Periarticular Calcifications: Clinical Features and Treatment Options
Abstract
1. Introduction
2. Mechanisms Leading to the Deposits and Pro-Inflammatory Effects of Apatite Crystals
3. Clinical Presentations
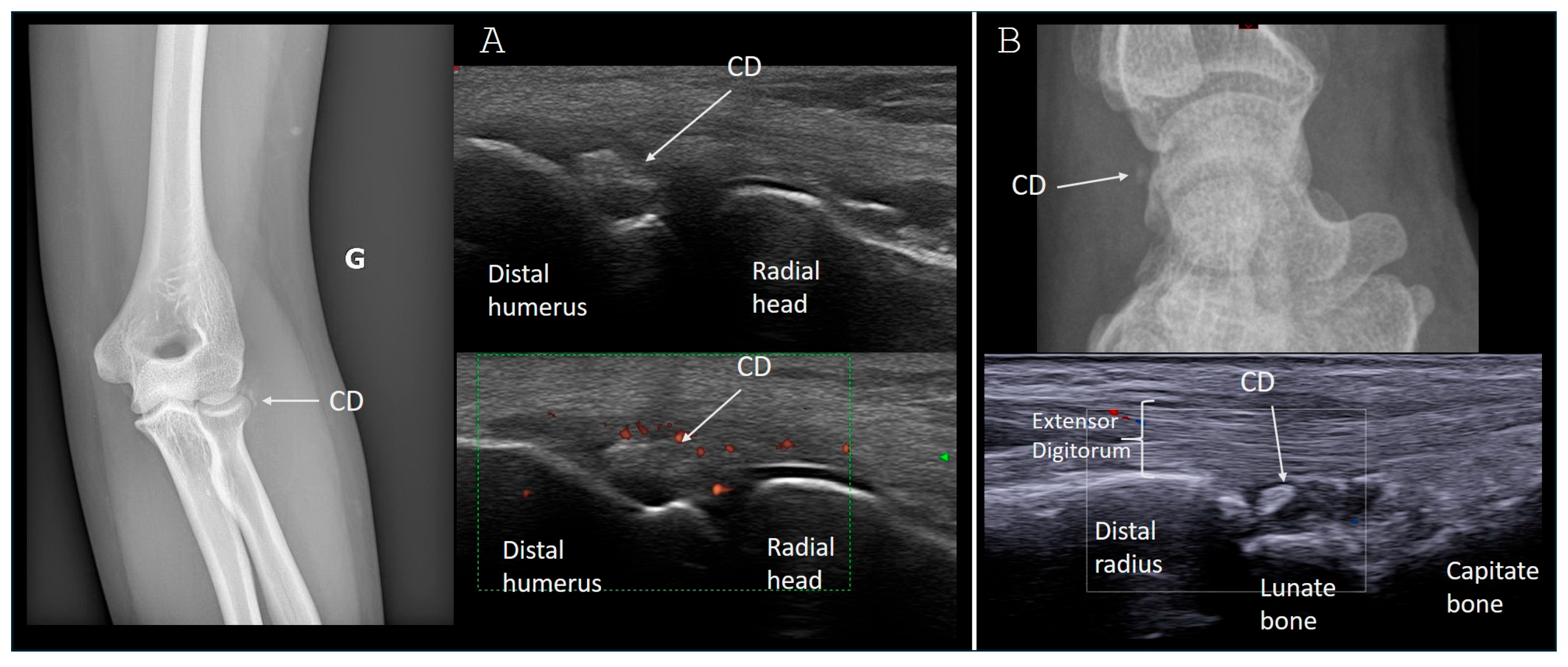
3.1. Upper Limb Involvement
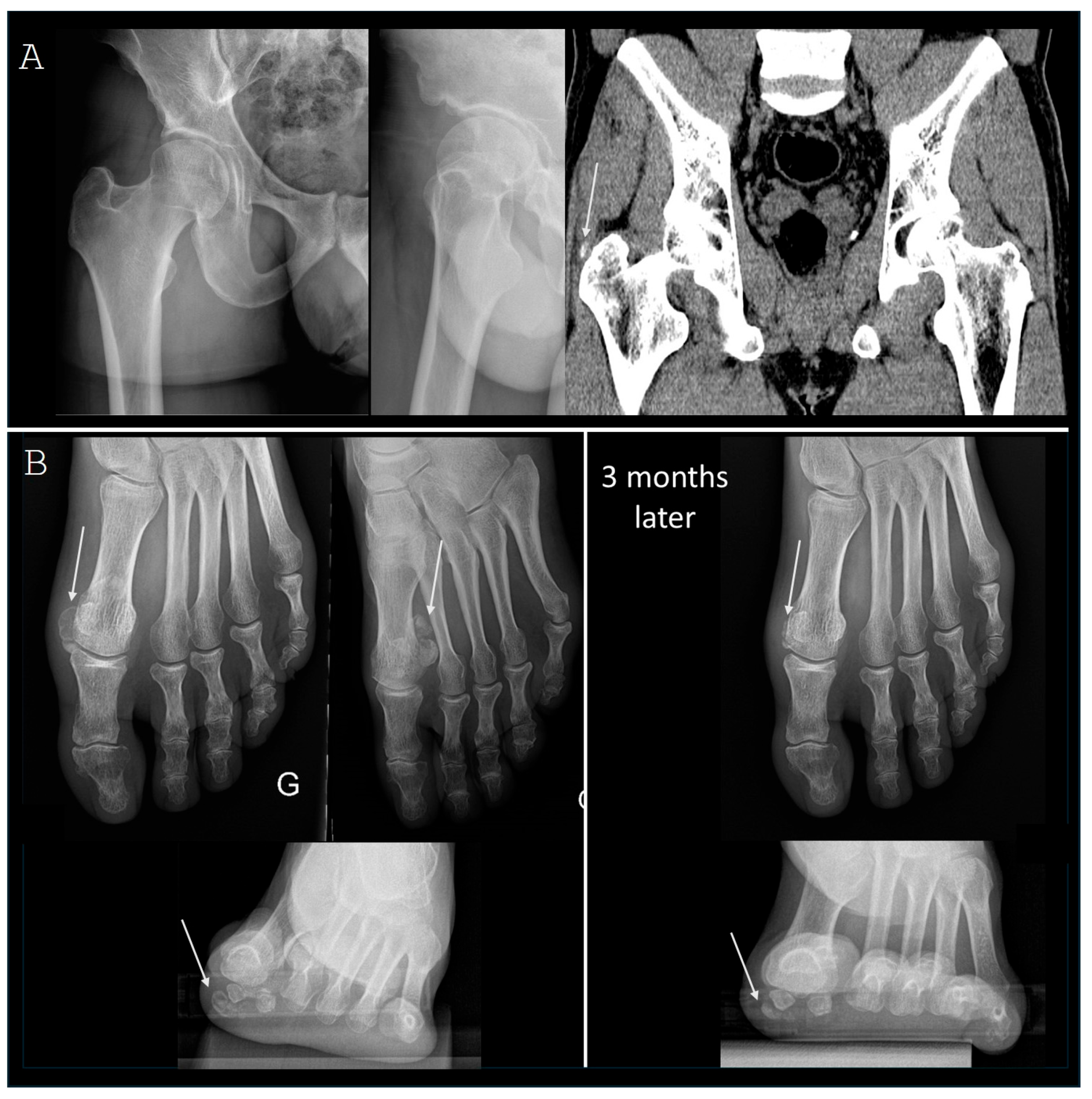
3.2. Lower Limb Involvement
3.3. Spinal Locations
3.4. Diffuse and Family Diseases
4. Treatments
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Su, Y.C.; Chung, C.H.; Ke, M.J.; Chen, L.C.; Chien, W.C.; Wu, Y.T. Increased risk of shoulder calcific tendinopathy in diabetes mellitus: A nationwide, population-based, matched cohort study. Int. J. Clin. Pract. 2021, 75, e14549. [Google Scholar] [CrossRef] [PubMed]
- Harvie, P.; Pollard, T.C.; Carr, A.J. Calcific tendinitis: Natural history and association with endocrine disorders. J. Shoulder Elbow Surg. 2007, 16, 169–173. [Google Scholar] [CrossRef]
- Grases, F.; Muntaner-Gimbernat, L.; Vilchez-Mira, M.; Costa-Bauzá, A.; Tur, F.; Prieto, R.M.; Torrens-Mas, M.; Vega, F.G. Characterization of deposits in patients with calcific tendinopathy of the supraspinatus. Role of phytate and osteopontin. J. Orthop. Res. 2015, 33, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Grases, F.; Prieto, R.M.; Sanchis, P.; Saus, C.; De Francisco, T. Role of phytate and osteopontin in the mechanism of soft tissue calcification. J. Nephrol. 2008, 21, 768–775. [Google Scholar]
- Canon, A.; Roy, L.; Chevalier, X.; Giraudier, S.; Eymard, F. Calcific tendinopathy: An unexpected side effect of tyrosine kinase inhibitor? Leuk. Lymphoma 2022, 63, 3175–3180. [Google Scholar] [CrossRef] [PubMed]
- Darrieutort-Laffite, C.; Arnolfo, P.; Garraud, T.; Adrait, A.; Couté, Y.; Louarn, G.; Trichet, V.; Layrolle, P.; Le Goff, B.; Blanchard, F. Rotator Cuff Tenocytes Differentiate into Hypertrophic Chondrocyte-like Cells to Produce Calcium Deposits in an Alkaline Phosphatase-Dependent Manner. J. Clin. Med. 2019, 8, 1544. [Google Scholar] [CrossRef]
- Uhthoff, H.K.; Loebr, J.W. Calcific tendinopathy of the rotator cuff: Pathogenesis, diagnosis, and management. J. Am. Acad. Orthop. Surg. 1997, 5, 183–191. [Google Scholar] [CrossRef]
- Hu, C.; Ma, L.; Gao, S.; Yang, M.Y.; Mu, M.D.; Chang, L.; Huang, P.; Ye, X.; Wang, W.; Tao, X.; et al. PPP1R3A inhibits osteogenesis and negatively regulates intracellular calcium levels in calcific tendinopathy. iScience 2023, 26, 107784. [Google Scholar] [CrossRef] [PubMed]
- Sougué, C.; Darrieutort-Laffite, C.; Maugars, Y.; Le Goff, B. Location of calcifications of rotator cuff on ultrasound in 74 patients: Near the junction between the supraspinatus and infraspinatus tendons in 96% of the cases. Jt. Bone Spine 2021, 88, 105–107. [Google Scholar] [CrossRef]
- Archer, R.S.; Bayley, J.I.; Archer, C.W.; Ali, S.Y. Cell and matrix changes associated with pathological calcification of the human rotator cuff tendons. J. Anat. 1993, 182, 1–11. [Google Scholar]
- Herman, J.; Le Goff, B.; De Lima, J.; Brion, R.; Chevalier, C.; Blanchard, F.; Darrieutort-Laffite, C. Pro-inflammatory effects of human apatite crystals extracted from patients suffering from calcific tendinopathy. Arthritis Res. Ther. 2021, 23, 131. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Frayssinet, P.; Pelker, R.; Cwirka, D.; Hu, B.; Vignery, A.; Eisenbarth, S.C.; Flavell, R.A. NLRP3 inflammasome plays a critical role in the pathogenesis of hydroxyapatite-associated arthropathy. Proc. Natl. Acad. Sci. USA 2011, 108, 14867–14872. [Google Scholar] [CrossRef] [PubMed]
- Molé, D.; Kempf, J.F.; Gleyze, P.; Rio, B.; Bonnomet, F.; Walch, G. Results of endoscopic treatment of non-broken tendinopathies of the rotator cuff. 2. Calcifications of the rotator cuff. Rev. Chir. Orthop. Reparatrice Appar. Mot. 1993, 79, 532–541. [Google Scholar] [PubMed]
- Gärtner, J.; Heyer, A. Calcific tendinitis of the shoulder. Orthopade 1995, 24, 284–302. [Google Scholar]
- Maier, M.; Schmidt-Ramsin, J.; Glaser, C.; Kunz, A.; Küchenhoff, H.; Tischer, T. Intra-and interobserver reliability of classification scores in calcific tendinitis using plain radiographs and CT scans. Acta Orthop. Belg. 2008, 74, 590–595. [Google Scholar] [PubMed]
- De Witte, P.B.; Van Adrichem, R.A.; Selten, J.W.; Nagels, J.; Reijnierse, M.; Nelissen, R.G.H.H. Radiological and clinical predictors of long-term outcome in rotator cuff calcific tendinitis. Eur. Radiol. 2016, 26, 3401–3411. [Google Scholar] [CrossRef] [PubMed]
- Louwerens, J.K.; Claessen, F.M.; Sierevelt, I.N.; Eygendaal, D.; Van Noort, A.; Van Den Bekerom, M. Radiographic assessment of calcifying tendinitis of the rotator cuff: An inter- and intraobserver study. Acta Orthop. Belg. 2020, 86, 525–531. [Google Scholar]
- Louwerens, J.K.; Sierevelt, I.N.; van Hove, R.P.; van den Bekerom, M.P.; van Noort, A. Prevalence of calcific deposits within the rotator cuff tendons in adults with and without subacromial pain syndrome: Clinical and radiologic analysis of 1219 patients. J. Shoulder Elbow Surg. 2015, 24, 1588–1593. [Google Scholar] [CrossRef]
- Le Goff, B.; Berthelot, J.M.; Guillot, P.; Glémarec, J.; Maugars, Y. Assessment of calcific tendonitis of rotator cuff by ultrasonography: Comparison between symptomatic and asymptomatic shoulders. Jt. Bone Spine 2010, 77, 258–263. [Google Scholar] [CrossRef]
- Paruthikunnan, S.M.; Boily, M.; Martin, M.H.; Assaf, A.; Jaffer, R. Intra-osseous migration in calcific rotator cuff tendinopathy- a novel depiction of temporal evolution on multimodality imaging. BJR Case Rep. 2022, 8, 20210156. [Google Scholar] [CrossRef]
- Zampa, V.; Aringhieri, G.; Rossi, P.; Capanna, R.; Caramella, D. Humeral greater tuberosity osteolysis as a complication of intraosseous calcification migration: Natural history depicted by imaging. Acta Biomed. 2021, 92, e2021052. [Google Scholar]
- Iovane, A.; Terrasi, M.; Iovane, E.M.; Mantia, C.; Messina, G.; Mantia, F. Intramuscular migration of calcium deposits into the deltoid muscle: Two cases of a rare complication of rotator cuff calcific tendinopathy. J. Ultrasound 2023, 26, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Cahir, J.; Saifuddin, A. Calcific tendonitis of pectoralis major: CT and MRI findings. Skeletal Radiol. 2005, 34, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Vaishya, R.; Siddalingeshwara, G.I.; Vaishya, A. Calcific Tendonitis of the Elbow in an Adult—A Case Report and Review of the Literature. J. Orthop. Case Rep. 2020, 10, 57–60. [Google Scholar]
- Kim, J.K.; Park, E.S. Acute calcium deposits in the hand and wrist; comparison of acute calcium peritendinitis and acute calcium periarthritis. J. Hand Surg. (Eur. Vol.) 2014, 39, 436–439. [Google Scholar] [CrossRef]
- Nadarajah, C.V.; Weichert, I. Milwaukee shoulder syndrome. Case Rep. Rheumatol. 2014, 2014, 458708. [Google Scholar] [CrossRef]
- Park, S.M.; Baek, J.H.; Ko, Y.B.; Lee, H.J.; Park, K.J.; Ha, Y.C. Management of acute calcific tendinitis around the hip joint. Am. J. Sports Med. 2014, 42, 2659–2665. [Google Scholar] [CrossRef]
- Yang, W.B.; Xu, Q.K.; Liu, X.H.; Bakhshi, P.; Wang, H.; Shao, Z.W.; Meng, C.Q.; Huang, W. Arthroscopic Treatment of Calcific Tendinitis of Gemellus Superior and Gemellus Inferior: A Case Report and Literature Review. Orthop. Surg. 2022, 14, 621–627. [Google Scholar] [CrossRef]
- Watura, K.; Greenish, D.; Williams, M.; Webb, J. Acute calcific periarthiritis of the knee presenting with calcification within the lateral collateral ligament. BMJ Case Rep. 2015, 2015, bcr2014209041. [Google Scholar] [CrossRef]
- Turner, V.L.; Martinez, C.; Rocha, J.; Valenzuela, A. Acute calcific tendinitis of the longus colli: A case report. Radiol. Case Rep. 2024, 19, 2650–2653. [Google Scholar] [CrossRef] [PubMed]
- Reitz, I.; Allen, C.; Rappaport, D.E. An Unusual Cause of Fever, Neck Pain, and Neck Stiffness: Acute Calcific Tendinitis of the Longus Colli Muscle. J. Emerg. Med. 2023, 65, e307–e309. [Google Scholar] [CrossRef] [PubMed]
- Urrutia, J.; Contreras, O. Calcium hydroxyapatite crystal deposition with intraosseous penetration involving the posterior aspect of the cervical spine: A previously unreported cause of neck pain. Eur. Spine J. 2017, 26 (Suppl. S1), 53–57. [Google Scholar] [CrossRef] [PubMed]
- Amor, B.; Cherot, A.; Delbarre, F. Hydroxyapatite rheumatism (multiple tendon calcification disease). I.-Clinical study. Rev. Rhum. Mal. Osteoartic. 1977, 44, 301–308. [Google Scholar] [PubMed]
- Guanabens, N.; Mumm, S.; Moller, I.; González-Roca, E.; Peris, P.; Demertzis, J.L.; Whyte, M. Calcific periarthritis as the only clinical manifestation of hypophosphatasia in middle-aged sisters. J. Bone Miner. Res. 2014, 29, 929–934. [Google Scholar] [CrossRef]
- Cudrici, C.D.; Newman, K.A.; Ferrante, E.A.; Huffstutler, R.; Carney, K.; Betancourt, B.; Miettinen, M.; Siegel, R.; Katz, J.D.; Nesti, L.J.; et al. Multifocal calcific periarthritis with distinctive clinical and radiological features in patients with CD73 deficiency. Rheumatology 2021, 61, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Zufferey, P.; So, A. A pilot study of IL-1 inhibition in acute calcific periarthritis of the shoulder. Ann. Rheum. Dis. 2013, 72, 465–467. [Google Scholar] [CrossRef] [PubMed]
- Zufferey, P.; Valcov, R.; Thomas, M.; Dumusc, A.; Forien, M.; So, A.; Ottaviani, S. Efficacy of anakinra in acute hydroxyapatite calcification-induced joint pain: A retrospective study of 23 cases. Jt. Bone Spine 2019, 86, 83–88. [Google Scholar] [CrossRef]
- Maugars, Y.; Varin, S.; Gouin, F.; Huguet, D.; Rodet, D.; Nizard, J.; N’Guyen, J.M.; Guillot, P.; Glémarec, J.; Passutti, N.; et al. Treatment of shoulder calcifications of the cuff: A controlled study. Jt. Bone Spine 2009, 76, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Bannuru, R.R.; Flavin, N.E.; Vaysbrot, E.; Harvey, W.; McAlindon, T. High-energy extracorporeal shock-wave therapy for treating chronic calcific tendinitis of the shoulder: A systematic review. Ann. Intern. Med. 2014, 160, 542–549. [Google Scholar] [CrossRef]
- Darrieutort-Laffite, C.; Varin, S.; Coiffier, G.; Albert, J.D.; Planche, L.; Maugars, Y.; Cormier, G.; Le Goff, B. Are corticosteroid injections needed after needling and lavage of calcific tendinitis? Randomised, double-blind, non-inferiority trial. Ann. Rheum. Dis. 2019, 78, 837–843. [Google Scholar] [CrossRef]
- Dumoulin, N.; Cormier, G.; Varin, S.; Coiffier, G.; Albert, J.D.; Le Goff, B.; Darrieutort-Laffite, C. Factors associated with clinical improvement and the disappearance of calcifications after ultrasound-guided percutaneous lavage of rotator cuff calcific tendinopathy: A post hoc analysis of a randomized controlled trial. Am. J. Sports Med. 2021, 49, 883–891. [Google Scholar] [CrossRef]
- Dalla-Torre, R.; Fouasson-Chailloux, A.; Le Goff, B.; Darrieutort-Laffite, C. Development of a new radiographic score for the follow-up of calcific tendinopathy of the rotator cuff. Clin. Exp. Rheumatol. 2024, 42, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Farin, P.U.; Räsänen, H.; Jaroma, H.; Harju, A. Rotator cuff calcifications: Treatment with ultrasound-guided percutaneous needle aspiration and lavage. Skeletal Radiol. 1996, 25, 551–554. [Google Scholar] [CrossRef]
- De Conti, G.; Marchioro, U.; Dorigo, A.; Boscolo, N.; Vio, S.; Trevisan, M.; Meneghini, A.; Baldo, V.; Angelini, F. Percutaneous ultrasound-guided treatment of shoulder tendon calcifications: Clinical and radiological follow-up at 6 months. J. Ultrasound 2010, 13, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Darrieurtort-Laffite, C.; Bertrand-Vasseur, A.; Garraud, T.; Planche, L.; Le Goff, B. Tolerance and effect of sodium thiosulfate in calcific tendinitis of the rotator cuff. Clin. Rheumatol. 2020, 39, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Albano, D.; Viglino, U.; Messina, C.; Fusco, S.; Gitto, S.; Lacelli, F.; Sconfienza, L.M. US-guided percutaneous irrigation of extra-shoulder calcific tendinitis. Br. J. Radiol. 2024, 97, 267–273. [Google Scholar] [CrossRef] [PubMed]
- de Witte, P.B.; Kolk, A.; Overes, F.; Nelissen, R.G.H.H.; Reijnierse, M. Rotator Cuff Calcific Tendinitis: Ultrasound-Guided Needling and Lavage Versus Subacromial Corticosteroids: Five-Year Outcomes of a Randomized Controlled Trial. Am. J. Sports Med. 2017, 45, 3305–3314. [Google Scholar] [CrossRef] [PubMed]
- Del Castillo-González, F.; Ramos-Alvarez, J.J.; Rodríguez-Fabián, G.; González-Pérez, J.; Jiménez-Herranz, E.; Varela, E. Extracorporeal shockwaves versus ultrasound-guided percutaneous lavage for the treatment of rotator cuff calcific tendinopathy: A randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2016, 52, 145–151. [Google Scholar] [PubMed]
- Marder, R.A.; Heiden, E.A.; Kim, S. Calcific tendonitis of the shoulder: Is subacromial decompression in combination with removal of the calcific deposit beneficial? J. Shoulder Elbow Surg. 2011, 20, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Deng, Z.; Liu, Y.; Chen, R.; Chen, K.; Xu, J. Arthroscopic Surgery Versus Nonoperative Treatment for Calcific Tendinitis of the Shoulder: A Retrospective Cohort Study. Am. J. Sports Med. 2024, 52, 461–473. [Google Scholar] [CrossRef]
- Angileri, H.S.; Gohal, C.; Comeau-Gauthier, M.; Owen, M.M.; Shanmugaraj, A.; Terry, M.A.; Tjong, V.K.; Khan, M. Chronic calcific tendonitis of the rotator cuff: A systematic review and meta-analysis of randomized controlled trials comparing operative and nonoperative interventions. J. Shoulder Elbow Surg. 2023, 32, 1746–1760. [Google Scholar] [CrossRef] [PubMed]
- Serafini, G.; Sconfienza, L.M.; Lacelli, F.; Silvestri, E.; Aliprandi, A.; Sardanelli, F. Rotator cuff calcific tendonitis: Short-term and 10-year outcomes after two-needle us-guided percutaneous treatment—Nonrandomized controlled trial. Radiology 2009, 252, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Ogon, P.; Suedkamp, N.P.; Jaeger, M.; Izadpanah, K.; Koestler, W.; Maier, D. Prognostic factors in nonoperative therapy for chronic symptomatic calcific tendinitis of the shoulder. Arthritis Rheum. 2009, 60, 2978–2984. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Gout, Hyperuricemia and Crystal Associated Disease Network. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalla-Torre, R.; Le Goff, B.; Darrieutort-Laffite, C. Periarticular Calcifications: Clinical Features and Treatment Options. Gout Urate Cryst. Depos. Dis. 2024, 2, 266-274. https://doi.org/10.3390/gucdd2030020
Dalla-Torre R, Le Goff B, Darrieutort-Laffite C. Periarticular Calcifications: Clinical Features and Treatment Options. Gout, Urate, and Crystal Deposition Disease. 2024; 2(3):266-274. https://doi.org/10.3390/gucdd2030020
Chicago/Turabian StyleDalla-Torre, Romain, Benoit Le Goff, and Christelle Darrieutort-Laffite. 2024. "Periarticular Calcifications: Clinical Features and Treatment Options" Gout, Urate, and Crystal Deposition Disease 2, no. 3: 266-274. https://doi.org/10.3390/gucdd2030020
APA StyleDalla-Torre, R., Le Goff, B., & Darrieutort-Laffite, C. (2024). Periarticular Calcifications: Clinical Features and Treatment Options. Gout, Urate, and Crystal Deposition Disease, 2(3), 266-274. https://doi.org/10.3390/gucdd2030020




