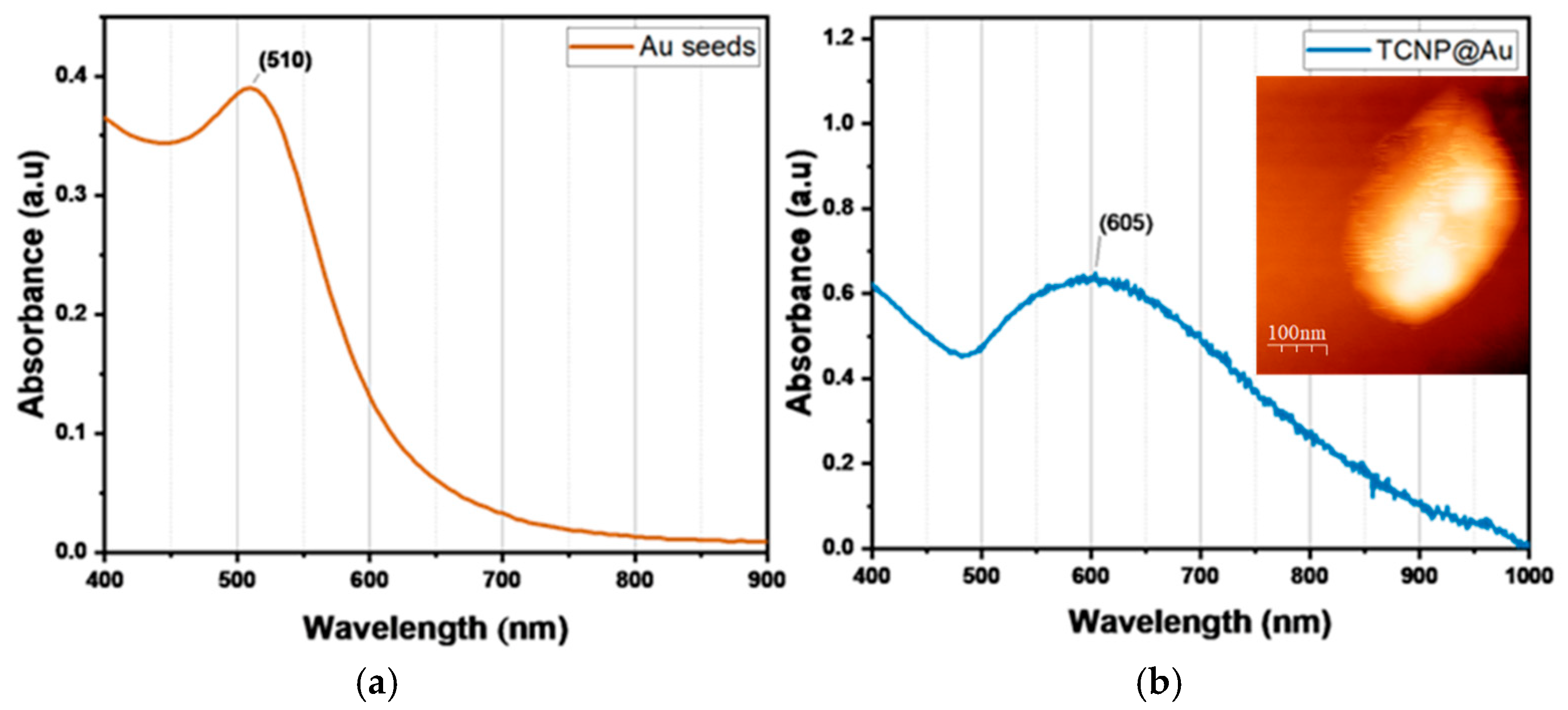
Then, TCNPs were used as templates for the growth of gold shells, mediated by gold seeds. First, gold seeds were added to TCNPs solution and allowed sufficient time (16 h) to anchor onto the surface of TCNPs. After this period, the gold shells growth was initiated by the sequential addition of Au+3 and ascorbic acid (AA). The gold shell formation was monitored by UV-Vis spectroscopy, where the appearance of a broader SPR band in the wavelength range of 600–1000 nm confirmed the successful gold shell growth. Figure 3 shows the characteristic SPR of TCNP core-gold shell nanoparticles (TCNP@Au), with the SPR absorption band appearing in the NIR region, showing a maximum wavelength of approximately 605 nm. The particle size and zeta potential of TCNP@Au were measured as 415 ± 15 nm (PDI = 0.380) and 7 ± 2 mV, respectively. These results are consistent with previous reports [27,31,32,33]. The morphology of TCNP@Au, recorded using AFM, showed an ovoidal shape (inset in Figure 3b), with a size comparable to that observed by DLS.
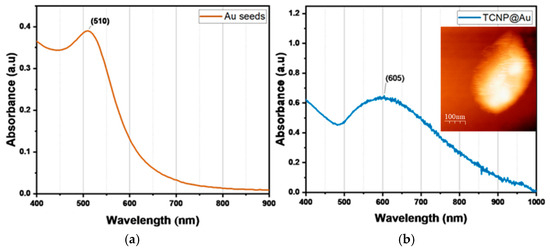
Figure 3.
UV-Vis spectra comparison of (a) gold colloid and (b) UV-Vis-NIR spectra of TCNP@Au. The inset shows an AFM image of TCNP@Au.
Photothermal Conversion Efficiency of TCNP@Au
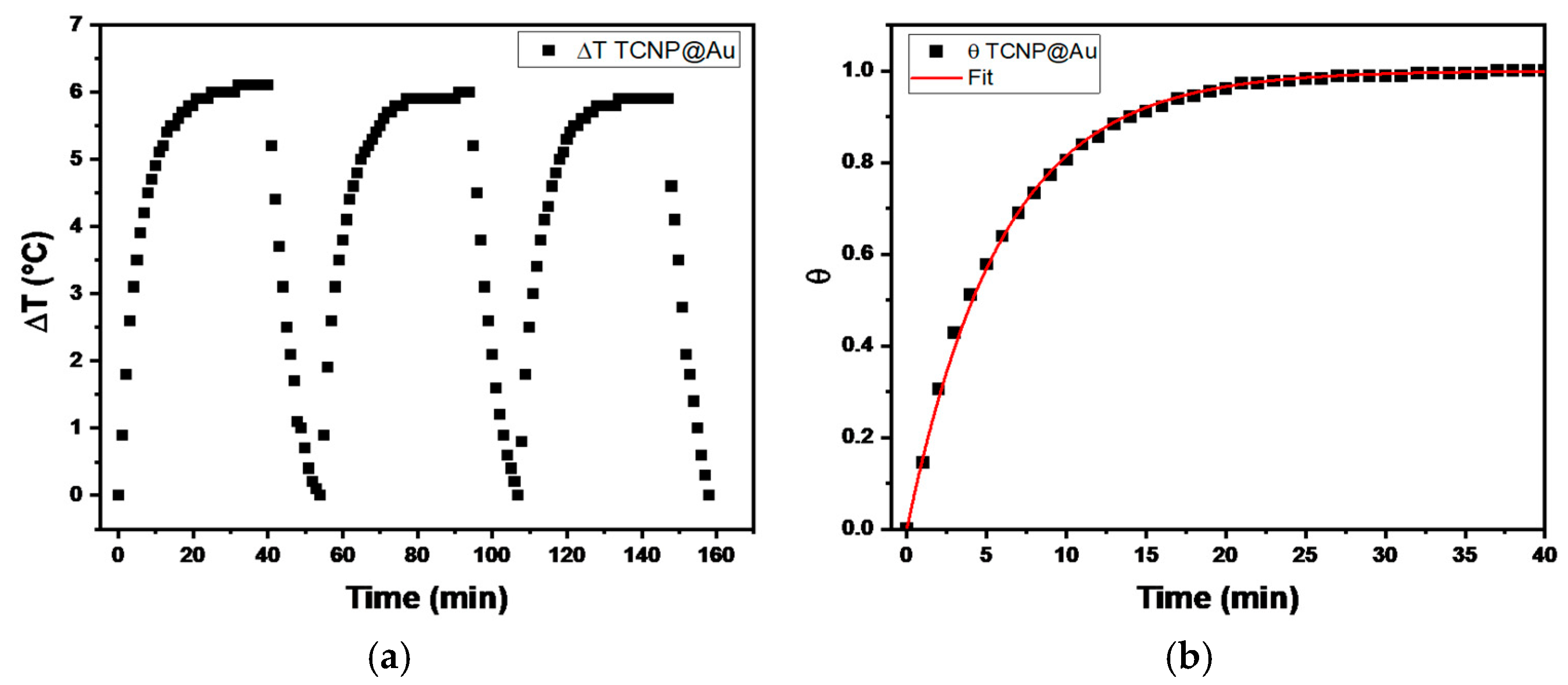
The temperature changes profiles are shown in Figure 4. It can be observed that the maximum temperature increase (ΔT) during the three irradiation cycles was 6 °C, above room temperature (20 °C), indicating the thermal stability of TCNP@Au throughout the irradiation process. Then, the photothermal conversion efficiency (η), determined by equation 1, was approximately 31%, a value comparable with previously reported results [34,35,36]. These findings suggest that the photothermal properties of TCNP@Au hold promise for biological applications.
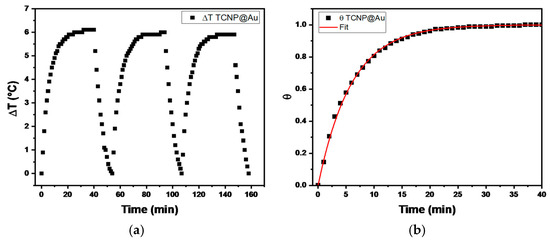
Figure 4.
(a) ∆T profile recorded for TCNPs@Au over three laser on/off cycles (λ = 808 nm radiation, 1 W). (b) θ as function of time, where the red line represents the fitted data according to Equation (3).
2.3. Photothermal Effect of TCNP@Au on the Viability of Gram-Positive and Gram-Negative Bacteria
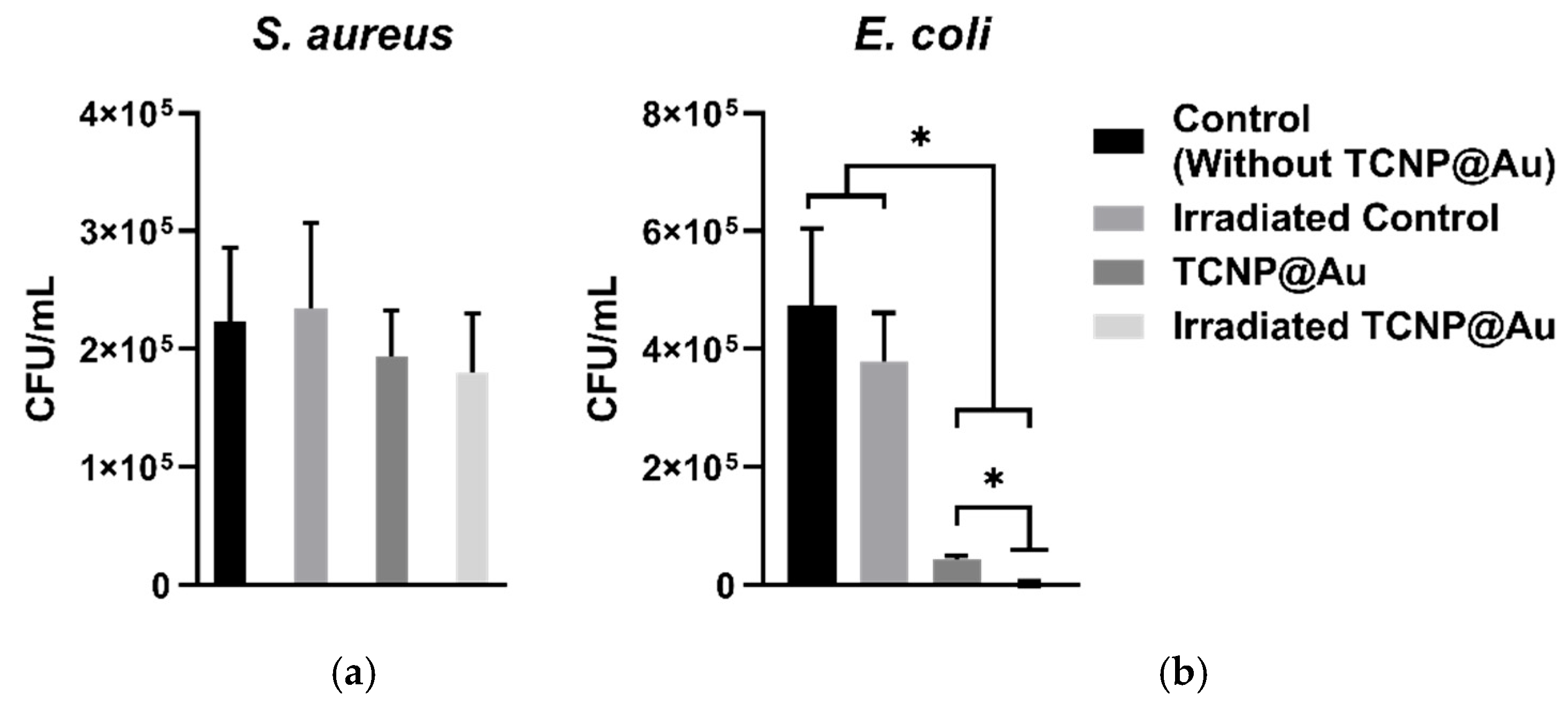
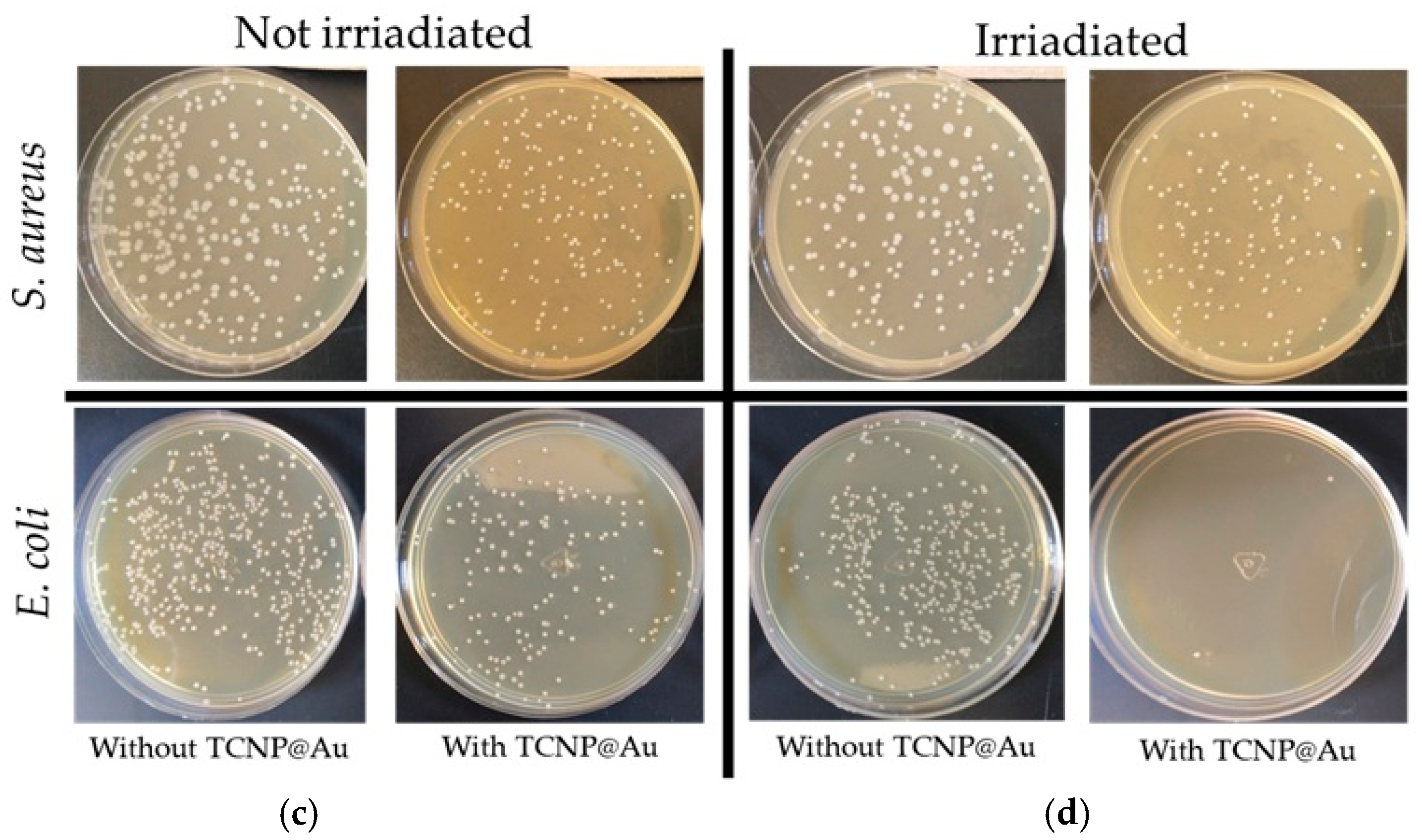
The potential of TCNP@Au as a photothermal agent for bacterial clearance was evaluated against both Gram-positive and Gram-negative bacteria, using Staphylococcus aureus (ATCC 25923) and Escherichia coli (ATCC 25922). The photothermal effect on bacterial viability was assessed after 15 min of laser irradiation (4.76 W/cm2, 808 nm) in the presence of TCNP@Au. Bacterial viability following irradiation was compared to controls samples incubated under the same conditions but without laser irradiation, as shown in Figure 5. Figure 5a shows the effect of TCNP@Au on the viability of S. aureus under both non-laser irradiated and laser-irradiated conditions. As observed, the presence of TCNP@Au alone slightly affected S. aureus viability reducing it to 86.8%. Upon laser irradiation, viability further decreased to 76.8%, although this reduction was not statistically significant (p > 0.05). The resilience of S. aureus to photothermal treatment can be attributed to its intrinsic tolerance to adverse conditions, including high temperatures, high salt concentrations, and osmotic pressure. Notably, S. aureus can grow within a temperature range of 7–48 °C, with an optimum at 37 °C. Moreover, it has been shown to withstand heat treatments exceeding 60 °C for up to 30 min [37]. The survival of S. aureus post-irradiation can be due to its thick peptidoglycan layer, which forms a robust structural barrier around the lipid membrane. This peptidoglycan layer serves as a protective shield against the photothermal action of TCNP@Au. These findings suggest that further optimization of photothermal treatment is necessary for effective S. aureus eradication. Potential strategies include increasing laser irradiation time or incorporating antibiotic compounds into the TCNP core to create a synergistic effect with photothermal therapy. On the other hand, Figure 5b shows that E. coli viability was significantly affected by the presence of TCNP@Au, with or without irradiation. A repeated measures one-way ANOVA followed by Tukey’s post hoc test revealed a significant reduction in viability compared to control conditions (p = 0.0250 for non-irradiated TCNP@Au and p = 0.0166 for irradiated TCNP@Au). Moreover, irradiation of TCNP@Au led to a statistically significant enhancement in the bactericidal effect compared to the non-irradiated condition (p = 0.0106). These findings confirm the intrinsic antibacterial activity of TCNP@Au and demonstrate the potentiation of its bactericidal properties via photothermal activation. The same statistical analysis was performed for S. aureus, but no statistically significant differences were observed between conditions (p > 0.05), supporting the notion that S. aureus exhibits greater tolerance to photothermal treatment under the tested parameters. The heightened susceptibility of E. coli to photothermal treatment can be attributed to the structural differences between Gram-negative and Gram-positive bacteria. Unlike S. aureus, E. coli possesses a lipid bilayer with a thin peptidoglycan layer, making it more vulnerable to thermal damage induced by TCNP@A [38,39,40].
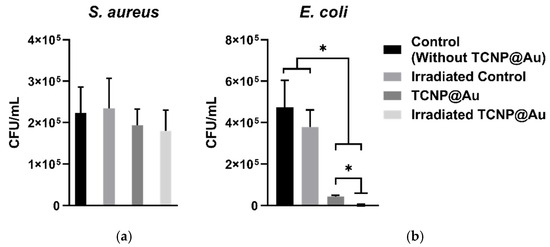
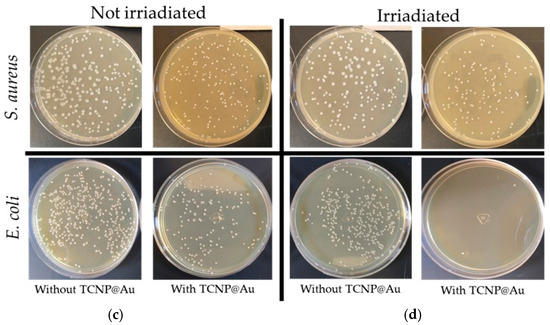
Figure 5.
Photothermal effect of TCNP@Au against bacterial strains: (a) S. aureus, (b) E. coli. Bacterial survival expressed as CFU/mL after 15 min of incubation with TCNP@Au nanoparticles, with and without 808 nm laser irradiation (4.76 W/cm2). Control groups include untreated bacteria and bacteria exposed to irradiation without nanoparticles. Data represents the mean ± standard deviation from two independent experiments, each performed in triplicate (n = 6 per group). Statistical analysis was performed using repeated measures one-way ANOVA followed by Tukey’s post hoc test, with significance considered at * p < 0.05. (c) and (d) Photographs of agar plates with bacterial growth after photothermal assay. Treated bacterial samples were diluted 1:100; 100 µL of diluted samples were plated on Mueller-Hinton agar and incubated at 37 °C for 18 h. Colony counts were expressed as CFU/mL considering the plated volume and dilution factor (colonies × 1000).
These results are consistent with the antibacterial activity reported for various photothermal nanosystems, such as spherical gold nanoparticles (SGNP) and gold nano rods (GNR) and GNSs. For instance, Millenbaugh et al. evaluated the photothermal effect of SGNP, both conjugated and non-conjugated with antibodies specific to S. aureus. Interestingly, bacteria survival remained relatively high (75%) when exposed to laser irradiation in the presence of non-functionalized SGNPs, whereas antibody conjugated SGNPs exhibited a significantly stronger antibacterial effect, reducing bacterial survival to 36 % [41]. An innovative antibacterial photothermal platform was reported by Uusitalo et al., who immobilized GNRs on the surface of titanium and glass substrates. To evaluate the photothermal effect on the viability of S. aureus and E. coli, the GNR-substrates were placed in direct contact with bacterial cultures on solid agar culture and irradiated with NIR laser at varying irradiance levels (0–20 W/cm2). Both the GNP-titanium substrate and GNR-glass substrate significantly reduced bacterial viability. Specifically, the GNP-titanium substrate decreased S. aureus viability to 18 CFU/cm2 at 10 W/cm2, while the GNR-glass substrate reduced the viability of both E. coli and S. aureus to nearly undetectable levels at 20 W/cm2. The enhanced activity observed in Uusitalo’s study is due to the considerably higher irradiation power applied [42]. In other study, Ma et al. reported that GNRs were capable of eliminating approximately ~99% of E. coli and 88% of S. aureus under NIR irradiation. This high antibacterial efficacy was attributed to the strong photothermal conversion (η > 60%) and the positively charged double layer of hydroxide coating GNR, which can promotes electrostatic interaction with the bacteria cell wall [43]. Manivasagan et al. investigated thiolated chitosan-wrapped gold nanoshells for their photothermal antibacterial effect against S. aureus. They report a negligible bacterial viability when cells were treated with 115 μg/mL of chitosan-wrapped GNSs and subjected to NIR irradiation for just 5 min [44]. The enhanced antibacterial performance in their study may be attributed to the use of silica nanoparticles as templates, which enable the formation of continuous gold shell layer capable of generating temperatures exceeding 55 °C upon irradiation.
3. Materials and Methods
The Chitosan (CS, low molecular weight average 120 kDa, 79% degree of deacetylation), sodium triphosphate pentabasic (TPP), 3-mercaptopropionic acid (3-MPA), acetic acid, gold (III) chloride trihydrate, L-ascorbic acid, NaBH4, NHS, EDAC, DMF all where from Sigma Aldrich (Missouri, MO, USA). Sodium citrate dihydrate granular from J.T. Barker. Spectra/Pro 1 dialysis Tubing, 6–8 kD MWCO, 23 mm flat width, 14.6 mm diameter. Bacteria stains (Escherichia coli and Staphylococcus aureus) were purchased from American Type Culture Collection (ATCC).
3.1. Thiolated Chitosan (TCs)
Thiolated chitosan was synthesized following the method described by Ko et al., with slight modifications, involving a two-step reaction [45]. First, solutions of EDAC, 3-MPA and NHS were prepared in DMF (2.0 mmol). 2.0 mL of EDAC was added to 2.0 mL of 3-MPA solution and the mixture was stirred magnetically for 1 h. After this period, 2.0 mL of NHS was gradually added to the EDAC-3-MPA mixture and stirred at room temperature overnight (solution-1). In the second step, a chitosan (CS) solution (2% w/v) was prepared using HCl solution (0.1 M). The pH of the CS solution was then adjusted to 4.0 using NaOH solution (1 M), and solution-1 was immediately added dropwise to the CS solution under magnetic stirring. The mixture was continuously stirred for 12 h at room temperature. The thiolated chitosan (TCS) was purified through an exhaustive dialysis (dialysis membrane 6–8 kD MWCO) process using deionized water. Then, the purified product was lyophilized and characterized by spectroscopic techniques.
FTIR-ATR spectroscopy was used to confirm the successful modification of chitosan with 3-MPA. The FTIR-ATR spectra were recorded using PerkinElmer spectrophotometer (Waltham, MA, USA). The spectral range was scanned from 4000 to 400 cm−1 with a resolution of 2 cm−1.
3.2. Synthesis of Thiolated Chitosan Nanoparticles (TCsNp)
Thiolated chitosan nanoparticles (TCSNPs) were synthesized using the well-established ionic gelation method, with TPP as a crosslinker, [46]. To obtain spherical TCSNPs with a size below 200 nm, different TCS solutions were adjusted to various pHs (4.5, 4.8, 5.0 and 5.2) and tested different TPP:C ratio (1.2:1, 1:1, 0.8:1 and 0.6:1 w/w). Under these conditions, 10 mL of TCS solution (0.5 mg/mL, in 50 mM acetic acid) was heated in a water bath at 60 °C for 10 min under constant stirring. The solution was then rapidly cooled in an ice bath (4 °C), and 2 mL TPP solution (2 mg/mL) was immediately added to the CS solution, followed by stirring for an additional 15 min. The resulting colloidal suspension was centrifuged at 7607× g for 30 min at 15 °C. After the centrifugation, the supernatant was discarded, and the sediment was resuspended in 5 mL in Milli-Q water.
3.3. Synthesis of Gold-Shell on TCSNPs
The gold-shell on the surface of TCSNPs (TCNP@Au) was synthesized using an in-situ seed-growth mediated method, consisting into consecutive steps. First, the gold seeds were synthesized following the method described by Topete et al. lightly modified, [47]. Briefly, an HAuCl4 (14.7 mM) and sodium citrate solution (1 mM) were prepared. Then 0.102 mL of gold solution was mixed with 2.25 mL of citrate solution and 2.6 mL of pure water was added to get a total volume solution of 5 mL, stirring constantly at room temperature. The gold seed was attained once of the addition of 50 μL of cold NaBH4 solution (0.1 M). The seeds were diluted 1:4 in sodium citrate before the second step.
20 μL of the diluted gold seed was added to the TCSNP solution, and the mixture was stirred at room temperature for 16-h. After this period, 50 μL of the Au3+ (1.47 mM) added to TCSNP suspension, followed immediately by the addition of 20 μL of ascorbic acid (10 mM), maintaining continuous stirring. After 90 min of reaction, an additional 50 μL of Au3+ and 20 μL of ascorbic acid were added. This procedure was repeated four times. Then, the colloidal suspension gradually changed from an opalescent to blue-green color. Finally, the mixture was purified by dialysis (dialysis membrane 6–8 kD MWCO) for four hours in deionized water.
3.4. Characterization
3.4.1. Nanoparticles Hydrodynamic Size (DH) and Zeta Potential (ζP)
The hydrodynamic diameter (DH) and zeta potential (ζP) of the nanoparticles were determined using a Zetasizer Nano ZS (Malvern instruments, UK) equipped with a 633 nm red laser (He-Ne, 4 mW). For dynamic light scattering (DLS), 0.6 mL of each sample was placed into disposable square cuvette. For the zeta potential measurements 1.2 mL of each sample was loaded in a folded capillary (U-shape) cell. All measurements were performed in triplicate at a constant temperature of 25 °C. Results are reported as mean ± standard deviation.
3.4.2. Atomic Force Microscopy
The morphology of TCSNPs and TCNP@Aus was analyzed by AFM (JSPM-4210, JEOL, Tokyo, Japan). Samples were prepared as follow: an aliquot of the nanoparticle suspension was placed onto the surface of freshly cleaved mica. After a minute, the excess water was adsorbed with paper and then left until the sample dries.
3.4.3. UV-Vis Spectroscopy
The gold-shell growth was followed using UV-Vis spectroscopy (Lambda 365 PerkinElmer, Hopkinton, MA, USA). The spectra were recorded on the wavelength range of 400–1000 nm.
3.5. Photothermal Conversion of TCNP@Au
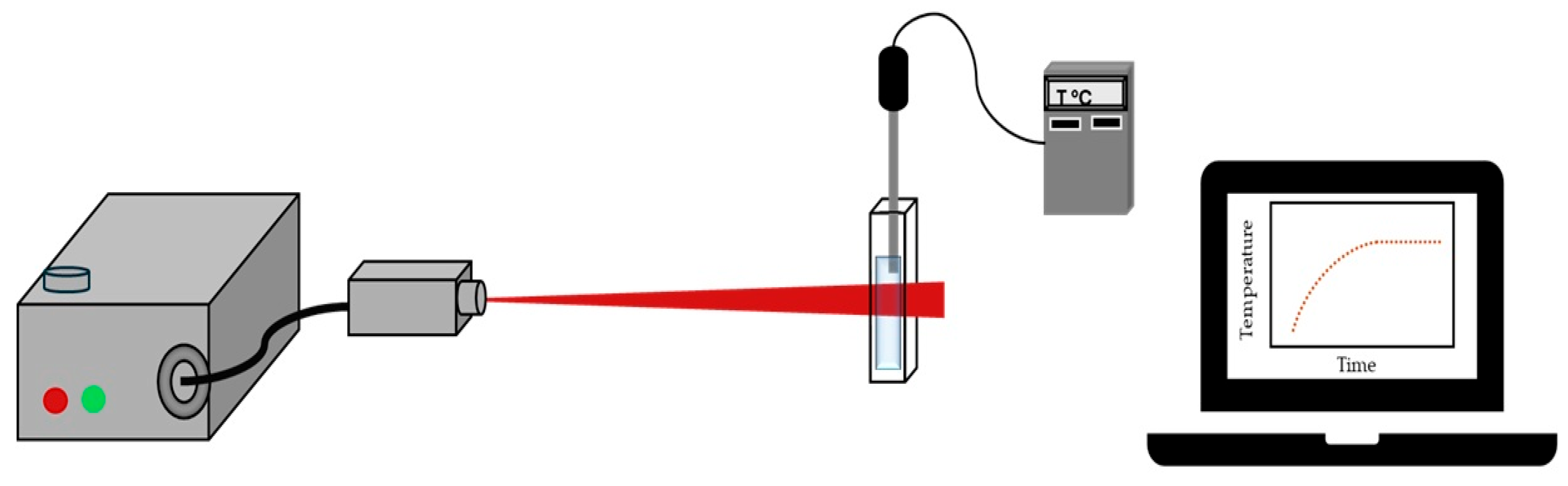
The photothermal conversion of TCNP@Au was evaluated under laser irradiation (4.76 W/cm2, λ = 808 nm, source diode module). The samples were placed in a measurement cell and were subjected to on/off cycles irradiation for 40 min and the temperature increase was recorded using a thermocouple every 60 s, Figure 6. Each experiment was performed in triplicate for reproducibility.
Figure 6.
Set up of the photothermal measurement assay.
To determine the photothermal conversion efficiency (η), the temperature data were plotted as a function of time to the theoretical Roper equation [48]:
were h is the heat transfer coefficient, A is the quartz cell area, Aλ is the absorbance value of the TCNP@Au suspension at 808 nm, Q0 is the system represents the system heat contribution (considering, water, TCNP@Au, and quartz), and I is the laser power. The value of h is calculated using the following equation [9]:
where is the total heat capacity of the system (Q0), A is the quartz cell area, and τs is the cooling rate constant. The parameter τs is determined from the increase in temperature during the laser turn-on period. The temperature data are fitted to the following equation:
where , a dimensional driving force temperature, T0 and Tmax, represent the room temperature and the maximum temperature, respectively. Tt is the temperature at time t [48].
3.6. Photothermal Effect on the Bacterial Growth
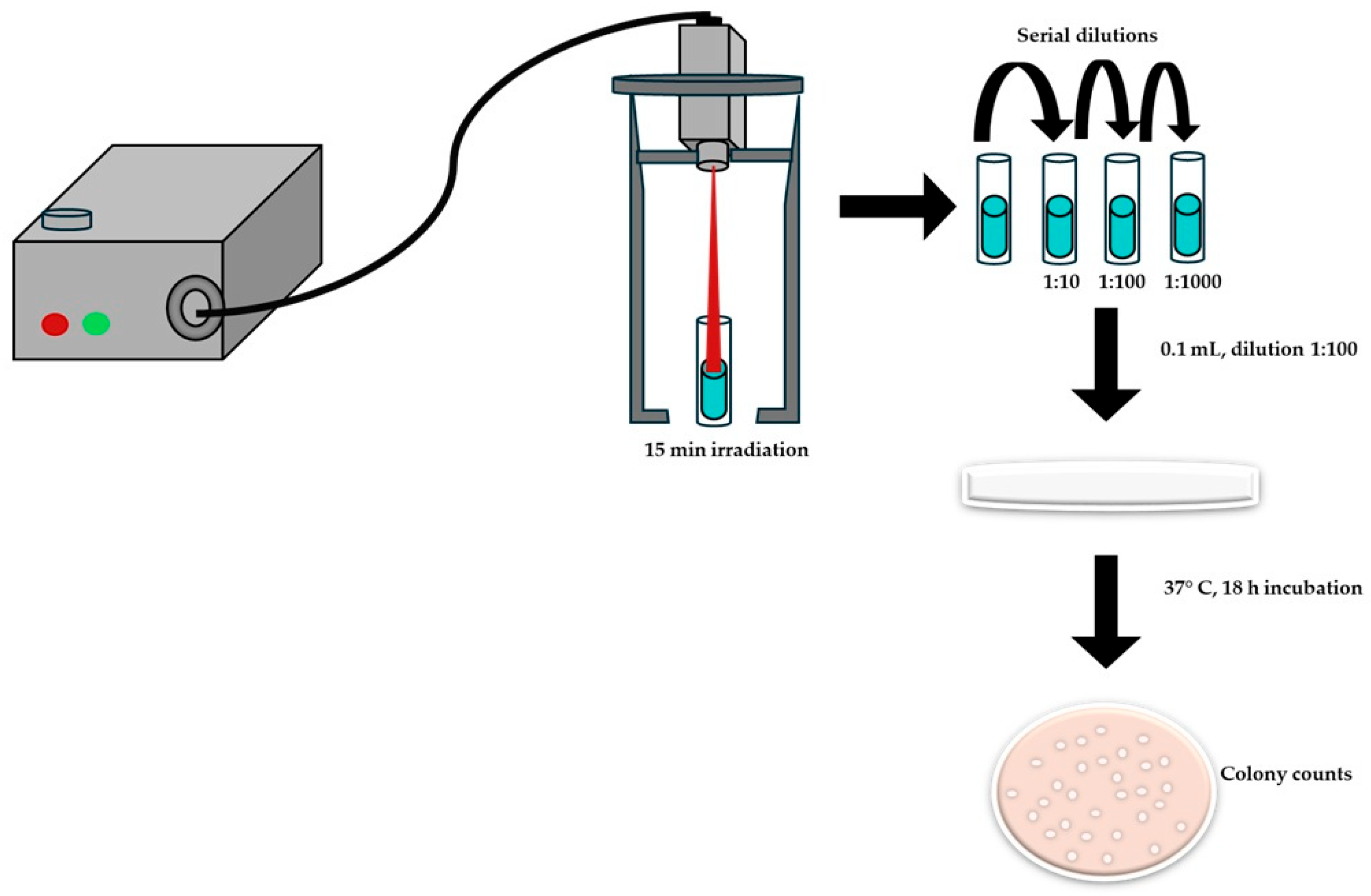
The photothermal effect on bacterial growth inhibition was evaluated using Escherichia coli (ATCC 25922) and Staphylococcus aureus (ATCC25923) as Gram-negative and Gram-positive bacterial models, respectively. The antibacterial activity of TCNP@Au was assessed using the macrodilution method, following the guidelines of Clinical and Laboratory Standards Institute (CLSI) [46]. Briefly, a bacterial suspension at a concentration of 500,000 CFU/mL was added to 2 mL of suspension of the TCNP@Au in a test tube. The upper part of the test tube was then irradiated for 15 min with a CW laser (808 nm, 4.76 W/cm2). After irradiation, serial dilutions (1:10, 1:100, 1:1000) were prepared, and 100 μL of each diluted samples were plated onto agar petri dishes. Bacterial viability was determined by counting the CFUs after 18 h of incubation at 37 °C, Figure 7.
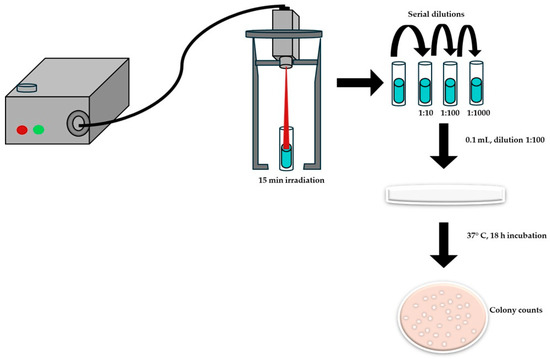
Figure 7.
Scheme of the photothermal effect measurements on the viability of bacteria by macrodilution method.
4. Conclusions
In summary, TCNPs with sizes under 200 nm were successfully prepared using a reproducible and straightforward approach, in which the solution pH and TPP:TCS ratio were systematically varied. The optimal experimental formulation was obtained at pH 4.8 with a TPP:TCS ratio of 0.8:1.0. Importantly, the resulting TCNPs can serve as effective templates for anchoring gold seeds due to the presence of surface-exposed -SH groups, which acted as nucleation sites for the growth the gold nanoshells. TCNP@Au showed excellent values η, reaching values comparable to previously reported one making them promising photothermal agent for the eradication of Gram-negative bacteria. E. coli, a Gram-negative bacterium, was strongly affected by the presence of TCNP@Au, with bacterial viability decreasing to 8.5%. Furthermore, after laser irradiation, E. coli was effectively eradicated from the culture medium. Interestingly, S. aureus, a Gram-positive bacterium, exhibited resistance to photothermal treatment, highlighting its ability to withstand harsh environmental conditions. This finding underscores the need to reassess strategies for combating S. aureus to achieve effective bacterial elimination. Despite this limitation, TCNP@Aus demonstrate promising potential as photothermal agents and for the design of novel devices with applications in antibacterial biomaterials.
Author Contributions
Conceptualization, J.J. and P.D.M.-F.; methodology, P.D.M.-F., M.G.B.-M., P.M.-P. and M.G.-C.; validation, P.D.M.-F., M.A.L.-M., G.G.-G., G.E.R.-M. and M.G.B.-M.; formal analysis, J.J.; investigation, J.J.; resources, J.J.; data curation, J.J.; writing—original draft preparation, J.J., P.D.M.-F.; writing—review and editing, J.J., M.A.L.-M., G.G.-G. and G.E.R.-M.; visualization, J.J.; supervision, J.J., M.A.L.-M., G.G.-G. and G.E.R.-M.; project administration, J.J.; funding acquisition, J.J. All authors have read and agreed to the published version of the manuscript.
Funding
Project CF-2023-G-41 and CV SECIHTI 1009922.
Data Availability Statement
All data supporting the finding of this study are included in the present manuscript.
Acknowledgments
To Universidad de Sonora for infrastructure support and to SECIHTI for economic support under the project CF-2023-G-41.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| Cs | Chitosan |
| TPP | Sodium Triphosphate Pentabasic |
| 3-MPA | 3-mercaptopropionic acid |
| EDAC | N-(3-Dimethylaninopropyl)-N-ethylcarbodiimide hydrochloride |
| NHS | N-Hydroxysuccinimide |
| DMF | N,N-Dimethylformamide |
| NaBH4 | Sodium borohydride |
| TCs | Thiolated Chitosan |
| FTIR-ATR | Fourier Transform Infrared Spectroscopy-Attenuated Total Reflectance |
| TCsNPs | Thiolated Chitosan Nanoparticles |
| TCs@AuNp | Core-Shell Chitosan-Gold Nanoparticles |
| AFM | Atomic Force Microscopy |
| AuSD | Gold Seeds |
| AA | Ascorbic Acid |
| LSPR | Localized Surface Plasmonic Resonance |
| PDI | Polydispersity Index |
References
- Karnwal, A.; Jassim, A.Y.; Mohammed, A.A.; Mohammad Said Al-Tawaha, A.R.; Selvaraj, M.; Malik, T. Addressing the global challenge of bacterial drug resistance: Insights, strategies, and future directions. Front. Microbiol. 2025, 16, 1517772. [Google Scholar] [CrossRef] [PubMed]
- Ajulo, S.; Awosile, B. Global antimicrobial resistance and use surveillance system (GLASS 2022): Investigating the relationship between antimicrobial resistance and antimicrobial consumption data across the participating countries. PLoS ONE 2024, 19, e0297921. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, A.; Countryman, A.; Laurence, T.; Gulliver, S.; Drake, T.; Edwards, S.; Kenny, C.; Lamberti, O.; Morton, A.; Shafira, A.; et al. Forecasting the Fallout from AMR: Economic Impacts of Antimicrobial Resistance in Humans; World Organisation for Animal Health and World Bank: Paris, France; Washington, DC, USA, 2024. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Ding, T.; Liu, J.; Zhao, H. Multifunctional Gold Nanoparticles: A Novel Nanomaterial for Various Medical Applications and Biological Activities. Front. Bioeng. Biotechnol. 2020, 8, 990. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.; Sharma, P.; Hasan, M.R.; Singh, S.; Thakur, D.; Narang, J. Gold nanomaterials—The golden approach from synthesis to applications. Mater. Sci. Energy Technol. 2022, 5, 375–390. [Google Scholar] [CrossRef]
- Kumar, P.P.P.; Lim, D.K. Photothermal Effect of Gold Nanoparticles as a Nanomedicine for Diagnosis and Therapeutics. Pharmaceutics 2023, 15, 2349. [Google Scholar] [CrossRef]
- Milan, J.; Niemczyk, K.; Kus-Liśkiewicz, M. Treasure on the Earth—Gold Nanoparticles and Their Biomedical Applications. Materials 2022, 15, 3355. [Google Scholar] [CrossRef]
- Tatsuno, I.; Niimi, Y.; Tomita, M.; Terashima, H.; Hasegawa, T.; Matsumoto, T. Mechanism of transient photothermal inactivation of bacteria using a wavelength-tunable nanosecond pulsed laser. Sci. Rep. 2021, 11, 22310. [Google Scholar] [CrossRef]
- Cole, J.R.; Mirin, N.A.; Knight, M.W.; Goodrich, G.P.; Halas, N.J. Photothermal efficiencies of nanoshells and nanorods for clinical therapeutic applications. J. Phys. Chem. C 2009, 113, 12090–12094. [Google Scholar] [CrossRef]
- Topete, A.; Alatorre-Meda, M.; Villar-Álvarez, E.M.; Cambón, A.; Barbosa, S.; Taboada, P.; Mosquera, V. Simple control of surface topography of gold nanoshells by a surfactant-less seeded-growth method. ACS Appl. Mater. Interfaces 2014, 6, 11142–11157. [Google Scholar] [CrossRef]
- Liu, Y.; Kangas, J.; Wang, Y.; Khosla, K.; Pasek-Allen, J.; Saunders, A.; Oldenburg, S.; Bischof, J. Photothermal conversion of gold nanoparticles for uniform pulsed laser warming of vitrified biomaterials. Nanoscale 2020, 12, 12346–12356. [Google Scholar] [CrossRef]
- De Torre-miranda, N.; Reilly, L.; Eloy, P.; Poleunis, C.; Hermans, S. Thiol functionalized activated carbon for gold thiosulfate recovery, an analysis of the interactions between gold and sulfur functions. Carbon N. Y. 2023, 204, 254–267. [Google Scholar] [CrossRef]
- Inkpen, M.S.; Liu, Z.-F.; Li, H.; Campos, L.M.; Neaton, J.B.; Venkataraman, L. Non-chemisorbed gold–sulfur binding prevails in self-assembled monolayers. Nat. Chem. 2019, 11, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Skwarczynska, A.; Kaminska, M.; Owczarz, P.; Bartoszek, N.; Walkowiak, B.; Modrzejewska, Z. The structural (FTIR, XRD, and XPS) and biological studies of thermosensitive chitosan chloride gels with β-glycerophosphate disodium. J. Appl. Polym. Sci. 2018, 135, 46459. [Google Scholar] [CrossRef]
- El-araby, A.; El Ghadraoui, L.; Errachidi, F. Usage of biological chitosan against the contamination of post-harvest treatment of strawberries by Aspergillus niger. Front. Sustain. Food Syst. 2022, 6, 881434. [Google Scholar] [CrossRef]
- Cheng, J.; Zhu, H.; Huang, J.; Zhao, J.; Yan, B.; Ma, S.; Zhang, H.; Fan, D. The physicochemical properties of chitosan prepared by microwave heating. Food Sci. Nutr. 2020, 8, 1987–1994. [Google Scholar] [CrossRef]
- Hussein, M.A.M.; Grinholc, M.; Dena, A.S.A.; El-Sherbiny, I.M.; Megahed, M. Boosting the antibacterial activity of chitosan–gold nanoparticles against antibiotic–resistant bacteria by Punicagranatum L. extract. Carbohydr. Polym. 2021, 256, 117498. [Google Scholar] [CrossRef]
- Pan, C.; Qian, J.; Zhao, C.; Yang, H.; Zhao, X.; Guo, H. Study on the relationship between crosslinking degree and properties of TPP crosslinked chitosan nanoparticles. Carbohydr. Polym. 2020, 241, 116349. [Google Scholar] [CrossRef]
- Hahn, T.; Tafi, E.; Paul, A.; Salvia, R.; Falabella, P.; Zibek, S. Current state of chitin purification and chitosan production from insects. J. Chem. Technol. Biotechnol. 2020, 95, 2775–2795. [Google Scholar] [CrossRef]
- Algharib, S.A.; Dawood, A.; Zhou, K.; Chen, D.; Li, C.; Meng, K.; Zhang, A.; Luo, W.; Ahmed, S.; Huang, L.; et al. Preparation of chitosan nanoparticles by ionotropic gelation technique: Effects of formulation parameters and in vitro characterization. J. Mol. Struct. 2022, 1252, 132129. [Google Scholar] [CrossRef]
- Sullivan, D.J.; Cruz-Romero, M.; Collins, T.; Cummins, E.; Kerry, J.P.; Morris, M.A. Synthesis of monodisperse chitosan nanoparticles. Food Hydrocoll. 2018, 83, 355–364. [Google Scholar] [CrossRef]
- Mudalige, T.; Qu, H.; Van Haute, D.; Ansar, S.M.; Paredes, A.; Ingle, T. Characterization of Nanomaterials: Tools and Challenges. In Nanomaterials for Food Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 313–353. [Google Scholar] [CrossRef]
- Yadav, P.; Yadav, A.B. Preparation and characterization of BSA as a model protein loaded chitosan nanoparticles for the development of protein-/peptide-based drug delivery system. Futur. J. Pharm. Sci. 2021, 7, 200. [Google Scholar] [CrossRef]
- Ha Pham, T.T.; Vu, X.H.; Dien, N.D.; Trang, T.T.; Van Truong, N.; Thanh, T.D.; Tan, P.M.; Ca, N.X. The structural transition of bimetallic Ag-Au from core/shell to alloy and SERS application. RSC Adv. 2020, 10, 24577–24594. [Google Scholar] [CrossRef] [PubMed]
- Dikkumbura, A.S.; Hamal, P.; Chen, M.; Babayode, D.A.; Ranasinghe, J.C.; Lopata, K.; Haber, L.H. Growth Dynamics of Colloidal Silver-Gold Core-Shell Nanoparticles Studied by in Situ Second Harmonic Generation and Extinction Spectroscopy. J. Phys. Chem. C 2021, 125, 25615–25623. [Google Scholar] [CrossRef] [PubMed]
- Alwhibi, M.S.; Ortashi, K.M.O.; Hendi, A.A.; Awad, M.A.; Soliman, D.A.; El-Zaidy, M. Green synthesis, characterization and biomedical potential of Ag@Au core–shell noble metal nanoparticles. J. King Saud Univ.—Sci. 2022, 34, 102000. [Google Scholar] [CrossRef]
- Gordel-Wójcik, M.; Pietrzak, M.; Kołkowski, R.; Zych, E. Silica-coated gold nanoshells: Surface chemistry, optical properties and stability. J. Lumin. 2024, 270, 120565. [Google Scholar] [CrossRef]
- Lee, S.; Lee, S.; Hwang, S.; Park, S.-J. Gold nanoshells with varying morphologies through templated surfactant-assisted seed-growth method. Bull. Korean Chem. Soc. 2023, 45, 486–494. [Google Scholar] [CrossRef]
- Dheyab, M.A.; Aziz, A.A.; Jameel, M.S.; Noqta, O.A.; Mehrdel, B. Synthesis and coating methods of biocompatible iron oxide/gold nanoparticle and nanocomposite for biomedical applications. Chin. J. Phys. 2020, 64, 305–325. [Google Scholar] [CrossRef]
- Horcas, I.; Fernandez, R.; Gomez-Rodriguez, J.M.; Colchero, J.; Gomez-Herrero, J.; Baró, A.M. WsxM 5.0. Rev. Sci. Instrum 2007, 78, 13705. [Google Scholar] [CrossRef]
- Liu, H.; Chen, D.; Tang, F.; Du, G.; Li, L.; Meng, X.; Liang, W.; Zhang, Y.; Teng, X.; Li, Y. Photothermal therapy of Lewis lung carcinoma in mice using gold nanoshells on carboxylated polystyrene spheres. Nanotechnology 2008, 19, 455101. [Google Scholar] [CrossRef]
- Sood, A.; Arora, V.; Shah, J.; Kotnala, R.K.; Jain, T.K. Ascorbic acid-mediated synthesis and characterisation of iron oxide/gold core–shell nanoparticles. J. Exp. Nanosci. 2016, 11, 370–382. [Google Scholar] [CrossRef]
- Grabowska-Jadach, I.; Kalinowska, D.; Drozd, M.; Pietrzak, M. Synthesis, characterization and application of plasmonic hollow gold nanoshells in a photothermal therapy—New particles for theranostics. Biomed. Pharmacother. 2019, 111, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Pattani, V.P.; Tunnell, J.W. Nanoparticle-mediated photothermal therapy: A comparative study of heating for different particle types. Lasers Surg. Med. 2012, 44, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Savchuk, O.A.; Carvajal, J.J.; Massons, J.; Aguiló, M.; Díaz, F. Determination of photothermal conversion efficiency of graphene and graphene oxide through an integrating sphere method. Carbon N. Y. 2016, 103, 134–141. [Google Scholar] [CrossRef]
- Ayala-orozco, C.; Urban, C.; Knight, M.W.; Urban, A.S.; Neumann, O.; Bishnoi, S.W.; Mukherjee, S.; Goodman, A.M.; Charron, H.; Mitchell, T.; et al. Au Nanomatryoshkas as E ffi cient Transducers for Cancer Treatment: Benchmarking against Nanoshells. ACS Nano 2014, 8, 6372–6381. [Google Scholar] [CrossRef]
- Hassan, H.; Iskandar, C.F.; Hamzeh, R.; Malek, N.J.; El Khoury, A.; Abiad, M.G. Heat resistance of Staphylococcus aureus, Salmonella sp., and Escherichia coli isolated from frequently consumed foods in the Lebanese market. Int. J. Food Prop. 2022, 25, 2435–2444. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, Z.; Li, F.; Xiao, Y.; Zhang, Y.; Bu, T.; Jia, P.; Zhe, T.; Wang, L. Multifunctional Magnetic Copper Ferrite Nanoparticles as Fenton-like Reaction and Near-Infrared Photothermal Agents for Synergetic Antibacterial Therapy. ACS Appl. Mater. Interfaces 2019, 11, 31649–31660. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, Q.; Wang, F.; Xiao, Z.; He, L.; He, D.; Deng, L. Gold-Platinum Nanodots with High-Peroxidase-like Activity and Photothermal Conversion Efficiency for Antibacterial Therapy. ACS Appl. Mater. Interfaces 2021, 13, 37535–37544. [Google Scholar] [CrossRef]
- Mao, C.; Xiang, Y.; Liu, X.; Zheng, Y.; Yeung, K.W.K.; Cui, Z.; Yang, X.; Li, Z.; Liang, Y.; Zhu, S.; et al. Local Photothermal/Photodynamic Synergistic Therapy by Disrupting Bacterial Membrane to Accelerate Reactive Oxygen Species Permeation and Protein Leakage. ACS Appl. Mater. Interfaces 2019, 11, 17902–17914. [Google Scholar] [CrossRef]
- Millenbaugh, N.J.; Baskin, J.B.; DeSilva, M.N.; Elliott, W.R.; Glickman, R.D. Photothermal killing of Staphylococcus aureus using antibody-targeted gold nanoparticles. Int. J. Nanomed. 2015, 10, 1953–1960. [Google Scholar] [CrossRef]
- Uusitalo, M.; Eriksson, G.; Hulander, M.; Andersson, M. Gold Nanorods as Photothermal Antibacterial Materials. ACS Appl. Nano Mater. 2025, 8, 3661–3670. [Google Scholar] [CrossRef]
- Ma, K.; Li, Y.; Wang, Z.; Chen, Y.; Zhang, X.; Chen, C.; Yu, H.; Huang, J.; Yang, Z.; Wang, X.; et al. Core-Shell Gold Nanorod@Layered Double Hydroxide Nanomaterial with Highly Efficient Photothermal Conversion and Its Application in Antibacterial and Tumor Therapy. ACS Appl. Mater. Interfaces 2019, 11, 29630–29640. [Google Scholar] [CrossRef] [PubMed]
- Manivasagan, P.; Khan, F.; Hoang, G.; Mondal, S.; Kim, H.; Hoang Minh Doan, V.; Kim, Y.M.; Oh, J. Thiol chitosan-wrapped gold nanoshells for near-infrared laser-induced photothermal destruction of antibiotic-resistant bacteria. Carbohydr. Polym. 2019, 225, 115228. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.B.; Cho, H.Y.; Kim, T.H.; Yea, C.H.; Choi, J.W. Cell chip with a thiolated chitosan self-assembled monolayer to detect the effects of anticancer drugs on breast normal and cancer cells. Colloids Surfaces B Biointerfaces 2013, 112, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 9th ed.; Approved Standard; CLSI: Wayne, PA, USA, 2012; Volume 32, ISBN 1562387839. [Google Scholar]
- Topete, A.; Alatorre-Meda, M.; Iglesias, P.; Villar-Alvarez, E.M.; Barbosa, S.; Costoya, J.A.; Taboada, P.; Mosquera, V. Fluorescent drug-loaded, polymeric-based, branched gold nanoshells for localized multimodal therapy and imaging of tumoral cells. ACS Nano 2014, 8, 2725–2738. [Google Scholar] [CrossRef]
- Roper, D.K.; Ahn, W.; Hoepfner, M. Microscale heat transfer transduced by surface plasmon resonant gold nanoparticles. J. Phys. Chem. C 2007, 111, 3636–3641. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).