Evaluation of the Efficacy of Three Newcastle Disease Vaccines Produced at the National Veterinary Institute, Bishoftu, Ethiopia, at Different Temperature Storage Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Vaccine Selection and Storage
2.2. Animals, Vaccines and Treatment Groups
2.3. Stability of the ND Vaccine
2.4. Blood Sample Collection
2.5. Hemagglutination Inhibition Test
2.6. Challenge and Clinical Monitoring of Vaccinated Chickens
2.7. Evaluation of Viral Shade after the Vaccination and Challenge
2.8. Data Management and Analysis
3. Results
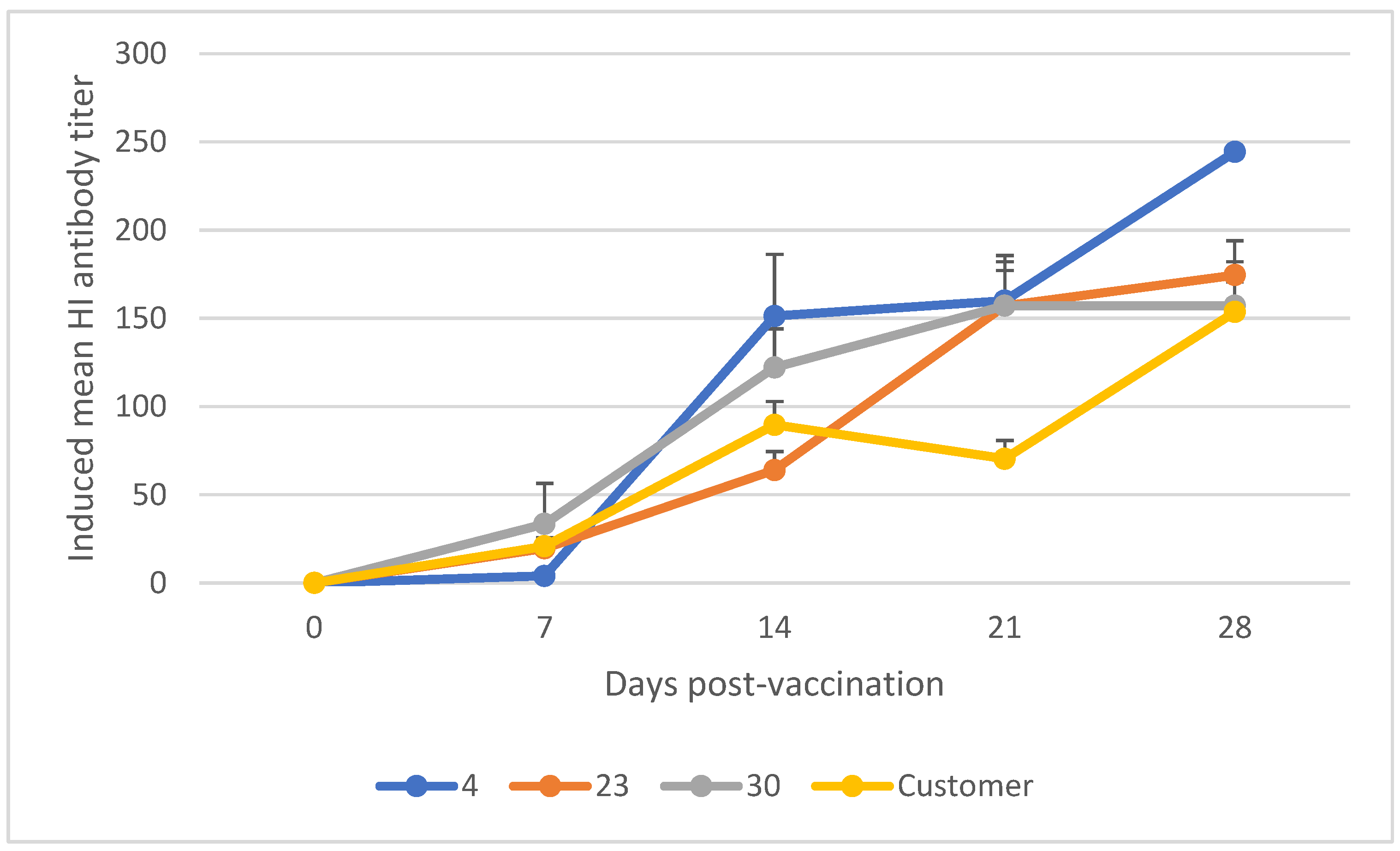
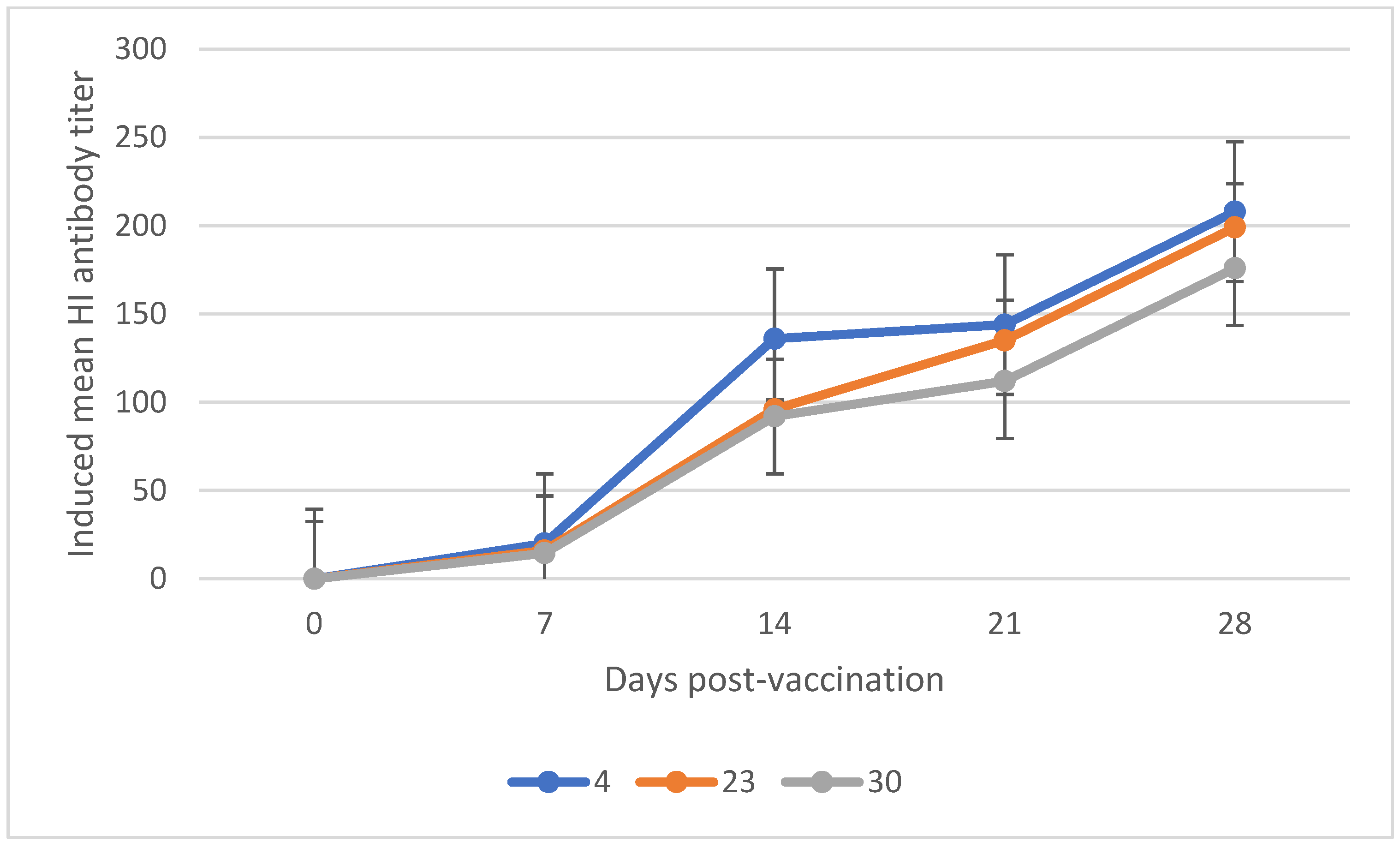
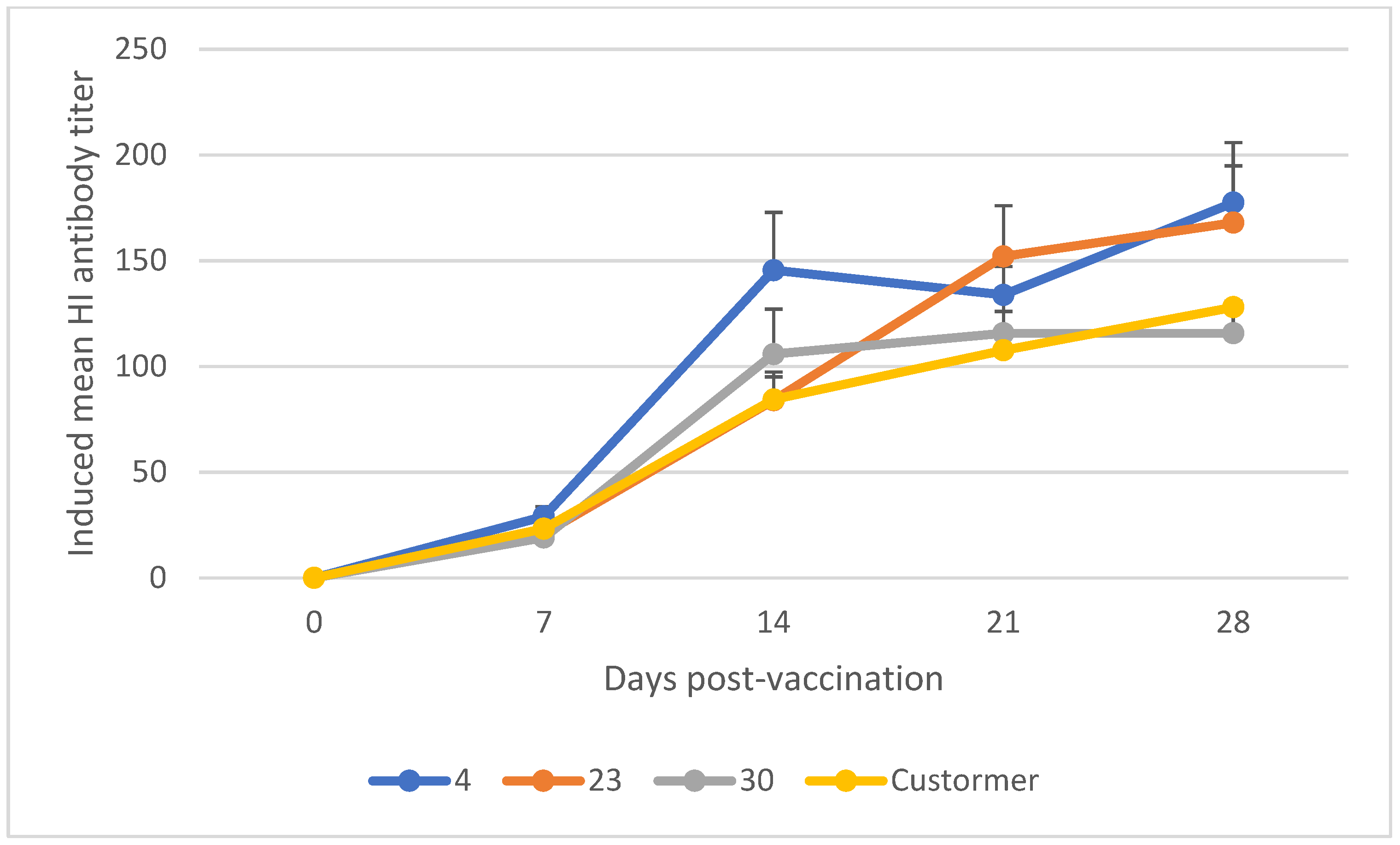
3.1. Mean Induced Antibody Titer of Chickens Vaccinated with Newcastle Disease Vaccine
3.2. Challenge of Vaccinated and Control Chickens
3.3. Viable Virus Titer of the Vaccines
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haque, M.H.; Hossain, M.T.; Islam, M.T.; Zinnah, M.A.; Khan, M.S.R.; Islam, M.A. Islam, Isolation and detection of Newcastle disease virus from field outbreaks in broiler and layer chickens by reverse transcription-Polymerase chain reaction. J. Vet. Med. 2010, 8, 87–92. [Google Scholar]
- Orsi, M.A.; Doretto, L., Jr.; Camillo, S.C.A.; Reischak, D.; Ribeiro, S.A.M.; Ramazzoti, A.; Mendonça, A.O.; Spilki, F.R.; Buzinaro, M.G.; Ferreira, H.L.; et al. Prevalence of Newcastle disease virus in Broiler chickens (Gallus gallus) in Brazil. Braz. J. Microbiol. 2010, 41, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Zegeye, A.; Temesgen, W.; Molla, W.; Setotaw, H.; Lakew, M. Epidemiology of Newcastle disease in chickens of Ethiopia: A systematic review and meta-analysis. Trop. Anim. Health Prod. 2022, 54, 328. [Google Scholar] [CrossRef] [PubMed]
- Butcher, G.D.; Yegani, M. Investigating Vaccination Failure in Poultry Flocks; VM174/VM136, 11/2008; EDIS: Gainesville, FL, USA, 2009. [Google Scholar] [CrossRef]
- Müller, H.; Mundt, E.; Eterradossi, N.; Islam, M.R. Current status of vaccines against infectious bursal disease. Avian Pathol. 2012, 41, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, K.M.; Afonso, C.L.; Yu, Q.; Miller, P.J. Newcastle disease vaccines—A solved problem or a continuous challenge? Vet. Microbiol. 2017, 206, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Ezema, W.S.; Okoye, J.O.A.; Nwanta, J.A. LaSota vaccination may not protect against the lesions of velogenic Newcastle disease virus against the lesions of velogenic Newcastle disease in chickens. Trop. Anim. Health Product. 2009, 41, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Dortmans, J.C.; Peeters, B.P.; Koch, G. Newcastle disease virus outbreaks: Vaccine mismatch or inadequate application? Vet. Microbiol. 2012, 160, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, K.M.; Lee, D.H.; Williams-Coplin, D.; Olivier, T.L.; Miller, P.J.; Afonso, C.L. Newcastle Disease Viruses Causing Recent Outbreaks Worldwide Show Unexpectedly High Genetic Similarity to Historical Virulent Isolates from the 1940s. J. Clin. Microbiol. 2015, 54, 122. [Google Scholar] [CrossRef] [PubMed]
- Samuel, A.; Nayak, B.; Paldurai, A.; Xiao, S.; Aplogan, G.L.; Awoume, K.A.; Webby, R.J.; Ducatez, M.F.; Collins, P.L.; Samal, S.K. Phylogenetic and pathotypic characterization of Newcastle disease viruses circulating in West Africa and the efficacy of a current vaccination. J. Clin. Microbiol. 2013, 51, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Roohani, K.; Tan, S.W.; Yeap, S.K.; Ideris, A.; Bejo, M.H.; Omar, A.R. Characterisation of genotype VII Newcastle disease virus (NDV) isolated from NDV vaccinated chickens, and the efficacy of LaSota and recombinant genotype VII vaccines against challenge velogenic virus. J. Vet. Sci. 2015, 16, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Wajid, A.; Basharat, A.; Bibi, T.; Rehmani, S.F. Comparison of protection and viral shedding following vaccination with Newcastle disease virus strains of different genotypes used in vaccine formulation. Trop. Anim. Health Prod. 2018, 50, 1645–1651. [Google Scholar] [CrossRef] [PubMed]
- Boumart, Z.; Hamdi, J.; Daouam, S.; Elarkam, A.; Tadlaoui, K.O.; El Harrak, M. Thermal Stability Study of Five Newcastle Disease Attenuated Vaccine Strains. Avian Dis. 2016, 60, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Sieng, S.; Walkden-Brown, S.W.; Kerr, J. Variation in storage temperatures for foot and mouth disease in Cambodia. Int. J. Environ. Rural Dev. 2016, 7, 24–31. [Google Scholar]
- El-Sayed, E.; El-Din, W.M.G.; Rizk, S.A.; Abd El-Aty, M. Effect of different storage temperatures on the efficacy of the bivalent foot and mouth disease oil vaccine. J. Adv. Vet. Res. 2012, 2, 198–205. [Google Scholar]
- Bordoloi, S.; Nayak, A.; Singh, A.P.; Singh, R.V.; Dubey, A.; Jadav, K.; Upadhyay, A.; Himani, K.; Lade, A. Evaluation of Newcastle Disease Antibody Titre in Broiler Poultry. Int. J. Curr. Microbiol. App. Sci. 2021, 10, 1839–1844. [Google Scholar] [CrossRef]
- National Veterinary Institute. NVI, 20 September 2009. Available online: https://www.nvi.com.et/products/vaccines-against/poultry-diseases/live-newcastle/ (accessed on 1 October 2023).
- WOAH Technical Manual. Newcastle Disease (Infection with Newcastle Disease Virus, Online). ROME. 2021. Available online: https://www.woah.org/fileadmin/Home/fr/Health_standards/tahm/3.03.14_NEWCASTLE_DIS.pdf (accessed on 12 October 2023).
- Young, M.; Alders, R.; Grimes, S.; Spradbrow, P.; Dias, P.; da Silva, A.; Lobo, Q. Controlling ND in village chickens. ACIAR Monogr. 2001, 82, 112. [Google Scholar]
- Abdi, R.D.; Amsalu, K.; Merera, O.; Asfaw, Y.; Gelaye, E.; Yami, M.; Sori, T. Serological response and protection level evaluation in chickens exposed to grains coated with I2 Newcastle disease virus for effective oral vaccination of village chickens. BMC Vet. Res. 2016, 12, 150. [Google Scholar]
- Wise, M.G.; Suarez, D.L.; Seal, B.S.; Pedersen, J.C.; Senne, D.A.; King, D.J.; Kapczynski, D.R.; Spackman, E. Development of a real-time reverse-transcription PCR for detection of new-castle disease virus RNA in clinical samples. J. Clin. Microbiol. 2004, 42, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Collett, S.R.; Smith, J.A.; Boulianne, M.; Owen, R.L.; Gingerich, E.; Singer, R.S.; Johnson, T.J.; Hofacre, C.L.; Berghaus, R.D.; Stewart-Brown, B. Principles of Disease Prevention: Diagnosis and control. In Diseases of Poultry; Calnek, B.W., Ed.; Wiley: New York, NY, USA, 2019; pp. 3–78. [Google Scholar]
| Group | No. of Chickens | Temperature °C | Type of Vaccine | Duration of Storage | Route of Administration |
|---|---|---|---|---|---|
| Groups 1–4 | 10 chicks each | Customer vaccine (−20) | ND-HB1 | 72 h | Intraocular |
| +4 | |||||
| +23 | |||||
| +30 | |||||
| Groups 5–8 | 10 chicks each | −20 | ND-Lasota | 72 h | Intraocular |
| +4 | |||||
| +23 | |||||
| +30 | |||||
| Groups 8–12 | 10 chicks each | Customer vaccine (−20) | ND-TH-I2 | 72 h | Intraocular |
| +4 | |||||
| +23 | |||||
| +30 | |||||
| Groups 13 | 7 chicks | Control |
| Storage Temperature | Days Post-Vaccination | Mean HI Antibody Titer | SE | CI | p-Value |
|---|---|---|---|---|---|
| NCD-HB1 (+4, +23, +30) | 7 | 15.57 | 6.03 | [−14.66, 49.48] | 0.14 |
| 14 | 85.39 | 19.00 | [−4.24, 217.72] | 0.05 | |
| 21 | 108.81 | 22.01 | [53.96, 183.92] | 0.01 | |
| 28 | 145.72 | 21.13 | [112.62, 341.32] | 0.20 | |
| NCD-Lasota (+4, +23, +30) | 7 | 16.83 | 1.64 | [20.62, 21.07] | 0.00 |
| 14 | 108.00 | 14.05 | [51.07, 232.53] | 0.03 | |
| 21 | 130.37 | 9.54 | [−16.78, 318.20] | 0.05 | |
| 28 | 194.34 | 9.53 | [48.24, 381.15] | 0.03 | |
| NCD-TH-I2 (+4, +23, +30) | 7 | 23.05 | 3.07 | [13.12, 34.92] | 0.01 |
| 14 | 111.76 | 17.98 | [19.99, 188.35] | 0.03 | |
| 21 | 133.83 | 10.48 | [72.70, 173.94] | 0.01 | |
| 28 | 153.71 | 19.21 | [59.34, 234.79] | 0.02 |
| Vaccines and Storage Conditions | Survived | Dead |
|---|---|---|
| Control | 0 | 8 |
| ND-HB1 + 23 | 11 | 0 |
| ND-HB1 + 30 | 11 | 0 |
| ND-HB1 + 4 | 11 | 0 |
| ND-HB1 cu | 10 | 0 |
| ND-L + 23 | 9 | 0 |
| ND-L + 30 | 8 | 0 |
| ND-L + 4 | 8 | 0 |
| ND-Thermostable-I2 cu | 11 | 0 |
| ND-Thermostable-I2 + 23 | 8 | 0 |
| ND-Thermostable-I2 + 30 | 13 | 0 |
| ND-Thermostable-I2 + 4 | 11 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Hellenic Society for Microbiology. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Degefa, T.; Birehanu, M.; Mulugeta, D.; Ferede, H.; Girma, E.; Alemu, A.; Muleta, D.; Aga, A.M.; Shimeket, D.; Woldemichael, D.N.; et al. Evaluation of the Efficacy of Three Newcastle Disease Vaccines Produced at the National Veterinary Institute, Bishoftu, Ethiopia, at Different Temperature Storage Conditions. Acta Microbiol. Hell. 2024, 69, 212-223. https://doi.org/10.3390/amh69040020
Degefa T, Birehanu M, Mulugeta D, Ferede H, Girma E, Alemu A, Muleta D, Aga AM, Shimeket D, Woldemichael DN, et al. Evaluation of the Efficacy of Three Newcastle Disease Vaccines Produced at the National Veterinary Institute, Bishoftu, Ethiopia, at Different Temperature Storage Conditions. Acta Microbiologica Hellenica. 2024; 69(4):212-223. https://doi.org/10.3390/amh69040020
Chicago/Turabian StyleDegefa, Teferi, Mahlet Birehanu, Demise Mulugeta, Henok Ferede, Endalkachew Girma, Anberber Alemu, Dassalegn Muleta, Abebe Mengesha Aga, Debebe Shimeket, Dereje Nigussie Woldemichael, and et al. 2024. "Evaluation of the Efficacy of Three Newcastle Disease Vaccines Produced at the National Veterinary Institute, Bishoftu, Ethiopia, at Different Temperature Storage Conditions" Acta Microbiologica Hellenica 69, no. 4: 212-223. https://doi.org/10.3390/amh69040020
APA StyleDegefa, T., Birehanu, M., Mulugeta, D., Ferede, H., Girma, E., Alemu, A., Muleta, D., Aga, A. M., Shimeket, D., Woldemichael, D. N., Akalu, M., & Woldemariyam, F. T. (2024). Evaluation of the Efficacy of Three Newcastle Disease Vaccines Produced at the National Veterinary Institute, Bishoftu, Ethiopia, at Different Temperature Storage Conditions. Acta Microbiologica Hellenica, 69(4), 212-223. https://doi.org/10.3390/amh69040020






