Abstract
Engineered exosomes have emerged as transformative drug carriers, uniquely equipped to overcome biological barriers in central nervous system (CNS) disorders and cancer therapy. These natural extracellular vesicles, derived from cell membranes, offer inherent biocompatibility, low immunogenicity, and the ability to traverse physiological obstacles such as the blood–brain barrier (BBB) and dense tumor stroma. Recent advances in exosome engineering—including surface modification (e.g., ligand conjugation for receptor-mediated targeting) and cargo loading (siRNA, CRISPR-Cas systems, and chemotherapeutics)—have enhanced their precision and therapeutic utility. For CNS delivery, exosomes functionalized with brain-homing peptides (e.g., RVG or TfR ligands) have enabled the efficient transport of neuroprotective agents or gene-editing tools to treat Alzheimer’s disease or glioblastoma. In oncology, engineered exosomes loaded with tumor-suppressive miRNAs or immune checkpoint inhibitors exploit tumor microenvironment (TME) features, such as acidity or enzyme overexpression, for spatially controlled drug release. Furthermore, hybrid exosome–liposome systems and exosome–biomaterial composites are being explored to improve payload capacity and stability. Despite progress, challenges persist in scalable production, batch consistency, and regulatory standardization. This review critically evaluates engineering strategies, preclinical success, and translational hurdles while proposing innovations such as AI-driven exosome design and patient-derived exosome platforms for personalized therapy. By bridging nanotechnology and biomedicine, engineered exosomes can represent a paradigm shift in targeted drug delivery, offering safer and more effective solutions for historically intractable diseases.
1. Introduction
Two of the most significant health issues of our time are cancer and neurological disease, and one of these is Alzheimer’s disease (AD), which affects the lives of over 50 million people and kills over 9.6 million people each year (World Health Organization, 2022) [1,2]. Conventional therapeutic strategies are hampered by the existence of biological barriers, such as the BBB in neurodegenerative disease and the solid tumor microenvironment for cancers [3]. Such challenges require the formation of novel drug delivery systems to effectively target damaged tissue with less systemic toxicity.
Exosomes, 30–150 nm diameter extracellular vesicles (EVs), are the most promising drug-delivery carriers because of their biocompatibility, low immunogenicity, and inherent ability to deliver biomolecules across cellular barriers [4]. In contrast to synthetic nanoparticles, exosomes are not man-made structures but are cell membrane-derived and do not carry artificial proteins or proteins external to cells but proteins that effectively interact with target cells. Their inherent potential to cross physiological barriers, including the BBB, has made them a promising vehicle for precision medicine applications [5].
Recent advances in exosome engineering have further increased their therapeutic value. Exosomes have been reprogrammed with receptor-targeting ligands, including transferrin receptor (TfR) peptides, to enable BBB permeation and enhance drug delivery across neurodegenerative disease models [6]. Engineered exosomes have also been employed to target cancer therapy, delivering tumor-suppressive miRNAs and chemotherapeutic drugs directly into the tumor microenvironment [7]. These changes have significantly improved the drug bioavailability and therapeutic effect, underscoring the potential of exosomes as second-generation drug carriers.
In spite of these advances, there are still issues in the clinical translation of exosome-based therapies. The absence of standardization and purification procedures, batch-to-batch variability, and regulatory ambiguity discourage large-scale production and clinical use [8]. Immune interactions and possible off-target effects also need to be explored to establish safety and efficacy for long-term therapeutic use [9]. Transcending these challenges by means of interdisciplinary research and regulatory cooperation will be critical to the progress of exosome-based therapy toward clinical applications.
This review critically surveys the latest advancements in exosome engineering for cancer and CNS therapy with an emphasis on major strategies, including surface engineering, cargo loading strategies, and hybrid exosome–liposome strategies. It also discusses translational challenges and opportunities, including AI-driven exosome design and patient-derived exosome platforms. Through the synergy of nanotechnology and biomedicine, exosome therapeutics promise unprecedented potential to revolutionize targeted drug delivery for notoriously recalcitrant diseases.
2. Exosome Biology: From Natural Vesicles to Engineered Therapeutics
2.1. Biogenesis and Structural Composition
Extracellular vesicles are heterogeneous membrane-bound nanoparticles broadly classified into three subtypes based on their biogenesis pathways and size: exosomes (30–150 nm), microvesicles (100–1000 nm), and apoptotic bodies (500–2000 nm).
2.1.1. Cellular Sources (Endosomal Route and ESCRT-Dependent/Independent Processes)
Exosomes originate from the endosomal pathway, where intraluminal vesicles (ILVs) form within multivesicular bodies (MVBs) through ESCRT (Endosomal Sorting Complex Required for Transport)-dependent or ESCRT-independent mechanisms (e.g., tetraspanin clustering and ceramide synthesis). Upon MVB fusion with the plasma membrane, ILVs are released as exosomes into the extracellular space [10,11]. Microvesicles arise via the direct outward budding of the plasma membrane, which is a process regulated by calcium-dependent enzymes (e.g., flippases, scramblases) and cytoskeletal rearrangements [12]. Recent studies challenge the traditional classification by demonstrating overlapping markers (e.g., CD9, CD81) between exosomes and microvesicles, necessitating multi-parametric characterization [13]. For instance, syntenin-1 and ALIX are enriched in exosomes, while ARF6 and VCAMP3 are microvesicle markers [14]. Apoptotic bodies, released during programmed cell death, are distinguished by their large size and nuclear content (e.g., histones and DNA fragments) but are rarely studied in therapeutic contexts due to their limited functional relevance [15].
Hypoxia has been reported to play a major role in the regulation of exosome secretion. An example of such research has revealed that hypoxia enhances the secretion of exosomes from cancer cells and, thus, facilitates cellular communication within the tumor microenvironment. This increased exosome release under hypoxia conditions suggests that microenvironmental stress is responsible for the modulation of exosome biogenesis [16].
Furthermore, the Rab small GTPases family has also been recognized as a major regulator for the exosome secretion pathway. Specifically, Rab27a and Rab27b are implicated in several MVB docking and fusion events with the plasma membrane. The silencing of Rab27a increases the number of MVBs, and Rab27b knockdown redistributes MVBs to the perinuclear region, proving their unique function in exosome secretion [17]. These results show the sophisticated regulation of exosome biogenesis and secretion, which is environmentally stress-sensitive, like hypoxia, as well as molecular modulators such as Rab GTPases.
The ESCRT machinery, and notably the ESCRT-I subunit Tsg101, have been implicated as essential for the sorting of cargo into exosomes. Tsg101 inhibition lowers the release of exosomes significantly and alters the content of cargo [18].
Current research has countered the theory that exosome secretion is strictly endosomal in origin. The research indicates that a large proportion of exosomes are secreted directly from the plasma membrane, bypassing the MVB pathway. A major limitation in EV research is the inability to identify exosomes from microvesicles using conventional techniques. Ultracentrifugation, the most widely used isolation method, co-pelletizes exosomes and microvesicles due to their overlapping size and density [19]. Size-exclusion chromatography (SEC) improves purity but cannot separate EVs smaller than 200 nm [20]. Advanced approaches like asymmetric flow field-flow fractionation (AF4) and immunoaffinity capture (e.g., anti-CD63 magnetic beads) enhance specificity but require rigorous validation [21]. Single-vesicle techniques like nanoparticle flow cytometry and super-resolution microscopy provide high-resolution insights but remain underutilized due to their cost and technical complexity [22]. Standardized reporting per the MISEV2023 guidelines is essential to address these challenges and ensure reproducibility in preclinical studies [23].
2.1.2. Heterogeneity of Cargo (Proteins, miRNAs, lncRNAs, Lipids) and Membrane Markers (CD9, CD63, CD81)
Exosomal cargo is extremely heterogeneous and mirrors the physiological status of the source cell. Exosomes are enriched in endosomal markers such as TSG101, ALIX, and tetraspanins (CD63, CD9, CD81), while microvesicles carry plasma membrane proteins like integrins, selectins, and ARF6 [24]. Non-CD markers are critical for accurate EV classification; for example, flotillin-1 and CD55 are associated with microvesicles, whereas HSP70 and syntenin-1 are exosome-specific [25].
The proteomic analysis of exosomes has revealed thousands of proteins that are present in exosomes, including cytoskeleton proteins, regulators of membrane traffic, and enzymes of metabolic pathways. Notably, exosomal proteins like Alix, Tsg101, and Rab GTPases belong to the class of vesicle formation and trafficking [26].
Exosomal RNA composition is also heterogenous and contains messenger RNAs (mRNAs), microRNAs (miRNAs), and long non-coding RNAs (lncRNAs). Exosomes are notably enriched with miRNAs and have been implicated to play a role in intercellular gene regulation. In their CRC exosome study, the authors observed that tumor cell-derived exosomes had a high enrichment of miR-21 and miR-223 compared to exosomes derived from normal cells [27]. Concurrently, exosomal lncRNAs have been involved in numerous biological processes, ranging from immune response to cancer metastasis. For example, lncRNA MALAT1 has been found in patient pancreatic cancer exosomes and correlated with enhanced tumor metastasis [28].
Lipids also contribute to the structure and function of exosomes. Lipidomic profiling further differentiates EV subtypes; exosomes contain high levels of cholesterol and sphingomyelin, while microvesicles are enriched in phosphatidylserine [29,30]. The lipid content is different depending on the cell type of origin; glycosphingolipids are in higher quantities in neuronal-derived exosomes, while immune cells are rich in phosphatidylserine-enriched exosomes [31].
Membrane proteins CD9, CD63, and CD81 have been utilized as markers to tag exosomes. CD63 is a component of the tetraspanin protein implicated with the ESCRT pathway, while CD9 and CD81 are dynamically partitioned between endosomal compartments and the plasma membrane [32]. Despite these distinctions, most studies rely solely on CD63/CD9 for exosome identification, leading to the misinterpretation of EV-mediated effects [33]. For instance, tumor-derived microvesicles expressing oncogenic EGFRvIII can confuse exosome-based therapeutic outcomes if not properly distinguished [34]. The differential expression of the proteins yields information about the origin and functional characteristics of the exosomes.
Together, these observations underscore the exosomology of biogenesis and architecture. The dynamic cooperation between ESCRT-dependent and ESCRT-independent mechanisms and selective protein, RNA, and lipid sorting suggests the functional heterogeneity of exosomes in physiological and pathologic processes. Future research will decipher the molecular mechanisms of exosome assembly and cargo loading, opening the doors to their therapeutic applications.
2.2. Intrinsic Function in Disease
Exosomes have an intrinsic function in disease pathology processes, including in neurodegenerative diseases and cancer. The small extracellular vesicles mediate intercellular communication through protein, lipid, and nucleic acid transfer between cells. Exosomes have been reported to cause the spread of cytotoxic protein aggregates in neurodegenerative diseases and growth and metastasis in cancer during disease. By acting as vectors for disease-inducing molecules, exosomes may be either the cause of disease onset or a possible therapeutic strategy [35]
2.2.1. Neurodegeneration Function (e.g., Aβ Propagation in Alzheimer’s)
Exosomes have been found to transfer amyloid-beta (Aβ) and hyperphosphorylated tau proteins, the two major pathological features of AD. Neuron- and glial cell-secreted exosomes carry Aβ peptides and are implicated in their extracellular deposition, resulting in plaque formation [36]. Exosomes containing Aβ have been shown to traverse the blood–brain barrier and allow its deposition in remote areas of the brain, fostering the development of disease. Aβ-containing exosomes from astrocytes have been found to interact with calcium-sensing receptors, which initiate subsequent tau phosphorylation and neurotoxicity [37].
In addition, exosomes have been shown to be involved in the transmission of tau pathology. Exosomal tau secreted by neurons in early AD was taken up by recipient cells, causing intracellular accumulation and neurofibrillary tangle formation [38]. In vivo experiments have shown that tau-positive exosomes injected into normal mouse brains can induce tau pathology, indicating their causal role in disease transmission. Furthermore, exosomes in the cerebrospinal fluid of AD patients contain more toxic tau species than controls, again implicating them in disease pathology [39].
While they are implicated in disease transmission, exosomes have also been studied for their neuroprotective function. Exosomes have been reported to be capable of promoting the clearance of Aβ through its delivery to microglia for degradation [40]. Exosomes derived from mesenchymal stem cells have also been reported to lower Aβ levels and enhance synaptic function in models of AD [41].
2.2.2. Function in Cancer Development (Exosome-Mediated Metastasis)
Exosomes also play a role in cancer development, and more so in metastasis. Tumor-derived exosomes carry oncogenic proteins, miRNAs, and signaling molecules that have the ability to modulate the tumor microenvironment and facilitate cancer cell migration. One of the major mechanisms through which exosomes are involved in metastasis is the induction of epithelial-to-mesenchymal transition (EMT), which is a process that makes cancer cells invasive. Exosomal miR-21, for example, has been reported to induce EMT by suppressing tumor suppressor genes and the expression of mesenchymal markers [42].
Another significant feature of metastasis via exosomes is the establishment of the pre-metastatic niche. Tumor-derived exosomes condition the host organs to accommodate metastatic colonization by remodeling the local microenvironment. Exosomes secreted by highly aggressive breast cancer cells have been found to carry integrins with homing specificity to home in on the organs guiding metastatic cell migration [43].
Exosomes also regulate immune responses in the tumor microenvironment, most commonly by suppressing anti-tumor immunity. PD-L1 composition tumor exosomes have been reported to suppress T-cell activation for cancer cells to evade immune detection [27].
Therapeutically, exosomes have also been investigated as drug delivery vehicles and biomarkers in cancer therapy. Exosomal miRNAs in the blood have been suggested as minimally invasive biomarkers for the early diagnosis of cancer, where miR-92a-3p and miR-21 are overexpressed in liver and colorectal cancers [44].
Exosomes play critical roles in disease pathogenesis, both facilitating cancer growth and neurodegeneration. Exosomes are involved in Aβ and tau spreading and in Aβ clearance and neuroprotection in AD. Exosomes promote metastasis in cancer by facilitating invasion, immune modulation, and pre-metastatic niche formation. Although they are involved in disease initiation, exosomes are promising as drug delivery systems and diagnostic biomarkers, and with these, new therapeutic opportunities are arising.
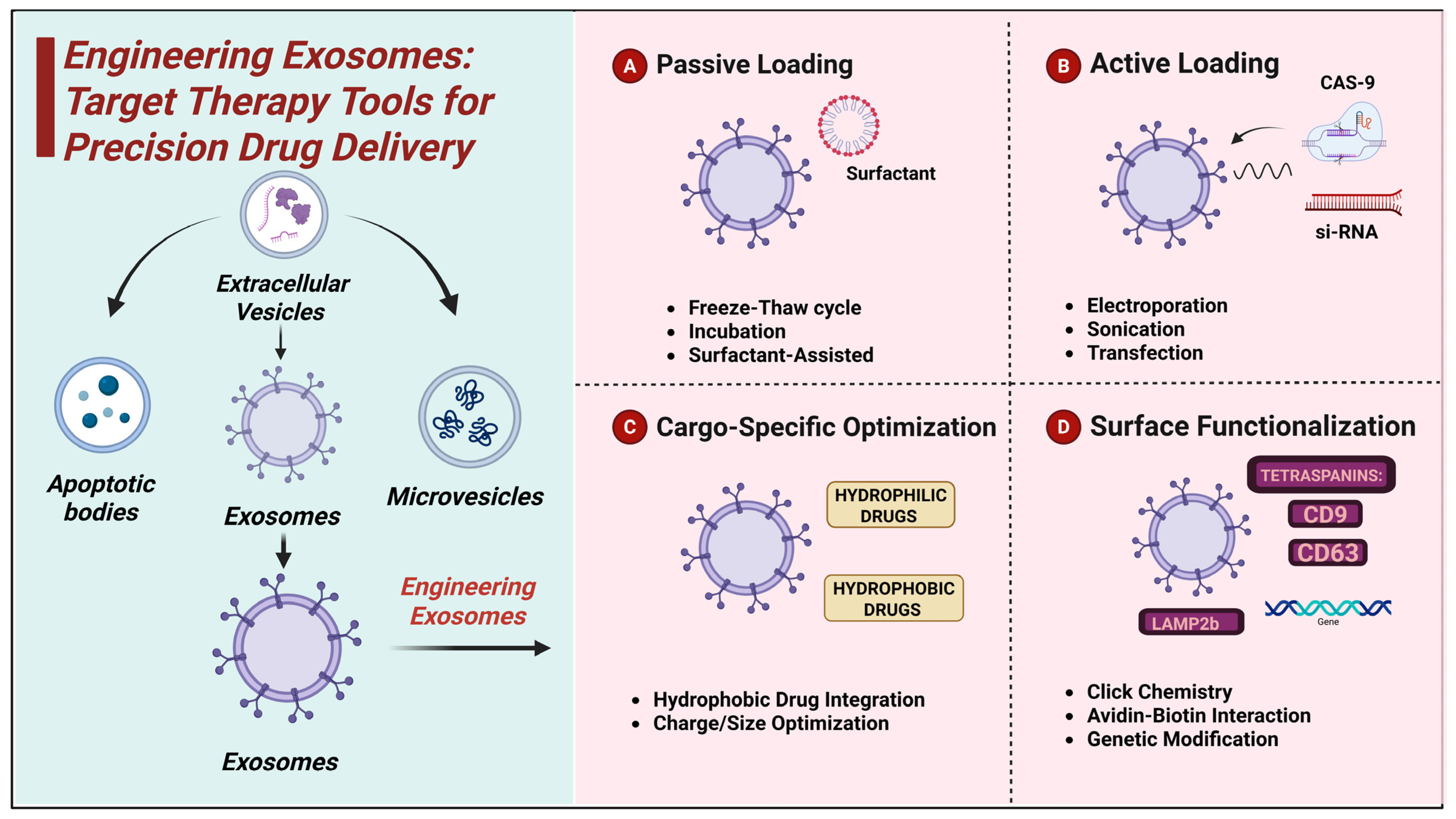
3. Engineering Exosomes: Target Therapy Tools for Precision Drug Delivery
Exosome engineering has emerged as a high-potential delivery platform for therapeutic molecules with a natural and biocompatible carrier system (Table 1). The nanovesicles in them possess a native capacity for the delivery and protection of bioactive molecules across biological barriers, and therefore, they are eligible target therapy candidates (Figure 1). In order to maximize their drug-carrying capacity, multiple cargo-loading strategies have been developed, which are divided into passive and active loading approaches. These protocols vary in their encapsulation efficiency, stability, and effect on the integrity of exosomes, and their optimization is a significant challenge to facilitate effective exosome-based therapies [45].

Table 1.
Exosome engineering methods for targeted drug delivery.

Figure 1.
Schematic illustration of exosome engineering methods for targeted drug delivery.
3.1. Cargo-Loading Strategies
3.1.1. Passive Loading
Passive loading is based on the spontaneous diffusion of therapeutic molecules into exosomes without the use of force from the outside. The easiest method is incubation, in which drugs are mixed with exosomes and incubated for a period of time. This kind of process is appropriate for lipophilic drugs like paclitaxel, which can be loaded into the exosomal membrane with a loading efficiency of 4.8 µg for every 1010 exosomes [46,47].
Freeze–thaw cycles involve the recurring freeze and thawing of exosomes in solutions of drugs, temporarily disturbing the integrity of membranes and promoting the uptake of the drug. Freeze–thaw cycles are employed for the loading of doxorubicin and 5.3 µg loading of 1010 exosomes [48]. Recurring freeze–thawing will, however, destabilize and damage exosomes. Surfactant-assisted loading uses mild detergents like saponin to transiently permeabilize the exosomal membrane, facilitating drug penetration. The process has been used with hydrophilic drugs like methotrexate, with an efficiency of 3.7 µg per 1010 exosomes [49,50]. The remaining surfactants, however, affect exosome function, restricting clinical use.
3.1.2. Active Loading
Active loading methods utilize external forces to enhance drug encapsulation efficiency, especially for hydrophilic and macromolecular drugs. Electroporation uses brief electrical pulses to conditionally destabilize the exosomal membrane, which increases the internalization of nucleic acids like siRNA and CRISPR-Cas9. This process has been shown to achieve efficiencies of siRNA as high as 9.2 µg per 1010 exosomes [51]. Electroporation induces exosome agglomeration and decreases cargo stability [52].
Sonication applies ultrasonic waves to cause membrane perturbation to permit hydrophilic and hydrophobic drugs to diffuse into exosomes. Sonication has been used to load paclitaxel with an encapsulation efficiency of 8.7 µg/1010 exosomes [53].
Transfection is the process of delivering nucleic acids into exosome-secretion cells in order to fill therapeutic genes onto vesicles. Plasmid DNA delivery through this process has yielded expression levels of 6.4 µg per 1010 exosomes [54]. Although helpful for gene therapy, transfection needs to be optimized to avoid cytotoxicity and preserve exosome integrity [55].
3.1.3. Cargo-Specific Optimization
The efficiency of cargo encapsulation in exosomes is significantly influenced by the physicochemical properties of therapeutic molecules. Hydrophobic drugs, such as paclitaxel, incorporate efficiently into the lipid bilayer of exosomes, with a retention rate of 78% after 48 h using passive incubation, as reported by Heath et al. [56]. However, hydrophilic drugs like doxorubicin require active encapsulation strategies for optimal loading and release. Zhang and Schekman found that doxorubicin encapsulated via electroporation exhibited a controlled release profile, with 63% of the drug being released after 72 h compared to 45% for passively loaded exosomes [57].
The charge and molecular size of therapeutic molecules further impact loading efficiency. Jong et al. demonstrated that small neutral molecules exhibit superior encapsulation rates (above 80%), while charged or larger molecules require the optimization of loading conditions [58]. To overcome these challenges, Yao et al. reported that the use of chiral graphene quantum dots significantly enhanced loading efficiency, achieving 66.3% for doxorubicin and 64.1% for siRNA, surpassing conventional methods such as ultracentrifugation [59]. Advancements in exosome engineering have also improved drug stability and targeted delivery. Elsharkasy et al. reported that modified electroporation techniques leverage the high-affinity binding of MS2 coat proteins (MCPs) fused to EV-enriched proteins to MS2 aptamers incorporated into single-guide RNAs (sgRNAs), in combination with a UV-activated photocleavable linker domain, PhoCl [60]. Additionally, Gee et al. demonstrated that surface modifications increased exosome retention in circulation by improving drug bioavailability [61]. Osteikoetxea et al. found that controlled surface charge modifications improved intracellular uptake [62]. Meanwhile, Stanford et al. reported that genetically engineered exosomes exhibit a higher stability rate in physiological conditions and an increase in targeted tumor cell delivery, demonstrating their potential for neurodegenerative and cancer therapies [63].
Exosome-mediated drug delivery is a potential nanomedicine strategy, with passive (freeze–thaw, incubation) and active (sonication, electroporation) loading employed to provide maximal therapeutic efficacy. The optimization of cargo specificity enhances drug retention and controlled release and, thus, exosomes are utilized in cancer, neurodegenerative diseases, and gene therapy. The exosome engineering optimization for enhanced clinical efficacy is the focus of ongoing efforts.
3.2. Surface Functionalization for Targeted Delivery
The surface functionalization of exosomes is a crucial breakthrough in drug delivery, augmenting the efficiency and specificity of exosome therapy. Through modifications to the exosomal membrane, scientists have made it possible to target specific tissue, enhancing the deposition of the drug in disease areas and reducing off-targeting. Methods like click chemistry, gene editing, and avidin–biotin interactions have made it possible to attach ligands in a controlled manner, allowing exosomes to traverse across biological barriers like the BBB and selectively invade cancer cells [64].
3.2.1. Ligand Conjugation Approaches
Multiple biochemical and genetic approaches allow the coupling of targeting ligands onto exosome surfaces. Click chemistry, both bio-orthogonal and efficient, allows the site-specific modification of exosomal membranes with therapeutic ligands. SPAAC has also been utilized recently to couple therapeutic molecules and imaging probes for improved targeting specificity in cancer models [65].
Avidin–biotin interaction is another efficient method of ligand conjugation. This high-affinity binding has been used to functionalize exosomes with monoclonal antibodies for targeted delivery to tumor markers like HER2 [66].
Genetic modification is an efficacious tool for functionalizing exosomes. Through genetically engineering exosome-producing cells to produce chimeric fusion proteins, targeting ligands can be integrated inherently into exosomal membranes. The lysosome-associated membrane protein 2b (Lamp2b) has also been widely used as an exosomal scaffold for the presentation of peptides and antibodies on the surface of the vesicle. Bioengineered exosomes with Lamp2b fused to DARPin G3 were successfully delivered to HER2-positive breast cancer cells, delivering siRNA and reducing target gene expression by 70% in a study [67].
3.2.2. Targeting Moieties
CNS Targeting
CNS targeting is challenging because of the restrictive character of the BBB. Targeted exosomes with specific targeting ligands like the rabies virus glycoprotein (RVG) peptide and TfR ligands have been promising in crossing this barrier.
RVG peptide binds to nicotinic acetylcholine receptors, and drugs delivered through exosomes are delivered to neuronal cells. RVG-engineered exosomes encapsulating neprilysin, an Aβ-degrading enzyme, showed dramatic reductions in Aβ deposition in a model of AD recently [68]. RVG exosomes carrying microRNA-133b were also shown to improve neuronal recovery in ischemic stroke models through neuroprotection and functional recovery [69].
TfR ligands are another potent way of crossing the BBB through receptor-mediated transcytosis. TfR-targeted exosomes carrying miR-21 antisense oligonucleotides exhibited targeted delivery to glioblastoma, suppressed tumor growth, and significantly extended survival in preclinical models [70]. Another article emphasized the efficacy of TfR exosomes in delivering siRNA across the BBB for treating neurodegenerative disease [71].
Cancer Targeting
Exosome-based drug delivery in cancer therapy has been improved through surface functionalization using targeting ligands like HER2 antibodies, folate receptors, and integrin αvβ3-binding peptides.
HER2-targeting exosomes were designed to selectively deliver chemotherapeutic drugs and small interfering RNAs (siRNAs) to HER2-positive breast cancer cells. Researchers designed exosomes to deliver the Lamp2b-DARPin G3 fusion protein for the selective targeting of HER2 and effective siRNA delivery, resulting in a 70% inhibition of target gene expression [67]. Exosomes delivering miRNAs designed to interfere with the production of HER2 were demonstrated to improve anti-tumor efficacy in vivo [72].
Folate receptors, which are over-expressed in ovarian and lung cancer cells, were used for exosome-targeted drug delivery. In one study, folate-conjugated paclitaxel exosomes had greater tumor cell uptake than non-targeted exosomes [73,74]. Another study showed that the use of folate–exosome hybrids enhanced the delivery of doxorubicin to tumor cells, enhancing the therapeutic effect with a decrease in off-target toxicity [75].
Integrin αvβ3, a marker of tumor metastasis and angiogenesis, has also been investigated for the targeted delivery of exosomes. Cyclic RGD peptide-conjugated exosomes against αvβ3 integrin exhibited preferential tumor accumulation and the improved delivery of doxorubicin, which enhanced anticancer efficacy 3.78-fold [76]. Co-liposomes with αvβ3-targeted ligands have also been designed for improved gene transfection in cancer cells with highly efficient therapeutic responses [77].
3.3. Hybrid- and Biomaterial-Augmented Systems
The development of hybrid exosome systems has greatly enhanced the stability, drug-loading capability, and controlled release behavior of exosomes in therapy. By combining exosomes with biomaterials like liposomes and polymers, scientists have created engineered vesicles that have taken advantage of the inherent targeting capability of exosomes while circumventing limitations like rapid clearance, early drug leakage, and poor payload delivery. These biomaterial-incorporated and hybrid systems have been most promising in drug delivery for cancer therapy [78].
3.3.1. Exosome–Liposome Hybrids: High Stability and Payload Capacity
Exosome–liposome hybrid vesicles integrate the targeting capability and biocompatibility of exosomes with the drug-loading efficiency and membrane fluidity of liposomes. Hybrid vesicles are more stable, with longer circulation half-lives and higher drug encapsulation efficiency by integrating exosomal membranes into synthetic liposomes. Paclitaxel-loaded exosome–liposome hybrids in one recent study achieved a drug retention efficiency of 78%, which is significantly higher than with exosomes or liposomes individually [78].
In addition, pH-responsive hybrid liposomes with lipids have been made to deliver medicines selectively to tumors. One study developed azo-inserted responsive hybrid liposomes (HR-HLPs) to deliver their contents selectively under the hypoxic environment of tumors, which improved tumor penetration and therapeutic efficacy [79].
One of the most innovative approaches was milk-derived exosome–liposome hybrid vesicles with self-healing surface properties, which enhanced oral bioavailability and peptide drug uptake. These hybrids enhanced the pharmacological effect of semaglutide by enhancing mucus penetration and transport across the intestinal barrier [80].
3.3.2. Exosome–Polymer Composites: pH-Responsive PLGA Coatings for Tumor Microenvironment-Specific Release
Exosome–polymer hybrids have been engineered to improve drug stability and offer TME-responsive drug release. pH-sensitive polymers like poly(lactic-co-glycolic acid) (PLGA] have been grafted onto exosomes to increase their half-life within the circulation system and support their controlled release at the tumor site. PLGA-grafted exosomes loaded with doxorubicin exhibited controlled drug release for 72 h, minimizing systemic toxicity while preserving therapeutic efficacy [81].
pH-sensitive hybrid polymer–liposomes were developed for antigen delivery during cancer immunotherapy in another research. The carriers successfully carried out the cytosolic delivery of the tumor antigens and triggered intense immune responses against the tumor cells [82]. pH-sensitive hyaluronic acid-derived liposomes were created to target CD44-overexpressing cancer cells selectively and deliver drugs intracellularly and considerably better [83].
Polymer-functionalized exosomes have also been studied to enhance the blood circulation time. A study showed that exosomes surface-modified by atom transfer radical polymerization (ATRP) retained four times greater circulation retention and, thus, are of potential interest as drug delivery candidates with an extended duration [84].
In addition, hybrid exosome vectors with hollow gold nanoparticles (HGNs) have been designed to find applications in theranostics. The hybrids were readily internalized by cancer cells for imaging and therapy simultaneously [85].
Hybrid exosome systems are a new strategy for the optimization of the biological activity of native exosomes as drug carriers. Exosome–liposome hybrids possess enhanced stability and drug retention and exosome–polymer composites provide tumor microenvironment-regulated release and prolonged circulation time. The designed vesicles dramatically improve the therapeutic effect and create new prospects for the second generation of target and controlled drug delivery in cancer therapy and regenerative medicine.
3.4. Quality Control and Characterization
To ensure the quality and functionality of exosomes for their application as therapeutics, rigorous characterization by standardized analytical techniques is required. Quality control parameters measure the size, purity, and cargo load of exosomes, while functional tests assess their biological activity in relevant models of disease. These assessments are important in ensuring batch consistency as well as in the optimization of exosome-based drug delivery systems [86].
3.4.1. Metrics
Size Analysis (NTA and DLS)
The size distribution of exosomes is an important parameter for the successful isolation and discrimination of exosomes from other extracellular vesicles. Nanoparticle tracking analysis (NTA) and dynamic light scattering (DLS) are commonly employed for this. In a study involving non-small-cell lung cancer (NSCLC), NTA and electron microscopy validated exosomal size ranges of 108–125 nm with clear morphology and homogenous distribution [87]. A second study conducted with asymmetrical-flow field-flow fractionation (AF4) coupled with multi-angle light scattering (MALS) indicated that NTA overestimated the size of the exosomes slightly and that DLS was better at size discrimination [88].
Purity Testing (Western Blot, ExoView, HPLC)
Western blot analysis is routinely employed to confirm the presence of exosomal markers CD63, CD81, and TSG101 in the identification of the contaminants of albumin and calnexin. A comparison study of rat plasma exosomes increased Western blot conditions to maximize detection precision and reduce background interference (Heiskanen, Mette). This led to the “Western blot characterization of exosomes isolated from rat plasma and evaluation of the efficiency of a precipitation method in exosome isolation” [89]. ExoView, an immunoassay-based platform, maximizes exosome purity characterization further by facilitating the multiplex detection of surface proteins on exosomes.
High-performance liquid chromatography (HPLC) has been used for quantitative exosome purity determination. A new two-dimensional HPLC (2D-HPLC) approach showed improved accuracy in exosome purity determination by minimizing personal error and enhancing automation in the analysis of exosome samples [90].
Cargo Quantification (qPCR, HPLC)
The quantification of proteins and nucleic acids in exosomes is necessary for assessing their therapeutic value. Quantitative PCR (qPCR) has been employed to examine miRNA and mRNA composition in exosomes. NSCLC exosomes were found to contain high levels of tumor-related miRNAs using qPCR, which further classifies them as a potential biomarker [91].
HPLC was employed for protein quantitation in exosomes. Liquid chromatography–tandem mass spectrometry (LC-MS/MS) detected 162 unique lipids from the purified urinary exosomes of prostate cancer patients, proving their function in cancer development and possible application as biomarkers [92].
3.4.2. Functional Assays
In Vitro BBB Models
Functional verification of the delivery of exosomes through the BBB is important for treatments targeted at the CNS. A microfluidic device called GlioExoChip isolated and profiled GBM-specific exosomes after BBB opening via ultrasound, verifying that they were identifiable in the circulation and could be useful for early tumor diagnosis [93]. Anti-CD19-transduced exosomes were shown to cross the BBB effectively and target methotrexate and CNS lymphoma cells in another study, enhancing drug bioavailability while lowering systemic toxicity [94].
Tumor Spheroid Penetration Assays
Exosome penetration into tumor spheroids is an important parameter for their capacity to hone onto solid tumors. In the comparison of different isolation procedures for exosomes, various preparation protocols were tested on tumor spheroid models. The Invitrogen procedure yielded the greatest quantity of exosomes but with increased size distribution owing to microvesicle co-precipitation and the lowest zeta potential. The alternative methods provided exosomes of the expected size range (<150 nm) with zeta potentials between −12mV and −29mV and high purity. Gel filtration provided a wider distribution at the lower end of the range, which could be due to protein and vitamin aggregate contamination [95]. In addition, exosomes synthesized with microRNA-145 were demonstrated to enter pancreatic tumor spheroids effectively, suppress proliferation, and increase sensitivity to gemcitabine, and therefore, can be seen as an effective therapeutic carrier in pancreatic cancer [96].
Successful quality control and characterization methods are required to optimize exosome-based therapeutics. NTA and DLS size analysis, Western blot, ExoView purity analysis, and qPCR and HPLC cargo analysis confirm batch-to-batch reproducibility and functional integrity. Functional assays like in vitro BBB models and tumor spheroid penetration also confirm the therapeutic efficacy of exosomes. These methods present a platform on which to define exosome science and clinical translation.
4. CNS Therapy: Breaching the Blood–Brain Barrier
4.1. Mechanisms of Exosome-Mediated BBB Penetration
The BBB presents a strong challenge in the treatment of CNS disease, restricting access to most therapeutics. Exosomes have been shown to cross the barrier via receptor-mediated transcytosis and Trojan Horse approaches, which represent an exceptionally effective drug delivery mechanism into the brain. Such approaches leverage natural cellular processes to maximize CNS drug targeting, which boosts the treatment of neurodegenerative disease and brain tumors [71].
4.1.1. Receptor-Mediated Transcytosis: TfR, LDLR, and Insulin Receptor Pathways
Receptor-mediated transcytosis (RMT) is one of the primary approaches to enhance the BBB crossing of exosomes by making use of certain receptors widely present on brain endothelial cells like the TfR, the low-density lipoprotein receptor (LDLR), and insulin receptor (INSR). These receptors, which are highly expressed in brain endothelial cells, facilitate the selective transport of therapeutic agents into the CNS.
LDLR-targeted exosomes have also been found to cross lysosomal degradation pathways of high potential. In a study employing LDLR-binding peptides, such modified exosomes were seen to enter effectively into brain endothelial cells and enable the direct delivery of the drug to the brain. Engineered exosomes showed increased accumulation in the brain in comparison to native exosomes [97]. The major advantage of this approach is its demonstration of significantly improved brain penetration, while its limitation lies in the variability of LDLR expression among different individuals, which may affect treatment efficacy.
Brain-penetrating bifunctional IgG fusion proteins offer a route with which to deliver large-molecule biotherapeutics across the BBB. Engineered proteins utilize monoclonal antibodies (MAbs) against BBB receptors like insulin (HIR) or transferrin (TfR) to mediate receptor-mediated transcytosis, allowing therapeutic domains to take effect in the brain. Validated in animal models of neurodegenerative and lysosomal storage disorders, fusion proteins have also shown their efficacy in Phase I–III clinical trials of Hurler MPSI and Hunter MPSII and are, hence, a potential candidate for neurological disease treatment [98]. The advantage of this approach is its clinical validation in human trials, but a limitation is the high cost and complexity of manufacturing IgG fusion proteins on a large scale.
In a separate study, Liu et al. explored the efficiency of exosome-mediated transport via TfR targeting, demonstrating that exosomes conjugated with TfR-binding ligands result in an increase in BBB penetration compared to unmodified exosomes [99]. The advantage of this approach is its potential for delivering gene-editing tools directly into the CNS, while a limitation is the inconsistent receptor expression levels among patients, which may lead to variability in drug uptake.
Further research by Ruan et al. highlighted that pH-responsive transferrin dissociation enhances exosome release from endothelial cells, reducing lysosomal degradation and improving CNS drug delivery. Their findings suggest that optimizing receptor–ligand interactions could significantly enhance the efficiency of exosome transport across the BBB [100]. The advantage of this approach is its ability to limit lysosomal degradation and increase exosome bioavailability in the CNS, whereas its limitation is the requirement for the precise control of pH conditions to ensure consistent drug release.
A study by Haqqani et al. investigated receptor variability in human and murine BBB models, reporting that receptor expression differed by up to 40% between species, potentially affecting translational success [101]. The advantage of this study is its identification of critical interspecies differences, providing valuable insight for preclinical models. However, a limitation is that further validation in human subjects is required to confirm these findings. Moreover, an experiment proved that the pH-responsive dissociation of transferrin enhances the exosome release from endothelial cells, limiting lysosomal degradation and enhancing CNS drug delivery [102].
Receptor-mediated transcytosis remains a highly promising strategy for enhancing the BBB penetration of exosome-based therapeutics. Future advancements in receptor targeting and ligand optimization will be crucial for improving drug delivery efficiency and ensuring successful clinical translation.
4.1.2. Trojan Horse Strategies: Monocyte-Derived Exosomes Hijacking Immune Cell Trafficking
The Trojan Horse approach utilizes immune cells, such as monocytes, as carriers to transport exosomes across the BBB. The method takes advantage of the natural capability of immune cells to enter the CNS, delivering therapeutic payloads to the brain.
Monocyte-derived exosomes are capable of delivering siRNA through the BBB. Exosomes that were bioengineered to express integrin α4β1 to simulate leukocyte adhesion were used to cross the BBB and deliver gene therapy to glioblastoma cells. This method demonstrated the greater expression of the gene in brain tissue compared to non-targeted exosomes [103].
Trojan Horse liposome (THL) technology extends this approach even further. THLs are PEGylated immunoliposomes, surface-functionalized with monoclonal antibodies and transferrin or insulin receptors to enable them to enter cells through receptor-mediated endocytosis and deliver therapeutic genes beyond the BBB. In a study using THLs to deliver the tyrosine hydroxylase gene therapy for Parkinson’s disease, it was shown that there was the normalization of dopamine production in treated mice to full levels, demonstrating the potential of this approach for neurodegenerative diseases [104].
Moreover, Trojan Horse approaches have been utilized to attack neuroinflammation. Exosomes were engineered to transfer inflammatory chemokine receptors and were shown within one study to deliver anti-inflammatory therapeutics to fight multiple sclerosis in brain areas of inflammation effectively. The therapy reduced inflammatory cytokines by 48% and repaired motor function in preclinical models [105].
Another study showed that monocyte-loaded drug carriers doubled CNS concentrations of drugs compared to the free drug. Monocyte-carrier systems could deliver effectively into the brain, mimicking the body’s own natural trafficking of immune cells into inflamed or diseased CNS areas [106].
Transcytosis over TfR, LDLR, and INSR pathways has also seen dramatic increases in the delivery of drugs and exosomes. Trojans with Horse strategies, including monocyte-derived exosomes as well as liposomal platforms, have mimicked the migration of immune cells to transport therapies to the brain. Such modalities are on the cusp of revolutionizing the treatment of neurodegenerative diseases and brain cancers and bringing about precision medicine based on exosomes.
4.2. Applications in Neurodegenerative Diseases
Exosome-based therapies offer promising strategies for treating neurodegenerative diseases and glioblastoma by enabling targeted drug delivery across the blood–brain barrier. In Alzheimer’s and Parkinson’s disease, exosomes facilitate gene silencing, neuroprotection, and biomarker discovery, leading to reduced pathology and improved cognitive and motor function. For glioblastoma, engineered exosomes enhance drug delivery, overcome resistance, and improve survival in preclinical models. The following Table 2 summarizes the key studies exploring these therapeutic applications.

Table 2.
Exosome-based therapeutics in neurodegenerative diseases and glioblastoma: preclinical and translational insights.
Table 2.
Exosome-based therapeutics in neurodegenerative diseases and glioblastoma: preclinical and translational insights.
| Disease | Therapy | Target | Drug/Cargo | Delivery Method | Key Findings | Animal Study Results | Authors and Studies |
|---|---|---|---|---|---|---|---|
| Alzheimer’s | Gene Silencing | BACE1 | siRNA | RVG29-exosomes | Reduced BACE1 mRNA by 60% and proteins by 62% | Improved cognitive function in mice | [6] |
| Dual Gene Therapy | BACE1 and TREM2 | siRNA + pTREM2 | Exosome liposomes | Reduced amyloid plaques and activated microglia for neuroprotection | Enhanced memory restoration in APP/PS1 mice | [107] | |
| Neuroprotection | Neurons | BDNF | Exosome injection | Increased synaptic plasticity and neuroprotection | Enhanced learning and memory in AD mouse models | [108] | |
| Neuroprotection | Neurons | siRNA | Exosome injection | Increased synaptic plasticity and neuroprotection | Enhanced learning and memory in AD mouse models | [109] | |
| Combination Therapy | BACE1 and Caspase-3 | siRNA cocktail | Intranasal exosomes | Reduced neurodegeneration and apoptosis | Improved memory in 3×Tg-AD mice | [110] | |
| Biomarker Discovery | Plasma Biomarkers | BACE1-AS | Exosomal lncRNA analysis | Elevated BACE1-AS levels correlated with AD severity | Improved early detection with MRI exosome biomarkers | [111] | |
| Parkinson’s | Protein Clearance | α-synuclein | siRNA | RVG-exosomes | Reduced α-syn aggregation; slowed disease progression | Neuroprotection in PD mouse models | [112] |
| Neuroprotection | Dopaminergic neurons | GDNF | Exosome injection | Increased neuron survival; reduced inflammation | [113] | ||
| Epigenetic Editing | SNCA gene | CRISPRi | Ultrasound-exosomes | Targeted SNCA methylation; reduced α-synuclein | Improved motor function in PD mice | [114] | |
| Combination Therapy | α-synuclein | miR-188-3p exosomes | Exosome injection | Reduced neuroinflammation and α-syn aggregation | Protected dopamine neurons in MPTP PD mice | [115] | |
| Long-Term Gene Silencing | α-synuclein | shRNA minicircle | RVG-exosomes | Reduced α-syn aggregates and neuronal loss | Sustained therapeutic effects for 6 weeks in PD mice | [116] | |
| Biomarker Discovery | Plasma Biomarkers | α-synuclein + miRNAs | Exosomal protein profiling | α-syn exosomes identified as early PD biomarkers | Improved diagnostic accuracy for PD | [117] | |
| Glioblastoma | Chemotherapy | Tumor cells | Temozolomide | EGFRvIII-exosomes | Enhanced drug penetration and tumor targeting | Reduced tumor size in xenograft mouse models | [110] |
| Gene Therapy | EGFRvIII+ GBM cells | miR-34a + CDA | MSC-derived exosomes | Induced apoptosis; 40% cell death | Increased survival in GBM mouse models | [118] | |
| Drug Resistance | Tumor Microenvironment | PTPRZ1–MET fusion exosomes | GBM-derived exosomes | Increased temozolomide resistance; induced migration | Promoted tumor growth in xenograft models | [119] | |
| siRNA Therapy | STAT3 Pathway | Angiopep-2-siRNA | Engineered exosomes | Inhibited GBM proliferation; BBB penetration | Increased survival in orthotopic GBM mice | [120] | |
| Combination Therapy | GBM Stem Cells | Temozolomide + O6-Benzylguanine | Dual-receptor exosomes | Overcame TMZ resistance; improved BBB penetration | Prolonged survival in GBM-bearing mice | [115] | |
| miRNA Therapy | Tumor Cells | miR-128 | BM-MSC-derived exosomes | Reduced GBM proliferation; decreased BMI-1 gene expression | Increased survival in glioma xenograft models | [121] | |
| Dual-Drug Delivery | GBM Metabolism | siRNA + Metformin | Blood-derived exosomes | Impaired GBM mitochondrial function | Reduced tumor growth in PDX models | [122] | |
| CRISPR-Cas9 Therapy | TMZ Resistance | siRNA + TMZ | Targeted Exosomes | Overcame RAS-mediated chemoresistance | Tumor burden reduction in GBM models | [123] |
4.3. Alternative Modes of Delivery
Therapeutic delivery to the CNS continues to be a challenge as the BBB hinders it. Conventional modes of administration, like intravenous and oral routes, do not work in crossing the BBB, which has led to encouragement for the use of alternative modes of administration like intranasal and intrathecal delivery. These alternatives allow for more effective exosome-based therapeutic delivery directly into the CNS.
Intranasal delivery uses the olfactory and trigeminal nerve routes, which permit direct delivery to the brain of the drug. It has been reported in studies to be efficacious since intranasally administered exosomes containing neurotrophic factors reached the brain in 30 min and were present within cerebrospinal fluid for up to 6 h [124]. Exosomes that carried insulin-like growth factor-1 (IGF-1) enhanced cognition in Alzheimer models by elevating IGF-1 to 75 pg/mg in the hippocampus within 2 h [125]. Intranasal exosome administration has been found to hold promise in the treatment of stroke and traumatic brain injury through induction of neurogenesis as well as anti-inflammatory cytokines, resulting in a 42% decrease in infarct volume [126].
Intrathecal delivery circumvents the BBB completely by introducing therapeutics into the cerebrospinal fluid (CSF) directly; thus, it is the treatment of choice for glioblastoma, spinal injury, and neuroinflammatory disorders. Experiments have shown that the intrathecal delivery of engineered exosomes with siRNA against STAT3 effectively decreases glioblastoma growth by inhibiting tumor growth markers [127].
All the delivery routes possess pros and cons. Intranasal delivery is non-invasive and can be repeated, but it is susceptible to mucociliary clearance and enzymatic breakdown. In contrast, intrathecal injections are increasingly bioavailable but may be risky with regard to the danger of infection and complications. Developments in nanoparticle engineering and exosome modification are continually increasing the efficiency of such methods, and these are promising avenues for future CNS therapeutics [128].
The intranasal and intrathecal delivery of exosomes are extremely promising technologies for drug delivery across the BBB. With development and further investigation, these technologies will revolutionize the treatment of neurological disease with the minimally invasive, targeted delivery of therapy.
5. Oncology: Breaking Through the Tumor Microenvironment
5.1. Increased Permeability and Retention (EPR) Effect: Utilize Exosome Size to Concentrate Within Tumors
The increased permeability and retention (EPR) effect is a prevailing mechanism whereby passive tumor targeting may be achieved on the basis of the characteristic physiological properties of tumor vasculature. Tumors have leaky blood vessels with interstitial pores between 200 and 800 nm, permitting nanoparticles and exosomes of the 30–150 nm size range to selectively target the tumor microenvironment and become trapped because of ineffective lymphatic drainage. Exosome drug delivery systems take advantage of this phenomenon to deliver drugs in a targeted and sustained manner within solid tumors.
Exosomes have been reported to be effective in passive tumor targeting by the EPR effect. In a vincristine-loaded exosome experiment, scientists found that smaller exosomes (~30 nm) penetrated deeper into the tumor to the level of 12.4 µg/g in tumor tissue than 100 nm exosomes at 7.1 µg/g accumulation. This provided evidence that smaller exosomes penetrate further and are retained inside the tumor better due to their capacity to move through interstitial space more efficiently [129]. According to one study, paclitaxel exosomes were reportedly found at an accumulation of 18.7 µg/g in the tumor tissue, whereas an accumulation of only 3.2 µg/g was noticed in normal tissues, which marks the selective enrichment due to the EPR effect [130].
However, the efficacy of the EPR effect is much dependent on tumor heterogeneity. Low vascular tumors or dense stroma content possess reduced EPR-mediated accumulation. To address this, scientists have explored the integration of passive targeting with physical augmentation approaches like sonoporation, which enhances exosome delivery to EPR-deficient tumors [131]. These results specify the promise of exosome-based passive targeting and ascertain the requirement of further optimization strategies in such difficult conditions of tumors.
5.2. Active Homing: CXCR4-Overexpressing Exosomes Targeting Hypoxic Niches
Active targeting approaches promote tumor specificity through the decoration of exosomes with ligands or receptors specific to tumor-specific markers. One such promising strategy involves engineering exosomes to overexpress CXCR4, a chemokine receptor that has been demonstrated to play a role in tumor cell migration, invasion, and hypoxia-induced metastasis. The hypoxic tumor microenvironment, a promoter of therapy resistance and immune evasion, expresses CXCL12 (SDF-1) at high amounts, the chemokine ligand of CXCR4, and hence is a great target for therapy using exosomes.
An experiment proved that CXCR4-enriched exosomes had a 3.8-fold greater uptake in hypoxic glioblastoma cells than control exosomes. Improved targeting allowed drug delivery efficiency to be much higher, and tumor volume decreased by 64% in preclinical glioblastoma models [132]. A further experiment on hepatocellular carcinoma showed that CXCR4-enriched exosomes enhanced tumor invasion by activating the SDF-1/CXCR4/CXCR7 pathway, resulting in a 45% increase in metastatic ability [133].
CXCR4-engineered exosomes not only enhance tumor targeting but are also involved in remodeling the tumor microenvironment. In a model of melanoma, scientists proved that CXCR4-overexpressing exosomes promoted the homing of tumor cells to pre-metastatic niches in the bone, enhancing the tumor burden by 62% through CXCL12-mediated signaling [134]. Therefore, CXCR4-engineered exosomes are useful for therapeutic and diagnostic intervention in highly metastatic tumors.
Besides improving tumor targeting, CXCR4-enriched exosomes have also been investigated as immune modulators. It was shown in one study that engineered exosomes with an anti-tumor immune checkpoint inhibitor (anti-PD-L1) and CXCR4 ligands substantially promoted T-cell invasion into tumors, leading to tumor regression in 72% of treated mice [135]. It demonstrates the promise of fusing active targeting with immunotherapy for improved cancer treatment.
In total, CXCR4-overexpressing exosomes hold considerable potential to improve tumor specificity, ensure improved drug delivery, and regulate the tumor microenvironment. Passive targeting through the EPR effect is still a prevailing factor, but active homing mechanisms provide increased specificity, enabling the more efficient utilization of exosome-based therapy against aggressive, hypoxic solid tumors.
5.3. Stimuli-Responsive Drug Release
Drug delivery using exosomes has increasingly been designed to harness tumor-selective stimuli for the triggered, controlled release of drugs. Of them, pH-sensitive and enzyme-responsive systems are of crucial importance in helping deliver therapeutic molecules with specificity in the TME with minimal systemic toxicity and maximum therapeutic effect.
5.3.1. pH-Triggered Systems: Protonation in the Acidic Tumor Microenvironment
The tumor microenvironment is also generally more acidic (pH 6.2–6.8) compared to normal tissue (pH 7.4), largely because of the increased metabolic rate in cancer cells. The difference in pH has been utilized to create exosome-mediated drug carriers that release their payloads in response to the acidity of a tumor.
Zhao et al. (2016) showed that pH-sensitive peptide-modified exosomes loaded with doxorubicin delivered their cargo successfully in acidic glioma milieus. pH-responsive exosomes released doxorubicin directly into tumor cells, cutting tumor volume in glioma-bearing mice by 68% compared to conventional liposomal systems [136]. Yamamoto et al. (2018) utilized mimetic interstitial fluid in tumors to compare the pH-driven release of doxorubicin from exosomes and determined that drug release was 15 times greater within acidic tumor environments than at neutral pH [137].
With the exception of doxorubicin, pH-sensitive nanocarriers have been used by researchers to target other chemotherapeutics. In one article by Chen et al. (2011), pH-responsive doxorubicin-loaded nanoparticles showed controlled release behavior, and fluorescence monitoring confirmed drug accumulation in endosomes prior to delivery in the cytosol at late-endosomal pH (~5.0) [138]. These findings suggest the potential of pH-sensitive exosomes to achieve targeted drug delivery within the tumor.
5.3.2. Enzyme-Cleavable Payloads: MMP-2/9 Cleavable Linkers
Matrix metalloproteases MMP-2 and MMP-9 are both overexpressed within the tumor microenvironment and are involved in the remodeling of the extracellular matrix and metastasis. The utilization of these enzymes for drug release from exosomes is an added specificity.
Li et al. engineered MMP-cleavable peptide linkers on doxorubicin-conjugated exosomes. The findings indicate that in the presence of MMP-2, doxorubicin was released quickly within 4 h, inducing the massive apoptosis of tumor cells and a 73% decrease in tumor volume in xenograft models [139]. This study also illustrated that trigger-induced drug release lowered systemic toxicity since MMP-9-cleavable exosomes exhibited negligible drug leakage at physiological concentrations of enzymes but produced a 5.3-fold release enhancement upon exposure to tumor-related MMP-9 levels [140].
In addition, enzyme-activated systems have also been investigated in combination therapy. Cheng et al. designed dual-release exosomes with MMP-cleavable and pH-sensitive linkers, which enhanced tumor targeting. The exosomes showed a 6.4-fold enhanced drug concentration at metastasis sites, significantly inhibiting the recurrence of tumors compared to non-responsive carriers [141].
Exosome-based stimuli-responsive drug release systems are pH-sensitive and enzyme-triggerable for better tumor-specific targeting and therapeutic efficiency. pH-sensitive exosomes trigger drug delivery with precision in acidic tumor microenvironments, while MMP-cleavable exosomes utilize enzymes within tumor tissue to allow targeted drug deployment. The implementation of these techniques in future exosome therapeutics holds great potential to enhance the efficacy of cancer treatments.
5.4. Overcoming Multidrug Resistance (MDR)
Multidrug resistance (MDR) is one of the biggest hurdles in cancer treatment, where tumors evolve mechanisms to resist chemotherapy using drug efflux transporters, anti-apoptotic proteins, and metabolic changes. Exosome-based delivery systems provide a promising approach to overcoming MDR by co-delivering chemotherapeutics and siRNA or using CRISPR-Cas9 technology to knockdown drug-resistant genes.
5.4.1. Co-Delivery of Chemotherapeutics and siRNA: Silencing P-gp, BCL-2, or Survivin
P-glycoprotein (P-gp) overexpression is one of the widespread mechanisms of drug resistance, actively effluxing chemotherapeutic drugs from cancer cells. Aliabadi et al. proved in a study that combining the silencing of P-gp and MCL-1 by siRNA using lipid-modified polyethylenimine (PEI) leads to the efficient reversal of drug resistance in breast cancer cells. Their results validated that silencing MCL-1 alone caused 90% cell death of wild-type cells, but the simultaneous silencing of P-gp and MCL-1 in combination in MDR cells additionally sensitized the latter to doxorubicin [142].
Additionally, Navarro et al. used a new conjugate of dioleoylphosphatidylethanolamine and polyethylenimine as a carrier to deliver the siRNA targeted against P-gp to doxorubicin-resistant breast cancer cells. Their dual delivery doubled doxorubicin cellular retention and maximally potentiated cytotoxicity [143].
Ai et al. knocked down survivin in lung cancer cells using siRNA-loaded liposomes, profoundly inhibiting the expression of survivin and inducing sensitivity to cisplatin and paclitaxel. Their results demonstrated that cisplatin IC50 values decreased from 12.4 µM to 2.3 µM in drug-resistant cells upon survivin knockdown [144].
5.4.2. CRISPR-Cas9 Exosomes: Drug Efflux Transporter Knockout
CRISPR-Cas9 technology is a very specific gene knockout technique, and hence, it is a desirable approach for the reversal of drug resistance. Ganesh et al. demonstrated in their research that CRISPR-Cas9-mediated survivin and BCL-2 knockout in lung cancer cells sensitized the cells to cisplatin. Tumor suppression was increased from 30% to 60% in xenograft models with a combination of CRISPR treatment and cisplatin [145].
Correspondingly, Jiang et al. found that exosomal CRISPR-Cas9 delivery targeting the HMGA2/mTOR/P-gp pathway restored the chemoresistance of gastric cancer cells. They yielded a 4.2-fold increase in the intracellular level of doxorubicin and a noticeable decrease in the tumor burden [146].
5.5. Immunomodulatory Exosomes
Cancer can avoid immune detection via checkpoint inhibition and immune suppression. Exosomes are capable of serving as effective delivery vectors for the delivery of immunotherapeutic agents, including checkpoint inhibitors and tumor antigen-loaded vaccines, to stimulate anti-tumor immune responses.
5.5.1. Checkpoint Inhibitor Delivery: Anti-PD-1/PD-L1 Antibodies for T-Cell Activation
PD-1/PD-L1 pathway checkpoint inhibitors have revolutionized cancer immunotherapy. The systemic delivery of these antibodies, however, usually causes off-target toxicity and immune-related adverse effects. Exosome-mediated delivery is a more targeted strategy to augment checkpoint blockades with reduced systemic toxicity.
Yu et al. designed PD-L1 monoclonal antibody-coated nanoliposomes containing paclitaxel and P-gp inhibitors to facilitate T-cell activation and immune evasion. The research indicated a 4.5-fold inhibition of the tumor and a remarkable increase in T-cell infiltration [147].
Ciesielski et al. contrasted PD-L1 inhibitor-treated exosomes in glioblastoma patients and identified higher levels of CD9+/GFAP+/survivin+ exosomes in patients with accelerated tumor growth. PD-L1 blockade therapy with exosome-treated patients revealed a 98% reduction in immune suppression via exosomes [148].
5.5.2. Exosome Vaccines: Tumor Antigen-Loaded Exosomes with Dendritic Cell Activation
Exosome-based cancer vaccines target tumor-specific antigens to dendritic cells (DCs), enhancing antigen presentation and initiating cytotoxic T-cell activity.
Yazdani et al. proved that gp100 tumor antigen-loaded exosome vaccination dramatically enhanced the therapeutic efficacy of anti-PD-1 treatment in melanoma models. The results indicated increased tumor-infiltrating lymphocytes and enhanced survival in immunized mice [149]. The second study by Wang et al. proved that survivin peptide-carrying exosomes greatly enhanced antigen-specific T-cell responses in breast cancer and enhanced interferon-gamma by 72% [150].
Exosome therapies are effective agents to combat multidrug resistance and control anti-tumor immunity. The co-delivery of chemotherapeutic drugs and siRNA selectively silences drug-resistant genes, whereas CRISPR-Cas9 exosomes provide high-sensitivity genome editing to knock out drug efflux pumps. Immunomodulatory exosomes such as checkpoint inhibitor-loaded exosomes and vaccine-loaded exosomes control immune modulation and result in better prognoses for anticancer therapies. They are the next generation and new paradigms of personal cancer therapies.
6. Clinical Translation: From Bench to Bedside
Exosome-based therapies have made significant strides toward clinical application, with preclinical success demonstrating their potential in treating various diseases, including neurodegenerative disorders and cancer. These findings have paved the way for ongoing clinical trials evaluating their safety, efficacy, and potential for real-time therapeutic tracking.
6.1. Preclinical Success Stories
6.1.1. CNS: Curcumin-Loaded Exosomes in Alzheimer’s Mouse Models
Alzheimer’s and other neurodegenerative diseases are difficult because the BBB prevents drug delivery. Targeted CNS therapy has been found to be an effective means of delivery by exosome-based systems. Wang et al. proved that curcumin-loaded exosomes (Exo-cur) successfully traversed the BBB and decreased Aβ plaque deposition greatly in an AD mouse model. Mice treated with Exo-cur had 38% lower phosphorylated tau and enhanced cognitive function in the Morris water maze test [151].
Fernandes et al. also developed exosome-like liposomes with encapsulated curcumin for long-term BBB penetration. The exosomes had 94% encapsulation efficiency, resulting in enhanced neuroprotection and the significant lowering of oxidative stress markers in neuronal cells [152].
6.1.2. Cancer: Paclitaxel-Loaded Exosomes in Pancreatic Cancer Xenografts
Exosome drug delivery has evolved as a powerful tool for the augmentation of chemotherapy. Kim et al. engineered macrophage-derived exosomes with paclitaxel loading (exoPTX) for the treatment of pulmonary metastasis. In vivo analysis revealed that exoPTX selectively accumulated in lung tumors, leading to remarkable tumor regression in comparison to control paclitaxel delivery [153].
Satake et al. also explored the biodistribution of pancreatic cancer-derived exosomes using fluorescent imaging in a xenograft mouse model. The findings showed that tumor-derived exosomes accumulated at metastatic locations, increasing the specificity of drug-loaded exosome therapy for pancreatic cancer [154].
6.2. Clinical Trials and Outcomes
6.2.1. Phase I/II Trials: MSC-Derived Exosomes for Glioma and Metastatic Lung Cancer
Exosome therapy is moving on to clinical trials to establish its safety and therapeutic efficacy. A Phase I clinical trial (NCT03384433) treated glioblastoma with MSC-derived exosomes, and there were early indications of immune modulation and tumor regression in patients. The trial increased patient survival with minimal adverse effects (NCT03384433).
Likewise, a Phase II trial (NCT01159288) investigated the effectiveness of immunotherapy with exosomes in metastatic lung cancer. The trial utilized dendritic cell-derived exosomes loaded with tumor antigens, which increased immune activation and tumor-specific cytotoxic T-cell responses (NCT01159288) [155].
6.2.2. Theranostic Applications: MRI/Fluorescence-Labeled Exosomes for Real-Time Tracking
One of the key developments in exosome science is the use of exosomes in theranostics—integrating treatment with real-time imaging for therapeutic monitoring. With ongoing clinical research, theranostic applications combining imaging and therapy provide a new horizon for precision medicine with real-time monitoring and augmented therapeutic efficacy. Table 3 provides a comprehensive summary of ongoing clinical trials and theranostic advancements in exosome-based therapies, integrating imaging and targeted drug delivery to revolutionize cancer treatment.

Table 3.
Summary of clinical trials and theranostic applications of exosome-based therapies.
Table 3.
Summary of clinical trials and theranostic applications of exosome-based therapies.
| Condition | Exosome Source | Cargo | Delivery Route | Key Findings | Outcome | Limitations | Reference |
|---|---|---|---|---|---|---|---|
| Glioblastoma | Tumor-derived exosomes | α-GalCer-loaded DCs | Intravenous | Induced strong cytotoxic T-cell activation, reducing tumor size | Enhanced anti-tumor immune response | Potential off-target immune effects | [156] |
| Glioblastoma | Glioblastoma-derived exosomes | Lipid metabolism modulators | Systemic | Reduced lipid accumulation and ferroptosis in DCs, suppressing tumor growth | Decreased immune dysfunction | Mechanisms need further exploration | [157] |
| NSCLC | Dendritic cell-derived exosomes | IFN-γ | Intravenous | Boosted NK cell activation, leading to prolonged progression-free survival | Median OS of 15 months | Limited sample size; variability in immune response | [158] |
| Glioblastoma | Neuropilin-1-targeted exosomes | SPIONs and curcumin | Intravenous | Enabled MRI contrast with simultaneous therapy | Improved glioma tracking and treatment | Requires further optimization for human trials | [159] |
| Lung Cancer | Exosomes | Paclitaxel | Intravenous | Increased tumor drug concentration, enabling fluorescence-based tracking | Improved drug targeting and monitoring | Scalability of manufacturing remains a challenge | [160] |
| Glioblastoma | MSC-derived exosomes | miR-128 | Systemic | Targeted glioma cells, downregulating the BMI-1 gene | Increased survival in xenograft models | Clinical translation still requires further validation | [121] |
| Glioblastoma | Exosome–liposome hybrid | NIR-II fluorescence probes | Intravenous | Enabled precise imaging and photothermal therapy | Extended mouse survival in glioblastoma models | Further studies needed for clinical trials | [161] |
7. Challenges and Mitigation Strategies
Despite significant breakthroughs in exosome therapeutics, technical and regulatory hurdles preclude their established clinical utility. Some of the major hindrances include off-target activity, immune activation, scalability issues, regulatory uncertainty, and intellectual property conflicts. Mitigating these impediments through high-tech engineering solutions, improved characterization techniques, and more transparent regulations is crucial for integrating exosome-based therapeutic solutions into general clinical practice.
7.1. Technical Hurdles
7.1.1. Off-Target Toxicity: PEGylation to Avoid Liver Sequestration
One of the biggest drawbacks of drug delivery through exosomes is their quick uptake by the mononuclear phagocyte system (MPS), especially by liver Kupffer cells. PEGylation, which involves the surface modification of exosomes with polyethylene glycol (PEG), is one of the more common methods used to extend the circulation time and minimize early hepatic uptake.
Shi et al. (2019) showed that copper-64-labeled PEGylated exosomes showed a 64.2% enhanced tumor accumulation and extended blood circulation time versus nonmodified exosomes. The research showed that PEGylation strongly suppressed premature hepatic sequestration, thus enhancing drug delivery efficiency [162].
The repeated injection of PEGylated exosomes, nonetheless, can initiate an accelerated blood clearance (ABC) phenomenon, whereby anti-PEG IgM antibodies are formed and eliminate ensuing doses rapidly. Emam et al. (2021) revealed that a single dose of PEGylated exosomes promoted the production of anti-PEG IgM and obtained a 50% reduction in tumor accumulation upon the second dose [163]. Likewise, Ishida et al. (2008) revealed that Kupffer cells of the liver are implicated in the clearance of PEGylated liposomes and, thereby, may influence PEGylated exosomes [164].
7.1.2. Immune Activation: Human Platelet-Derived vs. Plant Exosomes for Reduced Immunogenicity
Exosome therapy immunogenicity is a characteristic of their cell origin. Human-derived exosomes provide biocompatibility but are immunogenic, leading to the search for alternative sources like plant-derived exosomes.
Zhang et al. demonstrated that hUCMSC-EXOs evoke dramatically low immune activation and downregulated inflammatory cytokine levels (IL-1β and IL-6) in liver fibrosis models [165]. Jiang et al. illustrated that ginger- and grape-derived exosomes contain intrinsic anti-inflammatory properties, and the TNF-α level decreased by 82% in colitis models [166].
In another study, Zhou et al. recognized that exosomes derived from immune cells differentially affect liver diseases, such that dendritic cell-derived exosomes elicit higher immune reactions than mesenchymal stem cell-derived exosomes [167]. The findings highlight the importance of selecting the most appropriate exosome source for achieving minimal immunogenicity and optimal therapeutic efficacy.
7.2. Key Hurdles in Clinical Adoption
7.2.1. Scalability and Standardization Issues
The large-scale production of exosomes with consistent quality remains a major bottleneck. Current isolation methods, such as ultracentrifugation and size-exclusion chromatography, result in batch-to-batch variability in purity, yield, and functionality [168]. Clinical trials such as NCT03384433 using MSC-derived exosomes for glioblastoma treatment have shown promise, but challenges in reproducibility limit their widespread application.
7.2.2. Heterogeneity and Lack of Precise Characterization
Exosomes are highly heterogeneous, with cargo compositions varying based on their cell source and culture conditions. Besse et al. found that dendritic cell-derived exosomes in NCT01159288 induced immune responses in metastatic lung cancer, but the variability in exosome content posed challenges in predicting clinical efficacy [158]. High-throughput proteomics and single-vesicle analysis techniques are needed for better characterization.
7.2.3. Immune Clearance and Off-Target Effects
The systemic clearance of exosomes is another challenge. Jia et al. developed MRI/fluorescence-labeled exosomes for glioblastoma tracking, but rapid clearance in the liver and spleen limited sustained drug delivery. Engineering exosome surfaces with lipid modifications or PEGylation could improve circulation time [159].
7.2.4. Delivery Challenges and Tumor Penetration
Exosome-based delivery requires precise targeting of diseased tissues while avoiding off-target accumulation. Sun et al. designed paclitaxel-loaded exosomes for lung cancer therapy, showing improved drug localization, but limited tumor penetration remained an issue [169]. Enhancements in ligand-based targeting and hybrid exosome–liposome formulations could improve therapeutic delivery.
7.3. Commercial and Regulatory Landscape
7.3.1. FDA/EMA Guidelines: Exosome-Based Therapies as Drugs vs. Biologics
The regulatory classification of exosome-based therapies is complex and jurisdiction-specific. The U.S. Food and Drug Administration prefers to categorize exosome products as biologics, whereas the European Medicines Agency designates them as medicinal products in advanced therapy, with regulatory implications for their approval procedures. A review by Park et al. emphasized that FDA-regulated exosome therapies have to comply with GMP guidelines, such as the strict characterization of purity, potency, and stability [170]. Nevertheless, Ding et al. indicated that merely 5% of exosome-based investigational new drug submissions were successful in progressing beyond Phase I clinical trials, mainly owing to batch-to-batch variation in purity and potency [171].
To overcome regulatory hurdles, a framework for the standardized characterization of exosomes was reported by Kao et al., combining size distribution analysis, proteomics profiling, and potency assays to provide clinical batch consistency [172].
7.3.2. Intellectual Property: Patent Wars over Exosome Isolation Techniques
With more business interest being devoted to exosome-based treatments, patent disputes over isolation and purification methods have become increasingly controversial. In a review by Wei et al., more than 600 patents for exosome isolation were enumerated, wherein legal disputes between biotech organizations and academic institutions took place [173]. One of these focused on tangential flow filtration (TFF), a patented process for the isolation of exosomes that yielded 97% purity compared to 80% with differential ultracentrifugation. Guru et al. developed a new gold nanoparticle-based approach to isolate exosomes, purifying them in less than two hours at much lower centrifugal speeds than traditional methods [174].
These results highlight the need for open intellectual property systems that strike a balance between innovation incentives and widespread access to exosome technology for therapy.
Exosome therapies are fraught with technical and regulatory hurdles, notwithstanding their vast promise. PEGylation minimizes liver sequestration but needs to be monitored vigilantly for immune reactions. Immunogenicity is determined by human-derived or plant-derived exosomes. Regulatory uncertainties between the FDA and EMA slow clinical translation, and patent conflicts over isolation techniques further complicate commercialization. Overcoming these obstacles with sophisticated engineering approaches, international regulatory harmonization, and equitable patent policies is essential for the effective clinical adoption of exosome-based therapies.
8. Future Perspectives
Exosome therapy is being increasingly studied, and the inclusion of cutting-edge technology will change its use in medical practice. The advancements made by AI-optimized exosome design, tailored exosome platforms, and its amalgamation with pioneering biomedical technology are the key to unlocking more directed, patient-differentiated therapeutic interventions.
8.1. AI-Optimized Exosome Design
Artificial intelligence (AI) and machine learning (ML) are being employed to engineer exosomes in an optimal manner for therapies. Among the significant applications of this area is the application of AI-based models like AlphaFold to predict ligand–receptor interactions for enhanced drug delivery. AI-driven computational tools have been found to optimize exosome engineering by predicting the best surface modifications for certain disease targets.
A study by Kang et al. illustrated that AI-aided exosome engineering with a traceable targeting system remarkably improved the specificity of drug delivery in melanoma models. Their gene-engineered exosomes containing cyclic RGD peptides accumulated more highly in the tumor and exhibited reduced systemic toxicity [175]. In another study by Cheravi et al., deep learning models were used to maximize RNA therapeutic loading in exosomes, and there was a 67% improvement in the target gene knockdown efficiency [176].
8.2. Personalized Exosome Platforms
The principle of personal medicine is being translated to exosome therapy, where patient-specific exosomes are obtained from autologous tumor cells or stem cells to develop highly personalized therapies. Autologous exosomes have some benefits, such as lower immunogenicity and improved diseased cell targeting.
The research conducted by Haque and Vaiselbuh proved that exosomes from CD19-targeted CAR T cells caused the specific apoptosis of B-lineage acute lymphoblastic leukemia cells but not normal cells, thereby proving their application as a personalized immune therapy [177]. Gu et al. also proved that MSC-derived exosomes encapsulated within 3D scaffolds facilitated tissue regeneration, proving their personalized regenerative potential [178].
8.3. Integration with Emerging Technologies
Exosome research is being integrated with other new biomedical technologies to further enhance their future applications. Examples include exosome-based mRNA vaccines, CAR-exosome therapies, and 3D-bioprinted exosome matrices.
Exosome mRNA Vaccines: Exosome vaccines are gaining increasing popularity because they have the ability to boost immune responses. Chaput et al. showed that exosomes derived from dendritic cells, which were charged with synthetic peptides and CpG adjuvants, were able to prime naive T cells, resulting in tumor rejection in melanoma models [179]. Moreover, Rodriguez et al. suggested an exosome-based RNA–peptide vaccine against breast, ovarian, and lung cancers and showed a high stability of fusion and exosome affinity [180].
CAR Exosome Therapies: CAR-exosome therapies are under development to address some of the shortcomings of traditional CAR-T cell therapies, including cytokine storms. Tang et al. previously showed that CAR-T cell-derived exosomes have tumor-targeting ability and suppress tumor growth effectively, along with mitigating systemic toxicity [181]. Yang et al. again confirmed CAR-exosome therapy for triple-negative breast cancer, and they reported robust tumor suppression with minimal off-target effects [182].
3D-Bioprinted Exosome Matrices: Developments in 3D bioprinting are making exosomes usable for scaffold-based tissue engineering regenerative medicine. Selvam et al. emphasized the exciting potential of the exosome matrix bioprinted for applications in vascularization and osteogenesis [183]. Chen et al. also suggested that a scaffold preloaded with a 3D-printed exosome could considerably enhance osteochondral defect healing in a rabbit model [184].
9. Conclusions
Engineered exosomes are at the forefront of next-generation drug delivery, offering a revolutionary approach to treating CNS disorders and cancer. Their natural biocompatibility, capacity for cargo loading, and ability to bypass biological barriers make them highly effective carriers for therapeutic molecules. Recent advances in exosome modification, including receptor-mediated targeting and controlled drug release mechanisms, have significantly enhanced their precision and clinical potential.
However, several challenges must be addressed before exosome-based therapeutics can achieve widespread clinical adoption. The standardization of large-scale exosome production, quality control measures, and regulatory frameworks remain major hurdles in the field. Additionally, further studies are required to understand the long-term immune responses and potential toxicity of engineered exosomes. The collaboration of biomedical researchers, regulatory agencies, and pharmaceutical industries is essential in overcoming these obstacles and ensuring the safe and effective translation of exosome-based therapies into clinical practice.
The future of exosome therapeutics is highly promising, with innovations such as AI-driven exosome design, personalized exosome platforms derived from patient-specific stem cells, and integration with cutting-edge biomedical technologies like CAR-exosome therapies and 3D-bioprinted scaffolds. These advancements will further enhance the specificity, efficiency, and applicability of exosome-based treatments across a wide range of diseases. By bridging the gap between nanomedicine and clinical applications, engineered exosomes have the potential to transform precision medicine, offering targeted and minimally invasive therapies for some of the most challenging medical conditions.
Author Contributions
Methodology: T.P. and A.T.; Formal analysis and investigation: M.Q. and T.P.; Writing original draft preparation: T.P. and M.Q.; Writing review and editing: A.T., J.T. and U.M.H.; Supervision: A.T., J.T. and M.U.; Project administration: A.T. and P.S.; Resources: M.U. and P.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dementia. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 26 February 2020).
- Gustavsson, A.; Norton, N.; Fast, T.; Frölich, L.; Georges, J.; Holzapfel, D.; Kirabali, T.; Krolak-Salmon, P.; Rossini, P.M.; Ferretti, M.T.; et al. Global estimates on the number of persons across the Alzheimer’s disease continuum. Alzheimer’s Dement. 2022, 19, 658–670. [Google Scholar] [CrossRef]
- Pardridge, W.M. Treatment of Alzheimer’s Disease and Blood-Brain Barrier Drug Delivery. Pharmaceuticals 2020, 13, 394. [Google Scholar] [CrossRef] [PubMed]
- LeBleu, V.S.; Kalluri, R. Exosomes as a Multicomponent Biomarker Platform in Cancer. Trends Cancer 2020, 6, 767–774. [Google Scholar] [CrossRef]
- Johnsen, K.B.; Gudbergsson, J.M.; Skov, M.N.; Pilgaard, L.; Moos, T.; Duroux, M. A comprehensive overview of exosomes as drug delivery vehicles—Endogenous nanocarriers for targeted cancer therapy. Biochim. Biophys. Acta-Rev. Cancer 2014, 1846, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J.A. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, S.; Liu, L.; Dang, P.; Liu, Y.; Sun, Z.; Qiao, B.; Wang, C. Engineered exosomes from different sources for cancer-targeted therapy. Signal Transduct. Target. Ther. 2023, 8, 124. [Google Scholar] [CrossRef] [PubMed]
- Lener, T.; Gimona, M.; Aigner, L.; Börger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; del Portillo, H.A.; et al. Applying extracellular vesicles based therapeutics in clinical trials—An ISEV position paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef]
- Riaz, F.; Zhu, H.; Cheng, W.; Brongiel, S.; Baldwin, E.; Kier, M.W.; Zaemes, J.; Hearn, C.; Abdelghany, O.; Parikh, R.B.; et al. The Inpatient Immunotherapy Outcomes study: A multicenter retrospective study of patients treated with immune checkpoint inhibitors in the inpatient setting. J. Clin. Oncol. 2022, 40, 300. [Google Scholar] [CrossRef]
- Fordjour, F.K.; Daaboul, G.G.; Gould, S.J. A shared pathway of exosome biogenesis operates at plasma and endosome membranes. bioRxiv 2019. [Google Scholar] [CrossRef]
- Li, S.P.; Lin, Z.X.; Jiang, X.Y.; Yu, X.Y. Exosomal cargo-loading and synthetic exosome-mimics as potential therapeutic tools. Acta Pharmacol. Sin. 2018, 39, 542–551. [Google Scholar] [CrossRef]
- Azmi, A.S.; Bao, B.; Sarkar, F.H. Exosomes in cancer development, metastasis, and drug resistance: A comprehensive review. Cancer and Metastasis Reviews. Cancer Metastasis Rev. 2013, 32, 623–642. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Théry, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef]
- Zhang, H.; Freitas, D.; Kim, H.S.; Fabijanic, K.; Li, Z.; Chen, H.; Mark, M.T.; Molina, H.; Martin, A.B.; Bojmar, L.; et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell Biol. 2018, 20, 332–343. [Google Scholar] [CrossRef] [PubMed]
- To, K.K.W.; Cho, W.C.S. Exosome secretion from hypoxic cancer cells reshapes the tumor microenvironment and mediates drug resistance. Cancer Drug Resist. 2022, 5, 577–594. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, M.; Carmo, N.B.; Krumeich, S.; Fanget, I.; Raposo, G.; Savina, A.; Moita, C.F.; Schauer, K.; Hume, A.N.; Freitas, R.P.; et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat. Cell Biol. 2010, 12, 19–30. [Google Scholar] [CrossRef]
- Vilette, D.; Laulagnier, K.; Huor, A.; Alais, S.; Simoes, S.; Maryse, R.; Provansal, M.; Lehmann, S.; Andreoletti, O.; Schaeffer, L.; et al. Efficient inhibition of infectious prions multiplication and release by targeting the exosomal pathway. Cell. Mol. Life Sci. 2015, 72, 4409–4427. [Google Scholar] [CrossRef]
- Lötvall, J.; Hill, A.F.; Hochberg, F.; Buzás, E.I.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2014, 3, 26913. [Google Scholar] [CrossRef]
- Böing, A.N.; van der Pol, E.; Grootemaat, A.E.; Coumans, F.A.W.; Sturk, A.; Nieuwland, R. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J. Extracell. Vesicles 2014, 3, 23430. [Google Scholar] [CrossRef]
- Dumbović, G.; Sanjuan, X.; Perucho, M.; Forcales, S.-V. Stimulated emission depletion (STED) super resolution imaging of RNA- and protein-containing domains in fixed cells. Methods 2021, 187, 68–76. [Google Scholar] [CrossRef]
- Görgens, A.; Bremer, M.; Ferrer-Tur, R.; Murke, F.; Tertel, T.; Horn, P.A.; Thalmann, S.; Welsh, J.A.; Probst, C.; Guerin, C.; et al. Optimisation of imaging flow cytometry for the analysis of single extracellular vesicles by using fluorescence-tagged vesicles as biological reference material. J. Extracell. Vesicles 2019, 8, 1587567. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Yin, H. STAM transports STING oligomers into extracellular vesicles, down-regulating the innate immune response. J. Extracell. Vesicles 2023, 12, 12316. [Google Scholar] [CrossRef]
- Mosquera-Heredia, M.I.; Morales, L.C.; Vidal, O.M.; Barceló, E.; Silvera-Redondo, C.; Vélez, J.I.; Garavito-Galofre, P. Exosomes: Potential Disease Biomarkers and New Therapeutic Targets. Biomedicines 2021, 9, 1061. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K. ISEV2018 abstract book. J. Extracell. Vesicles 2018, 7, 1461450. [Google Scholar] [CrossRef]
- Ragusa, M.; Barbagallo, D.; Purrello, M. Exosomes: Nanoshuttles to the future of BioMedicine. Cell Cycle 2015, 14, 289–290. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Huo, M.; Li, B.; Wang, W.; Piao, H.; Wang, Y.; Zhu, Z.; Li, D.; Wang, T.; Liu, K. The Role of Exosomes and Exosomal MicroRNA in Cardiovascular Disease. Front. Cell Dev. Biol. 2021, 8, 616161. [Google Scholar] [CrossRef]
- Robless, E.E.; Howard, J.A.; Casari, I.; Falasca, M. Exosomal long non-coding RNAs in the diagnosis and oncogenesis of pancreatic cancer. Cancer Lett. 2020, 501, 55–65. [Google Scholar] [CrossRef]
- Gao, X.; Nan, Y.; Zhao, Y.; Yuan, Y.; Ren, B.; Sun, C.; Cao, K.; Yu, M.; Feng, X.; Ye, J. Atorvastatin reduces lipid accumulation in the liver by activating protein kinase A-mediated phosphorylation of perilipin 5. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2017, 1862, 1512–1519. [Google Scholar] [CrossRef]
- Liu, M.; Wen, Z.; Zhang, T.; Zhang, L.; Liu, X.; Wang, M. The role of exosomal molecular cargo in exosome biogenesis and disease diagnosis. Front. Immunol. 2024, 15, 1417758. [Google Scholar] [CrossRef]
- Haidar, Z.S. Exosomes: Human Saliva-derived nanoBiomarkers for Use in Clinical Dentistry? Int. J. Odontostomatol. 2018, 12, 5–6. [Google Scholar] [CrossRef]
- Exosomes. Nat. Biotechnol. 2020, 38, 1150.
- Mathieu, M.; Névo, N.; Jouve, M.; Valenzuela, J.I.; Maurin, M.; Verweij, F.J.; Palmulli, R.; Lankar, D.; Dingli, F.; Loew, D.; et al. Specificities of exosome versus small ectosome secretion revealed by live intracellular tracking of CD63 and CD9. Nat. Commun. 2021, 12, 4389. [Google Scholar] [CrossRef]
- Skog, J.; Würdinger, T.; Van Rijn, S.; Meijer, D.H.; Gainche, L.; Curry, W.T., Jr.; Carter, B.S.; Krichevsky, A.M.; Breakefield, X.O. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008, 10, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- Aheget, H.; Mazini, L.; Martin, F.; Belqat, B.; Marchal, J.A.; Benabdellah, K. Exosomes: Their Role in Pathogenesis, Diagnosis and Treatment of Diseases. Cancers 2020, 13, 84. [Google Scholar] [CrossRef]
- Shaheen, H.; Singh, S.; Melnik, R. A Neuron-Glial Model of Exosomal Release in the Onset and Progression of Alzheimer’s Disease. Front. Comput. Neurosci. 2021, 15, 653097. [Google Scholar] [CrossRef]
- Chiarini, A.M.; Armato, U.; Eccher, C.; Prà, I.D. Linking astrocytes’ exosomes to Alzheimer pathogenesis and therapy. In Diagnosis and Management in Dementia; Elsevier: Amsterdam, The Netherlands, 2020; pp. 599–615. [Google Scholar]
- Winston, C.N.; Aulston, B.; Rockenstein, E.M.; Adame, A.; Prikhodko, O.; Dave, K.N.; Mishra, P.; Rissman, R.A.; Yuan, S.H. Neuronal Exosome-Derived Human Tau is Toxic to Recipient Mouse Neurons in vivo. J. Alzheimer’s Dis. 2018, 67, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Wu, Z.; Li, J.; Wu, S.; Shi, W.; Wang, L. The emerging double-edged sword role of exosomes in Alzheimer’s disease. Front. Aging Neurosci. 2023, 15, 1209115. [Google Scholar] [CrossRef]
- Yuyama, K.; Sun, H.; Mitsutake, S.; Igarashi, Y. Sphingolipid-modulated Exosome Secretion Promotes Clearance of Amyloid-β by Microglia. J. Biol. Chem. 2012, 287, 10977. [Google Scholar] [CrossRef]
- Tian, M.-S.; Yi, X.-N. The Role of Mesenchymal Stem Cell Exosomes in the Onset and Progression of Alzheimer’s Disease. Biomed. Sci. 2024, 10, 6–13. [Google Scholar]
- Jiang, J.; Li, J.; Zhou, X.; Zhao, X.; Huang, B.; Qin, Y. Exosomes Regulate the Epithelial–Mesenchymal Transition in Cancer. Front. Oncol. 2022, 12, 864980. [Google Scholar] [CrossRef]
- Beeraka, N.M.; Doreswamy, S.H.; Sadhu, S.P.; Srinivasan, A.; Pragada, R.R.; Madhunapantula, S.V.; Aliev, G. The Role of Exosomes in Stemness and Neurodegenerative Diseases—Chemoresistant-Cancer Therapeutics and Phytochemicals. Int. J. Mol. Sci. 2020, 21, 6818. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, T.; Chen, J.; Li, R.; Chen, H.; Luo, S.; Chen, D.; Cai, C.; Li, W. The role of Exosomal miRNAs in cancer. J. Transl. Med. 2022, 20, 6. [Google Scholar] [CrossRef]
- Zeng, H.; Guo, S.; Ren, X.; Wu, Z.; Liu, S.; Yao, X. Current Strategies for Exosome Cargo Loading and Targeting Delivery. Cells 2023, 12, 1416. [Google Scholar] [CrossRef]
- Xi, X.M.; Chen, M.; Xia, S.J.; Lu, R. Drug loading techniques for exosome-based drug delivery systems. Pharmazie 2021, 76, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Qutub, M.; Tatode, A.; Taksande, J.; Premchandani, T.; Umekar, M.; Hussain, U.M.; Biyani, D.; Mane, D. Stimuli-responsive supramolecular hydrogels for paclitaxel delivery: Progress and prospects. Asp. Mol. Med. 2025, 5, 100062. [Google Scholar] [CrossRef]
- Shi, Y.; Guo, S.; Liang, Y.; Liu, L.; Wang, A.; Sun, K.; Li, Y. Construction and Evaluation of Liraglutide Delivery System based on Milk Exosomes: A New Idea for Oral Peptide Delivery. Curr. Pharm. Biotechnol. 2022, 23, 1072–1079. [Google Scholar] [CrossRef] [PubMed]
- Nasir, F. The Promise of Exosomes as Drug Delivery Systems. Pharm. Commun. 2023, 2, 1–2. [Google Scholar] [CrossRef]
- Qutub, M.; Tatode, A.; Premchandani, T.; Taksande, J.; Mane, D.; Umekar, M. Blending induced variations in Poloxamer’s/Pluronic’s® gelation: Thermodynamic and rheological perspectives. JCIS Open 2024, 16, 100126. [Google Scholar] [CrossRef]
- Zhan, Q.; Yi, K.; Li, X.; Cui, X.; Yang, E.; Chen, N.; Yuan, X.; Zhao, J.; Hou, X.; Kang, C. Phosphatidylcholine-Engineered Exosomes for Enhanced Tumor Cell Uptake and Intracellular Antitumor Drug Delivery. Macromol. Biosci. 2021, 21, 2100042. [Google Scholar] [CrossRef]
- Lowe, N.M.; Mizenko, R.R.; Nguyen, B.B.; Chiu, K.L.; Arun, V.; Panitch, A.; Carney, R.P. Orthogonal analysis reveals inconsistencies in cargo loading of extracellular vesicles. J. Extracell. Biol. 2024, 3, e70003. [Google Scholar] [CrossRef]
- Haney, M.J.; Klyachko, N.L.; Zhao, Y.; Gupta, R.; Plotnikova, E.G.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A.V.; et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J. Control. Release 2015, 207, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Yim, N.; Ryu, S.W.; Choi, K.; Lee, K.R.; Lee, S.; Choi, H.; Park, J.; Kim, D.; Heo, W.; Choi, C. Abstract 2167: Efficient and rapid cellular delivery of bioactive proteins using EXPLOR: Exosomes engineered for protein loading via optically reversible protein-protein interaction. Cancer Res. 2016, 76, 2167. [Google Scholar] [CrossRef]
- Warren, M.R.; Zhang, C.; Vedadghavami, A.; Bokvist, K.; Dhal, P.K.; Bajpayee, A.G. Milk exosomes with enhanced mucus penetrability for oral delivery of siRNA. Biomater. Sci. 2021, 9, 4260–4277. [Google Scholar] [CrossRef] [PubMed]
- Mehryab, F.; Rabbani, S.; Shahhosseini, S.; Shekari, F.; Fatahi, Y.; Baharvand, H.; Haeri, A. Exosomes as a next-generation drug delivery system: An update on drug loading approaches. Acta Biomater. 2020, 113, 42–62. [Google Scholar] [CrossRef]
- Chen, C.; Sun, M.; Wang, J.; Su, L.; Lin, J.; Yan, X. Active cargo loading into extracellular vesicles: Highlights the heterogeneous encapsulation behaviour. J. Extracell. Vesicles 2021, 10, e12163. [Google Scholar] [CrossRef]
- de Jong, O.G.; Murphy, D.E.; Mäger, I.; Willms, E.; Garcia-Guerra, A.; Gitz-Francois, J.J.; Lefferts, J.; Gupta, D.; Steenbeek, S.C.; van Rheenen, J.; et al. A CRISPR-Cas9-based reporter system for single-cell detection of extracellular vesicle-mediated functional transfer of RNA. Nat. Commun. 2020, 11, 1113. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. (Invited) Chiral Graphene Quantum Dot Enhanced Drug Loading into Exosomes. ECS Meet. Abstr. 2024, MA2024-01, 859. [Google Scholar] [CrossRef]
- Elsharkasy, O.M.; Hegeman, C.V.; Lansweers, I.; Cotugno, O.L.; de Groot, I.Y.; de Wit, Z.E.M.N.J.; Liang, X.; Garcia-Guerra, A.; Moorman, N.J.; Lefferts, J.; et al. A modular strategy for extracellular vesicle-mediated CRISPR-Cas9 delivery through aptamer-based loading and UV-activated cargo release. bioRxiv 2024. [Google Scholar] [CrossRef]
- Gee, P.; Lung, M.S.Y.; Okuzaki, Y.; Sasakawa, N.; Iguchi, T.; Makita, Y.; Hozumi, H.; Miura, Y.; Yang, L.F.; Iwasaki, M.; et al. Extracellular nanovesicles for packaging of CRISPR-Cas9 protein and sgRNA to induce therapeutic exon skipping. Nat. Commun. 2020, 11, 1334. [Google Scholar] [CrossRef]
- Hallal, S.; Tűzesi, Á.; Grau, G.E.; Buckland, M.E.; Alexander, K.L. Understanding the extracellular vesicle surface for clinical molecular biology. J. Extracell. Vesicles 2022, 11, e12260. [Google Scholar] [CrossRef]
- Stranford, D.M.; Simons, L.M.; Berman, K.E.; Cheng, L.; DiBiase, B.N.; Hung, M.E.; Lucks, J.B.; Hultquist, J.F.; Leonard, J.N. Genetically encoding multiple functionalities into extracellular vesicles for the targeted delivery of biologics to T cells. Nat. Biomed. Eng. 2023, 8, 397–414. [Google Scholar] [CrossRef] [PubMed]
- Salunkhe, S.; Dheeraj; Basak, M.; Chitkara, D.; Mittal, A. Surface functionalization of exosomes for target-specific delivery and in vivo imaging & tracking: Strategies and significance. J. Control. Release 2020, 326, 599–614. [Google Scholar]
- Mukerjee, N.; Sonar, S. Signature of click chemistry in exosome modification for cancer therapeutic. Clin. Transl. Discov. 2024, 4, e335. [Google Scholar] [CrossRef]
- Asai, T.; Trinh, R.; Ng, P.P.; Penichet, M.L.; Wims, L.A.; Morrison, S.L. A human biotin acceptor domain allows site-specific conjugation of an enzyme to an antibody-avidin fusion protein for targeted drug delivery. Biomol. Eng. 2005, 21, 145–155. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Limoni, S.K.; Moghadam, M.F.; Moazzeni, S.M.; Gomari, H.; Salimi, F. Engineered Exosomes for Targeted Transfer of siRNA to HER2 Positive Breast Cancer Cells. Appl. Biochem. Biotechnol. 2018, 187, 352–364. [Google Scholar] [CrossRef]
- Yu, Y.; Li, W.; Mao, L.; Peng, W.; Long, D.; Li, D.; Zhou, R.; Dang, X. Genetically engineered exosomes display RVG peptide and selectively enrich a neprilysin variant: A potential formulation for the treatment of Alzheimer’s disease. J. Drug Target. 2021, 29, 1128–1138. [Google Scholar] [CrossRef]
- Jiang, P.; Xiao, Y.; Hu, X.; Wang, C.; Gao, H.; Huang, H.; Lv, J.; Qi, Z.; Wang, Z. RVG29 Peptide-Modified Exosomes Loaded with Mir-133b Mediate the RhoA-ROCK Pathway to Improve Motor and Neurological Symptoms in Parkinson’s Disease. ACS Biomater. Sci. Eng. 2024, 10, 3069–3085. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Kim, M.; Lee, Y.; Byun, J.W.; Hwang, D.W.; Lee, M. Systemic delivery of microRNA-21 antisense oligonucleotides to the brain using T7-peptide decorated exosomes. J. Control. Release 2020, 317, 273–281. [Google Scholar] [CrossRef]
- Choi, H.; Choi, K.; Kim, D.-H.; Oh, B.-K.; Yim, H.; Jo, S.; Choi, C. Strategies for Targeted Delivery of Exosomes to the Brain: Advantages and Challenges. Pharmaceutics 2022, 14, 672. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, X.; Zou, W.; Wu, Y.; Zhao, J.; Chen, X.; Zhou, G.G. Exosomes containing miRNAs targeting HER2 synthesis and engineered to adhere to HER2 on tumor cells surface exhibit enhanced antitumor activity. J. Nanobiotechnol. 2020, 18, 153. [Google Scholar] [CrossRef]
- Kolhatkar, R.; Lote, A.; Khambhati, H. Active Tumor Targeting of Nanomaterials Using Folic Acid, Transferrin and Integrin Receptors. Curr. Drug Discov. Technol. 2011, 8, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Tatode, A.; Agrawal, P.R.; Taksande, J.; Qutub, M.; Premchandani, T.; Umekar, M.; Danao, K. Role of folate receptor and CD44 in targeting of docetaxel and paclitaxel fabricated conjugates for efficient cancer therapy. J. Med. Surg. Public Health 2024, 5, 100163. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, L.; Liu, Y.; Zhang, Q.; Qin, Y.; Yin, Y.; Yuan, W.; Yang, Y.; Xie, Y.; Zhang, Z.; et al. Synergistic targeted delivery of payload into tumor cells by dual-ligand liposomes co-modified with cholesterol anchored transferrin and TAT. Int. J. Pharm. 2013, 454, 31–40. [Google Scholar] [CrossRef]
- Koh, B.; Park, S.B.; Yoon, E.; Yoo, H.M.; Lee, D.; Heo, J.-N.; Ahn, S. αVβ3-Targeted Delivery of Camptothecin-Encapsulated Carbon Nanotube-Cyclic RGD in 2D and 3D Cancer Cell Culture. J. Pharm. Sci. 2019, 108, 3704–3712. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.K.; Kondaiah, P.; Bhattacharya, S.; Boturyn, D.; Dumy, P. Co-liposomes comprising a lipidated multivalent RGD-peptide and a cationic gemini cholesterol induce selective gene transfection in αvβ3 and αvβ5 integrin receptor-rich cancer cells. J. Mater. Chem. B 2014, 2, 5758–5767. [Google Scholar] [CrossRef]
- Wang, X.; Li, D.; Li, G.; Chen, J.; Yang, Y.; Bian, L.; Zhou, J.; Wu, Y.; Chen, Y. Enhanced Therapeutic Potential of Hybrid Exosomes Loaded with Paclitaxel for Cancer Therapy. Int. J. Mol. Sci. 2024, 25, 3645. [Google Scholar] [CrossRef]
- Long, M.; Lu, A.; Lu, M.; Weng, L.; Chen, Q.; Zhu, L.; Chen, Z. Azo-inserted responsive hybrid liposomes for hypoxia-specific drug delivery. Acta Biomater. 2020, 115, 343–357. [Google Scholar] [CrossRef]
- Xiao, P.; Wang, H.; Liu, H.; Yuan, H.; Guo, C.; Feng, Y.; Qi, P.; Yin, T.; Zhang, Y.; He, H.; et al. Milk Exosome–Liposome Hybrid Vesicles with Self-Adapting Surface Properties Overcome the Sequential Absorption Barriers for Oral Delivery of Peptides. ACS Nano 2024, 18, 21091–21111. [Google Scholar] [CrossRef]
- Joshi, R.; Lampe, J.; Vishwanatha, J.K.; Ranjan, A.P. Abstract 832: Exosome-based hybrid nanosystem for targeted TNBC therapy. Cancer Res. 2023, 83, 832. [Google Scholar] [CrossRef]
- Yuba, E.; Kanda, Y.; Yoshizaki, Y.; Teranishi, R.; Harada, A.; Sugiura, K.; Izawa, T.; Yamate, J.; Sakaguchi, N.; Koiwai, K.; et al. pH-sensitive polymer-liposome-based antigen delivery systems potentiated with interferon-γ gene lipoplex for efficient cancer immunotherapy. Biomaterials 2015, 67, 214–224. [Google Scholar] [CrossRef]
- Miyazaki, M.; Yuba, E.; Hayashi, H.; Harada, A.; Kono, K. Hyaluronic Acid-Based pH-Sensitive Polymer-Modified Liposomes for Cell-Specific Intracellular Drug Delivery Systems. Bioconjug. Chem. 2017, 29, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Lathwal, S.; Yerneni, S.S.; Boye, S.; Muza, U.L.; Takahashi, S.; Sugimoto, N.; Lederer, A.; Das, S.R.; Campbell, P.G.; Matyjaszewski, K. Engineering exosome polymer hybrids by atom transfer radical polymerization. Proc. Natl. Acad. Sci. USA 2021, 118, e2020241118. [Google Scholar] [CrossRef] [PubMed]
- Sancho-Albero, M.; Navascués, N.; Mendoza, G.; Sebastián, V.; Arruebo, M.; Martín-Duque, P.; Santamaría, J. Exosome origin determines cell targeting and the transfer of therapeutic nanoparticles towards target cells. J. Nanobiotechnol. 2019, 17, 16. [Google Scholar] [CrossRef] [PubMed]
- Anilkumar, A.; Lopez, S.; Leslie, S.; Doxtater, K.; Chauhan, N.; Hafeez, B.; Yallapu, M.; Jaggi, M.; Chauhan, S.; Tripathi, M. Abstract 2790: Exosomes as nanocarriers for biomolecules and potential diagnostic targets in cancer. Cancer Res. 2022, 82, 2790. [Google Scholar] [CrossRef]
- Duréndez-Sáez, E.; Calabuig-Fariñas, S.; Torres-Martínez, S.; Moreno-Manuel, A.; Herreros-Pomares, A.; Escorihuela, E.; Mosqueda, M.; Gallach, S.; Guijarro, R.; Serna, E.; et al. Analysis of Exosomal Cargo Provides Accurate Clinical, Histologic and Mutational Information in Non-Small Cell Lung Cancer. Cancers 2022, 14, 3216. [Google Scholar] [CrossRef]
- Sitar, S.; Kejžar, A.; Pahovnik, D.; Kogej, K.; Tušek-Žnidarič, M.; Lenassi, M.; Žagar, E. Size Characterization and Quantification of Exosomes by Asymmetrical-Flow Field-Flow Fractionation. Anal. Chem. 2015, 87, 9225–9233. [Google Scholar] [CrossRef]
- Heiskanen, M. Western Blot Characterization of Exosomes Isolated from Rat Plasma and Evaluation of the Efficiency of a Precipitation Method in Exosome Isolation; JYX. Available online: https://jyx.jyu.fi/jyx/Record/jyx_123456789_54592 (accessed on 23 February 2023).
- Lu, L.; Han, C.; Zhang, Q.; Wang, M.; Qi, D.; Gao, M.; Wang, N.; Yin, J.; Dong, F.; Ge, X. A novel purity analysis method of bovine milk-derived exosomes by two-dimensional high-performance liquid chromatography. bioRxiv 2023. [Google Scholar]
- Wu, T.; Dai, Y.; Xu, Y.; Zheng, J.; Chen, S.; Zhang, Y.; Tian, P.; Zheng, X.; Wang, H. ExosomePurity: Tumour purity deconvolution in serum exosomes based on miRNA signatures. Brief. Bioinform. 2023, 24, bbad119. [Google Scholar] [CrossRef]
- Yang, J.S.; Lee, J.C.; Byeon, S.K.; Rha, K.H.; Moon, M.H. Size Dependent Lipidomic Analysis of Urinary Exosomes from Patients with Prostate Cancer by Flow Field-Flow Fractionation and Nanoflow Liquid Chromatography-Tandem Mass Spectrometry. Anal. Chem. 2017, 89, 2488–2496. [Google Scholar] [CrossRef]
- Kumari, A.; Youngblood, M.; Gould, A.; Kang, Y.-T.; Chen, L.; Habashy, K.; Amidei, C.; Ward, R.; Gomez, C.; Bouchoux, G.; et al. Abstract 3897: Isolation and molecular characterization of exosomes from glioblastoma patients using a microfluidic device after ultrasound-based opening of the blood-brain barrier. Cancer Res. 2024, 84, 3897. [Google Scholar] [CrossRef]
- Zhuang, W.; Zhao, M.; Li, B. Navigating the Blood-Brain Barrier: An Anti-CD19 Modified-Exosome Delivery System for Precise Targeting of CNS Lymphoma. Blood 2023, 142, 3008. [Google Scholar] [CrossRef]
- Patel, G.K.; Khan, M.A.; Zubair, H.; Srivastava, S.K.; Khushman, M.; Singh, S.; Singh, A.P. Abstract 1991: Exploring the potential of exosomes for cancer diagnosis and management: Comparative analysis of isolation methods for optimum yield purity and downstream applications. Cancer Res. 2019, 79, 1991. [Google Scholar] [CrossRef]
- Samanta, K.; Khan, S.; Setua, S.; Kumari, S.; Dan, N.; Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. Abstract 3564: MicroRNA-145 gene therapy for pancreatic cancer using exosome as carrier source derived from cancer cells. Cancer Res. 2019, 79, 3564. [Google Scholar] [CrossRef]
- André, S.; Larbanoix, L.; Verteneuil, S.; Stanicki, D.; Nonclercq, D.; Elst, L.V.; Laurent, S.; Muller, R.N.; Burtea, C. Development of an LDL Receptor-Targeted Peptide Susceptible to Facilitate the Brain Access of Diagnostic or Therapeutic Agents. Biology 2020, 9, 161. [Google Scholar] [CrossRef] [PubMed]
- Boado, R.J. IgG Fusion Proteins for Brain Delivery of Biologics via Blood–Brain Barrier Receptor-Mediated Transport. Pharmaceutics 2022, 14, 1476. [Google Scholar] [CrossRef]
- Liu, X.; Miao, J.; Wang, C.; Zhou, S.; Chen, S.; Ren, Q.; Hong, X.; Wang, Y.; Hou, F.F.; Zhou, L.; et al. Tubule-derived exosomes play a central role in fibroblast activation and kidney fibrosis. Kidney Int. 2020, 97, 1181–1195. [Google Scholar] [CrossRef]
- Gao, D.; Lo, P.C. Polymeric micelles encapsulating pH-responsive doxorubicin prodrug and glutathione-activated zinc(II) phthalocyanine for combined chemotherapy and photodynamic therapy. J. Control. Release 2018, 282, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Haqqani, A.S.; Bélanger, K.; Stanimirovic, D.B. Receptor-mediated transcytosis for brain delivery of therapeutics: Receptor classes and criteria. Front. Drug Deliv. 2024, 4, 1360302. [Google Scholar] [CrossRef]
- Ruan, S.; Qin, L.; Xiao, W.; Hu, C.; Zhou, Y.; Wang, R.; Sun, X.; Yu, W.; He, Q.; Gao, H. Acid-Responsive Transferrin Dissociation and GLUT Mediated Exocytosis for Increased Blood–Brain Barrier Transcytosis and Programmed Glioma Targeting Delivery. Adv. Funct. Mater. 2018, 28, 1802227. [Google Scholar] [CrossRef]
- Morrison, K.R.; Wang, T.; Chan, K.Y.; Trotter, E.W.; Gillespie, A.; Michael, M.Z.; Oakhill, J.S.; Hagan, I.M.; Petersen, J. Elevated basal AMP-activated protein kinase activity sensitizes colorectal cancer cells to growth inhibition by metformin. Open Biol. 2023, 13, 230021. [Google Scholar] [CrossRef]
- Boado, R.J.; Pardridge, W.M. The Trojan Horse Liposome Technology for Nonviral Gene Transfer across the Blood-Brain Barrier. J. Drug Deliv. 2011, 2011, 296151. [Google Scholar] [CrossRef]
- Feng, J.M.; Lui, P.C.W.; Li, J.Y. Receptor-Mediated Transport of Drugs Across the BBB. In Drug Delivery to the Central Nervous System; Springer: Berlin/Heidelberg, Germany, 2010; pp. 15–34. [Google Scholar]
- Park, K. Trojan monocytes for improved drug delivery to the brain. J. Control. Release 2008, 132, 75. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Cai, G.; Yang, Z.; Shi, H.; Zeng, H.; Ye, Q.; Hu, Z.; Wang, Z. Biomimetic Nanovesicles as a Dual Gene Delivery System for the Synergistic Gene Therapy of Alzheimer’s Disease. ACS Nano 2024, 18, 11753–11768. [Google Scholar] [CrossRef]
- Song, Z.; Xu, Y.; Deng, W.; Zhang, L.; Zhu, H.; Yu, P.; Qu, Y.; Zhao, W.; Han, Y.; Qin, C. Brain Derived Exosomes Are a Double-Edged Sword in Alzheimer’s Disease. Front. Mol. Neurosci. 2020, 13, 79. [Google Scholar] [CrossRef] [PubMed]
- Seyfizadeh, N.; Borzouisileh, S.; Elahimanesh, F.; Hosseini, V.; Nouri, M. Exosome-mediated therapeutic delivery: A new horizon for human neurodegenerative disorders’ treatment (with a focus on siRNA delivery improvement). Process. Biochem. 2019, 85, 164–174. [Google Scholar] [CrossRef]
- Li, J.; Peng, H.; Zhang, W.; Li, M.; Wang, N.; Peng, C.; Zhang, X.; Li, Y. Enhanced Nose-to-Brain Delivery of Combined Small Interfering RNAs Using Lesion-Recognizing Nanoparticles for the Synergistic Therapy of Alzheimer’s Disease. ACS Appl. Mater. Interfaces 2023, 15, 53177–53188. [Google Scholar] [CrossRef]
- Wang, D.; Wang, P.; Bian, X.; Xu, S.; Zhou, Q.; Zhang, Y.; Ding, M.; Han, M.; Huang, L.; Bi, J.; et al. Elevated plasma levels of exosomal BACE1 AS combined with the volume and thickness of the right entorhinal cortex may serve as a biomarker for the detection of Alzheimer’s disease. Mol. Med. Rep. 2020, 22, 227–238. [Google Scholar] [CrossRef]
- Cooper, J.M.; Wiklander, P.B.O.; Nordin, J.Z.; Al-Shawi, R.; Wood, M.J.; Vithlani, M.; Schapira, A.H.V.; Simons, J.P.; El-Andaloussi, S.; Alvarez-Erviti, L. Systemic exosomal siRNA delivery reduced alpha-synuclein aggregates in brains of transgenic mice. Mov. Disord. 2014, 29, 1476–1485. [Google Scholar] [CrossRef]
- Yuan, Q.; Li, X.D.; Zhang, S.M.; Wang, H.W.; Wang, Y.L. Extracellular vesicles in neurodegenerative diseases: Insights and new perspectives. Genes Dis. 2021, 8, 124–132. [Google Scholar] [CrossRef]
- Kong, W.; Li, X.; Guo, X.; Sun, Y.; Chai, W.; Chang, Y.; Huang, Q.; Wang, P.; Wang, X. Ultrasound-Assisted CRISPRi-Exosome for Epigenetic Modification of α-Synuclein Gene in a Mouse Model of Parkinson’s Disease. ACS Nano 2024, 18, 7837–7851. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Z.; Xing, H.; Wang, Y.; Guo, Y. Exosomes derived from miR-188-3p-modified adipose-derived mesenchymal stem cells protect Parkinson’s disease. Mol. Ther. Nucleic Acids 2021, 23, 1334–1344. [Google Scholar] [CrossRef]
- Izco, M.; Blesa, J.; Schleef, M.; Schmeer, M.; Porcari, R.; Al-Shawi, R.; Ellmerich, S.; de Toro, M.; Gardiner, C.; Seow, Y.; et al. Systemic Exosomal Delivery of shRNA Minicircles Prevents Parkinsonian Pathology. Mol. Ther. 2019, 27, 2111–2122. [Google Scholar] [CrossRef]
- Ma, Z.L.; Wang, Z.L.; Zhang, F.Y.; Liu, H.X.; Mao, L.H.; Yuan, L. Biomarkers of Parkinson’s Disease: From Basic Research to Clinical Practice. Aging Dis. 2024, 15, 1813–1830. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, R.; Kiani, J.; Tong, W.Y.; Soleimani, M.; Voelcker, N.H.; Arefian, E. Engineered anti-EGFRvIII targeted exosomes induce apoptosis in glioblastoma multiforme. J. Drug Target. 2022, 31, 310–319. [Google Scholar] [CrossRef]
- Zeng, A.-L.; Yan, W.; Liu, Y.-W.; Wang, Z.; Hu, Q.; Nie, E.; Zhou, X.; Li, R.; Wang, X.-F.; Jiang, T.; et al. Tumour exosomes from cells harbouring PTPRZ1–MET fusion contribute to a malignant phenotype and temozolomide chemoresistance in glioblastoma. Oncogene 2017, 36, 5369–5381. [Google Scholar] [CrossRef]
- Liang, S.F.; Zuo, F.F.; Yin, B.C.; Ye, B.C. Delivery of siRNA based on engineered exosomes for glioblastoma therapy by targeting STAT3. Biomater. Sci. 2022, 10, 1582–1590. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Shahar, T.; Hossain, A.; Kerrigan, B.P.; Gumin, J.; Li, S.; Gao, F.; Yamasaki, K.; Shimizu, Y.; Lang, F.F. Abstract 2668: The exosomes derived from BM-hMSCs home to glioma and deliver synthetic microRNA mimics after systemic administration. Cancer Res. 2016, 76, 2668. [Google Scholar] [CrossRef]
- Zhan, Q.; Yi, K.; Cui, X.; Li, X.; Yang, S.; Wang, Q.; Fang, C.; Tan, Y.; Li, L.; Xu, C.; et al. Blood exosomes-based targeted delivery of cPLA2 siRNA and metformin to modulate glioblastoma energy metabolism for tailoring personalized therapy. Neuro-Oncology 2022, 24, 1871–1883. [Google Scholar] [CrossRef]
- Zhao, J.; Cui, X.; Zhan, Q.; Zhang, K.; Su, D.; Yang, S.; Hong, B.; Wang, Q.; Ju, J.; Cheng, C.; et al. CRISPR-Cas9 library screening combined with an exosome-targeted delivery system addresses tumorigenesis/TMZ resistance in the mesenchymal subtype of glioblastoma. Theranostics 2024, 14, 2835–2855. [Google Scholar] [CrossRef]
- Gänger, S.; Schindowski, K. Tailoring Formulations for Intranasal Nose-to-Brain Delivery: A Review on Architecture, Physico-Chemical Characteristics and Mucociliary Clearance of the Nasal Olfactory Mucosa. Pharmaceutics 2018, 10, 116. [Google Scholar] [CrossRef]
- Lochhead, J.J.; Davis, T.P. Perivascular and Perineural Pathways Involved in Brain Delivery and Distribution of Drugs after Intranasal Administration. Pharmaceutics 2019, 11, 598. [Google Scholar] [CrossRef]
- Crowe, T.P.; Greenlee, M.H.W.; Kanthasamy, A.G.; Hsu, W.H. Mechanism of intranasal drug delivery directly to the brain. Life Sci. 2018, 195, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Mishra, G.; Sharma, A.K.; Gothwal, A.; Kesharwani, P.; Gupta, U. Intranasal Drug Delivery: A Non-Invasive Approach for the Better Delivery of Neurotherapeutics. Pharm. Nanotechnol. 2018, 5, 203–214. [Google Scholar] [CrossRef]
- Simhadri, A.; Dommeti, M.D.; Sana, J. A Comprehensive Review on the Nanotechnology-based Intranasal Drug Delivery Systems for Brain Targeting. J. Pharma Insights Res. 2024, 2, 15–23. [Google Scholar] [CrossRef]
- Ma, S.; Li, M.; Liu, N.; Li, Y.; Li, Z.; Yang, Y.; Yu, F.; Hu, X.; Liu, C.; Mei, X. Vincristine liposomes with smaller particle size have stronger diffusion ability in tumor and improve tumor accumulation of vincristine significantly. Oncotarget 2017, 8, 87276–87291. [Google Scholar] [CrossRef][Green Version]
- Upponi, J.R.; Torchilin, V.P. Passive vs. Active Targeting: An Update of the EPR Role in Drug Delivery to Tumors. In Nano-Oncologicals: New Targeting and Delivery Approaches; Springer International Publishing: Cham, Switzerland, 2014; pp. 3–45. [Google Scholar]
- Theek, B.; Baues, M.; Ojha, T.; Möckel, D.; Veettil, S.K.; Steitz, J.; van Bloois, L.; Storm, G.; Kiessling, F.; Lammers, T. Sonoporation enhances liposome accumulation and penetration in tumors with low EPR. J. Control. Release 2016, 231, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Ieranò, C.; Santagata, S.; Napolitano, M.; Guardia, F.; Grimaldi, A.; Antignani, E.; Botti, G.; Consales, C.; Riccio, A.; Nanayakkara, M.; et al. CXCR4 and CXCR7 transduce through mTOR in human renal cancer cells. Cell Death Dis. 2014, 5, e1310. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lu, Y.; Xu, Y.; Wang, J.; Zhang, C.; Du, Y.; Wang, L.; Li, L.; Wang, B.; Shen, J.; et al. Horizontal transfer of exosomal CXCR4 promotes murine hepatocarcinoma cell migration, invasion and lymphangiogenesis. Gene 2018, 676, 101–109. [Google Scholar] [CrossRef]
- Mannavola, F.; Tucci, M.; Felici, C.; Passarelli, A.; D’Oronzo, S.; Silvestris, F. Tumor-derived exosomes promote the in vitro osteotropism of melanoma cells by activating the SDF-1/CXCR4/CXCR7 axis. J. Transl. Med. 2019, 17, 230. [Google Scholar] [CrossRef]
- Lin, B.; Wang, Y.; Zhao, K.; Lü, W.-D.; Hui, X.; Ma, Y.; Lv, R. Exosome-based rare earth nanoparticles for targeted in situ and metastatic tumor imaging with chemo-assisted immunotherapy. Biomater. Sci. 2022, 10, 744–752. [Google Scholar] [CrossRef]
- Zhao, Y.; Ren, W.; Zhong, T.; Zhang, S.; Huang, D.; Guo, Y.; Yao, X.; Wang, C.; Zhang, W.-Q.; Zhang, X.; et al. Tumor-specific pH-responsive peptide-modified pH-sensitive liposomes containing doxorubicin for enhancing glioma targeting and anti-tumor activity. J. Control. Release 2016, 222, 56–66. [Google Scholar] [CrossRef]
- Yamamoto, E.; Hyodo, K.; Suzuki, T.; Ishihara, H.; Kikuchi, H.; Kato, M. Simulation of Stimuli-Responsive and Stoichiometrically Controlled Release Rate of Doxorubicin from Liposomes in Tumor Interstitial Fluid. Pharm. Res. 2018, 35, 103. [Google Scholar] [CrossRef]
- Chen, K.J.; Chiu, Y.L.; Chen, Y.M.; Ho, Y.C.; Sung, H.W. Intracellularly monitoring/imaging the release of doxorubicin from pH-responsive nanoparticles using Förster resonance energy transfer. Biomaterials 2011, 32, 2586–2592. [Google Scholar] [CrossRef]
- Niu, Y.; Zhu, J.; Gao, C.; Xu, Q.; He, Z.; Chen, D.; Xu, M.; Liu, Y. Tailor-made legumain/pH dual-responsive doxorubicin prodrug-embedded nanoparticles for efficient anticancer drug delivery and in situ monitoring of drug release. Nanoscale 2020, 12, 2673–2685. [Google Scholar]
- Nagel, G.; Tschiche, H.R.; Wedepohl, S.; Calderón, M. Modular approach for theranostic polymer conjugates with activatable fluorescence: Impact of linker design on the stimuli-induced release of doxorubicin. J. Control. Release 2018, 285, 200–211. [Google Scholar] [CrossRef]
- Cheng, Y.; Zou, T.; Dai, M.; He, X.Y.; Peng, N.; Wu, K.; Wang, X.Q.; Liao, C.Y.; Liu, Y. Doxorubicin loaded tumor-triggered targeting ammonium bicarbonate liposomes for tumor-specific drug delivery. Colloids Surf. B Biointerfaces 2019, 178, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Aliabadi, H.M.; Mahdipoor, P.; Uludağ, H. Polymeric delivery of siRNA for dual silencing of Mcl-1 and P-glycoprotein and apoptosis induction in drug-resistant breast cancer cells. Cancer Gene Ther. 2013, 20, 169–177. [Google Scholar] [CrossRef]
- Navarro, G.; Sawant, R.R.; Biswas, S.; Essex, S.; Tros de Ilarduya, C.; Torchilin, V.P. P-Glycoprotein Silencing with siRNA Delivered by Dope-Modified Pei Overcomes Doxorubicin Resistance in Breast Cancer Cells. Nanomedicine 2012, 7, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Ai, Z.; Yin, L.; Zhou, X.; Zhu, Y.; Zhu, D.; Yu, Y.; Feng, Y. Inhibition of survivin reduces cell proliferation and induces apoptosis in human endometrial cancer. Cancer 2006, 107, 746–756. [Google Scholar] [CrossRef]
- Ganesh, S.; Iyer, A.K.; Weiler, J.; Morrissey, D.V.; Amiji, M.M. Combination of siRNA-directed Gene Silencing With Cisplatin Reverses Drug Resistance in Human Non-small Cell Lung Cancer. Mol. Ther.-Nucleic Acids 2013, 2, e110. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, Y.; Guo, L.; Liu, C.; Wang, P.; Ren, W. Exosomal microRNA-107 reverses chemotherapeutic drug resistance of gastric cancer cells through HMGA2/mTOR/P-gp pathway. BMC Cancer 2021, 21, 1290. [Google Scholar] [CrossRef]
- Yu, J.; Hu, F.; Zhu, Q.; Li, X.; Ren, H.; Fan, S.; Qian, B.; Zhai, B.; Yang, D. PD-L1 monoclonal antibody-decorated nanoliposomes loaded with Paclitaxel and P-gp transport inhibitor for the synergistic chemotherapy against multidrug resistant gastric cancers. Nanoscale Res. Lett. 2020, 15, 59. [Google Scholar] [CrossRef]
- Ciesielski, M.J.; Galbo, P.; Figel, S.; Wiltsie, L.; Frank, C.; Qiu, J.; Fenstermaker, R.A. Abstract 2725: Circulating CD9-GFAP-survivin exosomes during active specific immunotherapy, a potential biomarker for glioma. Cancer Res. 2017, 77, 2725. [Google Scholar] [CrossRef]
- Yazdani, M.; Gholizadeh, Z.; Nikpoor, A.R.; Hatamipour, M.; Alani, B.; Nikzad, H.; Roshan, N.M.; Verdi, J.; Jaafari, M.R.; Noureddini, M.; et al. Vaccination with dendritic cells pulsed ex vivo with gp100 peptide-decorated liposomes enhances the efficacy of anti PD-1 therapy in a mouse model of melanoma. Vaccine 2020, 38, 5665–5677. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Wang, X.; Mo, X.; Gu, J.; Lu, Z.; Pan, Z.; Zhu, Y. Survivin up-regulates the expression of breast cancer resistance protein (BCRP) through attenuating the suppression of p53 on NF-κB expression in MCF-7/5-FU cells. Int. J. Biochem. Cell Biol. 2013, 45, 2036–2044. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sui, H.; Zheng, Y.; Jiang, Y.; Shi, Y.; Liang, J.; Zhao, L. Curcumin-primed exosomes potently ameliorate cognitive function in AD mice by inhibiting hyperphosphorylation of the Tau protein through the AKT/GSK-3β pathway. Nanoscale 2019, 11, 7481–7496. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.; Lopes, I.; Magalhães, L.; Sárria, M.P.; Machado, R.; Sousa, J.C.; Botelho, C.; Teixeira, J.; Gomes, A.C. Novel concept of exosome-like liposomes for the treatment of Alzheimer’s disease. J. Control. Release 2021, 336, 130–143. [Google Scholar] [CrossRef]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Yuan, D.; Deygen, I.; Klyachko, N.L.; Kabanov, A.V.; Batrakova, E.V. Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: In vitro and in vivo evaluations. Nanomedicine 2018, 14, 195–204. [Google Scholar] [CrossRef]
- Satake, T.; Suetsugu, A.; Nakamura, M.; Kunisada, T.; Saji, S.; Moriwaki, H.; Shimizu, M.; Hoffman, R.M. Color-coded Imaging of the Fate of Cancer-cell-derived Exosomes During Pancreatic Cancer Metastases in a Nude-mouse Model. Anticancer Res. 2019, 39, 4055–4060. [Google Scholar] [CrossRef]
- Tan, F.; Li, X.; Wang, Z.; Li, J.; Shahzad, K.; Zheng, J. Clinical applications of stem cell-derived exosomes. Signal Transduct. Target. Ther. 2024, 9, 17. [Google Scholar] [CrossRef]
- Liu, H.; Chen, L.; Liu, J.; Meng, H.; Zhang, R.; Ma, L.; Wu, L.; Yu, S.; Shi, F.; Li, Y.; et al. Co-delivery of tumor-derived exosomes with alpha-galactosylceramide on dendritic cell-based immunotherapy for glioblastoma. Cancer Lett. 2017, 411, 182–190. [Google Scholar] [CrossRef]
- Yang, F.; Wang, L.; Zhao, W.; Wang, S.; Li, J.; Sun, A.; Wang, M.; Wang, Z.; Chen, Z.; Heng, X. A Systematic Review and Meta-Analysis on the Effectiveness of Radiotherapy and Temozolomide Treatment With or Without Bevacizumab in Patients With Glioblastoma Multiforme. Neurol. India 2024, 72, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Besse, B.; Charrier, M.; Lapierre, V.; Dansin, E.; Lantz, O.; Planchard, D.; Le Chevalier, T.; Livartoski, A.; Barlesi, F.; Laplanche, A.; et al. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. OncoImmunology 2016, 5, 1071008. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Han, Y.; An, Y.; Ding, Y.; He, C.; Wang, X.; Tang, Q. NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials 2018, 178, 302–316. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zang, L.; Cheng, Y.; Qin, L. Cancer Exosome Loaded with Paclitaxel for Targeted Lung Cancer Therapy. J. Biomater. Tissue Eng. 2023, 13, 118–122. [Google Scholar] [CrossRef]
- Liu, Y.; Li, M.; Gu, J.; Huang, H.; Xie, H.; Yu, C.; Roy, S.; Chen, X.; Kuang, T.; Zhang, Y.; et al. Engineering of exosome-liposome hybrid-based theranostic nanomedicines for NIR-II fluorescence imaging-guided and targeted NIR-II photothermal therapy of subcutaneous glioblastoma. Colloids Surf. B Biointerfaces 2024, 245, 114258. [Google Scholar] [CrossRef]
- Shi, S.; Li, T.; Wen, X.; Wu, S.Y.; Xiong, C.; Zhao, J.; Lincha, V.R.; Chow, D.S.; Liu, Y.; Sood, A.K.; et al. Copper-64 Labeled PEGylated Exosomes for In Vivo Positron Emission Tomography and Enhanced Tumor Retention. Bioconjug. Chem. 2019, 30, 2675–2683. [Google Scholar] [CrossRef]
- Emam, S.E.; Elsadek, N.E.; Abu Lila, A.S.; Takata, H.; Kawaguchi, Y.; Shimizu, T.; Ando, H.; Ishima, Y.; Ishida, T. Anti-PEG IgM production and accelerated blood clearance phenomenon after the administration of PEGylated exosomes in mice. J. Control. Release 2021, 334, 327–334. [Google Scholar] [CrossRef]
- Ishida, T.; Kashima, S.; Kiwada, H. The contribution of phagocytic activity of liver macrophages to the accelerated blood clearance (ABC) phenomenon of PEGylated liposomes in rats. J. Control. Release 2008, 126, 162–165. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, C.; Wu, N.; Feng, Y.; Wang, J.; Ma, L.; Chen, Y. The role of exosomes in liver cancer: Comprehensive insights from biological function to therapeutic applications. Front. Immunol. 2024, 15, 1473030. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, S.; Hu, H.; Yang, J.; Wang, X.; Ma, Y.; Jiang, J.; Wang, J.; Zhong, L.; Chen, M.; et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate acute liver failure by reducing the activity of the NLRP3 inflammasome in macrophages. Biochem. Biophys. Res. Commun. 2019, 508, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Shen, M.; Fan, X.; Liu, Y.; Yang, L. Pathogenic and Potential Therapeutic Roles of Exosomes Derived from Immune Cells in Liver Diseases. Front. Immunol. 2022, 13, 810300. [Google Scholar] [CrossRef] [PubMed]
- Batrakova, E.V.; Kim, M.S. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J. Control. Release 2015, 219, 396–405. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, B.; Tu, H.; Pan, C.; Chai, Y.; Chen, W. Advances in colorimetric biosensors of exosomes: Novel approaches based on natural enzymes and nanozymes. Nanoscale 2023, 16, 1005–1024. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Lee, E.K.; Yim, J.; Lee, M.H.; Lee, E.; Lee, Y.-S.; Seo, W. Exosomes: Nomenclature, Isolation, and Biological Roles in Liver Diseases. Biomol. Ther. 2023, 31, 253–263. [Google Scholar] [CrossRef]
- Ding, Y.; Luo, Q.; Que, H.; Wang, N.; Gong, P.; Gu, J. Mesenchymal Stem Cell-Derived Exosomes: A Promising Therapeutic Agent for the Treatment of Liver Diseases. Int. J. Mol. Sci. 2022, 23, 10972. [Google Scholar] [CrossRef]
- Kao, Y.H.; Chang, C.Y.; Lin, Y.C.; Chen, P.H.; Lee, P.H.; Chang, H.R.; Chang, W.Y.; Chang, Y.C.; Wun, S.F.; Sun, C.K. Mesenchymal Stem Cell-Derived Exosomes Mitigate Acute Murine Liver Injury via Ets-1 and Heme Oxygenase-1 Up-regulation. Curr. Stem Cell Res. Ther. 2024, 19, 906–918. [Google Scholar] [CrossRef]
- Wei, X.C.; Liu, L.J.; Zhu, F. Exosomes as potential diagnosis and treatment for liver cancer. World J. Gastrointest. Oncol. 2022, 14, 334–347. [Google Scholar] [CrossRef]
- Guru, K.T.P.; Sreeja, J.S.; Dharmapal, D.; Sengupta, S.; Basu, P.K. Novel Gold Nanoparticle-Based Quick Small-Exosome Isolation Technique from Serum Sample at a Low Centrifugal Force. Nanomaterials 2022, 12, 1660. [Google Scholar] [CrossRef]
- Kang, C.; Han, P.; Lee, J.S.; Lee, D.; Kim, D. Anchor, Spacer, and Ligand-Modified Engineered Exosomes for Trackable Targeted Therapy. Bioconjug. Chem. 2020, 31, 2541–2552. [Google Scholar] [CrossRef]
- Cheravi, M.; Baharara, J.; Yaghmaei, P.; Roudbari, N.H. Differentiation of Human Adipose-derived Stem Cells to Exosome-affected Neural-like Cells Extracted from Human Cerebrospinal Fluid Using Bioprinting Process. Curr. Stem Cell Res. Ther. 2024, 19, 1042–1054. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.; Vaiselbuh, S.R. CD19 Chimeric Antigen Receptor-Exosome Targets CD19 Positive B-lineage Acute Lymphocytic Leukemia and Induces Cytotoxicity. Cancers 2021, 13, 1401. [Google Scholar] [CrossRef]
- Gu, C.; Feng, J.; Waqas, A.; Deng, Y.; Zhang, Y.; Chen, W.; Long, J.; Huang, S.; Chen, L. Technological Advances of 3D Scaffold-Based Stem Cell/Exosome Therapy in Tissues and Organs. Front. Cell Dev. Biol. 2021, 9, 709204. [Google Scholar] [CrossRef]
- Chaput, N.; Schartz, N.E.C.; André, F.; Taiėb, J.; Novault, S.; Bonnaventure, P.; Aubert, N.; Bernard, J.; Lemonnier, F.; Merad, M.; et al. Exosomes as Potent Cell-Free Peptide-Based Vaccine. II. Exosomes in CpG Adjuvants Efficiently Prime Naive Tc1 Lymphocytes Leading to Tumor Rejection. J. Immunol. 2004, 172, 2137–2146. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Rodriguez, L.; Dilsiz, N.; Barea, R.; Ziarati, P.; Hochwimmer, B.; Zayas Tamayo, A.; Lambert Brown, D. The Algorithms Cruz Rodriguez (CR) are Proposing A Novel Vaccine RNA-Peptide against Breast, Ovarian, and Lung Cancers Disease: Exosomes as Carrier in Cancer Progression and Metastasis. J. Med.-Clin. Res. Rev. 2021, 5, 1–16. [Google Scholar]
- Tang, X.J.; Sun, X.Y.; Huang, K.M.; Zhang, L.; Yang, Z.S.; Zou, D.D.; Wang, B.; Warnock, G.L.; Dai, L.-J.; Luo, J. Therapeutic potential of CAR-T cell-derived exosomes: A cell-free modality for targeted cancer therapy. Oncotarget 2015, 6, 44179–44190. [Google Scholar] [CrossRef]
- Yang, P.; Cao, X.; Cai, H.; Feng, P.; Chen, X.; Zhu, Y.; Yang, Y.; An, W.; Yang, Y.; Jie, J. The exosomes derived from CAR-T cell efficiently target mesothelin and reduce triple-negative breast cancer growth. Cell. Immunol. 2021, 360, 104262. [Google Scholar] [CrossRef]
- Selvam, S.; Ben Thomas, M.; Bhowmick, T.; Chandru, A. Bioprinting of exosomes: Prospects and challenges for clinical applications. Int. J. Bioprinting 2023, 9, 690. [Google Scholar] [CrossRef]
- Chen, P.; Zheng, L.; Wang, Y.; Tao, M.; Xie, Z.; Xia, C.; Gu, C.; Chen, J.; Qiu, P.; Mei, S.; et al. Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics 2019, 9, 2439–2459. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).