Abstract
Pollinators play important roles in providing pollination services, maintaining biodiversity, and boosting crop production. Even though pollinators are essential to the environment and agriculture, their decline has been noted across multiple studies in the recent past. Both natural and anthropogenic factors have contributed to their decline. Much of the focus has been placed on climate change, habitat loss, pests and pathogens, and synthetic pesticides, but relatively little is known about the effects of biopesticides. Biopesticides are biological control agents derived from living organisms and are classified into three groups: microbial, biochemical, and plant-incorporated protectant-based products. Biopesticides are formulated similarly to their synthetic counterparts and are readily available and used within urban and agricultural settings by pest management experts and household residents. The general public and much scientific literature support the prevailing idea that biopesticides are environmentally safe and pollinator friendly in comparison with synthetic versions. However, such generalizations are based on studies with a few key pollinator species and may not be relevant to several other species that provide crop pollination services. Studies focused on native pollinators have shown that some biopesticides have lethal and sublethal effects. Because each biopesticide exhibits varying effects across pollinator species, it could be dangerous to generalize their non-toxicity across taxa and environmental settings. In this article, recent research in this direction is discussed.
1. Introduction
Flower pollination is essential for the reproduction of plants, seed/crop production, and maintenance of ecosystem biodiversity [1]. A variety of animals pollinate, including reptiles, mammals, and insects [2,3,4,5]. Of the many pollinator groups, insect pollinators are considered the most important in nature and agriculture [6]. This is because insect interactions with flowering plants directly affect the stability of ecoregions and terrestrial biodiversity [6]. The loss of even a single bee species has been shown to reduce plant reproduction efficacy and floral fidelity [7], ultimately leading to a decline in biodiversity. Additionally, an approximated 75% of leading global food crops are dependent on insect-mediated pollination [8]. Production of various economically important crops depends on different species of pollinators [9,10]. Crops, such as those from the Rosaceae plant family, have a noticeable increase in fruit production when pollinated by a wide range of native insect pollinators [11]. A decline in insect pollinators would negatively impact agricultural production [12].
Even though insect pollinators play important roles in ecosystem pollination services and agricultural produce output, it is widely accepted that insect pollinators are on the decline [6,13,14,15]. Some studies suggest that pollinators such as bees are especially at risk of local and regional extinction [1,13]. The causes believed to contribute to global insect pollinator and other beneficial insect decline are grouped into two categories: (1) natural and (2) anthropogenic. Natural causes include parasites, pathogens, and intraspecific/interspecific competition [7,16]. Anthropogenic causes are climate change, habitat destruction/alteration, pesticide use, and many others. Climate change and agriculture are thought to be the two causes most responsible for insect pollinator decline [17,18,19] because both can lead to alteration/loss of habitat, foraging biodiversity loss, and the introduction of exotic species. Studies show that for every 1 °C change in climate, insect flight patterns and plant flowering dates are altered by four days [1]. The altered phenology typically affects 17–50% of pollinators and their foraging supply (floral food resources) due to temporal mismatch [20]. Pesticides have also gained interest due to their prolific use in agricultural/urban settings and their effects on the environment.
Synthetic pesticides have been commercially available since the 1940s [21] and are widely used for pest management in different ecosystems. Concerns for the environment and human health started to appear in the 1960s, leading to the ban on chlorinated organic pesticides [22]. Chlorinated organic pesticides, such as DDT (dichlorodiphenyltrichloroethane), have half-lives of 4–30 years in soil, accumulate in animals and plants for human consumption, are toxic to most forms of life [23], and are likely to contaminate soil for a longer duration [24,25,26]. Even though bans have been implemented to limit the use of extremely harmful pesticides, the increase in commercial agricultural production has also increased the application of pesticides in the environment [27]. While pesticides are an integral part of pest management, increased volumes of pesticides have a higher probability of contaminating soil, air, and water through runoff, leaching, volatilization, and erosion. The perceived hazardous nature of synthetic pesticides has led to the creation of movements such as The Pesticide Risk Reduction Program and organic farming, which aim to limit the use of synthetic pesticides to maximize animal welfare [28]. These programs assume that organic biopesticides, agents derived from living organisms, are minimally harmful to the environment and pollinators; this assumption aligns with the general public’s view [28]. Many synthetic pesticides, especially systemic insecticides, and other active ingredients are well known to cause lethal and sub-lethal impacts on pollinators [29]. While biopesticides are generally not as harmful in comparison to their synthetic counterparts, biopesticides may still pose a significant threat to beneficial non-target insects such as different pollinator species [30,31]. In commercial production, biopesticides are relatively new, accounting for only 5% of total pesticide use [32]. Their small contribution to total pesticide use potentially helps to mask their negative effects in comparison to more established factors such as climate change, land use, and synthetic pesticide application, which have been present for decades. Studies show that biopesticides have an annual growth of 10% in their use and are expected to surpass synthetic pesticides by the mid to late 2050s [33]. At this point, biopesticides will potentially represent a greater threat to pollinators and other non-target insects due to more extensive use and may have unforeseen sub-lethal and detrimental effects. Therefore, it is important to examine biopesticide effects on non-target species.
The guidelines for testing pesticide toxicity set forth by the United States Environmental Protection Agency (US EPA) are divided into three tiers. Tier I is meant to quickly assess a pesticide’s risk level. These tests are performed in a controlled setting and look at acute oral toxicity, chronic oral toxicity, adult acute contact toxicity, and the toxicity of residue on foliage to determine LD50 levels, sublethal/lethal effects, and if residues are still toxic to 25% or more of the adult insects tested. Tiers II and III are reserved for compounds that show potential risk. Tier II focuses on a colony (if eusocial) and is performed in semi-field conditions. Tier II looks at three metrics: (1) the effect of the compound on a pollinator colony in an enclosed space, (2) the effect of the compound on insects in a field setting when fed predetermined amounts, and (3) exposure effects when the compound is applied at the recommended field rate. Tier III focuses on the long-term effects of compounds in real-world settings (application by experts or non-experts, proper use, environmental variables, etc.), including agricultural and urban environments [34]. In this context, we discuss the toxicity of current biopesticides and exposure risk to pollinators in urban and agricultural settings.
2. Biopesticides: Types of Biopesticides, Their Formulations, and Modes of Action
Biopesticides are classified into three groups based on the ingredients used or extraction sources: (1) microbial pesticides, (2) biochemical pesticides, and (3) plant-incorporated protectants (PIPs). Microbial pesticides are derivatives of bacteria, viruses, and fungi [35].
2.1. Microbial Pesticides
Bacteria are unicellular organisms ranging in length from <1 μm to a few μm in size. Most entomopathogenic bacteria are found in five bacterial families [36]. The most common example of bacteria used for pest management is the bacteria Bacillus thuringiensis [Bacilli: Bacillaceae] (Bt). Bt as a biopesticide has dominated the biocontrol market. In 1990, Bt accounted for 90% of all biopesticide sales [37]. This is because different strains of Bt are effective against a few target species. For example, the Bt strains kurstaki and aizawai target lepidopterans, while the strain israelensis targets mosquitoes [38], making them environmentally safe. The mechanisms of action for bacteria such as Bt are ingestion and the subsequent release of toxins to kill the pests [39]. A virus is a complex of genetic material housed in a protective protein/lipoprotein capsule [40]. Entomoviruses are split into two categories: those that release inclusion bodies (IVs) and those that do not (NIVs). IVs are further broken down into polyhedron viruses (PVs) and granulosis viruses (GVs) based on the inclusion bodies released. These PVs can then inhabit either the cytoplasm (CPVs) or the nucleus (NPVs) [41]. The virus family, Baculovirus, is the most studied for commercial biopesticide use [42]. This virus family is characterized by its supercoiled, double-strand DNA arranged circularly and ranging from 80 to 180 kbp [41]. The virus works by infecting its target, replicating exponentially, and eventually killing the pest between 3 days to 4 weeks [38]. Fungi are single cell or multi-celled organisms that drain nutrients from their environment to survive [43]. Currently, more than 700 species of fungi are considered entomopathogenic [44]. The two major fungi used as insect biopesticides are Beauveria bassiana [Hypocreales: Cordycipitaceae] and Metarhizium anisopliae [Hypocreales: Clavicipittaceae] [45]. Fungi begin the infection process by penetrating the integument, forming germinative tubes that release enzymes and eventually kill the host [38].
2.2. Biochemical Pesticides
Biochemical pesticides are derived from naturally present compounds such as insect pheromones and plant byproducts. These compounds act in non-toxic ways to eliminate target pests, are target-specific compared to synthetic pesticides, and can be classified further into three distinct subgroups: insect pheromones, plant-based extracts, and insect growth regulators (IGRs) [46].
Insect pheromones are used for communication and elicit a behavioral response [47], such as attracting other insects, finding mates, locating areas for ovipositing, and trail-following behavior [48]. The first sex pheromone was identified in 1959 from the silk moth [49] and since then, large amounts of progress in the field have been made. The three major reasons pheromones, especially sex pheromones, potentially make great biopesticides is because they are highly species specific, require small concentrations to be effective, and many are not known to be toxic to non-target organisms (NTOs) due to their specificity [50]. Additionally, pheromone biopesticides induce a decrease in pest populations over long periods of application while conventional pesticides do not [51,52]. Pheromone-based biopesticides are used commonly in two strategies: detection/monitoring of a pest and mating disruption [48,50]. Detection/monitoring involves traps with the pest’s pheromone-based attractant as a lure [53]. A relationship is then extrapolated between the number of males caught, the amount of damage per insect, and the estimated pest population to determine thresholds [54]. This same strategy can be changed into a mass trapping strategy by simply adding more traps to an area to reduce pest density [55,56]. This control strategy is particularly effective against pests with slow reproductive life cycles and when both sexes aggregate to the same attractant [57]. The successful use of this strategy was implemented against bark beetles that cause damage to coniferous forests. A field study showed that the tree mortality rate of a 2000 ha forest infested with Ips duplicatus [Coleoptera: Curculionidae] (double-spined spruce bark engraver) was down to 17% when using a pheromone blend trap of E-myrcenol and ipsdienol [58]. Other successful uses of mass trapping against pests include red palm weevils, olive fruit fly, cotton boll weevil, and the Japanese beetle, among others [50].
Mating disruption involves the release of volatile sex pheromones in large quantities, causing either sensory overload or “false trails” that prevent pests from finding partners and thus mating [59]. Currently, this strategy is the most developed in terms of disrupting moth pests [60]. The sex pheromones are emitted via aerial dispensers, microencapsulation, matrix formulations, or hand application [61]. A variety of factors determine the overall effectiveness of mating disruption. First, the physiological state of an insect and consequently its age and species determine if a response occurs. For example, Agrotis ipsilon [Lepidoptera: Noctuidae] (black cutworm) juveniles will not respond to sex pheromones. On the other hand, males only mate once every night and do not respond to sex pheromones temporarily afterward [62], making mating disruption less effective. Other factors include the density of the pest population. For example, mating disruption is ineffective in orchards heavily infested with Platynota idaeusalis [Lepidoptera: Totricidae] (tufted apple bud moth) [63] and forests with a high density of spongy moths [64]. The mating disruption approach can further be changed into a push–pull tactic by incorporating both attractants and repellents [65]. The repellents keep (or push) pests away from target crops while the attractants lure (or pull) pests to traps or trap crops. The attractant blend may also lure natural enemies, compounding the pest control effects of the biopesticide. For example, a three-year study focused on alfalfa fields and aphids showed that the aphid’s pheromone blend attracted parasitoids and significantly reduced the aphid’s abundance [66].
Since at least 4000 years ago, botanicals have been used to control pests [67]. This is because the plant kingdom is recognized as the leading source of biochemical compounds effective at controlling insects [68], and the use of locally available plants simplified pest control in ancient times [69]. Now, many botanical extracts exist as biopesticides since they induce anti-feeding behavior, toxicity, and repellence towards various pests [70,71]. Botanical extracts used for biopesticides are classified as secondary metabolites and are further subdivided into three groups: phenolics, terpenes, and nitrogen-containing compounds [72]. Some of the more prominent secondary metabolites often used include pyrethrins, nicotine, and rotenone [73]. Pyrethrum is the most heavily used botanical biopesticide and is derived from Tanacetum cinerariifolium [Asteraceae] flowers. The cost to produce natural pyrethrins and their instability under environmental conditions make the natural form seldom used [69,74]. However, their synthetic counterparts, pyrethroids, are more toxic, long-lasting, and do not break down too quickly under environmental conditions [75].
Nicotine is a synaptic poison that mimics acetylcholine, an important neurotransmitter in insects [76]. Nicotine is an aqueous extract from the tobacco plant (Nicotiana spp.), which is used extensively. However, its high toxicity to humans has mostly phased it out in Europe and North America [77]. Nicotinoids are synthetic versions of nicotine and exhibit similar properties and modes of action. Nicotinoids include the popular biopesticide imidacloprid, as well as thiamethoxam, acetamiprid, and dinotefuran [69]. Rotenone is a botanical biopesticide declining in agricultural use but is still on the market for organic farming, home, and gardens. Rotenone is isolated from the rhizomes of plants of the genera Derris, Lonchocarpus, and Tephrosia. It is commonly found in dust formulations for urban applications or liquid formulations for organic farming operations [78]. It acts as a contact or ingested cytotoxin that disrupts vital respiratory enzymes in insects [79].
The first recorded use of an IGR was in 1956 when juvenile hormone was isolated from the abdominal crude extract of male cecropia moths. Its topical application prevented further insect multiplication [80]. However, it was not until 1965 that the “paper effect” was observed in Pyrrhocoris apterus [Hemiptera: Pyrrhocoridae] during an unrelated study. It was this accidental discovery that sparked industrial interest in IGRs [80]. Now, a large variety of IGR formulations exist, such as buprofezin for scale insects and lufenuron for fleas [81,82]. Currently, work is ongoing to control insects by either disrupting reproduction or metamorphosis. For example, chitin synthesis inhibitors (CSIs) disrupt the production of chitin. Chitin is a major component of the inset cuticle separating the insect from the external environment and provides support for major physiological systems such as the gut lining, respiratory system, and some gland/reproductive glands [80]. Since CSIs disrupt chitin production, they are most effective as larvicides, the insect life-cycle stage that requires large amounts of chitin production and molting and makes insects extremely vulnerable to failed or abnormal molts, resulting in subsequent death [83]. Juvenile hormone analogs (JHA) are another major IGR. Juvenile hormone (JH) is responsible for many vital physiological processes in insects, such as embryogenesis, reproduction, communication, molting, and metamorphosis, among others [84]. JHAs work to disrupt the JH equilibrium and therefore disrupt the physiological processes preventing the insect from successfully reproducing or reaching its adult form. JHAs are also often used as ovicides or larvicides due to their fatal effects in early life stages [85]. The first JHA released for market use was methoprene. It is primarily used for household pests due to its low residual activity [86]. However, other more effective JHA pesticides have been developed and are available for use.
2.3. Plant-Incorporated Protectants
PIPs are unique biopesticides because target genetic material is incorporated into the plant’s genome. The plant then produces the compound associated with the genetic material, boosting its pest resistance [46]. The first transgenic crop was reported in 1987 and expressed Cry protein biotoxins from B. thuringiensis [87,88]. Today, the main transgenic crops are soybean, maize, and cotton, each making up 77%, 26%, and 49% of their total respective crop type [89]. Many of them express Bt PIP and produce Cry proteins. In insects, Cry toxins are ingested along with plant material, interact with epithelial cell membranes, and cause lysis through the formation of transmembrane pores [90]. Different Cry proteins are used against different insect orders, such as Cry3 for Coleopterans and Cry1 for Lepidopterans [88]. As of June 2017, the U.S. Environmental Protection Agency (EPA) approved a new class of PIPs known as RNA interference (RNAi) [91]. RNAi works by targeting specific gene pairings and then suppresses their expression at the post-transcriptional level, preventing protein synthesis [92]. Like Cry proteins, the double-stranded RNAi is ingested and transported to target cells. From there, dsRNAi is cleaved into smaller RNAi molecules that disrupt the insect’s mRNA translation, eventually causing insect mortality [91].
2.4. Biopesticide Formulations
Biopesticide formulations are typically similar to those used for synthetic pesticides. They can either be dry (e.g., dusts, powders, granules/micro-granules) or liquid (emulsions or suspension concentrations) [93,94]. Dusts are formulated by having their active ingredient absorbed into a finely ground mineral powder. The size of dust particles usually ranges from 50 to 100 μm and dust formulations can be directly applied via manual or mechanical methods [95]. Granules are formulated in a similar manner except they are composed of larger particles; 100–600 microns and 100–1000 microns for micro-granules and granules, respectively. Biopesticides formulated in the form of granules are applied directly to soil where they gradually release their active compound [96,97]. Powders for seed treatment are typically formulated by mixing a fine active ingredient with an inert ingredient that facilitates adherence to seeds. The seeds are then tumbled with the mixture [98] and processed for further use. Water-dispersible granules are intended to be used with water, and they break up into a homogenous suspension. The suspension is then applied with conventional or specialized spray equipment. Biopesticides in granular form are formulated using extrusion granulation, fluid bed granulation, spray drying, and other processing techniques [99]. Emulsions are a mixture of an immiscible liquid with liquid droplets of the active ingredient dispersed within. They are designed to be mixed with water and then applied [100]. Wettable powders are similar to water-dispersed granules except the particles of wettable powders are smaller in size (5 microns) [101]. Suspension concentrates are formulated with a mixture of a liquid (commonly water) and a finely-ground active ingredient. The mixture needs to be agitated to homogenously disperse the solid active ingredient [96,98]. Oil dispersions are formulated in the same manner as a suspension concentrate. The difference is that oil dispersions use non-aqueous liquids such as oils derived from plants [100]. While a few other formulations do exist, these are the most common in use for biopesticides today.
3. Biopesticide Uses and Routes of Exposure in Urban and Agricultural Ecosystems
The world’s population continues to grow exponentially and is predicted to reach 8–10 billion people by 2050 [102]. According to the United Nations Department of Economic and Social Affairs [103], 55% of the world’s population resides in urban areas. That number is expected to reach 68%, or 5–7 billion people, in 2050. The expansion in population and urbanization has decreased natural habitats while increasing the need for food production [104]. Consequently, these changes have also increased the number of pests and pesticide use in urban areas. Common examples of pests are wasps in urban structures, ant hills in gardens, housefly invasions, and cockroach infestations. To lessen the negative impact caused by the overuse of pesticides, integrated pest management (IPM) programs were created [105]. These IPM programs incorporate chemical, biological, and cultural methods to reduce pest levels below the economic injury threshold in both agricultural and urban settings [37,105]. For example, Kass et al. [106] focused on mice and cockroach infestations in New York urban settings. The study compared the effectiveness of standard pesticide applications to the incorporation of an IPM. In this case, the IPM involved denying food sources, water sources, movement between buildings, and nesting sites, as well as improving the structural and sanitary conditions of the building. Simultaneously, the IPM reduced the volume of pesticide applied and outperformed regular pesticide applications in mitigating the abundance of these pests. The reduced application of pesticides makes it less likely that non-target organisms will be negatively affected. Unfortunately, IPM knowledge diffusion and proper incorporation can be slow. It is typically sponsored by universities, government/non-government agencies, or private companies and involves knowledge dispersion ranging from non-intensive, learning-intensive, to cost-intensive sessions, mass media campaigns, or a series of educational meetings, making it unattractive to some [107]. Even though IPM strategies call for more environmentally friendly control methods such as biopesticides, non-target pollinators are still exposed in various ways to the active ingredients when applied. The three common modes of biopesticide application in urban and agricultural landscapes are topical spray applications, dust/particle dispersal, and PIPs integrated into a plant population [45]. These modes of application, therefore, determine possible direct or indirect exposure of pollinators and other non-target species to biopesticides.
3.1. Biopesticides in Urban Settings
In urban settings, the use of synthetic pesticides has increased because residents are reluctant to cohabitate with pests [104] and may be unaware of IPM strategies. Pesticides can be easily applied by both household residents and pest management experts, making them extremely convenient for use. The combination of convenience and intolerance toward insect pests in urban structures has led to pesticide resistance in urban insects [108]. Pesticides in urban areas also pose a threat to non-target species such as bees as they are exposed to pesticide residues [109]. Due to growing concerns about synthetic pesticides, there has been a surge in biopesticide incorporation. Like synthetic pesticides, biopesticides are available in ready-to-use or ready-to-spray formulations. Biopesticides have been shown to effectively reduce pest populations in urban settings. For example, two studies [110,111] released Hyphomycetes fungi targeting mosquitos. This resulted in mosquito mortality after blood feeding and lowered malarial survivorship. Another study compared EcoRaider (biopesticide) to Temprid SC (synthetic pesticide) in the eradication of bed bugs [112]. No significant difference was noted in the bed bug mortality rate, meaning that biopesticides could effectively replace synthetic pesticides in urban settings in this study. Some common biopesticides readily available for purchase and in urban use are GrowSafe, Regalia, and Rango. These biopesticides are advertised as safe for beneficial insects (bees, ladybugs, etc.), safer for the environment and human health, and effective at reducing or eliminating the broad range of pests they target, which include powdery mildew, blight, mites, and pest insects [113,114,115]. Yet studies [116,117,118] show that biopesticides can incur lethal and sublethal effects on non-target organisms. This is important to note because urban agriculture is gaining popularity. Approximately 15–20% of food production takes place in urban settings or closely surrounding areas [119]. Depending on the level of urbanization intensification, urbanization may foster pollinator biodiversity [120]. Specifically, hymenopteran richness and abundance increase whilst those of dipterans, lepidopterans, and coleopterans typically see a small decrease [121]. The increase in hymenopteran richness and abundance is supported by (1) Patenković et al. [120], who noticed significant survivorship and genetic variation amongst honey bees in urban settings; and (2) Samuelson et al. [122], who observed an increase in colony size and food storage with a decrease in parasite load in Bombus terrestris [Hymenoptera: Apidae]. This is due to initial urban sprawl, the first step in urbanization, which creates areas with impervious surfaces (IS) of 50% or less [119]. These areas are typically composed of intermittent gardens, parks, green areas, and pockets of natural land giving insects an abundance of floral resources and nesting areas in comparison to agricultural land [123]. In contrast, urban areas with >50% or more IS do not foster biodiversity and are composed of abiotic, non-flowering surfaces such as sidewalks, car parks, buildings, etc., reducing overall floral resources and nesting habitats while increasing risk factors such as pollution, noise, and artificial light [120]. This is supported by Baldock et al.’s. [124] study showing that bees, hoverflies, and non-syrphid flies composed 90% of the biodiversity in their urban test site. Most of the biodiversity aggregated around allotments or gardens while the least biodiversity was found on manmade structures such as car parks. Unfortunately, urban residents are more likely to improperly use biopesticides in gardens, and therefore, pollinators have a greater chance of encountering the active ingredient [125] in different exposure scenarios. For example, grooming behavior exhibited by honey bees and other eusocial pollinators could lead to the entire colony becoming exposed through contact after the return of foragers [126]. Additionally, visiting pollinators risk ingesting the biopesticide as they feed on contaminated flowers or risk contaminating the brood if it is for provisions. Depending on the biopesticide used, pollinators could suffer a decline in survival or an alteration in behavior [127] in different ways (Figure 1). Eusocial bees, such as honey bees, can also be exposed to biopesticides when urban beekeepers spray hives for parasites and hive pests. The direct application exposes both adults and broods topically and when contaminated food is ingested [30]. Many biopesticides have been reported to have sublethal effects on different species of bees (Table 1), and exposure to these biopesticides can affect bee learning and behavior. For instance, exposure to B. bassiana-based biopesticide formulations altered the guard behavior of stingless bees (Tetragonisca angustula [Hymeoptera: Apidae]) [116]. In this study, the guard bees prevented exposed, but otherwise healthy, individuals from entering the colony. A similar study performed by Cappa et al. [117] showed that B. bassiana altered the behavior and recognition abilities of honey bees as well. The guard bees antagonized their contaminated nestmates and prevented them from entering the colony, lowering the number of returning individuals and pollination services. Interestingly, a separate study [118] also exposed honey bees to B. bassiana to test honey bees’ response to sucrose, and the study found that the bees’ response was altered, causing suboptimal foraging. However, the actual impact of such exposure on colony foraging performance was not examined in this study.
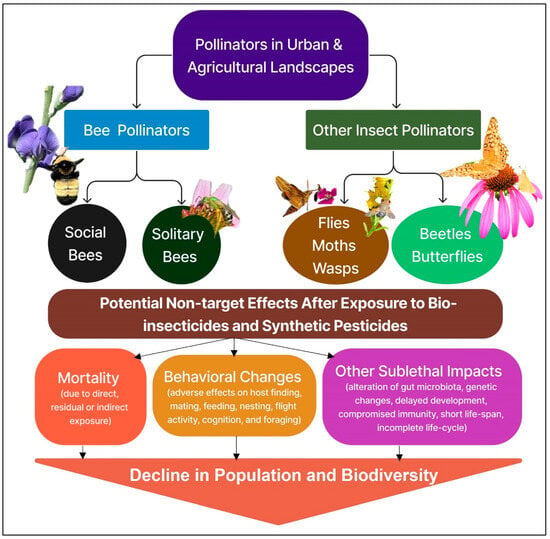
Figure 1.
Potential impacts of insect pollinator exposure to biopesticides used for pest management in urban and agricultural landscapes. Picture by N. Joshi.
3.2. Biopesticides in Agricultural Settings
In the agricultural setting, biopesticides are also gaining popularity [128] due to growing concerns about environmental and health security. Organic farming has grown and aims to maximize animal welfare, limit the use of synthetic products including pesticides, and increase soil health over time [129]. To help farmers across the globe, IPMs have been implemented. In commercial agriculture, one of the most successful IPMs is for soybeans grown in Brazil. The IPM program incorporates biopesticides derived from viruses, fungi, and bacteria, lowering the amount of synthetic insecticide use by 50% [37]. Biopesticides commonly used in agriculture are Bt, Trichoderma spp., Trichogramma spp., neem, and baculovirus [130]. They help to control lepidopterans, storage pests, and infections [45]. Depending on the species and mode of application, pollinators can be exposed to various biopesticides by being used as entomovectors, through contact by foragers, ingestion, or from within or near their nests when applied for parasite control [30,131]. The concept of pollinators as entomovectors to distribute biopesticides [131] has been in use since the 1990s and involves using insects to disperse biological control agents to target plants; however, it was not until 2007 that it was officially acknowledged in the literature [30,131]. In this case, pollinators directly contact the biopesticide via anthropological application. This can easily contaminate other pollinator species when contaminated pollinators visit flowers and leave behind short-lived biopesticide residues. In the case of spore-based, viral-based, or bacterial-based biopesticides, the microorganisms may attach themselves to pollinators when they visit, contaminating entire populations of varying insect species [126].
Another example of biopesticide use in agricultural settings is essential oils. Essential oils, such as neem, are a type of biopesticide that is topically applied to crops to deter pests [132]. During the flowering season, pollinators such as bees are attracted to crop flowers, collect pollen/nectar, and may be exposed to pesticide residue found in floral food resources [133]. If that floral food is meant for the brood, then the brood is exposed to the active ingredients as well [134]. Additionally, upon contact with sprayed flowers while foraging, the pollinator becomes exposed to the biopesticide. A common biopesticide application method with similar exposure routes as topical application is seed treatment. This method is performed by dipping crop seeds in a mixture of the active ingredient and other inert compounds to coat the seeds [135]. This helps to mitigate the number of soil-born pest interactions with the seeds. Furthermore, seeds can be primed to increase the population load of the active ingredient if the active ingredient is a living organism (bacteria, virus, fungi, nematode, etc.) to further increase protection [136]. Despite the treated seeds being buried, the ecology of some pollinator species, such as ground-nesting bees and syrphid flies, also determines how exposure occurs. Ground-nesting bees typically build their nests in well-drained soil near other ground-nesting bees and food sources [135], which include crop fields and urban building garden plots. When biopesticides are applied, the soil surrounding the bees’ nests becomes contaminated. As the bees leave to forage or the larvae emerge, they become exposed to the active ingredient through contact. In some cases, such as biopesticides with nematodes as the active ingredient, exposure can parasitize ground-nesting bee larvae [136].
Syrphid flies encounter a similar problem. The adults exclusively feed on pollen, nectar, and honey, while the larvae feed on plant-sucking insects such as aphids. The syrphid’s dual ecology makes it likely that adults and larvae reside near crops [137], raising the chances that they become disrupted when biopesticides are applied. Exposure can occur through contact, ingestion, or direct application when the biopesticide is administered. Beneficial lepidopteran pollinators are also at high risk of being collateral. Biopesticides such as Bt are designed to target pest lepidopterans that damage crops [37]. Bt biopesticides are incorporated into crops through genetic modification or spray application. Even though the biopesticide is target-specific, beneficial lepidopterans may not be differentiated enough from pest lepidopterans [30]. When collecting nectar, beneficial lepidopterans may ingest Bt toxins, leading to their exposure and consequential death since Bt is broadly effective against lepidopterans.
The same biopesticides and methods are also used in commercial organic farming in combination with other pest management tools used in conventional farming. IPM programs can be further refined to incorporate pollinator health under the framework of integrated pest and pollinator management (IPPM) and by modifying pesticide programs in different crops [138,139,140]. While some IPM programs have beneficial synergistic effects on pollinators, others do not. For example, the application of a biopesticide during pollinator flight times or the use of exclusion netting to prevent pests from reaching a crop may also disrupt pollinators [140]. Consequently, pest crop damage is reduced but so is crop output due to reduced pollination services since most yields are directly correlated with both pest damage and insect-mediated pollination [109]. By adding a pollinator management facet to an IPM, the newly refined IPM incorporates cultural, physical, and mechanical controls that purposefully mitigate the non-target effects between pest and pollinator management strategies [126].
Compared to an agricultural setting, urban land is likely to have a higher biodiversity of pollinators based on previous studies [141]. However, it varies by the type of agricultural land. For example, agricultural land dominated by forest or grassland has a higher abundance and richness of pollinators in comparison to apple orchards [142] due to wider floral and habitat resources. Similarly, land used for animal husbandry has lower pollinator richness and abundance in comparison to arable land [138]. This may, in part, be due to the short flowering period of monoculture crop plots or continuous grazing by farm animals leaving pollinators with insufficient food sources year-round [142]. Studies concurrently show that agricultural land with a high degree of fertilizer application sees lower abundance and richness of hymenopterans, lepidopterans and coleopterans see no significant change, and most dipterans see a positive increase [138]. It is unsurprising to see a lower biodiversity of hymenopterans and lepidopterans, some of which are specialists as adults or larvae, in agricultural land since the land reduces all flower resources [143]. The increase in dipterans is partly due to nitrogen saturation, animal manure, and their generalist characteristics. Agricultural land sees greater risks for the comparatively fewer pollinator taxa due to the consistent use of pesticides targeting crops, the main floral resource for pollinators in the area [120].
4. Biopesticide Effects on Various Pollinator Taxa and Mitigation Recommendations
4.1. Honey Bees
Honey bees are eusocial insects that live in large colonies, are orange-brownish in color, have fuzzy bodies, and are considered the most important pollinators [144,145]. Honey bees are both wild and managed for commercial purposes, and because they are vital to the maintenance of wild plants, crops, and the economy, honey bees are pervasive in most environments [146,147]. Their pervasiveness exposes them to a wide range of biopesticides with potentially lethal and/or sublethal effects.
For example, spinosad, a bacterial-based biopesticide derived from Saccharopolyspora spinosa [Pseudonocardiales: Psuedonocardiceae], is extremely toxic to honey bees through oral ingestion and contact at recommended spray levels in laboratory settings [30,148,149]. At sublethal concentrations, spinosad induces behavioral changes in foragers such as reluctance to collect contaminated food, reluctance to convey contaminated food source locations [150], and reduced walking distance [151]; genetic changes including lower expression of enzymes associated with metabolism and detoxification [152]; and structural changes such as less columnar epithelium and regenerative cells in the midgut of honey bees [151]. This indicates that implementing spinosad when honey bees are prolific (such as flowering season for any particular plant) could be detrimental to them [126]. However, more recent studies such that by as Christen et al. [152] support the notion that spinosad is harmless to pollinators when the residue dries. To mitigate spinosad’s potential effects on pollinators, it is recommended to not apply it during bloom season when honey bees are present in high densities. If its application during bloom season cannot be avoided, spinosad should be applied in the late afternoon/early evening when bee activity is reduced. This gives spinosad residue time to dry and become harmless to beneficial insects. Managed honey bee hive entrances can also be closed and fed artificially during spray days to prevent exposure while spinosad remains harmful [153].
Another biopesticide shown to have negative effects on honey bees is azadirachtin (AZ), a botanical pesticide derived from the neem tree [30]. AZ acts as both a repellent and IGR to its target pests. One major pest that AZ is implemented against is the honey bee ectoparasite, the Varroa mite [154]. AZ is either mixed into artificial feed for honey bees or applied directly into the hive. Peng et al. [155] reported that AZ concentrations > 18 μg/mL significantly increased mite and honey bee mortality. Interestingly, honey bees infested with mites have a slightly higher tolerance (oral LC50 is 13.69 μg/mL) for AZ than honey bees that are not infested (oral LC50 is 10.87 μg/mL). Unfortunately, the LC50 for Varroa mites remains much higher at 41.86 μg/mL [155]. Another study [156] supports these findings. The study reports a positive relationship between neem extract concentrations and honey bee brood mortality. Specifically, the most affected stages were eggs and first instar larvae. Sublethal effects have also been reported in hives. Thompson et al. [157] reported that four out of five hives treated with AZ failed to overwinter despite no apparent brood effects. Outside the hive, crops treated with AZ lower the honey bee forager visitation rate to the treated field [158]. To limit the effects of AZ (and other related IGRs) on non-target honey bees, it is recommended not to use AZ during the spring/early summer to prevent overwintering failure. Instead, AZ should be applied in the later months (August, September, October) to lessen the number of obscure sublethal effects and impact on the honey bee brood (honey bee brood production slows) [157]. Furthermore, lower concentrations of AZ should be applied when little to no mite load is present. A 4% neem concentration has been shown to repel mites from honey bees, therefore breaking their life cycle [156]. If higher concentrations must be applied, the bee hive infestation should be moderate to provide most bee individuals with a slightly higher tolerance to AZ [155].
Entomopathogenic fungi, such as B. bassiana, are another class of biopesticide to control Varroa mites on honey bees. In the case of B. bassiana, studies agree that overall colony health remains unaffected in terms of colony weight, growth, and survivorship among other metrics [159,160]. Other entomopathogenic fungi such as Hirsutella spp. and Verticillium lecanii [Hypocreales: Cordycipitaceae] are toxic to honey bees [161]. Sublethal effects and effects on individual honey bee workers induced by B. bassiana exist and include altered AchE activity, reduced weight, reduced individual longevity, and altered sucrose response [162,163]. To mitigate the impact of entomopathogenic fungi on honey bees, non-toxic or less harmful fungi species, such as B. bassiana, should be chosen. Consecutive daily or short-term applications should be avoided where possible since lethal cumulative effects are possible [163,164]. It is further recommended to monitor treated hives since sublethal effects may not be immediately apparent as effects vary among individuals yet may still impact colony survivorship.

Table 1.
Examples of bioinsecticides and their impacts on different species of bees.
Table 1.
Examples of bioinsecticides and their impacts on different species of bees.
| Bioinsecticides | Active Ingredient | Non-Target Bee Species | Effects Reported | Citations |
|---|---|---|---|---|
| BotaniGard (Bt) | Beauveria bassiana | Bombus terrestris | Reduced longevity | [164] |
| Abamectin | Apis mellifera | Very toxic Adverse effects | [165] | |
| Nostalgist® | Beauveria bassiana | Bombus terrestris | Queen emergence times affected | [166] |
| Priority® | Paecilomyces fumosoroseus | Bombus terrestris | Lower number of workers | [166] |
| Nimbecidine | Azadirachtin | Bombus terrestris | Queen emergence times affected Competition point alteration Increase in male production Lower food intake | [166] |
| Emamectin Benzoate | Apis mellifera | Extremely toxic | [165] | |
| Spinetoram | Bombus impatiens | Very toxic | [167] |
4.2. Bumblebees
Bumblebees are a species of pollinating hymenopterans that are both wild and commercialized [166]. Like honey bees, bumblebees are eusocial and live in a caste-based colony hive. Bumblebees engage in a unique form of foraging called buzzed pollination, making them vital to the reproduction of wild plants and crops [166,167,168]. Their efficiency as pollinators and biology as ground-nesters exposes them to a wide range of biopesticides.
Bacterial-based biopesticides such as Bt are commonly deployed through sprays or transgenic crops. Bumblebees are then exposed orally (contaminated pollen/nectar) or through contact [169]. Depending on the Cry toxin, bacterial species, bee species, and/or mode of application, bacterial-based biopesticides can be lethal or have sublethal effects. For example, Bombus impatiens [Hymenoptera: Apidae] showed sublethal effects (lower production of reproductive and slower ovipositing) when orally exposed to Bacillus amyloliquefaciens [Bacillales: Bacillaceae]. Adversely, no effects were reported when exposed topically [170]. When B. terrestris was exposed to B. amyloliquefaciens, no immediate effects were observed. However, after several weeks, the colony experienced a high mortality rate [171]. The same bumblebee species exposed to Bt strain kurstaki and Bt strain aizawai used against Lepidopterans showed no negative effects when exposed through contact or oral ingestion (pollen). Oral ingestion of nectar with Bt strain aizawai had a 100% mortality rate while the kurstaki strain showed no effect [172]. Studies such as that by Malone et al. [173] have also looked at the effects of Bt transgenic crops on bumblebees. It is suggested that Bt corn pollen is safe for bumblebees to consume with no negative effects [174]. To lessen the impact of bacteria-based biopesticides on bumblebees, it is suggested to choose pathogens or derivatives that are minimally harmful to the local bumblebee assemblage. The mode of application should also be considered. Sprays may inadvertently contaminate nectar sources, proving to be lethal to some bumblebee species. The incorporation of transgenic Bt corn is the safest option based on current information.
Azadirachtin is a major botanical biopesticide repellent and IGR classified as safe for bees. However, studies have focused on honey bees and made generalizations based on those studies. Studies focusing on bumblebees such as that by Koskor et al. [174] show that chronic neem extract applications alter forager behavior, potentially lowering individual and colony survivorship. Another study [173] found similar results and established a negative relationship between AZ concentration and bumblebee survivorship and that bumblebees are minimally repelled by neem extract even at maximum field recommendation levels. This implies that bumblebees are unable to purposefully avoid contaminated floral resources and are more likely to be contaminated themselves. Their eusociality and grooming behavior further enhance the chance of cross-contamination within the hive. In the same study [175], a negative impact on oogenesis and drone production was observed. Currently, it is not recommended to use neem or its derivatives in greenhouses where bumblebees are managed or fields with high densities of bumblebees until gaps in the research are filled.
Many entomopathogenic fungi, such as V. lecanii, Trichoderma atroviride [Hypocreales: Hypocreaceae], and Ampelomyces quisqualis [Pleosporales: Phaeasphaeriaceae], have been tested on bumblebees and show little to no effects [171,176,177]. The more widely used M. anisopliae and B. bassiana have both been shown to have toxic effects on bumblebees [178,179]. It is important to note that for M. anisopliae, exposed B. terristris nests showed no negative effects except when M. anisopliae was present in higher densities than the field use recommendation [180]. For B. bassiana, sublethal effects were present in the form of less foraging and rearing of reproductive when it was applied for dissemination through an entomovector (in this case, bumblebees) [171,181]. Lethal effects were present in the form of mortality and lifespan [164,178]. Conversely, a study [182] reported that no negative effects on colony health occurred when bumblebees vectored B. bassiana. Furthermore, the oral administration of B. bassiana showed no adverse effects on bumblebee colony health [171]. Unfortunately, fewer studies on the effects of entomopathogenic fungi have been conducted on bumblebee species compared to honey bees. The studies that have been conducted show bee species-specific reactions to various fungal pathogens and have mostly mimicked managed colony environments rather than feral colony environments. Feral colonies could have factors that boost fungal pathogenicity such as more humidity or more optimal incubation temperatures, making findings hard to generalize across species and settings [30]. Based on the information available, mitigation efforts should include an understanding of local bumblebee biodiversity to select fungal biopesticides that are harmless or minimally harmful to as many bumblebee species as possible. Following recommended use levels should also be followed as higher densities may prove toxic while otherwise harmless. Lastly, the mode of application should be considered. Using bumblebees as entomovectors has been shown to be harmful to some species. Direct application to plants should be used to lessen fungal loads on bumblebees.
4.3. Other Bees
There are over 20,000 bee species and most are non-Apis, non-Bombus species [183]. Yet Apis and Bombus species receive the most attention due to their easy management, general pollination biology, and economic value. At the same time, other bees remain neglected despite their diversity, ecological importance, and function [183]. For example, mason bees (Osmia spp.) are solitary pollinators prolifically found in orchards, are known to be more efficient pollinators in orchards compared to honey bees [184], and are exposed to the same levels of biopesticides as honey bees in orchards.
The relatively few studies that have been performed show that biopesticide toxicity to other bees can occur and that it is species dependent. For example, B. bassiana proved toxic to Melipona scutellaris [Hymenoptera: Apidae] [185] but remained minimally toxic (<30% mortality) to T. angustula, Scaptotrigona Mexicana [Hymenoptera: Apidae], and Melipona beecheii [Hymenoptera: Apidae] [186]. Megachile rotundata [Hymenoptera: Megachilidae] was also shown to have twice the mortality in comparison to honey bees when exposed to B. bassiana [187]. Entomopathogenic biopesticides may also incur sublethal effects on other bee species, as shown by Almedia et al. [116]. Guard T. angustula bees can detect and repel otherwise healthy colony members exposed to B. bassiana.
Bacteria-based biopesticides such as spinosad and its derivatives have also proved toxic to M. rotundata across all life stages [167] and toxic to adult Tetragonisca fiebrigi [Hymenoptera: Apidae] [188], Plebeia lucii [Hymenoptera: Apidae] [189], and Plebeia emerina [Hymenoptera: Apidae] stingless bees [190]. Comparative studies [191,192] have also demonstrated a higher sensitivity to spinosad in M. rotundata and Osmia lignaria [Hymenoptera: Megachilidae] compared to honey bees and bumblebees, suggesting a greater biohazard than previously believed from flagship studies. Studies that focus on or observed sublethal effects in other bees report a delay in development [193], impaired locomotion [188,189], and antifeeding behavior [194] across various bee species.
Following the trend, azadirachtin is toxic to adult Partamona Hhelleri [Hymenoptera: Apidae] and Scaptotrigona xanthotricha [Hymenoptera: Apidae], albeit minimally [195]. M. quadrifasciata larvae exposed to AZ exhibited a positive dose-dependent mortality rate [196]. Melipona quadrifasciata [Hymenoptera: Apidae] larvae also exhibited sublethal effects concerning body mass, deformation, and suppression of immune-related genes [196,197]. P. helleri exhibited a dose-dependent antifeeding behavior and delayed/deformed larvae, affecting colony fitness over time [198,199]. A large gap between the effects of botanical biopesticides (such as AZ) and non-Apis, non-Bombus bees exist. Based on the few studies available, biopesticides affect bee species differently based on the formulation, mode of action, application method, and active ingredient concentration (Table 2). The limited information in combination with ground-dwelling bee biology makes it difficult to give generalizable and effective mitigation strategies for solitary bees. Future research is needed in this area of study to better inform sustainable and effective mitigation strategies.
4.4. Lepidopterans
Lepidopterans are a taxonomic order consisting of over 168,000 species of butterflies and moths [200]. Their scaled wings provide bright coloration as adults. Adult lepidopterans typically feed on nectar and exhibit pollination behavior. Immature lepidopterans retain chewing mouthparts and feed on plant material [201], making them pests to many cultivated plants. Due to their pestiferous nature, many insecticides target lepidopterans without discriminating between pestiferous and beneficial species, causing steep declines [202,203,204]. The result is that many studies focus on the lethality of pesticides on pestiferous lepidopterans but disregard the effects on beneficial lepidopterans.
The studies focusing on non-target beneficial lepidopterans unsurprisingly show the negative impacts of biopesticides [205]. A study on Bt cotton expressing the Cry1Ac protein resulted in less beneficial lepidopteran abundance. The study concluded that Bt cotton was safer than synthetic pesticides but was still harmful in comparison to non-pesticide fields [205]. More recent studies [206,207,208] have investigated the effects of Bt on a limited number of beneficial lepidopterans, showing reduced weight and feeding with high toxicity and mortality. Unfortunately, other classes of biopesticides, such as entomopathogenic fungi, nematodes, and viruses, have been neglected regarding beneficial lepidopterans. Only generalizations based on pestiferous lepidopterans can be made. Consequently, no suitable mitigation recommendations exist except that biopesticides may be safer for lepidopterans compared to synthetic pesticides [205].
4.5. Dipterans (Syrphids)
Dipterans are a diverse group of 120,000 fly species. Dipterans are characterized by a pair of functional wings and a pair of modified wings (halteres) used for stabilization. Many dipterans are known as pests and disease vectors, but syrphid flies, a subset of dipterans, are important biological control agents and pollinators. Their unique biology potentially exposes them to biopesticides across their whole life cycle [209,210].
Moens et al. [211] explored one such biopesticide, spinosad, on hoverfly larvae. The results were a 60% mortality rate and a cessation in successful oviposition. In combination, hoverfly populations would quickly decline locally. Other studies, such as that by Jansen et al. [212], observed the effects of biopesticides at the community level. A reduction in hoverfly populations was described, but more detailed information could not be obtained due to the scope of the study. Some studies [213,214] show the potential safety of biopesticides such as M. anisopliae and B. bassiana, citing efficiency against pests with no significant decrease in hoverfly populations. More studies are needed in this area to recommend robust mitigation strategies. However, safe biopesticides regarding dipterans do exist in the literature. Based on the limited information, it is recommended to incorporate safe biopesticides into fields or to incorporate harmful pesticides when hoverfly densities are low to lessen the impact of population levels.

Table 2.
Examples of bioinsecticides and their impacts on insect pollinators other than honey bees and bumblebees.
Table 2.
Examples of bioinsecticides and their impacts on insect pollinators other than honey bees and bumblebees.
| Bioinsecticides | Active Ingredient | Non-Target Species | Effect Reported | Citation |
|---|---|---|---|---|
| ESALQ bacterial culture | Beauveria bassiana strain ESALQ-PL63 | Tetragonisca angustula | Guard bee behavior change | [116] |
| Biofungi 1 | Beauveria bassiana | Melipona scutellaris | High toxicity | [185] |
| Mycotrol | Beauveria bassiana | Megachile rotundata | Toxicity | [189] |
| Azadirachtin | Azadirachtin | Partamona helleri | Feeding avoidance behavior Reduced feeding | [198] |
| Cursor | Azadirachtin | Partamona helleri | Delayed development Deformed larvae | [199] |
| Bt Cotton | Cry1Ac | Lepidopterans | Reduced populations | [205] |
| MON810 | Cry1Ab | Inachis io | Reduced larval population | [206] |
| MON89034 × MON88017 | CryA.105 × Cry2Ab2 | Aglais urticae | Feeding impairment Slower development Increased mortality | [207] |
| MON810 Bt Maize | Cry1Ab | Aglais urticae | Reduced feeding Slower development | [208] |
| Tracer | Spinosad | Episyrphus balteatus | High mortality Failure to oviposit | [211] |
5. Public Perception and Current Scenario of Biopesticide Risk Assessment
The recent recognition that intensive synthetic pesticide application harms the environment and human health has pressured governments, organizations, and farmers to seek more eco-friendly methods for agriculture [27]. Even so, most developing countries depend on large-scale agriculture to feed their populations, usually at the cost of sustainability and environmental quality. This is reflected by a study in China that incentivized rice farmers to incorporate biopesticides through digital advertising, government subsidies, and free training [215]. Only 14% of the farmers incorporated biopesticides for a variety of reasons: poor storage stability, expensive production methods, and efficacy problems under varying environmental conditions. Public pressure for greener methods has also had an impact. The public community perceives biopesticides as being more environmentally friendly, target specific, decompose quickly without leaving harmful residue, and can be effective in smaller doses [37,128]. Some of farming community members argue that biopesticides require more applications, have a narrow spectrum, cannot be easily stored or obtained, require additional training, more specific timing, and sometimes lack the efficacy of their synthetic counterparts [215]. Current research ventures to improve formulations and active ingredients to improve their shelf life, efficacy, and eco-friendliness.
Biopesticides are considered pollinator friendly, but this is a generalization and is based on risk assessment research that focuses on only a few commercial pollinator species, such as honey bees and bumble bees [30]. This is problematic since different pollinator species differ in individual size, flight range, food sources, ecology, etc., affecting lethal dosage, sublethal dosage, mode of contamination, and amount of contamination. For example, a study testing the effect of the entomopathic fungi, B. bassiana, on honey bees found that the overall health of the hive was unaffected and it was therefore considered to be pollinator safe [181]. Yet, some individual bees in the same study displayed developmental and physiological abnormalities (<1%). Spinosad, another popular biopesticide, has been shown to be lethal to various pollinators, including honey bees, bumblebees, and solitary bees [126,188,196]. Studies focusing on non-commercial native bees have found that different biopesticides have varying effects on some pollinator species but are benign to others. For example, the stingless bee species, T. angustula, was exposed to B. bassiana [115]. The contaminated bees were not recognized by the guard bees near the entrance and were chased away. Another study compared the effects of B. bassiana on M. rotundata leafcutter bees to those on honey bees. The study found that M. rotundata had a lower lethal dose and exhibited double the mortality rate compared to honey bees [187]. These studies centered around a single biopesticide’s exposure across multiple bee species and found that each species responded differently. It appears that biopesticides have the potential to be detrimental to some pollinator species, making generalizations across taxa dangerous. In addition, specific biopesticide risk assessment procedures are non-existent [30]. The countries involved with the Organization for Economic Co-operation and Development (OECD) depend on the existing procedures established for synthetic pesticides, which are further limited to only a small number of bee species [30].
6. Conclusions and Recommendations
In general, synthetic pesticides are potentially detrimental to the environment and human health. This has led to a push for more eco-friendly agriculture and pest management, resulting in eco-regulatory bodies, organic agriculture, IPMs and IPPMs, and the development of biopesticides. Biopesticides are derived from living organisms such as bacteria, fungi, and viruses or are naturally occurring products such as essential oils. Biopesticides are generally considered safer for the environment, pollinator friendly, and effective in comparison with many types of synthetic pesticides. Even so, biopesticides might still pose a risk to non-target pollinators (Table 1) and could add to the decline in pollinators alone or in combination with other environmental stressors.
Current research focuses on increasing the efficacy and bettering the formulation of biopesticides to make them more desirable. Future research should be aimed at risk assessment between individual biopesticides and non-commercial pollinators. Additionally, the detrimental effects of biopesticides should be compared to their synthetic counterparts within the same context and ecosystem. Non-commercial pollinators also play an important role in pollination services, yet most of the pesticide risk assessment research focuses on commercial pollinators such as honey bees and bumblebees. The effects of biopesticides vary between pollinator species and creating generalizations based on flagship species could be dangerous. Pollinator habitat is also an important consideration. Urban regions may hold higher biodiversity of pollinators in comparison to agricultural land due higher floral and habitat resources. The risks that pollinators face also differ by consistency and proper use within the two settings. Lastly, harmful biopesticides should be regulated until further studies establish the toxicity risks they pose to pollinators, and the need to establish specific risk assessment procedures for biopesticides is necessary to standardize the data across multiple studies. The standardization of biopesticide risk assessment could aid in the preservation of pollinators by accurately identifying biohazardous biopesticides during the testing phase. Biopesticides are a promising alternative to synthetic pesticides but require in-depth observations to mitigate their sublethal effects on non-target species such as pollinators. Based on available information, the biopesticide with the least risk to pollinators in agricultural and urban settings is Bt. A review on this topic by Raymond and Wright [216] outlines 27 separate studies on various Bt toxins showing no effects on bees. Furthermore, a meta-analysis consisting of 25 unrelated studies also shows no effects on honeybees from Bt [30], possibly because bees lack the appropriate receptors for Bt toxins. In general, plant-based biopesticides have short half-lives and are less likely to contaminate non-target areas surrounding farms and urban environments, causing less harm to non-target organisms including pollinators. However future studies in this direction are needed to examine the potential non-target effects of such products.
Author Contributions
Conceptualization, J.C. and N.K.J.; methodology, J.C. and N.K.J.; investigation, J.C.; resources, N.K.J.; writing—original draft preparation, J.C.; writing—review and editing, J.C. and N.K.J.; visualization, N.K.J.; supervision, N.K.J.; project administration, N.K.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable as no new data were created or analyzed in this review article.
Acknowledgments
Authors are thankful to the UA System Division of Agriculture for support. The views and opinions expressed in this publication are those of the authors. Mention of companies or commercial products does not imply recommendation or endorsement over others not mentioned. Product names are mentioned solely to report factually on available data and to provide specific information.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Memmott, J.; Craze, P.G.; Waser, N.M.; Price, M.V. Global warming and the disruption of plant-pollinator interactions. Ecol. Lett. 2007, 10, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Cozien, R.J.; van der Niet, T.; Johnson, S.D.; Steenhuisen, S.L. Saurian surprise: Lizards pollinate South Africa’s enigmatic hidden Flower. Ecology 2019, 100, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Gomes, V.G.N.; Quirino, Z.G.M.; Machado, I.C. Pollination and seed dispersal of Melocactus elocactus ernestii (Cactaceae) by lizards: An example of double mutualism. Plant Biol. 2014, 16, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Kay, K.M.; Sargent, R.D. The role of animal pollination in plant speciation: Integrating ecology, geography, and genetics. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 637. [Google Scholar] [CrossRef]
- Free, J.B. Insect Pollination of Crops, 2nd ed.; Academic Press: Cambridge, MA, USA, 1993. [Google Scholar]
- David, T.I.; Storkey, J.; Stevens, C.J. Understanding how changing soil nitrogen affects plant-pollinator interactions. Arthropod-Plant Interact. 2019, 13, 671–684. [Google Scholar] [CrossRef]
- Brosi, B.J.; Briggs, H.M. Single pollinator species losses reduce floral fidelity and plant reproductive function. Proc. Natl. Acad. Sci. USA 2013, 110, 13044–13048. [Google Scholar] [CrossRef] [PubMed]
- Earaerts, M.; Clymans, R.; van Kerckvoorde, V.; Beliën, T. Nesting material, phenology and landscape complexity influence nesting success and parasite infestation of a trap nesting bee. Agric. Ecosyst. Environ. 2022, 332, 107951. [Google Scholar] [CrossRef]
- Reilly, J.R.; Artz, D.R.; Biddinger, D.; Bobiwash, K.; Boyle, N.K.; Brittain, C.; Brokaw, J.; Campbell, J.W.; Daniels, J.; Elle, E.; et al. Crop production in the USA is frequently limited by a lack of pollinators. Proc. R. Soc. B 2020, 287, 20200922. [Google Scholar] [CrossRef]
- Allen-Perkins, A.; Magrach, A.; Dainese, M.; Garibaldi, L.A.; Kleijn, D.; Rader, R.; Reilly, J.R. CropPol: A dynamic, open and global database on crop pollination. Ecology 2022, 103, e3614. [Google Scholar] [CrossRef]
- Kraemer, M.E.; Favi, F.D. Flower phenology and pollen choice of Osmia lignaria (Hymenoptera: Megachilidae) in Central Virginia. Environ. Entomol. 2005, 34, 1593–1605. [Google Scholar] [CrossRef]
- Gallai, N.; Salles, J.M.; Settele, J.; Vaissière, B.E. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 2009, 68, 810–821. [Google Scholar] [CrossRef]
- Kovács-Hostyánszki, A.; Földesi, R.; Báldi, A.; Endrédi, A.; Jordán, F. The vulnerability of plant-pollinator communities to honey bee decline: A comparative network analysis in different habitat types. Ecol. Indic. 2019, 97, 35–50. [Google Scholar] [CrossRef]
- Hung, K.L.J.; Kingston, J.M.; Albrecht, M.; Holway, D.A.; Kohn, J.R. The worldwide importance of honey bees as pollinators in natural habitats. Proc. R. Soc. B Biol. Sci. 2018, 285, 20172140. [Google Scholar] [CrossRef]
- Belsky, J.; Joshi, N.K. Assessing role of major drivers in recent decline of monarch butterfly population in North America. Front. Environ. Sci. 2018, 6, 86. [Google Scholar] [CrossRef]
- Belsky, J.; Joshi, N.K. Impact of biotic and abiotic stressors on managed and feral bees. Insects 2019, 10, 233. [Google Scholar] [CrossRef] [PubMed]
- Steffan-Dewenter, I. Importance of Habitat Area and Landscape Context for Species Richness of Bees and Wasps in Fragmented Orchard Meadows. Conserv. Biol. 2003, 17, 1036–1044. [Google Scholar] [CrossRef]
- Gómez-Ruiz, E.P.; Lacher, T.E. Climate change, range shifts, and the disruption of a pollinator-plant complex. Sci. Rep. 2019, 1, 14048. [Google Scholar] [CrossRef] [PubMed]
- Vasiliev, D.; Greenwood, S. The role of climate change in pollinator decline across the Northern Hemisphere is underestimated. Sci. Total Environ. 2021, 775, 145788. [Google Scholar] [CrossRef]
- Hegland, S.J.; Nielsen, A.; Lázaro, A.; Bjerknes, A.L.; Totland, Ø. How does climate warming affect plant-pollinator interactions? Ecol. Lett. 2009, 12, 184–195. [Google Scholar] [CrossRef]
- Van der Werf, H.M.G. Assessing the impact of pesticides on the environment. Ecosyst. Environ. 1996, 60, 81–96. [Google Scholar] [CrossRef]
- Fontcuberta, M.; Arqués, J.F.; Villalbí, J.R.; Martínez, M.; Centrich, F.; Serrahima, E.; Pineda, L.; Duran, J.; Casas, C. Chlorinated organic pesticides in marketed food: Barcelona, 2001–2006. Sci. Total Environ. 2008, 389, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Yadav, I.C.; Devi, N.L.; Syed, J.H.; Cheng, Z.; Li, J.; Zhang, G.; Jones, K.C. Current status of persistent organic pesticides residues in air, water, and soil, and their possible effect on neighboring countries: A comprehensive review of India. Sci. Total Environ. 2015, 511, 123–137. [Google Scholar] [CrossRef]
- Yu, H.; Liu, Y.; Shu, X.; Ma, L.; Pan, Y. Assessment of the spatial distribution of organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) in urban soil of China. Chemosphere 2020, 243, 125392. [Google Scholar] [CrossRef] [PubMed]
- Kafaei, R.; Arfaeinia, H.; Savari, A.; Mahmoodi, M.; Rezaei, M.; Rayani, M.; Sorial, G.A.; Fattahi, N.; Ramavandi, B. Organochlorine pesticides contamination in agricultural soils of southern Iran. Chemosphere 2020, 240, 124983. [Google Scholar] [CrossRef]
- Alshemmari, H.; Al-Shareedah, A.E.; Rajagopalan, S.; Talebi, L.A.; Hajeyah, M. Pesticides driven pollution in Kuwait: The first evidence of environmental exposure to pesticides in soils and human health risk assessment. Chemosphere 2021, 273, 129688. [Google Scholar] [CrossRef] [PubMed]
- Konda, L.N.; Czinkota, I.; Füleky, G.; Morovján, G. Modeling of Single-Step and Multistep Adsorption Isotherms of Organic Pesticides on Soil. J. Agric. Food Chem. 2002, 50, 7326–7331. [Google Scholar] [CrossRef]
- Bahlai, C.A.; Xue, Y.; McCreary, C.M.; Schaafsma, A.W.; Hallett, R.H. Choosing Organic Pesticides over Synthetic Pesticides May Not Effectively Mitigate Environmental Risk in Soybeans. PLoS ONE 2010, 5, e11250. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef]
- Cappa, F.; Baracchi, D.; Cervo, R. Biopesticides and insect pollinators: Detrimental effects, outdated guidelines, and future directions. Sci. Total Environ. 2022, 837, 155714. [Google Scholar] [CrossRef] [PubMed]
- Erler, S.; Eckert, J.H.; Steinert, M.; Alkassab, A.T. Impact of microorganisms and entomopathogenic nematodes used for plant protection on solitary and social bee pollinators: Host range, specificity, pathogenicity, toxicity, and effects of experimental parameters. Environ. Pollut. 2022, 302, 119051. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Singh, A. Biopesticides: Present Status and the Future Prospects. J. Biofertil. Biopestic. 2015, 6, e129. [Google Scholar] [CrossRef]
- Damalas, C.A.; Koutroubas, S.D. Current status and recent developments in biopesticide use. Agriculture 2018, 8, 13. [Google Scholar] [CrossRef]
- How We Assess Risks to Pollinators. Available online: https://www.epa.gov/pollinator-protection/how-we-assess-risks-pollinators (accessed on 14 January 2023).
- Kumar, J.; Ramlal, A.; Mallick, D.; Mishra, V. An overview of some biopesticides and their importance in plant protection for commercial acceptance. Plants 2021, 10, 1185. [Google Scholar] [CrossRef] [PubMed]
- Tanda, Y.; Kaya, H.K. Insect Pathology; Academic Press Inc.: Cambridge, MA, USA; Harcourt Brace Jovanovich Publishers: San Diego, CA, USA, 1993. [Google Scholar]
- Samada, L.H.; Tambunan, U.S.F. Biopesticides as Promising Alternatives to Chemical Pesticides: A Review of Their Current and Future Status. J. Biol. Sci. 2020, 20, 66–76. [Google Scholar] [CrossRef]
- Kachhawa, D. Microorganisms as a biopesticides. J. Entomol. Zool. Stud. 2017, 5, 468–473. [Google Scholar]
- Gupta, S.; Dikshit, A.K. Biopesticides: An ecofriendly approach for pest control. J. Biopestic. 2010, 3, 186. [Google Scholar]
- Srivastava, K.P.; Dhaliwal, G.S. A Textbook of Applied Entomology; Kalyani Publishers: New Delhi, India, 2010; p. 113. [Google Scholar]
- Rohrmann, G.F. Baculovirus Molecular Biology, 3rd ed.; National Center for Biotechnology Information: Bethesda, MD, USA, 2013. Available online: http://www.ncbi.nlm.nih.gov/books/NBK114593/ (accessed on 15 November 2023).
- Beas-Catena, A.; Sánchez-Mirón, A.; García-Camacho, F.; Contreras-Gómez, A.; Molina-Grima, E. Baculovirus Biopesticides: An Overview. J. Anim. Plant Sci. 2014, 24, 362–373. [Google Scholar]
- Dar, S.A.; Wani, S.H.; Mir, S.H.; Showkat, A.; Dolkar, T.; Dawa, T. Biopesticides: Mode of Action, Efficacy and Scope in Pest Management. J. Adv. Res. Biochem. Pharmacol. 2021, 4, 1–8. [Google Scholar]
- Hajek, A.E.; St. Leger, R.J. Interactions between fungal pathogens and insect hosts. Annu. Rev. Entomol. 1994, 39, 293–322. [Google Scholar] [CrossRef]
- Berini, F.; Katz, C.; Gruzdev, N.; Casartelli, M.; Tettamanti, G.; Marinelli, F. Microbial and viral chitinases: Attractive biopesticides for integrated pest management. Biotechnol. Adv. 2018, 36, 818–838. [Google Scholar] [CrossRef]
- Kumar, S. Biopesticides: A Need for Food and Environmental Safety. Biofertil. Biopestic. 2012, 3, e107. [Google Scholar] [CrossRef]
- Benton, R.; Vannice, K.S.; Vosshall, L.B. An essential role for a CD36-related receptor in pheromone detection in Drosophila. Nature 2007, 450, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.V.; Guerrero, A. New Pheromones and Insect Control Strategies. Vitam. Horm. 2010, 83, 493–520. [Google Scholar] [CrossRef] [PubMed]
- Butenandt; Beckmann, R.; Stamm, D.; Hecker, E. Über den Sexual-Lockstoff des Seidensspinners Bombyx mori. Reindarstellung und Konstitution. Z. Naturforsch 1959, 14, 283–284. [Google Scholar]
- Witzgall, P.; Kirsch, P.; Cork, A. Sex Pheromones and Their Impact on Pest Management. J. Chem. Ecol. 2010, 36, 80–100. [Google Scholar] [CrossRef] [PubMed]
- Witzgall, P.; Bäckman, A.-C.; Svensson, M.; Koch, U.; Rama, F.; El-Sayed, A.; Brauchli, J.; Arn, H.; Bengtsson, M.; Löfqvist, J. Behavioral observations of codling moth, Cydia pomonella, in orchards permeated with synthetic pheromone. BioControl 1999, 44, 211–237. [Google Scholar] [CrossRef]
- Weddle, P.W.; Welter, S.C.; Thomson, D. History of IPM in California pears—50 years of pesticide use and the transition to biologically intensive IPM. Pest Manag. Sci. 2009, 65, 1287–1292. [Google Scholar] [CrossRef]
- Joshi, N.K.; Hull, L.A.; Rajotte, E.G.; Krawczyk, G.; Bohnenblust, E. Evaluating sex-pheromone-and kairomone-based lures for attracting codling moth adults in mating disruption versus conventionally managed apple orchards in Pennsylvania. Pest Manag. Sci. 2011, 67, 1332–1337. [Google Scholar] [CrossRef]
- Smart, L.E.; Aradottir, G.I.; Bruce, T.J.A. Role of Semiochemicals in Integrated Pest Management. In Integrated Pest Management; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar] [CrossRef]
- Jones, O.T. Practical applications of pheromones and other semiochemicals. In Insect Pheromones and Their Use in Pest Management; Howse, P., Stevens, I., Jones, O., Eds.; Chapman and Hall: London, UK, 1998; pp. 261–355. [Google Scholar]
- Trematerra, P.; Colacci, M. Recent advances in management by pheromones of Thaumetopoea moths in urban parks and woodland recreational areas. Insects 2019, 10, 395. [Google Scholar] [CrossRef]
- Byers, J.A. Modelling female mating success during mass trapping and natural competitive attraction of searching males or females. Entomol. Exp. Appl. 2012, 145, 228–237. [Google Scholar] [CrossRef]
- Schlyter, F. A successful Case of Pheromone Mass Trapping of the Bark Beetle Ips duplicatus in a Forest Island, Analysed by 20-year Time-Series Data. Integr. Pest Manag. Rev. 2001, 6, 185–196. [Google Scholar] [CrossRef]
- Howse, P.; Stevens, I.; Jones, O. Insect Pheromones and Their Use in Pest Management; Chapman & Hill: London, UK, 1998; p. 639. [Google Scholar]
- Lance, D.R.; Leonard, D.S.; Mastro, V.C.; Walters, M.L. Mating Disruption as a Suppression Tactic in Programs Targeting Regulated Lepidopteran Pests in US. J. Chem. Ecol. 2016, 42, 590–605. [Google Scholar] [CrossRef]
- Welter, S.C.; Pickel, C.; Millar, J.; Cave, F.; van Steenwyk, R.A.; Dunley, J. Pheromone mating disruption offers selective management options for key pests. Calif. Agric. 2005, 59, 16–22. [Google Scholar] [CrossRef]
- Barrozo, R.B.; Gadenne, C.; Anton, S. Switching attraction to inhibition: Mating-induced reversed role of sex pheromone in an insect. J. Exp. Biol. 2010, 213, 2933–2939. [Google Scholar] [CrossRef]
- Borchert, D.M.; Walgenbach, J.F. Comparison of Pheromone-Mediated Mating Disruption and Conventional Insecticides for Management of Tufted Apple Bud Moth (Lepidoptera: Tortricidae). J. Econ. Entomol. 2000, 93, 769–776. [Google Scholar] [CrossRef]
- Onufrieva, K.S.; Thorpe, K.W.; Hickman, A.D.; Leonard, D.S.; Mastro, V.C.; Roberts, E.A. Gypsy moth mating disruption in open landscapes. Agric. For. Entomol. 2008, 10, 175–179. [Google Scholar] [CrossRef]
- Rizvi, S.A.H.; George, J.; Reddy, G.V.P.; Zeng, X.; Guerrero, A. Latest developments in insect sex pheromone research and its application in agricultural pest management. Insects 2021, 12, 484. [Google Scholar] [CrossRef]
- Nakashima, Y.; Ida, T.Y.; Powell, W.; Pickett, J.A.; Birkett, M.A.; Taki, H.; Takabayashi, J. Field evaluation of synthetic aphid sex pheromone in enhancing suppression of aphid abundance by their natural enemies. BioControl 2016, 61, 485–496. [Google Scholar] [CrossRef][Green Version]
- Bezabih, G.; Satheesh, N.; Workneh Fanta, S.; Wale, M.; Atlabachew, M. Reducing Postharvest Loss of Stored Grains Using Plant-Based Biopesticides: A Review of Past Research Efforts. Adv. Agric. 2022, 2022, 6946916. [Google Scholar] [CrossRef]
- Khursheed, A.; Rather, M.A.; Jain, V.; Wani, R.; Rasool, S.; Nazir, R.; Malik, A.; Majid, A. Plant based natural products as potential ecofriendly and safer biopesticides: A comprehensive overview of their advantages over conventional pesticides, limitations and regulatory aspects. Microb. Pathog. 2022, 173, 105854. [Google Scholar] [CrossRef]
- Khater, H.F. Prospects of Botanical Biopesticides in Insect Pest Management. Pharmacologia 2012, 3, 641–656. [Google Scholar]
- Prakash, A.; Rao, J. Botanical Insecticides in Agriculture, 1st ed.; CRC Press Inc.: Baton Rouge, FL, USA, 1997; p. 461. [Google Scholar]
- Prakash, A.; Rao, J. Evaluation of plant products as antifeedants against the rice storage pests. In Proceedings of the Symposium on Residues and Environmental Pollution, Muzaffarnagar, India, 2 October 1986; pp. 201–205. [Google Scholar]
- Jones, W.P.; Kinghorn, D. Extraction of Plant Secondary Metabolites. In Natural Products Isolation, 2nd ed.; Sarker, S.D., Latif, Z., Gray, A.I., Eds.; Methods in Biotechnology Volume 20; Humana Press Inc.: Totowa, NJ, Canada, 2006. [Google Scholar]
- Lopes, A.I.F.; Monteiro, M.; Araújo, A.R.L.; Rodrigues, A.R.O.; Castanheira, E.M.S.; Pereira, D.M.; Olim, P.; Fortes, A.G.; Gonçalves, M.S.T. Cytotoxic Plant Extracts towards Insect Cells: Bioactivity and Nanoencapsulation Studies for Application as Biopesticides. Molecules 2020, 25, 5855. [Google Scholar] [CrossRef] [PubMed]
- Casida, J.E.; Quistad, G.B. Pyrethrum Flowers Production, Chemistry, Toxicology and Uses; Oxford University Press: New York, NY, USA, 1995; p. 356. ISBN 0195082109. [Google Scholar]
- Singh, A.; Srivastava, V.K. Toxic Effect of Synthetic Pyrethroid Permethrin on the Enzyme System of the Freshwater Fish Channa striatus. Pergamon Chemosphere 1999, 39, 1951–1956. [Google Scholar] [CrossRef]
- Hayes, W.J. Pesticides Studied in Man; Williams and Wilkins: Baltimore, MD, USA, 1982; p. 672. ISBN 9780683038965. [Google Scholar]
- Morse, S.; Ward, A.; Mcnamara, N.; Denholm, I. Exploring the Factors that Influence the Uptake of Botanical Insecticides by Farmers: A Case Study of Tobacco-Based Products in Nigeria. Exp. Agric. 2022, 38, 469–479. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical Insecticides, Deterrents, and Repellents in Modern Agriculture and an Increasingly Regulated World. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef]
- Ware, G.W.; Whitacre, D.M. A Division of Meister Media Worldwide; MeisterPro Information Resources: Willoughby, OH, USA, 2004. [Google Scholar]
- Tunaz, H. Insect Growth Regulators for Insect Pest Control. Turk. J. Agric. For. 2004, 28, 377–387. [Google Scholar]
- Asai, T.; Kajihara, O.; Fukada, M.; Maekawa, S. Studies no the Mode of Action of Buprofezin II. Effects on Reproduction of the Brown Planthopper, Nilaparvata lugens (Homoptera: Delphacidae). Appl. Entomol. Zool. 1985, 20, 111–117. [Google Scholar] [CrossRef]
- Ellsworth, P.C.; Martinez-Carillo, J.L. IPM for Bemisia tabaci: A case study from North America. Crop Prot. 2001, 20, 853–869. [Google Scholar] [CrossRef]
- Miyamoto, J.; Hirano, M.; Takimoto, Y.; Hatakoshi, M. Insect growth regulators for pest control, with emphasis on juvenile hormone analogs: Present status and future prospects. ACS Symp. Ser. 1993, 524, 144–168. [Google Scholar]
- Eto, M. Biochemical mechanism of insecticidal activities. In Chemistry of Plant Protection; Haug, G., Hoffman, H., Eds.; Springer: Berlin/Heidelberg, Germany, 1990; Volume 6, pp. 65–107. [Google Scholar]
- Koçak, E.; Kilincer, N. Investigations on the effects of juvenile hormone analogue methoprene to of cotton leafworm [Spodoptera littoralis (Boisd.) (Lep.: Noctuidae)]: I. Effects on pupae and eggs. Bitki Koruma Bul. 1997, 37, 163–172. [Google Scholar]
- Smith, C.A. Searching for safe methods of flea control. J. Am. Vet. Med. Assoc. 1995, 206, 1137–1143. [Google Scholar] [CrossRef]
- Barton, K.A.; Whiteley, H.R.; Yang, N.S. Bacillus thuringiensis 6-Endotoxin Expressed in Transgenic Nicotiana tabacum Provides Resistance to Lepidopteran Insects. Plant Physiol. 1987, 85, 1103–1109. [Google Scholar] [CrossRef]
- Clark, B.W.; Phillips, T.A.; Coats, J.R. Environmental Fate and Effects of Bacillus thuringiensis (Bt) Proteins from Transgenic Crops: A Review. J. Agric. Food Chem. 2005, 53, 4643–4653. [Google Scholar] [CrossRef]
- Raymond Park, J.; McFarlane, I.; Hartley Phipps, R.; Ceddia, G. The role of transgenic crops in sustainable development. Plant Biotechnol. J. 2011, 9, 2–21. [Google Scholar] [CrossRef]
- Bravo, A.; Gill, S.S.; Soberón, M. Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon 2007, 49, 423–435. [Google Scholar] [CrossRef]
- EPA Registers Innovative Tool to Control Corn Rootworm|US EPA. Epa.Gov. Available online: https://www.epa.gov/pesticide-registration/epa-registers-innovative-tool-control-corn-rootworm (accessed on 13 January 2024).
- Li, X.; Liu, X.; Lu, W.; Yin, X.; An, S. Application progress of plant-mediated RNAi in pest control. Front. Bioeng. Biotechnol. 2022, 10, 963026. [Google Scholar] [CrossRef]
- Gašić, S.; Tanović, B. Biopesticide Formulations, Possibility of Application, and Future Trends. Pestic. Phytomed. 2013, 28, 97–102. [Google Scholar] [CrossRef]
- Butu, M.; Rodino, S.; Butu, A. Biopesticide formulations-current challenges and future perspectives. In Biopesticides; Woodhead Publishing: Cambridge, UK, 2022; pp. 19–29. [Google Scholar]
- Knowles, A. Trends in Pesticide Formulations; Agrow Reports; PJB Publications Ltd.: London, UK, 2001; Volume D215, pp. 89–92. [Google Scholar]
- Knowles, A. New Developments in Crop Protection Product Formulation; Agrow Report: London, UK, 2005; pp. 153–156. [Google Scholar]
- Lyn, M.E.; Burnett, D.; Garcia, A.R.; Gray, R. Interaction of Water with Three Granular Biopesticide Formulations. J. Agric. Food Chem. 2010, 58, 1804–1814. [Google Scholar] [CrossRef] [PubMed]
- Woods, T.S. Pesticide Formulations. In Encyclopedia of Agrochemicals; AGR 185; Wiley & Sons: New York, NY, USA, 2003; pp. 1–11. [Google Scholar]
- Knowles, A. Recent developments of safer formulations of agrochemicals. Environmentalist 2008, 28, 35–44. [Google Scholar] [CrossRef]
- Vernner, R.; Bauer, P. Q-TEO, a formulation concept that overcomes the incompatibility between water and oil. Pfalz. Nachrichten Bayer 2007, 60, 7–26. [Google Scholar]
- Brar, S.K.; Verma, M.; Tyagi, R.D.; Valero, J.R. Recent advances in downstream processing and formulations of Bacillus thuringiensis-based biopesticides. Process Biochem. 2006, 41, 323–342. [Google Scholar] [CrossRef]
- Lutz, W.; KC, S. Dimensions of global population projections: What do we know about future population trends and structures? Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2779–2791. [Google Scholar] [CrossRef] [PubMed]
- 68% of the World Population Projected to Live in Urban Areas by 2050, Says UN. Available online: https://www.un.org/development/desa/en/news/population/2018-revision-of-world-urbanization-prospects.html (accessed on 14 October 2023).
- Understanding Global Change. Available online: https://ugc.berkeley.edu/background-content/population-growth/ (accessed on 14 October 2023).
- Zhu, F.; Lavine, L.; O’neal, S.; Lavine, M.; Foss, C.; Walsh, D. Insecticide Resistance and Management Strategies in Urban Ecosystems. Insects 2016, 7, 2. [Google Scholar] [CrossRef]
- Kass, D.; McKelvey, W.; Carlton, E.; Hernandez, M.; Chew, G.; Nagle, S.; Garfinkel, R.; Clarke, B.; Tiven, J.; Espino, C.; et al. Effectiveness of an integrated pest management intervention in controlling cockroaches, mice, and allergens in New York City public housing. Environ. Health Perspect. 2009, 117, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Ricker-Gilbert, J.; Norton, G.W.; Alwang, J.; Miah, M.; Feder, G. Cost- Effectiveness of Alternative Integrated Pest Management Extension Methods: An Example from Bangladesh. Appl. Econ. Perspect. Policy 2008, 30, 252–269. [Google Scholar] [CrossRef]
- Arthropods Resistant to Pesticides Database (ARPD). Available online: http://www.pesticideresistance.org (accessed on 13 October 2023).
- Zawislak, J.; Lorenz, G.; Adamczyk, J.; Wiedenmann, R.; Joshi, N.K. Proportion of commodity crop pollens and pesticide contamination in honey bee diets in two different landscapes. Environ. Adv. 2021, 5, 100116. [Google Scholar] [CrossRef]
- Blanford, S.; Chan, B.H.K.; Jenkins, N.; Sim, D.; Turner, R.J.; Read, A.F.; Thomas, M.B. Fungal pathogen reduces potential for malaria transmission. Science 2005, 308, 1638–1641. [Google Scholar] [CrossRef]
- Scholte, E.-J.; Ng’habi, K.; Kihonda, J.; Takken, W.; Paaijmans, K.; Abdulla, S.; Killeen, G.F.; Knols, B.G.J. An entomopathogenic fungus for control of adult African malaria mosquitoes. Science 2005, 308, 1641–1642. [Google Scholar] [CrossRef]
- Wang, C.; Singh, N.; Cooper, R. Efficacy of an essential oil-based pesticide for controlling bed bug (Cimex lectularius) infestations in apartment buildings. Insects 2014, 5, 849–859. [Google Scholar] [CrossRef]
- Regalia® Biofungicide—Control Powdery Mildew, Increase Crop Performance. Available online: https://marronebio.com/products/regalia/#efficacy\ (accessed on 14 October 2023).
- GrowSafe Bio-Pesticide. Available online: https://www.agromagen.com/product-overview/ (accessed on 14 October 2023).
- Rango Broad Range Control. Available online: https://www.terrameraagriculture.com/rango (accessed on 14 October 2023).
- Almeida, F.C.R.; Magalhães, D.M.; Favaris, A.P.; Rodríguez, J.; Azevedo, K.E.X.; Bento, J.M.; Alves, D.A. Side effects of a fungus-based biopesticide on stingless bee guarding behavior. Chemosphere 2022, 287, 132147. [Google Scholar] [CrossRef]
- Cappa, F.; Petrocelli, I.; Dani, F.R.; Dapporto, L.; Giovannini, M.; Silva-Castellari, J.; Turillazzi, S.; Cervo, R. Natural biocide disrupts nestmate recognition in honeybees. Sci. Rep. 2019, 9, 3171. [Google Scholar] [CrossRef]
- Carlesso, D.; Smargiassi, S.; Sassoli, L.; Cappa, F.; Cervo, R.; Baracchi, D. Exposure to a biopesticide interferes with sucrose responsiveness and learning in honey bees. Sci. Rep. 2020, 10, 19929. [Google Scholar] [CrossRef]
- Wenzel, A.; Grass, I.; Belavadi, V.V.; Tscharntke, T. How urbanization is driving pollinator diversity and pollination—A systematic review. Biol. Conserv. 2020, 241, 108321. [Google Scholar] [CrossRef]
- Patenković, A.; Tanasković, M.; Erić, P.; Erić, K.; Mihajlović, M.; Stanisavljević, L.; Davidović, S. Urban ecosystem drives genetic diversity in feral honey bee. Sci. Rep. 2022, 12, 17692. [Google Scholar] [CrossRef] [PubMed]
- Theodorou, P.; Radzevičiūtė, R.; Lentendu, G.; Kahnt, B.; Husemann, M.; Bleidorn, C.; Settele, J.; Schweiger, O.; Grosse, I.; Wubet, T.; et al. Urban areas as hotspots for bees and pollination but not a panacea for all insects. Nat. Commun. 2020, 11, 576. [Google Scholar] [CrossRef]
- Samuelson, A.E.; Gill, R.J.; Brown, M.J.F.; Leadbeater, E. Lower bumblebee colony reproductive success in agricultural compared with urban environments. Proc. R. Soc. B 2018, 285, 20180807. [Google Scholar] [CrossRef] [PubMed]
- Samuelson, A.E.; Schürch, R.; Leadbeater, E. Dancing bees evaluate central urban forage resources as superior to agricultural land. J. Appl. Ecol. 2022, 59, 79–88. [Google Scholar] [CrossRef]
- Baldock, K.C.R.; Goddard, M.A.; Hicks, D.M.; Kunin, W.E.; Mitschunas, N.; Morse, H.; Osgathorpe, L.M.; Potts, S.G.; Robertson, K.M.; Scott, A.V.; et al. A systems approach reveals urban pollinator hotspots and conservation opportunities. Nat. Ecol. Evol. 2019, 3, 363. [Google Scholar] [CrossRef] [PubMed]
- Siviter, H.; Pardee, G.L.; Baert, N.; McArt, S.; Jha, S.; Muth, F. Wild bees are exposed to low levels of pesticides in urban grasslands and community gardens. Sci. Total Environ. 2023, 858, 159839. [Google Scholar] [CrossRef] [PubMed]
- Challa, G.K.; Firake, D.M.; Behere, G.T. Bio-pesticide applications may impair the pollination services and survival of foragers of honey bee, Apis cerana Fabricius in oilseed brassica. Environ. Pollut. 2019, 249, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Hansen, B.; Alrøe, H.F.; Kristensen, E.S. Approaches to assess the environmental impact of organic farming with particular regard to Denmark. Agric. Ecosyst. Environ. 2001, 83, 11–26. [Google Scholar] [CrossRef]
- Liu, X.; Cao, A.; Yan, D.; Ouyang, C.; Wang, Q.; Li, Y. Overview of mechanisms and uses of biopesticides. Int. J. Pest Manag. 2021, 67, 65–72. [Google Scholar] [CrossRef]
- Biddinger, D.; Rajotte, E.G.; Joshi, N.K. Integrating pollinator health into tree fruit IPM—A case study of Pennsylvania apple production. In The Pollination of Cultivated Plants: A Compendium for Practitioners, 2nd ed.; FAO: Rome, Italy, 2018; Volume 1, pp. 69–83. [Google Scholar]
- Joshi, N.K.; Leslie, T.; Rajotte, E.G.; Biddinger, D.J. Environmental impacts of reduced-risk and conventional pesticide programs differ in commercial apple orchards, but similarly influence pollinator community. Chemosphere 2020, 240, 124926. [Google Scholar] [CrossRef] [PubMed]
- Belien, T.; Raymaekers, S.; Eeraerts, M.; Mommaerts, V.; Claus, G.; Bogen, C.; Piot, N.; Smagghe, G.; Spanoghe, P.; Bylemans, D. Towards Integrated Pest and Pollinator Management in Intensive Pear Cultivation: A Case Study from Belgium. Insects 2021, 12, 901. [Google Scholar] [CrossRef] [PubMed]
- Flöhr, A.; Stenberg, J.A.; Egan, P.A. The Joint Economic Impact Level (jEIL): A Decision Metric for Integrated Pest and Pollinator Management. In Integrative Biological Control; Progress in Biological Control; Springer: Cham, Switzerland, 2020; pp. 17–38. [Google Scholar] [CrossRef]
- Raine, N.E.; Rundlöf, M. Pesticide Exposure and Effects on Non-Apis Bees. Annu. Rev. Entomol. 2024, 69, 551–576. [Google Scholar] [CrossRef] [PubMed]
- Egan, P.A.; Dicks, L.V.; Hokkanen, H.M.T.; Stenberg, J.A. Delivering Integrated Pest and Pollinator Management (IPPM). Trends Plant Sci. 2020, 25, 577–589. [Google Scholar] [CrossRef]
- Senapathi, D.; Goddard, M.A.; Kunin, W.E.; Baldock, K.C.R. Landscape impacts on pollinator communities in temperate systems: Evidence and knowledge gaps. Funct. Ecol. 2016, 31, 26–37. [Google Scholar] [CrossRef]
- Le Féon, V.; Burel, F.; Chifflet, R.; Henry, M.; Vaissière, A.R.; Vaissière, B.E.; Baudry, J. Solitary bee abundance and species richness in dynamic agricultural landscapes. Agric. Ecosyst. Environ. 2013, 166, 94–101. [Google Scholar] [CrossRef]
- Millard, J.; Outhwaite, C.L.; Kinnersley, R.; Freeman, R.; Gregory, R.D.; Adedoja, O.; Gavini, S.; Kioko, E.; Kuhlmann, M.; Ollerton, J.; et al. Global effects of land-use intensity on local pollinator biodiversity. Nat. Commun. 2021, 12, 2902. [Google Scholar] [CrossRef]
- Wezel, A. Agroecological Practices for Sustainable Agriculture: Principles, Applications, and Making the Transition; World Scientific: Singapore, 2017. [Google Scholar]
- Giunti, G.; Benelli, G.; Palmeri, V.; Laudani, F.; Ricupero, M.; Ricciardi, R.; Maggi, F.; Lucchi, A.; Guedes, R.N.C.; Desneux, N.; et al. Non-target effects of essential oil-based biopesticides for crop protection: Impact on natural enemies, pollinators, and soil invertebrates. Biol. Control. 2022, 176, 105071. [Google Scholar] [CrossRef]
- Goerzen, D.; Erlandson, M.; Moore, K. Effect of two insect viruses and two entomo-pathogenic fungi on larval and pupal development in the alfalfa leafcutting bee, Megachile rotundata (Hymenoptera: Megachilidae). Can. Entomol. 1990, 122, 1039–1040. [Google Scholar] [CrossRef]
- Park, M.; Danforth, B.; Losey, J.; Agnello, A.; Biddinger, D.; Rajotte, E.; Vaughan, M.; Dollar, J. IPM Resource: Wild Pollinators of Eastern Apple Orchards and How to Conserve Them. 2012. Available online: http://www.northeastipm.org/park2012 (accessed on 19 February 2024).
- Dutka, A.; McNulty, A.; Williamson, S.M. A new threat to bees? Entomopathogenic nematodes used in biological pest control cause rapid mortality in Bombus terrestris. Peer J. 2015, 3, 1413. [Google Scholar] [CrossRef]
- Honey Bees. Available online: https://extension.umd.edu/resource/honey-bees/ (accessed on 16 January 2024).
- Siregar, E.H.; Atmowidi, T.; Kahono, S. Diversity and Abundance of Insect Pollinators in Different Agricultural Lands in Jambi, Sumatera. HAYATI J. Biosci. 2016, 23, 13–17. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Kremen, C.; Williams, N.M.; Aizen, M.A.; Gemmill-Herren, B.; Lebuhn, G.; Minckley, R.; Packer, L.; Potts, S.G.; Roulston, T.; Steffan-Dewenter, I.; et al. Pollination and other ecosystem services produced by mobile organisms: A conceptual framework for the effects of land-use change. Ecol. Lett. 2007, 10, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Miles, M.J. The effects of spinosad, a naturally derived insect control agent, to the honeybee (Apis melifera) The effects of spinosad, a naturally derived insect control agent to the honeybee. Bull. Insectol. 2003, 56, 119–124. [Google Scholar]
- Miles, J.M.; Alix, A.; Schmitzer, S.; Bourgouin, C. Effects of spinosad on honey bees (Apis mellifera): Findings from over ten years of testing and commercial use. In Proceedings of the 11th International Symposium of the ICP-BR Bee Protection Group, Wageningen, The Netherlands, 2–4 November 2011. [Google Scholar] [CrossRef]
- Bailey, J.; Scott-Dufree, C.; Harris, R.; Tolman, J.; Harris, B. Contact and oral toxicity to honey bees (Apis mellifera) of agents registered for use for sweet corn insect control in Ontario, Canada. Apidologie 2005, 36, 623–633. [Google Scholar] [CrossRef]
- Cabrera-Marín, N.V.; Liedo, P.; Vandame, R.; Sánchez, D. Foraging Allocation in the Honey Bee, Apis mellifera L. (Hymenoptera, Apidae), Tuned by the Presence of the Spinosad-Based Pesticide GF-120. Neotrop. Entomol. 2015, 44, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.P.; Fernandes, K.M.; Tomé, H.V.V.; Gonçalves, W.G.; Miranda, F.R.; Serrão, J.E.; Martins, G.F. Spinosad-mediated effects on the walking ability, midgut, and Malpighian tubules of Africanized honey bee workers. Pest Manag. Sci. 2018, 74, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Christen, V.; Krebs, J.; Bünter, I.; Fent, K. Biopesticide spinosad induces transcriptional alterations in genes associated with energy production in honey bees (Apis mellifera) at sublethal concentrations. J. Hazard. Mater. 2019, 378, 120736. [Google Scholar] [CrossRef]
- Xavier, V.M.; Message, D.; Picanço, M.C.; Chediak, M.; Santana Jú, P.A.; Ramos, R.S.; Lio, J.; Martins, C. Acute Toxicity and Sublethal Effects of Botanical Insecticides to Honey Bees. J. Insect Sci. 2015, 15, 137. [Google Scholar] [CrossRef]
- Ramsey, S.D.; Ochoa, R.; Bauchan, G.; Gulbronson, C.; Mowery, J.D.; Cohen, A.; Lim, D.; Joklik, J.; Cicero, J.M.; Ellis, J.D.; et al. Varroa destructor feeds primarily on honey bee fat body tissue and not hemolymph. Proc. Natl. Acad. Sci. USA 2019, 116, 1792–1801. [Google Scholar] [CrossRef]
- S Peng, C.Y.; Trinh, S.; Lopez, J.E.; Mussen, E.C.; Hung, A.; Chuang, R. The effects of azadirachtin on the parasitic mite, Varroa jacobsoni and its host honey bee (Apis mellifera). J. Apic. Res. 2015, 39, 159–168. [Google Scholar] [CrossRef]
- González-Gómez, R.; Otero-Colina, G.; Villanueva-Jiménez, J.A.; Santillán-Galicia, M.T.; Beatriz Peña-Valdivia, C.; Santizo-Rincón, J.A.; Santizo, J.A. Effects of neem (Azadriachta indica) on honey bee workers and queens, while applied to control Varroa destructor. J. Apic. Res. 2016, 55, 413–421. [Google Scholar] [CrossRef]
- Thompson, H.M.; Wilkins, S.; Battersby, A.H.; Waite, R.J.; Wilkinson, D. The Effects of Four Insect Growth-Regulating (IGR) Insecticides on Honeybee (Apis mellifera L.) Colony Development, Queen Rearing and Drone Sperm Production. Ecotoxicology 2005, 14, 757–769. [Google Scholar] [CrossRef] [PubMed]
- Tschoeke, P.H.; Oliveira, E.; Dalcin, M.S.; Cristina, M.; Silveira-Tschoeke, A.C.; Sarmento, R.A.; Santos, G.R. Botanical and synthetic pesticides alter the flower visitation rates of pollinator bees in Neotropical melon fields. Environ. Pollut. 2019, 251, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Meikle, W.G.; Mercadier, G.; Holst, N.; Girod, V. Impact of two treatments of a formulation of Beauveria bassiana (Deuteromycota: Hyphomycetes) conidia on Varroa mites (Acari: Varroidae) and on honeybee (Hymenoptera: Apidae) colon. Exp. Appl. Acarol. 2008, 46, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Meikle, W.G.; Mercadier, G.; Guermache, F.; Bon, M.-C. Pseudomonas contamination of a fungus-based biopesticide: Implications for honey bee (Hymenoptera: Apidae) health and Varroa mite (Acari: Varroidae) control. Biol. Control 2012, 60, 312–320. [Google Scholar] [CrossRef]
- Shaw, K.E.; Davidson, G.; Clark, S.J.; Ball, B.V.; Pell, J.K.; Chandler, D.; Sunderland, K.D. Laboratory bioassays to assess the pathogenicity of mitosporic fungi to Varroa destructor (Acari: Mesostigmata), an ectoparasitic mite of the honeybee, Apis mellifera. Biol. Control 2002, 24, 266–276. [Google Scholar] [CrossRef]
- Butt, T.; Ibrahim, L.; Ball, B.; Clark, S. Pathogenicity of the entomogenous fungi Metarhizium anisopliae and Beauveria bassiana against crucifer pests and the honey bee. Biocontrol Sci. Technol. 1994, 4, 207–214. [Google Scholar] [CrossRef]
- Abdel Rasoul, M.A.; Eid KS, A.; Marei GI, K. Impacts of multiple applications with Biofly (Beauveria bassiana) and Spintor® (Spinosad) on Honey Bee (Apis mellifera) Larvae. J. Plant Prot. Pathol. 2013, 4, 49–66. [Google Scholar] [CrossRef]
- Karise, R.; Muljar, R.; Smagghe, G.; Kaart, T.; Kuusik, A.; Dreyersdorff, G.; Williams, I.H.; Mänd, M. Sublethal effects of kaolin and the biopesticides Prestop-Mix and BotaniGard on metabolic rate, water loss and longevity in bumble bees (Bombus terrestris). J. Pest Sci. 2016, 89, 171–178. [Google Scholar] [CrossRef]
- Abdel razik, M.A.A. Toxicity and side effects of some insecticides applied incotton fields on Apis mellifera. Environ. Sci. Pollut. Res. 2019, 26, 4987–4996. [Google Scholar] [CrossRef]
- Demirozer, O.; Uzun, A.; Gosterit, A. Lethal and sublethal effects of different biopesticides on Bombus terrestris (Hymenoptera: Apidae). Apidologie 2022, 53, 24. [Google Scholar] [CrossRef]
- Gradish, A.E.; Scott-Dupree, C.D.; Frewin, A.J.; Cutler, G.C. Lethal and sublethal effects of some insecticides recommended for wild blueberry on the pollinator Bombus impatiens. Can. Entomol. 2012, 144, 478–486. [Google Scholar] [CrossRef]
- Cressman, A.; Boyle, N.; Amsalem, E. The Bumble Bee Lifestyle; PennState Extension: State College, PA, USA, 2021. [Google Scholar]
- Konrad, R.; Connor, M.; Ferry, N.; Gatehouse, A.M.R.; Babendreier, D. Impact of transgenic oilseed rape expressing oryzacystatin-1 (OC-1) and of insecticidal proteins on longevity and digestive enzymes of the solitary bee Osmia bicornis. J. Insect Physiol. 2009, 55, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Ramanaidu, K.; Cutler, G.C. Different toxic and hormetic responses of Bombus impatiens to Beauveria bassiana, Bacillus subtilis and spirotetramat. Pest Manag. Sci. 2013, 69, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Mommaerts, V.; Sterk, G.; Hoffmann, L.; Smagghe, G. A laboratory evaluation to determine the compatibility of microbiological control agents with the pollinator Bombus terrestris. Pest Manag. Sci. 2009, 65, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Mommaerts, V.; Jans, K.; Smagghe, G. Impact of Bacillus thuringiensis strains on survival, reproduction and foraging behaviour in bumblebees (Bombus terrestris). Pest Manag. Sci. 2010, 66, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Malone, L.A.; Scott-Dupree, C.D.; Todd, J.H.; Ramankutty, P. No sub-lethal toxicity to bumblebees, Bombus terrestris, exposed to Bt-corn pollen, captan and novaluron. N. Z. J. Crop Hortic. Sci. 2010, 35, 435–439. [Google Scholar] [CrossRef]
- Koskor, E.; Muljar, R.; Drenkhan, K.; Karise, R.; Bender, A.; Viik, E.; Luik, A.; Mänd, M. The chronic effect of the botanical insecticide Neem EC on the pollen forage of the bumble bee Bombus terrestris L. Agron. Res. 2009, 7, 341–346. [Google Scholar]
- Barbosa, W.F.; de Meyer, L.; Guedes, R.N.C.; Smagghe, G. Lethal and sublethal effects of azadirachtin on the bumblebee Bombus terrestris (Hymenoptera: Apidae). Ecotoxicology 2015, 24, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Akkoç, S.; Karaca, İ.; Karaca, G. Effects of Some Entomopathogen Fungi on Apis mellifera L. and Bombus terrestris L. Süleyman Demirel Univ. J. Nat. Appl. Sci. 2019, 23, 433–439. [Google Scholar] [CrossRef]
- Demirozer, O.; Uzun, A.; Yanik, G.; Bulus, I.Y.; Gosterit, A. Investigation of the efficacy of some biopesticides by food exposure on Bombus terrestris L. (Hymenoptera: Apidae). J. Apic. Res. 2023, 62, 1153–1157. [Google Scholar] [CrossRef]
- Hokkanen, H.M.; Zeng, Q.-Q.; Menzler-Hokkanen, I. Assessing the impacts of Metarhizium and Beauveria on bumblebees. In Environmental Impacts of Microbial Insecticides; Springer: Dordrecht, The Netherlands, 2003; pp. 63–71. [Google Scholar]
- Kapongo, J.P.; Shipp, L.; Kevan, P.; Sutton, J.C. Co-vectoring of Beauveria bassiana and Clonostachys rosea by bumble bees (Bombus impatiens) for control of insect pests and suppression of grey mould in greenhouse tomato and sweet pepper. Biol. Control 2008, 46, 508–514. [Google Scholar] [CrossRef]
- Smagghe, G.; De Meyer, L.; Meeus, I.; Mommaerts, V. Safety and acquisition potential of Metarhizium anisopliae in entomovectoring with bumble bees, Bombus terrestris. J. Econ. Entomol. 2013, 106, 277–282. [Google Scholar] [CrossRef]
- Al Mazra’awi, M.; Shipp, J.; Broadbent, A.; Kevan, P. Dissemination of Beauveria bassiana by honey bees (Hymenoptera: Apidae) for control of tarnished plant bug (Hemiptera: Miridae) on canola. Environ. Entomol. 2006, 35, 1569–1577. [Google Scholar] [CrossRef]
- Yale Environment 360. Scientists Create the First Global Map of Bee Distribution—Yale E360; Yale School of Environment: New Haven, CT, USA, 2020. [Google Scholar]
- Voulgari-Kokota, A.; McFrederick, Q.S.; Steffan-Dewenter, I.; Keller, A. Drivers, Diversity, and Functions of the Solitary-Bee Microbiota. Trends Microbiol. 2019, 27, 1034–1044. [Google Scholar] [CrossRef] [PubMed]
- Kline, O.; Phan, N.T.; Porras, M.F.; Chavana, J.; Little, C.Z.; Stemet, L.; Acharya, R.S.; Biddinger, D.J.; Reddy, G.V.P.; Rajotte, E.G.; et al. Biology, Genetic Diversity, and Conservation of Wild Bees in Tree Fruit Orchards. Biology 2023, 12, 31. [Google Scholar] [CrossRef]
- de Conceição, P.J.; de Neves, C.M.L.; da Sodré, G.S.; de Carvalho, C.A.L.; Souza, A.V.; Ribeiro, G.S.; de Pereira, R.C. Susceptibility of Melipona scutellaris latreille, 1811 (Hymenoptera: Apidae) to Beauveria bassiana (Bals.) Vuill. Sociobiology 2014, 61, 184–188. [Google Scholar] [CrossRef]
- Toledo-Hernández, R.A.; Ruíz-Toledo, J.; Toledo, J.; Sánchez, D. Effect of three entomopathogenic fungi on three species of stingless bees (Hymenoptera: Apidae) under laboratory conditions. J. Econ. Entomol. 2016, 109, 1015–1019. [Google Scholar] [CrossRef]
- James, R.; McGuire, M.; Leland, J.E. Susceptibility of adult alfalfa leaf-cutting bee and honey bees to a microbial control agent, Beauveria bassiana. Southwest Entomol. 2012, 37, 13–21. [Google Scholar] [CrossRef]
- Piovesan, B.; Padilha, A.C.; Morais, M.C.; Botton, M.; Grützmacher, A.D.; Zotti, M.J. Effects of insecticides used in strawberries on stingless bees Melipona quadrifasciata and Tetragonisca fiebrigi (Hymenoptera: Apidae). Environ. Sci. Pollut. Res. 2020, 27, 42472–42480. [Google Scholar] [CrossRef]
- Marques, R.D.; Lima, M.A.P.; Marques, R.D.; Bernardes, R.C. A Spinosad-Based Formulation Reduces the Survival and Alters the Behavior of the Stingless Bee Plebeia lucii. Neotrop. Entomol. 2020, 49, 578–585. [Google Scholar] [CrossRef]
- Padilha, A.C.; Piovesan, B.; Morais, M.C.; Arioli, C.J.; Zotti, M.J.; Grützmacher, A.D.; Botton, M. Toxicity, attraction, and repellency of toxic baits to stingless bees Plebeia emerina (Friese) and Tetragonisca fiebrigi (Schwarz) (Hymenoptera: Apidae: Meliponini). Ecotoxicol. Environ. Saf. 2019, 183, 109490. [Google Scholar] [CrossRef]
- Mayer, D.F.; Kovacs, G.; Brett, B.L.; Bisabri, B.L. The effects of spinosad insecticide to adults of Apis mellifera, Megachile rotundata, and Nomia melanderi (Hymenoptera: Apidae). Int. J. Hortic. Sci. 2001, 7, 93–97. [Google Scholar] [CrossRef]
- Scott-Dupree, C.D.; Conroy, L.; Harris, C. Impact of currently used or potentially useful insecticides for canola agroecosystems on Bombus impatiens (Hymenoptera: Apidae), Megachile rotundata (Hymentoptera: Megachilidae), and Osmia lignaria (Hymenoptera: Megachilidae). J. Econ. Entomol. 2009, 102, 177–182. [Google Scholar] [CrossRef] [PubMed]
- dos Araujo, R.S.; Lopes, M.P.; Barbosa, W.F.; Gonçalves, W.G.; Fernandes, K.M.; Martins, G.F.; Tavares, M.G. Spinosad-mediated effects on survival, overall group activity and the midgut of workers of Partamona helleri (Hymenoptera: Apidae). Ecotoxicol. Environ. Saf. 2019, 175, 148–154. [Google Scholar] [CrossRef]
- Gómez-Escobar, E.; Liedo, P.; Montoya, P.; Méndez-Villarreal, A.; Guzmán, M.; Vandame, R.; Sánchez, D. Effect of GF-120 (Spinosad) aerial sprays on colonies of the stingless bee Scaptotrigona mexicana (Hymenoptera: Apidae) and the honey bee (Hymenoptera: Apidae). J. Econ. Entomol. 2018, 111, 1711–1715. [Google Scholar] [CrossRef] [PubMed]
- Tomé, H.V.V.; Barbosa, W.F.; Corrêa, A.S.; Gontijo, L.M.; Martins, G.F.; Guedes, R.N.C. Reduced-risk insecticides in neotropical stingless bee species: Impact on survival and activity. Ann. Appl. Biol. 2015, 167, 186–196. [Google Scholar] [CrossRef]
- Barbosa, W.F.; Tomé, H.V.V.; Bernardes, R.C.; Siqueira, M.A.L.; Smagghe, G.; Guedes, R.N.C. Biopesticide-induced behavioral and morphological alterations in the stingless bee Melipona quadrifasciata. Environ. Toxicol. Chem. 2015, 34, 2149–2158. [Google Scholar] [CrossRef]
- Viana, T.A.; Barbosa, W.F.; Lourenço, A.P.; Santana, W.C.; Campos, L.O.; Martins, G.F. Changes in innate immune response and detoxification in Melipona quadrifasciata (Apinae: Meliponini) on oral exposure to azadirachtin and spinosad. Apidologie 2021, 52, 252–261. [Google Scholar] [CrossRef]
- Bernardes, R.C.; Tomé, H.V.V.; Barbosa, W.F.; Guedes, R.N.C.; Lima, M.A.P. Azadirachtin-induced antifeeding in Neotropical stingless bees. Apidologie 2017, 48, 275–285. [Google Scholar] [CrossRef]
- Bernardes, R.C.; Barbosa, W.F.; Martins, G.F.; Lima, M.A.P. The reduced-risk insecticide azadirachtin poses a toxicological hazard to stingless bee Partamona helleri (Friese, 1900) queens. Chemosphere 2018, 201, 550–556. [Google Scholar] [CrossRef]
- Lepidoptera—INHS Environmental Education. University of Illinois Urbana-Champaign. Available online: https://publish.illinois.edu/inhseducation/biodiversity/what-is-an-animal/arthropods/insects/lepidoptera/ (accessed on 16 January 2024).
- Introduction to the Lepidoptera. University of California Berkeley. Available online: https://ucmp.berkeley.edu/arthropoda/uniramia/lepidoptera.html?sa=X&ved=0CC4Q9QEwBmoVChMI3t6Jzun3xgIVRpQNCh3lawj1 (accessed on 16 January 2024).
- Sánchez-Bayo, F.; Wyckhuys, K.A.G. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]
- Fox, R. The decline of moths in Great Britain: A review of possible causes. Insect Conserv. Divers. 2013, 6, 5–19. [Google Scholar] [CrossRef]
- Miller, J.C. Monitoring the effects of Bacillus thuringiensis kurstaki on nontarget Lepidoptera in woodlands and forests of western Oregon. In Nontarget Effects of Biological Control; Springer: Boston, MA, USA, 2000; pp. 277–286. [Google Scholar]
- Marvier, M.; Regetz, J.; Kareiva, P.; McCreedy, C. A meta-analysis of effects of Bt cotton and maize on nontarget invertebrates. Science 2007, 316, 1475–1477. [Google Scholar] [CrossRef]
- Holst, N.; Lang, A.; Lövei, G.; Otto, M. Increased mortality is predicted of inachis io larvae caused by bt-maize pollen in European farmland. Ecol. Model. 2013, 250, 126–133. [Google Scholar] [CrossRef]
- Schuppener, M.; Muehlhause, J.; Müller, A.K.; Rauschen, S. Environmental risk assessment for the small tortoiseshell Aglais urticae and a stacked Bt-maize with combined resistances against Lepidoptera and Chrysomelidae in central European agrarian landscapes. Mol. Ecol. 2012, 21, 4646–4662. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.-K.; Schuppener, M.; Rauschen, S. Assessing the impact of Cry1Ab expressing corn pollen on larvae of Aglais urticae in a laboratory bioassay. IOBC/Wprs Bull. 2012, 73, 55–60. [Google Scholar]
- Introduction to the Diptera. University of California Berkeley. Available online: https://ucmp.berkeley.edu/arthropoda/uniramia/diptera.html (accessed on 17 January 2024).
- Dunn, L.; Lequerica, M.; Reid, C.R.; Latty, T. Dual ecosystem services of syrphid flies (Diptera: Syrphidae): Pollinators and biological control agents. Pest Manag. Sci. 2020, 76, 1973–1979. [Google Scholar] [CrossRef]
- Moens, J.; de Clercq, P.; Tirry, L. Side effects of pesticides on the larvae of the hoverfly Episyrphus balteatus in the laboratory. Phytoparasitica 2011, 39, 1–9. [Google Scholar] [CrossRef]
- Jansen, J.P.; San, G.; Gomez, M.Y. A field study to assess the effects of insecticides used to control the Colorado beetle in potato on aphid antagonists. Pestic. Benef. Org. IOBC-WPRS Bull. 2014, 103, 29–36. [Google Scholar]
- Boopathi, T.; Pathak, K.A. Ischiodon scutellaris (Fabricius) in broccoli ecosystem. J. Biol. Control. 2011, 25, 294–297. [Google Scholar]
- Dwivedi, S.A.; Singh, R.S. Synthetic Insecticides and Bio-pesticide Affect Natural Enemies of Aphid (Lipaphis erysimi Kalt) in Mustard. Indian J. Agric. Res. 2022, 56, 717–725. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Z.; Luo, X.; Liu, D. Biopesticide Extension and Rice Farmers’ Adoption Behavior: A Survey from Rural China. Res. Sq. 2021. preprint. [Google Scholar] [CrossRef]
- Raymond, B.; Wright, D.J. Resistance management of transgenic insect-Resistant crops: Ecological factors. In Environmental Impact of Genetically Modified Crops; CABI Publishing: Wallingford, UK, 2009; pp. 101–114. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).