Potential Involvement of Protein Phosphatase PPP2CA on Protein Synthesis and Cell Cycle During SARS-CoV-2 Infection: A Meta-Analysis Investigation
Abstract
:1. Introduction
2. Results and Discussion
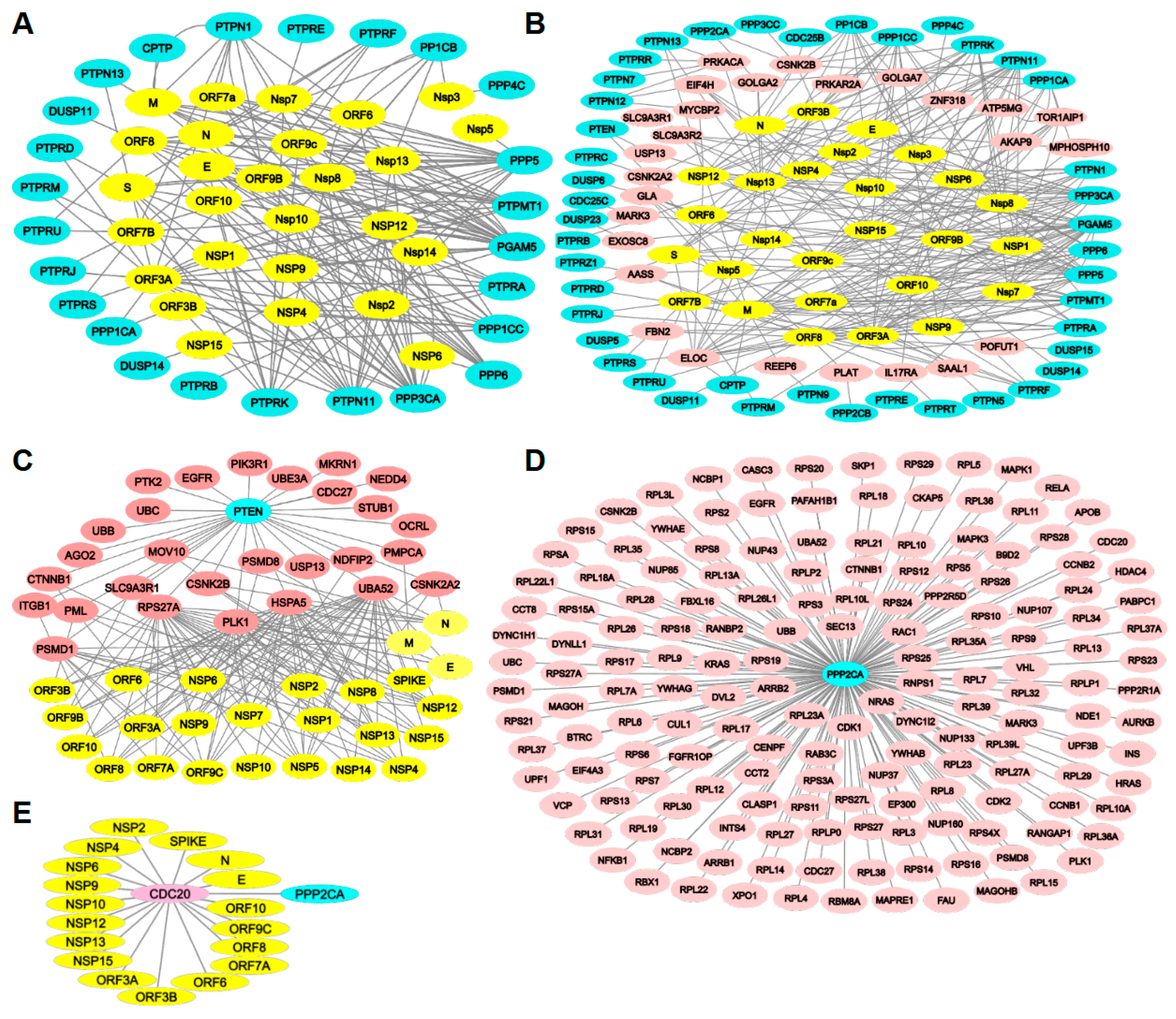
2.1. Expression Levels of Protein Phosphatases During SARS-CoV-2 Infection
2.2. Protein Phosphatases Interact Directly with Viral Proteins
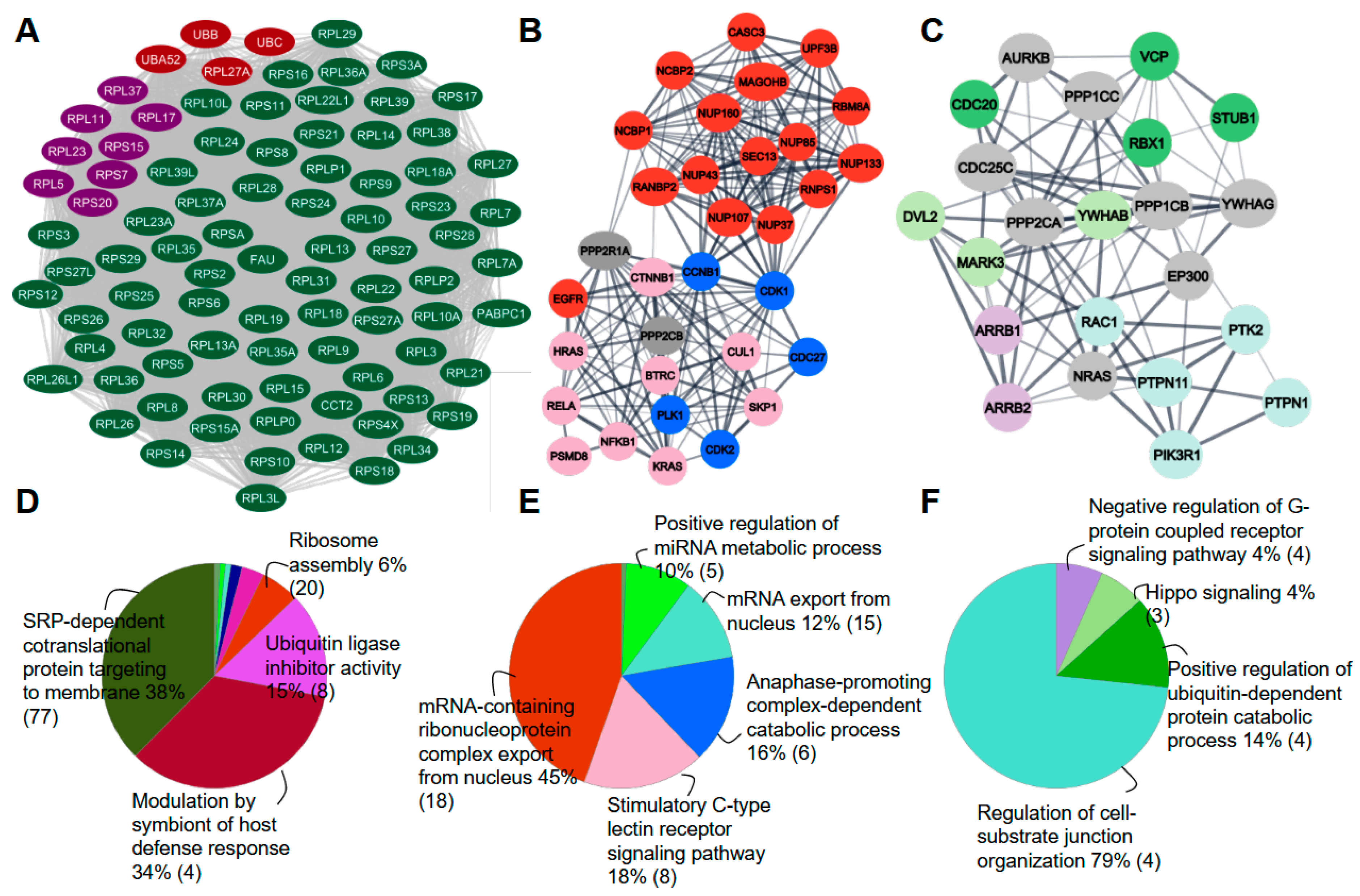
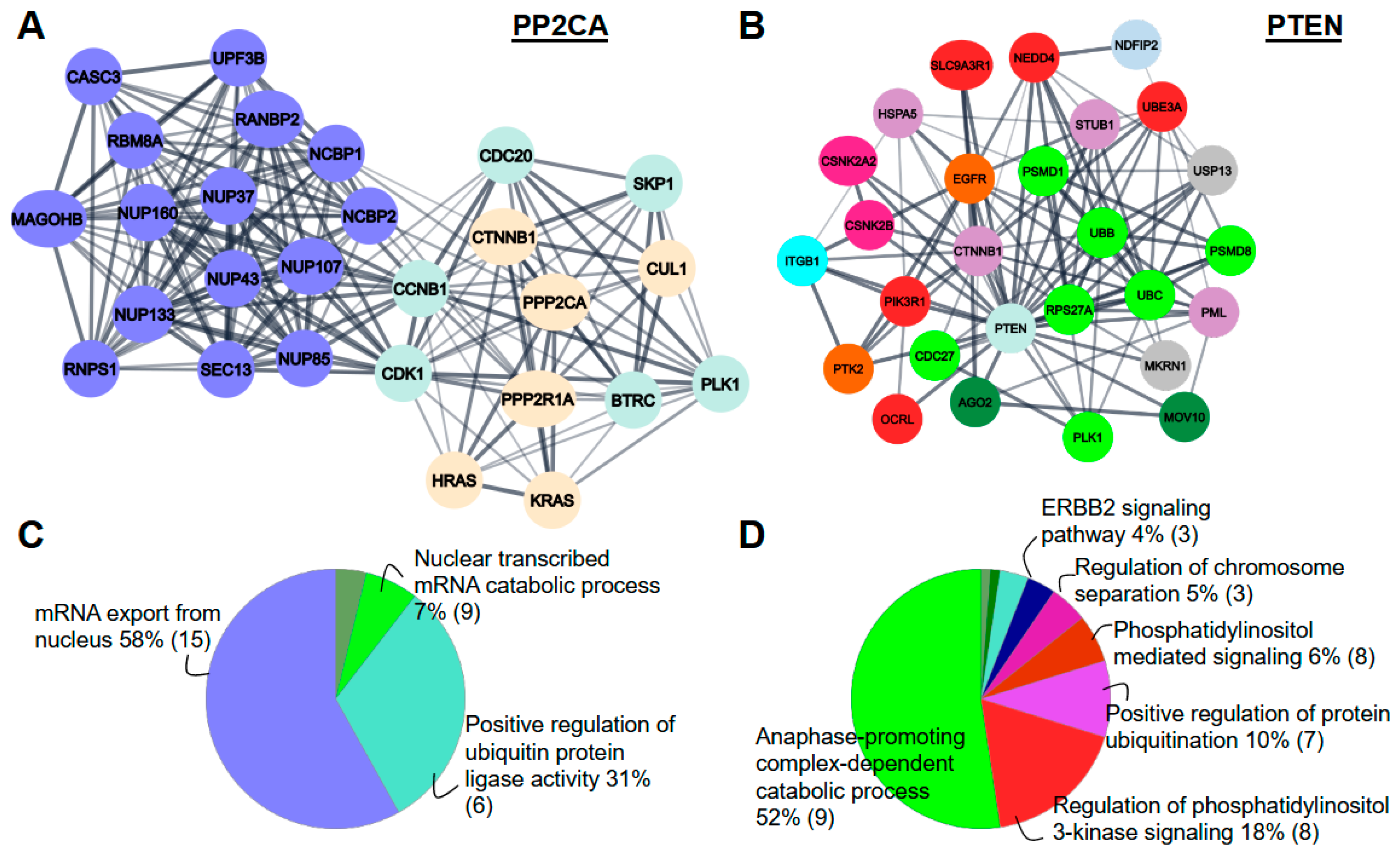
2.3. Protein Phosphatases Interact with Intermediate Proteins
2.4. Phosphorylation Level of Intermediate Proteins
2.5. PPP2CA Recognizes a Conserved Motif in Their Substrates
3. Conclusions
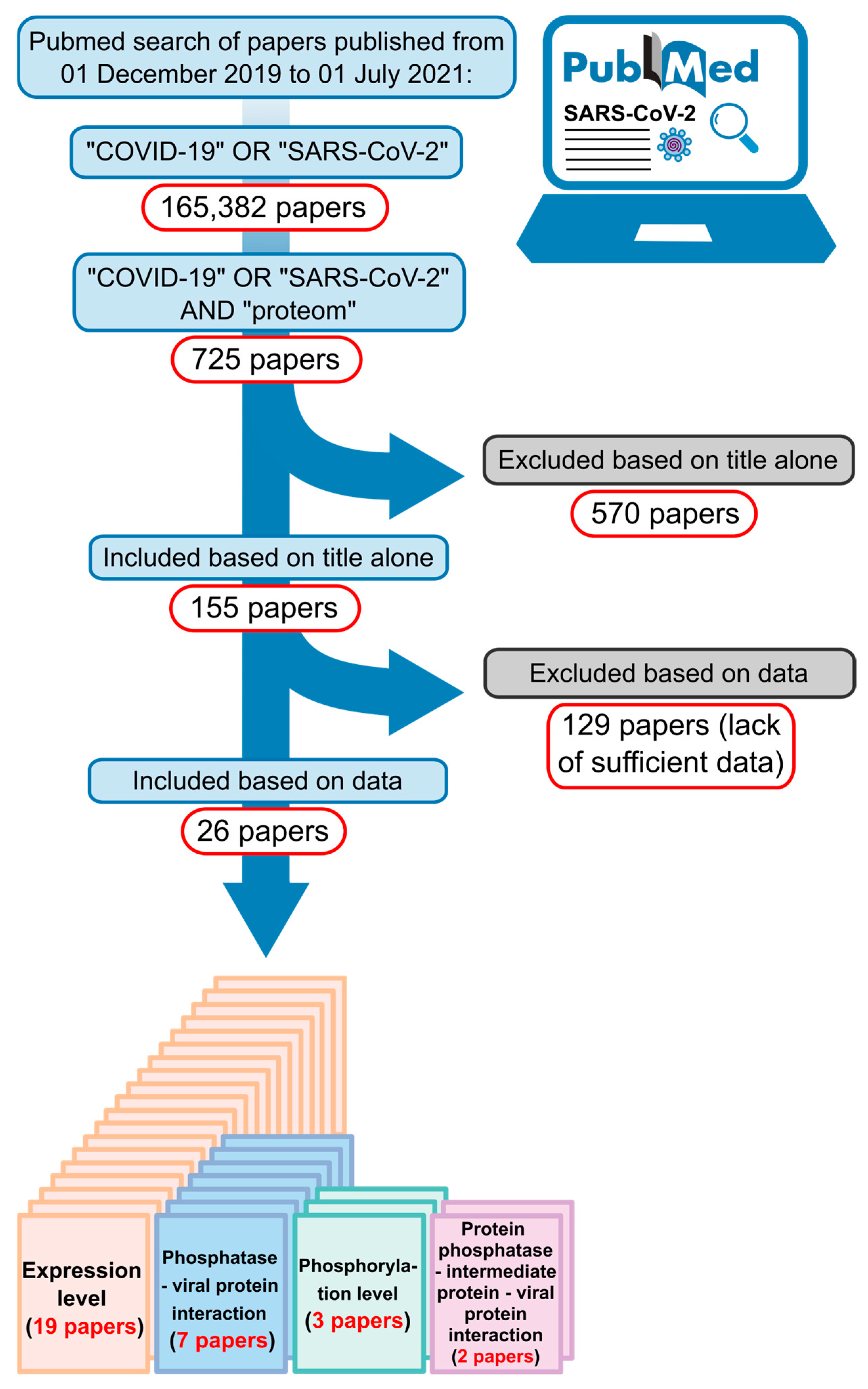
4. Material and Methods
4.1. In Silico Analysis
4.2. Protein–Protein Interaction Network
4.3. Statistical Analysis
4.4. Docking
4.5. Molecular Dynamics
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- COVID-19 Map. In Johns Hopkins Coronavirus Resource Center. Available online: https://coronavirus.jhu.edu/map.html (accessed on 11 December 2024).
- Naqvi, A.A.T.; Fatima, K.; Mohammad, T.; Fatima, U.; Singh, I.K.; Singh, A.; Atif, S.M.; Hariprasad, G.; Hasan, G.M.; Hassan, M.I. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: Structural genomics approach. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165878. [Google Scholar] [CrossRef] [PubMed]
- Malik, Y.A. Properties of Coronavirus and SARS-CoV-2. Malays. J. Pathol. 2020, 42, 3–11. [Google Scholar] [PubMed]
- Harrison, A.G.; Lin, T.; Wang, P. Mechanisms of SARS-CoV-2 Transmission and Pathogenesis. Trends Immunol. 2020, 41, 1100–1115. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.; Cao, R.; Ma, J.; Mou, D.; Zhu, Y.; Li, W.; Lv, L.; Gao, D.; Zhang, S.; Gong, F.; et al. Pathological features of COVID-19-associated lung injury: A preliminary proteomics report based on clinical samples. Signal Transduct. Target. Ther. 2020, 5, 240. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guo, M.; Tian, X.; Wang, X.; Yang, X.; Wu, P.; Liu, C.; Xiao, Z.; Qu, Y.; Yin, Y.; et al. Virus-Host Interactome and Proteomic Survey Reveal Potential Virulence Factors Influencing SARS-CoV-2 Pathogenesis. Med 2021, 2, 99–112.e7. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Liu, H.; Sun, W.; Ding, B.; Zhao, Y.; Chen, P.; Zhu, L.; Li, Z.; Li, N.; et al. Urine proteome of COVID-19 patients. Urine 2020, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bojkova, D.; Klann, K.; Koch, B.; Widera, M.; Krause, D.; Ciesek, S.; Cinatl, J.; Münch, C. Proteomics of SARS-CoV-2-infected host cells reveals therapy targets. Nature 2020, 583, 469–472. [Google Scholar] [CrossRef]
- Bouhaddou, M.; Memon, D.; Meyer, B.; White, K.M.; Rezelj, V.V.; Correa Marrero, M.; Polacco, B.J.; Melnyk, J.E.; Ulferts, S.; Kaake, R.M.; et al. The Global Phosphorylation Landscape of SARS-CoV-2 Infection. Cell 2020, 182, 685–712.e19. [Google Scholar] [CrossRef]
- Appelberg, S.; Gupta, S.; Svensson Akusjärvi, S.; Ambikan, A.T.; Mikaeloff, F.; Saccon, E.; Végvári, Á.; Benfeitas, R.; Sperk, M.; Ståhlberg, M.; et al. Dysregulation in Akt/mTOR/HIF-1 signaling identified by proteo-transcriptomics of SARS-CoV-2 infected cells. Emerg. Microbes Infect. 2020, 9, 1748–1760. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.-L.; Chen, D.; Yan, J.; Yang, Q.; Han, Q.-Q.; Li, S.-S.; Cheng, L. Proteomic characteristics of bronchoalveolar lavage fluid in critical COVID-19 patients. FEBS J. 2020, 288, 5190–5200. [Google Scholar] [CrossRef]
- Gisby, J.; Clarke, C.L.; Medjeral-Thomas, N.; Malik, T.H.; Papadaki, A.; Mortimer, P.M.; Buang, N.B.; Lewis, S.; Pereira, M.; Toulza, F.; et al. Longitudinal proteomic profiling of dialysis patients with COVID-19 reveals markers of severity and predictors of death. Elife 2021, 10, e64827. [Google Scholar] [CrossRef] [PubMed]
- Filbin, M.R.; Mehta, A.; Schneider, A.M.; Kays, K.R.; Guess, J.R.; Gentili, M.; Fenyves, B.G.; Charland, N.C.; Gonye, A.L.; Gushterova, I.; et al. Longitudinal proteomic analysis of severe COVID-19 reveals survival-associated signatures, tissue-specific cell death, and cell-cell interactions. CR Med. 2021, 2, 100287. [Google Scholar] [CrossRef] [PubMed]
- Stukalov, A.; Girault, V.; Grass, V.; Karayel, O.; Bergant, V.; Urban, C.; Haas, D.A.; Huang, Y.; Oubraham, L.; Wang, A.; et al. Multilevel proteomics reveals host perturbations by SARS-CoV-2 and SARS-CoV. Nature 2021, 594, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; John Wherry, E. T cell responses in patients with COVID-19. Nat. Rev. Immunol. 2020, 20, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Rümke, L.W.; Smit, W.L.; Bossink, A.; Limonard, G.J.M.; Muilwijk, D.; Haas, L.E.M.; Reusken, C.; van der Wal, S.; Thio, B.J.; van Os, Y.M.G.; et al. Impaired SARS-CoV-2 specific T-cell response in patients with severe COVID-19. Front. Immunol. 2023, 14, 1046639. [Google Scholar] [CrossRef] [PubMed]
- Klann, K.; Bojkova, D.; Tascher, G.; Ciesek, S.; Münch, C.; Cinatl, J. Growth Factor Receptor Signaling Inhibition Prevents SARS-CoV-2 Replication. Mol. Cell 2020, 80, 164–174.e4. [Google Scholar] [CrossRef]
- Novak, B.; Kapuy, O.; Domingo-Sananes, M.R.; Tyson, J.J. Regulated protein kinases and phosphatases in cell cycle decisions. Curr. Opin. Cell Biol. 2010, 22, 801–808. [Google Scholar] [CrossRef]
- Smoly, I.; Shemesh, N.; Ziv-Ukelson, M.; Ben-Zvi, A.; Yeger-Lotem, E. An Asymmetrically Balanced Organization of Kinases versus Phosphatases across Eukaryotes Determines Their Distinct Impacts. PLoS Comput. Biol. 2017, 13, e1005221. [Google Scholar] [CrossRef] [PubMed]
- Fischer, E.H. Cell signaling by protein tyrosine phosphorylation. Adv. Enzyme Regul. 1999, 39, 359–369. [Google Scholar] [CrossRef]
- Hornberg, J.J.; Bruggeman, F.J.; Binder, B.; Geest, C.R.; de Vaate, A.J.M.B.; Lankelma, J.; Heinrich, R.; Westerhoff, H.V. Principles behind the multifarious control of signal transduction. ERK phosphorylation and kinase/phosphatase control. FEBS J. 2005, 272, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Hunter, T. Protein kinases and phosphatases: The yin and yang of protein phosphorylation and signaling. Cell 1995, 80, 225–236. [Google Scholar] [CrossRef]
- Corda, P.O.; Bollen, M.; Ribeiro, D.; Fardilha, M. Emerging roles of the Protein Phosphatase 1 (PP1) in the context of viral infections. Cell Commun. Signal 2024, 22, 65. [Google Scholar] [CrossRef]
- Sontag, E. Protein phosphatase 2A: The Trojan Horse of cellular signaling. Cell Signal 2001, 13, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Ouellet, M.; Barbeau, B.; Tremblay, M.J. Protein tyrosyl phosphatases in T cell activation: Implication for human immunodeficiency virus transcriptional activity. Prog. Nucleic Acid Res. Mol. Biol. 2003, 73, 69–105. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Pan, W.; Wang, S.; Pan, C.; Ning, H.; Huang, S.; Chiu, S.-H.; Chen, J.-L. Protein Tyrosine Phosphatase SHP2 Suppresses Host Innate Immunity against Influenza A Virus by Regulating EGFR-Mediated Signaling. J. Virol. 2021, 95, e02001-20. [Google Scholar] [CrossRef] [PubMed]
- Devi, P.; Ota, S.; Punga, T.; Bergqvist, A. Hepatitis C Virus Core Protein Down-Regulates Expression of Src-Homology 2 Domain Containing Protein Tyrosine Phosphatase by Modulating Promoter DNA Methylation. Viruses 2021, 13, 2514. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhao, Y.; Gao, Y.; Hu, W.; Qu, Y.; Lou, N.; Zhu, Y.; Zhang, X.; Yang, H. Hepatitis C virus NS3 protein enhances hepatocellular carcinoma cell invasion by promoting PPM1A ubiquitination and degradation. J. Exp. Clin. Cancer Res. 2017, 36, 42. [Google Scholar] [CrossRef]
- Hu, Z.; Ban, H.; Zheng, H.; Liu, M.; Chang, J.; Guo, J.-T. Protein phosphatase 1 catalyzes HBV core protein dephosphorylation and is co-packaged with viral pregenomic RNA into nucleocapsids. PLoS Pathog. 2020, 16, e1008669. [Google Scholar] [CrossRef] [PubMed]
- Gerlt, V.; Mayr, J.; Del Sarto, J.; Ludwig, S.; Boergeling, Y. Cellular Protein Phosphatase 2A Regulates Cell Survival Mechanisms in Influenza A Virus Infection. Int. J. Mol. Sci. 2021, 22, 11164. [Google Scholar] [CrossRef]
- He, C.; Qiu, Y.; Han, P.; Chen, Y.; Zhang, L.; Yuan, Q.; Zhang, T.; Cheng, T.; Yuan, L.; Huang, C.; et al. ER stress regulating protein phosphatase 2A-B56γ, targeted by hepatitis B virus X protein, induces cell cycle arrest and apoptosis of hepatocytes. Cell Death Dis. 2018, 9, 762. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Thomas, T.; Dzieciatkowska, M.; Hill, R.C.; Francis, R.O.; Hudson, K.E.; Zimring, J.C.; Hod, E.A.; Spitalnik, S.L.; Hansen, K.C. Serum Proteomics in COVID-19 Patients: Altered Coagulation and Complement Status as a Function of IL-6 Level. J. Proteome Res. 2020, 19, 4417–4427. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, N.; Lareau, C.A.; Keshishian, H.; Ganskih, S.; Schneider, C.; Hennig, T.; Melanson, R.; Werner, S.; Wei, Y.; Zimmer, M.; et al. The SARS-CoV-2 RNA-protein interactome in infected human cells. Nat. Microbiol. 2021, 6, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.D.; Kitchen, R.R.; Guseh, J.S.; McNeill, J.N.; Aid, M.; Martinot, A.J.; Yu, A.; Platt, C.; Rhee, J.; Weber, B.; et al. Plasma Proteomics of COVID-19-Associated Cardiovascular Complications: Implications for Pathophysiology and Therapeutics. JACC Basic. Transl. Sci. 2022, 7, 425–441. [Google Scholar] [CrossRef]
- Nie, X.; Qian, L.; Sun, R.; Huang, B.; Dong, X.; Xiao, Q.; Zhang, Q.; Lu, T.; Yue, L.; Chen, S.; et al. Multi-organ proteomic landscape of COVID-19 autopsies. Cell 2021, 184, 775–791.e14. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wu, X.; Zhang, X.; Hou, X.; Liang, T.; Wang, D.; Teng, F.; Dai, J.; Duan, H.; Guo, S.; et al. SARS-CoV-2 Proteome Microarray for Mapping COVID-19 Antibody Interactions at Amino Acid Resolution. ACS Central Sci. 2020, 6, 2238–2249. [Google Scholar] [CrossRef] [PubMed]
- Almasy, K.M.; Davies, J.P.; Plate, L. Comparative Host Interactomes of the SARS-CoV-2 Nonstructural Protein 3 and Human Coronavirus Homologs. Mol. Cell. Proteom. 2021, 20, 100120. [Google Scholar] [CrossRef]
- Vanderboom, P.M.; Mun, D.-G.; Madugundu, A.K.; Mangalaparthi, K.K.; Saraswat, M.; Garapati, K.; Chakraborty, R.; Ebihara, H.; Sun, J.; Pandey, A. Proteomic Signature of Host Response to SARS-CoV-2 Infection in the Nasopharynx. Mol. Cell. Proteom. 2021, 20, 100134. [Google Scholar] [CrossRef]
- Xu, B.; Lei, Y.; Ren, X.; Yin, F.; Wu, W.; Sun, Y.; Wang, X.; Sun, Q.; Yang, X.; Wang, X.; et al. SOD1 is a Possible Predictor of COVID-19 Progression as Revealed by Plasma Proteomics. ACS Omega 2021, 6, 16826–16836. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Chen, Y.; Xia, H.; Wang, C.; Tan, C.Y.; Cai, X.; Liu, Y.; Ji, F.; Xiong, P.; Liu, R.; et al. Transcriptional and proteomic insights into the host response in fatal COVID-19 cases. Proc. Natl. Acad. Sci. USA 2020, 117, 28336–28343. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, W.; Zhu, Z.; Qiao, N.; Ling, Y.; Guo, M.; Yin, T.; Fang, H.; Xu, X.; Lu, G.; et al. Integrating longitudinal clinical laboratory tests with targeted proteomic and transcriptomic analyses reveal the landscape of host responses in COVID-19. Cell Discov. 2021, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhao, L.; Zhan, X. Quantitative proteomics reveals a broad-spectrum antiviral property of ivermectin, benefiting for COVID-19 treatment. J. Cell. Physiol. 2021, 236, 2959–2975. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.E.; Jang, G.M.; Bouhaddou, M.; Xu, J.; Obernier, K.; White, K.M.; O’Meara, M.J.; Rezelj, V.V.; Guo, J.Z.; Swaney, D.L.; et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 2020, 583, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Karunakaran, K.B.; Balakrishnan, N.; Ganapathiraju, M.K. Interactome of SARS-CoV-2/nCoV19 modulated host proteins with computationally predicted PPIs. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Nadeau, R.; Shahryari Fard, S.; Scheer, A.; Hashimoto-Roth, E.; Nygard, D.; Abramchuk, I.; Chung, Y.-E.; Bennett, S.A.L.; Lavallée-Adam, M. Computational Identification of Human Biological Processes and Protein Sequence Motifs Putatively Targeted by SARS-CoV-2 Proteins Using Protein-Protein Interaction Networks. J. Proteome Res. 2020, 19, 4553–4566. [Google Scholar] [CrossRef] [PubMed]
- Bamberger, C.; Pankow, S.; Martínez-Bartolomé, S.; Diedrich, J.K.; Park, R.S.K.; Yates, J.R. Analysis of the Tropism of SARS-CoV-2 Based on the Host Interactome of the Spike Protein. J. Proteome Res. 2023, 22, 3742–3753. [Google Scholar] [CrossRef]
- Meyers, J.M.; Ramanathan, M.; Shanderson, R.L.; Beck, A.; Donohue, L.; Ferguson, I.; Guo, M.G.; Rao, D.S.; Miao, W.; Reynolds, D.; et al. The proximal proteome of 17 SARS-CoV-2 proteins links to disrupted antiviral signaling and host translation. PLoS Pathog. 2021, 17, e1009412. [Google Scholar] [CrossRef] [PubMed]
- Perfetto, L.; Pastrello, C.; Del-Toro, N.; Duesbury, M.; Iannuccelli, M.; Kotlyar, M.; Licata, L.; Meldal, B.; Panneerselvam, K.; Panni, S.; et al. The IMEx coronavirus interactome: An evolving map of Coronaviridae-host molecular interactions. Database 2020, 2020, baaa096. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Y.; Gupta, S.; Paramo, M.I.; Hou, Y.; Mao, C.; Luo, Y.; Judd, J.; Wierbowski, S.; Bertolotti, M.; et al. A comprehensive SARS-CoV-2-human protein-protein interactome reveals COVID-19 pathobiology and potential host therapeutic targets. Nat. Biotechnol. 2023, 41, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Rodriguez-Bravo, V.; Kim, H.; Datta, S.; Foley, E.A. The PP2AB56 phosphatase promotes the association of Cdc20 with APC/C in mitosis. J. Cell Sci. 2017, 130, 1760–1771. [Google Scholar] [CrossRef] [PubMed]
- Fujimitsu, K.; Yamano, H. PP2A-B56 binds to Apc1 and promotes Cdc20 association with the APC/C ubiquitin ligase in mitosis. EMBO Rep. 2020, 21, e48503. [Google Scholar] [CrossRef]
- Labit, H.; Fujimitsu, K.; Bayin, N.S.; Takaki, T.; Gannon, J.; Yamano, H. Dephosphorylation of Cdc20 is required for its C-box-dependent activation of the APC/C. EMBO J. 2012, 31, 3351–3362. [Google Scholar] [CrossRef]
- Craney, A.; Kelly, A.; Jia, L.; Fedrigo, I.; Yu, H.; Rape, M. Control of APC/C-dependent ubiquitin chain elongation by reversible phosphorylation. Proc. Natl. Acad. Sci. USA 2016, 113, 1540–1545. [Google Scholar] [CrossRef] [PubMed]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.-H.; Pagès, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef]
- Kuss, S.K.; Mata, M.A.; Zhang, L.; Fontoura, B.M.A. Nuclear Imprisonment: Viral Strategies to Arrest Host mRNA Nuclear Export. Viruses 2013, 5, 1824–1849. [Google Scholar] [CrossRef]
- Melenotte, C.; Silvin, A.; Goubet, A.-G.; Lahmar, I.; Dubuisson, A.; Zumla, A.; Raoult, D.; Merad, M.; Gachot, B.; Hénon, C.; et al. Immune responses during COVID-19 infection. Oncoimmunology 2020, 9, 1807836. [Google Scholar] [CrossRef] [PubMed]
- Song, M.S.; Carracedo, A.; Salmena, L.; Song, S.J.; Egia, A.; Malumbres, M.; Pandolfi, P.P. Nuclear PTEN regulates the APC-CDH1 tumor-suppressive complex in a phosphatase-independent manner. Cell 2011, 144, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.H.; Pagano, M.; Huang, C.; Dai, W. Cdh1, a substrate-recruiting component of anaphase-promoting complex/cyclosome (APC/C) ubiquitin E3 ligase, specifically interacts with phosphatase and tensin homolog (PTEN) and promotes its removal from chromatin. J. Biol. Chem. 2014, 289, 17951–17959. [Google Scholar] [CrossRef] [PubMed]
- Pesin, J.A.; Orr-Weaver, T.L. Regulation of APC/C Activators in Mitosis and Meiosis. Annu. Rev. Cell Dev. Biol. 2008, 24, 475–499. [Google Scholar] [CrossRef]
- Shao, C.; Li, Z.; Ahmad, N.; Liu, X. Regulation of PTEN degradation and NEDD4-1 E3 ligase activity by Numb. Cell Cycle 2017, 16, 957–967. [Google Scholar] [CrossRef]
- Pilecki, M.; Grzyb, A.; Zień, P.; Sekuła, O.; Szyszka, R. Yeast protein phosphatase active with acidic ribosomal proteins. J. Basic Microbiol. 2000, 40, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-S.; Kim, H.D.; Shin, H.-S.; Kim, J. Phosphorylation status of nuclear ribosomal protein S3 is reciprocally regulated by protein kinase Cδ and protein phosphatase 2A. J. Biol. Chem. 2009, 284, 21201–21208. [Google Scholar] [CrossRef]
- Andres, J.L.; Johansen, J.W.; Maller, J.L. Identification of protein phosphatases 1 and 2B as ribosomal protein S6 phosphatases in vitro and in vivo. J. Biol. Chem. 1987, 262, 14389–14393. [Google Scholar] [CrossRef] [PubMed]
- Andres, J.L.; Maller, J.L. Purification and characterization of a novel protein phosphatase highly specific for ribosomal protein S6. J. Biol. Chem. 1989, 264, 151–156. [Google Scholar] [CrossRef]
- Hutchinson, J.A.; Shanware, N.P.; Chang, H.; Tibbetts, R.S. Regulation of ribosomal protein S6 phosphorylation by casein kinase 1 and protein phosphatase 1. J. Biol. Chem. 2011, 286, 8688–8696. [Google Scholar] [CrossRef] [PubMed]
- Hirano, K.; Ito, M.; Hartshorne, D.J. Interaction of the ribosomal protein, L5, with protein phosphatase type 1. J. Biol. Chem. 1995, 270, 19786–19790. [Google Scholar] [CrossRef] [PubMed]
- Brewer, A.; Sathe, G.; Pflug, B.E.; Clarke, R.G.; Macartney, T.J.; Sapkota, G.P. Mapping the substrate landscape of protein phosphatase 2A catalytic subunit PPP2CA. iScience 2024, 27, 109302. [Google Scholar] [CrossRef]
- Yu, H. Regulation of APC-Cdc20 by the spindle checkpoint. Curr. Opin. Cell Biol. 2002, 14, 706–714. [Google Scholar] [CrossRef]
- Qiao, R.; Weissmann, F.; Yamaguchi, M.; Brown, N.G.; VanderLinden, R.; Imre, R.; Jarvis, M.A.; Brunner, M.R.; Davidson, I.F.; Litos, G.; et al. Mechanism of APC/CCDC20 activation by mitotic phosphorylation. Proc. Natl. Acad. Sci. USA 2016, 113, E2570–E2578. [Google Scholar] [CrossRef]
- Barski, M.S.; Minnell, J.J.; Maertens, G.N. PP2A Phosphatase as an Emerging Viral Host Factor. Front. Cell Infect. Microbiol. 2021, 11, 725615. [Google Scholar] [CrossRef]
- Wang, X.; Bajaj, R.; Bollen, M.; Peti, W.; Page, R. Expanding the PP2A Interactome by Defining a B56-Specific SLiM. Structure 2016, 24, 2174–2181. [Google Scholar] [CrossRef]
- Hertz, E.P.T.; Kruse, T.; Davey, N.E.; López-Méndez, B.; Sigurðsson, J.O.; Montoya, G.; Olsen, J.V.; Nilsson, J. A Conserved Motif Provides Binding Specificity to the PP2A-B56 Phosphatase. Mol. Cell 2016, 63, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Garvanska, D.H.; Nasa, I.; Ueki, Y.; Zhang, G.; Kettenbach, A.N.; Peti, W.; Nilsson, J.; Page, R. A dynamic charge-charge interaction modulates PP2A:B56 substrate recruitment. Elife 2020, 9, e55966. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Fang, Y.; Gong, S.; Xue, T.; Wang, P.; She, L.; Huang, J. Deep learning-based network pharmacology for exploring the mechanism of licorice for the treatment of COVID-19. Sci. Rep. 2023, 13, 5844. [Google Scholar] [CrossRef]
- Deutsch, E.W.; Bandeira, N.; Sharma, V.; Perez-Riverol, Y.; Carver, J.J.; Kundu, D.J.; García-Seisdedos, D.; Jarnuczak, A.F.; Hewapathirana, S.; Pullman, B.S.; et al. The ProteomeXchange consortium in 2020: Enabling “big data” approaches in proteomics. Nucleic Acids Res. 2020, 48, D1145–D1152. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- UniProt Consortium. UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar] [CrossRef] [PubMed]
- Bindea, G.; Galon, J.; Mlecnik, B. CluePedia Cytoscape plugin: Pathway insights using integrated experimental and in silico data. Bioinformatics 2013, 29, 661–663. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef]
- de Castro, E.; Sigrist, C.J.A.; Gattiker, A.; Bulliard, V.; Langendijk-Genevaux, P.S.; Gasteiger, E.; Bairoch, A.; Hulo, N. ScanProsite: Detection of PROSITE signature matches and ProRule-associated functional and structural residues in proteins. Nucleic Acids Res. 2006, 34, W362–W365. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.J.; Headd, J.J.; Moriarty, N.W.; Prisant, M.G.; Videau, L.L.; Deis, L.N.; Verma, V.; Keedy, D.A.; Hintze, B.J.; Chen, V.B.; et al. MolProbity: More and better reference data for improved all-atom structure validation. Protein Sci. 2018, 27, 293–315. [Google Scholar] [CrossRef]
- Nguyen, H.; Roe, D.R.; Simmerling, C. Improved Generalized Born Solvent Model Parameters for Protein Simulations. J. Chem. Theory Comput. 2013, 9, 2020–2034. [Google Scholar] [CrossRef]
- Anandakrishnan, R.; Aguilar, B.; Onufriev, A.V. H++ 3.0: Automating pK prediction and the preparation of biomolecular structures for atomistic molecular modeling and simulations. Nucleic Acids Res. 2012, 40, W537–W541. [Google Scholar] [CrossRef]
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. ff14SB: Improving the Accuracy of Protein Side Chain and Backbone Parameters from ff99SB. J Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Joung, I.S.; Cheatham, T.E. Molecular dynamics simulations of the dynamic and energetic properties of alkali and halide ions using water-model-specific ion parameters. J. Phys. Chem. B 2009, 113, 13279–13290. [Google Scholar] [CrossRef] [PubMed]
- Josino, L.P.C.; Alves, C.N.; Lima, A.H. A molecular model to study FosA enzyme inhibition. J. Mol. Graph. Model. 2021, 107, 107978. [Google Scholar] [CrossRef] [PubMed]
- Bonetta, R.; Hunter, G.J.; Trinh, C.H.; Borowski, T.; Fenech, A.G.; Kulp, M.; Tabares, L.C.; Un, S.; Hunter, T. Substitution of histidine 30 by asparagine in manganese superoxide dismutase alters biophysical properties and supports proliferation in a K562 leukemia cell line. Eur. Biophys. J. 2021, 50, 571–585. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Merz, K.M. Taking into Account the Ion-induced Dipole Interaction in the Nonbonded Model of Ions. J. Chem. Theory Comput. 2014, 10, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Case, D.A.; Aktulga, H.M.; Belfon, K.; Cerutti, D.S.; Cisneros, G.A.; Cruzeiro, V.W.D.; Forouzesh, N.; Giese, T.J.; Götz, A.W.; Gohlke, H.; et al. AmberTools. J. Chem. Inf. Model. 2023, 63, 6183. [Google Scholar] [CrossRef]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007, 126, 014101. [Google Scholar] [CrossRef] [PubMed]
- Åqvist, J.; Wennerström, P.; Nervall, M.; Bjelic, S.; Brandsdal, B.O. Molecular dynamics simulations of water and biomolecules with a Monte Carlo constant pressure algorithm. Chem. Phys. Lett. 2004, 384, 288–294. [Google Scholar] [CrossRef]
- Hopkins, C.W.; Le Grand, S.; Walker, R.C.; Roitberg, A.E. Long-Time-Step Molecular Dynamics through Hydrogen Mass Repartitioning. J. Chem. Theory Comput. 2015, 11, 1864–1874. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, S.; Kollman, P.A. Settle: An analytical version of the SHAKE and RATTLE algorithm for rigid water models. J. Comput. Chem. 1992, 13, 952–962. [Google Scholar] [CrossRef]
- Salomon-Ferrer, R.; Götz, A.W.; Poole, D.; Le Grand, S.; Walker, R.C. Routine Microsecond Molecular Dynamics Simulations with AMBER on GPUs. 2. Explicit Solvent Particle Mesh Ewald. J. Chem. Theory Comput. 2013, 9, 3878–3888. [Google Scholar] [CrossRef] [PubMed]
- Grant, B.J.; Rodrigues, A.P.C.; ElSawy, K.M.; McCammon, J.A.; Caves, L.S.D. Bio3d: An R package for the comparative analysis of protein structures. Bioinformatics 2006, 22, 2695–2696. [Google Scholar] [CrossRef]
- Roe, D.R.; Cheatham, T.E. PTRAJ and CPPTRAJ: Software for Processing and Analysis of Molecular Dynamics Trajectory Data. J. Chem. Theory Comput. 2013, 9, 3084–3095. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; Available online: https://ggplot2.tidyverse.org/authors.html#citation (accessed on 31 October 2024).
- RStudio Team. RStudio: Integrated Development for R. In Posit Support. 2020. Available online: https://support.posit.co/hc/en-us/articles/206212048-Citing-RStudio (accessed on 31 October 2024).



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otvos, L.P.; Garrito, G.I.M.; Jara, G.E.; Lopes-de-Oliveira, P.S.; Machado, L.E.S.F. Potential Involvement of Protein Phosphatase PPP2CA on Protein Synthesis and Cell Cycle During SARS-CoV-2 Infection: A Meta-Analysis Investigation. Kinases Phosphatases 2025, 3, 4. https://doi.org/10.3390/kinasesphosphatases3010004
Otvos LP, Garrito GIM, Jara GE, Lopes-de-Oliveira PS, Machado LESF. Potential Involvement of Protein Phosphatase PPP2CA on Protein Synthesis and Cell Cycle During SARS-CoV-2 Infection: A Meta-Analysis Investigation. Kinases and Phosphatases. 2025; 3(1):4. https://doi.org/10.3390/kinasesphosphatases3010004
Chicago/Turabian StyleOtvos, Luca P., Giulia I. M. Garrito, Gabriel E. Jara, Paulo S. Lopes-de-Oliveira, and Luciana E. S. F. Machado. 2025. "Potential Involvement of Protein Phosphatase PPP2CA on Protein Synthesis and Cell Cycle During SARS-CoV-2 Infection: A Meta-Analysis Investigation" Kinases and Phosphatases 3, no. 1: 4. https://doi.org/10.3390/kinasesphosphatases3010004
APA StyleOtvos, L. P., Garrito, G. I. M., Jara, G. E., Lopes-de-Oliveira, P. S., & Machado, L. E. S. F. (2025). Potential Involvement of Protein Phosphatase PPP2CA on Protein Synthesis and Cell Cycle During SARS-CoV-2 Infection: A Meta-Analysis Investigation. Kinases and Phosphatases, 3(1), 4. https://doi.org/10.3390/kinasesphosphatases3010004





