Contributions to the Palaeobiodiversity of Psocodea (‘Psocoptera’) from Lebanese Amber: A Review
Abstract
1. Introduction
1.1. Overview
1.2. Phylogeny and Classification
1.3. Studies on Fossil Taxa
| Species | Family | Type Locality | Remarks |
|---|---|---|---|
| Archaeatropos randatae (Azar & Nel, 2004) | Empheriidae (Trogiomorpha) | Jouar-Ess-Souss (Jezzine) outcrop | |
| Asphaeropsocites neli Azar, Engel & Grimaldi, 2010 | Sphaeropsocidae (Troctomorpha) | Hammana–Mdeyrij outcrop | |
| Bcharreglaris amunobi Azar & Nel, 2004 | Empheriidae (Trogiomorpha) | Bcharreh outcrop | |
| Cretacetrocta libanella Hakim & Azar, 2024 | Pachytroctidae (Troctomorpha) | Bqaatouta outcrop | The assignation to Pachytroctidae is tentative; authors noted some similarities to electrentomoids as well. |
| Libaneuphoris jantopi Azar, Huang, Cai & Nel, 2015 | Pachytroctidae (Troctomorpha) | Falougha outcrop | |
| Libanoglaris chehabi Azar & Nel, 2004 | Empheriidae (Trogiomorpha) | Hammana–Mdeyrij outcrop | |
| Libanoglaris mouawadi Azar, Perrichot, Néraudeau & Nel, 2003 | Empheriidae (Trogiomorpha) | Hammana–Mdeyrij outcrop | |
| Libanomphientomum nudus Choufani, Azar & Nel, 2011 | Family Incertae sedis (Amphientometae, Troctomorpha) | Hammana–Mdeyrij outcrop | |
| Libanopsyllipsocus alexanderasnitsyni Azar & Nel, 2011 | Psyllipsocidae (Trogiomorpha) | Hammana–Mdeyrij outcrop | Belongs to Pachytroctidae (Troctomorpha) according to Mockford et al. (2013) [73] |
| Palaeosiamoglaris hammanaensis Hakim, Huang & Azar, 2022 | Prionoglarididae (Trogiomorpha) | Hammana–Mdeyrij outcrop | |
| Paramesopsocus lu Azar, Hajar, Indary & Nel, 2008 | Electrentomidae (Troctomorpha) | Hammana–Mdeyrij outcrop | |
| Setoglaris reemae Azar & Nel, 2004 | Empheriidae (Trogiomorpha) | Hammana–Mdeyrij outcrop | |
| Sphaeropsocites lebanensis Grimaldi & Engel, 2006 | Sphaeropsocidae (Troctomorpha) | Jouar-Ess-Souss (Jezzine) outcrop | |
| Sp. 1 (Nymph) | Family Incertae sedis | Ain Dara outcrop |
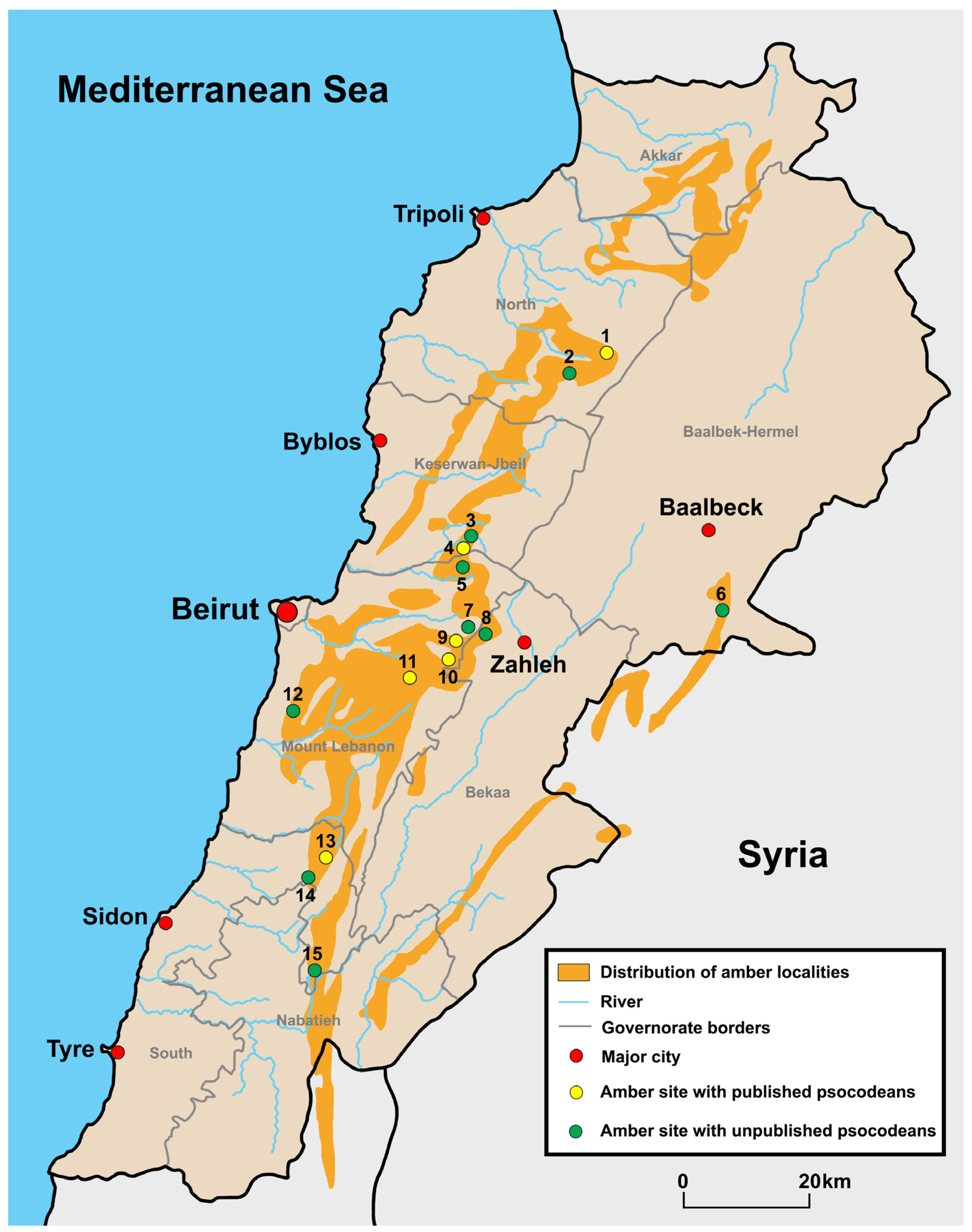
2. Lebanese Amber
2.1. Locality and Age
2.2. Scientific Significance of Lebanese Amber
2.3. Lebanese Amber Collections
3. Systematic Paleontology
- Order Psocodea Hennig, 1966 [89]
- Suborder Trogiomorpha Roesler, 1940 [90]
- Family Psyllipsocidae Kolbe, 1884 [92]
- Genus Libanopsyllipsocus Azar and Nel, 2011 [93]
- Type species. Libanopsyllipsocus alexanderasnitsyni Azar and Nel, 2011 [93]; by original designation.
- Libanopsyllipsocus alexanderasnitsyni Azar & Nel, 2011 [93] (figs 1–12)
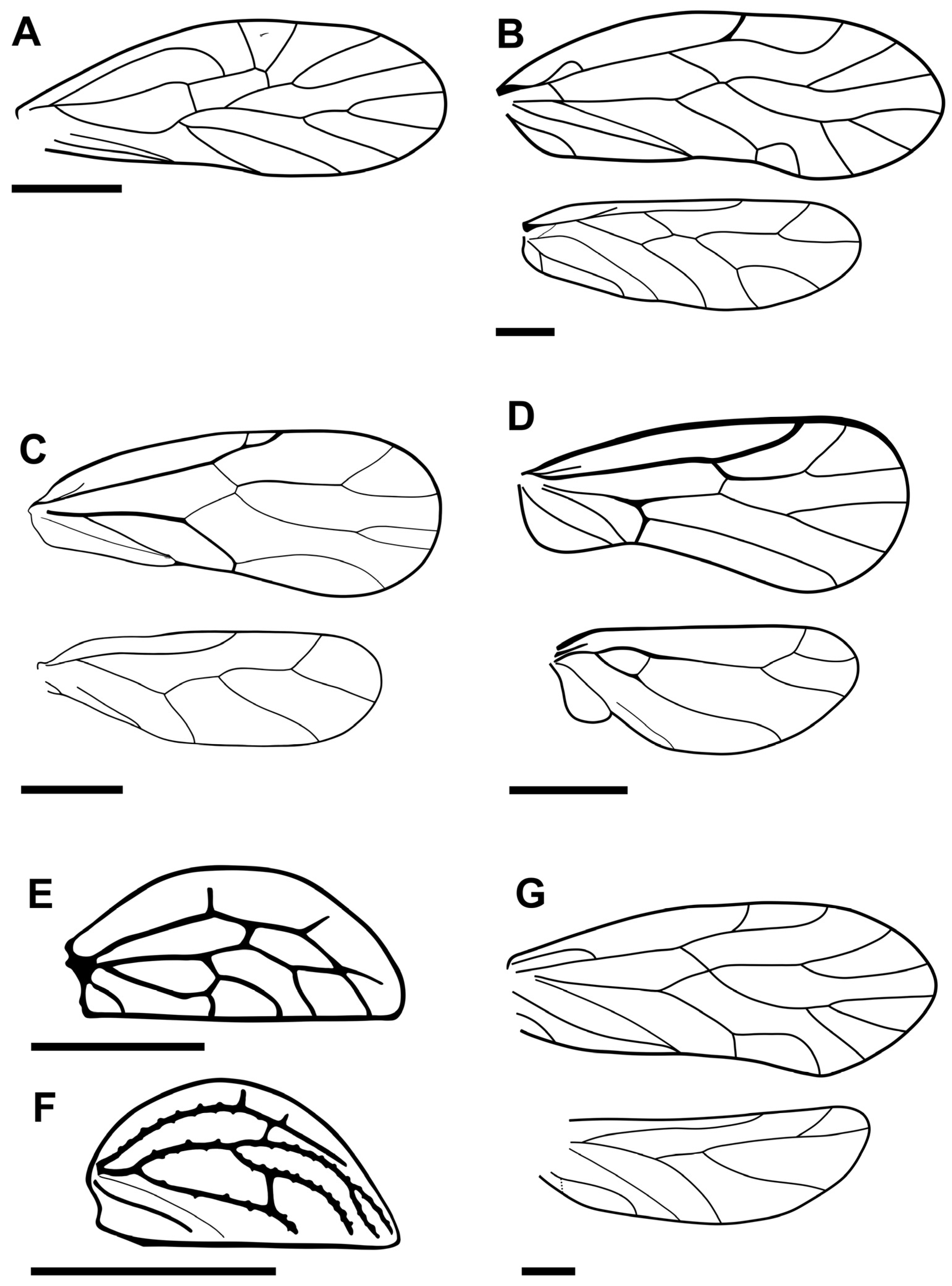
- (Figure 2A)
- Material. Specimen 30 (holotype); Azar collection, currently deposited in the Natural History Museum of the Lebanese University, Faculty of Sciences II, Fanar, Lebanon.
- Locality and age. Hammana–Mdeyrij, Baabda District (= Caza), Mount Lebanon Governorate (= Mohafazat), Central Lebanon; early Barremian, Early Cretaceous [79].
- Diagnostic remarks. Libanopsyllipsocus alexanderasnitsyni is mainly differentiated from fossil psyllipsocids by their wing traits (forewing with pterostigma absent, and M two-branched; hind wing with R1 absent, and M simple). It is noteworthy that Concavapsocus parallelus Wang, Li, Ren and Yao, 2019 also lacks a pterostigma in the forewing and has vein M simple in the hind wing, but the latter species differs from L. alexanderasnitsyni in multiple other venation traits (e.g., R2+3 forked, M simple, CuA simple, and veins CuP and A fused forming a long Y-shape in the forewings; R simple and basi-radial cell absent in hind wings) [94]. In terms of the wing venation, L. alexanderasnitsyni is closer to Pachytroctidae. The forewing venation of L. alexanderasnitsyni appears closest to that of pachytroctid Libaneuphoris jantopi Azar, Huang, Cai and Nel, 2015 [95]. Nonetheless, it is distinguished from it in the absence of the anal lobe, longer basi-radial cell, and shorter common stem of Rs-M in the hind wing. It can also be differentiated from the other four fossil pachytroctids by the absence of the pterostigma in the forewing (present in the rest) and the presence of the basi-radial cell in the hind wing (absent in the rest), as well as some other wing and body features that vary depending on the taxa.
- Azar and Nel (2011) described this taxon as a highly unusual psyllipsocid with wing characteristics very similar to Pachytroctidae [93]. They assigned it to Psyllipsocidae mainly due to the presence of ‘two distinct sclerotized filaments on the hypopharynx’, ‘nodulus in the forewings’, and ‘Pearman’s coxal organ in hind legs’, all features characteristically absent from Pachytroctidae. They also identified an ‘anal spine on each paraproct’ and stated the specimen to be male with the ‘phallosome having two curved arms not fused anteriorly’. However, the classification of this species remains under debate. Mockford et al. (2013) rejected this attribution, stating that these traits, as presented in the illustrations, are absent and/or incorrectly identified [73]. They advocated for the specimen being female and moved the species to the Pachytroctidae. The original authors (D.A. and A.N.) rejected this decision and reinstated the taxon back to Psyllipsocidae in Azar et al. (2015) and later in Hakim et al. (2018) [95,96]. To date, Libanopsyllipsocus remains monospecific and assigned to Psyllipsocidae; the discovery of new well-preserved individuals belonging to L. alexanderasnitsyni or other closely related species is crucial to solving this polemic of opposing opinions and finally clarifying the true nature of the structures involved for a better understanding of the appropriate classification.
- Family Prionoglarididae Karny, 1930 [97]
- Subfamily Prionoglaridinae Karny, 1930 [97]
- Tribe Siamoglaridini Azar, Huang and Nel, 2017 [98]
- Genus Palaeosiamoglaris Azar, Huang and Nel, 2017 [98]
- Type species. Palaeosiamoglaris lienhardi Azar, Huang and Nel, 2017 [98]; by original designation.
- Palaeosiamoglaris hammanaensis Hakim, Huang and Azar, 2022 [99] (figs 1–6)
- (Figure 2B)
- Material. Specimen 1308 (holotype), specimen 164C, specimen 904A, specimen 1095B, specimen 1428A, specimen 728A (paratypes); Azar collection, deposited at the Natural History Museum of the Lebanese University, Faculty of Sciences II, Fanar, Lebanon.
- Locality and age. Hammana–Mdeyrij, Baabda District, Mount Lebanon Governorate, Central Lebanon; early Barremian, Early Cretaceous [79].
- Diagnostic remarks. This species is mainly differentiated from other taxa of the genus by the features of the wings and, with some, the absence of a conical sensillum in the maxillary palpomeres. The wing venation patterns of Palaeosiamoglaris hammanaensis are very similar to those of Palaeosiamoglaris lienhardi Azar, Huang and Nel, 2017; both species clearly have CuP and A reaching the margin separately while the nodulus is present [98] (fig. 3B) [99] (fig. 3C). The illustration of the nodulus of Palaeosiamoglaris burmica Azar, Huang and Nel, 2017 [98] (fig. 8B) does not clearly show whether CuP and A meet or reach the margin separately due to the overlapping of the hind wing margin; thus, we can only speculate that it is possibly the same as in P. burmica and P. hammanaensis. Species Palaeosiamoglaris inexpectata Azar, Huang and Nel, 2017 and Palaeosiamoglaris hkamtiensis Jouault, Yoshizawa, Hakim, Huang and Nel, 2021 are both described as having a nodulus—CuP and A joining at the margin in P. hkamtiensis stated in the text—but no detailed pictures for the nodulus area are presented [47,98].
- Additional remarks. This genus also includes four fossil species from Burmese amber: the three species P. burmica, P. inexpectata, and P. lienhardi from Kachin amber, and the one species P. hkamtiensis from Hkamti amber.
- Infraorder Atropetae Pearman, 1936 [100]
- Family †Empheriidae Kolbe, 1884 [92]
- (= †Archaeatropidae Baz and Ortuño, 2000) [101]
- Remarks. Bcharreglaris, Libanoglaris and Setoglaris were initially not classified in a family and were suggested for attribution to either Prionoglarididae or Archaeatropidae by the original authors [43,102]. Mockford et al. (2013) later placed the genera and their respective species in Archaeatropidae based on the presence of macropterous setose wings—without scales – with the forewings having vein Sc curved apically joining R1, and the nodulus present [73]. For Setoglaris, they also listed the presence of well-developed female valvulae.
- Genus Archaeatropos Baz and Ortuño, 2000 [101]
- Type species. Archaeatropos alavensis Baz and Ortuño, 2000 [101]; by original designation.
- Archaeatropos randatae (Azar and Nel, 2004) [102] (figs 4–14)
- (Figure 2C)
- Material. Specimen JS 203/2 BM 904 (holotype); Acra collection, currently deposited in the Natural History Museum of the Lebanese University, Faculty of Sciences II, Fanar, Lebanon. Specimen JG 21/12 BM 753, specimen JG 21/7 BM 698, specimen JG 21/4 BM 738, specimen JG 21/8 BM 678, specimen JG 21/3 BM 669 A, specimen JG 21/3 BM 669 B, specimen JS 108 BM 35, specimen JG 21/10 BM 709 (paratypes); Acra collection, deposited in the American Museum of Natural History, New York, NY, USA.
- Locality and age. Jouar-Ess-Souss (Jezzine), Jezzine District, South Lebanon Governorate, Southern Lebanon; early Barremian, Early Cretaceous [79].
- Diagnostic remarks. This species is mainly distinguished from those of Bcharreglaris, Libanoglaris and Setoglaris by varying combinations of features of the wings (e.g., shape of the pterostigma, and presence/absence of setae in pterostigmal and anal areas) and antennae (number of flagellomeres, and presence/absence of secondary annulations). Azar and Nel (2004) originally assigned Archaeatropos randatae to Libanoglaris due to the absence of secondary annulations on flagellomeres and the presence of a distinct preapical tooth on the claws [102]. Mockford et al. (2013) transferred A. randatae to Archaeatropos due to the vein Sc’ being strongly curved backwards, reaching to the wing margin, a trait observed in Archaeatropos alavensis Baz and Ortuño, 2000, while the taxa of Libanoglaris have Sc’ more perpendicular to the wing margin [43,73,101,102,103]. Species A. randatae also differs from A. alavensis in the absence of a fringe on both wings (the presence of setae on the wing margins is not stated in the description of A. randatae nor illustrated in [102] (figs 12 and 13)), and the length and orientation of R1 in the forewing [101,102,103].
- Additional remarks. A. alavensis is the only other species included in this genus, described from inclusions in Spanish amber of different outcrops [103].
- Genus Bcharreglaris Azar and Nel, 2004 [102]
- Type species. Bcharreglaris amunobi Azar and Nel, 2004 [102]; by original designation.
- Bcharreglaris amunobi Azar and Nel, 2004 [102] (figs 1–3)
- (Figure 2D)
- Material. Specimen 21a (holotype), specimen 21b (paratype); Estephan collection, deposited in the American Museum of Natural History, New York, NY, USA.
- Locality and age. Bcharreh, Bcharreh District, North Lebanon Governorate, Northern Lebanon; early Barremian, Early Cretaceous [79].
- Diagnostic remarks. This species is mainly characterized from other Cretaceous empheriids by its triangular (three-angled) pterostigma.
- Additional remarks. Two additional species Bcharreglaris amooni Kaddumi, 2007 and Bcharreglaris haddadini Kaddumi, 2007 have been described from the co-eval Jordanian amber [104]. However, these species are considered by several psocodean experts to be nomen nudum since the work is not peer-reviewed and the type material is not accessible to the public or experts for examination.
- Genus Libanoglaris Azar, Perrichot, Néraudeau and Nel, 2003 [43]
- Type species. Libanoglaris mouawadi Azar, Perrichot, Néraudeau and Nel, 2003 [43]; by original designation.
- Remarks. In addition to the taxa described from Lebanese amber, this genus also includes Libanoglaris hespericus Álvarez-Parra, Peñalver, Nel and Delclòs, 2022 discovered from Spanish amber [103].
- Libanoglaris chehabi Azar and Nel, 2004 [102] (figs 15–22)
- (Figure 2E)
- Material. Specimen 117B (holotype), specimen 1194A (paratype); Azar collection, currently deposited in the Natural History Museum of the Lebanese University, Faculty of Sciences II, Fanar, Lebanon.
- Locality and age. Hammana–Mdeyrij, Baabda District, Mount Lebanon Governorate, Central Lebanon; early Barremian, Early Cretaceous [79].
- Diagnostic remarks. This species is mainly distinguished from others assigned to Libanoglaris by the characteristics of the forewings, e.g., the presence of three setae arranged in a triangle in the pterostigmal cell (vs. no setae), Sc’ weakly directed backwards toward wing base and R1 almost perpendicular to margin (vs. Sc’ perpendicular to wing margin and R1 directed forwards), and the fork of CuA1 and CuA2 occurring before the fork of Rs and M (vs. fork of CuA1 and CuA2 almost at the same level as the fork of Rs and M in L. hespericus) [43,102,103]. Refer to Álvarez-Parra et al. (2022) for comparative drawings of the forewings of the three Libanoglaris species [103] (fig. 8).
- Libanoglaris mouawadi Azar, Perrichot, Néraudeau and Nel, 2003 [43] (figs 10–19)
- (Figure 2F)
- Material. Specimen 420 (holotype), specimen 58, specimen 713A, specimen 714, specimen 423, specimen 378 (paratypes); Azar collection, currently deposited in the Natural History Museum of the Lebanese University, Faculty of Sciences II, Fanar, Lebanon.
- Locality and age. Hammana–Mdeyrij, Baabda District, Mount Lebanon Governorate, Central Lebanon; early Barremian, Early Cretaceous [79].
- Diagnostic remarks. Refer to the discussion above for the differences from L. chehabi. Species L. mouawadi also differs from L. hespericus by having the fork of CuA1 and CuA2 occurring before the fork of Rs and M (vs. almost at the same level).
- Genus Setoglaris Azar and Nel, 2004 [102]
- Type species. Setoglaris reemae Azar and Nel, 2004 [102]; by original designation.
- Setoglaris reemae Azar and Nel, 2004 [102] (figs 23–27)
- (Figure 3A)
- Material. Specimen 1197 (holotype); Azar collection, currently deposited in the Natural History Museum of the Lebanese University, Faculty of Sciences II, Fanar, Lebanon.
- Locality and age. Hammana–Mdeyrij, Baabda District, Mount Lebanon Governorate, Central Lebanon; early Barremian, Early Cretaceous [79].
- Diagnostic remarks. This species is distinguished from other empheriids in Lebanese amber in terms of the characteristics of the antennae and forewing venation. In particular, the main known differences from Libanoglaris are the presence of secondary annulations on the flagellomeres, the forewing vein Rs basally perpendicular (not oblique), and the presence of a single seta in the pterostigmal area [43,102,103], which might not be enough to support a separate genus. Setoglaris is monospecific, based on a singular individual. Therefore, the discovery of new material is crucial for assessing the true value of the diagnostic characters and retaining the validity of the generic and specific taxa.
- Suborder Troctomorpha Roesler, 1940 [90]
- Infraorder Amphientometae Pearman, 1936 [100]
- Family Electrentomidae Enderlein, 1911 [70]
- (= †Paramesopsocidae Azar, Hajar, Indary and Nel, 2008) [48]
- Remarks. Lienhard and Smithers (2002) synonymized Manicapsocidae with Electrentomidae as well, but Azar et al. (2017) suggested to continue following the traditional classification, referring to Manicapsocidae and Electrentomidae as distinct families, until this synonymy is backed up by phylogenetic analyses [41,105].
- Genus Paramesopsocus Azar, Hajar, Indary and Nel, 2008 [48]
- Type species. Paramesopsocus lu Azar, Hajar, Indary and Nel, 2008 [48]; by original designation.
- Paramesopsocus lu Azar, Hajar, Indary and Nel, 2008 [48] (figs 1–19)
- (Figure 3B)
- Material. Specimen 746C (holotype), specimen 422, specimen 427A, specimen 886 (paratypes); Azar collection, currently deposited in the Natural History Museum of the Lebanese University, Faculty of Sciences II, Fanar, Lebanon.
- Locality and age. Hammana–Mdeyrij, Baabda District, Mount Lebanon Governorate, Central Lebanon; early Barremian, Early Cretaceous [79].
- Diagnostic remarks. Azar et al. (2008) described this species as possessing characteristics of both Troctomorpha (e.g., antennae with 13 flagellomeres) and Psocomorpha (e.g., presence of thickened and sclerotized pterostigma) [48]. Ultimately, they assigned it to Psocomorpha—while noting some similarities to Mesopsocidae—mainly due to the presence of a thickened and sclerotized pterostigma with nodus, and a hooked nodulus in the forewing. They discussed the fact that the characteristics shared with Troctomorpha are either plesiomorphic or found in both suborders, but they noted that excluding the characters of the pterostigma would place the genus near the electrentomoids. Mockford et al. (2013) later transferred Paramesopsocus—which was assigned by the original authors to a new family, Paramesopsocidae—to Electrentomidae, thus synonymizing the two families based on the presence of several characteristics of Troctomorpha in Paramesopsocus, notably the forewing venation pattern (presence of a well-developed Sc and A2) [73], stating that the character ‘thickened and sclerotized pterostigma with nodus’ cannot be used as an autapomorphy of Psocomorpha as it was also detected in some extant and fossil Troctomorpha [73] (p. 14–15, remarks section of Arcantipsocus).
- Additional remarks. This genus also includes one other species, Paramesopsocus adibi Azar, Hajar, Indary and Nel, 2008, preserved as adpressions from the Late Jurassic of Karatau, South Kazakhstan [48].
- Family Pachytroctidae Enderlein, 1905 [106]
- Subfamily Tapinellinae Enderlein, 1908 [107]
- Remarks. The subfamily also includes the three fossil species Atapinella garroustei Azar, Huang, Cai and Nel, 2015 and Burmipachytrocta singularis Azar, Huang, Cai and Nel, 2015 from the Cretaceous Burmese amber, and Tapinella eocenica Nel, Prokop, De Ploëg and Millet, 2005 from the Eocene amber of Oise (France) [55,95].
- Genus Cretacetrocta Hakim and Azar, 2024 [108]
- Type species. Cretacetrocta libanella Hakim and Azar, 2024 [108]; by original designation.
- Cretacetrocta libanella Hakim and Azar, 2024 [108] (figs 2–7)
- (Figure 3C)
- Material. Specimen BKT-12A (holotype); Maalouf collection, deposited in the Natural History Museum of the Lebanese University, Faculty of Sciences II, Fanar, Lebanon.
- Locality and age. Bqaatouta, Kesserouan District, Jbeil—Kesserouan Governorate, Central Lebanon; early Barremian, Early Cretaceous [79].
- Diagnostic remarks. Hakim and Azar (2024) tentatively placed the taxon in Pachytroctidae while noting that it does not fit well in any family of Troctomorpha [108]. They discussed that the presence of a nodulus (CuP and A joining at margin) is not typical in the family, but it is rather common in Amphientometae. However, unlike Amphientometae, e.g., electrentomoid families, the taxon has forewings’ Sc not joining R, vein M-two branched, and A2 seemingly absent. The genus is currently monospecific.
- Subfamily Libanophorinae Azar, Huang, Cai and Nel, 2015 [95]
- Genus Libaneuphoris Azar, Huang, Cai and Nel, 2015 [95]
- Type species. Libaneuphoris jantopi Azar, Huang, Cai and Nel, 2015 [95]; by original designation.
- Libaneuphoris jantopi Azar, Huang, Cai and Nel, 2015 [95] (figs 4–17)
- (Figure 3D)
- Material. Specimen FAL-11A (holotype); Azar collection, deposited in the Natural History Museum of the Lebanese University, Faculty of Sciences II, Fanar, Lebanon.
- Locality and age. Falougha, Baabda District, Mount Lebanon Governorate, Central Lebanon; early Barremian, Early Cretaceous [79].
- Diagnostic remarks. Azar et al. (2015) mainly distinguished this species from other pachytroctids by characters relevant to the shape and venation of the hind wings [95]. They also noted the presence of the female T-shaped sclerite, a feature shared with the subfamily Tapinellinae. To date, the subfamily and genus remain monospecific. The discovery of more material belonging to this group from Lebanese amber or other deposits is necessary for further cementing the validity of the subfamily.
- Family Sphaeropsocidae Kolbe, 1883 [109]
- Genus Asphaeropsocites Azar, Engel and Grimaldi, 2010 [110]
- Type species. Asphaeropsocites neli Azar, Engel and Grimaldi, 2010 [110]; by original designation.
- Asphaeropsocites neli Azar, Engel and Grimaldi, 2010 [110] (figs 1 and 2)
- (Figure 3E)
- Material. Specimen 1513-A (holotype); Azar collection, currently deposited in the Natural History Museum of the Lebanese University, Faculty of Sciences II, Fanar, Lebanon.
- Locality and age. Hammana–Mdeyrij, Baabda District, Mount Lebanon Governorate, Central Lebanon; early Barremian, Early Cretaceous [79].
- Diagnostic remarks. To date, the genus remains monospecific. The species shares multiple characteristics of the head and wings with Sphaeropsocites lebanensis Grimaldi and Engel, 2006, but is clearly distinguished from the latter by others traits (e.g., presence of a distinct frontal suture, areolate surface of the head and wings, absence of a claval furrow, and all the veins reaching the wing margin except R) [110,111]. Consequently, Azar et al. (2010) suggested a more derived placement of Asphaeropsocites neli compared to S. lebanensis, not as sister taxa, although A. neli still retains multiple plesiomorphic traits to warrant a basal position, albeit related, to the other Sphaeropsocidae [110].
- Genus Sphaeropsocites Grimaldi and Engel, 2006 [111]
- Type species. Sphaeropsocites lebanensis Grimaldi and Engel, 2006 [111]; by original designation.
- Sphaeropsocites lebanensis Grimaldi and Engel, 2006 [111] (figs 2E and 6)
- (Figure 3F)
- Material. Specimen AMNH JS284 (holotype); Acra collection, deposited in the American Museum of Natural History, New York, NY, USA.
- Locality and age. Jouar Ess-Souss (Jezzine), Jezzine District, South Lebanon Governorate, Southern Lebanon; early Barremian, Early Cretaceous [79].
- Diagnostic remarks. To date, the genus remains monospecific. Grimaldi and Engel (2006) proposed Sphaeropsocites lebanensis to be a sister group to all the Sphaeropsocidae as it retains plesiomorphic features in the wings in comparison to the other taxa [111]. Also refer to the previous discussion on Asphaeropsocites neli.
- Family Incertae sedis
- Genus Libanomphientomum Choufani, Azar and Nel, 2011 [112]
- Type species. Libanomphientomum nudus Choufani, Azar and Nel, 2011 [112]; by original designation.
- Libanomphientomum nudus Choufani, Azar and Nel, 2011 [112] (figs 1–8)
- (Figure 3G)
- Material. Specimen 1646 (holotype); Azar collection, currently deposited in the Natural History Museum of the Lebanese University, Faculty of Sciences II, Fanar, Lebanon.
- Locality and age. Hammana–Mdeyrij, Baabda District, Mount Lebanon Governorate, Central Lebanon; early Barremian, Early Cretaceous [79].
- Diagnostic remarks. Libanomphientomum nudus is distinguished by the combination of different features of the head, body and wings, particularly the lack of scales, 14 antennomeres, forewing venation pattern (e.g., Sc joining R distally, presence of a well-developed pterostigma, and two separated anal veins), and ventral row of ctenidiobothria on the basal tarsomere [112]. Choufani et al. (2011) assigned the species to Amphientometae but refrained from placing the taxon in any family, suggesting multiple possible options “when considering different sets of characters” [112]. Mockford et al. (2013) agreed with the choice of infraorder by further placing L. nudus in the superfamily Electrentomoidea [73]. To date, the genus remains monospecific.
- Sp. 1 (immature) [72] (figs 1–3)
- Material. Specimen AD-33; Azar collection, deposited in the Natural History Museum of the Lebanese University, Faculty of Sciences II, Fanar, Lebanon.
- Locality and age. Ain Dara, Aley District, Mount Lebanon Governorate, Central Lebanon; early Barremian, Early Cretaceous [79].
- Remarks. This specimen is the first and only immature psocodean described from Lebanese amber thus far. While the authors could not classify it, they were able to investigate the camouflage behavior, common among immatures in modern taxa, as the individual was carrying some debris, leaving the dorsal side of the abdomen exposed for examination of the involved structures.
- 1-
- Compound eyes reduced; forewings elytriform, hemispherical, with inner margins touching for entire length and not divergent apically, wing surface with dense areolation or punctation; hind wings absent .…………………………………………………… 2Compound eyes normal; forewings elongated, wing surface membranous without any areolation or punctation; hind wings present ……………………………………… 3
- 2-
- Developed frontal suture present, surface of head and wings areolate, claval furrow absent, all veins reaching margin except R ………………………… Asphaeropsocites neliFrontal suture absent, surface of head not areolate, surface of wings punctate, claval furrow present, all veins not reaching margin ….…………… Sphaeropsocites lebanensis
- 3-
- Forewing Sc not joining R, vein M two-branched .………...……………………………. 4Forewing Sc joining R, vein M three-branched ..………………...………………………. 6
- 4-
- Forewing with pterostigma; hind wing with vein R1 present, basiradial cell absent .………………………………………………………………………… Cretacetrocta libanellaForewing without pterostigma; hind wing with vein R1 absent, basiradial cell present ………………………………………………………………………………………………… 5
- 5-
- Forewings tinted; hind wings with anal lobe protruding and well developed, Rs and M fused for a length …………………………………………………. Libaneuphoris jantopiForewings not tinted; hind wings with anal lobe normal, not protruding, Rs and M fused briefly ………………………………….……... Libanopsyllipsocus alexanderasnitsyni
- 6-
- Forewing Sc short, crossvein r1-rs absent ………...……………………………………… 7Forewing Sc very elongate, crossvein r1-rs present ..……………………………………. 8
- 7-
- Fifteen antennomeres; forewing with pterostigma thickened and sclerotized; hind wing with M branched …………………………………………………. Paramesopsocus luFourteen antennomeres; forewing with pterostigma not thickened or sclerotized; hind wing with M simple .………………………………………….. Libanomphientomum nudus
- 8-
- Forewings with setae on veins and (sometimes) membrane, base of Sc straight, one anal vein present …………………………………………………………………………… 9Forewings without setae on veins or membrane, base of Sc sigmoidal, two anal veins present .…………………………………………………… Palaeosiamoglaris hammanaensis
- 9-
- Forewing pterostigma four-angled, axillar and anal area without long setae .……… 10Forewing pterostigma three-angled, axillar and anal area with long setae ………………………………………………………………………… Bcharreglaris amunobi
- 10-
- Forewing Sc’ strongly arched backwards, three aligned setae present in pterostigmal cell ……...…….……………………………………………………… Archaeatropos randataeForewing Sc’ weakly curved and directed forwards or backwards (almost perpendicular to margin), different setation in pterostigmal cell (see below) …………………… 11
- 11-
- Without secondary annulations on flagellomeres; forewings with base of Rs strongly or weakly oblique …………………...……………………………………………………. 12With secondary annulations on flagellomeres; forewings with base of Rs perpendicular, one seta present in pterostigmal cell …….…………………………. Setoglaris reemae
- 12-
- Forewing Sc’ slightly directed backwards, three setae present, disposed in triangle in pterostigmal cell ………………………………………………………. Libanoglaris chehabiForewing Sc’ slightly directed forwards, no setae present in pterostigmal cell …………………………………………………………………………. Libanoglaris mouawadi
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lienhard, C. Psocoptères Euro-Méditerranéen; Faune de France; Fédération Française des Sociétés de Sciences Naturelles: Paris, France, 1998; Volume 83. [Google Scholar]
- Huang, D.Y.; Bechly, G.; Nel, P.; Engel, M.S.; Prokop, J.; Azar, D.; Cai, C.Y.; van de Kamp, T.; Staniczek, A.H.; Garrouste, R.; et al. New fossil insect order Permopsocida elucidates major radiation and evolution of suction feeding in hemimetabolous insects (Hexapoda: Acercaria). Sci. Rep. 2016, 6, 23004. [Google Scholar] [CrossRef]
- Gillott, C. Chapter 8—The Hemipteroid orders. In Entomology, 2nd ed.; Gillott, C., Ed.; Springer: Dordrecht, The Netherlands, 1995; pp. 195–232. [Google Scholar] [CrossRef]
- Grimaldi, D.; Engel, M.S. Evolution of the Insects; Cambridge University Press: New York, NY, USA, 2005; pp. xv + 755. [Google Scholar]
- Mashaya, N. Population dynamics of Liposcelis entomophila (Enderlein) (Psocoptera: Liposcelidae) in farm tobacco processing buildings. J. Stored Prod. Res. 1999, 35, 355–368. [Google Scholar] [CrossRef]
- Kučerová, Z. Weight losses of wheat grains caused by psocid infestation (Liposcelis bostrychophila: Liposcelididae: Psocoptera). Plant Prot. Sci. 2002, 38, 103–107. [Google Scholar] [CrossRef]
- Kučerová, Z.; Carvalho, M.O.; Stejskal, V. Faunistic records of new stored product psocids (Psocoptera: Liposcelididae) for Portugal. In Proceedings of the Ninth International Conference on Stored-Product Protection; Lorini, I., Bacaltchuk, B., Beckel, H., Deckers, E., Sundfeld, E., dos Santos, J.P., Biagi, J.D., Celaro, J.C., Faroni, L.R.D., de Bortolini, L.O.F., et al., Eds.; ABRAPOS: Campinas, Brazil, 2006; pp. 1104–1107. [Google Scholar]
- Ahmedani, M.S.; Shagufta, N.; Aslam, M.; Hussain, S.A. Psocid: A new risk for global food security and safety. Appl. Entomol. Zool. 2010, 45, 89–100. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Opit, G.P.; Throne, J.E. Influence of commodity type, percentage of cracked kernels, and wheat class on population growth of stored-product psocids (Psocoptera: Liposcelidae). J. Econ. Entomol. 2010, 103, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.G.; Opit, G.P.; Giles, K.L.; Adam, B. Weight loss and germination failure caused by psocids in different wheat varieties. J. Econ. Entomol. 2013, 106, 491–498. [Google Scholar] [CrossRef]
- Nayak, M.K.; Collins, P.J.; Throne, J.E.; Wang, J.J. Biology and management of psocids infesting stored products. Annu. Rev. Entomol. 2014, 59, 279–297. [Google Scholar] [CrossRef] [PubMed]
- Turner, B.; Staines, N.A.; Brostoff, J.; Howe, C.A.; Cooper, K. Allergy to Psocids. In Proceedings of the Second International Conference on Urban Pests in the Urban Environment; Wildey, K.B., Ed.; International Conference on Urban Pests: Edinburgh, Scotland, 1996; p. 609. [Google Scholar]
- Patil, M.P.; Niphadkar, P.V.; Bapat, M.M. Psocoptera spp. (book louse): A new major household allergen in Mumbai. Ann. Allergy Asthma Immunol. 2001, 87, 151–155. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chan, M.L.; Ko, C.W.; Hseih, M.Y. Nail infestation by Liposcelis bostrychophila Badonnel. Clin. Exp. Dermatol. 2004, 29, 620–621. [Google Scholar] [CrossRef]
- Perotin, J.M.; Scherer, P.; Leduc, V.; Bouchet, F.; Deslee, G.; Lavaud, F. Allergic asthma to psocids, a new indoor allergen of ecological building materials. Allergy 2011, 66, 1257–1258. [Google Scholar] [CrossRef]
- Fukutomi, Y.; Kawakami, Y.; Taniguchi, M.; Saito, A.; Fukuda, A.; Yasueda, H.; Nakazawa, T.; Hasegawa, M.; Nakamura, H.; Akiyama, K. Allergenicity and cross-reactivity of booklice (Liposcelis bostrichophila): A common household insect pest in Japan. Int. Arch. Allergy Immunol. 2012, 157, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Marco, G.; Pelta, R.; Carnés, J.; Iraola, V.; Zambrano, G.; Baeza, M.L. Occupational allergic asthma induced by Liposcelis decolor. Allergol. Int. 2016, 65, 210–211. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Veraldi, S.; Brena, M.; Süss, L. Occupational allergy to Psocoptera species. Contact Dermat. 2019, 81, 306–307. [Google Scholar] [CrossRef] [PubMed]
- Pearman, J.V. Some African Psocoptera found on rats. Entomologist 1960, 93, 246–250. [Google Scholar]
- Mockford, E.L. Some Psocoptera from plumage of birds. Proc. Entomol. Soc. Wash. 1967, 69, 307–309. [Google Scholar]
- Mockford, E.L. Psocoptera from sleeping nests of the dusky-footed wood rat in southern California (Psocoptera: Atropidae, Psoquillidae, Liposcelidae). Pan-Pac. Entomol. 1971, 47, 127–140. [Google Scholar]
- Baz, A. Psocoptera from weaver bird nests (Aves: Ploceidae) in Equatorial Guinea (West-Africa). Ann. Soc. Entomol. Fr. 1990, 26, 33–38. [Google Scholar] [CrossRef]
- Durden, L.A. Chapter 7—Lice (Phthiraptera). In Medical and Veterinary Entomology, 3rd ed.; Mullen, G.R., Durden, L.A., Eds.; Academic Press: London, UK, 2019; pp. 79–106. [Google Scholar] [CrossRef]
- Amanzougaghene, N.; Fenollar, F.; Raoult, D.; Mediannikov, O. Where are we with human lice? A review of the current state of knowledge. Front. Cell. Infect. Microbiol. 2020, 9, 474. [Google Scholar] [CrossRef] [PubMed]
- Mediannikov, O.; Bechah, Y.; Amanzougaghene, N.; Lepidi, H.; Bassene, H.; Sambou, M.; Lienhard, C.; Benkacimi, L.; Dieme, C.; Sokhna, C.; et al. Booklice Liposcelis bostrychophila naturally infected by Rickettsia felis cause fever and experimental Pneumonia in mammals. J. Infect. Dis. 2022, 226, 1075–1083. [Google Scholar] [CrossRef]
- Johnson, K.P.; Yoshizawa, K.; Smith, V.S. Multiple origins of parasitism in lice. Proc. R. Soc. Lond. B Biol. Sci. 2004, 271, 1771–1776. [Google Scholar] [CrossRef]
- Johnson, K.P.; Dietrich, C.H.; Friedrich, F.; Beutel, R.G.; Wipfler, B.; Peters, R.S.; Allen, J.M.; Petersen, M.; Donath, A.; Walden, K.K.O.; et al. Phylogenomics and the evolution of hemipteroid insects. Proc. Natl. Acad. Sci. USA 2018, 115, 12775–12780. [Google Scholar] [CrossRef] [PubMed]
- Murell, A.; Baker, S.C. Multiple origins of parasitism in lice: Phylogenetic analysis of SSU rDNA indicates that the Phthiraptera and Psocoptera are not monophyletic. Parasitol. Res. 2005, 97, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, D.; Engel, M.S. Fossil Liposcelididae and the lice ages (Insecta: Psocodea). Proc. R. Soc. Lond. B Biol. Sci. 2006, 273, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, K.; Lienhard, C.; Johnson, K.P. Molecular systematics of the suborder Trogiomorpha (Insecta: Psocodea: ‘Psocoptera’). Zool. J. Linn. Soc. 2006, 146, 287–299. [Google Scholar] [CrossRef]
- Yoshizawa, K.; Johnson, K.P.; Sweet, A.D.; Yao, I.; Ferreira, R.L.; Cameron, S.L. Mitochondrial phylogenomics and genome rearrangements in the barklice (Insecta: Psocodea). Mol. Phylogenet. Evol. 2018, 119, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, K.; Lienhard, C. In search of the sister group of the true lice: A systematic review of booklice and their relatives with an updated checklist of Liposcelididae (Insecta: Psocodea). Arthropod Syst. Phylogeny 2010, 68, 181–195. [Google Scholar] [CrossRef]
- Yoshizawa, K.; Johnson, K.P. Phylogenetic position of Phthiraptera (Insecta: Paraneoptera) and elevated rate of evolution in mitochondrial 12S and 16S rDNA. Mol. Phylogenet. Evol. 2003, 29, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, K.; Johnson, K.P. How stable is the “Polyphyly of Lice” hypothesis (Insecta: Psocodea)?: A comparison of phylogenetic signal in multiple genes. Mol. Phylogenet. Evol. 2010, 55, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, K.; Johnson, K.P. Phylogeny of the suborder Psocomorpha: Congruence and incongruence between morphology and molecular data (Insecta: Psocodea: ‘Psocoptera’). Zool. J. Linn. Soc. 2014, 171, 716–731. [Google Scholar] [CrossRef]
- de Moya, R.S.; Yoshizawa, K.; Walden, K.K.O.; Sweet, A.D.; Dietrich, C.H.; Johnson, K.P. Phylogenomics of parasitic and non-parasitic lice (Insecta: Psocodea): Combining sequence data and exploring compositional bias solutions in next generation datasets. Syst. Biol. 2021, 70, 719–738. [Google Scholar] [CrossRef]
- Álvarez-Parra, S.; Nel, A.; Perrichot, V.; Jouault, C. Unravelling the mishmash: A new phylogeny for the family Empheriidae (Psocodea, Trogiomorpha) with a new genus and species from Cretaceous Charentese amber. Arthropod Syst. Phylo. 2024, 82, 183–199. [Google Scholar] [CrossRef]
- Saenz Manchola, O.F.; Herrera, S.V.; D’Alessio, L.M.; Yoshizawa, K.; García Aldrete, A.N.; Johnson, K.P. Mitochondrial genomes within bark lice (Insecta: Psocodea: Psocomorpha) reveal novel gene rearrangements containing phylogenetic signal. Syst. Entomol. 2021, 46, 938–951. [Google Scholar] [CrossRef]
- Saenz Manchola, O.F.; Herrera, S.V.; D’Alessio, L.M.; Yoshizawa, K.; García Aldrete, A.N.; Johnson, K.P. Phylogenomics of the family Lachesillidae (Insecta: Psocodea: Psocomorpha). Syst. Entomol. 2022, 48, 316–327. [Google Scholar] [CrossRef]
- Liang, F.Y.; Liu, X.Y. Systematic revision and molecular phylogenetics refine the generic classification of the bark louse family Stenopsocidae (Insecta: Psocodea: Psocomorpha). Arthropod Syst. Phylo. 2024, 82, 433–446. [Google Scholar] [CrossRef]
- Lienhard, C.; Smithers, C.N. Psocoptera (Insecta): World Catalogue and Bibliography; Instrumenta Biodiversitatis V; Muséum d’Histoire Naturelle de Genève: Geneva, Switzerland, 2002. [Google Scholar]
- Yoshizawa, K.; Lienhard, C. †Cormopsocidae: A new family of the suborder Trogiomorpha (Insecta: Psocodea) from Burmese amber. Entomol. Sci. 2020, 23, 208–215. [Google Scholar] [CrossRef]
- Perrichot, V.; Azar, D.; Néraudeau, D.; Nel, A. New Psocoptera in the Lower Cretaceous ambers of southwestern France and Lebanon (Insecta: Psocoptera: Trogiomorpha). Geol. Mag. 2003, 140, 669–683. [Google Scholar] [CrossRef]
- Nel, A.; Roques, P.; Nel, P.; Prokin, A.A.; Bourgoin, T.; Prokop, J.; Szwedo, J.; Azar, D.; Desutter-Grandcolas, L.; Wappler, T.; et al. The earliest known holometabolous insects. Nature 2013, 503, 257–261. [Google Scholar] [CrossRef]
- Tillyard, R.J. Upper Permian insects of New South Wales. III. The order Copeognatha. Proc. Linn. Soc. N. S. W. 1935, 60, 265–279. [Google Scholar]
- Nel, A.; Prokop, J.; Nel, P.; Grandcolas, P.; Huang, D.Y.; Roques, P.; Guilbert, E.; Dostál, O.; Szwedo, J. Traits and evolution of wing venation pattern in paraneopteran insects. J. Morphol. 2012, 273, 480–506. [Google Scholar] [CrossRef]
- Jouault, C.; Yoshizawa, K.; Hakim, M.; Huang, D.Y.; Nel, A. New psocids (Psocodea: Prionoglarididae, Psyllipsocidae) from Cretaceous Burmese amber deposits. Cretac. Res. 2021, 126, 104890. [Google Scholar] [CrossRef]
- Azar, D.; Hajar, L.; Indary, C.; Nel, A. Paramesopsocidae, a new Mesozoic psocid family (Insecta: Psocodea “Psocoptera”: Psocomorpha). Ann. Soc. Entomol. Fr. 2008, 44, 459–470. [Google Scholar] [CrossRef]
- Álvarez-Parra, S.; Peñalver, E.; Nel, A.; Delclòs, X. The oldest representative of the extant barklice genus Psyllipsocus (Psocodea: Trogiomorpha: Psyllipsocidae) from the Cenomanian amber of Myanmar. Cretac. Res. 2020, 113, 104480. [Google Scholar] [CrossRef]
- Álvarez-Parra, S.; Peñalver, E.; Nel, A.; Delclòs, X. Barklice (Insecta: Psocodea) from Early Cretaceous resiniferous forests of Iberia (Spanish amber): New Troctomorpha and a possible Psocomorpha. Cretac. Res. 2023, 148, 105544. [Google Scholar] [CrossRef]
- Hakim, M.; Huang, D.Y.; Azar, D. New fossil psocids from Cretaceous Siberian ambers (Psocodea: Trogiomorpha: Atropetae). Palaeoentomology 2021, 4, 186–198. [Google Scholar] [CrossRef]
- Ross, A. Complete checklist of Burmese (Myanmar) amber taxa 2023. Mesozoic 2024, 1, 21–57. [Google Scholar] [CrossRef]
- Azar, D. Tertiary bark lice (Insecta: Psocodea) from the Insect Limestone (Bembridge Marls, Late Eocene) of the Isle of Wight, UK. Earth Environ. Sci. Trans. R. Soc. Edinb. 2014, 104, 307–316. [Google Scholar] [CrossRef]
- Nel, A.; de Ploëg, G.; Azar, D. The oldest Liposcelididae in the lowermost Eocene amber of the Paris Basin (Insecta: Psocoptera). Geol. Acta 2004, 2, 31–36. [Google Scholar]
- Nel, A.; Prokop, J.; de Ploëg, G.; Millet, J. New Psocoptera (Insecta) from the lowermost Eocene amber of Oise, France. J. Syst. Palaeontol. 2005, 3, 371–391. [Google Scholar] [CrossRef]
- Engel, M.S.; Perkovsky, E.E. Sphaeropsocus kuenowii Hagen in Rovno amber from the Ukraine (Psocoptera: Sphaeropsocidae). Entomol. News 2006, 117, 243–245. [Google Scholar] [CrossRef]
- Engel, M.S. A new species of the booklouce genus Embidopsocus in Baltic amber (Psocoptera: Liposcelididae). Novit. Paleoentomologicae 2016, 1–9. [Google Scholar] [CrossRef][Green Version]
- Zhang, Q.; Nel, A.; Azar, D.; Wang, B. New Chinese psocids from Eocene Fushun amber (Insecta: Psocodea). Alcheringa 2016, 40, 366–372. [Google Scholar] [CrossRef]
- Azar, D.; Maksoud, S.; Nammour, C.; Nel, A.; Wang, B. A new trogiid genus from lower Eocene Fushun amber (Insecta: Psocodea: Trogiomorpha). Geobios 2018, 51, 101–106. [Google Scholar] [CrossRef]
- Álvarez-Parra, S.; Nel, A. A new genus of setose-winged barklice (Psocodea: Trogiomorpha: Lepidopsocidae) from the Eocene amber of Oise with notes on the biogeography of Thylacellinae. Hist. Biol. 2023, 35, 1136–1145. [Google Scholar] [CrossRef]
- Mockford, E.L. Fossil insects of the order Psocoptera from Tertiary Amber of Chiapas, Mexico. J. Paleontol. 1969, 43, 1267–1273. [Google Scholar]
- Nel, A.; Waller, A.; Poinar, G.O., Jr. The first Myopsocidae (Psocoptera) in Dominican amber. Zootaxa 2006, 1349, 63–68. [Google Scholar] [CrossRef]
- Mockford, E.L.; Garcia-Aldrete, A.N. A new genus and two new species, one extant and one fossil, in the family Troctopsocidae (Psocodea: ‘Psocoptera’: Troctomorpha: Amphientometae: Electrentomoidea). Zootaxa 2014, 3869, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Mockford, E.L. A New Species of Belaphopsocus Badonnel (Psocodea: ‘Psocoptera’: Troctomorpha: Liposcelididae: Embidopsocinae) from Costa Rican Amber. Life Excit. Biol. 2015, 3, 207–212. [Google Scholar] [CrossRef]
- Hakim, M.; Huang, D.Y.; Azar, D. First lepidopsocid from the mid Miocene Dominican amber (Psocodea: Trogiomorpha: Lepidopsocidae). Palaeoentomology 2018, 1, 58–64. [Google Scholar] [CrossRef]
- Wappler, T.; Smith, V.S.; Dalgleish, R.C. Scratching an ancient itch: An Eocene bird louse fossil. Proc. R. Soc. Lond. B Biol. Sci. 2004, 271, S255–S258. [Google Scholar] [CrossRef]
- Dalgleish, R.C.; Palma, R.L.; Price, R.D.; Smith, V.S. Fossil lice (Insecta: Phthiraptera) reconsidered. Syst. Entomol. 2006, 31, 648–651. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Rasnitsyn, A.P.; Zhang, W.W.; Song, F.; Shih, C.K.; Ren, D.; Wang, Y.J.; Li, H.; Gao, T.P. Stem chewing lice on Cretaceous feathers preserved in amber. Curr. Biol. 2024, 34, 916–922. [Google Scholar] [CrossRef]
- Hagen, H. Psocinorum et Embidonorum synopsis synonymica. Verh. Zool.-Bot. Ges. Wien 1866, 16, 201–222. [Google Scholar]
- Enderlein, G. Die fossilen Copeognathen und ihre Phylogenie. Palaeontographica 1911, 58, 279–360. [Google Scholar]
- Azar, D.; Nel, A.; Waller, A. Two new Ptiloneuridae from Colombian copal (Psocodea: Psocomorpha). Denisia 2009, 26, 21–28. [Google Scholar]
- Hakim, M.; Huang, D.Y.; Azar, D. Debris-carrying psocodean nymph from Lebanese amber. Palaeoentomology 2022, 5, 222–225. [Google Scholar] [CrossRef]
- Mockford, E.L.; Lienhard, C.; Yoshizawa, K. Revised classification of ‘Psocoptera’ from Cretaceous amber, a reassessment of published information. Insecta Matsumurana 2013, 69, 1–26. [Google Scholar]
- Maksoud, S.; Azar, D. Lebanese amber: A fantastic journey into the time of dinosaurs. J. Gems Gemmol. 2023, 25, 136–145. [Google Scholar]
- Azar, D.; Gèze, R.; El-Samrani, A.; Maalouly, J.; Nel, A. Jurassic amber in Lebanon. Acta Geol. Sin. (Engl. Ed.) 2010, 84, 977–983. [Google Scholar] [CrossRef]
- Nohra, Y.; Azar, D.; Gèze, R.; Maksoud, S.; El-Samrani, A.; Perrichot, V. New Jurassic outcrops from Lebanon. Terr. Arthropod Rev. 2013, 6, 27–51. [Google Scholar] [CrossRef]
- Granier, B.; Toland, C.; Gèze, R.; Azar, D.; Maksoud, S. Some steps toward a new story for the Jurassic—Cretaceous transition in Mount Lebanon. Carnets Géol. 2016, 16, 247–269. [Google Scholar] [CrossRef]
- Maksoud, S.; Azar, D.; Granier, B.; Gèze, R. New data on the age of the Lower Cretaceous amber outcrops of Lebanon. Palaeoworld 2017, 26, 331–338. [Google Scholar] [CrossRef]
- Maksoud, S.; Granier, B.R.C.; Azar, D. Palaeoentomological (fossil insects) outcrops in Lebanon. Carnets Géol. 2022, 22, 699–743. [Google Scholar] [CrossRef]
- Azar, D.; Dejax, J.; Masure, E. Palynological analysis of amber-bearing clay from the Lower Cretaceous of Central Lebanon. Acta Geol. Sin. (Engl. Ed.) 2011, 85, 942–949. [Google Scholar] [CrossRef]
- Maksoud, S.; Iskandar-Tabib, D.; Azar, D. Tannoura: A new early Barremian fossiliferous amber outcrop from South Lebanon. Mesozoic 2024, 1, 90–98. [Google Scholar] [CrossRef]
- Azar, D.; Szwedo, J.; Maalouf, M.; Maalouf, R.; Maksoud, S. Libanonemopalpus grimaldii, a new genus and species of Bruchomyiinae from Lower Cretaceous Lebanese amber (Diptera: Psychodidae). Palaeoentomology 2022, 5, 569–578. [Google Scholar] [CrossRef]
- Sendi, H.; Vršanský, P.; Azar, D. Jordanian—Lebanese—Syrian cockroaches s.s. from Lower Cretaceous amber—Monograph. Biologia 2023, 78, 1447–1541. [Google Scholar] [CrossRef]
- Ulmer, J.M.; Janšta, P.; Azar, D.; Krogmann, L. At the dawn of megadiversity—Protoitidae, a new family of Chalcidoidea (Hymenoptera) from Lower Cretaceous Lebanese amber. J. Hymenopt. Res. 2023, 96, 879–924. [Google Scholar] [CrossRef]
- Azar, D.; Nel, A.; Huang, D.Y.; Engel, M.S. The earliest fossil mosquito. Curr. Biol. 2023, 33, 5240–5246. [Google Scholar] [CrossRef]
- Pielowska-Ceranowska, A.; Azar, D.; Szwedo, J. A new genus and species of Leptoconopinae (Diptera: Ceratopogonidae) from Lower Cretaceous Baskinta amber outcrop in Lebanon. Eur. J. Taxon. 2023, 908, 27–38. [Google Scholar] [CrossRef]
- Azar, D.; Maksoud, S.; Huang, D.Y.; Maalouf, M.; Cai, C.Y. A new fossil psychodomorphan fly from Lower Barremian Lebanese amber elucidates the relationship of the Tanyderinae stat. nov. within the Psychodidae. Carnets Géol. 2024, 24, 113–126. [Google Scholar] [CrossRef]
- Azar, D. Preservation and accumulation of biological inclusions in Lebanese amber and their significance. Comptes Rendus Palevol 2007, 6, 151–156. [Google Scholar] [CrossRef]
- Hennig, W. Phylogenetic Systematics; University of Illinois Press: Urbana, IL, USA, 1966. [Google Scholar]
- Roesler, R. Neue und wenig bekannte Copeognathengattungen I. Zool. Anz. 1940, 129, 225–243. [Google Scholar]
- Smithers, C.N. The classification and phylogeny of the Psocoptera. Mem. Aust. Mus. 1972, 14, 1–349. [Google Scholar] [CrossRef]
- Kolbe, H.J. Der Entwickelungsgang der Psociden im Individuum und in der Zeit. Berl. Entomol. Z. 1884, 28, 35–38. [Google Scholar]
- Azar, D.; Nel, A. The oldest psyllipsocid booklice, in Lower Cretaceous amber from Lebanon (Psocodea, Trogiomorpha, Psocathropetae, Psyllipsocidae). ZooKeys 2011, 130, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.Q.; Li, S.; Ren, D.; Yao, Y.Z. New genus and species of the Psyllipsocidae (Psocodea: Trogiomorpha) from mid-Cretaceous Burmese amber. Cretac. Res. 2019, 104, 104178. [Google Scholar] [CrossRef]
- Azar, D.; Huang, D.Y.; Cai, C.Y.; Nel, A. The earliest records of pachytroctid booklice from Lebanese and Burmese Cretaceous ambers (Psocodea, Troctomorpha, Nanopsocetae, Pachytroctidae). Cretac. Res. 2015, 52, 336–347. [Google Scholar] [CrossRef]
- Hakim, M.; Azar, S.; Maksoud, S.; Huang, D.Y.; Azar, D. New polymorphic psyllipsocids from Burmese amber (Psocodea: Psyllipsocidae). Cretac. Res. 2018, 84, 389–400. [Google Scholar] [CrossRef]
- Karny, H.H. Zur Systematik der Orthopteroiden Insekten. Zweiter Teil. Treubia 1930, 12, 431–461. [Google Scholar]
- Azar, D.; Huang, D.Y.; El-Hajj, L.; Cai, C.; Nel, A.; Maksoud, S. New Prionoglarididae from Burmese amber (Psocodea: Trogiomorpha: Prionoglaridetae). Cretac. Res. 2017, 75, 146–156. [Google Scholar] [CrossRef]
- Hakim, M.; Huang, D.Y.; Azar, D. Earliest record of Prionoglarididae from the Lower Cretaceous Lebanese amber (Psocodea; Trogiomorpha). Cretac. Res. 2022, 132, 105121. [Google Scholar] [CrossRef]
- Pearman, J.V. The taxonomy of the Psocoptera: Preliminary sketch. Proc. R. Entomol. Soc. Lond. B Taxon. 1936, 5, 58–62. [Google Scholar] [CrossRef]
- Baz, A.; Ortuño, V.M. Archaeatropidae, a new family of Psocoptera from the Cretaceous amber of Alava, Northern Spain. Ann. Entomol. Soc. Am. 2000, 93, 367–373. [Google Scholar] [CrossRef]
- Azar, D.; Nel, A. Four new Psocoptera from Lebanese amber (Insecta: Psocomorpha: Trogiomorpha). Ann. Soc. Entomol. Fr. 2004, 40, 185–192. [Google Scholar] [CrossRef]
- Álvarez-Parra, S.; Peñalver, E.; Nel, A.; Delclòs, X. New bark lice (Psocodea, Trogiomorpha) from Lower Cretaceous Spanish amber. Pap. Palaeontol. 2022, 8, e1436. [Google Scholar] [CrossRef]
- Kaddumi, H.F. Amber of Jordan: The Oldest Prehistoric Insects in Fossilized Resin, 3rd ed.; Eternal River Museum of Natural History: Amman, Jordan, 2007; 298p. [Google Scholar]
- Azar, D.; Hakim, M.; Huang, D.Y.; Cai, C.Y.; Nel, A. New fossil booklice from the Cretaceous amber of Myanmar (Psocodea: Troctomorpha: Amphientometae: Manicapsocidae). Cretac. Res. 2017, 70, 8–14. [Google Scholar] [CrossRef]
- Enderlein, G. Morphologie, Systematik und Biologie der Atropiden und Troctiden, sowie Zusammenstellung aller bisher bekannten recenten und fossilen Formen. In Results of the Swedish Zoological Expedition to Egypt and the White Nile, 1901; Jägerskiöld, L., Ed.; The Library of the Royal University of Uppsala: Uppsala, Sweden, 1905; 58p. [Google Scholar]
- Enderlein, G. Die Copeognathenfauna der Insel Formosa. Zool. Anz. 1908, 33, 759–779. [Google Scholar]
- Hakim, M.; Azar, S. Cretacetrocta, a new genus of barklice from the Early Cretaceous Lebanese amber. Palaeoentomology 2024, 7, 395–406. [Google Scholar] [CrossRef]
- Kolbe, H.J. Neue Beiträge zur Kenntniss der Psociden der Bernstein-Fauna. Stettiner Entomol. Z. 1883, 44, 186–191. [Google Scholar]
- Azar, D.; Engel, M.S.; Grimaldi, D.A. A new genus of sphaeropsocid bark lice from the Early Cretaceous amber of Lebanon (Psocodea: Sphaeropsocidae). Ann. Soc. Entomol. Fr. 2010, 46, 103–107. [Google Scholar] [CrossRef]
- Grimaldi, D.; Engel, M.S. Extralimital fossils of the “Gondwanan” family Sphaeropsocidae (Insecta: Psocodea). Am. Mus. Novit. 2006, 1–18. [Google Scholar] [CrossRef]
- Choufani, J.; Azar, D.; Nel, A. The oldest amphientomete booklouce from Lower Cretaceous amber of Lebanon (Psocodea: Troctomorpha). Insect Syst. Evol. 2011, 42, 149–159. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hakim, M.; Azar, D. Contributions to the Palaeobiodiversity of Psocodea (‘Psocoptera’) from Lebanese Amber: A Review. Foss. Stud. 2024, 2, 160-176. https://doi.org/10.3390/fossils2030008
Hakim M, Azar D. Contributions to the Palaeobiodiversity of Psocodea (‘Psocoptera’) from Lebanese Amber: A Review. Fossil Studies. 2024; 2(3):160-176. https://doi.org/10.3390/fossils2030008
Chicago/Turabian StyleHakim, Marina, and Dany Azar. 2024. "Contributions to the Palaeobiodiversity of Psocodea (‘Psocoptera’) from Lebanese Amber: A Review" Fossil Studies 2, no. 3: 160-176. https://doi.org/10.3390/fossils2030008
APA StyleHakim, M., & Azar, D. (2024). Contributions to the Palaeobiodiversity of Psocodea (‘Psocoptera’) from Lebanese Amber: A Review. Fossil Studies, 2(3), 160-176. https://doi.org/10.3390/fossils2030008







