Recent Development of Organic Afterglow Probes for Diagnosis and Treatment of Cancer
Abstract
:1. Introduction
2. Photo-Afterglow Probes
3. Sono-Afterglow Probes
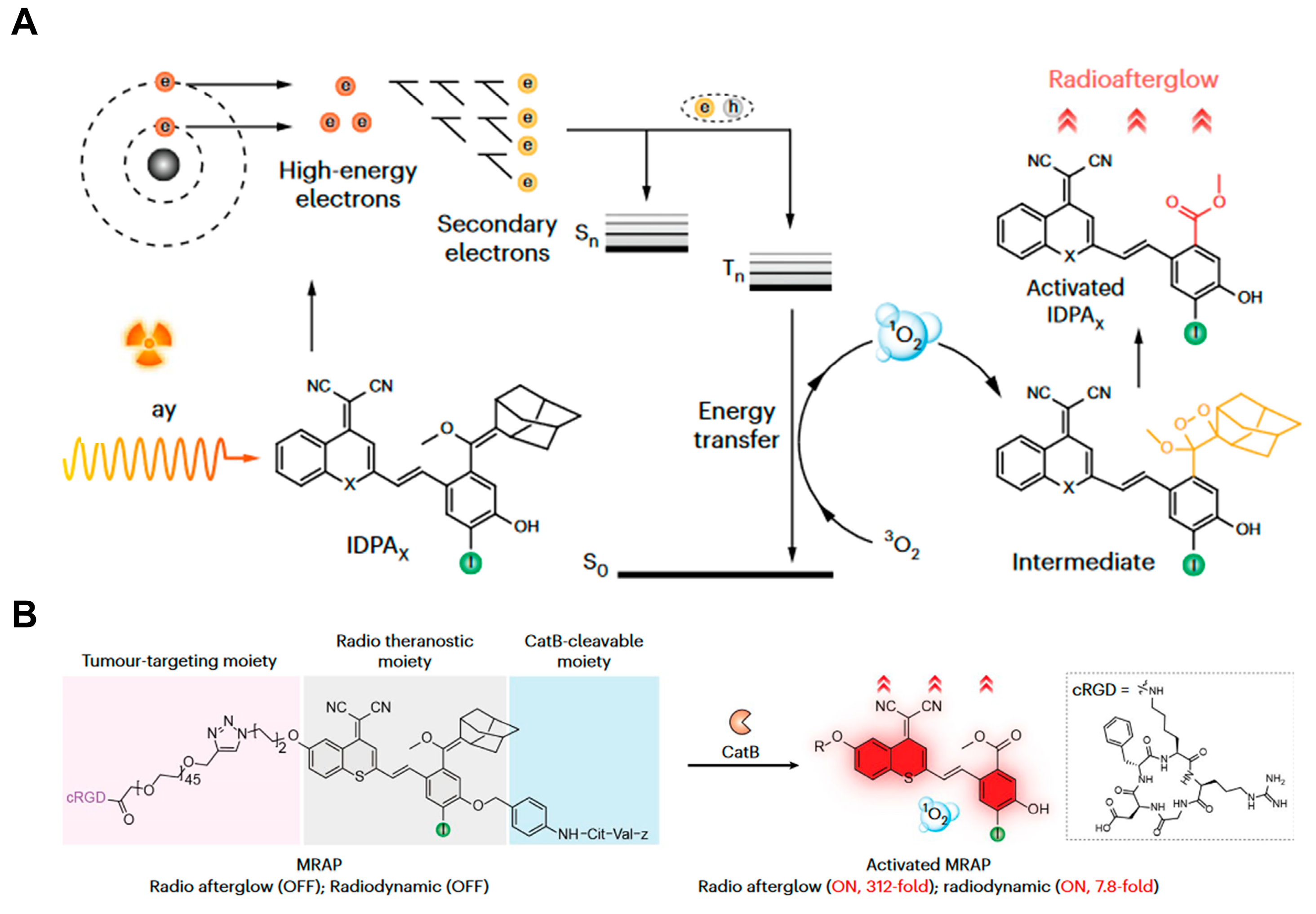
4. Radio-Afterglow Probes
5. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Winkler, R.; Ciria, M.; Ahmad, M.; Plank, H.; Marcuello, C. A Review of the Current State of Magnetic Force Microscopy to Unravel the Magnetic Properties of Nanomaterials Applied in Biological Systems and Future Directions for Quantum Technologies. Nanomaterials 2023, 13, 2585. [Google Scholar] [CrossRef] [PubMed]
- Luthra, R.; Chen, H.; Roy-Chowdhuri, S.; Singh, R.R. Next-Generation Sequencing in Clinical Molecular Diagnostics of Cancer: Advantages and Challenges. Cancers 2015, 7, 2023–2036. [Google Scholar] [CrossRef] [PubMed]
- Razgulin, A.; Ma, N.; Rao, J. Strategies for in vivo imaging of enzyme activity: An overview and recent advances. Chem. Soc. Rev. 2011, 40, 4186–4216. [Google Scholar] [CrossRef]
- Zhang, J.; Chai, X.; He, X.P.; Kim, H.J.; Yoon, J.; Tian, H. Fluorogenic Probes for Disease-Relevant Enzymes. Chem. Soc. Rev. 2019, 48, 683–722. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, Y.; Zhou, C.; Mei, W.; Zeng, C. PI3K/Akt/mTOR Pathway and Its Role in Cancer Therapeutics: Are We Making Headway? Front. Oncol. 2022, 12, 819128. [Google Scholar] [CrossRef]
- Huang, J.; Li, J.; Lyu, Y.; Miao, Q.; Pu, K. Molecular Optical Imaging Probes for Early Diagnosis of Drug-Induced Acute Kidney Injury. Nat. Mater. 2019, 18, 1133–1143. [Google Scholar] [CrossRef]
- Wang, S.; Fan, Y.; Li, D.; Sun, C.; Lei, Z.; Lu, L.; Wang, T.; Zhang, F. Anti-Quenching NIR-II Molecular Fluorophores for in Vivo High-Contrast Imaging and pH Sensing. Nat. Commun. 2019, 10, 1058–1068. [Google Scholar] [CrossRef]
- Moore, C.; Chen, F.; Wang, J.; Jokerst, J.V. Listening for the Therapeutic Window: Advances in Drug Delivery Utilizing Photoacoustic Imaging. Adv. Drug Deliv. Rev. 2019, 144, 78–89. [Google Scholar] [CrossRef]
- Huang, Z.; Yu, P.; Tang, J. Characterization of Triple-Negative Breast Cancer MDA-MB-231 Cell Spheroid Model. OncoTargets Ther. 2020, 13, 5395–5405. [Google Scholar] [CrossRef]
- Shuhendler, A.J.; Pu, K.; Cui, L.; Uetrecht, J.P.; Rao, J. Real-time imaging of oxidative and nitrosative stress in the liver of live animals for drug-toxicity testing. Nat. Biotechnol. 2024, 32, 373–380. [Google Scholar] [CrossRef]
- Badr, C.E.; Tannous, B.A. Bioluminescence imaging: Progress and applications. Trends Biotechnol. 2011, 29, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Nagai, T. Recent progress in expanding the chemiluminescent toolbox for bioimaging. Curr. Opin. Biotechnol. 2017, 48, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Pu, K. Multimodal Biophotonics of Semiconducting Polymer Nanoparticles. Acc. Chem. Res. 2018, 51, 1840–1849. [Google Scholar] [CrossRef]
- Sun, S.K.; Wang, H.F.; Yan, X.P. Engineering Persistent Luminescence Nanoparticles for Biological Applications: From Biosensing/Bioimaging to Theranostics. Acc. Chem. Res. 2018, 51, 1131–1143. [Google Scholar] [CrossRef]
- Xu, Y.; Li, C.; An, J.; Ma, X.; Yang, J.; Luo, L.; Deng, Y.; Kim, J.S.; Sun, Y. Construction of a 980 nm laser-activated Pt(II) metallacycle nanosystem for efficient and safe photo-induced bacteria sterilization. Sci. China Chem. 2023, 66, 155–163. [Google Scholar] [CrossRef]
- Duan, Q.; Zhao, Z.; Zhang, Y.; Fu, L.; Yuan, Y.; Du, J.; Wang, J. Activatable fluorescent probes for real-time imaging-guided tumor therapy. Adv. Drug Deliv. Rev. 2023, 196, 114793. [Google Scholar] [CrossRef]
- Zeng, W.; Wu, L.; Ishigaki, Y.; Harimoto, T.; Hu, Y.; Sun, Y.; Wang, Y.; Suzuki, T.; Chen, H.; Ye, D. An Activatable Afterglow/MRI Bimodal Nanoprobe with Fast Response to H2S for In Vivo Imaging of Acute Hepatitis. Angew. Chem. Int. Ed. 2022, 134, e202111759. [Google Scholar] [CrossRef]
- Yang, J.; Yin, W.; Van, R.; Yin, K.; Wang, P.; Zheng, C.; Zhu, B.; Ran, K.; Zhang, C.; Kumar, M.; et al. Turn-on Chemiluminescence Probes and Dual-Amplification of Signal for Detection of Amyloid Beta Species in Vivo. Nat. Commun. 2020, 11, 4052. [Google Scholar] [CrossRef]
- Zhen, X.; Xie, C.; Pu, K. Temperature-Correlated Afterglow of a Semiconducting Polymer Nanococktail for Imaging-Guided Photothermal Therapy. Angew. Chem. Int. Ed. 2018, 57, 3938–3942. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, T.; Tuo, W.; Liu, Y.; Umar, T.; Chen, Y.; Wu, Z.; Zhou, Q.; Li, X.; Deng, G.; et al. Rising interest in the accurate and controllable anticancer strategy: Based on photon-evoked pyroptosis engineering perspective. Coord. Chem. Rev. 2024, 501, 215588. [Google Scholar] [CrossRef]
- Xu, Y.; Pang, Y.; Luo, L.; Sharma, A.; Yang, J.; Li, C.; Liu, S.; Zhan, J.; Sun, Y. De Novo Designed Ru(II) Metallacycle as a Microenvironment-Adaptive Sonosensitizer and Sonocatalyst for Multidrug-Resistant Biofilms Eradication. Angew. Chem. Int. Ed. 2024, 63, E202319966. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ye, H.; Cai, F.; Sun, Y. Recent advances on the construction of long-wavelength emissive supramolecular coordination complexes for photo-diagnosis and therapy. Dalton Trans. 2023, 52, 15193–15202. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Phua, S.Z.F.; Li, Y.; Zhou, X.; Jana, D.; Liu, G.; Lim, W.Q.; Ong, W.K.; Yang, C.; Zhao, Y. Ultralong Room Temperature Phosphorescence from Amorphous Organic Materials toward Confidential Information Encryption and Decryption. Sci. Adv. 2018, 4, eaas9732. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Zhen, X.; Miao, Q.; Lyu, Y.; Pu, K. Self-Assembled Semiconducting Polymer Nanoparticles for Ultrasensitive Near-Infrared Afterglow Imaging of Metastatic Tumors. Adv. Mater. 2018, 30, 1801331. [Google Scholar] [CrossRef]
- Li, C.; Tu, L.; Xu, Y.; Li, M.; Du, J.; Stang, P.J.; Sun, Y.; Sun, Y. A NIR-Light-Activated and Lysosomal-Targeted Pt(II) Metallacycle for Highly Potent Evoking of Immunogenic Cell Death that Potentiates Cancer Immunotherapy of Deep-Seated Tumors. Angew. Chem. Int. Ed. 2024, 63, e202406392. [Google Scholar] [CrossRef]
- Li, Y.; Gecevicius, M.; Qiu, J. Long Persistent Phosphors-from Fundamentals to Applications. Chem. Soc. Rev. 2016, 45, 2090–2136. [Google Scholar] [CrossRef]
- Chuang, Y.J.; Zhen, Z.; Zhang, F.; Liu, F.; Mishra, J.P.; Tang, W.; Chen, H.; Huang, X.; Wang, L.; Chen, X.; et al. Photostimulable Near-Infrared Persistent Luminescent Nanoprobes for Ultrasensitive and Longitudinal Deep-Tissue Bio-Imaging. Theranostics 2014, 4, 1112–1122. [Google Scholar] [CrossRef]
- Wu, S.; Yang, C.; Yan, X. A dual-functional persistently luminescent nanocomposite enables engineering of mesenchymal stem cells for homing and gene therapy of glioblastoma. Adv. Funct. Mater. 2017, 27, 1604992. [Google Scholar] [CrossRef]
- Zhou, H.; Zeng, X.; Li, A.; Zhou, W.; Tang, L.; Hu, W.; Fan, Q.; Meng, X.; Deng, H.; Duan, L.; et al. Upconversion NIR-II Fluorophores for Mitochondria-Targeted Cancer Imaging and Photothermal Therapy. Nat. Commun. 2020, 11, 6183. [Google Scholar] [CrossRef]
- Ren, J.; He, J.; Zhang, H.; Xia, Y.; Hu, Z.; Loughran, P.; Billiar, T.; Huang, H.; Tsung, A. Platelet TLR4-ERK5 Axis Facilitates NET-Mediated Capturing of Circulating Tumor Cells and Distant Metastasis after Surgical Stress. Cancer Res. 2021, 81, 2373–2385. [Google Scholar] [CrossRef]
- Ma, L.; Fei, B. Comprehensive review of surgical microscopes: Technology development and medical applications. J. Biomed. Opt. 2021, 26, 010901. [Google Scholar] [CrossRef] [PubMed]
- Bortot, B.; Mangogna, A.; Lorenzo, G.; Stabile, G.; Ricci, G.; Biffi, S. Image-guided cancer surgery: A narrative review on imaging modalities and emerging nanotechnology strategies. J. Nanobiotechnol. 2023, 21, 155. [Google Scholar] [CrossRef] [PubMed]
- Miao, Q.; Xie, C.; Zhen, X.; Lyu, Y.; Duan, H.; Liu, X.; Jokerst, J.V.; Pu, K. Molecular afterglow imaging with bright, biodegradable polymer nanoparticles. Nat. Biotechnol. 2017, 35, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Pei, P.; Chen, Y.; Sun, C.; Fan, Y.; Yang, Y.; Liu, X.; Lu, L.; Zhao, M.; Zhang, H.; Zhao, D.; et al. X-ray-activated persistent luminescence nanomaterials for NIR-II imaging. Nat. Nanotechnol. 2021, 16, 1011–1018. [Google Scholar] [CrossRef]
- Hananya, N.; Shabat, D. A Glowing Trajectory between Bio- and Chemiluminescence: From Luciferin-Based Probes to Triggerable Dioxetanes. Angew. Chem. Int. Ed. 2017, 56, 16454–16463. [Google Scholar] [CrossRef]
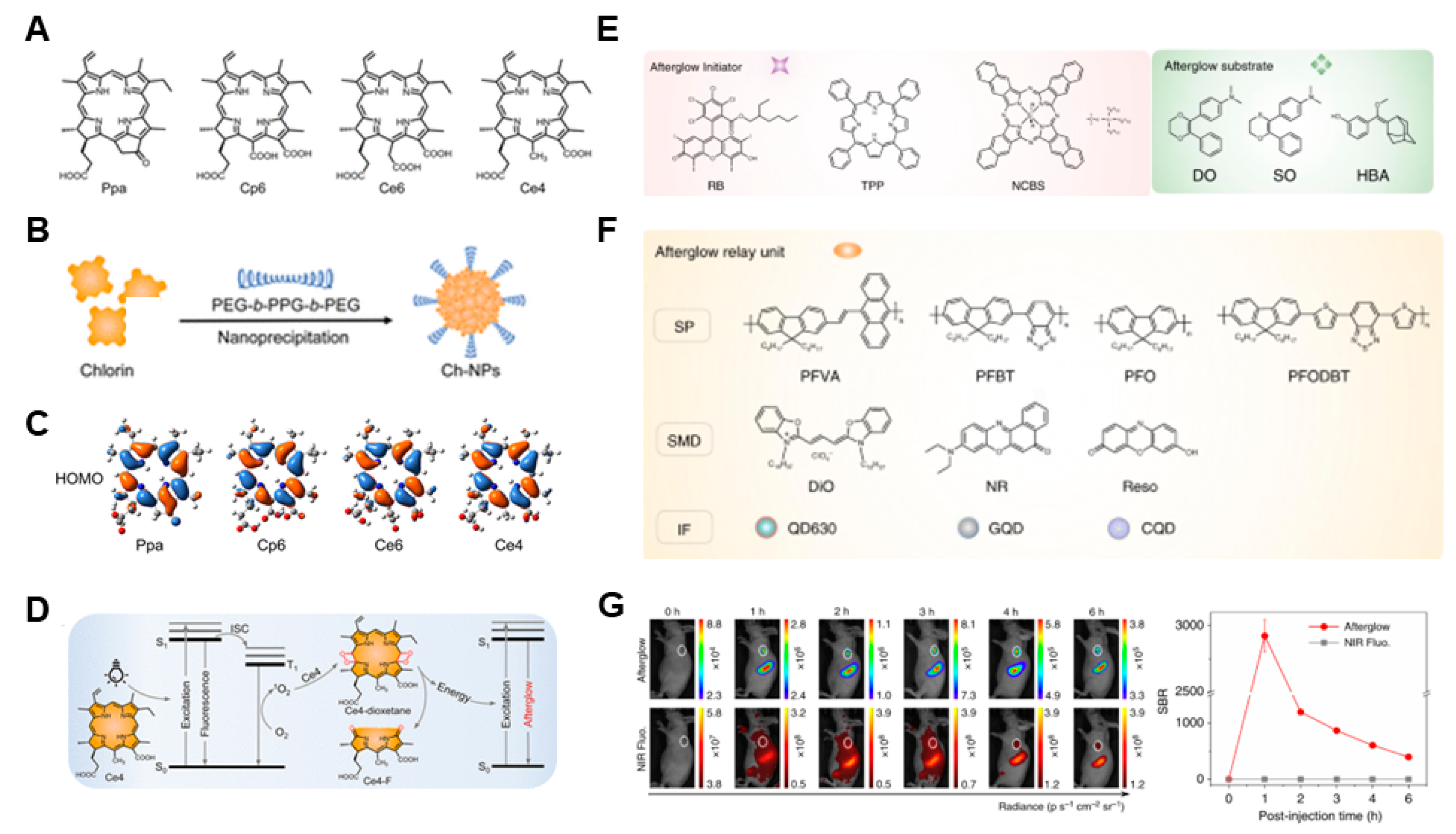
- Wan, C.; Zhang, Y.; Li, Q.; Jiang, Y.; Zhou, H.; Liu, Y.; Miao, Q.; Gao, M. Near-Infrared Afterglow Luminescence of Chlorin Nanoparticles for Ultrasensitive In Vivo Imaging. J. Am. Chem. Soc. 2022, 144, 6719–6726. [Google Scholar]
- Jiang, Y.; Huang, J.; Zhen, X.; Li, J.; Xie, C.; Miao, Q.; Chen, J.; Chen, P.; Pu, K. A generic approach towards afterglow luminescent nanoparticles for ultrasensitive in vivo imaging. Nat. Commun. 2019, 10, 2064. [Google Scholar] [CrossRef]
- Niu, G.; Zhang, R.; Shi, X.; Park, H.; Xie, S.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. AIE Luminogens as Fluorescent Bioprobes. TrAC. Trends Anal. Chem. 2020, 123, 115769. [Google Scholar] [CrossRef]
- Ni, X.; Zhang, X.; Duan, X.; Zheng, H.L.; Xue, X.S.; Ding, D. Near-Infrared Afterglow Luminescent Aggregation-Induced Emission Dots with Ultrahigh Tumor-to-Liver Signal Ratio for Promoted Image-Guided Cancer Surgery. Nano Lett. 2019, 19, 318–330. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, W.; Yao, D.; Bian, K.; Zeng, W.; Liu, K.; Wang, D.; Zhang, B. An Aggregation-Induced Emission Dye-Powered Afterglow Luminogen for Tumor Imaging. Chem. Sci. 2019, 11, 419–428. [Google Scholar] [CrossRef]
- Li, J.; Zhang, G.; Zhang, Y.; Tang, Y.; Ding, D.; Li, W.; Liu, Q. Building Highly Light-Harvesting Near-Infrared AIEgens Using Triazole-Based Luminescent Core for Improved Intravital Afterglow Imaging. Adv. Funct. Mater. 2023, 33, 2212380. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, M.; Chen, W.; Zhu, J.; Xu, W.; Li, Q.; Pu, K.; Miao, Q. A Highly Bright Near-Infrared Afterglow Luminophore for Activatable Ultrasensitive In Vivo Imaging. Angew. Chem. Int. Ed. 2024, 63, e202313117. [Google Scholar] [CrossRef] [PubMed]
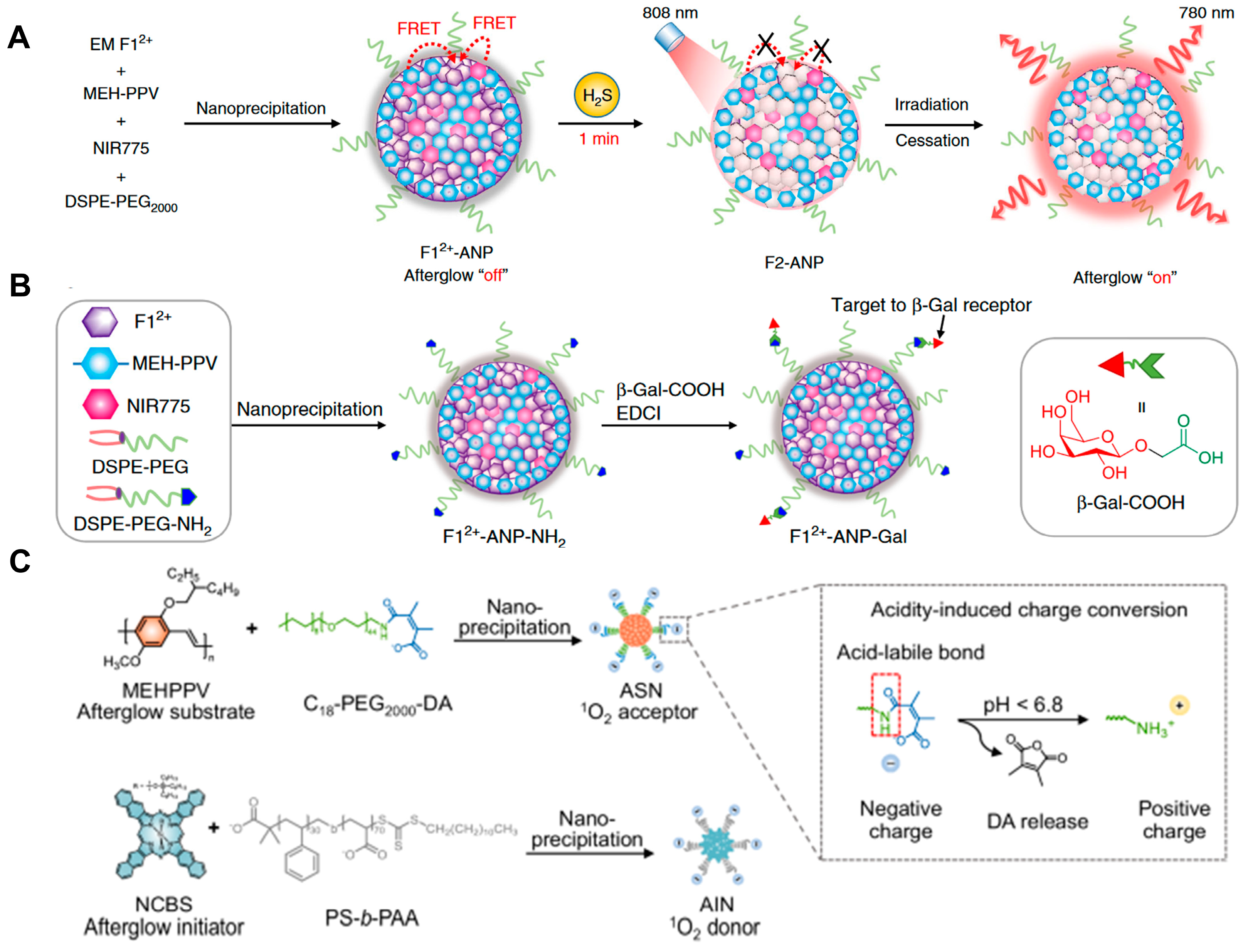
- Wu, L.; Ishigaki, Y.; Hu, Y.; Sugimoto, K.; Zeng, W.; Harimoto, T.; Sun, Y.; He, J.; Suzuki, T.; Jiang, X.; et al. H2S-Activatable near-Infrared Afterglow Luminescent Probes for Sensitive Molecular Imaging in Vivo. Nat. Commun. 2020, 11, 446. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, M.; Miao, J.; Chen, W.; Zhang, Y.; Miao, M.; Yang, L.; Li, Q.; Miao, Q. Acidity-activatable upconversion afterglow luminescence cocktail nanoparticles for ultrasensitive in vivo imaging. Nat. Commun. 2024, 15, 2124. [Google Scholar] [CrossRef]
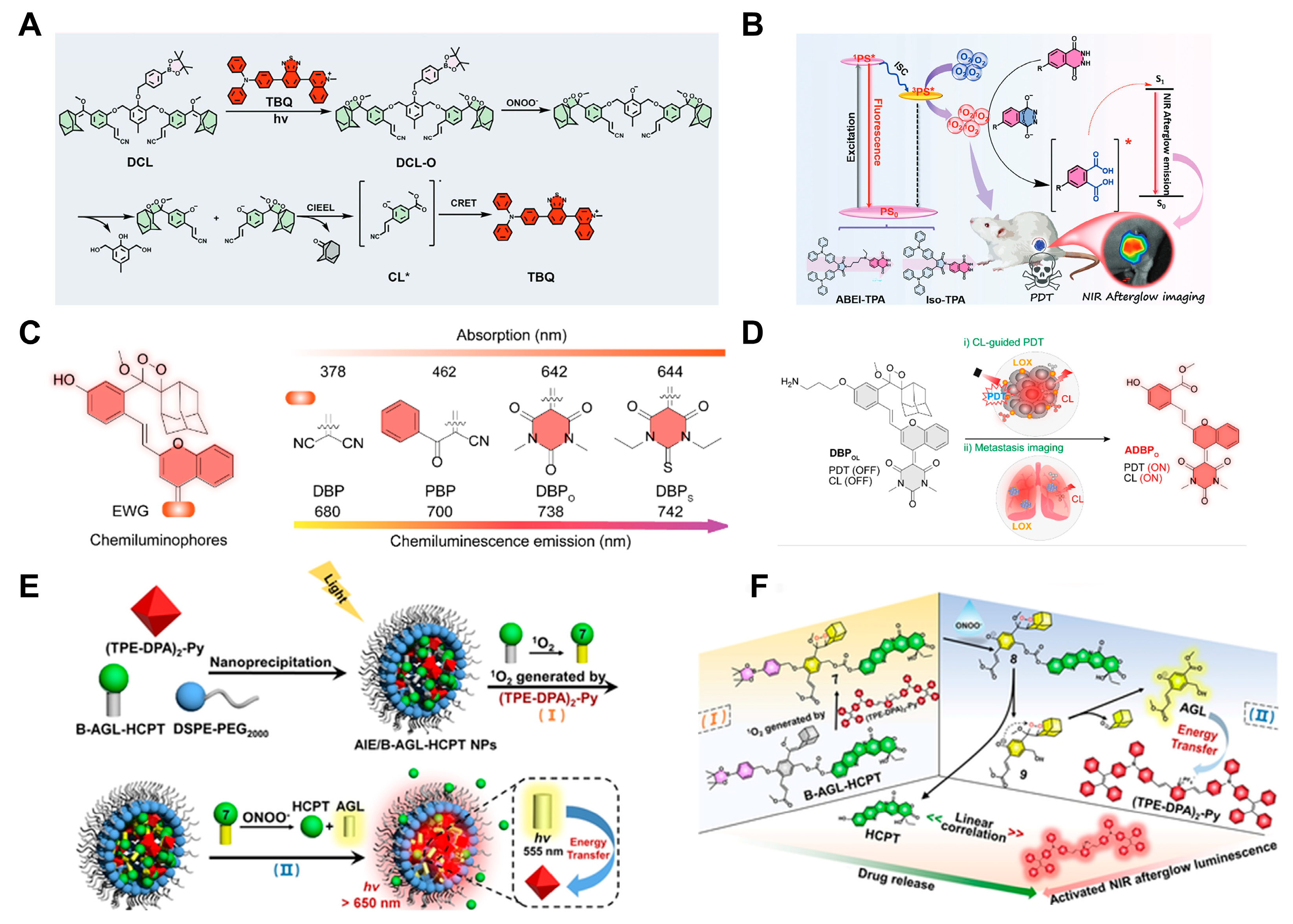
- Huang, S.; Bai, S.; Luo, T.; Zheng, F.; Fan, D.; Zhou, Y.; Dong, J.; Chen, F.; Zeng, W. Chemiluminescent Afterglow Material for Enhanced Tumor Diagnosis and Photodynamic Therapy. Adv. Funct. Mater. 2024. [Google Scholar] [CrossRef]
- Li, Y.; He, D.; Zheng, Q.; Tang, R.; Wan, Q.; Tang, B.; Wang, Z. Single-Component Photochemical Afterglow Near-Infrared Luminescent Nano-Photosensitizers: Bioimaging and Photodynamic Therapy. Adv. Healthc. Mater. 2024, 13, 2304392. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, C.; Wang, X.; Wei, X.; Pu, K. Near-Infrared Photodynamic Chemiluminescent Probes for Cancer Therapy and Metastasis Detection. Angew. Chem. Int. Ed. 2023, 62, e202303982. [Google Scholar] [CrossRef]
- Gao, Z.; Jia, S.; Ou, H.; Hong, Y.; Shan, K.; Kong, X.; Wang, Z.; Feng, G.; Ding, D. An Activatable Near-Infrared Afterglow Theranostic Prodrug with Self-Sustainable Magnification Effect of Immunogenic Cell Death. Angew. Chem. Int. Ed. 2022, 61, e202209793. [Google Scholar] [CrossRef]
- Tong, Y.; Li, M.; Huang, H.; Long, S.; Sun, W.; Du, J.; Fan, J.; Wang, L.; Liu, B.; Peng, X. Urea-Bond Scission Induced by Therapeutic Ultrasound for Biofunctional Molecule Release. J. Am. Chem. Soc. 2022, 144, 16799–16807. [Google Scholar] [CrossRef]
- Dou, Y.; Wang, Y.; Tian, S.; Song, Q.; Deng, Y.; Zhang, Z.; Chen, P.; Sun, Y. Metal–organic framework (MOF)-based materials for pyroptosis-mediated cancer therapy. Chem. Commun. 2024, 60, 6476–6487. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, N.; Wang, Z.; Wu, M.; Chen, Y.; Ma, M.; Chen, H.; Shi, J. Endogenous Catalytic Generation of O2 Bubbles for In Situ Ultrasound-Guided High Intensity Focused Ultrasound Ablation. ACS Nano 2017, 11, 9093–9102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, X.; Yang, H.; Yu, L.; Xu, Y.; Sharma, A.; Yin, P.; Li, X.; Kim, J.S.; Sun, Y. Advanced Biotechnology-Assisted Precise Sonodynamic Therapy. Chem. Soc. Rev. 2021, 50, 11227–11248. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Yao, Z.; Chen, Z.; Ge, X.; Su, L.; Wang, S.; Wu, Y.; Song, J. Ultrasound-Activated NIR Chemiluminescence for Deep Tissue and Tumor Foci Imaging. Anal. Chem. 2023, 95, 11219–11226. [Google Scholar] [CrossRef]
- Yang, Z.; Shen, X.; Jin, J.; Jiang, X.; Pan, W.; Wu, C.; Yu, D.; Li, P.; Feng, W.; Chen, Y. Sonosynthetic Cyanobacteria Oxygenation for Self-Enhanced Tumor-Specific Treatment. Adv. Sci. 2024, 11, 2400251. [Google Scholar] [CrossRef]
- De Visser, K.E.; Eichten, A.; Coussens, L.M. Paradoxical Roles of the Immune System during Cancer Development. Nat. Rev. Cancer 2006, 6, 24–37. [Google Scholar] [CrossRef]
- Schmidt, R.E.; Grimbacher, B.; Witte, T. Autoimmunity and Primary Immunodeficiency: Two Sides of the Same Coin? Nat. Rev. Rheumatol. 2018, 14, 7–18. [Google Scholar] [CrossRef]
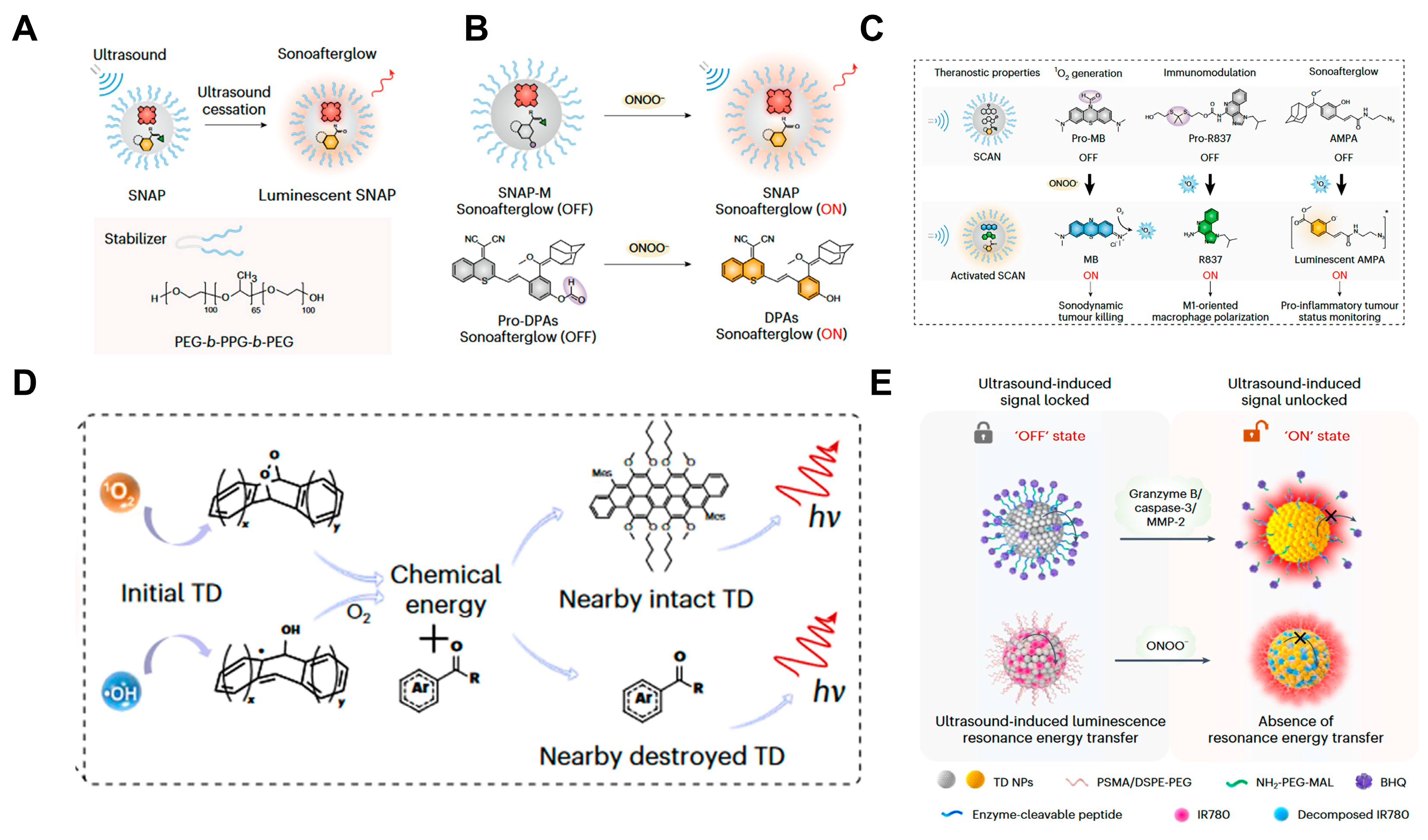
- Xu, C.; Huang, J.; Jiang, Y.; He, S.; Zhang, C.; Pu, K. Nanoparticles with Ultrasound-Induced Afterglow Luminescence for Tumour-Specific Theranostics. Nat. Biomed. Eng. 2022, 7, 298–312. [Google Scholar] [CrossRef]
- Wang, Y.; Yi, Z.; Guo, J.; Liao, S.; Li, Z.; Xu, S.; Yin, B.; Liu, Y.; Feng, Y.; Rong, Q.; et al. In Vivo Ultrasound-Induced Luminescence Molecular Imaging. Nat. Photonics 2024, 18, 334–343. [Google Scholar] [CrossRef]
- Xu, J.; Chen, Q.; Tian, K.; Liang, R.; Chen, T.; Gong, A.; Mathy, N.; Yu, T.; Chen, X. m6A methyltransferase METTL3 maintains colon cancer tumorigenicity by suppressing SOCS2 to promote cell proliferation. Oncol. Rep. 2020, 44, 973–986. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, N.; Ma, M.; Luo, Y.; Chen, H. Transferrin Receptor-Mediated Sequential Intercellular Nanoparticles Relay for Tumor Deep Penetration and Sonodynamic Therapy. Adv. Ther. 2019, 2, 1800152. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, J. Using Nanoparticles to Enable Simultaneous Radiation and Photodynamic Therapies for Cancer Treatment. J. Nanosci. Nanotechnol. 2006, 6, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Liu, Y.; Zhang, H.; Wang, Z. Electrochemiluminescence Biosensor for Nucleolin Imaging in a Single Tumor Cell Combined with Synergetic Therapy of Tumor. ACS Sens. 2020, 5, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, W.; Shi, H.; Ma, H.; Niu, G.; Li, Y.; Zhi, J.; Yao, X.; Song, Z.; Chen, L.; et al. Organic Phosphorescent Nanoscintillator for Low-Dose X-Ray-Induced Photodynamic Therapy. Nat. Commun. 2022, 13, 5091. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Song, J.; Chen, X.; Yang, H. X-Ray-Activated Nanosystems for Theranostic Applications. Chem. Soc. Rev. 2019, 48, 3073–3101. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, L.; Manoharan, D.; Lee, C.; Wu, L.; Huang, W.; Huang, E.; Su, C.; Sheu, H.; Yeh, C. Low Dose of X-Ray-Excited Long-Lasting Luminescent Concave Nanocubes in Highly Passive Targeting Deep-Seated Hepatic Tumors. Adv. Mater. 2019, 31, 1905087. [Google Scholar] [CrossRef]
- Shi, T.; Sun, W.; Qin, R.; Li, D.; Feng, Y.; Chen, L.; Liu, G.; Chen, X.; Chen, H. X-Ray-Induced Persistent Luminescence Promotes Ultrasensitive Imaging and Effective Inhibition of Orthotopic Hepatic Tumors. Adv. Funct. Mater. 2020, 30, 2001166. [Google Scholar] [CrossRef]
- Huang, K.; Le, N.; Wang, J.S.; Huang, L.; Zeng, L.; Xu, W.; Li, Z.; Li, Y.; Han, G. Designing Next Generation of Persistent Luminescence: Recent Advances in Uniform Persistent Luminescence Nanoparticles. Adv. Mater. 2022, 34, 2107962. [Google Scholar] [CrossRef]
- Huang, J.; Su, L.; Xu, C.; Ge, X.; Zhang, R.; Song, J.; Pu, K. Molecular Radio Afterglow Probes for Cancer Radiodynamic Theranostics. Nat. Mater. 2023, 22, 1421–1429. [Google Scholar] [CrossRef]
- Wilkinson, F.; Helman, W.P.; Ross, A.B. Rate Constants for the Decay and Reactions of the Lowest Electronically Excited Singlet State of Molecular Oxygen in Solution: An Expanded and Revised Compilation. J. Phys. Chem. Ref. Data 1995, 24, 663–677. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, C.; Wang, P.; Zhao, Y.; Yang, Y.; Wang, Y.; Yuan, H.; Qu, S.; Zhang, X.; Song, G.; et al. Light-free Generation of Singlet Oxygen through Manganese-Thiophene Nanosystems for pH-Responsive Chemiluminescence Imaging and Tumor Therapy. Chem 2020, 6, 2314–2334. [Google Scholar] [CrossRef]



| Excitation Source | Name of Afterglow Probe | Afterglow Emission | Half-Life |
|---|---|---|---|
| Photo-afterglow Probe | NPs-Ce4 | 680 nm | 1.5 h |
| PFVA-N-DO | 780 nm | - | |
| AGL-AIE | 550–850 nm | 48 min | |
| TPT-DCM | 630 nm | - | |
| TPP-DO | 670 nm | - | |
| F12+-ANP | 780 nm | 6.6 min | |
| DBPOL | 720 nm | - | |
| B-AGL-HCPT | 400–650 nm | 118.5 min | |
| Sono-afterglow Probe | PNCL | 710 nm | - |
| NPs-Ce6 | 680 nm | - | |
| NCBS/DPAs SNAP | 780 nm | 110 s | |
| TD | 635–650 nm | 180 s | |
| Radio-afterglow Probe | IDPAs | 624–792 nm | 9.2–196.8 min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Tu, L.; Wang, H.; Li, J.; Sun, Y. Recent Development of Organic Afterglow Probes for Diagnosis and Treatment of Cancer. Targets 2024, 2, 327-340. https://doi.org/10.3390/targets2040019
Li M, Tu L, Wang H, Li J, Sun Y. Recent Development of Organic Afterglow Probes for Diagnosis and Treatment of Cancer. Targets. 2024; 2(4):327-340. https://doi.org/10.3390/targets2040019
Chicago/Turabian StyleLi, Meiqin, Le Tu, Huiling Wang, Junrong Li, and Yao Sun. 2024. "Recent Development of Organic Afterglow Probes for Diagnosis and Treatment of Cancer" Targets 2, no. 4: 327-340. https://doi.org/10.3390/targets2040019
APA StyleLi, M., Tu, L., Wang, H., Li, J., & Sun, Y. (2024). Recent Development of Organic Afterglow Probes for Diagnosis and Treatment of Cancer. Targets, 2(4), 327-340. https://doi.org/10.3390/targets2040019






