Review of Hydrogen Sulfide Based on Its Activity Mechanism and Fluorescence Sensing
Abstract
1. Introduction
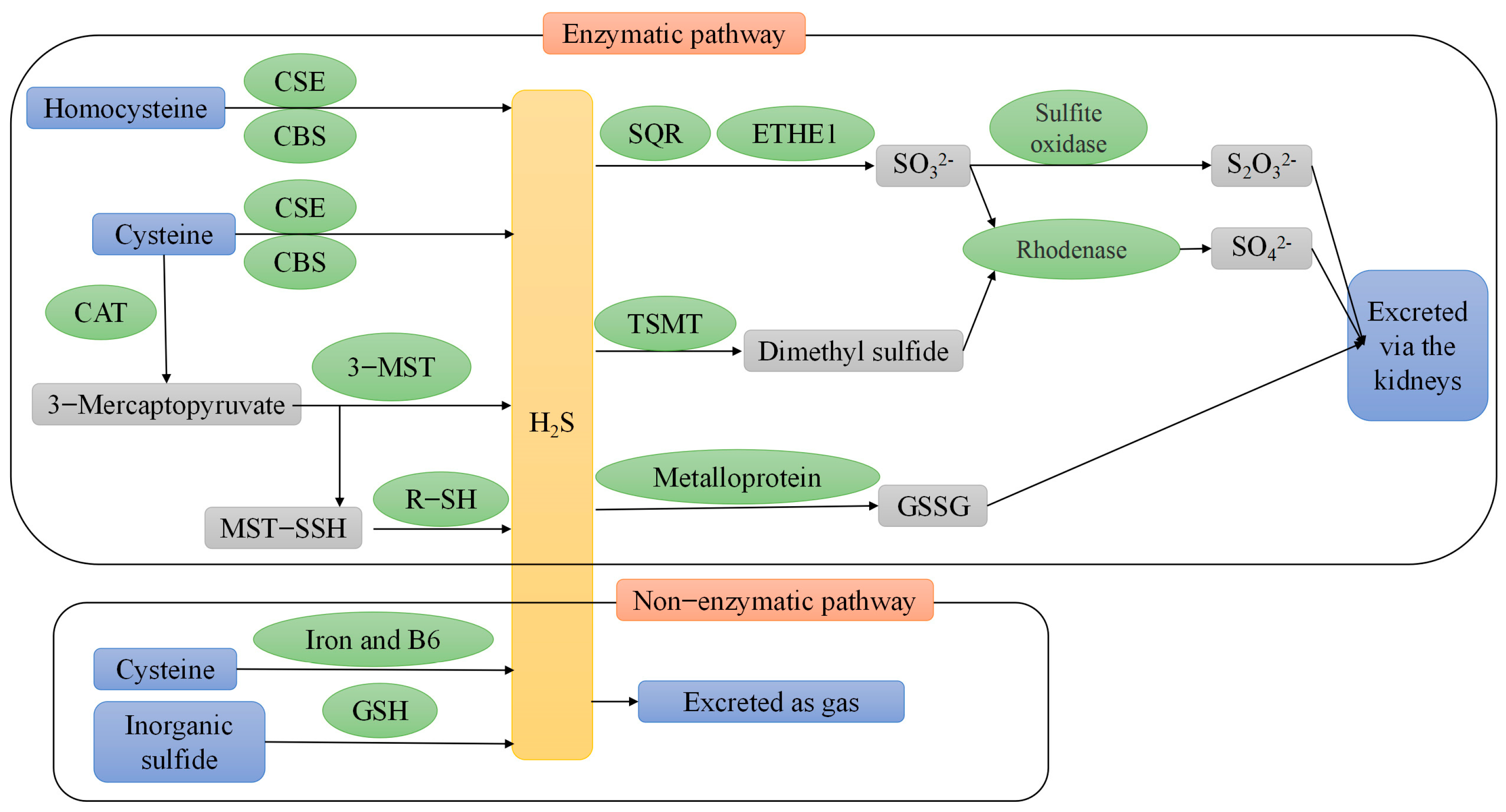
2. Endogenous H2S Production
2.1. Enzymatic Pathway
2.2. Non-Enzymatic Pathways
3. Metabolism of Endogenous H2S
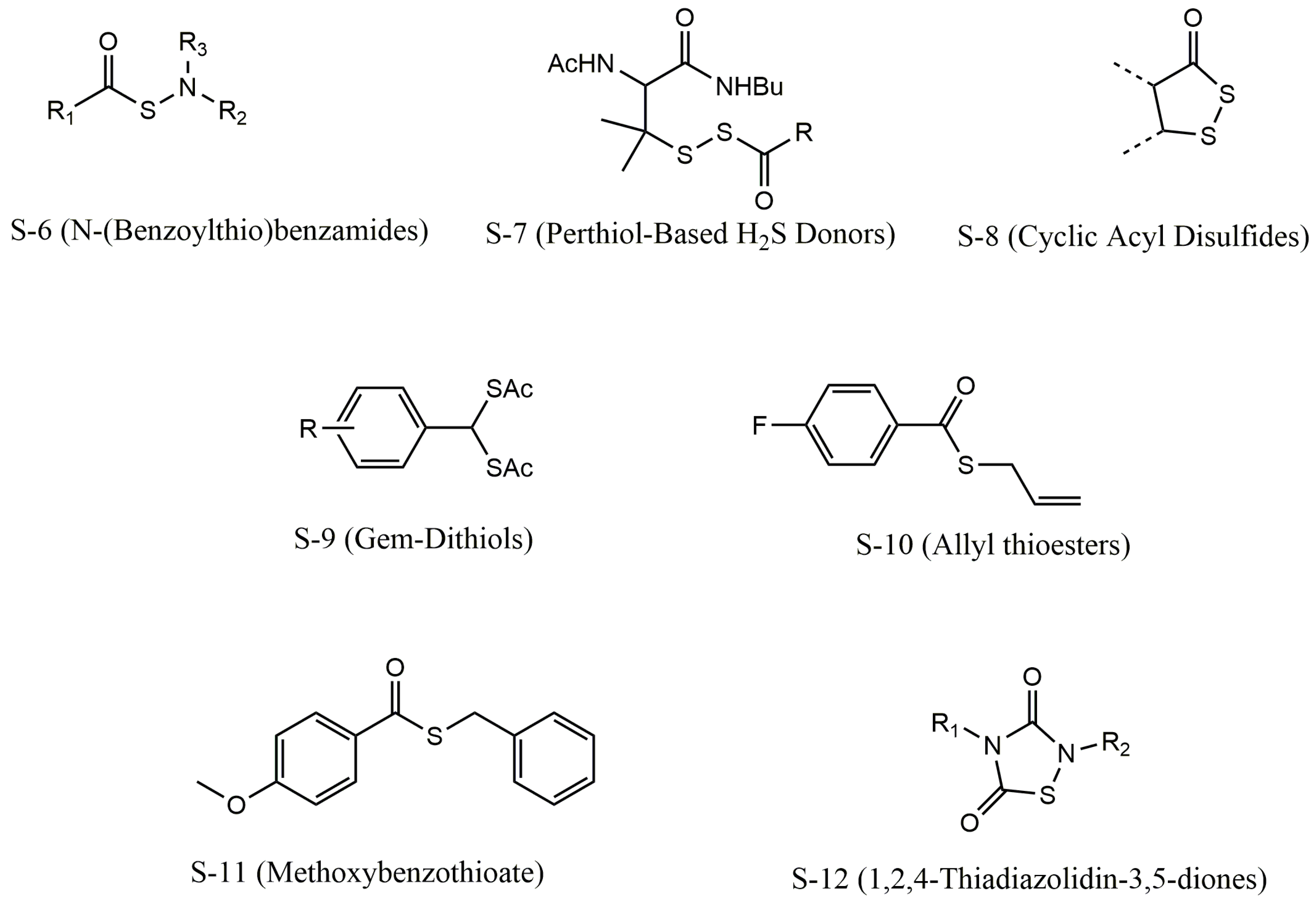
4. Donor Categories of H2S
4.1. Inorganic H2S Donors
4.2. Naturally Derived H2S Donors
4.3. Hydrolysis-Triggered H2S Donors
4.4. Thiol-Triggered H2S Donors
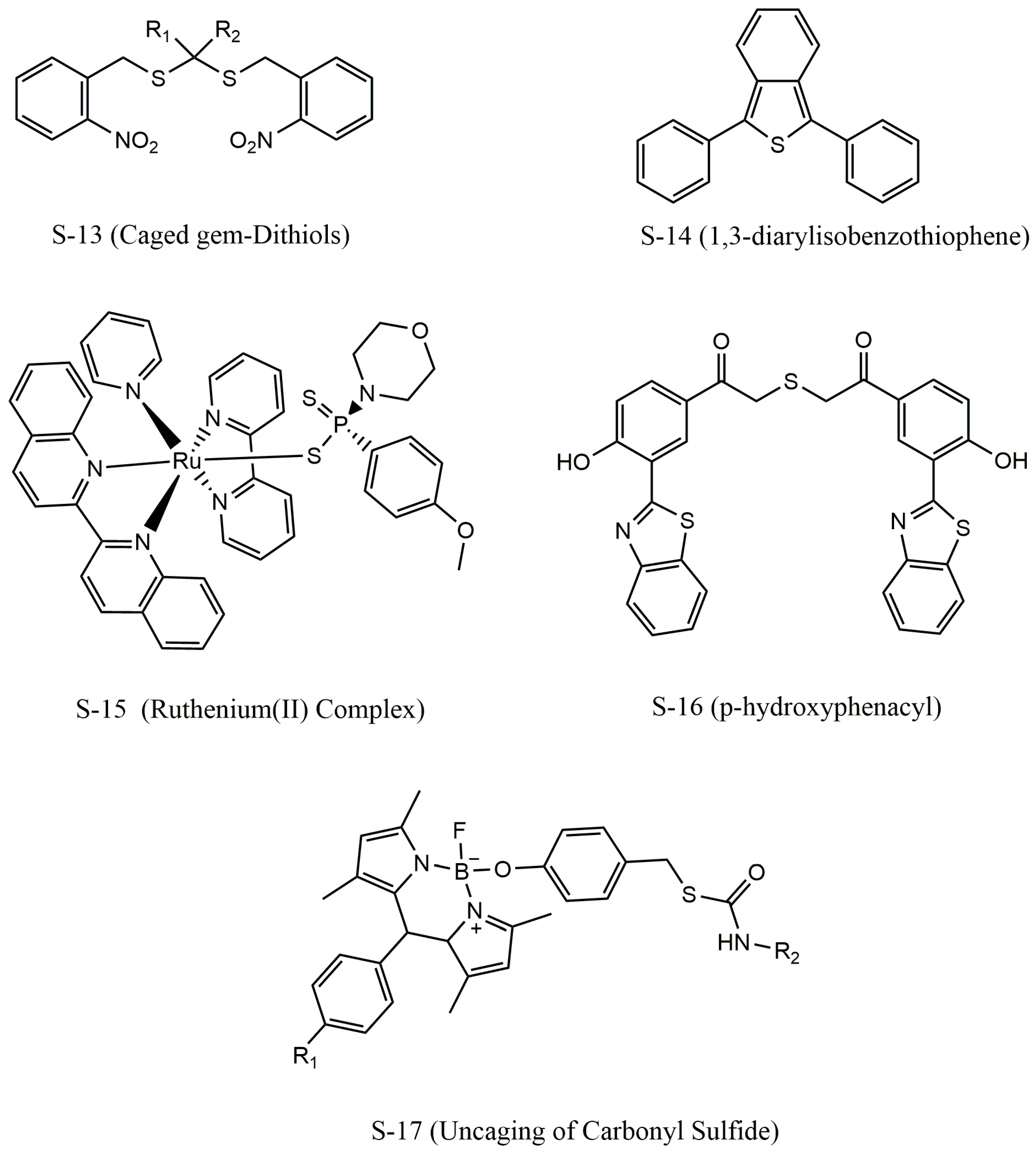
4.5. Light-Triggered H2S Donors
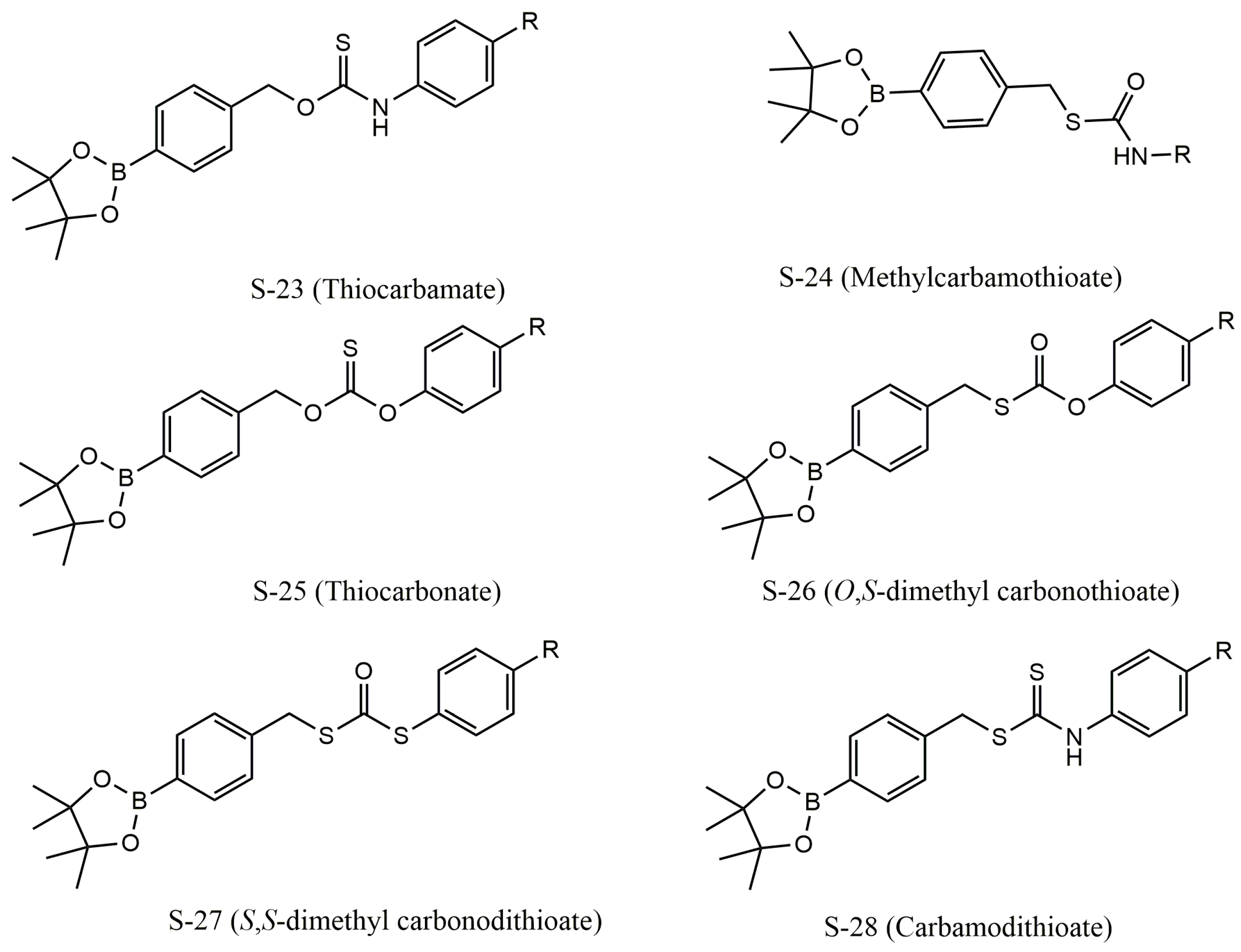
4.6. Enzyme-Triggered H2S Donors
4.7. Reactive Oxygen Species-Triggered H2S Donor
5. Physiological Activity of Endogenous H2S
5.1. Antioxidative Stress
5.2. Anti-Inflammatory
5.3. Protection against Myocardial Damage
5.4. Related to Liver Disease
5.5. Related to Cancer
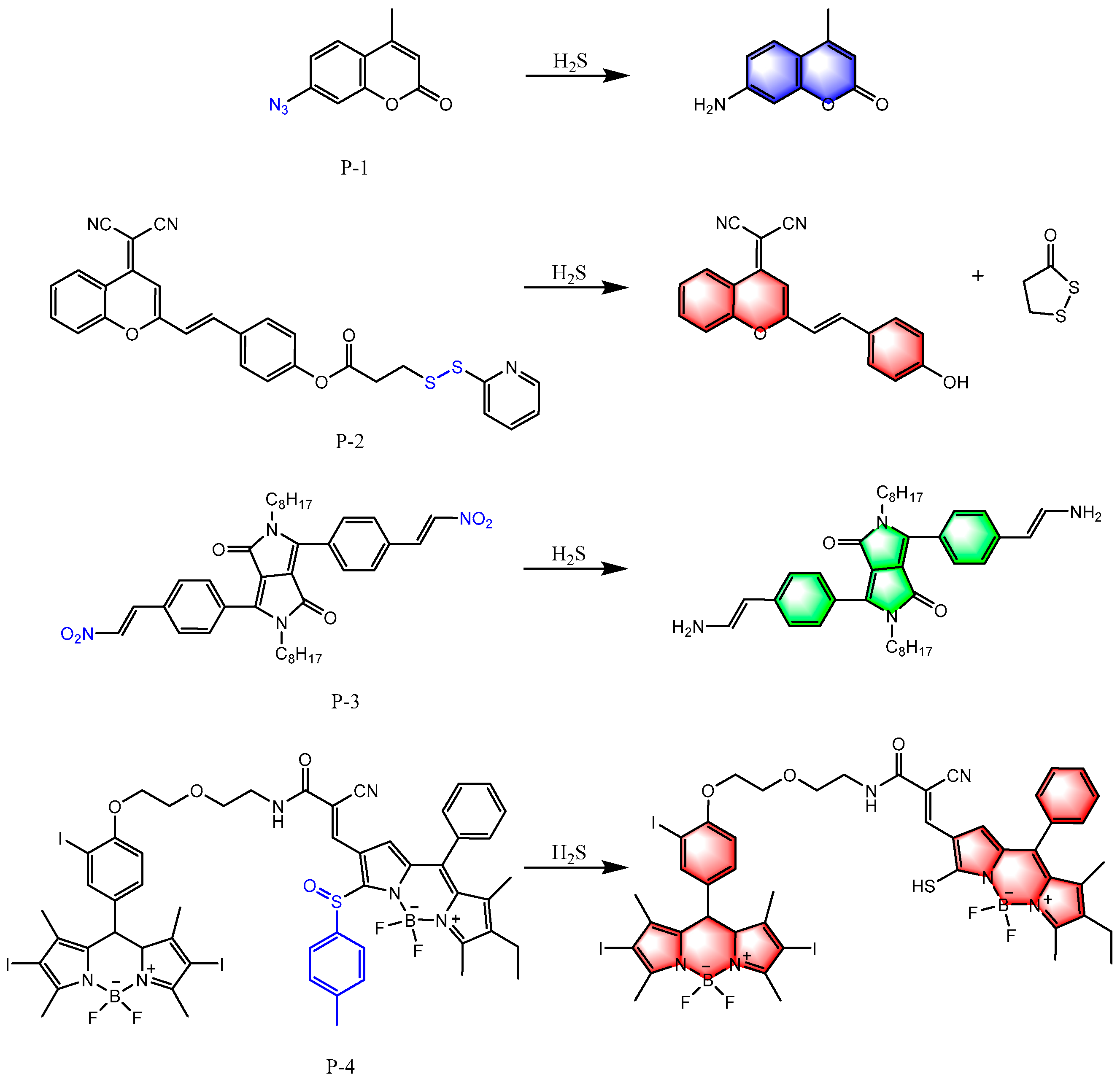
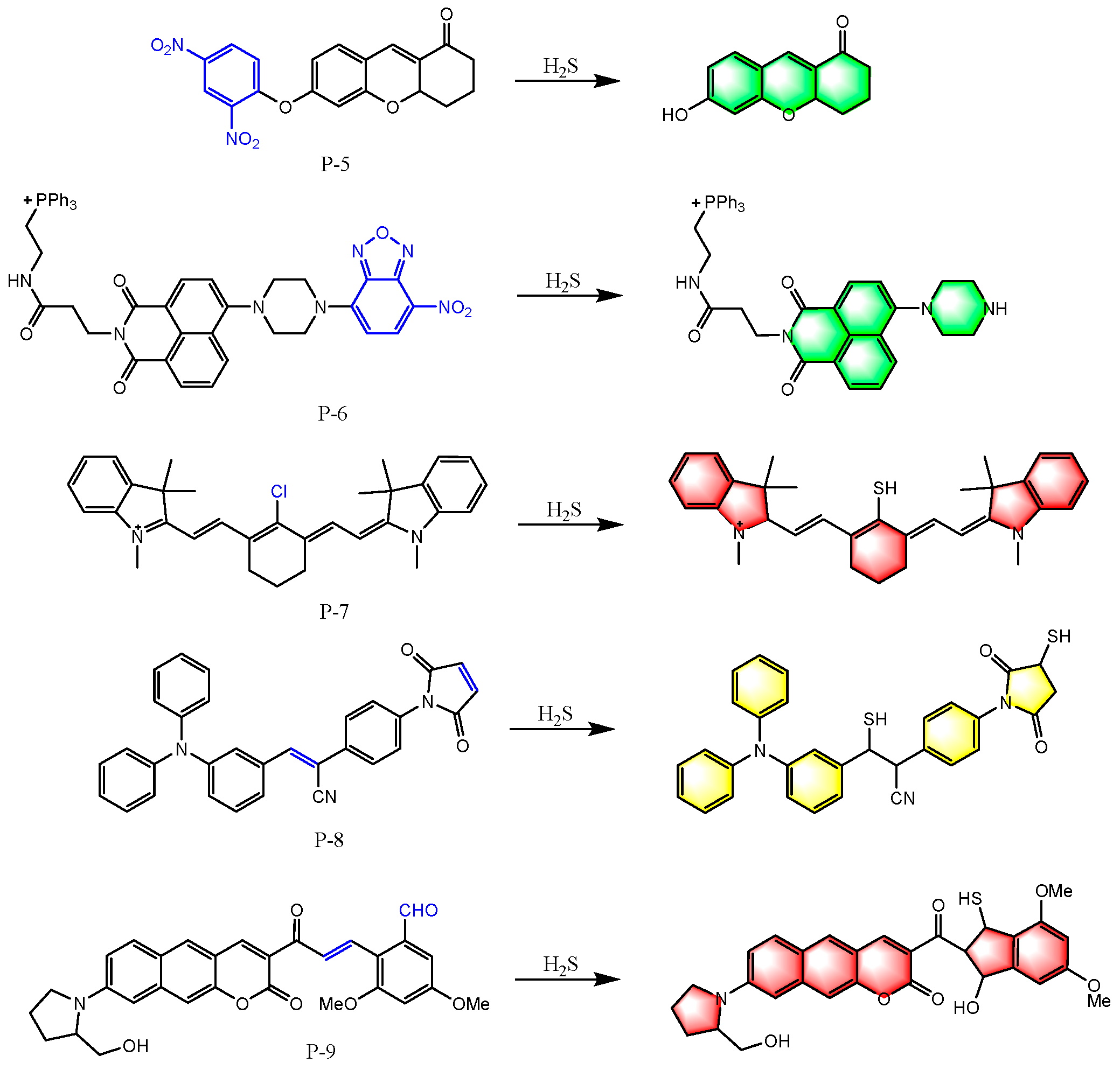
6. Fluorescent Probes for Detecting H2S
6.1. Based on Reducibility
6.2. Based on Nucleophilicity
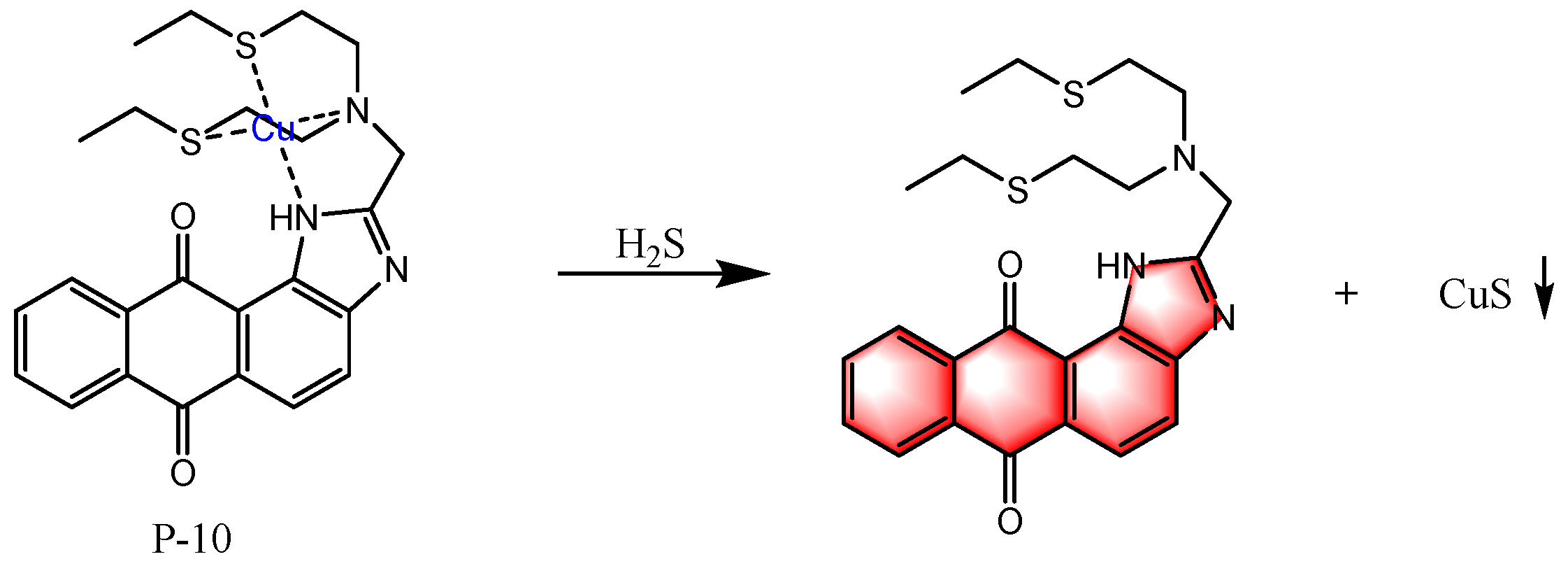
6.3. Based on Metal Coordination
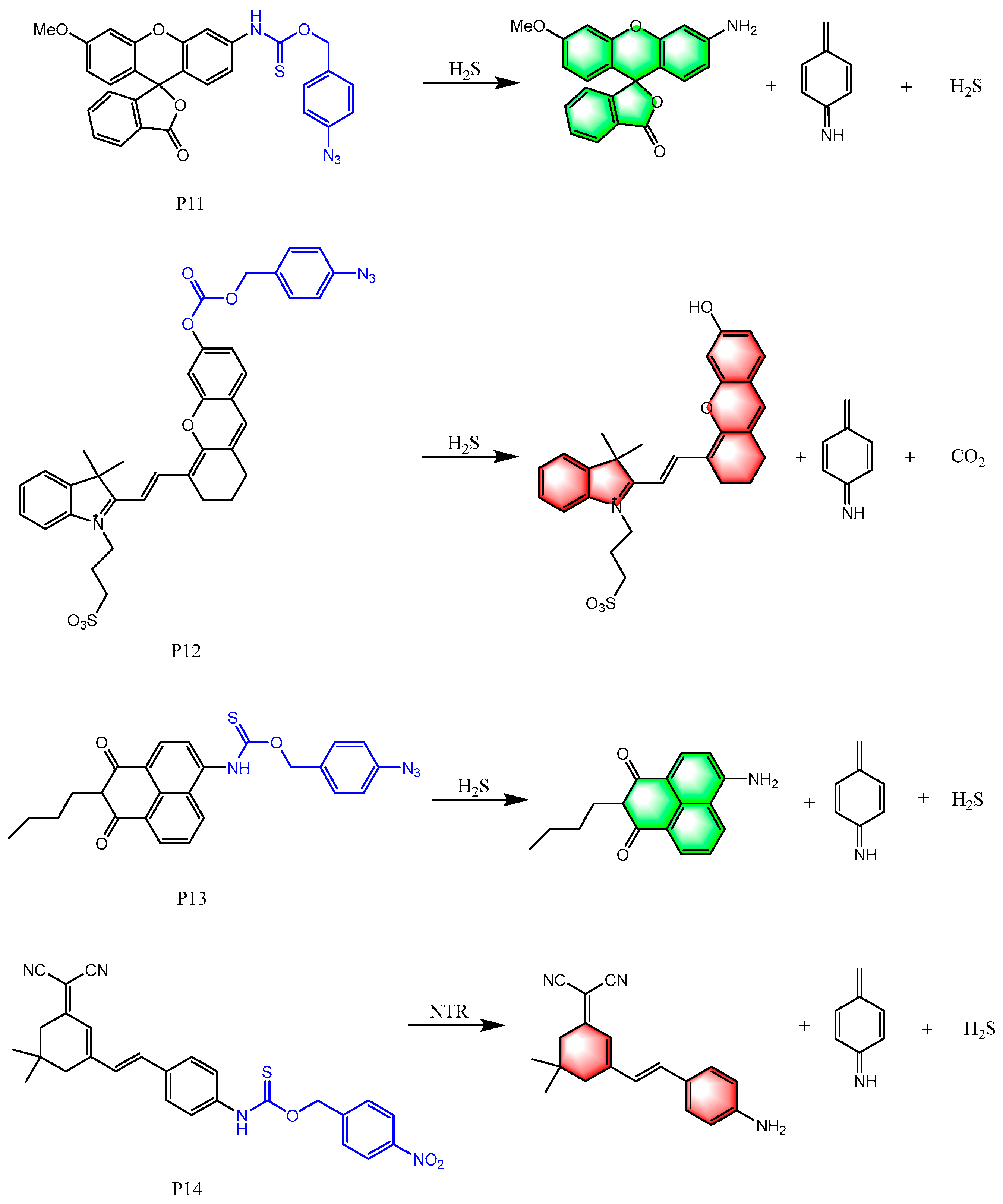
6.4. Based on Self-Immolation Reaction
7. H2S Scavenging Agents
8. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Araujo-Alvarez, J.M.; Trujillo-Ferrara, J.G. De morbis artificum diatriba 1700–2000. Salud Pública de México 2002, 44, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Szabo, C. A timeline of hydrogen sulfide (H2S) research: From environmental toxin to biological mediator. Biochem. Pharmacol. 2018, 149, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Warenycia, M.W.; Goodwin, L.R.; Benishin, C.G.; Reiffenstein, R.; Francom, D.M.; Taylor, J.D.; Dieken, F.P. Acute hydrogen sulfide poisoning: Demonstration of selective uptake of sulfide by the brainstem by measurement of brain sulfide levels. Biochem. Pharmacol. 1989, 38, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, L.R.; Francom, D.; Dieken, F.P.; Taylor, J.D.; Warenycia, M.W.; Reiffenstein, R.; Dowling, G. Determination of sulfide in brain tissue by gas dialysis/ion chromatography: Postmortem studies and two case reports. J. Anal. Toxicol. 1989, 13, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Savage, J.; Gould, D. Determination of sulfide in brain tissue and rumen fluid by ion-interaction reversed-phase high-performance liquid chromatography. J. Chromatogr. Biomed. Appl. 1990, 526, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Kimura, H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 1996, 16, 1066–1071. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, R. H2S-induced vasorelaxation and underlying cellular and molecular mechanisms. Am. J. Physiol.-Heart Circ. Physiol. 2002, 283, H474–H480. [Google Scholar] [CrossRef]
- Chakraborty, P.K.; Xiong, X.; Mustafi, S.B.; Saha, S.; Dhanasekaran, D.; Mandal, N.A.; McMeekin, S.; Bhattacharya, R.; Mukherjee, P. Role of cystathionine beta synthase in lipid metabolism in ovarian cancer. Oncotarget 2015, 6, 37367. [Google Scholar] [CrossRef]
- Kanagy, N.L.; Szabo, C.; Papapetropoulos, A. Vascular biology of hydrogen sulfide. Am. J. Physiol.-Cell Physiol. 2017, 312, C537–C549. [Google Scholar] [CrossRef]
- Zhao, Z.-Z.; Wang, Z.; Li, G.-H.; Wang, R.; Tan, J.-M.; Cao, X.; Suo, R.; Jiang, Z.-S. Hydrogen sulfide inhibits macrophage-derived foam cell formation. Exp. Biol. Med. 2011, 236, 169–176. [Google Scholar] [CrossRef]
- Wang, C.; Du, J.; Du, S.; Liu, Y.; Li, D.; Zhu, X.; Ni, X. Endogenous H2S resists mitochondria-mediated apoptosis in the adrenal glands via ATP5A1 S-sulfhydration in male mice. Mol. Cell. Endocrinol. 2018, 474, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Szabo, C. Both the H2S biosynthesis inhibitor aminooxyacetic acid and the mitochondrially targeted H2S donor AP39 exert protective effects in a mouse model of burn injury. Pharmacol. Res. 2016, 113, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Filipovic, M.R. Persulfidation (S-sulfhydration) and H2S. Handb. Exp. Pharmacol 2015, 230, 29–59. [Google Scholar] [PubMed]
- Kabil, O.; Banerjee, R. Enzymology of H2S biogenesis, decay and signaling. Antioxid. Redox Signal. 2014, 20, 770–782. [Google Scholar] [CrossRef] [PubMed]
- Badiei, A.; Chambers, S.; Gaddam, R.; Bhatia, M. Cystathionine-γ-lyase gene silencing with siRNA in monocytes/macrophages attenuates inflammation in cecal ligation and puncture-induced sepsis in the mouse. J. Biosci. 2016, 41, 87–95. [Google Scholar] [CrossRef]
- Lu, Y.; O’Dowd, B.F.; Orrego, H.; Israel, Y. Cloning and nucleotide sequence of human liver cDNA encoding for cystathionine γ-lyase. Biochem. Biophys. Res. Commun. 1992, 189, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Tripatara, P.; SA Patel, N.; Collino, M.; Gallicchio, M.; Kieswich, J.; Castiglia, S.; Benetti, E.; Stewart, K.N.; Brown, P.A.; Yaqoob, M.M. Generation of endogenous hydrogen sulfide by cystathionine γ-lyase limits renal ischemia/reperfusion injury and dysfunction. Lab. Investig. 2008, 88, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Dominy, J.E.; Stipanuk, M.H. New roles for cysteine and transsulfuration enzymes: Production of H2S, a neuromodulator and smooth muscle relaxant. Nutr. Rev. 2004, 62, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Banerjee, R. PLP-dependent H2S biogenesis. Biochim. et Biophys. Acta (BBA)-Proteins Proteom. 2011, 1814, 1518–1527. [Google Scholar] [CrossRef]
- Singh, S.; Padovani, D.; Leslie, R.A.; Chiku, T.; Banerjee, R. Relative contributions of cystathionine β-synthase and γ-cystathionase to H2S biogenesis via alternative trans-sulfuration reactions. J. Biol. Chem. 2009, 284, 22457–22466. [Google Scholar] [CrossRef]
- Stipanuk, M.H.; Beck, P.W. Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. Biochem. J. 1982, 206, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Chiku, T.; Padovani, D.; Zhu, W.; Singh, S.; Vitvitsky, V.; Banerjee, R. H2S biogenesis by human cystathionine γ-lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. J. Biol. Chem. 2009, 284, 11601–11612. [Google Scholar] [CrossRef] [PubMed]
- Giuffrè, A.; Vicente, J.B. Hydrogen sulfide biochemistry and interplay with other gaseous mediators in mammalian physiology. Oxidative Med. Cell. Longev. 2018, 2018, 6290931. [Google Scholar] [CrossRef] [PubMed]
- Kuo, M.M.; Kim, D.H.; Jandu, S.; Bergman, Y.; Tan, S.; Wang, H.; Pandey, D.R.; Abraham, T.P.; Shoukas, A.A.; Berkowitz, D.E. MPST but not CSE is the primary regulator of hydrogen sulfide production and function in the coronary artery. Am. J. Physiol.-Heart Circ. Physiol. 2016, 310, H71–H79. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, W.; Li, S.; Han, Y.; Zhang, P.; Meng, G.; Xiao, Y.; Xie, L.; Wang, X.; Sha, J. Hydrogen sulfide as a potential target in preventing spermatogenic failure and testicular dysfunction. Antioxid. Redox Signal. 2018, 28, 1447–1462. [Google Scholar] [CrossRef]
- Liang, R.; Yu, W.-d.; Du, J.-b.; Yang, L.-j.; Shang, M.; Guo, J.-z. Localization of cystathionine β synthase in mice ovaries and its expression profile during follicular development. Chin. Med. J. 2006, 119, 1877–1883. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, M.A.; Navarro, M.A.; Carnicer, R.; Sarría, A.J.; Acín, S.; Arnal, C.; Muniesa, P.; Surra, J.C.; Arbonés-Mainar, J.M.; Maeda, N. Cystathionine β-synthase is essential for female reproductive function. Hum. Mol. Genet. 2006, 15, 3168–3176. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, R.; Otsuguro, K.-i.; Yamaguchi, S.; Ito, S. Neuronal regulation of expression of hydrogen sulfide-producing enzyme cystathionine β-synthase in rat spinal cord astrocytes. Neurosci. Res. 2015, 97, 52–59. [Google Scholar] [CrossRef]
- Lee, M.; Schwab, C.; Yu, S.; McGeer, E.; McGeer, P.L. Astrocytes produce the antiinflammatory and neuroprotective agent hydrogen sulfide. Neurobiol. Aging 2009, 30, 1523–1534. [Google Scholar] [CrossRef] [PubMed]
- Szabó, C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov. 2007, 6, 917–935. [Google Scholar] [CrossRef]
- Mustafa, A.K.; Gadalla, M.M.; Snyder, S.H. Signaling by gasotransmitters. Sci. Signal. 2009, 2, re2. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jhee, K.-H.; Kruger, W.D. Production of the neuromodulator H2S by cystathionine β-synthase via the condensation of cysteine and homocysteine. J. Biol. Chem. 2004, 279, 52082–52086. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, S.; Kang, L.; Kimura, T.; Niki, I. Hydrogen sulphide protects mouse pancreatic β-cells from cell death induced by oxidative stress, but not by endoplasmic reticulum stress. Br. J. Pharmacol. 2011, 162, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Fräsdorf, B.; Radon, C.; Leimkühler, S. Characterization and interaction studies of two isoforms of the dual localized 3-mercaptopyruvate sulfurtransferase TUM1 from humans. J. Biol. Chem. 2014, 289, 34543–34556. [Google Scholar] [CrossRef] [PubMed]
- Nagahara, N.; Ito, T.; Kitamura, H.; Nishino, T. Tissue and subcellular distribution of mercaptopyruvate sulfurtransferase in the rat: Confocal laser fluorescence and immunoelectron microscopic studies combined with biochemical analysis. Histochem. Cell Biol. 1998, 110, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, N.; Mikami, Y.; Kimura, Y.; Nagahara, N.; Kimura, H. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J. Biochem. 2009, 146, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, N.; Koike, S.; Tanaka, M.; Ishigami-Yuasa, M.; Kimura, Y.; Ogasawara, Y.; Fukui, K.; Nagahara, N.; Kimura, H. A novel pathway for the production of hydrogen sulfide from D-cysteine in mammalian cells. Nat. Commun. 2013, 4, 1366. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Minkler, P.; Grove, D.; Wang, R.; Willard, B.; Dweik, R.; Hine, C. Non-enzymatic hydrogen sulfide production from cysteine in blood is catalyzed by iron and vitamin B6. Commun. Biol. 2019, 2, 194. [Google Scholar] [CrossRef] [PubMed]
- Benavides, G.A.; Squadrito, G.L.; Mills, R.W.; Patel, H.D.; Isbell, T.S.; Patel, R.P.; Darley-Usmar, V.M.; Doeller, J.E.; Kraus, D.W. Hydrogen sulfide mediates the vasoactivity of garlic. Proc. Natl. Acad. Sci. USA 2007, 104, 17977–17982. [Google Scholar] [CrossRef]
- DeLeon, E.R.; Stoy, G.F.; Olson, K.R. Passive loss of hydrogen sulfide in biological experiments. Anal. Biochem. 2012, 421, 203–207. [Google Scholar] [CrossRef]
- Powell, C.R.; Dillon, K.M.; Matson, J.B. A review of hydrogen sulfide (H2S) donors: Chemistry and potential therapeutic applications. Biochem. Pharmacol. 2018, 149, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Carlström, M.; Borniquel, S.; Jädert, C.; Kevil, C.G.; Lundberg, J.O. Microbial regulation of host hydrogen sulfide bioavailability and metabolism. Free Radic. Biol. Med. 2013, 60, 195–200. [Google Scholar] [CrossRef]
- Furne, J.; Saeed, A.; Levitt, M.D. Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2008, 295, R1479–R1485. [Google Scholar] [CrossRef] [PubMed]
- Vitvitsky, V.; Kabil, O.; Banerjee, R. High turnover rates for hydrogen sulfide allow for rapid regulation of its tissue concentrations. Antioxid. Redox Signal. 2012, 17, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Curtis, C.; Bartholomew, T.; Rose, F.; Dodgson, K. Detoxication of sodium 35S-sulphide in the rat. Biochem. Pharmacol. 1972, 21, 2313–2321. [Google Scholar] [CrossRef] [PubMed]
- Bartholomew, T.C.; Powell, G.M.; Dodgson, K.S.; Curtis, C.G. Oxidation of sodium sulphide by rat liver, lungs and kidney. Biochem. Pharmacol. 1980, 29, 2431–2437. [Google Scholar] [CrossRef] [PubMed]
- Donnarumma, E.; Trivedi, R.K.; Lefer, D.J. Protective actions of H2S in acute myocardial infarction and heart failure. Compr. Physiol. 2011, 7, 583–602. [Google Scholar]
- Vitvitsky, V.; Yadav, P.K.; Kurthen, A.; Banerjee, R. Sulfide oxidation by a noncanonical pathway in red blood cells generates thiosulfate and polysulfides. J. Biol. Chem. 2015, 290, 8310–8320. [Google Scholar] [CrossRef]
- Bostelaar, T.; Vitvitsky, V.; Kumutima, J.; Lewis, B.E.; Yadav, P.K.; Brunold, T.C.; Filipovic, M.; Lehnert, N.; Stemmler, T.L.; Banerjee, R. Hydrogen sulfide oxidation by myoglobin. J. Am. Chem. Soc. 2016, 138, 8476–8488. [Google Scholar] [CrossRef]
- Ruetz, M.; Kumutima, J.; Lewis, B.E.; Filipovic, M.R.; Lehnert, N.; Stemmler, T.L.; Banerjee, R. A distal ligand mutes the interaction of hydrogen sulfide with human neuroglobin. J. Biol. Chem. 2017, 292, 6512–6528. [Google Scholar] [CrossRef]
- Wagner, F.; Wagner, K.; Weber, S.; Stahl, B.; Knöferl, M.W.; Huber-Lang, M.; Seitz, D.H.; Asfar, P.; Calzia, E.; Senftleben, U. Inflammatory effects of hypothermia and inhaled H2S during resuscitated, hyperdynamic murine septic shock. Shock 2011, 35, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Whiteman, M.; Cheung, N.S.; Zhu, Y.-Z.; Chu, S.H.; Siau, J.L.; Wong, B.S.; Armstrong, J.S.; Moore, P.K. Hydrogen sulphide: A novel inhibitor of hypochlorous acid-mediated oxidative damage in the brain? Biochem. Biophys. Res. Commun. 2005, 326, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L.; Vong, L.; McKnight, W.; Dicay, M.; Martin, G.R. Endogenous and exogenous hydrogen sulfide promotes resolution of colitis in rats. Gastroenterology 2009, 137, 569–578.e1. [Google Scholar] [CrossRef] [PubMed]
- Pluth, M.D.; Bailey, T.S.; Hammers, M.D.; Hartle, M.D.; Henthorn, H.A.; Steiger, A.K. Natural products containing hydrogen sulfide releasing moieties. Synlett 2015, 26, 2633–2643. [Google Scholar] [CrossRef]
- Rahman, M.S. Allicin and other functional active components in garlic: Health benefits and bioavailability. Int. J. Food Prop. 2007, 10, 245–268. [Google Scholar] [CrossRef]
- Rana, S.; Pal, R.; Vaiphei, K.; Sharma, S.K.; Ola, R. Garlic in health and disease. Nutr. Res. Rev. 2011, 24, 60–71. [Google Scholar] [CrossRef]
- Butt, M.S.; Sultan, M.T.; Butt, M.S.; Iqbal, J. Garlic: Nature’s protection against physiological threats. Crit. Rev. Food Sci. Nutr. 2009, 49, 538–551. [Google Scholar] [CrossRef]
- Brodnitz, M.H.; Pascale, J.V.; Van Derslice, L. Flavor components of garlic extract. J. Agric. Food Chem. 1971, 19, 273–275. [Google Scholar] [CrossRef]
- Guo, W.; Cheng, Z.-y.; Zhu, Y.-z. Hydrogen sulfide and translational medicine. Acta Pharmacol. Sin. 2013, 34, 1284–1291. [Google Scholar] [CrossRef]
- Citi, V.; Martelli, A.; Testai, L.; Marino, A.; Breschi, M.C.; Calderone, V. Hydrogen sulfide releasing capacity of natural isothiocyanates: Is it a reliable explanation for the multiple biological effects of Brassicaceae? Planta Medica 2014, 80, 610–613. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, T.; Ertas, E.; Mert, O. Use of Lawesson’s reagent in organic syntheses. Chem. Rev. 2007, 107, 5210–5278. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Whiteman, M.; Guan, Y.Y.; Neo, K.L.; Cheng, Y.; Lee, S.W.; Zhao, Y.; Baskar, R.; Tan, C.-H.; Moore, P.K. Characterization of a novel, water-soluble hydrogen sulfide–releasing molecule (GYY4137) new insights into the biology of hydrogen sulfide. Circulation 2008, 117, 2351–2360. [Google Scholar] [CrossRef]
- Kang, J.; Li, Z.; Organ, C.L.; Park, C.-M.; Yang, C.-T.; Pacheco, A.; Wang, D.; Lefer, D.J.; Xian, M. pH-controlled hydrogen sulfide release for myocardial ischemia-reperfusion injury. J. Am. Chem. Soc. 2016, 138, 6336–6339. [Google Scholar] [CrossRef] [PubMed]
- Caliendo, G.; Cirino, G.; Santagada, V.; Wallace, J.L. Synthesis and biological effects of hydrogen sulfide (H2S): Development of H2S-releasing drugs as pharmaceuticals. J. Med. Chem. 2010, 53, 6275–6286. [Google Scholar] [CrossRef]
- Wallace, J.L.; Caliendo, G.; Santagada, V.; Cirino, G.; Fiorucci, S. Gastrointestinal safety and anti-inflammatory effects of a hydrogen sulfide–releasing diclofenac derivative in the rat. Gastroenterology 2007, 132, 261–271. [Google Scholar] [CrossRef]
- Xu, S.; Yang, C.-T.; Meng, F.-H.; Pacheco, A.; Chen, L.; Xian, M. Ammonium tetrathiomolybdate as a water-soluble and slow-release hydrogen sulfide donor. Bioorg. Med. Chem. Lett. 2016, 26, 1585–1588. [Google Scholar] [CrossRef]
- Zhang, J.; Duan, D.; Song, Z.L.; Liu, T.; Hou, Y.; Fang, J. Small molecules regulating reactive oxygen species homeostasis for cancer therapy. Med. Res. Rev. 2021, 41, 342–394. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H.; Xian, M. Cysteine-activated hydrogen sulfide (H2S) donors. J. Am. Chem. Soc. 2011, 133, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Bhushan, S.; Yang, C.; Otsuka, H.; Stein, J.D.; Pacheco, A.; Peng, B.; Devarie-Baez, N.O.; Aguilar, H.C.; Lefer, D.J. Controllable hydrogen sulfide donors and their activity against myocardial ischemia-reperfusion injury. ACS Chem. Biol. 2013, 8, 1283–1290. [Google Scholar] [CrossRef]
- Kang, J.; Ferrell, A.J.; Chen, W.; Wang, D.; Xian, M. Cyclic acyl disulfides and acyl selenylsulfides as the precursors for persulfides (RSSH), selenylsulfides (RSeSH), and hydrogen sulfide (H2S). Org. Lett. 2018, 20, 852–855. [Google Scholar] [CrossRef]
- Zhao, Y.; Kang, J.; Park, C.-M.; Bagdon, P.E.; Peng, B.; Xian, M. Thiol-activated gem-dithiols: A new class of controllable hydrogen sulfide donors. Org. Lett. 2014, 16, 4536–4539. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Luo, S.; Liu, J.; Xie, S.; Liu, Y.; Xu, J.; Zhu, Z.; Xu, S. Controllable thioester-based hydrogen sulfide slow-releasing donors as cardioprotective agents. Chem. Commun. 2019, 55, 6193–6196. [Google Scholar] [CrossRef] [PubMed]
- Barresi, E.; Nesi, G.; Citi, V.; Piragine, E.; Piano, I.; Taliani, S.; Da Settimo, F.; Rapposelli, S.; Testai, L.; Breschi, M.C. Iminothioethers as hydrogen sulfide donors: From the gasotransmitter release to the vascular effects. J. Med. Chem. 2017, 60, 7512–7523. [Google Scholar] [CrossRef] [PubMed]
- Severino, B.; Corvino, A.; Fiorino, F.; Luciano, P.; Frecentese, F.; Magli, E.; Saccone, I.; Di Vaio, P.; Citi, V.; Calderone, V. 1, 2, 4-Thiadiazolidin-3, 5-diones as novel hydrogen sulfide donors. Eur. J. Med. Chem. 2018, 143, 1677–1686. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Moralès, O.; Mordon, S.; Delhem, N.; Boleslawski, E. Could Photodynamic Therapy Be a Promising Therapeutic Modality in Hepatocellular Carcinoma Patients? A Critical Review of Experimental and Clinical Studies. Cancers 2021, 13, 5176. [Google Scholar] [CrossRef] [PubMed]
- Devarie-Baez, N.O.; Bagdon, P.E.; Peng, B.; Zhao, Y.; Park, C.-M.; Xian, M. Light-induced hydrogen sulfide release from “caged” gem-dithiols. Org. Lett. 2013, 15, 2786–2789. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Nair, M.; Chauhan, P.; Gupta, K.; Saini, D.K.; Chakrapani, H. Visible-light-triggered uncaging of carbonyl sulfide for hydrogen sulfide (H2S) release. Org. Lett. 2017, 19, 4822–4825. [Google Scholar] [CrossRef] [PubMed]
- Woods, J.J.; Cao, J.; Lippert, A.R.; Wilson, J.J. Characterization and biological activity of a hydrogen sulfide-releasing red light-activated ruthenium (ii) complex. J. Am. Chem. Soc. 2018, 140, 12383–12387. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.Y.; Moon, Y.K.; Kim, S.; Kim, S.; Park, G.; Kim, J.J.; You, Y. Visible light-driven photogeneration of hydrogen sulfide. Chem. Commun. 2017, 53, 11830–11833. [Google Scholar] [CrossRef]
- Venkatesh, Y.; Das, J.; Chaudhuri, A.; Karmakar, A.; Maiti, T.K.; Singh, N.P. Light triggered uncaging of hydrogen sulfide (H 2 S) with real-time monitoring. Chem. Commun. 2018, 54, 3106–3109. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, B.; Ji, K.; Pan, Z.; Chittavong, V.; Wang, B. Esterase-sensitive prodrugs with tunable release rates and direct generation of hydrogen sulfide. Angew. Chem. Int. Ed. 2016, 55, 4514–4518. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.; Khodade, V.S.; SharathChandra, M.; Chauhan, P.; Mishra, S.; Siddaramappa, S.; Pradeep, B.E.; Singh, A.; Chakrapani, H. “On demand” redox buffering by H2S contributes to antibiotic resistance revealed by a bacteria-specific H2S donor. Chem. Sci. 2017, 8, 4967–4972. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.; Bora, P.; Ravikumar, G.; Jos, S.; Chakrapani, H. Esterase activated carbonyl sulfide/hydrogen sulfide (H2S) donors. Org. Lett. 2017, 19, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Shyaka, C.; Xian, M.; Park, C.-M. Esterase-sensitive trithiane-based hydrogen sulfide donors. Org. Biomol. Chem. 2019, 17, 9999–10003. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Li, X.; Shen, T.-L.; Qian, W.-J.; Xian, M. A sweet H2S/H2O2 dual release system and specific protein S-persulfidation mediated by thioglucose/glucose oxidase. J. Am. Chem. Soc. 2021, 143, 13325–13332. [Google Scholar] [CrossRef] [PubMed]
- Parvez, S.; Long, M.J.; Poganik, J.R.; Aye, Y. Redox signaling by reactive electrophiles and oxidants. Chem. Rev. 2018, 118, 8798–8888. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Sies, H. Oxidative eustress: On constant alert for redox homeostasis. Redox Biol. 2021, 41, 101867. [Google Scholar] [CrossRef]
- Scammahorn, J.J.; Nguyen, I.T.; Bos, E.M.; Van Goor, H.; Joles, J.A. Fighting oxidative stress with sulfur: Hydrogen sulfide in the renal and cardiovascular systems. Antioxidants 2021, 10, 373. [Google Scholar] [CrossRef]
- Yang, X.; Wang, C.; Zhang, X.; Chen, S.; Chen, L.; Lu, S.; Lu, S.; Yan, X.; Xiong, K.; Liu, F. Redox regulation in hydrogen sulfide action: From neurotoxicity to neuroprotection. Neurochem. Int. 2019, 128, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Corsello, T.; Komaravelli, N.; Casola, A. Role of hydrogen sulfide in NRF2-and sirtuin-dependent maintenance of cellular redox balance. Antioxidants 2018, 7, 129. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Pluth, M.D. Hydrogen sulfide donors activated by reactive oxygen species. Angew. Chem. 2016, 128, 14858–14862. [Google Scholar] [CrossRef]
- Chauhan, P.; Jos, S.; Chakrapani, H. Reactive oxygen species-triggered tunable hydrogen sulfide release. Org. Lett. 2018, 20, 3766–3770. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Han, Y.; Li, L.; Lu, H.; Meng, G.; Li, X.; Shirhan, M.; Peh, M.T.; Xie, L.; Zhou, S. The hydrogen sulfide donor, GYY 4137, exhibits anti-atherosclerotic activity in high fat fed apolipoprotein E−/− mice. Br. J. Pharmacol. 2013, 169, 1795–1809. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.; Li, H.; Untereiner, A.; Wu, L.; Yang, G.; Austin, R.C.; Dickhout, J.G.; Lhoták, Š.; Meng, Q.H.; Wang, R. Decreased endogenous production of hydrogen sulfide accelerates atherosclerosis. Circulation 2013, 127, 2523–2534. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, X.; Jin, H.; Wei, H.; Li, W.; Bu, D.; Tang, X.; Ren, Y.; Tang, C.; Du, J. Role of hydrogen sulfide in the development of atherosclerotic lesions in apolipoprotein E knockout mice. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Guo, C.; Wu, D.; Zhang, A.; Gu, T.; Wang, L.; Wang, C. Hydrogen sulfide inhibits the development of atherosclerosis with suppressing CX3CR1 and CX3CL1 expression. PLoS ONE 2012, 7, e41147. [Google Scholar] [CrossRef] [PubMed]
- Fransen, M.; Nordgren, M.; Wang, B.; Apanasets, O. Role of peroxisomes in ROS/RNS-metabolism: Implications for human disease. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2012, 1822, 1363–1373. [Google Scholar] [CrossRef]
- Kneeshaw, S.; Keyani, R.; Delorme-Hinoux, V.; Imrie, L.; Loake, G.J.; Le Bihan, T.; Reichheld, J.-P.; Spoel, S.H. Nucleoredoxin guards against oxidative stress by protecting antioxidant enzymes. Proc. Natl. Acad. Sci. USA 2017, 114, 8414–8419. [Google Scholar] [CrossRef]
- Lv, H.; Liu, Q.; Wen, Z.; Feng, H.; Deng, X.; Ci, X. Xanthohumol ameliorates lipopolysaccharide (LPS)-induced acute lung injury via induction of AMPK/GSK3β-Nrf2 signal axis. Redox Biol. 2017, 12, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Vandervliet, A.; Eiserich, J.P.; Oneill, C.A.; Halliwell, B.; Cross, C.E. Tyrosine modification by reactive nitrogen species: A closer look. Arch. Biochem. Biophys. 1995, 319, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Whiteman, M.; Armstrong, J.S.; Chu, S.H.; Jia-Ling, S.; Wong, B.S.; Cheung, N.S.; Halliwell, B.; Moore, P.K. The novel neuromodulator hydrogen sulfide: An endogenous peroxynitrite ‘scavenger’? J. Neurochem. 2004, 90, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Goto, Y.-I.; Kimura, H. Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxid. Redox Signal. 2010, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yang, G.; Jia, X.; Wu, L.; Wang, R. Activation of KATP channels by H2S in rat insulin-secreting cells and the underlying mechanisms. J. Physiol. 2005, 569, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.D.; Augustine, L.M.; Maher, J.M.; Nelson, D.M.; Slitt, A.L.; Klaassen, C.D.; Lehman-McKeeman, L.D.; Cherrington, N.J. Induction of drug-metabolizing enzymes by garlic and allyl sulfide compounds via activation of constitutive androstane receptor and nuclear factor E2-related factor 2. Drug Metab. Dispos. 2007, 35, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Seki, S.; Hiramoto, K.; Naganuma, E.; Kobayashi, E.H.; Yamaoka, A.; Baird, L.; Takahashi, N.; Sato, H.; Yamamoto, M. Hyperactivation of Nrf2 in early tubular development induces nephrogenic diabetes insipidus. Nat. Commun. 2017, 8, 14577. [Google Scholar] [CrossRef] [PubMed]
- Tanito, M.; Agbaga, M.-P.; Anderson, R.E. Upregulation of thioredoxin system via Nrf2-antioxidant responsive element pathway in adaptive-retinal neuroprotection in vivo and in vitro. Free Radic. Biol. Med. 2007, 42, 1838–1850. [Google Scholar] [CrossRef]
- Zhu, H.; Itoh, K.; Yamamoto, M.; Zweier, J.L.; Li, Y. Role of Nrf2 signaling in regulation of antioxidants and phase 2 enzymes in cardiac fibroblasts: Protection against reactive oxygen and nitrogen species-induced cell injury. FEBS Lett. 2005, 579, 3029–3036. [Google Scholar] [CrossRef]
- Calvert, J.W.; Jha, S.; Gundewar, S.; Elrod, J.W.; Ramachandran, A.; Pattillo, C.B.; Kevil, C.G.; Lefer, D.J. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ. Res. 2009, 105, 365–374. [Google Scholar] [CrossRef]
- Xu, Z.; Xu, L.; Li, W.; Jin, X.; Song, X.; Chen, X.; Zhu, J.; Zhou, S.; Li, Y.; Zhang, W. Innate scavenger receptor-A regulates adaptive T helper cell responses to pathogen infection. Nat. Commun. 2017, 8, 16035. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, Y.; Tsutsui, H.; Kawada, N.; Suzuki, H.; Kataoka, M.; Kodama, T.; Yano, I.; Kaneda, K.; Kobayashi, K. Macrophage scavenger receptor down-regulates mycobacterial cord factor-induced proinflammatory cytokine production by alveolar and hepatic macrophages. Microb. Pathog. 2006, 40, 171–176. [Google Scholar] [CrossRef]
- Beamer, C.A.; Holian, A. Scavenger receptor class A type I/II (CD204) null mice fail to develop fibrosis following silica exposure. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2005, 289, L186–L195. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Sakashita, N.; Okuma, T.; Terasaki, Y.; Tsujita, K.; Suzuki, H.; Kodama, T.; Nomori, H.; Kawasuji, M.; Takeya, M. Class A scavenger receptor (CD204) attenuates hyperoxia-induced lung injury by reducing oxidative stress. J. Pathol. A J. Pathol. Soc. Great Br. Irel. 2007, 212, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Kimura, H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J. 2004, 18, 1165–1167. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Dargusch, R.; Schubert, D.; Kimura, H. Hydrogen sulfide protects HT22 neuronal cells from oxidative stress. Antioxid. Redox Signal. 2006, 8, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Pattison, D.J.; Winyard, P.G. Dietary antioxidants in inflammatory arthritis: Do they have any role in etiology or therapy? Nat. Clin. Pract. Rheumatol. 2008, 4, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Laggner, H.; Muellner, M.K.; Schreier, S.; Sturm, B.; Hermann, M.; Exner, M.; Laggner, H.; Muellner, M.K.; Schreier, S.; Sturm, B. Hydrogen sulphide: A novel physiological inhibitor of LDL atherogenic modification by HOCl. Free Radic. Res. 2007, 41, 741–747. [Google Scholar] [CrossRef]
- Muellner, M.K.; Schreier, S.M.; Laggner, H.; Hermann, M.; Esterbauer, H.; Exner, M.; Gmeiner, B.M.; Kapiotis, S. Hydrogen sulfide destroys lipid hydroperoxides in oxidized LDL. Biochem. J. 2009, 420, 277–281. [Google Scholar] [CrossRef]
- Schreier, S.M.; Muellner, M.K.; Steinkellner, H.; Hermann, M.; Esterbauer, H.; Exner, M.; Gmeiner, B.M.; Kapiotis, S.; Laggner, H. Hydrogen sulfide scavenges the cytotoxic lipid oxidation product 4-HNE. Neurotox. Res. 2010, 17, 249–256. [Google Scholar] [CrossRef]
- Tyagi, N.; Moshal, K.S.; Sen, U.; Vacek, T.P.; Kumar, M.; Hughes Jr, W.M.; Kundu, S.; Tyagi, S.C. H2S protects against methionine–induced oxidative stress in brain endothelial cells. Antioxid. Redox Signal. 2009, 11, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Muzaffar, S.; Shukla, N.; Bond, M.; Newby, A.C.; Angelini, G.D.; Sparatore, A.; Del Soldato, P.; Jeremy, J.Y. Exogenous hydrogen sulfide inhibits superoxide formation, NOX-1 expression and Rac1 activity in human vascular smooth muscle cells. J. Vasc. Res. 2008, 45, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Rossoni, G.; Sparatore, A.; Lee, L.C.; Del Soldato, P.; Moore, P.K. Anti-inflammatory and gastrointestinal effects of a novel diclofenac derivative. Free Radic. Biol. Med. 2007, 42, 706–719. [Google Scholar] [CrossRef]
- Fiorucci, S.; Antonelli, E.; Distrutti, E.; Rizzo, G.; Mencarelli, A.; Orlandi, S.; Zanardo, R.; Renga, B.; Di Sante, M.; Morelli, A. Inhibition of hydrogen sulfide generation contributes to gastric injury caused by anti-inflammatory nonsteroidal drugs. Gastroenterology 2005, 129, 1210–1224. [Google Scholar] [CrossRef]
- Zanardo, R.C.; Brancaleone, V.; Distrutti, E.; Fiorucci, S.; Cirino, G.; Wallace, J.L.; Zanardo, R.C.; Brancaleone, V.; Distrutti, E.; Fiorucci, S. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 2006, 20, 2118–2120. [Google Scholar] [CrossRef]
- Venardos, K.M.; Kaye, D.M. Myocardial ischemia-reperfusion injury, antioxidant enzyme systems, and selenium: A review. Curr. Med. Chem. 2007, 14, 1539–1549. [Google Scholar] [CrossRef]
- Kang, K.W.; Lee, S.J.; Kim, S.G. Molecular mechanism of nrf2 activation by oxidative stress. Antioxid. Redox Signal. 2005, 7, 1664–1673. [Google Scholar] [CrossRef]
- Pan, L.-L.; Liu, X.-H.; Gong, Q.-H.; Wu, D.; Zhu, Y.-Z. Hydrogen sulfide attenuated tumor necrosis factor-α-induced inflammatory signaling and dysfunction in vascular endothelial cells. PLoS ONE 2011, 6, e19766. [Google Scholar] [CrossRef]
- Pan, T.-T.; Feng, Z.-N.; Lee, S.W.; Moore, P.K.; Bian, J.-S. Endogenous hydrogen sulfide contributes to the cardioprotection by metabolic inhibition preconditioning in the rat ventricular myocytes. J. Mol. Cell. Cardiol. 2006, 40, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.-L.; Huang, X.-W.; Wang, Y.-G.; Cao, Y.-X.; Zhang, C.-C.; Zhu, Y.-C. Hydrogen sulfide protects cardiomyocytes from hypoxia/reoxygenation-induced apoptosis by preventing GSK-3β-dependent opening of mPTP. Am. J. Physiol.-Heart Circ. Physiol. 2010, 298, H1310–H1319. [Google Scholar] [CrossRef]
- Hu, L.-F.; Pan, T.-T.; Neo, K.L.; Yong, Q.C.; Bian, J.-S. Cyclooxygenase-2 mediates the delayed cardioprotection induced by hydrogen sulfide preconditioning in isolated rat cardiomyocytes. Pflügers Arch.-Eur. J. Physiol. 2008, 455, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.; Cao, W.; Wu, L.; Wang, R. Hydrogen sulfide and the liver. Nitric Oxide 2014, 41, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Jeong, W.I.; Tian, Z. Liver: An organ with predominant innate immunity. Hepatology 2008, 47, 729–736. [Google Scholar] [CrossRef]
- Shi, D.; Chen, J.; Wang, J.; Yao, J.; Huang, Y.; Zhang, G.; Bao, Z. Circadian clock genes in the metabolism of non-alcoholic fatty liver disease. Front. Physiol. 2019, 10, 423. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhong, J.; Hu, L.; Li, R.; Du, Q.; Cai, J.; Li, Y.; Gao, Y.; Cui, X.; Yang, X. The role of xenobiotic receptors on hepatic glycolipid metabolism. Curr. Drug Metab. 2019, 20, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Zhao, M.; Jiang, H.; Tan, G.; Pan, S.; Sun, X. Role of hydrogen sulfide in hepatic ischemia-reperfusion–induced injury in rats. Liver Transplant. 2009, 15, 1306–1314. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xu, C.; Shi, J.; Ding, J.; Wan, X.; Chen, D.; Gao, J.; Li, C.; Zhang, J.; Lin, Y. Fatty acids promote fatty liver disease via the dysregulation of 3-mercaptopyruvate sulfurtransferase/hydrogen sulfide pathway. Gut 2018, 67, 2169–2180. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Li, Q.; Yin, X.-Y.; Lu, Y.; Liu, C.-F.; Hu, L.-F. Hydrogen sulfide: A therapeutic candidate for fibrotic disease? Oxidative Med. Cell. Longev. 2015, 2015, 458720. [Google Scholar] [CrossRef]
- Yin, P.; Zhao, C.; Li, Z.; Mei, C.; Yao, W.; Liu, Y.; Li, N.; Qi, J.; Wang, L.; Shi, Y. Sp1 is involved in regulation of cystathionine γ-lyase gene expression and biological function by PI3K/Akt pathway in human hepatocellular carcinoma cell lines. Cell. Signal. 2012, 24, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Andrade, R.J.; Chalasani, N.; Björnsson, E.S.; Suzuki, A.; Kullak-Ublick, G.A.; Watkins, P.B.; Devarbhavi, H.; Merz, M.; Lucena, M.I.; Kaplowitz, N. Drug-induced liver injury. Nat. Rev. Dis. Primers 2019, 5, 58. [Google Scholar] [CrossRef] [PubMed]
- Norris, E.J.; Culberson, C.R.; Narasimhan, S.; Clemens, M.G. The liver as a central regulator of hydrogen sulfide. Shock 2011, 36, 242. [Google Scholar] [CrossRef] [PubMed]
- Szabo, C.; Hellmich, M.R. Endogenously produced hydrogen sulfide supports tumor cell growth and proliferation. Cell Cycle 2013, 12, 2915–2916. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Gai, J.-W.; Wang, Y.; Jin, H.-F.; Du, J.-B.; Jin, J. Characterization of hydrogen sulfide and its synthases, cystathionine β-synthase and cystathionine γ-lyase, in human prostatic tissue and cells. Urology 2012, 79, 483.e1–483.e5. [Google Scholar] [CrossRef] [PubMed]
- Jurkowska, H.; Placha, W.; Nagahara, N.; Wróbel, M. The expression and activity of cystathionine-γ-lyase and 3-mercaptopyruvate sulfurtransferase in human neoplastic cell lines. Amino Acids 2011, 41, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.; Zatarain, J.R.; Ding, Y.; Coletta, C.; Mrazek, A.A.; Druzhyna, N.; Johnson, P.; Chen, H.; Hellmich, J.L.; Asimakopoulou, A. Cystathionine-β-synthase inhibition for colon cancer: Enhancement of the efficacy of aminooxyacetic acid via the prodrug approach. Mol. Med. 2016, 22, 361–379. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Saha, S.; Giri, K.; Lanza, I.R.; Nair, K.S.; Jennings, N.B.; Rodriguez-Aguayo, C.; Lopez-Berestein, G.; Basal, E.; Weaver, A.L. Cystathionine beta-synthase (CBS) contributes to advanced ovarian cancer progression and drug resistance. PLoS ONE 2013, 8, e79167. [Google Scholar] [CrossRef] [PubMed]
- Lee, Z.W.; Zhou, J.; Chen, C.-S.; Zhao, Y.; Tan, C.-H.; Li, L.; Moore, P.K.; Deng, L.-W. The slow-releasing hydrogen sulfide donor, GYY4137, exhibits novel anti-cancer effects in vitro and in vivo. PLoS ONE 2011, 6, e21077. [Google Scholar] [CrossRef]
- Wu, D.; Li, M.; Tian, W.; Wang, S.; Cui, L.; Li, H.; Wang, H.; Ji, A.; Li, Y. Hydrogen sulfide acts as a double-edged sword in human hepatocellular carcinoma cells through EGFR/ERK/MMP-2 and PTEN/AKT signaling pathways. Sci. Rep. 2017, 7, 5134. [Google Scholar] [CrossRef]
- Wu, D.; Si, W.; Wang, M.; Lv, S.; Ji, A.; Li, Y. Hydrogen sulfide in cancer: Friend or foe? Nitric Oxide 2015, 50, 38–45. [Google Scholar] [CrossRef]
- Wang, X.; An, L.; Tian, Q.; Cui, K. Recent progress in H 2 S activated diagnosis and treatment agents. RSC Adv. 2019, 9, 33578–33588. [Google Scholar] [CrossRef]
- Lin, V.S.; Chen, W.; Xian, M.; Chang, C.J. Chemical probes for molecular imaging and detection of hydrogen sulfide and reactive sulfur species in biological systems. Chem. Soc. Rev. 2015, 44, 4596–4618. [Google Scholar] [CrossRef] [PubMed]
- Agard, N.J.; Baskin, J.M.; Prescher, J.A.; Lo, A.; Bertozzi, C.R. A comparative study of bioorthogonal reactions with azides. ACS Chem. Biol. 2006, 1, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Li, X.; Zeng, F.; Zheng, F.; Wu, S. Carbon-dot-based ratiometric fluorescent sensor for detecting hydrogen sulfide in aqueous media and inside live cells. Chem. Commun. 2013, 49, 403–405. [Google Scholar] [CrossRef]
- Chen, B.; Li, W.; Lv, C.; Zhao, M.; Jin, H.; Jin, H.; Du, J.; Zhang, L.; Tang, X. Fluorescent probe for highly selective and sensitive detection of hydrogen sulfide in living cells and cardiac tissues. Analyst 2013, 138, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-L.; Zhu, H.-K.; Zhao, C.-C.; Gu, X.-F. A near-infrared fluorescent probe for monitoring fluvastatin-stimulated endogenous H2S production. Chin. Chem. Lett. 2017, 28, 218–221. [Google Scholar] [CrossRef]
- Wang, L.; Chen, X.; Cao, D. A nitroolefin functionalized DPP fluorescent probe for the selective detection of hydrogen sulfide. New J. Chem. 2017, 41, 3367–3373. [Google Scholar] [CrossRef]
- Wang, R.; Dong, K.; Xu, G.; Shi, B.; Zhu, T.; Shi, P.; Guo, Z.; Zhu, W.-H.; Zhao, C. Activatable near-infrared emission-guided on-demand administration of photodynamic anticancer therapy with a theranostic nanoprobe. Chem. Sci. 2019, 10, 2785–2790. [Google Scholar] [CrossRef]
- He, L.; Yang, X.; Xu, K.; Kong, X.; Lin, W. A multi-signal fluorescent probe for simultaneously distinguishing and sequentially sensing cysteine/homocysteine, glutathione, and hydrogen sulfide in living cells. Chem. Sci. 2017, 8, 6257–6265. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Lin, W.; Tan, L.; Cheng, D. A two-photon fluorescent probe with a large turn-on signal for imaging hydrogen sulfide in living tissues. Anal. Chim. Acta 2015, 853, 548–554. [Google Scholar] [CrossRef]
- Pak, Y.L.; Li, J.; Ko, K.C.; Kim, G.; Lee, J.Y.; Yoon, J. Mitochondria-targeted reaction-based fluorescent probe for hydrogen sulfide. Anal. Chem. 2016, 88, 5476–5481. [Google Scholar] [CrossRef]
- Ismail, I.; Wang, D.; Wang, Z.; Wang, D.; Zhang, C.; Yi, L.; Xi, Z. A julolidine-fused coumarin-NBD dyad for highly selective and sensitive detection of H2S in biological samples. Dye. Pigment. 2019, 163, 700–706. [Google Scholar] [CrossRef]
- Ismail, I.; Chen, Z.; Sun, L.; Ji, X.; Ye, H.; Kang, X.; Huang, H.; Song, H.; Bolton, S.G.; Xi, Z. Highly efficient H 2 S scavengers via thiolysis of positively-charged NBD amines. Chem. Sci. 2020, 11, 7823–7828. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, C.; Qu, X.; Cheng, S.; Xian, Y. Cationic cyanine chromophore-assembled upconversion nanoparticles for sensing and imaging H2S in living cells and zebrafish. Biosens. Bioelectron. 2019, 126, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Hu, X.; Yang, B.; Liu, B. Dual sites fluorescence probe for hydrogen sulfide: AIEE activity and supramolecular assembly with β-cyclodextrin. Sens. Actuators B Chem. 2019, 282, 743–749. [Google Scholar] [CrossRef]
- Ryu, H.G.; Singha, S.; Jun, Y.W.; Reo, Y.J.; Ahn, K.H. Two-photon fluorescent probe for hydrogen sulfide based on a red-emitting benzocoumarin dye. Tetrahedron Lett. 2018, 59, 49–53. [Google Scholar] [CrossRef]
- Hu, Y.; Kang, J.; Zhou, P.; Han, X.; Sun, J.; Liu, S.; Zhang, L.; Fang, J. A selective colorimetric and red-emitting fluorometric probe for sequential detection of Cu2+ and H2S. Sens. Actuators B Chem. 2018, 255, 3155–3162. [Google Scholar] [CrossRef]
- Steiger, A.K.; Pardue, S.; Kevil, C.G.; Pluth, M. Self-Immolative Thiocarbamates Provide Access to Triggered H2S Donors and Analyte Replacement Fluorescent Probes. J. Am. Chem. Soc. 2016, 138, 7256–7259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Mu, S.; Wang, Y.; Li, S.; Shi, X.; Liu, X.; Zhang, H. A water-soluble near-infrared fluorescent probe for monitoring change of hydrogen sulfide during cell damage and repair process. Anal. Chim. Acta 2022, 1195, 339457. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Mu, S.; Wang, W.; Sun, H.; Li, S.; Shi, X.; Liu, Y.; Liu, X.; Zhang, H. Design strategy for an analyte-compensated fluorescent probe to reduce its toxicity. Chem. Commun. 2022, 58, 9136–9139. [Google Scholar] [CrossRef]
- Zhang, J.; Han, T.; Sun, H.; Han, Z.; Shi, X.; Gao, J.; Liu, X.; Zhang, H. A self-immolative near-infrared fluorescent probe for identification of cancer cells and facilitating its apoptosis. Anal. Bioanal. Chem. 2024, 416, 1529–1540. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, Y.; Huo, F.; Chao, J.; Cheng, F.; Yin, C. A new strategy: Distinguishable multi-substance detection, multiple pathway tracing based on a new site constructed by the reaction process and its tumor targeting. J. Am. Chem. Soc. 2020, 142, 18706–18714. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, W.; Tang, J.; Li, P.; Weng, H.; Ye, Y.; Xian, M.; Tang, B.; Zhao, Y. A multi-signal mitochondria-targeted fluorescent probe for real-time visualization of cysteine metabolism in living cells and animals. Chem. Commun. 2018, 54, 11387–11390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, H.; Zhong, M.; Wang, S.; Xu, Q.; Cho, D.-H.; Qiu, H. A novel off-on fluorescent probe for specific detection and imaging of cysteine in live cells and in vivo. Chin. Chem. Lett. 2020, 31, 133–135. [Google Scholar] [CrossRef]
- Yang, C.T.; Wang, Y.; Marutani, E.; Ida, T.; Ni, X.; Xu, S.; Chen, W.; Zhang, H.; Akaike, T.; Ichinose, F. Data-driven identification of hydrogen sulfide scavengers. Angew. Chem. Int. Ed. 2019, 58, 10898–10902. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Jing, Q.; Gao, F.; Zhang, F.; Pei, D.; Di, D.; Hai, J. Review of Hydrogen Sulfide Based on Its Activity Mechanism and Fluorescence Sensing. Targets 2024, 2, 202-223. https://doi.org/10.3390/targets2030012
Zhang J, Jing Q, Gao F, Zhang F, Pei D, Di D, Hai J. Review of Hydrogen Sulfide Based on Its Activity Mechanism and Fluorescence Sensing. Targets. 2024; 2(3):202-223. https://doi.org/10.3390/targets2030012
Chicago/Turabian StyleZhang, Jinlong, Quan Jing, Fei Gao, Fuxin Zhang, Dong Pei, Duolong Di, and Jun Hai. 2024. "Review of Hydrogen Sulfide Based on Its Activity Mechanism and Fluorescence Sensing" Targets 2, no. 3: 202-223. https://doi.org/10.3390/targets2030012
APA StyleZhang, J., Jing, Q., Gao, F., Zhang, F., Pei, D., Di, D., & Hai, J. (2024). Review of Hydrogen Sulfide Based on Its Activity Mechanism and Fluorescence Sensing. Targets, 2(3), 202-223. https://doi.org/10.3390/targets2030012






