Epigenetic Rewiring of Protein Kinase Signalling in T-Cell Acute Lymphoblastic Leukaemia
Abstract
1. Introduction
| Kinase Classification | Kinase Targets | Epigenetic Signalling Involved and Biological Relevance | Organism and/or Cell Type | Inhibitors | Article(s) |
|---|---|---|---|---|---|
| Serine/threonine kinases | CDKN2B/p15INK4b |
| Human (paediatric and adult T-ALL) | / | [16,17,18,19] |
| DAPK |
| Human—Burkitt lymphoma and B-ALL | / | [20,21,22] | |
| MAP2K7 |
| Mouse and human (paediatric) T-ALL—lymphoblasts | / | [23] | |
| CDK2 |
| Mouse human T-ALL—T6E, 8946, and KOPT-K1 cells | / | [24] | |
| IRAK1 |
| Human T-ALL—Jurkat cells | / | [25,26,27,28] | |
| ERK |
| Mouse—lymph node cells | Indirect through MEK inhibitors: Selumetinib Trametinib | [29] | |
| Tyrosine kinases | EphB6 |
| Human—Jurkat cells | / | [30,31] |
| Mouse—thymocytes | [32,33] | |||
| EphB1 |
| Mouse—embryonic and adult thymic T cells | / | [34] | |
| EphB4 |
| Human—cell lines and bone marrow samples | / | [35] | |
| Human (paediatric)—newly diagnosed patients | [36] | |||
| EphA3 |
| Human—HSB2, Jurkat, MOLT4 cell lines and patients with high blast counts | / | [37] | |
| SYK |
| Human—T-ALL patients and pro-B-lineage ALL | / | [38,39] | |
| LCK |
| Mouse—lymph node cells | Indirect through ABL/SRC inhibitors: Imatinib Dasatinib | [29] | |
| ZAP70 |
| Mouse—lymph node cells | / | [29] | |
| Nucleotide kinase | dCK |
| Human T-ALL—cell lines | TRE-515 | [40,41,42] |
| RNA polymerase II kinase CTD Ser kinase | BRD4 |
| Human (paediatric)—primary cells T-ALL | / | [43,44,45] |
| Mouse T-ALL | [46] | |||
| Lipide kinase Ser/Thr kinase | PI3K/AKT(/mTOR) |
| Human (paediatric) T-ALL—patients and cells | PI3K: Buparlisib AKT: MK-2206 mTOR: Sirolimus Everolimus Temsirolimus | [47,48,49,50] |
| Human T-ALL—tissue | [51] | |||
| Human T-ALL—cell lines | [52] | |||
| Mouse—Pten-deficient T-ALL | [53] | |||
| Non-receptor tyrosine kinases | JAK/STAT |
| Human (paediatric) T-ALL—patients and DND-41, ALL-SIL cells | JAK: Ruxolitinib | [48] |
| Lipide kinase/phosphatase | PI3K/PTEN |
| Mouse and Human T-ALL | PI3K: Buparlisib | [54,55] |
2. Role of Protein Kinases in T-ALL
2.1. PI3K/AKT/mTOR Signalling in T-ALL
2.2. MAPK/ERK Signalling in T-ALL
2.3. IL7R/JAK/STAT Pathway Activation in T-ALL
2.4. Activation of ABL1 Kinase in T-ALL
2.5. Role of PIM Kinases in T-ALL
2.6. Src Family of Kinases
2.7. c-Jun NH2 Kinase (JNK) Signalling
2.8. Aurora B Kinase
3. Epigenetic DNA Methylation Regulation of Kinase Expression in T-ALL
3.1. DNA Methylation Programming of Kinase Signalling Pathways in T-ALL
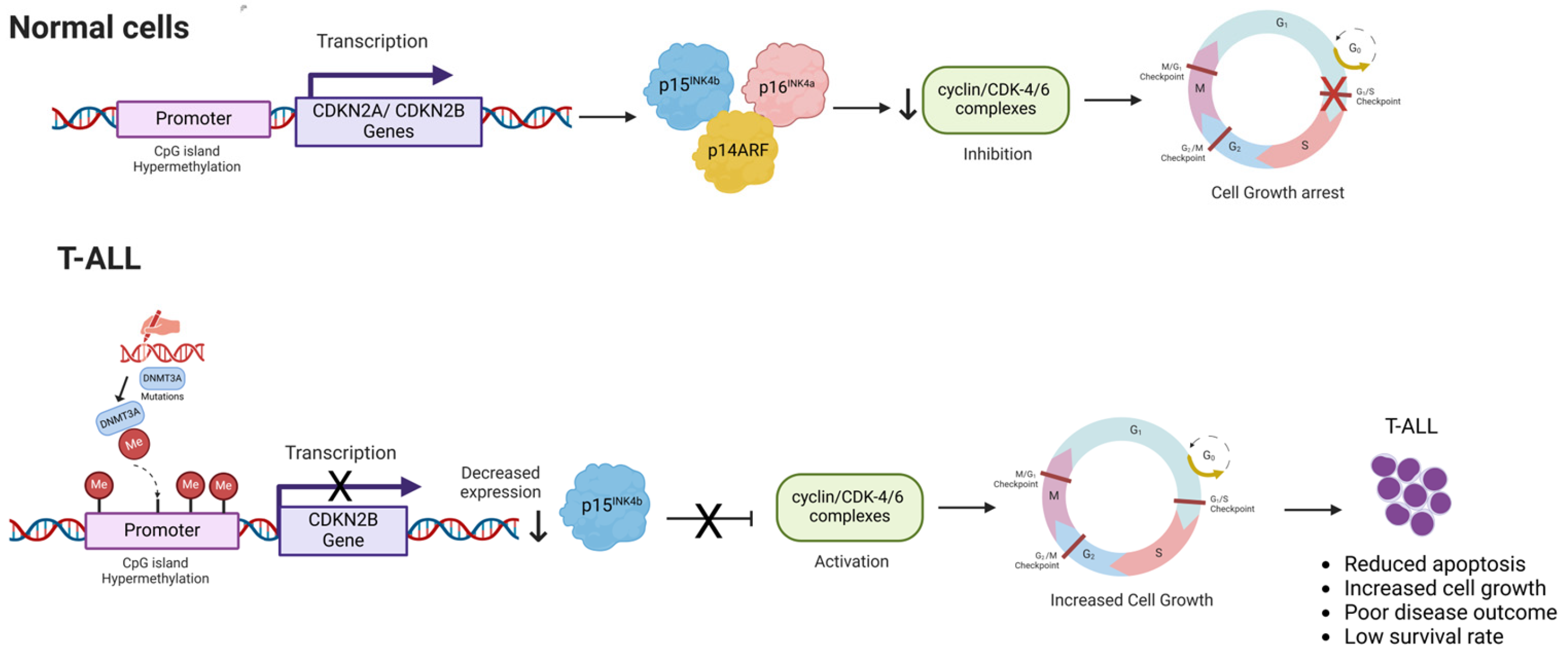
3.1.1. Hypermethylation of Cyclin-Dependent Kinase Inhibitor Gene Promoters
3.1.2. Differential Methylation Status of Ephrin Tyrosine Kinase Genes
3.1.3. Hypermethylation of Death-Associated Protein Kinase Gene Promoter
3.1.4. Hypermethylation of Spleen Tyrosine Kinase (SYK) Gene Promoter
3.1.5. Epigenetic Silencing of Krüppel-like Factor 4 and MAP2K7 Signalling
4. Histone Mark Regulation of Kinase Expression in T-ALL
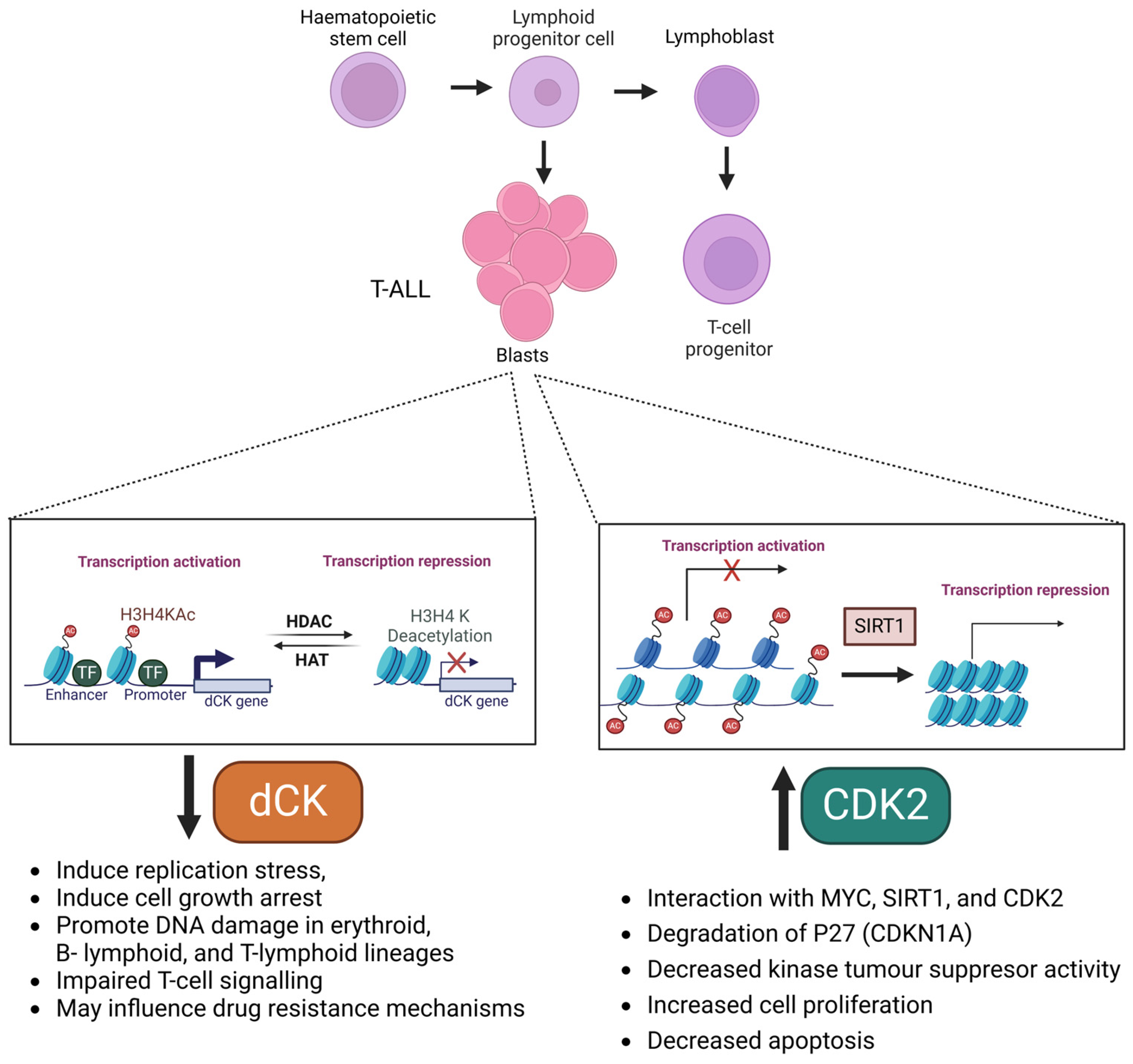
4.1. Histone Chromatin Regulation of Deoxycytidine Kinase (dCK) Expression in T-ALL
4.2. (Histone) Acetylation Regulation of Cyclin-Dependent Kinase 2 (CDK2) in T-ALL
4.3. Dual Chromatin Acetylase–Kinase Bromodomain Protein 4 (Brd4) Enhances c-Myc Expression in T-ALL
5. Epigenetic microRNA Regulation of Kinase Expression in T-ALL
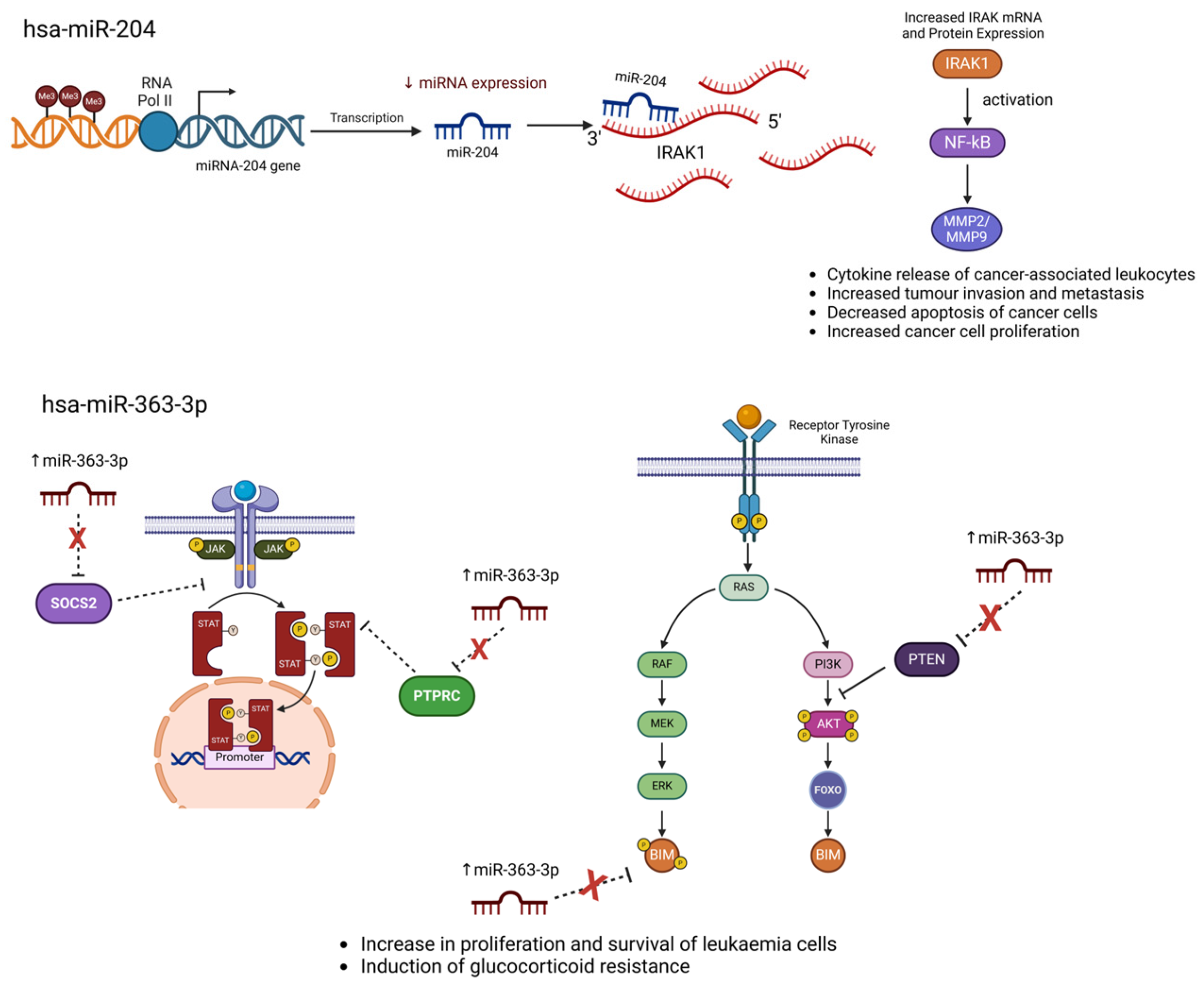
5.1. hsa-miR-363-3p Crosstalk with PI3K/AKT Signalling
5.2. hsa-miR-204 Crosstalk with IRAK Signalling
5.3. miR-653-5p Crosstalk with Circ-PRKDC and PI3K/AKT/mTOR Signalling Pathways
5.4. hsa-miR-150 Crosstalk with mTOR Signalling
5.5. miR-181 Crosstalk with LCK, ZAP70, and ERK Kinase Signalling Pathways
5.6. miR-19 Crosstalk with PI3K/PTEN Signalling
5.7. miR-26 Crosstalk with PI3K/AKT Signalling
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- van Vlierberghe, P.; Ferrando, A. The molecular basis of T cell acute lymphoblastic leukemia. J. Clin. Investig. 2012, 122, 3398–3406. [Google Scholar] [CrossRef] [PubMed]
- Litzow, M.R.; Ferrando, A.A. How I treat T-cell acute lymphoblastic leukemia in adults. Blood 2015, 126, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Cordo’, V.; van der Zwet, J.C.G.; Canté-Barrett, K.; Pieters, R.; Meijerink, J.P.P. T-cell Acute Lymphoblastic Leukemia: A Roadmap to Targeted Therapies. Blood Cancer Discov. 2021, 2, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Weng, A.P.; Ferrando, A.A.; Lee, W.; Morris, I.V.J.P.; Silverman, L.B.; Sanchez-Irizarry, C.; Blacklow, S.C.; Look, A.T.; Aster, J.C. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science 2004, 306, 269–271. [Google Scholar] [CrossRef]
- Robles-Valero, J.; Lorenzo-Martín, L.F.; Menacho-Márquez, M.; Fernández-Pisonero, I.; Abad, A.; Camós, M.; Toribio, M.L.; Espinosa, L.; Bigas, A.; Bustelo, X.R. A Paradoxical Tumor-Suppressor Role for the Rac1 Exchange Factor Vav1 in T Cell Acute Lymphoblastic Leukemia. Cancer Cell 2017, 32, 608–623.e9. [Google Scholar] [CrossRef]
- King, B.; Trimarchi, T.; Reavie, L.; Xu, L.; Mullenders, J.; Ntziachristos, P.; Aranda-Orgilles, B.; Perez-Garcia, A.; Shi, J.; Vakoc, C.; et al. The ubiquitin ligase FBXW7 modulates leukemia-initiating cell activity by regulating MYC stability. Cell 2013, 153, 1552–1566. [Google Scholar] [CrossRef]
- Neaga, A.; Jimbu, L.; Mesaros, O.; Bota, M.; Lazar, D.; Cainap, S.; Blag, C.; Zdrenghea, M. Why do children with acute lymphoblastic leukemia fare better than adults? Cancers 2021, 13, 3886. [Google Scholar] [CrossRef]
- Ling, Y.; Xie, Q.; Zhang, Z.; Zhang, H. Protein kinase inhibitors for acute leukemia. Biomark. Res. 2018, 6, 8. [Google Scholar] [CrossRef]
- Cordo’, V.; Meijer, M.T.; Hagelaar, R.; de Goeij-de Haas, R.R.; Poort, V.M.; Henneman, A.A.; Piersma, S.R.; Pham, T.V.; Oshima, K.; Ferrando, A.A.; et al. Phosphoproteomic profiling of T cell acute lymphoblastic leukemia reveals targetable kinases and combination treatment strategies. Nat. Commun. 2022, 13, 1048. [Google Scholar] [CrossRef]
- Cohen, P.; Cross, D.; Jänne, P.A. Kinase drug discovery 20 years after imatinib: Progress and future directions. Nat. Rev. Drug Discov. 2021, 20, 551–569. [Google Scholar] [CrossRef]
- Bhullar, K.S.; Lagarón, N.O.; McGowan, E.M.; Parmar, I.; Jha, A.; Hubbard, B.P.; Rupasinghe, H.P.V. Kinase-targeted cancer therapies: Progress, challenges and future directions. Mol. Cancer 2018, 17, 48. [Google Scholar] [CrossRef] [PubMed]
- Hunter, T.; Cooper, J.A. Protein-tyrosine kinases. Annu. Rev. Biochem. 1985, 54, 897–930. [Google Scholar] [CrossRef] [PubMed]
- Cicenas, J.; Zalyte, E.; Bairoch, A.; Gaudet, P. Kinases and cancer. Cancers 2018, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Angus, S.P.; Zawistowski, J.S.; Johnson, G.L. Epigenetic Mechanisms Regulating Adaptive Responses to Targeted Kinase Inhibitors in Cancer. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 209–229. [Google Scholar] [CrossRef]
- Li, J.; Gong, C.; Zhou, H.; Liu, J.; Xia, X.; Ha, W.; Jiang, Y.; Liu, Q.; Xiong, H. Kinase Inhibitors and Kinase-Targeted Cancer Therapies: Recent Advances and Future Perspectives. Int. J. Mol. Sci. 2024, 25, 5489. [Google Scholar] [CrossRef]
- Drexler, H.G. Review of alterations of the cyclin-dependent kinase inhibitor INK4 family genes p15, p16, p18 and p19 in human leukemia-lymphoma cells. Leukemia 1998, 12, 845–859. [Google Scholar] [CrossRef]
- Tsellou, E.; Troungos, C.; Moschovi, M.; Athanasiadou-Piperopoulou, F.; Polychronopoulou, S.; Kosmidis, H.; Kalmanti, M.; Hatzakis, A.; Dessypris, N.; Kalofoutis, A.; et al. Hypermethylation of CpG islands in the promoter region of the p15INK4B gene in childhood acute leukaemia. Eur. J. Cancer 2005, 41, 584–589. [Google Scholar] [CrossRef]
- Batova, A.; Diccianni, M.B.; Yu, J.C.; Nobori, T.; Link, M.P.; Pullen, J.; Yu, A.L. Frequent and selective methylation of p15 and deletion of both p15 and p16 in T-cell acute lymphoblastic leukemia. Cancer Res. 1997, 57, 832–836. [Google Scholar]
- Jang, W.; Park, J.; Kwon, A.; Choi, H.; Kim, J.; Lee, G.D.; Han, E.; Jekarl, D.W.; Chae, H.; Han, K.; et al. CDKN2B downregulation and other genetic characteristics in T-acute lymphoblastic leukemia. Exp. Mol. Med. 2019, 51, 1–15. [Google Scholar] [CrossRef]
- Kissil, J.L.; Feinstein, E.; Cohen, O.; Jones, P.A.; Tsai, Y.C.; Knowles, M.A.; Eydmann, M.E.; Kimchi, A. DAP-kinase loss of expression in various carcinoma and B-cell lymphoma cell lines: Possible implications for role as tumor suppressor gene. Oncogene 1997, 15, 403–407. [Google Scholar] [CrossRef]
- Shiramizu, B.; Mick, P. Epigenetic Changes in the DAP-Kinase CpG Island in Pediatric Lymphoma. Med. Pediatr. Oncol. 2003, 41, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Zöchbauer-Müller, S.; Fong, K.M.; Virmani, A.K.; Geradts, J.; Gazdar, A.F.; Minna, J.D. Aberrant promoter methylation of multiple genes in non-small cell lung cancers. Cancer Res. 2001, 61, 249–255. [Google Scholar] [PubMed]
- Shen, Y.; Park, C.S.; Suppipat, K.; Yamada, T.; Mistretta, T.-A.; Lacorazza, D. Krüppel-like Factor 4 (KLF4) Suppresses T-Cell Acute Lymphoblastic Leukemia By Inhibiting Expression of MAP2K7 and Expansion of Leukemia Initiating Cells. Blood 2014, 124, 3569. [Google Scholar] [CrossRef]
- Wang, F.; Li, Z.; Zhou, J.; Wang, G.; Zhang, W.; Xu, J.; Liang, A. SIRT1 regulates the phosphorylation and degradation of P27 by deacetylating CDK2 to promote T-cell acute lymphoblastic leukemia progression. J. Exp. Clin. Cancer Res. 2021, 40, 259. [Google Scholar] [CrossRef]
- Yin, J.J.; Liang, B.; Zhan, X.R. MicroRNA-204 inhibits cell proliferation in T-cell acute lymphoblastic leukemia by down-regulating SOX4. Int. J. Clin. Exp. Pathol. 2015, 8, 9189. [Google Scholar]
- Cao, Z.; Henzel, W.J.; Gao, X. IRAK: A kinase associated with the interleukin-1 receptor. Science 1996, 271, 1128–1131. [Google Scholar] [CrossRef]
- Lin, C.; Chen, D.; Xiao, T.; Lin, D.; Lin, D.; Lin, L.; Zhu, H.; Xu, J.; Huang, W.; Yang, T. DNA methylation-mediated silencing of microRNA-204 enhances T cell acute lymphoblastic leukemia by up-regulating MMP-2 and MMP-9 via NF-κB. J. Cell Mol. Med. 2021, 25, 2365–2376. [Google Scholar] [CrossRef]
- Dussiau, C.; Trinquand, A.; Lhermitte, L.; Latiri, M.; Simonin, M.; Cieslak, A.; Bedjaoui, N.; Villarèse, P.; Verhoeyen, E.; Dombret, H.; et al. Targeting IRAK1 in T-Cell acute lymphoblastic leukemia. Oncotarget 2015, 6, 18956–18965. [Google Scholar] [CrossRef][Green Version]
- Štefanová, I.; Hemmer, B.; Vergelli, M.; Martin, R.; Biddison, W.E.; Germain, R.N. TCR ligand discrimination is enforced by competing ERK positive and SHP-I negative feedback pathways. Nat. Immunol. 2003, 4, 248–254. [Google Scholar] [CrossRef]
- Luo, H.; Wan, X.; Wu, Y.; Wu, J. Cross-Linking of EphB6 Resulting in Signal Transduction and Apoptosis in Jurkat Cells. J. Immunol. 2001, 167, 1362–1370. [Google Scholar] [CrossRef]
- Shimoyama, M.; Matsuoka, H.; Tamekane, A.; Ito, M.; Nobuko, I.; Inoue, R.; Chihara, K.; Furuya, A.; Hanai, N.; Matsui, T. T-cell-specific expression of kinase-defective Eph-family receptor protein, EphB6 in normal as well as transformed hematopoietic cells. Growth Factors 2000, 18, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Bassiri, H.; Carding, S.R. A Requirement for IL-2/IL-2 Receptor Signaling in Intrathymic Negative Selection. J. Immunol. 2001, 166, 5945–5954. [Google Scholar] [CrossRef] [PubMed]
- Freywald, A.; Sharfe, N.; Rashotte, C.; Grunberger, T.; Roifman, C.M. The EphB6 receptor inhibits JNK activation in T lymphocytes and modulates T cell receptor-mediated responses. J. Biol. Chem. 2003, 278, 10150–10156. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Mao, J.; Wu, Y.; Luo, H.; Wu, J. Ephrin-B1 is critical in T-cell development. J. Biol. Chem. 2006, 281, 10222–10229. [Google Scholar] [CrossRef]
- Kuang, S.Q.; Bai, H.; Fang, Z.H.; Lopez, G.; Yang, H.; Tong, W.; Wang, Z.Z.; Garcia-Manero, G. Aberrant DNA methylation and epigenetic inactivation of Eph receptor tyrosine kinases and ephrin ligands in acute lymphoblastic leukemia. Blood 2010, 115, 2412–2419. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Chen, X.; Mai, H.; Li, C.; Wen, F. Aberrant EPHB4 gene methylation and childhood acute lymphoblastic leukemia. Oncol. Lett. 2017, 14, 4433–4440. [Google Scholar] [CrossRef]
- Dottori, M.; Down, M.; Hüttmann, A.; Fitzpatrick, D.R.; Boyd, A.W. Cloning and characterization of EphA3 (Hek) gene promoter: DNA methylation regulates expression in hematopoietic tumor cells. Blood 1999, 94, 2477–2486. [Google Scholar] [CrossRef]
- Goodman, P.A.; Burkhardt, N.; Juran, B.; Tibbles, H.E.; Uckun, F.M. Hypermethylation of the spleen tyrosine kinase promoter in T-lineage acute lymphoblastic leukemia. Oncogene 2003, 22, 2504–2514. [Google Scholar] [CrossRef]
- Goodman, P.A.; Wood, C.M.; Vassilev, A.; Mao, C.; Uckun, F.M. Spleen tyrosine kinase (Syk) deficiency in childhood pro-B cell acute lymphoblastic leukemia. Oncogene 2001, 20, 3969–3978. [Google Scholar] [CrossRef]
- Chen, B.Y.; Salas, J.R.; Trias, A.O.; Perez Rodriguez, A.; Tsang, J.E.; Guemes, M.; Le, T.M.; Galic, Z.; Shepard, H.M.; Steinman, L.; et al. Targeting deoxycytidine kinase improves symptoms in mouse models of multiple sclerosis. Immunology 2023, 168, 152–169. [Google Scholar] [CrossRef]
- Yoshida, K.; Fujita, A.; Narazaki, H.; Asano, T.; Itoh, Y. Drug resistance to nelarabine in leukemia cell lines might be caused by reduced expression of deoxycytidine kinase through epigenetic mechanisms. Cancer Chemother. Pharmacol. 2022, 89, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Choi, O.; Heathcote, D.A.; Ho, K.-K.; Müller, P.J.; Ghani, H.; Lam, E.W.-F.; Ashton-Rickardt, P.G.; Rutschmann, S. A Deficiency in Nucleoside Salvage Impairs Murine Lymphocyte Development, Homeostasis, and Survival. J. Immunol. 2012, 188, 3920–3927. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Jiang, Y.; Hong, Y.; Chu, X.; Zhang, Z.; Tao, Y.; Fan, Z.; Bai, Z.; Li, X.; Chen, Y.; et al. BRD4 PROTAC degrader ARV-825 inhibits T-cell acute lymphoblastic leukemia by targeting “Undruggable” Myc-pathway genes. Cancer Cell Int. 2021, 21, 230. [Google Scholar] [CrossRef] [PubMed]
- Delmore, J.E.; Issa, G.C.; Lemieux, M.E.; Rahl, P.B.; Shi, J.; Jacobs, H.M.; Kastritis, E.; Gilpatrick, T.; Paranal, R.M.; Qi, J.; et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell 2011, 146, 904–917. [Google Scholar] [CrossRef]
- Uppal, S.; Gegonne, A.; Chen, Q.; Thompson, P.S.; Cheng, D.; Mu, J.; Meerzaman, D.; Misra, H.S.; Singer, D.S. The Bromodomain Protein 4 Contributes to the Regulation of Alternative Splicing. Cell Rep. 2019, 29, 2450–2460.e5. [Google Scholar] [CrossRef]
- Piya, S.; Yang, Y.; Bhattacharya, S.; Sharma, P.; Ma, H.; Mu, H.; He, H.; Ruvolo, V.; Baran, N.; Davis, R.E.; et al. Targeting the NOTCH1-MYC-CD44 axis in leukemia-initiating cells in T-ALL. Leukemia 2022, 36, 1261–1273. [Google Scholar] [CrossRef]
- Wallaert, A.; Van Loocke, W.; Hernandez, L.; Taghon, T.; Speleman, F.; Van Vlierberghe, P. Comprehensive miRNA expression profiling in human T-cell acute lymphoblastic leukemia by small RNA-sequencing. Sci. Rep. 2017, 7, 7901. [Google Scholar] [CrossRef]
- Drobna-Śledzińska, M.; Maćkowska-Maślak, N.; Jaksik, R.; Kosmalska, M.; Szarzyńska, B.; Lejman, M.; Sędek, Ł.; Szczepański, T.; Taghon, T.; Van Vlierberghe, P.; et al. Multiomics to investigate the mechanisms contributing to repression of PTPRC and SOCS2 in pediatric T-ALL: Focus on miR-363-3p and promoter methylation. Genes Chromosomes Cancer 2022, 61, 720–733. [Google Scholar] [CrossRef]
- Reynolds, C.; Roderick, J.E.; LaBelle, J.L.; Bird, G.; Mathieu, R.; Bodaar, K.; Colon, D.; Pyati, U.; Stevenson, K.E.; Qi, J.; et al. Repression of BIM mediates survival signaling by MYC and AKT in high-risk T-cell acute lymphoblastic leukemia. Leukemia 2014, 28, 1819–1827. [Google Scholar] [CrossRef]
- van der Zwet, J.C.G.; Buijs-Gladdines, J.G.C.A.M.; Cordo’, V.; Debets, D.O.; Smits, W.K.; Chen, Z.; Dylus, J.; Zaman, G.J.R.; Altelaar, M.; Oshima, K.; et al. MAPK-ERK is a central pathway in T-cell acute lymphoblastic leukemia that drives steroid resistance. Leukemia 2021, 35, 3394–3405. [Google Scholar] [CrossRef]
- Chen, H.; Pei, L.; Xie, P.; Guo, G. Circ-prkdc contributes to 5-fluorouracil resistance of colorectal cancer cells by regulating mir-375/foxm1 axis and wnt/β-catenin pathway. Onco Targets Ther. 2020, 13, 5939–5953. [Google Scholar] [CrossRef] [PubMed]
- Podshivalova, K.; Wang, E.A.; Hart, T.; Salomon, D.R. Expression of the miR-150 tumor suppressor is restored by and synergizes with rapamycin in a human leukemia T-cell line. Leuk. Res. 2018, 74, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Yang, Y.; Chen, J.; Li, W.; Li, W.; Zhang, Q.; Mi, Y.; Goswami, R.S.; You, J.Q.; Lin, D.; et al. Regulation of PI3K signaling in T-cell acute lymphoblastic leukemia: A novel PTEN/Ikaros/miR-26b mechanism reveals a critical targetable role for PIK3CD. Leukemia 2017, 31, 2355–2364. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Li, C.; Olive, V.; Lykken, E.; Feng, F.; Sevilla, J.; Wan, Y.; He, L.; Li, Q.J. Molecular dissection of the miR-17-92 cluster’s critical dual roles in promoting Th1 responses and preventing inducible Treg differentiation. Blood 2011, 118, 5487–5497. [Google Scholar] [CrossRef]
- Mavrakis, K.J.; Wolfe, A.L.; Oricchio, E.; Palomero, T.; De Keersmaecker, K.; McJunkin, K.; Zuber, J.; James, T.; Chang, K.; Khan, A.A.; et al. Genome-wide RNA-mediated interference screen identifies miR-19 targets in Notch-induced T-cell acute lymphoblastic leukaemia. Nat. Cell Biol. 2010, 12, 372–379. [Google Scholar] [CrossRef]
- Dupont, C.; Armant, D.R.; Brenner, C.A. Epigenetics: Definition, mechanisms and clinical perspective. Semin. Reprod. Med. 2009, 27, 351–357. [Google Scholar] [CrossRef]
- Bonev, B.; Cavalli, G. Organization and function of the 3D genome. Nat. Rev. Genet. 2016, 17, 661–678. [Google Scholar] [CrossRef]
- Rowley, M.J.; Corces, V.G. Organizational principles of 3D genome architecture. Nat. Rev. Genet. 2018, 19, 789–800. [Google Scholar] [CrossRef]
- Pombo, A.; Dillon, N. Three-dimensional genome architecture: Players and mechanisms. Nat. Rev. Mol. Cell Biol. 2015, 16, 245–257. [Google Scholar] [CrossRef]
- van der Meulen, J.; van Roy, N.; van Vlierberghe, P.; Speleman, F. The epigenetic landscape of T-cell acute lymphoblastic leukemia. Int. J. Biochem. Cell Biol. 2014, 53, 547–557. [Google Scholar] [CrossRef]
- Huether, R.; Dong, L.; Chen, X.; Wu, G.; Parker, M.; Wei, L.; Ma, J.; Edmonson, M.N.; Hedlund, E.K.; Rusch, M.C.; et al. The landscape of somatic mutations in epigenetic regulators across 1000 paediatric cancer genomes. Nat. Commun. 2014, 5, 3630. [Google Scholar] [CrossRef] [PubMed]
- van Vlierberghe, P.; Palomero, T.; Khiabanian, H.; van der Meulen, J.; Castillo, M.; van Roy, N.; de Moerloose, B.; Philippé, J.; González-García, S.; Toribio, M.L.; et al. PHF6 mutations in T-cell acute lymphoblastic leukemia. Nat. Genet. 2010, 42, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Ntziachristos, P.; Tsirigos, A.; Vlierberghe, P.; van Nedjic, J.; Trimarchi, T.; Flaherty, M.S.; Ferres-Marco, D.; da Ros, V.; Tang, Z.; Siegle, J.; et al. Genetic inactivation of the polycomb repressive complex 2 in T cell acute lymphoblastic leukemia. Nat. Med. 2012, 18, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Valls, P.O.; Esposito, A. Signalling dynamics, cell decisions, and homeostatic control in health and disease. Curr. Opin. Cell Biol. 2022, 75, 102066. [Google Scholar] [CrossRef]
- Theivendren, P.; Kunjiappan, S.; Hegde, Y.M.; Vellaichamy, S.; Gopal, M.; Dhramalingam, S.R.; Kumar, S. Importance of Protein Kinase and Its Inhibitor: A Review. In Protein Kinases; Singh, R.K., Ed.; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar] [CrossRef]
- Logie, E.; Novo, C.P.; Driesen, A.; Van Vlierberghe, P.; Vanden Berghe, W. Phosphocatalytic kinome activity profiling of apoptotic and ferroptotic agents in multiple myeloma cells. Int. J. Mol. Sci. 2021, 22, 12731. [Google Scholar] [CrossRef]
- Manning, G.; Whyte, D.B.; Martinez, R.; Hunter, T.; Sudarsanam, S. The protein kinase complement of the human genome. Science 2002, 298, 1912–1934. [Google Scholar] [CrossRef]
- Bhanumathy, K.K.; Balagopal, A.; Vizeacoumar, F.S.; Vizeacoumar, F.J.; Freywald, A.; Giambra, V. Review protein tyrosine kinases: Their roles and their targeting in leukemia. Cancers 2021, 13, 184. [Google Scholar] [CrossRef]
- Liu, Y.; Easton, J.; Shao, Y.; Maciaszek, J.; Wang, Z.; Wilkinson, M.R.; McCastlain, K.; Edmonson, M.; Pounds, S.B.; Shi, L.; et al. The genomic landscape of pediatric and young adult T-lineage acute lymphoblastic leukemia. Nat. Genet. 2017, 49, 1211–1218. [Google Scholar] [CrossRef]
- Vicente, C.; Schwab, C.; Broux, M.; Geerdens, E.; Degryse, S.; Demeyer, S.; Lahortiga, I.; Elliott, A.; Chilton, L.; La Starza, R.; et al. Targeted sequencing identifies associations between IL7R-JAK mutations and epigenetic modulators in T-cell acute lymphoblastic leukemia. Haematologica 2015, 100, 1301–1310. [Google Scholar] [CrossRef]
- Graux, C.; Stevens-Kroef, M.; Lafage, M.; Dastugue, N.; Harrison, C.J.; Mugneret, F.; Bahloula, K.; Struski, S.; Grégoire, M.J.; Nadal, N.; et al. Heterogeneous patterns of amplification of the NUP214-ABL1 fusion gene in T-cell acute lymphoblastic leukemia. Leukemia 2008, 23, 125–133. [Google Scholar] [CrossRef]
- Verbeke, D.; Gielen, O.; Jacobs, K.; Boeckx, N.; de Keersmaecker, K.; Maertens, J.; Uyttebroeck, A.; Segers, H.; Cools, J. Ruxolitinib Synergizes with Dexamethasone for the Treatment of T-cell Acute Lymphoblastic Leukemia. Hemasphere 2019, 3, e310. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.P.; Reynolds, C.P.; Kang, M.H. Modulation of Glucocorticoid Resistance in Pediatric T-cell Acute Lymphoblastic Leukemia by Increasing BIM Expression with the PI3K/mTOR Inhibitor BEZ235. Clin. Cancer Res. 2016, 22, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Gazi, M.; Moharram, S.A.; Marhäll, A.; Kazi, J.U. Corrigendum to “The dual specificity PI3K/mTOR inhibitor PKI-587 displays efficacy against T-cell acute lymphoblastic leukemia (T-ALL)”. Cancer Lett. 2019, 461, 155. [Google Scholar] [CrossRef] [PubMed]
- de Smedt, R.; Morscio, J.; Reunes, L.; Roels, J.; Bardelli, V.; Lintermans, B.; van Loocke, W.; Almeida, A.; Cheung, L.C.; Kotecha, R.S.; et al. Targeting cytokine- And therapy-induced PIM1 activation in preclinical models of T-cell acute lymphoblastic leukemia and lymphoma. Blood 2020, 135, 1685–1695. [Google Scholar] [CrossRef]
- Spartà, A.M.; Bressanin, D.; Chiarini, F.; Lonetti, A.; Cappellini, A.; Evangelisti, C.; Evangelisti, C.; Melchionda, F.; Pession, A.; Bertaina, A.; et al. Therapeutic targeting of Polo-like kinase-1 and Aurora kinases in T-cell acute lymphoblastic leukemia. Cell Cycle 2014, 13, 2237–2247. [Google Scholar] [CrossRef]
- Akahane, K.; Li, Z.; Etchin, J.; Berezovskaya, A.; Gjini, E.; Masse, C.E.; Miao, W.; Rocnik, J.; Kapeller, R.; Greenwood, J.R.; et al. Anti-leukaemic activity of the TYK2 selective inhibitor NDI-031301 in T-cell acute lymphoblastic leukaemia. Br. J. Haematol. 2017, 177, 271–282. [Google Scholar] [CrossRef]
- Brown, K.K.; Toker, A. The phosphoinositide 3-kinase pathway and therapy resistance in cancer. F1000Prime Rep. 2015, 7, 13. [Google Scholar] [CrossRef]
- Bongiovanni, D.; Saccomani, V.; Piovan, E. Aberrant signaling pathways in t-cell acute lymphoblastic leukemia. Int. J. Mol. Sci. 2017, 18, 1904. [Google Scholar] [CrossRef]
- Juntilla, M.M.; Koretzky, G.A. Critical roles of the PI3K/Akt signaling pathway in T cell development. Immunol. Lett. 2008, 116, 104–110. [Google Scholar] [CrossRef]
- Martelli, A.M.; Chiarini, F.; Evangelisti, C.; Cappellini, A.; Buontempo, F.; Bressanin, D.; Fini, M.; McCubrey, J.A. Two hits are better than one: Targeting both phosphatidylinositol 3-kinase and mammalian target of rapamycin as a therapeutic strategy for acute leukemia treatment. Oncotarget 2012, 3, 371–394. [Google Scholar] [CrossRef]
- Palomero, T.; Sulis, M.L.; Cortina, M.; Real, P.J.; Barnes, K.; Ciofani, M.; Caparros, E.; Buteau, J.; Brown, K.; Perkins, S.L.; et al. Mutational loss of PTEN induces resistance to NOTCH1 inhibition in T-cell leukemia. Nat. Med. 2007, 13, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Barata, J.T.; Silva, A.; Abecasis, M.; Carlesso, N.; Cumano, A.; Cardoso, A.A. Molecular and functional evidence for activity of murine IL-7 on human lymphocytes. Exp. Hematol. 2006, 34, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Barata, J.T.; Silva, A.; Brandao, J.G.; Nadler, L.M.; Cardoso, A.A.; Boussiotis, V.A. Activation of PI3K is indispensable for interleukin 7-mediated viability, proliferation, glucose use, and growth of T cell acute lymphoblastic leukemia cells. J. Exp. Med. 2004, 200, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Martelli, A.M.; Paganelli, F.; Fazio, A.; Bazzichetto, C.; Conciatori, F.; McCubrey, J.A. The key roles of PTEN in T-cell acute lymphoblastic leukemia development, progression, and therapeutic response. Cancers 2019, 11, 629. [Google Scholar] [CrossRef]
- Thorpe, L.M.; Yuzugullu, H.; Zhao, J.J. PI3K in cancer: Divergent roles of isoforms, modes of activation and therapeutic targeting. Nat. Rev. Cancer 2015, 15, 7–24. [Google Scholar] [CrossRef]
- Silva, A.; Yunes, J.A.; Cardoso, B.A.; Martins, L.R.; Jotta, P.Y.; Abecasis, M.; Nowill, A.E.; Leslie, N.R.; Cardoso, A.A.; Barata, J.T. PTEN posttranslational inactivation and hyperactivation of the PI3K/Akt pathway sustain primary T cell leukemia viability. J. Clin. Investig. 2008, 118, 3762–3774. [Google Scholar] [CrossRef]
- Vazquez, F.; Ramaswamy, S.; Nakamura, N.; Sellers, W.R. Phosphorylation of the PTEN Tail Regulates Protein Stability and Function. Mol. Cell Biol. 2000, 20, 5010–5018. [Google Scholar] [CrossRef]
- Silva, A.; Gírio, A.; Cebola, I.; Santos, C.I.; Antunes, F.; Barata, J.T. Intracellular reactive oxygen species are essential for PI3K/Akt/mTOR-dependent IL-7-mediated viability of T-cell acute lymphoblastic leukemia cells. Leukemia 2011, 25, 960–967. [Google Scholar] [CrossRef]
- Hales, E.C.; Orr, S.M.; Gedman, A.L.; Taub, J.W.; Matherly, L.H. Notch1 receptor regulates AKT protein activation loop (Thr308) Dephosphorylation through modulation of the PP2A phosphatase in phosphatase and tensin homolog (PTEN)-null T-cell acute lymphoblastic leukemia cells. J. Biol. Chem. 2013, 288, 22836–22848. [Google Scholar] [CrossRef]
- Cardoso, B.A.; Martins, L.R.; Santos, C.I.; Nadler, L.M.; Boussiotis, V.A.; Cardoso, A.A.; Barata, J.T. Interleukin-4 stimulates proliferation and growth of T-cell acute lymphoblastic leukemia cells by activating mTOR signaling. Leukemia 2009, 23, 206–208. [Google Scholar] [CrossRef]
- Zenatti, P.P.; Ribeiro, D.; Li, W.; Zuurbier, L.; Silva, M.C.; Paganin, M.; Tritapoe, J.; Hixon, J.A.; Silveira, A.B.; Cardoso, B.A.; et al. Oncogenic IL7R gain-of-function mutations in childhood T-cell acute lymphoblastic leukemia. Nat. Genet. 2011, 43, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.; Korz, W. Translating an antagonist of chemokine receptor CXCR4: From bench to bedside. Clin. Cancer Res. 2008, 14, 7975–7980. [Google Scholar] [CrossRef] [PubMed]
- Rubinfeld, H.; Seger, R. The ERK cascade: A prototype of MAPK signaling. Mol. Biotechnol. 2005, 31, 151–174. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, M.C.; Jivan, A.; Shao, C.; Duan, L.; Goad, D.; Zaganjor, E.; Osborne, J.; McGlynn, K.; Stippec, S.; Earnest, S.; et al. The roles of MAPKs in disease. Cell Res. 2008, 18, 436–442. [Google Scholar] [CrossRef]
- Lapinski, P.E.; King, P.D. Regulation of Ras signal transduction during T cell development and activation. Am. J. Clin. Exp. Immunol. 2012, 1, 147. [Google Scholar]
- Jing, D.; Bhadri, V.A.; Beck, D.; Thoms, J.A.I.; Yakob, N.A.; Wong, J.W.H.; Knezevic, K.; Pimanda, J.E.; Lock, R.B. Opposing regulation of BIM and BCL2 controls glucocorticoid-induced apoptosis of pediatric acute lymphoblastic leukemia cells. Blood 2015, 125, 273–283. [Google Scholar] [CrossRef]
- Thomas, S.J.; Snowden, J.A.; Zeidler, M.P.; Danson, S.J. The role of JAK/STAT signalling in the pathogenesis, prognosis and treatment of solid tumours. Br. J. Cancer 2015, 113, 365–371. [Google Scholar] [CrossRef]
- Kim, M.S.; Chung, N.G.; Kim, M.S.; Yoo, N.J.; Lee, S.H. Somatic mutation of IL7R exon 6 in acute leukemias and solid cancers. Hum. Pathol. 2013, 44, 551–555. [Google Scholar] [CrossRef]
- Ferrando, A. Can one target T-cell ALL? Best. Pract. Res. Clin. Haematol. 2018, 31, 361–366. [Google Scholar] [CrossRef]
- Shouse, G.; Nikolaenko, L. Targeting the JAK/STAT Pathway in T Cell Lymphoproliferative Disorders. Curr. Hematol. Malig. Rep. 2019, 14, 570–576. [Google Scholar] [CrossRef]
- Kruh, G.D.; Perego, R.; Miki, T.; Aaronson, S.A. The complete coding sequence of arg defines the Abelson subfamily of cytoplasmic tyrosine kinases. Proc. Natl. Acad. Sci. USA 1990, 87, 5802–5806. [Google Scholar] [CrossRef] [PubMed]
- Goff, S.P.; Gilboa, E.; Witte, O.N.; Baltimore, D. Structure of the Abelson murine leukemia virus genome and the homologous cellular gene: Studies with cloned viral DNA. Cell 1980, 22, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Heisterkamp, N.; Groffen, J.; Stephenson, J.R. The human v-abl cellular homologue. J. Mol. Appl. Genet. 1983, 2, 57–68. [Google Scholar] [PubMed]
- Klein, A.d.; Kessel, A.G.v.; Grosveld, G.; Bartram, C.R.; Hagemeijer, A.; Bootsma, D.; Spurr, N.K.; Heisterkamp, N.; Groffen, J.; Stephenson, J.R. A cellular oncogene is translocated to the Philadelphia chromosome in chronic myelocytic leukaemia. Nature 1982, 300, 765–767. [Google Scholar] [CrossRef]
- Konopka, J.B.; Watanabe, S.M.; Singer, J.W.; Collins, S.J.; Witte, O.N. Cell lines and clinical isolates derived from Ph1-positive chronic myelogenous leukemia patients express c-abl proteins with a common structural alteration. Proc. Natl. Acad. Sci. USA 1985, 82, 1810–1814. [Google Scholar] [CrossRef]
- Greuber, E.K.; Smith-Pearson, P.; Wang, J.; Pendergast, A.M. Role of ABL family kinases in cancer: From leukaemia to solid tumours. Nat. Rev. Cancer 2013, 13, 559–571. [Google Scholar] [CrossRef]
- Gu, J.J.; Ryu, J.R.; Pendergast, A.M. Abl tyrosine kinases in T-cell signaling. Immunol. Rev. 2009, 228, 170–183. [Google Scholar] [CrossRef]
- Fattizzo, B.; Rosa, J.; Giannotta, J.A.; Baldini, L.; Fracchiolla, N.S. The Physiopathology of T-Cell Acute Lymphoblastic Leukemia: Focus on Molecular Aspects. Front. Oncol. 2020, 10, 273. [Google Scholar] [CrossRef]
- de Braekeleer, E.; Douet-Guilbert, N.; Rowe, D.; Bown, N.; Dé Ric Morel, F.; Berthou, C.; Fé Rec, C.; de Braekeleer, M. ABL1 fusion genes in hematological malignancies: A review. Eur. J. Haematol. 2011, 86, 361–371. [Google Scholar] [CrossRef]
- Hagemeijer, A.; Graux, C. ABLI rearrangements in T-cell acute lymphoblastic leukemia. Genes Chromosomes Cancer 2010, 49, 299–308. [Google Scholar] [CrossRef]
- Burmeister, T.; Gökbuget, N.; Reinhardt, R.; Rieder, H.; Hoelzer, D.; Schwartz, S. NUP214-ABL1 in adult T-ALL: The GMALL study group experience. Blood 2006, 108, 3556–3559. [Google Scholar] [CrossRef] [PubMed]
- Haberle, V.; Stark, A. Eukaryotic core promoters and the functional basis of transcription initiation. Nat. Rev. Mol. Cell Biol. 2018, 19, 621–637. [Google Scholar] [CrossRef] [PubMed]
- Qian, K.C.; Wang, L.; Hickey, E.R.; Studts, J.; Barringer, K.; Peng, C.; Kronkaitis, A.; Li, J.; White, A.; Mische, S.; et al. Structural basis of constitutive activity and a unique nucleotide binding mode of human Pim-1 kinase. J. Biol. Chem. 2005, 280, 6130–6137. [Google Scholar] [CrossRef] [PubMed]
- Nawijn, M.C.; Alendar, A.; Berns, A. For better or for worse: The role of Pim oncogenes in tumorigenesis. Nat. Rev. Cancer 2011, 11, 23–34. [Google Scholar] [CrossRef]
- Wang, Z.; Bhattacharya, N.; Weaver, M.; Petersen, K.; Meyer, M.; Gapter, L.; Magnuson, N.S. Pim-1: A serine/threonine kinase with a role in cell survival, proliferation, differentiation and tumorigenesis. J. Vet. Sci. 2001, 2, 167–179. [Google Scholar] [CrossRef]
- White, E. The pims and outs of survival signaling: Role for the Pim-2 protein kinase in the suppression of apoptosis by cytokines. Genes Dev. 2003, 17, 1813–1816. [Google Scholar] [CrossRef]
- Mikkers, H.; Nawijn, M.; Allen, J.; Brouwers, C.; Verhoeven, E.; Jonkers, J.; Berns, A. Mice Deficient for All PIM Kinases Display Reduced Body Size and Impaired Responses to Hematopoietic Growth Factors. Mol. Cell Biol. 2004, 24, 6104–6115. [Google Scholar] [CrossRef]
- Jackson, L.J.; Pheneger, J.A.; Pheneger, T.J.; Davis, G.; Wright, A.D.; Robinson, J.E.; Allen, S.; Munson, M.C.; Carter, L.L. The role of PIM kinases in human and mouse CD4+ T cell activation and inflammatory bowel disease. Cell Immunol. 2012, 272, 200–213. [Google Scholar] [CrossRef]
- Tay, R.E.; Richardson, E.K.; Toh, H.C. Revisiting the role of CD4+ T cells in cancer immunotherapy—New insights into old paradigms. Cancer Gene Ther. 2021, 28, 5–17. [Google Scholar] [CrossRef]
- Hüttmann, A.; Klein-Hitpass, L.; Thomale, J.; Deenen, R.; Carpinteiro, A.; Nückel, H.; Ebeling, P.; Führer, A.; Edelmann, J.; Sellmann, L.; et al. Gene expression signatures separate B-cell chronic lymphocytic leukaemia prognostic subgroups defined by ZAP-70 and CD38 expression status. Leukemia 2006, 20, 1774–1782. [Google Scholar] [CrossRef]
- Cohen, A.M.; Grinblat, B.; Bessler, H.; Kristt, D.A.; Kremer, A.; Shalom, S.; Schwartz, A.; Halperin, M.; Merkel, D.; Don, J. Increased expression of the hPim-2 gene in human chronic lymphocytic leukemia and non-Hodgkin lymphoma. Leuk. Lymphoma 2004, 45, 951–955. [Google Scholar] [CrossRef] [PubMed]
- la Starza, R.; Messina, M.; Gianfelici, V.; Pierini, V.; Matteucci, C.; Pierini, T.; Limongi, M.Z.; Vitale, A.; Roti, G.; Chiaretti, S.; et al. High PIM1 expression is a biomarker of T-cell acute lymphoblastic leukemia with JAK/STAT activation or t(6;7)(p21;q34)/TRB@-PIM1 rearrangement. Leukemia 2018, 32, 1807–1810. [Google Scholar] [CrossRef] [PubMed]
- de Smedt, R.; Peirs, S.; Morscio, J.; Matthijssens, F.; Roels, J.; Reunes, L.; Lintermans, B.; Goossens, S.; Lammens, T.; van Roy, N.; et al. Pre-clinical evaluation of second generation pim inhibitors for the treatment of t-cell acute lymphoblastic leukemia and lymphoma. Haematologica 2019, 104, e17–e20. [Google Scholar] [CrossRef] [PubMed]
- Arrouchi, H.; Lakhlili, W.; Ibrahimi, A. A review on PIM kinases in tumors. Bioinformation 2019, 15, 40–45. [Google Scholar] [CrossRef]
- Padi, S.K.R.; Luevano, L.A.; An, N.; Pandey, R.; Singh, N.; Song, J.H.; Aster, J.C.; Yu, X.Z.; Mehrotra, S.; Kraft, A.S. Targeting the PIM protein kinases for the treatment of a T-cell acute lymphoblastic leukemia subset. Oncotarget 2017, 8, 30199–30216. [Google Scholar] [CrossRef]
- Jamieson, C.A.M.; Yamamoto, K.R. Crosstalk pathway for inhibition of glucocorticoid-induced apoptosis by T cell receptor signaling. Proc. Natl. Acad. Sci. USA 2000, 97, 7319–7324. [Google Scholar] [CrossRef]
- Laukkanen, S.; Veloso, A.; Yan, C.; Oksa, L.; Alpert, E.J.; Do, D.; Hyvärinen, N.; McCarthy, K.; Adhikari, A.; Yang, Q. Therapeutic targeting of LCK tyrosine kinase and mTOR signaling in T-cell acute lymphoblastic leukemia. Blood J. Am. Soc. Hematol. 2022, 140, 1891–1906. [Google Scholar] [CrossRef]
- Hibi, M.; Lin, A.; Smeal, T.; Minden, A.; Karin, M. Identification of an oncoprotein-and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 1993, 7, 2135–2148. [Google Scholar] [CrossRef]
- Weston, C.R.; Davis, R.J. The JNK signal transduction pathway. Curr. Opin. Cell Biol. 2007, 19, 142–149. [Google Scholar] [CrossRef]
- Yu, C.; Minemoto, Y.; Zhang, J.; Liu, J.; Tang, F.; Bui, T.N.; Xiang, J.; Lin, A. JNK Suppresses Apoptosis via Phosphorylation of the Proapoptotic Bcl-2 Family Protein BAD. Mol. Cell 2004, 13, 329–340. [Google Scholar] [CrossRef]
- Cui, J.; Wang, Q.; Wang, J.; Lv, M.; Zhu, N.; Li, Y.; Feng, J.; Shen, B.; Zhang, J. Basal c-Jun NH2-terminal protein kinase activity is essential for survival and proliferation of T-cell acute lymphoblastic leukemia cells. Mol. Cancer Ther. 2009, 8, 3214–3222. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, J.; Li, J.; Volk, A.; Breslin, P.; Zhang, J.; Zhang, Z. The Synergistic Repressive Effect of NF-kB and JNK Inhibitor on the Clonogenic Capacity of Jurkat Leukemia Cells. PLoS ONE 2014, 9, e115490. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liou, J.T.; Lin, C.S.; Liao, Y.C.; Ho, L.J.; Yang, S.P.; Lai, J.H. JNK/AP-1 activation contributes to tetrandrine resistance in T-cell acute lymphoblastic leukaemia. Acta Pharmacol. Sin. 2017, 38, 1171–1183. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.; Gao, K.; Chu, L.; Zhang, R.; Yang, J.; Zheng, J. Aurora kinases: Novel therapy targets in cancers. Oncotarget 2017, 8, 23937–23954. [Google Scholar] [CrossRef]
- Willems, E.; Dedobbeleer, M.; Digregorio, M.; Lombard, A.; Lumapat, P.N.; Rogister, B. The functional diversity of Aurora kinases: A comprehensive review. Cell Div. 2018, 13, 7. [Google Scholar] [CrossRef]
- Nesbit, C.E.; Tersak, J.M.; Prochownik, E.V. MYC oncogenes and human neoplastic disease. Oncogene 1999, 18, 3004–3016. [Google Scholar] [CrossRef]
- Bonnet, M.; Loosveld, M.; Montpellier, B.; Navarro, J.M.; Quilichini, B.; Picard, C.; Cristofaro, J.D.; Bagnis, C.; Fossat, C.; Hernandez, L.; et al. Posttranscriptional deregulation of MYC via PTEN constitutes a major alternative pathway of MYC activation in T-cell acute lymphoblastic leukemia. Blood 2011, 117, 6650–6659. [Google Scholar] [CrossRef]
- Roderick, J.E.; Tesell, J.; Shultz, L.D.; Brehm, M.A.; Greiner, D.L.; Harris, M.H.; Silverman, L.B.; Sallan, S.E.; Gutierrez, A.; Look, A.T.; et al. c-Myc inhibition prevents leukemia initiation in mice and impairs the growth of relapsed and induction failure pediatric T-ALL cells. Blood 2014, 123, 1040–1050. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, J.; Yue, M.; Cai, X.; Wang, T.; Wu, C.; Su, H.; Wang, Y.; Han, M.; Zhang, Y.; et al. Direct Phosphorylation and Stabilization of MYC by Aurora B Kinase Promote T-cell Leukemogenesis. Cancer Cell 2020, 37, 200–215.e5. [Google Scholar] [CrossRef]
- Yang, J.; Kang, H.; Lyu, L.; Xiong, W.; Hu, Y. A target map of clinical combination therapies in oncology: An analysis of clinicaltrials.gov. Discov. Oncol. 2023, 14, 151. [Google Scholar] [CrossRef]
- Scott, E.C.; Baines, A.C.; Gong, Y.; Moore, R.; Pamuk, G.E.; Saber, H.; Subedee, A.; Thompson, M.D.; Xiao, W.; Pazdur, R.; et al. Trends in the approval of cancer therapies by the FDA in the twenty-first century. Nat. Rev. Drug Discov. 2023, 22, 625–640. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.V.; Lee, D.Y.; Li, B.; Quinlan, M.P.; Takahashi, F.; Maheswaran, S.; McDermott, U.; Azizian, N.; Zou, L.; Fischbach, M.A.; et al. A Chromatin-Mediated Reversible Drug-Tolerant State in Cancer Cell Subpopulations. Cell 2010, 141, 69–80. [Google Scholar] [CrossRef] [PubMed]
- East, M.P.; Johnson, G.L. Adaptive chromatin remodeling and transcriptional changes of the functional kinome in tumor cells in response to targeted kinase inhibition. J. Biol. Chem. 2022, 298, 101525. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Malik, S. The phosphorylation to acetylation/methylation cascade in transcriptional regulation: How kinases regulate transcriptional activities of DNA/histone-modifying enzymes. Cell Biosci. 2022, 12, 83. [Google Scholar] [CrossRef]
- Henikoff, S.; Greally, J.M. Epigenetics, cellular memory and gene regulation. Curr. Biol. 2016, 26, R644–R648. [Google Scholar] [CrossRef]
- Jones, P.A. Functions of DNA methylation: Islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 2012, 13, 484–492. [Google Scholar] [CrossRef]
- Maćkowska, N.; Drobna-śledzińska, M.; Witt, M.; Dawidowska, M. Dna methylation in t-cell acute lymphoblastic leukemia: In search for clinical and biological meaning. Int. J. Mol. Sci. 2021, 22, 1388. [Google Scholar] [CrossRef]
- Roels, J.; Thénoz, M.; Szarzyńska, B.; Landfors, M.; De Coninck, S.; Demoen, L.; Provez, L.; Kuchmiy, A.; Strubbe, S.; Reunes, L.; et al. Aging of Preleukemic Thymocytes Drives CpG Island Hypermethylation in T-cell Acute Lymphoblastic Leukemia. Blood Cancer Discov. 2020, 1, 274–289. [Google Scholar] [CrossRef]
- Kraszewska, M.D.; Dawidowska, M.; Larmonie, N.S.D.; Kosmalska, M.; Sędek, Ł.; Szczepaniak, M.; Grzeszczak, W.; Langerak, A.W.; Szczepański, T.; Witt, M. DNA methylation pattern is altered in childhood T-cell acute lymphoblastic leukemia patients as compared with normal thymic subsets: Insights into CpG island methylator phenotype in T-ALL. Leukemia 2012, 26, 367–371. [Google Scholar] [CrossRef]
- Sato, H.; Oka, T.; Shinnou, Y.; Kondo, T.; Washio, K.; Takano, M.; Takata, K.; Morito, T.; Huang, X.; Tamura, M.; et al. Multi-step aberrant CpG island hyper-methylation is associated with the progression of adult T-cell leukemia/lymphoma. Am. J. Pathol. 2010, 176, 402–415. [Google Scholar] [CrossRef]
- Hetzel, S.; Mattei, A.L.; Kretzmer, H.; Qu, C.; Chen, X.; Fan, Y.; Wu, G.; Roberts, K.G.; Luger, S.; Litzow, M.; et al. Acute lymphoblastic leukemia displays a distinct highly methylated genome. Nat. Cancer 2022, 3, 768–782. [Google Scholar] [CrossRef] [PubMed]
- Borssén, M.; Palmqvist, L.; Karrman, K.; Abrahamsson, J.; Behrendtz, M.; Heldrup, J.; Forestier, E.; Roos, G.; Degerman, S. Promoter DNA Methylation Pattern Identifies Prognostic Subgroups in Childhood T-Cell Acute Lymphoblastic Leukemia. PLoS ONE 2013, 8, e65373. [Google Scholar] [CrossRef] [PubMed]
- Ruas, M.; Peters, G. The p16(INK4a)/CDKN2A tumor suppressor and its relatives. Biochim. Biophys. Acta Rev. Cancer 1998, 1378, F115–F177. [Google Scholar] [CrossRef]
- Zhang, X.; Su, J.; Jeong, M.; Ko, M.; Huang, Y.; Park, H.J.; Guzman, A.; Lei, Y.; Huang, Y.H.; Rao, A.; et al. DNMT3A and TET2 compete and cooperate to repress lineage-specific transcription factors in hematopoietic stem cells. Nat. Genet. 2016, 48, 1014–1023. [Google Scholar] [CrossRef]
- Scourzic, L.; Couronné, L.; Pedersen, M.T.; della Valle, V.; Diop, M.; Mylonas, E.; Calvo, J.; Mouly, E.; Lopez, C.K.; Martin, N.; et al. DNMT3A R882H mutant and Tet2 inactivation cooperate in the deregulation of DNA methylation control to induce lymphoid malignancies in mice. Leukemia 2016, 30, 1388–1398. [Google Scholar] [CrossRef]
- Kikuchi, T.; Toyota, M.; Itoh, F.; Suzuki, H.; Obata, T.; Yamamoto, H.; Kakiuchi, H.; Kusano, M.; Issa, J.P.J.; Imai, K. Inactivation of p57KIP2 by regional promoter hypermethylation and histone deacetylation in human tumors. Oncogene 2002, 21, 2741–2749. [Google Scholar] [CrossRef][Green Version]
- Hatzimichael, E.; Dasoula, A.; Benetatos, L.; Makis, A.; Stebbing, J.; Crook, T.; Syrrou, M.; Bourantas, K.L. The absence of CDKN1C (p57KIP2) promoter methylation in myeloid malignancies also characterizes plasma cell neoplasms. Br. J. Haematol. 2008, 141, 557–558. [Google Scholar] [CrossRef]
- Kuang, S.Q.; Ling, X.; Sanchez-Gonzalez, B.; Yang, H.; Andreeff, M.; Garcia-Manero, G. Differential tumor suppressor properties and transforming growth factor-β responsiveness of p57KIP2 in leukemia cells with aberrant p57KIP2 promoter DNA methylation. Oncogene 2007, 26, 1439–1448. [Google Scholar] [CrossRef]
- Shen, L.L.; Toyota, M.; Kondo, Y.; Obata, T.; Daniel, S.; Pierce, S.; Imai, K.; Kantarjian, H.M.; Issa, J.P.J.; Garcia-Manero, G. Aberrant DNA methylation of p57KIP2 identifies a cell-cycle regulatory pathway with prognostic impact in adult acute lymphocytic leukemia. Blood 2003, 101, 4131–4136. [Google Scholar] [CrossRef]
- Himanen, J.P.; Saha, N.; Nikolov, D.B. Cell-cell signaling via Eph receptors and ephrins. Curr. Opin. Cell Biol. 2007, 19, 534–542. [Google Scholar] [CrossRef]
- Funk, S.D.; Orr, A.W. Ephs and ephrins resurface in inflammation, immunity, and atherosclerosis. Pharmacol. Res. 2013, 67, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Luo, H. Recent advances on T-cell regulation by receptor tyrosine kinases. Curr. Opin. Hematol. 2005, 12, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Darling, T.K.; Lamb, T.J. Emerging roles for Eph receptors and ephrin ligands in immunity. Front. Immunol. 2019, 10, 1473. [Google Scholar] [CrossRef] [PubMed]
- Shiuan, E.; Chen, J. Eph receptor tyrosine kinases in tumor immunity. Cancer Res. 2016, 76, 6452–6457. [Google Scholar] [CrossRef]
- Noberini, R.; Pasquale, E.B. Proliferation and Tumor Suppression: Not Mutually Exclusive for Eph Receptors. Cancer Cell 2009, 16, 452–454. [Google Scholar] [CrossRef]
- Herath, N.I.; Spanevello, M.D.; Sabesan, S.; Newton, T.; Cummings, M.; Duffy, S.; Lincoln, D.; Boyle, G.; Parsons, P.G.; Boyd, A.W. Over-expression of Eph and ephrin genes in advanced ovarian cancer: Ephrin gene expression correlates with shortened survival. BMC Cancer 2006, 6, 144. [Google Scholar] [CrossRef]
- Noren, N.K.; Foos, G.; Hauser, C.A.; Pasquale, E.B. The EphB4 receptor suppresses breast cancer cell tumorigenicity through an Abl-Crk pathway. Nat. Cell Biol. 2006, 8, 815–825. [Google Scholar] [CrossRef]
- Foubert, P.; Silvestre, J.S.; Souttou, B.; Barateau, V.; Martin, C.; Ebrahimian, T.G.; Leré-Déan, C.; Contreres, J.O.; Sulpice, E.; Levy, B.I.; et al. PSGL-1-mediated activation of EphB4 increases the proangiogenic potential of endothelial progenitor cells. J. Clin. Investig. 2007, 117, 1527–1537. [Google Scholar] [CrossRef]
- Pasquale, E.B. Eph-Ephrin Bidirectional Signaling in Physiology and Disease. Cell 2008, 133, 38–52. [Google Scholar] [CrossRef]
- El Zawily, A.; McEwen, E.; Toosi, B.; Vizeacoumar, F.S.; Freywald, T.; Vizeacoumar, F.J.; Freywald, A. The EphB6 receptor is overexpressed in pediatric T cell acute lymphoblastic leukemia and increases its sensitivity to doxorubicin treatment. Sci. Rep. 2017, 7, 14767. [Google Scholar] [CrossRef]
- Jiang, G.; Freywald, T.; Webster, J.; Kozan, D.; Geyer, R.; DeCoteau, J.; Narendran, A.; Freywald, A. In human leukemia cells ephrin-B-induced invasive activity is supported by Lck and is associated with reassembling of lipid raft signaling complexes. Mol. Cancer Res. 2008, 6, 291–305. [Google Scholar] [CrossRef] [PubMed]
- Kuang, S.Q.; Tong, W.G.; Yang, H.; Lin, W.; Lee, M.K.; Fang, Z.H.; Wei, Y.; Jelinek, J.; Issa, J.P.; Garcia-Manero, G. Genome-wide identification of aberrantly methylated promoter associated CpG islands in acute lymphocytic leukemia. Leukemia 2008, 22, 1529–1538. [Google Scholar] [CrossRef] [PubMed]
- Cohen, O.; Feinstein, E.; Kimchi, A. DAP-kinase is a Ca2+/calmodulin-dependent, cytoskeletal-associated protein kinase, with cell death-inducing functions that depend on its catalytic activity. EMBO J. 1997, 16, 998–1008. [Google Scholar] [CrossRef]
- Elbadawy, M.; Usui, T.; Yamawaki, H.; Sasaki, K. Novel functions of death-associated protein kinases through mitogen-activated protein kinase-related signals. Int. J. Mol. Sci. 2018, 19, 3031. [Google Scholar] [CrossRef]
- Cohen, O.; Kimchi, A. DAP-kinase: From functional gene cloning to establishment of its role in apoptosis and cancer. Cell Death Differ. 2001, 8, 6–15. [Google Scholar] [CrossRef]
- Borssén, M.; Nordlund, J.; Haider, Z.; Landfors, M.; Larsson, P.; Kanerva, J.; Schmiegelow, K.; Flaegstad, T.; Jónsson, O.G.; Frost, B.M.; et al. DNA methylation holds prognostic information in relapsed precursor B-cell acute lymphoblastic leukemia. Clin Epigenet. 2018, 10, 31. [Google Scholar] [CrossRef]
- Yang, Y.; Takeuchi, S.; Hofmann, W.K.; Ikezoe, T.; van Dongen, J.J.M.; Szczepański, T.; Bartram, C.R.; Yoshino, N.; Taguchi, H.; Koeffler, H.P. Aberrant methylation in promoter-associated CpG islands of multiple genes in acute lymphoblastic leukemia. Leuk. Res. 2006, 30, 98–102. [Google Scholar] [CrossRef]
- Gutierrez, M.I.; Siraj, A.K.; Bhargava, M.; Ozbek, U.; Banavali, S.; Chaudhary, M.A.; el Sohl, H.; Bhatia, K. Concurrent methylation of multiple genes in childhood ALL: Correlation with phenotype and molecular subgroup. Leukemia 2003, 17, 1845–1850. [Google Scholar] [CrossRef]
- Chu, D.H.; Morita, C.T.; Weiss, A. The Syk family of protein tyrosine kinases in T-cell activation and development. Immunol. Rev. 1998, 165, 167–180. [Google Scholar] [CrossRef]
- Chan, A.C.; van Oers, N.S.; Tran, A.; Turka, L.; Law, C.L.; Ryan, J.C.; Clark, E.A.; Weiss, A. Differential expression of ZAP-70 and Syk protein tyrosine kinases, and the role of this family of protein tyrosine kinases in TCR signaling. J. Immunol. 1994, 152, 4758–4766. [Google Scholar] [CrossRef]
- Li, Y.; Melnikov, A.A.; Levenson, V.; Guerra, E.; Simeone, P.; Alberti, S.; Deng, Y. A seven-gene CpG-island methylation panel predicts breast cancer progression. BMC Cancer 2015, 15, 417. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Kim, B.H.; Cho, N.Y.; Yoo, E.J.; Choi, M.; Shin, S.H.; Jang, J.J.; Suh, K.S.; Kim, Y.S.; Kang, G.H. Prognostic implications of and relationship between CpG island hypermethylation and repetitive DNA hypomethylation in hepatocellular carcinoma. Clin. Cancer Res. 2009, 15, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Ducler, A.; Gianino, N.; Villarroel-Espindola, F.; Desai, S.; Tang, D.; Zhao, H.; Syrigos, K.; Trepicchio, W.L.; Kannan, K.; Gregory, R.C.; et al. Tumor cell SYK expression modulates the tumor immune microenvironment composition in human cancer via TNF-α dependent signaling. J. Immunother. Cancer 2022, 10, e005113. [Google Scholar] [CrossRef] [PubMed]
- Ghaleb, A.M.; Yang, V.W. Krüppel-like factor 4 (KLF4): What we currently know. Gene 2017, 611, 27–37. [Google Scholar] [CrossRef]
- Hoffmeyer, K.; Raggioli, A.; Rudloff, S.; Hierholzer, A.; del Valle, I.; Hein, K.; Vogt, R.; Kemler, R. Wnt-Beta-catenin signaling regulates telomerase in stem cells and cancer cells. Science 2012, 336, 1549–1554. [Google Scholar] [CrossRef]
- Liu, Y.; Olanrewaju, Y.O.; Zheng, Y.; Hashimoto, H.; Blumenthal, R.M.; Zhang, X.; Cheng, X. Structural basis for Klf4 recognition of methylated DNA. Nucleic Acids Res. 2014, 42, 4859–4867. [Google Scholar] [CrossRef]
- Park, C.S.; Shen, Y.; Lewis, A.; Lacorazza, H.D. Role of the reprogramming factor KLF4 in blood formation. J. Leukoc. Biol. 2016, 99, 673–685. [Google Scholar] [CrossRef]
- Wei, Z.; Gao, F.; Kim, S.; Yang, H.; Lyu, J.; An, W.; Wang, K.; Lu, W. Klf4 organizes long-range chromosomal interactions with the OCT4 locus inreprogramming andpluripotency. Cell Stem Cell 2013, 13, 36–47. [Google Scholar] [CrossRef]
- Li, W.; Jiang, Z.; Li, T.; Wei, X.; Zheng, Y.; Wu, D.; Yang, L.; Chen, S.; Xu, B.; Zhong, M.; et al. Genome-wide analyses identify KLF4 as an important negative regulator in T-cell acute lymphoblastic leukemia through directly inhibiting T-cell associated genes. Mol. Cancer 2015, 14, 26. [Google Scholar] [CrossRef]
- Shen, Y.; Park, C.S.; Suppipat, K.; Mistretta, T.A.; Puppi, M.; Horton, T.M.; Rabin, K.; Gray, N.S.; Meijerink, J.P.P.; Lacorazza, H.D. Inactivation of KLF4 promotes T-cell acute lymphoblastic leukemia and activates the MAP2K7 pathway. Leukemia 2017, 31, 1314–1324. [Google Scholar] [CrossRef]
- Shen, Y.; Chen, T.J.; Lacorazza, H.D. Novel tumor-suppressor function of KLF4 in pediatric T-cell acute lymphoblastic leukemia. Exp. Hematol. 2017, 53, 16–25. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, A.D.; Hoff, F.W.; Qiu, Y.H.; Chandra, J.; Jabbour, E.; de Bont, E.S.J.M.; Horton, T.M.; Kornblau, S.M. Loss of H3K27 methylation identifies poor outcomes in adult-onset acute leukemia. Clin. Epigenet. 2021, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Kantarjian, H.; Jabbour, E.; Kanagal-Shamanna, R.; Patel, K.; Pierce, S.; Garcia-Manero, G.; Borthakur, G.; Ravandi, F.; O’Brien, S.; et al. Clinical characteristics of Philadelphia positive T-cell lymphoid leukemias—(De novo and blast phase CML). Am. J. Hematol. 2017, 92, E3–E4. [Google Scholar] [CrossRef] [PubMed]
- Einsiedel, H.G.; Kawan, L.; Eckert, C.; Witt, O.; Fichtner, I.; Henze, G.; Seeger, K. Histone deacetylase inhibitors have antitumor activity in two NOD/SCID mouse models of B-cell precursor childhood acute lymphoblastic leukemia. Leukemia 2006, 20, 1435–1436. [Google Scholar] [CrossRef]
- Okabe, S.; Tauchi, T.; Ohyashiki, K. Efficacy of MK-0457 and in combination with vorinostat against Philadelphia chromosome positive acute lymphoblastic leukemia cells. Ann. Hematol. 2010, 89, 1081–1087. [Google Scholar] [CrossRef]
- Nguyen, T.; Dai, Y.; Attkisson, E.; Kramer, L.; Jordan, N.; Nguyen, N.; Kolluri, N.; Muschen, M.; Grant, S. HDAC inhibitors potentiate the activity of the BCR/ABL kinase inhibitor KW-2449 in imatinib-sensitive or -resistant BCR/ABL+ leukemia cells in vitro and in vivo. Clin. Cancer Res. 2011, 17, 3219–3232. [Google Scholar] [CrossRef]
- Asano, T. Drug resistance in cancer therapy and the role of epigenetics. J. Nippon Med. Sch. 2020, 87, 244–251. [Google Scholar] [CrossRef]
- Dunsmore, K.P.; Winter, S.S.; Devidas, M.; Wood, B.L.; Esiashvili, N.; Chen, Z.; Eisenberg, N.; Briegel, N.; Hayashi, R.J.; Gastier-Foster, J.M.; et al. Children’s oncology group AALL0434: A phase III randomized clinical trial testing nelarabine in newly diagnosed t-cell acute lymphoblastic leukemia. J. Clin. Oncol. 2020, 38, 3282–3293. [Google Scholar] [CrossRef]
- Cooper, T.M. Role of nelarabine in the treatment of T-cell acute lymphoblastic leukemia and T-cell lymphoblastic lymphoma. Ther. Clin. Risk Manag. 2007, 3, 1135–1141. [Google Scholar]
- Weng, A.P.; Millholland, J.M.; Yashiro-Ohtani, Y.; Arcangeli, M.L.; Lau, A.; Wai, C.; del Bianco, C.; Rodriguez, C.G.; Sai, H.; Tobias, J.; et al. c-Myc is an important direct target of Notch1 in T-cell acute lymphoblastic leukemia/lymphoma. Genes Dev. 2006, 20, 2096–2109. [Google Scholar] [CrossRef]
- Mateo, F.; Vidal-laliena, M.; Canela, N.; Zecchin, A.; Martínez-balbás, M.; Agell, N.; Giacca, M.; Pujol, M.J.; Bachs, O. The transcriptional co-activator PCAF regulates cdk2 activity. Nucleic Acids Res. 2009, 37, 7072–7084. [Google Scholar] [CrossRef] [PubMed]
- Devaiah, B.N.; Case-Borden, C.; Gegonne, A.; Hsu, C.H.; Chen, Q.; Meerzaman, D.; Dey, A.; Ozato, K.; Singer, D.S. BRD4 is a histone acetyltransferase that evicts nucleosomes from chromatin. Nat. Struct. Mol. Biol. 2016, 23, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Kyriakidis, I.; Kyriakidis, K.; Tsezou, A. MicroRNAs and the Diagnosis of Childhood Acute Lymphoblastic Leukemia: Systematic Review, Meta-Analysis and Re-Analysis with Novel Small RNA-Seq Tools. Cancers 2022, 14, 3976. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.S.; Costa e Silva, M.; Coutinho, L.L.; Garcia Gomes, R.; Pedrosa, F.; Massaro, J.D.; Donadi, E.A.; Lucena-Silva, N. MicroRNA expression profiles discriminate childhood T- from B-acute lymphoblastic leukemia. Hematol. Oncol. 2019, 37, 103–112. [Google Scholar] [CrossRef]
- Dawidowska, M.; Jaksik, R.; Drobna, M.; Szarzyńska-Zawadzka, B.; Kosmalska, M.; Sędek, Ł.; Machowska, L.; Lalik, A.; Lejman, M.; Ussowicz, M.; et al. Comprehensive Investigation of miRNome Identifies Novel Candidate miRNA-mRNA Interactions Implicated in T-Cell Acute Lymphoblastic Leukemia. Neoplasia 2019, 21, 294–310. [Google Scholar] [CrossRef]
- Saunders, A.E.; Johnson, P. Modulation of immune cell signalling by the leukocyte common tyrosine phosphatase, CD45. Cell Signal. 2010, 22, 339–348. [Google Scholar] [CrossRef]
- Ling, Z.; Fang, Z.G.; Wu, J.Y.; Liu, J.J. The depletion of Circ-PRKDC enhances autophagy and apoptosis in T-cell acute lymphoblastic leukemia via microRNA-653-5p/Reelin mediation of the PI3K/AKT/mTOR signaling pathway. Kaohsiung J. Med. Sci. 2021, 37, 392–401. [Google Scholar] [CrossRef]
- Omorou, M.; Huang, Y.W.; Liu, N.; Bafei, S.E.C.; Gao, M.; Mu, C.X.; Zhang, L.; Hui, X. The emerging role of miR-653 in human cancer. Cancer Epidemiol. 2022, 79. [Google Scholar] [CrossRef]
- Liu, F.; Hu, L.; Pei, Y.; Zheng, K.; Wang, W.; Li, S.; Qiu, E.; Shang, G.; Zhang, J.; Zhang, X. Long non-coding RNA AFAP1-AS1 accelerates the progression of melanoma by targeting miR-653-5p/RAI14 axis. BMC Cancer 2020, 20, 258. [Google Scholar] [CrossRef]
- Williams, A.; Henao-Mejia, J.; Harman, C.C.D.; Flavell, R.A. miR-181 and metabolic regulation in the immune system. Cold Spring Harb. Symp. Quant. Biol. 2013, 78, 223–230. [Google Scholar] [CrossRef]
- Henao-Mejia, J.; Williams, A.; Goff, L.A.; Staron, M.; Licona-Limón, P.; Kaech, S.M.; Nakayama, M.; Rinn, J.L.; Flavell, R.A. The MicroRNA miR-181 Is a Critical Cellular Metabolic Rheostat Essential for NKT Cell Ontogenesis and Lymphocyte Development and Homeostasis. Immunity 2013, 38, 984–997. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.J.; Chau, J.; Ebert, P.J.R.; Sylvester, G.; Min, H.; Liu, G.; Braich, R.; Manoharan, M.; Soutschek, J.; Skare, P.; et al. miR-181a Is an Intrinsic Modulator of T Cell Sensitivity and Selection. Cell 2007, 129, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Pui, C.H.; Mullighan, C.G.; Evans, W.E.; Relling, M.V. Pediatric acute lymphoblastic leukemia: Where are we going and how do we get there? Blood 2012, 120, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Raetz, E.A.; Borowitz, M.J.; Devidas, M.; Linda, S.B.; Hunger, S.P.; Winick, N.J.; Camitta, B.M.; Gaynon, P.S.; Carroll, W.L. Reinduction Platform for Children With First Marrow Relapse of Acute Lymphoblastic Leukemia: A Children’s Oncology Group Study. J. Clin. Oncol. 2008, 26, 3971–3978. [Google Scholar] [CrossRef]
- Logie, E.; Chirumamilla, C.S.; Perez-novo, C.; Shaw, P.; Declerck, K.; Palagani, A.; Rangarajan, S.; Cuypers, B.; De Neuter, N.; Mobashar Hussain Urf Turabe, F.; et al. Covalent cysteine targeting of Bruton’s Tyrosine kinase (BTK) family by withaferin-A reduces survival of glucocorticoid-resistant multiple myeloma mm1 cells. Cancers 2021, 13, 1618. [Google Scholar] [CrossRef]
- Chirumamilla, C.S.; Fazil, M.H.U.T.; Perez-Novo, C.; Rangarajan, S.; de Wijn, R.; Ramireddy, P.; Verma, N.K.; Vanden Berghe, W. Profiling activity of cellular kinases in migrating T-cells. Methods Mol. Biol. 2019, 1930, 99–113. [Google Scholar] [CrossRef]
- Fazil, M.H.U.T.; Chirumamilla, C.S.; Perez-Novo, C.; Wong, B.H.S.; Kumar, S.; Sze, S.K.; Berghe, W.V.; Verma, N.K. The steroidal lactone withaferin A impedes T-cell motility by inhibiting the kinase ZAP70 and subsequent kinome signaling. J. Biol. Chem. 2021, 297, 101377. [Google Scholar] [CrossRef]
- Yang, Y.; Li, S.; Wang, Y.; Zhao, Y.; Li, Q. Protein tyrosine kinase inhibitor resistance in malignant tumors: Molecular mechanisms and future perspective. Signal Transduct. Target. Ther. 2022, 7, 329. [Google Scholar] [CrossRef]
- Wu, D.; Sun, Q.; Tang, H.; Xiao, H.; Luo, J.; Ouyang, L.; Sun, Q. Acquired resistance to tyrosine kinase targeted therapy: Mechanism and tackling strategies. Drug Resist. Updates 2025, 78, 101176. [Google Scholar] [CrossRef]
- Adebayo, A.K.; Bhat-Nakshatri, P.; Davis, C.; Angus, S.P.; Erdogan, C.; Gao, H.; Green, N.; Kumar, B.; Liu, Y.; Nakshatri, H. Oxygen tension-dependent variability in the cancer cell kinome impacts signaling pathways and response to targeted therapies. iScience 2024, 27, 110068. [Google Scholar] [CrossRef]
- Magliulo, D.; Bernardi, R. Hypoxic stress and hypoxia-inducible factors in leukemias. Front. Oncol. 2022, 12, 973978. [Google Scholar] [CrossRef] [PubMed]
- Chiarini, F.; Lonetti, A.; Evangelisti, C.; Buontempo, F.; Orsini, E.; Evangelisti, C.; Cappellini, A.; Neri, L.M.; McCubrey, J.A.; Martelli, A.M. Advances in understanding the acute lymphoblastic leukemia bone marrow microenvironment: From biology to therapeutic targeting. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 449–463. [Google Scholar] [CrossRef] [PubMed]
- Lyu, A.; Nam, S.H.; Humphrey, R.S.; Horton, T.M.; Ehrlich, L.I.R. Cells and signals of the leukemic microenvironment that support progression of T-cell acute lymphoblastic leukemia (T-ALL). Exp. Mol. Med. 2024, 56, 2337–2347. [Google Scholar] [CrossRef] [PubMed]
- Kaehler, M.; Osteresch, P.; Künstner, A.; Vieth, S.J.; Esser, D.; Möller, M.; Busch, H.; Vater, I.; Spielmann, M.; Cascorbi, I.; et al. Clonal evolution in tyrosine kinase inhibitor-resistance: Lessons from in vitro-models. Front. Oncol. 2023, 13, 1200897. [Google Scholar] [CrossRef]
- Waanders, E.; Gu, Z.; Dobson, S.M.; Antić, Ž.; Crawford, J.C.; Ma, X.; Edmonson, M.N.; Payne-Turner, D.; van de Vorst, M.; Jongmans, M.C.J.; et al. Mutational Landscape and Patterns of Clonal Evolution in Relapsed Pediatric Acute Lymphoblastic Leukemia. Blood Cancer Discov. 2020, 1, 96–111. [Google Scholar] [CrossRef]
- Yesilkanal, A.E.; Johnson, G.L.; Ramos, A.F.; Rosner, M.R. New strategies for targeting kinase networks in cancer. J. Biol. Chem. 2021, 297, 101128. [Google Scholar] [CrossRef]
- Joisa, C.U.; Chen, K.A.; Berginski, M.E.; Golitz, B.T.; Jenner, M.R.; Loeza, G.H.; Yeh, J.J.; Gomez, S.M. Integrated single-dose kinome profiling data is predictive of cancer cell line sensitivity to kinase inhibitors. PeerJ 2023, 11, e16342. [Google Scholar] [CrossRef]
- Biersack, B.; Polat, S.; Höpfner, M. Anticancer properties of chimeric HDAC and kinase inhibitors. Semin. Cancer Biol. 2022, 83, 472–486. [Google Scholar] [CrossRef]
- Ranganna, K.; Selvam, C.; Shivachar, A.; Yousefipour, Z. Histone deacetylase inhibitors as multitarget-directed epi-drugs in blocking pi3k oncogenic signaling: A polypharmacology approach. Int. J. Mol. Sci. 2020, 21, 8198. [Google Scholar] [CrossRef]
- Stazi, G.; Fioravanti, R.; Mai, A.; Mattevi, A.; Valente, S. Histone deacetylases as an epigenetic pillar for the development of hybrid inhibitors in cancer. Curr. Opin. Chem. Biol. 2019, 50, 89–100. [Google Scholar] [CrossRef]
- de Lera, A.R.; Ganesan, A. Epigenetic polypharmacology: From combination therapy to multitargeted drugs. Clin. Epigenet. 2016, 8, 105. [Google Scholar] [CrossRef] [PubMed]
- Doostmohammadi, A.; Jooya, H.; Ghorbanian, K.; Gohari, S.; Dadashpour, M. Potentials and future perspectives of multi-target drugs in cancer treatment: The next generation anti-cancer agents. Cell Commun. Signal. 2024, 22, 228. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Börgeling, Y.; Zardo, P.; Jonigk, D.; Borlak, J. Comprehensive transcriptome, miRNA and kinome profiling identifies new treatment options for personalized lung cancer therapy. Clin. Transl. Med. 2025, 15, e70177. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Novo, C.A.; Driesen, A.; Van Trimpont, M.; Theys, C.; Logie, E.; Van Vlierberghe, P.; Vanden Berghe, W. Epigenetic Rewiring of Protein Kinase Signalling in T-Cell Acute Lymphoblastic Leukaemia. Kinases Phosphatases 2025, 3, 7. https://doi.org/10.3390/kinasesphosphatases3020007
Pérez-Novo CA, Driesen A, Van Trimpont M, Theys C, Logie E, Van Vlierberghe P, Vanden Berghe W. Epigenetic Rewiring of Protein Kinase Signalling in T-Cell Acute Lymphoblastic Leukaemia. Kinases and Phosphatases. 2025; 3(2):7. https://doi.org/10.3390/kinasesphosphatases3020007
Chicago/Turabian StylePérez-Novo, Claudina A., Amber Driesen, Maaike Van Trimpont, Claudia Theys, Emilie Logie, Pieter Van Vlierberghe, and Wim Vanden Berghe. 2025. "Epigenetic Rewiring of Protein Kinase Signalling in T-Cell Acute Lymphoblastic Leukaemia" Kinases and Phosphatases 3, no. 2: 7. https://doi.org/10.3390/kinasesphosphatases3020007
APA StylePérez-Novo, C. A., Driesen, A., Van Trimpont, M., Theys, C., Logie, E., Van Vlierberghe, P., & Vanden Berghe, W. (2025). Epigenetic Rewiring of Protein Kinase Signalling in T-Cell Acute Lymphoblastic Leukaemia. Kinases and Phosphatases, 3(2), 7. https://doi.org/10.3390/kinasesphosphatases3020007







