Tracking the Threat, 50 Years of Laboratory-Acquired Infections: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Extraction
2.3. Inclusion and Exclusion Criteria
2.4. Analysis
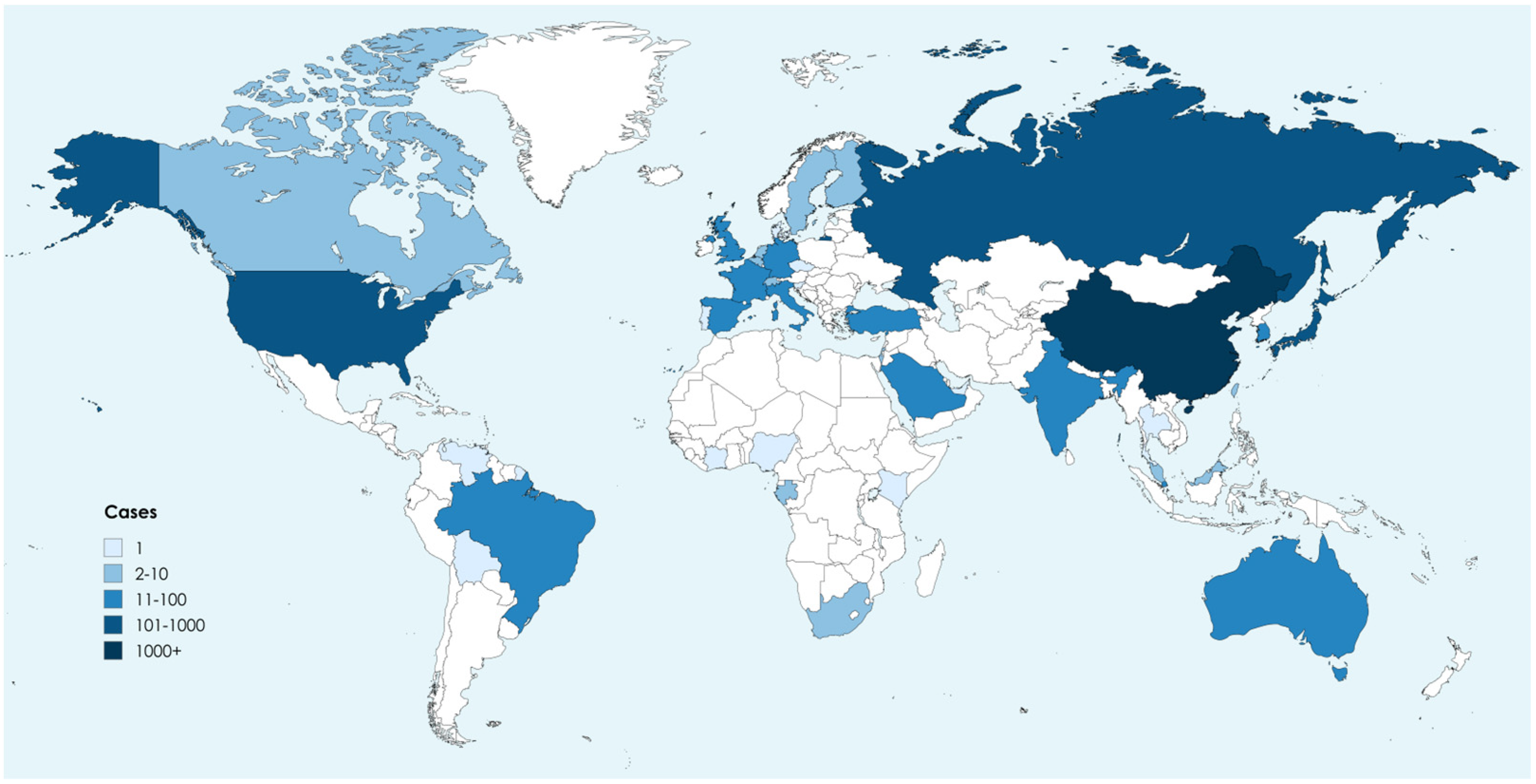
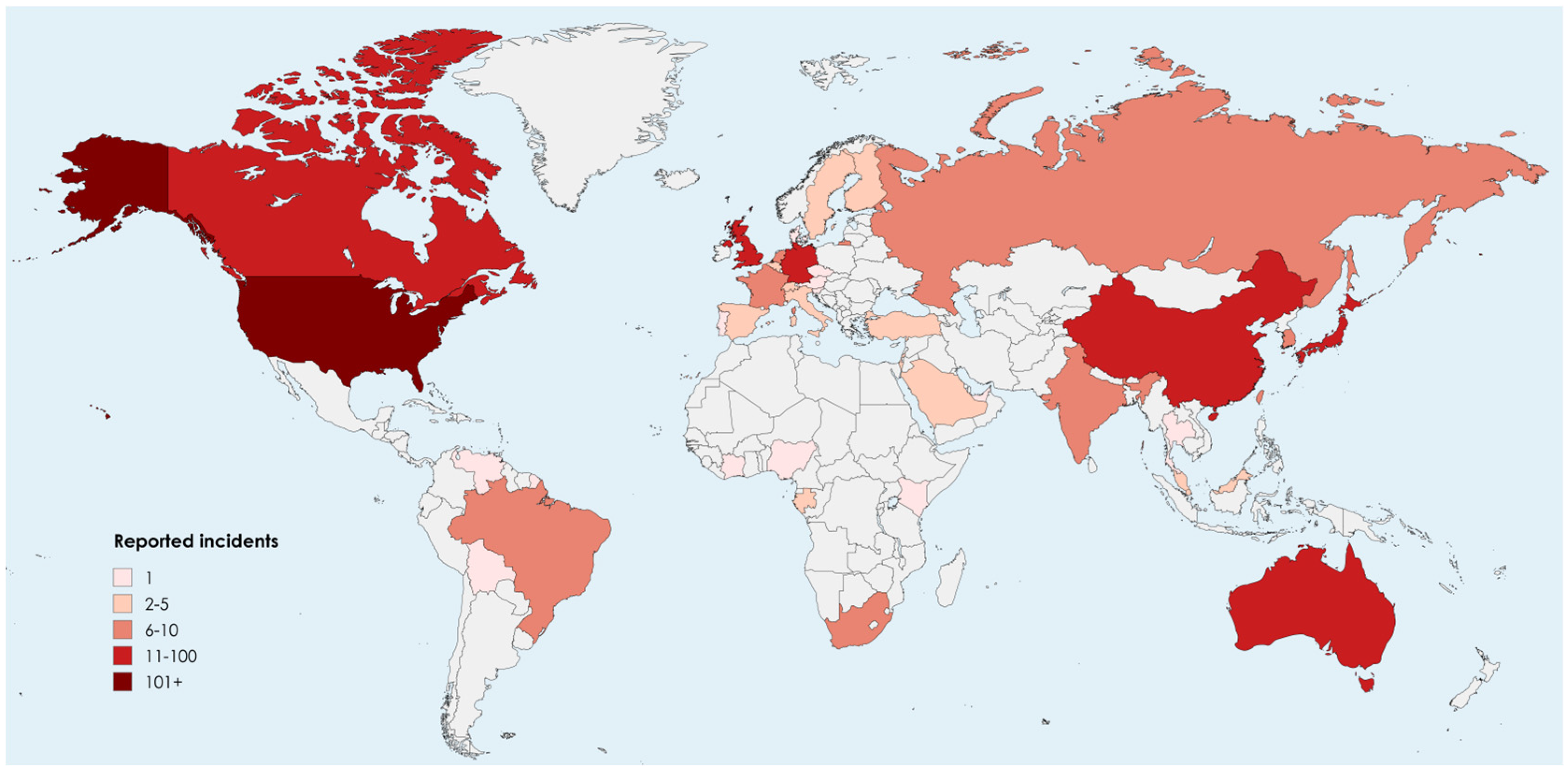
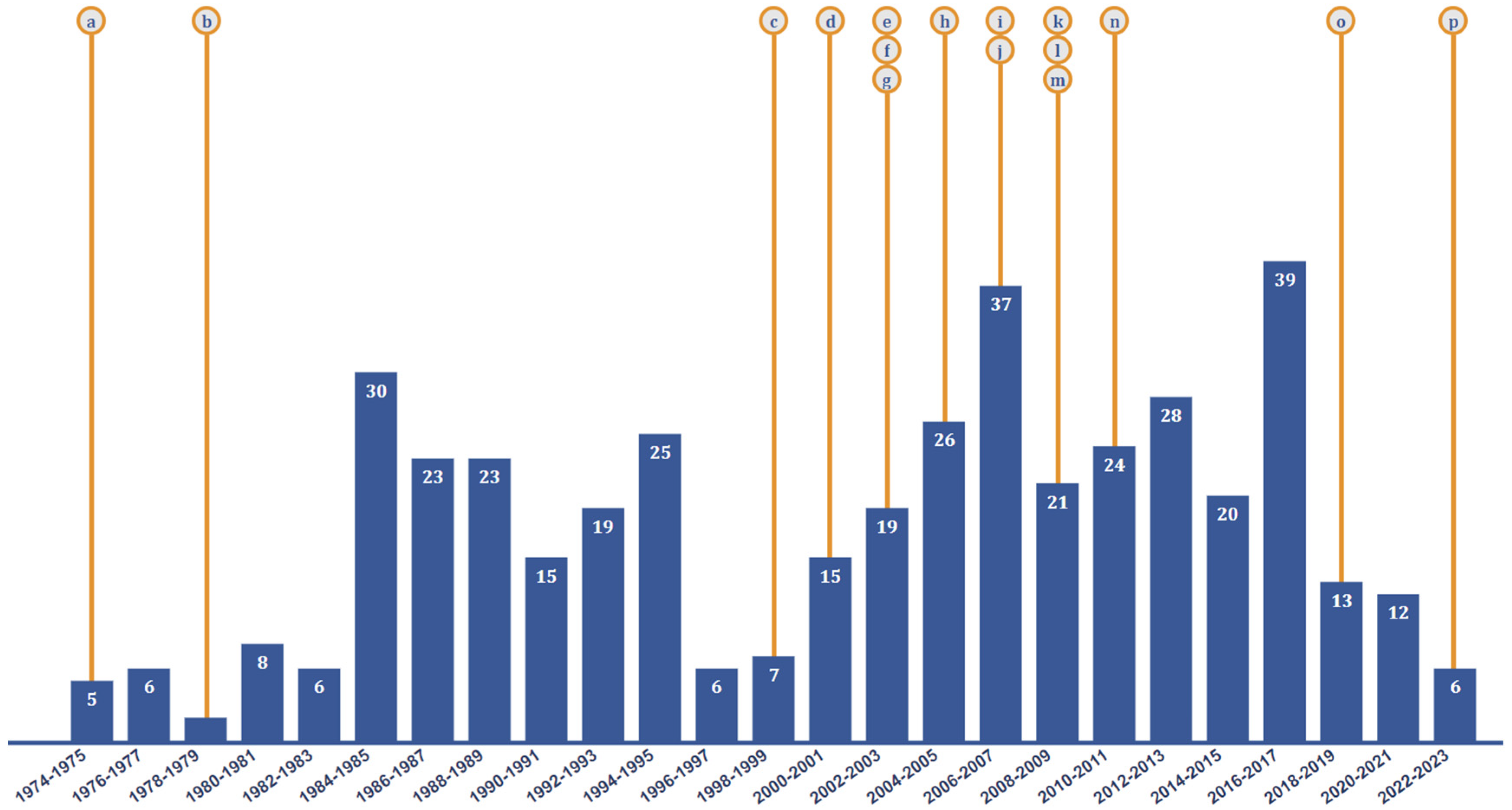
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Appelt, S.; Jacob, D.; Rohleder, A.-M.; Bråve, A.; Björndal, Å.S.; Di Caro, A.; Grunow, R. Assessment of Biorisk Management Systems in High Containment Laboratories, 18 Countries in Europe, 2016 and 2017. Eurosurveillance 2020, 25, 2000089. [Google Scholar] [CrossRef] [PubMed]
- Beeckman, D.S.; Rüdelsheim, P. Biosafety and Biosecurity in Containment: A Regulatory Overview. Front. Bioeng. Biotechnol. 2020, 8, 650. [Google Scholar] [CrossRef] [PubMed]
- Petts, D.; Wren, M.; Nation, B.; Guthrie, G.; Kyle, B.; Peters, L.; Mortlock, S.; Clarke, S.; Burt, C. A Short History of Occupational Disease: 1. Laboratory-Acquired Infections. Ulst. Med. J. 2021, 90, 28. [Google Scholar]
- Peng, H.; Bilal, M.; Iqbal, H.M. Improved Biosafety and Biosecurity Measures and/or Strategies to Tackle Laboratory-Acquired Infections and Related Risks. Int. J. Environ. Res. Public Health 2018, 15, 2697. [Google Scholar] [CrossRef]
- Gao, W.; Wu, Z.; Zuo, K.; Xiang, Q.; Zhang, L.; Chen, X.; Tan, F.; Liu, H. From Biosafety to National Security: The Evolution and Challenges of Biosafety Laboratories. Laboratories 2024, 1, 158–173. [Google Scholar] [CrossRef]
- Coelho, A.C.; García Díez, J. Biological Risks and Laboratory-Acquired Infections: A Reality That Cannot Be Ignored in Health Biotechnology. Front. Bioeng. Biotechnol. 2015, 3, 56. [Google Scholar] [CrossRef]
- Microbe, T.L. Searching for SARS-CoV-2 Origins: Confidence versus Evidence. Lancet Microbe 2023, 4, e200. [Google Scholar] [CrossRef]
- Willemarck, N.; Van Vaerenbergh, B.; Descamps, E.; Brosius, B.; Dai, C.; Leunda, A.; Baldo, A. Laboratory-Acquired Infections in Belgium; Technical report; Flemish Agency for Care and Health, Biosafety and Biotechnology Unit: Brussels, Belgium, 2015. [Google Scholar]
- Blacksell, S.D.; Dhawan, S.; Kusumoto, M.; Le, K.K.; Summermatter, K.; O’Keefe, J.; Kozlovac, J.P.; Almuhairi, S.S.; Sendow, I.; Scheel, C.M.; et al. Laboratory-Acquired Infections and Pathogen Escapes Worldwide between 2000 and 2021: A Scoping Review. Lancet Microbe 2024, 5, e194–e202. [Google Scholar] [CrossRef]
- Manheim, D.; Lewis, G. High-Risk Human-Caused Pathogen Exposure Events from 1975–2016. F1000Research 2021, 10, 752. [Google Scholar] [CrossRef]
- Balbontin, N.; Gauthier, A.; Abalos, C.; Davis, A.N.; Lister, M. Canadian Laboratory Incidents with Human Pathogens and Toxins: An Overview of Reports, 2016–2022. Can. Commun. Dis. Rep. 2024, 50, 144–152. [Google Scholar] [CrossRef]
- Ackelsberg, J.; Liddicoat, A.; Burke, T.; Szymczak, W.A.; Levi, M.H.; Ostrowsky, B.; Hamula, C.; Patel, G.; Kopetz, V.; Saverimuttu, J.; et al. Brucella Exposure Risk Events in 10 Clinical Laboratories, New York City, USA, 2015 to 2017. J. Clin. Microbiol. 2020, 58, e01096-19. [Google Scholar] [CrossRef] [PubMed]
- Bang, E.; Oh, S.; Chang, H.E.; Shin, I.S.; Park, K.U.; Kim, E.S. Zika Virus Infection During Research Vaccine Development: Investigation of the Laboratory-Acquired Infection via Nanopore Whole-Genome Sequencing. Front. Cell. Infect. Microbiol. 2022, 12, 819829. [Google Scholar] [CrossRef]
- Aebischer, O.; Meylan, P.; Kunz, S.; Lazor-Blanchet, C. Lymphocytic Choriomeningitis Virus Infection Induced by Percutaneous Exposure. Occup. Med. 2016, 66, 171–173. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. PLoS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef] [PubMed]
- WHO. Biorisk Management. In Laboratory Biosecurity Guidance; WHO: Geneva, Switzerland, 2006. [Google Scholar]
- National Research Council; Policy and Global Affairs; Development, Security, and Cooperation; Committee on Research Standards and Practices to Prevent the Destructive Application of Biotechnology. Biotechnology Research in an Age of Terrorism; National Academies Press: Washington, DC, USA, 2004; p. 10827. ISBN 978-0-309-08977-7. [Google Scholar]
- Convention on the Prohibition of the Development, Production and Stockpiling of Bacteriological (Biological) and Toxin Weapons and on Their Destruction. Main Page. Available online: https://legal.un.org/avl/ha/cpdpsbbtwd/cpdpsbbtwd.html (accessed on 18 July 2024).
- European Commission. EU Animal Health Strategy 2007–2013. Available online: https://food.ec.europa.eu/animals/animal-health/eu-animal-health-strategy-2007-2013_en (accessed on 18 July 2024).
- Executive Order 13486 Working Group on Strengthening the Biosecurity of the United States. Available online: https://www.phe.gov/s3/law/boards-committees/biosecurity-wg/Pages/default.aspx (accessed on 18 July 2024).
- Federal Select Agent Program. Available online: https://www.selectagents.gov/overview/history.htm (accessed on 18 July 2024).
- World Health Organization. Guidelines for the Collection of Clinical Specimens during Field Investigation of Outbreaks; WHO: Geneva, Switzerland, 2000. [Google Scholar]
- Laboratory Biorisk Management. CWA 15793. Available online: https://internationalbiosafety.org/wp-content/uploads/2019/08/CWA-15793-English.pdf (accessed on 18 July 2024).
- World Health Organization. Laboratory Biosafety Manual, 3rd ed.; World Health Organization: Geneva, Switzerland, 2004; ISBN 978-92-4-154650-8. [Google Scholar]
- Laboratory Biosafety Manual Fourth Edition. Available online: https://www.who.int/publications/i/item/9789240011311 (accessed on 18 July 2024).
- European Commission. National Action Plans. Available online: https://food.ec.europa.eu/plants/pesticides/sustainable-use-pesticides/national-action-plans_en (accessed on 18 July 2024).
- Presidential Policy Directive 2 (SGP-2). National Strategy to Counter Biological Threats. Available online: https://irp.fas.org/offdocs/ppd/ppd-2.pdf (accessed on 18 July 2024).
- World Health Organization. Public Health Response to Biological and Chemical Weapons: WHO Guidance, 2nd ed.; Robinson, J.P., Ed.; World Health Organization: Geneva, Switzerland, 2004; ISBN 978-92-4-154615-7. [Google Scholar]
- Goldson, S.L.; Frampton, E.R.; Ridley, G.S. The Effects of Legislation and Policy in New Zealand and Australia on Biosecurity and Arthropod Biological Control Research and Development. Biol. Control. 2010, 52, 241–244. [Google Scholar] [CrossRef]
- UN Security Council Resolution 1540 (2004)—UNODA. Available online: https://disarmament.unoda.org/wmd/sc1540/ (accessed on 25 November 2024).
- World Health Organization. WHO Guidance on Implementing Regulatory Requirements for Biosafety and Biosecurity in Biomedical Laboratories: A Stepwise Approach; World Health Organization: Geneva, Switzerland, 2020; ISBN 978-92-4-151626-6. [Google Scholar]
- Ali, K.; Meyer, E.; Kabir, F. Biorisk Management, Laboratory Acquired Infections and Clinical Containment, Volume II. Front. Public Health 2024, 12, 1368828. [Google Scholar] [CrossRef] [PubMed]
- Vennis, I.M.; Boskovic, M.; Bleijs, D.A.; Rutjes, S.A. Complementarity of International Instruments in the Field of Biosecurity. Front. Public Health 2022, 10, 894389. [Google Scholar] [CrossRef]
- Joseph, T. Management System Approach for Addressing Biosafety and Biosecurity of Emerging Pathogens in a Biosafety Level-3 Core Facility. Appl. Biosaf. 2021, 26, 210–220. [Google Scholar] [CrossRef]
- WHO. Pathogens Prioritization: A Scientific Framework for Epidemic and Pandemic Reserach Preparednesss; WHO: Geneva, Switzerland, 2024. [Google Scholar]
- Mallapaty, S. The Pathogens That Could Spark the Next Pandemic. Available online: https://nature.proxyucr.elogim.com/articles/d41586-024-02513-3 (accessed on 24 January 2025).
- Klotz, L.C.; Sylvester, E.J. The Consequences of a Lab Escape of a Potential Pandemic Pathogen. Front. Public Health 2014, 2, 116. [Google Scholar] [CrossRef]
- Merler, S.; Ajelli, M.; Fumanelli, L.; Vespignani, A. Containing the Accidental Laboratory Escape of Potential Pandemic Influenza Viruses. BMC Med. 2013, 11, 252. [Google Scholar] [CrossRef]
- Holmes, E.C.; Goldstein, S.A.; Rasmussen, A.L.; Robertson, D.L.; Crits-Christoph, A.; Wertheim, J.O.; Anthony, S.J.; Barclay, W.S.; Boni, M.F.; Doherty, P.C.; et al. The Origins of SARS-CoV-2: A Critical Review. Cell 2021, 184, 4848–4856. [Google Scholar] [CrossRef] [PubMed]
- Rozo, M.; Gronvall, G.K. The Reemergent 1977 H1N1 Strain and the Gain-of-Function Debate. mBio 2015, 6, e01013-15. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.L.; Kurup, A.; Gopalakrishna, G.; Chan, K.P.; Wong, C.W.; Ng, L.C.; Se-Thoe, S.Y.; Oon, L.; Bai, X.; Stanton, L.W.; et al. Laboratory-Acquired Severe Acute Respiratory Syndrome. N. Engl. J. Med. 2004, 350, 1740–1745. [Google Scholar] [CrossRef]
- Parry, J. Breaches of Safety Regulations Are Probable Cause of Recent SARS Outbreak, WHO Says. BMJ 2004, 328, 1222. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.-N.; Zhao, T.; Liu, Z.-J.; Guan, B.-Y.; He, X.; Liu, M.; Chen, Q.; Liu, G.-F.; Wu, J.; Huang, R.-G.; et al. Severe Acute Respiratory Syndrome—Retrospect and Lessons of 2004 Outbreak in China. Biomed. Environ. Sci. 2006, 19, 445–451. [Google Scholar] [PubMed]
- WHO. WHO-Convened Global Study of Origins of SARS-CoV-2: China Part; COVID-19: Animal-Human Interface and Food Safety; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Pappas, G. The Lanzhou Brucella Leak: The Largest Laboratory Accident in the History of Infectious Diseases? Clin. Infect. Dis. 2022, 75, 1845–1847. [Google Scholar] [CrossRef]
- CDC. Bioterrorism and Anthrax: The Threat. Available online: https://www.cdc.gov/anthrax/bioterrorism/index.html (accessed on 18 July 2024).
- Khorram-Manesh, A.; Brukle, F.M., Jr.; Goniewicz, K. Pandemics: Past, Present, and Future: Multitasking Challenges in Need of Cross-Disciplinary, Transdisciplinary, and Multidisciplinary Collaborative Solutions. PHRP 2024, 15, 267–285. [Google Scholar] [CrossRef]
- Khorram-Manesh, A.; Goniewicz, K.; Burkle, F.M. Unleashing the Global Potential of Public Health: A Framework for Future Pandemic Response. J. Infect. Public Health 2024, 17, 82–95. [Google Scholar] [CrossRef]
- Ward, R.J.; Mark Jjunju, F.P.; Kabenge, I.; Wanyenze, R.; Griffith, E.J.; Banadda, N.; Taylor, S.; Marshall, A. FluNet: An AI-Enabled Influenza-Like Warning System. IEEE Sens. J. 2021, 21, 24740–24748. [Google Scholar] [CrossRef]
- WHO. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report: 2022; Global Antimicrobial Resistance and Use Surveillance System (GLASS); WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Syrowatka, A.; Kuznetsova, M.; Alsubai, A.; Beckman, A.L.; Bain, P.A.; Craig, K.J.T.; Hu, J.; Jackson, G.P.; Rhee, K.; Bates, D.W. Leveraging Artificial Intelligence for Pandemic Preparedness and Response: A Scoping Review to Identify Key Use Cases. NPJ Digit. Med. 2021, 4, 1–14. [Google Scholar] [CrossRef]
- AlShammari, W.; Alhussain, H.; Rizk, N.M. Risk Management Assessments and Recommendations Among Students, Staffs, and Health Care Workers in Educational Biomedical Laboratories. Risk Manag. Healthc. Policy 2021, 14, 185–198. [Google Scholar] [CrossRef] [PubMed]
- El Jaouhari, M.; Striha, M.; Edjoc, R.; Bonti-Ankomah, S. Healthcare-Associated Infections & Antimicrobial Resistance: Laboratory-Acquired Infections in Canada from 2016 to 2021. Can. Commun. Dis. Rep. 2022, 48, 303. [Google Scholar] [PubMed]

| Safety Level | Description of Permissible Microorganisms |
|---|---|
| Biosafety Level 1 (BSL-1) | Agents unlikely to cause disease in healthy adults that pose minimal laboratory exposure and infection risk. |
| Biosafety Level 2 (BSL-2) | Agents that may cause moderate human disease and can result in severe illness or death through exposure via inhalation, ingestion, or skin absorption. |
| Biosafety Level 3 (BSL-3) | Agents that can cause serious or fatal human disease through inhalation, ingestion, or skin absorption. |
| Biosafety Level 4 (BSL-4) | Agents that cause extremely dangerous and fatal human diseases through airborne transmission. |
| Cause | Definition |
|---|---|
| Procedural error | Improper selection or use of personal protective equipment (PPE) or primary containment devices, inadequate training, incorrect techniques or procedures, or mishandling of specimens. |
| Accident | Unintended exposure to a pathogen despite adherence to adequate safety procedures through incidents such as cuts from glass shards, animal bites, needlestick injuries, or splashes and spills. |
| Intentional | Deliberate actions carried out with the intent to cause harm due to the intentional misuse of biological agents to compromise safety or security. |
| Not stated | The cause of the LAI is not mentioned or specified in the literature. |
| Multiple causes | A specific cause is not reported, but rather a combination of several factors that triggered the incident. |
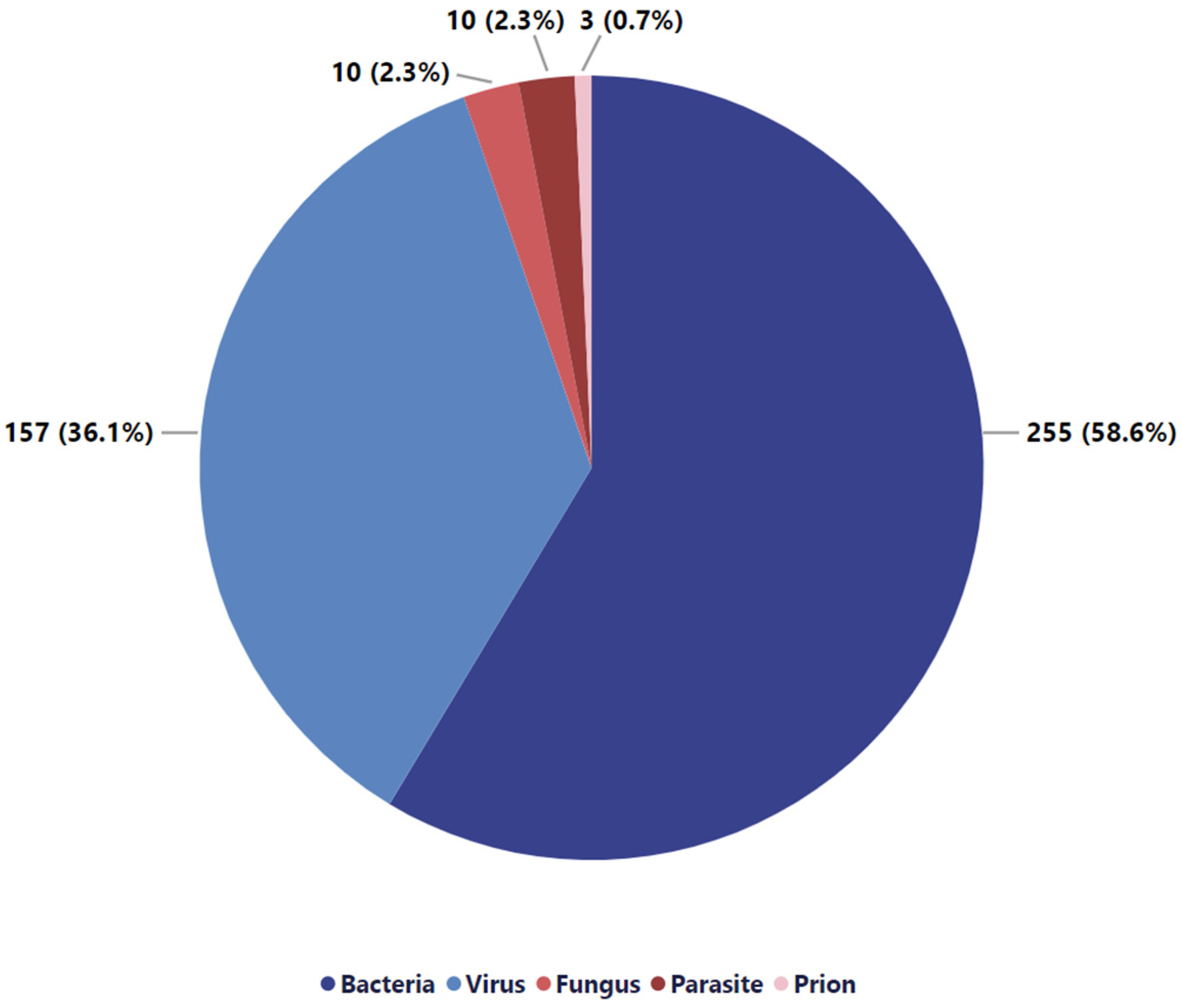
| Bacteria | Virus | Fungus | Parasite | Prion | |
|---|---|---|---|---|---|
| Accident | 22.5% | 48.7% | 30.0% | 50.0% | 33.3% |
| Procedural error | 38.3% | 18.0% | 10.0% | 20.0% | - |
| Multiple causes | 2.0% | 1.3% | - | - | - |
| Not stated | 36.0% | 32.1% | 60.0% | 30.0% | 66.7% |
| Intentional | 0.8% | - | - | - | - |
| Unknown | 0.4% | - | - | - | - |
| Pathogen | Total Case Number | Percentage (%) |
|---|---|---|
| Brucella spp. | 10,794 | 90.6% |
| Anthrax (Bacillus anthracis) | 205 | 1.7% |
| Coxiella burnetii | 149 | 1.6% |
| Hemorrhagic fever with renal syndrome virus | 140 | 1.2% |
| CCHF-like virus | 75 | 0.6% |
| Salmonella spp. | 70 | 0.6% |
| West Nile virus | 55 | 0.5% |
| SARS-CoV-2 | 54 | 0.5% |
| SARS-CoV | 34 | 0.3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Hellenic Society for Microbiology. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zavaleta-Monestel, E.; Rojas-Chinchilla, C.; Anchía-Alfaro, A.; Quesada-Loría, D.; García-Montero, J.; Arguedas-Chacón, S.; Hanley-Vargas, G. Tracking the Threat, 50 Years of Laboratory-Acquired Infections: A Systematic Review. Acta Microbiol. Hell. 2025, 70, 11. https://doi.org/10.3390/amh70020011
Zavaleta-Monestel E, Rojas-Chinchilla C, Anchía-Alfaro A, Quesada-Loría D, García-Montero J, Arguedas-Chacón S, Hanley-Vargas G. Tracking the Threat, 50 Years of Laboratory-Acquired Infections: A Systematic Review. Acta Microbiologica Hellenica. 2025; 70(2):11. https://doi.org/10.3390/amh70020011
Chicago/Turabian StyleZavaleta-Monestel, Esteban, Carolina Rojas-Chinchilla, Adriana Anchía-Alfaro, Diego Quesada-Loría, Jonathan García-Montero, Sebastián Arguedas-Chacón, and Georgia Hanley-Vargas. 2025. "Tracking the Threat, 50 Years of Laboratory-Acquired Infections: A Systematic Review" Acta Microbiologica Hellenica 70, no. 2: 11. https://doi.org/10.3390/amh70020011
APA StyleZavaleta-Monestel, E., Rojas-Chinchilla, C., Anchía-Alfaro, A., Quesada-Loría, D., García-Montero, J., Arguedas-Chacón, S., & Hanley-Vargas, G. (2025). Tracking the Threat, 50 Years of Laboratory-Acquired Infections: A Systematic Review. Acta Microbiologica Hellenica, 70(2), 11. https://doi.org/10.3390/amh70020011







