Influence of Microbiome Interactions on Antibiotic Resistance Development in the ICU Environment: Insights and Opportunities with Machine Learning
Abstract
:1. Introduction
2. Microbiome Interactions in the ICU
2.1. Composition of the ICU Microbiome
2.2. Factors Influencing Microbiome Dynamics
3. Mechanisms of Antibiotic Resistance Development via Microbiome Interactions
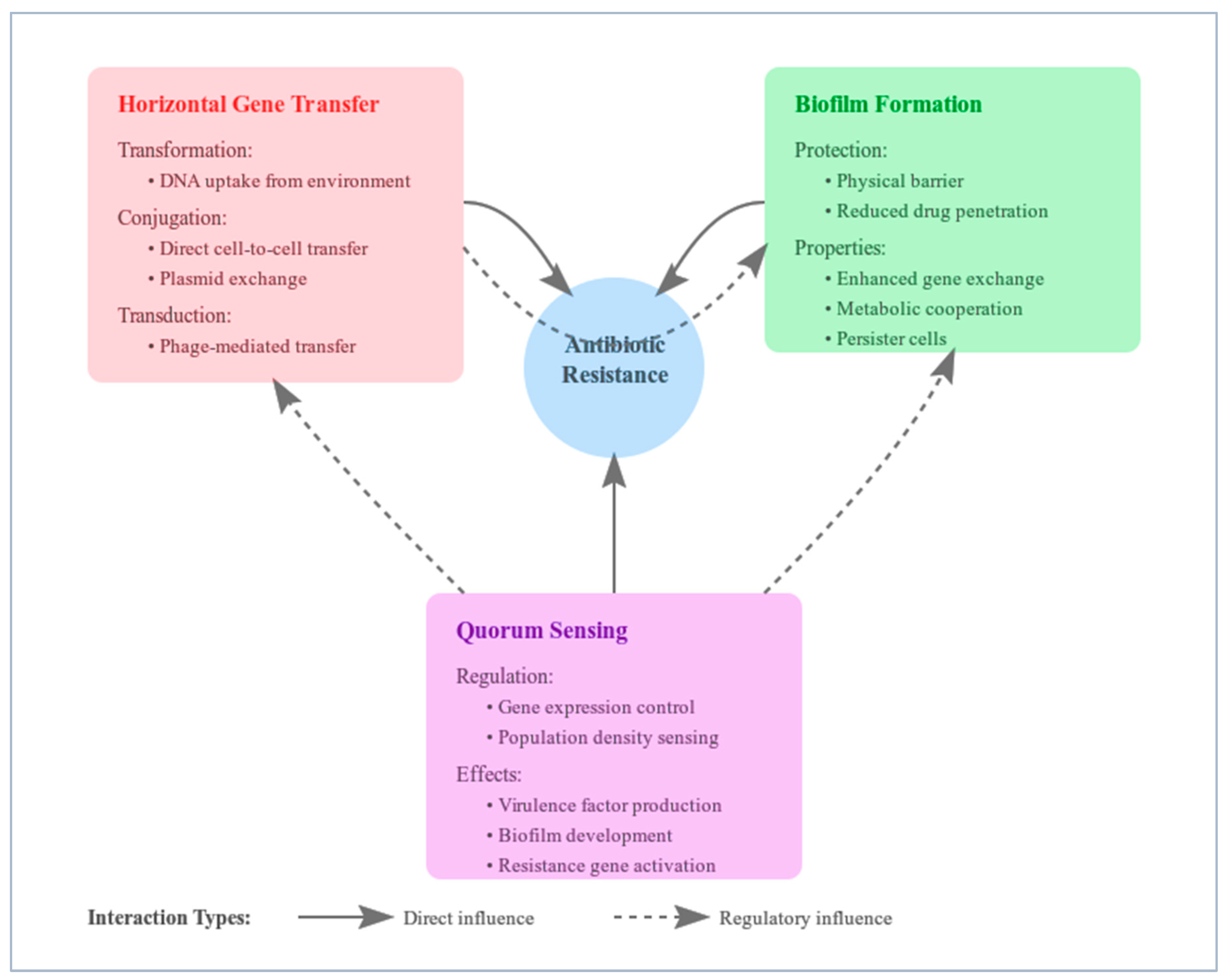
3.1. Horizontal Gene Transfer
- Transformation involves the uptake of free DNA fragments from the environment by competent bacterial cells [45]. In the ICU setting, the frequent use of antibiotics leads to bacterial cell lysis, releasing DNA into the surroundings. The dense microbial populations increase the likelihood that other bacteria will encounter and incorporate this genetic material, including ARGs [46]. Environmental stressors, such as antibiotic pressure, can induce competence in bacteria, enhancing their ability to take up external DNA [45].
- Transduction is mediated by bacteriophages, which are viruses that infect bacteria [45]. During the infection process, bacteriophages may inadvertently package fragments of the host bacterial DNA, including ARGs, into new viral particles. When these phages infect other bacteria, they introduce the acquired DNA into the new host’s genome [45]. The high diversity of bacteriophages in the ICU microbiome facilitates the transduction of ARGs among different bacterial populations [46].
- Conjugation involves direct cell-to-cell contact through pilus formation, allowing the transfer of plasmids and transposons carrying ARGs between bacteria [46]. Plasmids are extrachromosomal DNA elements that often carry multiple resistance genes, and their transfer can confer multidrug resistance to recipient bacteria [45]. The selective pressure exerted by antibiotics in the ICU environment promotes the survival and proliferation of bacteria capable of conjugative gene transfer [46]. Mobile genetic elements, like integrons and insertion sequences, can also facilitate the integration and dissemination of ARGs within bacterial genomes [19].
3.2. Biofilm Formation
- Reduced antibiotic penetration: The EPS matrix acts as a physical barrier that impedes the diffusion of antibiotics into the deeper layers of the biofilm [51]. This barrier can result in sub-inhibitory concentrations of antibiotics reaching the bacterial cells, which not only fail to eradicate the bacteria but may also promote the development of resistance [52].
- Altered microenvironment: Within biofilms, gradients of nutrients, oxygen, and waste products create heterogeneous microenvironments [51]. Bacteria in different regions of the biofilm may exhibit varied metabolic activities, with cells in nutrient-deprived zones entering a slow-growing or dormant state [52]. Antibiotics targeting active cellular processes are less effective against these dormant cells, allowing them to survive treatment [52].
- Enhanced HGT: The close proximity of cells within biofilms facilitates HGT [51]. The accumulation of extracellular DNA within the EPS matrix serves as a source of genetic material for transformation [51]. Additionally, the high cell density promotes conjugation events, enabling the transfer of plasmids carrying ARGs [51]. Biofilms can thus act as reservoirs for resistance genes and hotspots for genetic exchange [51].
- Protection from immune responses: The EPS matrix shields bacteria from phagocytosis and the action of antimicrobial peptides produced by the host immune system [51]. Biofilm-associated infections often lead to chronic inflammation, which can cause tissue damage and further compromise the immune response [51].
3.3. Quorum Sensing
- Antibiotic resistance mechanisms: Quorum sensing can modulate the expression of efflux pumps, enzymes that degrade or modify antibiotics, and other resistance factors [59]. For instance, the overexpression of efflux pumps can reduce intracellular antibiotic concentrations, diminishing drug efficacy [59].
- Enzymatic degradation of signaling molecules: Enzymes like lactonases and acylases can degrade autoinducers, interrupting the quorum sensing signal [60].
- Structural analogues of autoinducers: Molecules that mimic autoinducers can competitively inhibit receptor binding, blocking signal transduction [61].
- Antagonists of quorum sensing receptors: Designing molecules that bind to quorum sensing receptors without activating them can prevent signal transduction [62].
4. The ICU Environment as a Catalyst for Resistance
4.1. High Antibiotic Usage
4.2. Patient Susceptibility
4.3. Environmental Factors
5. Machine Learning Applications
5.1. Predicting Antibiotic Resistance Patterns
5.2. Analyzing Microbiome Data
5.3. Identifying Novel Therapeutic Targets
6. Current Challenges and Limitations
6.1. Data Availability and Quality
6.2. Interpretability of Models
- Use of explainable algorithms: Selecting algorithms that are inherently interpretable, such as decision trees or linear models, allows for a straightforward understanding of how input features contribute to the output [84]. While these models may be less powerful than complex neural networks, they offer transparency that is valuable in a clinical context [97].
- Model-agnostic explanation techniques: Methods like SHAP (Shapley Additive Explanations) values and LIME (Local Interpretable Model-Agnostic Explanations) provide insights into complex models by quantifying the contribution of each feature to a specific prediction [97]. These techniques enable clinicians to see which variables influenced the model’s decision, even in deep learning models [97].
- Visualization tools: Graphical representations of model outputs, such as heatmaps, feature importance plots, and decision pathways, can help clinicians grasp complex relationships within the data [97]. Visual tools make abstract concepts more tangible and can be integrated into user interfaces for clinical applications [97].
6.3. Ethical Considerations
- Informed consent: Patients must provide explicit consent for their data to be collected and used for specific purposes. Consent forms should be clear and comprehensive, explaining the scope of data usage, potential risks, and the right to withdraw consent [99].
7. Future Perspectives
7.1. Integrating ML into Clinical Practice
7.2. Personalized Medicine Approaches
- Evidence-based guidelines: Clinical guidelines incorporating personalized medicine principles are needed to standardize practices. Research must demonstrate the effectiveness and safety of personalized approaches to gain acceptance [87].
7.3. Policy Implications
- Antimicrobial stewardship programs: Policies should encourage stewardship programs tailored to ICU settings [65]. These programs can leverage microbiome data and ML insights to ensure appropriate antibiotic use, minimize resistance, and optimize patient outcomes [91]. Guidelines for ICU-specific interventions can be developed to improve prescribing practices.
- Infection control standards: ICU-specific infection control policies should incorporate insights from microbiome studies to limit the spread of resistant organisms. This includes advanced sterilization protocols, isolation measures, and monitoring of microbiome composition in patients.
- Data sharing and surveillance: Policies promoting data sharing across healthcare systems and research institutions are crucial. Establishing secure, standardized databases for microbiome and antibiotic resistance data can enhance machine learning applications and improve global surveillance of resistance trends [91].
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Antimicrobial Resistance. WHO Fact Sheets. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 1 October 2024).
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Aljeldah, M.M. Antimicrobial Resistance and Its Spread Is a Global Threat. Antibiotics 2022, 11, 1082. [Google Scholar] [CrossRef] [PubMed]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef]
- Michaelis, C.; Grohmann, E. Horizontal Gene Transfer of Antibiotic Resistance Genes in Biofilms. Antibiotics 2023, 12, 328. [Google Scholar] [CrossRef] [PubMed]
- Theodorakis, N.; Feretzakis, G.; Hitas, C.; Kreouzi, M.; Kalantzi, S.; Spyridaki, A.; Boufeas, I.Z.; Sakagianni, A.; Paxinou, E.; Verykios, V.S.; et al. Antibiotic Resistance in the Elderly: Mechanisms, Risk Factors, and Solutions. Microorganisms 2024, 12, 1978. [Google Scholar] [CrossRef] [PubMed]
- Sakagianni, A.; Koufopoulou, C.; Koufopoulos, P.; Feretzakis, G.; Kalles, D.; Paxinou, E.; Myrianthefs, P.; Verykios, V.S. The Synergy of Machine Learning and Epidemiology in Addressing Carbapenem Resistance: A Comprehensive Review. Antibiotics 2024, 13, 996. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The human microbiome project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef]
- Robinson, C.J.; Bohannan, B.J.M.; Young, V.B. From structure to function: The ecology of host-associated microbial communities. Microbiol. Mol. Biol. Rev. 2010, 74, 453–476. [Google Scholar] [CrossRef]
- Bassetti, M.; Righi, E.; Carnelutti, A.; Graziano, E.; Russo, A. Multidrug-resistant Klebsiella pneumoniae: Challenges for treatment, prevention and control. Expert Rev. Anti. Infect. Ther. 2018, 16, 749–761. [Google Scholar] [CrossRef]
- Ojima, M.; Motooka, D.; Shimizu, K.; Gotoh, K.; Shintani, A.; Yoshiya, K.; Nakamura, S.; Ogura, H.; Iida, T.; Shimazu, T. Metagenomic Analysis Reveals Dynamic Changes of Whole Gut Microbiota in the Acute Phase of Intensive Care Unit Patients. Dig. Dis. Sci. 2016, 61, 1628–1634. [Google Scholar] [CrossRef]
- Deo, R.C. Machine learning in medicine. Circulation 2015, 132, 1920–1930. [Google Scholar] [CrossRef] [PubMed]
- Sakagianni, A.; Koufopoulou, C.; Feretzakis, G.; Kalles, D.; Verykios, V.S.; Myrianthefs, P.; Fildisis, G. Using Machine Learning to Predict Antimicrobial Resistance―A Literature Review. Antibiotics 2023, 12, 452. [Google Scholar] [CrossRef] [PubMed]
- McDermott, J.; Bumgarner, R.E.; Bean, H.D. Emerging applications of machine learning in microbial ecology, human microbiome studies, and environmental monitoring. Comput. Struct. Biotechnol. J. 2021, 19, 1093–1107. [Google Scholar] [CrossRef]
- Azamfirei, L. The Human Microbiome in Intensive Care—A Journey Forward? J. Crit. Care Med. 2023, 9, 205–207. [Google Scholar] [CrossRef]
- Liu, H.Y.; Prentice, E.L.; Webber, M.A. Mechanisms of antimicrobial resistance in biofilms. NPJ Antimicrob. Resist. 2024, 2, 27. [Google Scholar] [CrossRef]
- Andreescu, M. Molecular Insights Into the Role of Gut Microbiota in Antibiotic Therapy Selection and Resistance Mitigation. Cureus 2023, 15, e50318. [Google Scholar] [CrossRef]
- Taner, F.; Baddal, B.; Theodoridis, L.; Petrovski, S. Biofilm Production in Intensive Care Units: Challenges and Implications. Pathogens 2024, 13, 954. [Google Scholar] [CrossRef]
- Zaborin, A.; Smith, D.; Garfield, K.; Quensen, J.; Shakhsheer, B.; Kade, M.; Tirrell, M.; Tiedje, J.; Gilbert, J.A.; Zaborina, O.; et al. Membership and behavior of ultra-low-diversity pathogen communities present in the gut of humans during prolonged critical illness. mBio 2014, 5, e01361-14. [Google Scholar] [CrossRef]
- Nguyen, T.Q.N.; Young, V.B. The Relationship Between the Microbiome and Antimicrobial Resistance. Clin. Infect. Dis. 2023, 77 (Suppl. 6), S479–S486. [Google Scholar] [CrossRef]
- Wolff, N.S.; Hugenholtz, F.; Wiersinga, W.J. The Emerging Role of the Microbiota in the ICU. Crit. Care 2018, 22, 78. [Google Scholar] [CrossRef]
- Patangia, D.V.; Ryan, C.A.; Dempsey, E.; Ross, R.P.; Stanton, C. Impact of Antibiotics on the Human Microbiome and Consequences for Host Health. Microbiol. Open 2022, 11, e1260. [Google Scholar] [CrossRef]
- Fromentin, M.; Ricard, J.-D.; Roux, D. Lung Microbiome in Critically Ill Patients. Life 2022, 12, 7. [Google Scholar] [CrossRef]
- Xiao, T.; Guo, Q.; Zhou, Y.; Shen, P.; Wang, Y.; Fang, Q.; Li, M.; Zhang, S.; Guo, L.; Yu, X.; et al. Comparative Respiratory Tract Microbiome Between Carbapenem-Resistant Acinetobacter baumannii Colonization and Ventilator Associated Pneumonia. Front. Microbiol. 2022, 13, 782210. [Google Scholar] [CrossRef]
- García-Muñoz Rodrigo, F.; Urquía Martí, L.; Siguero Onrubia, M.; Borges Luján, M.; Galán Henríquez, G.; Reyes Suárez, D. Lung Microbiota and Ventilator-Associated Pneumonia in the Neonatal Period. Pathogens 2024, 13, 220. [Google Scholar] [CrossRef] [PubMed]
- Chu, V.T.; Tsitsiklis, A.; Mick, E.; Ambroggio, L.; Kalantar, K.L.; Glascock, A.; Osborne, C.M.; Wagner, B.D.; Matthay, M.A.; DeRisi, J.L.; et al. The Antibiotic Resistance Reservoir of the Lung Microbiome Expands with Age in a Population of Critically Ill Patients. Nat. Commun. 2024, 15, 92. [Google Scholar] [CrossRef] [PubMed]
- Choy, A.; Freedberg, D.E. Impact of Microbiome-Based Interventions on Gastrointestinal Pathogen Colonization in the Intensive Care Unit. Ther. Adv. Gastroenterol. 2020, 13, 1756284820939447. [Google Scholar] [CrossRef]
- Zhang, Y.; Xue, G.; Wang, F.; Zhang, J.; Xu, L.; Yu, C. The Impact of Antibiotic Exposure on Antibiotic Resistance Gene Dynamics in the Gut Microbiota of Inflammatory Bowel Disease Patients. Front. Microbiol. 2024, 15, 1382332. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Sánchez, M.A.; Melgar, S.; O’Donoghue, K.; Martínez-Sánchez, M.A.; Fernández-Ruiz, V.E.; Ferrer-Gómez, M.; Ruiz-Alcaraz, A.J.; Ramos-Molina, B. Crohn’s Disease, Host–Microbiota Interactions, and Immunonutrition: Dietary Strategies Targeting Gut Microbiome as Novel Therapeutic Approaches. Int. J. Mol. Sci. 2022, 23, 8361. [Google Scholar] [CrossRef]
- Ducarmon, Q.R.; Grundler, F.; Le Maho, Y.; Wilhelmi de Toledo, F.; Zeller, G.; Habold, C.; Mesnage, R. Remodelling of the Intestinal Ecosystem During Caloric Restriction and Fasting. Trends Microbiol. 2023, 31, 832–844. [Google Scholar] [CrossRef]
- Dickson, R.P. The Microbiome and Critical Illness. Lancet Respir. Med. 2016, 4, 59–72. [Google Scholar] [CrossRef]
- Miniet, A.A.; Grunwell, J.R.; Coopersmith, C.M. The Microbiome and the Immune System in Critical Illness. Curr. Opin. Crit. Care 2021, 27, 157–163. [Google Scholar] [CrossRef]
- Dickson, R.P.; Singer, B.H.; Newstead, M.W.; Falkowski, N.R.; Erb-Downward, J.R.; Standiford, T.J.; Huffnagle, G.B. Enrichment of the lung microbiome with gut bacteria in sepsis and the acute respiratory distress syndrome. Nat. Microbiol. 2016, 1, 16113. [Google Scholar] [CrossRef]
- Zhou, X.; Liao, Y. Gut-Lung Crosstalk in Sepsis-Induced Acute Lung Injury. Front. Microbiol. 2021, 12, 779620. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.-W.; Lu, J.-L.; Dong, B.-Y.; Fang, M.-Y.; Xiong, X.; Qin, X.-J.; Fan, X.-M. Gut Microbiota and Its Metabolic Products in Acute Respiratory Distress Syndrome. Front. Immunol. 2024, 15, 1330021. [Google Scholar] [CrossRef]
- Orieux, A.; Enaud, R.; Imbert, S.; Boyer, P.; Begot, E.; Camino, A.; Boyer, A.; Berger, P.; Gruson, D.; Delhaes, L.; et al. The Gut Microbiota Composition Is Linked to Subsequent Occurrence of Ventilator-Associated Pneumonia in Critically Ill Patients. Microbiol. Spectr. 2023, 11, e0064123. [Google Scholar] [CrossRef] [PubMed]
- Ziaka, M.; Exadaktylos, A. Gut-Derived Immune Cells and the Gut-Lung Axis in ARDS. Crit. Care 2024, 28, 220. [Google Scholar] [CrossRef]
- Francino, M.P. Antibiotics and the human gut microbiome: Dysbioses and accumulation of resistances. Front. Microbiol. 2016, 6, 1543. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.; Di Ciaula, A.; Mahdi, L.; Jaber, N.; Di Palo, D.M.; Graziani, A.; Baffy, G.; Portincasa, P. Unraveling the Role of the Human Gut Microbiome in Health and Diseases. Microorganisms 2024, 12, 2333. [Google Scholar] [CrossRef]
- Zuo, W.; Wang, B.; Bai, X.; Luan, Y.; Fan, Y.; Michail, S.; Sun, F. 16S rRNA and Metagenomic Shotgun Sequencing Data Revealed Consistent Patterns of Gut Microbiome Signature in Pediatric Ulcerative Colitis. Sci. Rep. 2022, 12, 6421. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Yang, L.; Feng, J.; Tian, S.; Chen, L.; Huang, W.; Liu, J.; Wang, X. Integrated 16S rRNA Sequencing and Metagenomics Insights into Microbial Dysbiosis and Distinct Virulence Factors in Inflammatory Bowel Disease. Front. Microbiol. 2024, 15, 1375804. [Google Scholar] [CrossRef]
- Jiménez-Rojas, V.; Villanueva-García, D.; Miranda-Vega, A.L.; Aldana-Vergara, R.; Aguilar-Rodea, P.; López-Marceliano, B.; Reyes-López, A.; Alcántar-Curiel, M.D. Gut Colonization and Subsequent Infection of Neonates Caused by Extended-Spectrum Beta-Lactamase-Producing Escherichia coli and Klebsiella pneumoniae. Front. Cell. Infect. Microbiol. 2023, 13, 1322874. [Google Scholar] [CrossRef] [PubMed]
- Ruppé, E.; Burdet, C.; Grall, N.; de Lastours, V.; Lescure, F.X.; Andremont, A.; Armand-Lefèvre, L. Impact of antibiotics on the intestinal microbiota needs to be re-defined to optimize antibiotic usage. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2018, 24, 3–5. [Google Scholar] [CrossRef] [PubMed]
- von Wintersdorff, C.J.; Penders, J.; van Niekerk, J.M.; Mills, N.D.; Majumder, S.; van Alphen, L.B.; Savelkoul, P.H.; Wolffs, P.F. Dissemination of antimicrobial resistance in microbial ecosystems through horizontal gene transfer. Front. Microbiol. 2016, 7, 173. [Google Scholar] [CrossRef] [PubMed]
- Gillings, M.R. Evolutionary consequences of antibiotic use for the resistome, mobilome, and microbial pangenome. Front. Microbiol. 2013, 4, 4. [Google Scholar] [CrossRef]
- Smillie, C.S.; Smith, M.B.; Friedman, J.; Cordero, O.X.; David, L.A.; Alm, E.J. Ecology drives a global network of gene exchange connecting the human microbiome. Nature 2011, 480, 241–244. [Google Scholar] [CrossRef]
- Wurfel, M.M.; Gordon, A.C.; Holden, T.D.; Radella, F.; Strout, J.; Kajikawa, O.; Ruzinski, J.T.; Rona, G.; Black, R.A.; Stratton, S.; et al. Toll-Like Receptor 1 Polymorphisms Affect Innate Immune Responses and Outcomes in Sepsis. Am. J. Respir. Crit. Care Med. 2008, 178, 710–720. [Google Scholar] [CrossRef]
- Sun, M.; Lu, F.; Yu, D.; Wang, Y.; Chen, P.; Liu, S. Respiratory Diseases and Gut Microbiota: Relevance, Pathogenesis, and Treatment. Front. Microbiol. 2024, 15, 1358597. [Google Scholar] [CrossRef]
- Schinas, G.; Polyzou, E.; Spernovasilis, N.; Gogos, C.; Dimopoulos, G.; Akinosoglou, K. Preventing Multidrug-Resistant Bacterial Transmission in the Intensive Care Unit with a Comprehensive Approach: A Policymaking Manual. Antibiotics 2023, 12, 1255. [Google Scholar] [CrossRef]
- Kuczewski, E.; Henaff, L.; Regard, A.; Argaud, L.; Lukaszewicz, A.-C.; Rimmelé, T.; Cassier, P.; Fredenucci, I.; Loeffert-Frémiot, S.; Khanafer, N.; et al. Bacterial Cross-Transmission between Inanimate Surfaces and Patients in Intensive Care Units under Real-World Conditions: A Repeated Cross-Sectional Study. Int. J. Environ. Res. Public Health 2022, 19, 9401. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef]
- Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment. Microorganisms 2023, 11, 1614. [Google Scholar] [CrossRef] [PubMed]
- Grooters, K.E.; Ku, J.C.; Richter, D.M.; Krinock, M.J.; Minor, A.; Li, P.; Kim, A.; Sawyer, R.; Li, Y. Strategies for Combating Antibiotic Resistance in Bacterial Biofilms. Front. Cell. Infect. Microbiol. 2024, 14, 1352273. [Google Scholar] [CrossRef]
- Yaikhan, T.; Chukamnerd, A.; Singkhamanan, K.; Nokchan, N.; Chintakovid, N.; Chusri, S.; Pomwised, R.; Wonglapsuwan, M.; Surachat, K. Genomic Characterization of Mobile Genetic Elements Associated with Multidrug-Resistant Acinetobacter Non-baumannii Species from Southern Thailand. Antibiotics 2024, 13, 149. [Google Scholar] [CrossRef] [PubMed]
- Gedefie, A.; Demsis, W.; Ashagrie, M.; Kassa, Y.; Tesfaye, M.; Tilahun, M.; Bisetegn, H.; Sahle, Z. Acinetobacter baumannii Biofilm Formation and Its Role in Disease Pathogenesis: A Review. Infect. Drug Resist. 2021, 14, 3711–3719. [Google Scholar] [CrossRef]
- Naga, N.G.; Shaaban, M.I.; El-Metwally, M.M. An insight on the powerful of bacterial quorum sensing inhibition. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2024, 43, 2071–2081. [Google Scholar] [CrossRef]
- Rutherford, S.T.; Bassler, B.L. Bacterial quorum sensing: Its role in virulence and possibilities for its control. Cold Spring Harb. Perspect. Med. 2012, 2, a012427. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Bassler, B.L. Bacterial quorum sensing in complex and dynamically changing environments. Nat. Rev. Microbiol. 2019, 17, 371–382. [Google Scholar] [CrossRef]
- Hetta, H.F.; Ramadan, Y.N.; Rashed, Z.I.; Alharbi, A.A.; Alsharef, S.; Alkindy, T.T.; Alkhamali, A.; Albalawi, A.S.; Battah, B.; Donadu, M.G. Quorum Sensing Inhibitors: An Alternative Strategy to Win the Battle against Multidrug-Resistant (MDR) Bacteria. Molecules 2024, 29, 3466. [Google Scholar] [CrossRef]
- Naga, N.G.; El-Badan, D.E.; Ghanem, K.M.; Shaaban, M.I. It Is the Time for Quorum Sensing Inhibition as an Alternative Strategy of Antimicrobial Therapy. Cell Commun. Signal. 2023, 21, 133. [Google Scholar] [CrossRef]
- Cui, S.; Kim, E. Quorum Sensing and Antibiotic Resistance in Polymicrobial Infections. Commun. Integr. Biol. 2024, 17, 2415598. [Google Scholar] [CrossRef]
- Zilahi, G.; Artigas, A.; Martin-Loeches, I. What’s New in Multidrug-Resistant Pathogens in the ICU? Ann. Intensive Care 2016, 6, 96. [Google Scholar] [CrossRef]
- De Waele, J.J.; Martin-Loeches, I. Optimal Duration of Antibiotic Treatment in Gram-Negative Infections. Curr. Opin. Infect. Dis. 2018, 31, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Giamarellou, H.; Galani, L.; Karavasilis, T.; Ioannidis, K.; Karaiskos, I. Antimicrobial Stewardship in the Hospital Setting: A Narrative Review. Antibiotics 2023, 12, 1557. [Google Scholar] [CrossRef]
- Barnsteiner, S.; Baty, F.; Albrich, W.C.; Babouee Flury, B.; Gasser, M.; Plüss-Suard, C.; Schlegel, M.; Kronenberg, A.; Kohler, P.; Swiss Centre for Antibiotic Resistance (ANRESIS). Antimicrobial Resistance and Antibiotic Consumption in Intensive Care Units, Switzerland, 2009 to 2018. Euro Surveill. 2021, 26, 2001537. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.F.A.; Silveira, G.G.O.S.; Cândido, E.S.; Cardoso, M.H.; Carvalho, C.M.E.; Franco, O.L. Effects of Antibiotic Treatment on Gut Microbiota and How to Overcome Its Negative Impacts on Human Health. ACS Infect. Dis. 2020, 6, 2544–2559. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.; Rajendraprasad, S.S.; Philbrick, K.L.; Bauer, B.A.; Gajic, O.; Shah, A.; Laudanski, K.; Bakken, J.S.; Skalski, J.; Karnatovskaia, L.V. The Human Gut Microbiome in Critical Illness: Disruptions, Consequences, and Therapeutic Frontiers. J. Crit. Care 2024, 79, 154436. [Google Scholar] [CrossRef]
- Kraemer, S.A.; Ramachandran, A.; Perron, G.G. Antibiotic Pollution in the Environment: From Microbial Ecology to Public Policy. Microorganisms 2019, 7, 180. [Google Scholar] [CrossRef]
- Szychowiak, P.; Villageois-Tran, K.; Patrier, J.; Timsit, J.-F.; Ruppé, É. The Role of the Microbiota in the Management of Intensive Care Patients. Ann. Intensive Care 2022, 12, 3. [Google Scholar] [CrossRef]
- Stokes, H.W.; Gillings, M.R. Gene Flow, Mobile Genetic Elements, and the Recruitment of Antibiotic Resistance Genes into Gram-Negative Pathogens. FEMS Microbiol. Rev. 2011, 35, 790–819. [Google Scholar] [CrossRef]
- Memar, M.Y.; Yekani, M.; Celenza, G.; Poortahmasebi, V.; Naghili, B.; Bellio, P.; Bannazadeh Baghi, H. The central role of the SOS DNA repair system in antibiotics resistance: A new target for a new infectious treatment strategy. Life Sci. 2020, 262, 118562. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, S.; Tschudin-Sutter, S.; Egli, A.; Osthoff, M. Optimizing Antibiotic Therapies to Reduce the Risk of Bacterial Resistance. Eur. J. Intern. Med. 2022, 99, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Van Boeckel, T.P.; Gandra, S.; Ashok, A.; Caudron, Q.; Grenfell, B.T.; Levin, S.A.; Laxminarayan, R. Global Antibiotic Consumption 2000 to 2010: An Analysis of National Pharmaceutical Sales Data. Lancet Infect. Dis. 2014, 14, 742–750. [Google Scholar] [CrossRef]
- CDC. Core Elements of Hospital Antibiotic Stewardship Programs. Atlanta, GA: US Department of Health and Human Services, CDC. 2019. Available online: https://www.cdc.gov/antibiotic-use/healthcare/pdfs/hospital-core-elements-H.pdf (accessed on 2 September 2024).
- Schlechte, J.; Zucoloto, A.Z.; Yu, I.; Doig, C.J.; Dunbar, M.J.; McCoy, K.D.; McDonald, B. Dysbiosis of a Microbiota–Immune Metasystem in Critical Illness Is Associated with Nosocomial Infections. Nat. Med. 2023, 29, 1017–1027. [Google Scholar] [CrossRef]
- Salazar, C.; Giménez, M.; Riera, N.; Parada, A.; Puig, J.; Galiana, A.; Grill, F.; Vieytes, M.; Mason, C.E.; Antelo, V.; et al. Human Microbiota Drives Hospital-Associated Antimicrobial Resistance Dissemination in the Urban Environment and Mirrors Patient Case Rates. Microbiome 2022, 10, 208. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Moldawer, L.L.; Opal, S.M.; Reinhart, K.; Turnbull, I.R.; Vincent, J.-L. Sepsis and Septic Shock. Nat. Rev. Dis. Primers 2016, 2, 16045. [Google Scholar] [CrossRef]
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Kainer, M.A.; Lynfield, R.; Maloney, M.; McAllister-Hollod, L.; Nadle, J.; et al. Multistate Point-Prevalence Survey of Health Care–Associated Infections. N. Engl. J. Med. 2014, 370, 1198–1208. [Google Scholar] [CrossRef]
- Dancer, S.J. Controlling hospital-acquired infection: Focus on the role of the environment and new technologies for decontamination. Clin. Microbiol. Rev. 2014, 27, 665–690. [Google Scholar] [CrossRef]
- Otter, J.A.; Yezli, S.; French, G.L. The role played by contaminated surfaces in the transmission of nosocomial pathogens. Infect. Control Hosp. Epidemiol. 2011, 32, 687–699. [Google Scholar] [CrossRef]
- Leal, J.; Farkas, B.; Mastikhina, L.; Flanagan, J.; Skidmore, B.; Salmon, C.; Dixit, D.; Smith, S.; Tsekrekos, S.; Lee, B.; et al. Risk of Transmission of Respiratory Viruses During Aerosol-Generating Medical Procedures (AGMPs) Revisited in the COVID-19 Pandemic: A Systematic Review. Antimicrob. Resist. Infect. Control 2022, 11, 102. [Google Scholar] [CrossRef]
- Casini, B.; Tuvo, B.; Scarpaci, M.; Totaro, M.; Badalucco, F.; Briani, S.; Luchini, G.; Costa, A.L.; Baggiani, A. Implementation of an Environmental Cleaning Protocol in Hospital Critical Areas Using a UV-C Disinfection Robot. Int. J. Environ. Res. Public Health 2023, 20, 4284. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Improving Infection Prevention and Control at the Health Facility: Interim Practical Manual Supporting Implementation of the WHO Guidelines on Core Components of Infection Prevention and Control Programmes; WHO/HIS/SDS/2018.10; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Peters, A.; Schmid, M.N.; Parneix, P.; Lebowitz, D.; de Kraker, M.; Sauser, J.; Zingg, W.; Pittet, D. Impact of Environmental Hygiene Interventions on Healthcare-Associated Infections and Patient Colonization: A Systematic Review. Antimicrob. Resist. Infect. Control 2022, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Luo, J.; Huang, D.; Liu, Y.; Li, D.-d. Machine Learning Advances in Microbiology: A Review of Methods and Applications. Front. Microbiol. 2022, 13, 925454. [Google Scholar] [CrossRef] [PubMed]
- Beaudoin, M.; Kabanza, F.; Nault, V.; Valiquette, L. Evaluation of a Machine Learning Capability for a Clinical Decision Support System to Enhance Antimicrobial Stewardship Programs. Artif. Intell. Med. 2016, 68, 29–36. [Google Scholar] [CrossRef]
- Feretzakis, G.; Loupelis, E.; Sakagianni, A.; Kalles, D.; Martsoukou, M.; Lada, M.; Skarmoutsou, N.; Christopoulos, C.; Valakis, K.; Velentza, A.; et al. Using Machine Learning Techniques to Aid Empirical Antibiotic Therapy Decisions in the Intensive Care Unit of a General Hospital in Greece. Antibiotics 2020, 9, 50. [Google Scholar] [CrossRef]
- Feretzakis, G.; Sakagianni, A.; Loupelis, E.; Kalles, D.; Skarmoutsou, N.; Martsoukou, M.; Christopoulos, C.; Lada, M.; Petropoulou, S.; Velentza, A.; et al. Machine Learning for Antibiotic Resistance Prediction: A Prototype Using Off-the-Shelf Techniques and Entry-Level Data to Guide Empiric Antimicrobial Therapy. Healthc. Inform. Res. 2021, 27, 214–221. [Google Scholar] [CrossRef]
- Arango-Argoty, G.; Garner, E.; Pruden, A.; Heath, L.S.; Vikesland, P.; Zhang, L. DeepARG: A deep learning approach for predicting antibiotic resistance genes from metagenomic data. Microbiome 2018, 6, 23. [Google Scholar] [CrossRef]
- Sakagianni, A.; Koufopoulou, C.; Koufopoulos, P.; Kalantzi, S.; Theodorakis, N.; Nikolaou, M.; Paxinou, E.; Kalles, D.; Verykios, V.S.; Myrianthefs, P.; et al. Data-Driven Approaches in Antimicrobial Resistance: Machine Learning Solutions. Antibiotics 2024, 13, 1052. [Google Scholar] [CrossRef]
- Kim, J.I.; Maguire, F.; Tsang, K.K.; Gouliouris, T.; Peacock, S.J.; McAllister, T.A.; McArthur, A.G.; Beiko, R.G. Machine Learning for Antimicrobial Resistance Prediction: Current Practice, Limitations, and Clinical Perspective. Clin. Microbiol. Rev. 2022, 35, e00179-21. [Google Scholar] [CrossRef]
- Lewin-Epstein, O.; Baruch, S.; Hadany, L.; Stein, G.Y.; Obolski, U. Predicting Antibiotic Resistance in Hospitalized Patients by Applying Machine Learning to Electronic Medical Records. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2021, 72, e848–e855. [Google Scholar] [CrossRef]
- Thomas, T. Machine learning improves antibiotics use. Nature reviews. Urology 2021, 18, 3. [Google Scholar] [CrossRef]
- Tursunalieva, A.; Alexander, D.L.J.; Dunne, R.; Li, J.; Riera, L.; Zhao, Y. Making Sense of Machine Learning: A Review of Interpretation Techniques and Their Applications. Appl. Sci. 2024, 14, 496. [Google Scholar] [CrossRef]
- Ribeiro, M.T.; Singh, S.; Guestrin, C. “Why Should I Trust You?”: Explaining the Predictions of Any Classifier. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; Krishnapuram, B., Shah, M., Smola, A.J., Aggarwal, C.C., Shen, D., Rastogi, R., Eds.; ACM: New York, NY, USA, 2016; pp. 1135–1144. [Google Scholar] [CrossRef]
- Lundberg, S.M.; Lee, S.-I. A unified approach to interpreting model predictions. In Advances in Neural Information Processing Systems; Curran Associates, Inc.: Red Hook, NY, USA, 2017; Volume 30. [Google Scholar] [CrossRef]
- Price, W.N.; Cohen, I.G. Privacy in the age of medical big data. Nat. Med. 2019, 25, 37–43. [Google Scholar] [CrossRef] [PubMed]
- European Parliament and Council of the European Union. General Data Protection Regulation (GDPR). Off. J. Eur. Union 2016, 119, 1–88. Available online: http://data.europa.eu/eli/reg/2016/679/oj (accessed on 1 November 2024).
- World Health Organization (WHO). Ethics and Governance of Artificial Intelligence for Health: WHO Guidance; World Health Organization: Geneva, Switzerland, 2021; Available online: https://www.who.int/publications/i/item/9789240029200 (accessed on 1 November 2024).
- Topol, E.J. High-performance medicine: The convergence of human and artificial intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Rajpurkar, P.; Chen, E.; Banerjee, O.; Topol, E.J. AI in health and medicine. Nat. Med. 2022, 28, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Stokes, J.M.; Yang, K.; Swanson, K.; Jin, W.; Cubillos-Ruiz, A.; Donghia, N.M.; MacNair, C.R.; French, S.; Carfrae, L.A.; Bloom-Ackermann, Z.; et al. A Deep Learning Approach to Antibiotic Discovery. Cell 2020, 180, 688–702.e13. [Google Scholar] [CrossRef]
- Sinha, R.; Abnet, C.C.; White, O.; Knight, R.; Huttenhower, C. The microbiome quality control project: Baseline study design and future directions. Genome Biol. 2015, 16, 276. [Google Scholar] [CrossRef]
- Levy, R.; Magis, A.T.; Earls, J.C.; Manor, O.; Wilmanski, T.; Lovejoy, J.; Gibbons, S.M.; Omenn, G.S.; Hood, L.; Price, N.D. Longitudinal Analysis Reveals Transition Barriers Between Dominant Ecological States in the Gut Microbiome. Proc. Natl. Acad. Sci. USA 2020, 117, 13839–13845. [Google Scholar] [CrossRef]
- Lagier, J.C.; Dubourg, G.; Million, M.; Cadoret, F.; Bilen, M.; Fenollar, F.; Levasseur, A.; Rolain, J.M.; Fournier, P.E.; Raoult, D. Culturing the Human Microbiota and Culturomics. Nat. Rev. Microbiol. 2018, 16, 540–550. [Google Scholar] [CrossRef]
- Qu, A.; Brulc, J.M.; Wilson, M.K.; Law, B.F.; Theoret, J.R.; Joens, L.A.; Konkel, M.E.; Angly, F.; Dinsdale, E.A.; Edwards, R.A.; et al. Comparative Metagenomics Reveals Host-Specific Metavirulomes and Horizontal Gene Transfer Elements in the Chicken Cecum Microbiome. PLoS ONE 2008, 3, e2945. [Google Scholar] [CrossRef]
- Kamel, M.; Aleya, S.; Alsubih, M.; Aleya, L. Microbiome Dynamics: A Paradigm Shift in Combatting Infectious Diseases. J. Pers. Med. 2024, 14, 217. [Google Scholar] [CrossRef]
- Tudela, H.; Claus, S.P.; Saleh, M. Next Generation Microbiome Research: Identification of Keystone Species in the Metabolic Regulation of Host-Gut Microbiota Interplay. Front. Cell Dev. Biol. 2021, 9, 719072. [Google Scholar] [CrossRef]
- Ramirez, J.; Guarner, F.; Bustos Fernandez, L.; Maruy, A.; Sdepanian, V.L.; Cohen, H. Antibiotics as Major Disruptors of Gut Microbiota. Front. Cell. Infect. Microbiol. 2020, 10, 572912. [Google Scholar] [CrossRef]
- Gao, C.; Xu, L.; Montoya, L.; Madera, M.; Hollingsworth, J.; Chen, L.; Purdom, E.; Singan, V.; Vogel, J.; Hutmacher, R.B.; et al. Co-Occurrence Networks Reveal More Complexity than Community Composition in Resistance and Resilience of Microbial Communities. Nat. Commun. 2022, 13, 3867. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Luo, H.; Ji, B.; Nielsen, J. Machine Learning for Data Integration in Human Gut Microbiome. Microb. Cell Fact. 2022, 21, 241. [Google Scholar] [CrossRef] [PubMed]
- Sardar, P.; Almeida, A.; Pedicord, V.A. Integrating Functional Metagenomics to Decipher Microbiome–Immune Interactions. Immunol. Cell Biol. 2024, 102, 680–691. [Google Scholar] [CrossRef]
- Marcos-Zambrano, L.J.; Karaduzovic-Hadziabdic, K.; Loncar Turukalo, T.; Przymus, P.; Trajkovik, V.; Aasmets, O.; Berland, M.; Gruca, A.; Hasic, J.; Hron, K.; et al. Applications of Machine Learning in Human Microbiome Studies: A Review on Feature Selection, Biomarker Identification, Disease Prediction and Treatment. Front. Microbiol. 2021, 12, 634511. [Google Scholar] [CrossRef]
- Liu, W.; Cheng, M.; Li, J.; Zhang, P.; Fan, H.; Hu, Q.; Han, M.; Su, L.; He, H.; Tong, Y.; et al. Classification of the Gut Microbiota of Patients in Intensive Care Units During Development of Sepsis and Septic Shock. Genom. Proteom. Bioinform. 2020, 18, 696–707. [Google Scholar] [CrossRef]
- Maier, L.; Pruteanu, M.; Kuhn, M.; Zeller, G.; Telzerow, A.; Anderson, E.E.; Brochado, A.R.; Fernandez, K.C.; Dose, H.; Mori, H.; et al. Extensive Impact of Non-Antibiotic Drugs on Human Gut Bacteria. Nature 2018, 555, 623–628. [Google Scholar] [CrossRef]
- Lyu, R.; Qu, Y.; Divaris, K.; Wu, D. Methodological Considerations in Longitudinal Analyses of Microbiome Data: A Comprehensive Review. Genes 2024, 15, 51. [Google Scholar] [CrossRef]
- Martínez Arbas, S.; Busi, S.B.; Queirós, P.; de Nies, L.; Herold, M.; May, P.; Wilmes, P.; Muller, E.E.L.; Narayanasamy, S. Challenges, Strategies, and Perspectives for Reference-Independent Longitudinal Multi-Omic Microbiome Studies. Front. Genet. 2021, 12, 666244. [Google Scholar] [CrossRef]
- Chang, Q.; Yan, Z.; Zhou, M.; Qu, H.; He, X.; Zhang, H.; Baskaran, L.; Al’Aref, S.; Li, H.; Zhang, S.; et al. Mining Multi-Center Heterogeneous Medical Data with Distributed Synthetic Learning. Nat. Commun. 2023, 14, 5510. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- D’Urso, F.; Broccolo, F. Applications of Artificial Intelligence in Microbiome Analysis and Probiotic Interventions—An Overview and Perspective Based on the Current State of the Art. Appl. Sci. 2024, 14, 8627. [Google Scholar] [CrossRef]
- Ranallo, R.T.; McDonald, L.C.; Halpin, A.L.; Hiltke, T.; Young, V.B. The State of Microbiome Science at the Intersection of Infectious Diseases and Antimicrobial Resistance. J. Infect. Dis. 2021, 223 (Suppl. 2), S187–S193. [Google Scholar] [CrossRef] [PubMed]
- Lathakumari, R.H.; Vajravelu, L.K.; Satheesan, A.; Ravi, S.; Thulukanam, J. Antibiotics and the Gut Microbiome: Understanding the Impact on Human Health. Med. Microecol. 2024, 20, 100106. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, Y.; Huang, W.; Zhou, H.; Zhang, W. Drug–Microbiota Interactions: An Emerging Priority for Precision Medicine. Signal Transduct. Target. Ther. 2023, 8, 386. [Google Scholar] [CrossRef]
- Tu, V.; Ren, Y.; Tanes, C.; Mukhopadhyay, S.; Daniel, S.G.; Li, H.; Bittinger, K. A Quantitative Approach to Measure and Predict Microbiome Response to Antibiotics. mSphere 2024, 9, e00488-24. [Google Scholar] [CrossRef]
- Baron, S.A.; Diene, S.M.; Rolain, J.-M. Human Microbiomes and Antibiotic Resistance. Hum. Microbiome J. 2018, 10, 43–52. [Google Scholar] [CrossRef]
- Sakagianni, A.; Feretzakis, G.; Kalles, D.; Loupelis, E.; Rakopoulou, Z.; Fildisis, G. Discovering Association Rules in Antimicrobial Resistance in Intensive Care Unit. Stud. Health Technol. Inform. 2022, 295, 430–433. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Numan, A.; Cinti, S. Point-of-Care for Evaluating Antimicrobial Resistance through the Adoption of Functional Materials. Anal. Chem. 2022, 94, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Kasztura, M.; Richard, A.; Bempong, N.E.; Loncar, D.; Flahault, A. Cost-effectiveness of Precision Medicine: A Scoping Review. Int. J. Public Health 2019, 64, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. Government of the United Kin gdom and Wellcome Trust: London, UK, 2016. Available online: https://wellcomecollection.org/works/thvwsuba (accessed on 8 April 2021).
- Moulac, M.; Theuretzbacher, U. Antimicrobial Resistance—New Incentives to Improve the Accessibility and Availability of Antimicrobial Medicinal Products—HWG Workshop Proceedings, Publication for the Committee on Environment, Public Health and Food Safety, Policy Department for Economic, Scientific and Quality of Life Policies, European Parliament, Luxembourg, 2023. Available online: http://www.europarl.europa.eu/supporting-analyses (accessed on 1 November 2024).
- Walsh, T.R.; Gales, A.C.; Laxminarayan, R.; Dodd, P.C. Antimicrobial Resistance: Addressing a Global Threat to Humanity. PLoS Med. 2023, 20, e1004264. [Google Scholar] [CrossRef]
- Rogers Van Katwyk, S.; Hoffman, S.J.; Mendelson, M.; Taljaard, M.; Grimshaw, J.M. Strengthening the Science of Addressing Antimicrobial Resistance: A Framework for Planning, Conducting and Disseminating Antimicrobial Resistance Intervention Research. Health Res. Policy Syst. 2020, 18, 60. [Google Scholar] [CrossRef]

| Category | Description | Examples/Mechanisms | Proposed Interventions |
|---|---|---|---|
| ICU-Specific Factors | Environmental and clinical elements unique to ICU settings that drive resistance. | High antibiotic usage, invasive devices, immunosuppression. | Antibiotic stewardship, device management, infection control. |
| Microbiome Interactions | Processes within the microbiome that influence resistance gene dissemination. | Horizontal gene transfer, biofilm formation, quorum sensing. | Probiotics, biofilm disruptors, quorum sensing inhibitors. |
| Antibiotic Resistance Mechanisms | Genetic and physiological strategies employed by bacteria to evade antibiotics. | Efflux pumps, enzymatic degradation, target modifications. | Target-specific inhibitors, combination therapies. |
| ML Applications | Computational approaches to predict, analyze, and mitigate antibiotic resistance. | Predicting resistance, identifying therapeutic targets, optimizing treatments. | Integrating genomic and clinical data, personalized medicine. |
| Mechanism | Description | Key Features | Relevance in ICU | Mitigation Strategies |
|---|---|---|---|---|
| Horizontal Gene Transfer (HGT) | Transfer of genetic material between bacteria without reproduction. Includes transformation, transduction, and conjugation. |
| Dense microbial populations and biofilms promote HGT. Antibiotic pressure in ICU increases selective survival of resistant bacteria. | Antibiotic stewardship, infection control, agents targeting gene transfer mechanisms. |
| Biofilm Formation | Structured bacterial communities embedded in extracellular polymeric substance (EPS) matrix. |
| Biofilms form on medical devices (catheters, ventilators), leading to device-associated infections and resistance. | Anti-fouling materials, biofilm-disrupting agents, quorum sensing inhibitors, enzymatic degradation of biofilm components. |
| Quorum Sensing (QS) | Bacterial communication system regulating gene expression based on population density via autoinducers. |
| High bacterial densities in ICU intensify quorum sensing activities, influencing resistance and virulence traits. | Quorum sensing inhibitors (QSIs), enzymatic degradation of signaling molecules, structural analogues of autoinducers, antagonists of quorum sensing receptors. |
| Factor | Description | Mitigation Strategies |
|---|---|---|
| High Antibiotic Usage | Broad-spectrum antibiotics frequently used empirically; creates selective pressure favoring resistant organisms. |
|
| Patient Susceptibility | Critically ill patients have weakened immune systems, often requiring invasive devices, leading to higher infection risk. |
|
| Environmental Factors | Contaminated surfaces, inadequate cleaning, shared equipment, biofilm formation on devices, and poor air quality contribute to the spread of resistant microbes. |
|
| Antibiotic Pressure | Promotes genetic changes (mutations, HGT) in bacteria and encourages MDRO proliferation. |
|
| Healthcare Worker Role | Cross-transmission due to inadequate hand hygiene and improper use of personal protective equipment. |
|
| Biofilm Formation | Persistent bacterial communities on medical devices shield bacteria from antibiotics and host defenses. |
|
| Environmental Reservoir of Resistance | Antibiotics excreted by patients affect microbial communities on surfaces, enhancing resistance spread. |
|
| Delayed Pathogen Identification | Empirical antibiotic therapy without confirmation risks inappropriate treatment. |
|
| Application Area | Description | Techniques and Models Used | Challenges |
|---|---|---|---|
| Predicting Antibiotic Resistance | Analyze genomic and microbiome data to predict resistance patterns, aiding therapy selection. | Random forests, neural networks (CNNs, RNNs), stack ensembles, AutoML. | Data quality, model generalizability, interpretability, ethical concerns (data privacy, regulations). |
| Analyzing Microbiome Data | Process high-dimensional microbiome data to identify key species, interactions, and resistance mechanisms. | Clustering, network analysis, deep learning (taxonomic classification, resistance gene identification), functional metagenomics. | Standardization, computational demands, variability in sample collection, ensuring data privacy and ethical compliance. |
| Identifying Novel Therapeutic Targets | Discover critical nodes in microbial networks or resistance pathways for drug development. | Predictive modeling for essential genes/proteins, synergistic drug prediction, antimicrobial peptides (AMPs), phage therapy target identification, biofilm disruption pathways. | Complexity of biological systems, validation of predictions, need for extensive datasets, ethical concerns about environmental and health impacts. |
| Challenge | Details | Proposed Solutions |
|---|---|---|
| Data Availability and Quality |
|
|
|
| |
|
| |
| Interpretability of Models |
|
|
|
| |
| Ethical Considerations |
|
|
|
| |
|
| |
| Computational and Technical Barriers |
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Hellenic Society for Microbiology. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakagianni, A.; Koufopoulou, C.; Koufopoulos, P.; Feretzakis, G.; Anastasiou, A.; Theodorakis, N.; Myrianthefs, P. Influence of Microbiome Interactions on Antibiotic Resistance Development in the ICU Environment: Insights and Opportunities with Machine Learning. Acta Microbiol. Hell. 2025, 70, 14. https://doi.org/10.3390/amh70020014
Sakagianni A, Koufopoulou C, Koufopoulos P, Feretzakis G, Anastasiou A, Theodorakis N, Myrianthefs P. Influence of Microbiome Interactions on Antibiotic Resistance Development in the ICU Environment: Insights and Opportunities with Machine Learning. Acta Microbiologica Hellenica. 2025; 70(2):14. https://doi.org/10.3390/amh70020014
Chicago/Turabian StyleSakagianni, Aikaterini, Christina Koufopoulou, Petros Koufopoulos, Georgios Feretzakis, Athanasios Anastasiou, Nikolaos Theodorakis, and Pavlos Myrianthefs. 2025. "Influence of Microbiome Interactions on Antibiotic Resistance Development in the ICU Environment: Insights and Opportunities with Machine Learning" Acta Microbiologica Hellenica 70, no. 2: 14. https://doi.org/10.3390/amh70020014
APA StyleSakagianni, A., Koufopoulou, C., Koufopoulos, P., Feretzakis, G., Anastasiou, A., Theodorakis, N., & Myrianthefs, P. (2025). Influence of Microbiome Interactions on Antibiotic Resistance Development in the ICU Environment: Insights and Opportunities with Machine Learning. Acta Microbiologica Hellenica, 70(2), 14. https://doi.org/10.3390/amh70020014








