Safety and Feasibility of Lin- Cells Administration to ALS Patients: A Novel View on Humoral Factors and miRNA Profiles
Abstract
:1. Introduction
2. Results
2.1. Analysis of Included Individuals
2.2. Growth Factor Levels in CSF
2.3. Comparison of Systemic Levels of Neurotrophic Growth Factors in Blood Plasma from ALS Patients
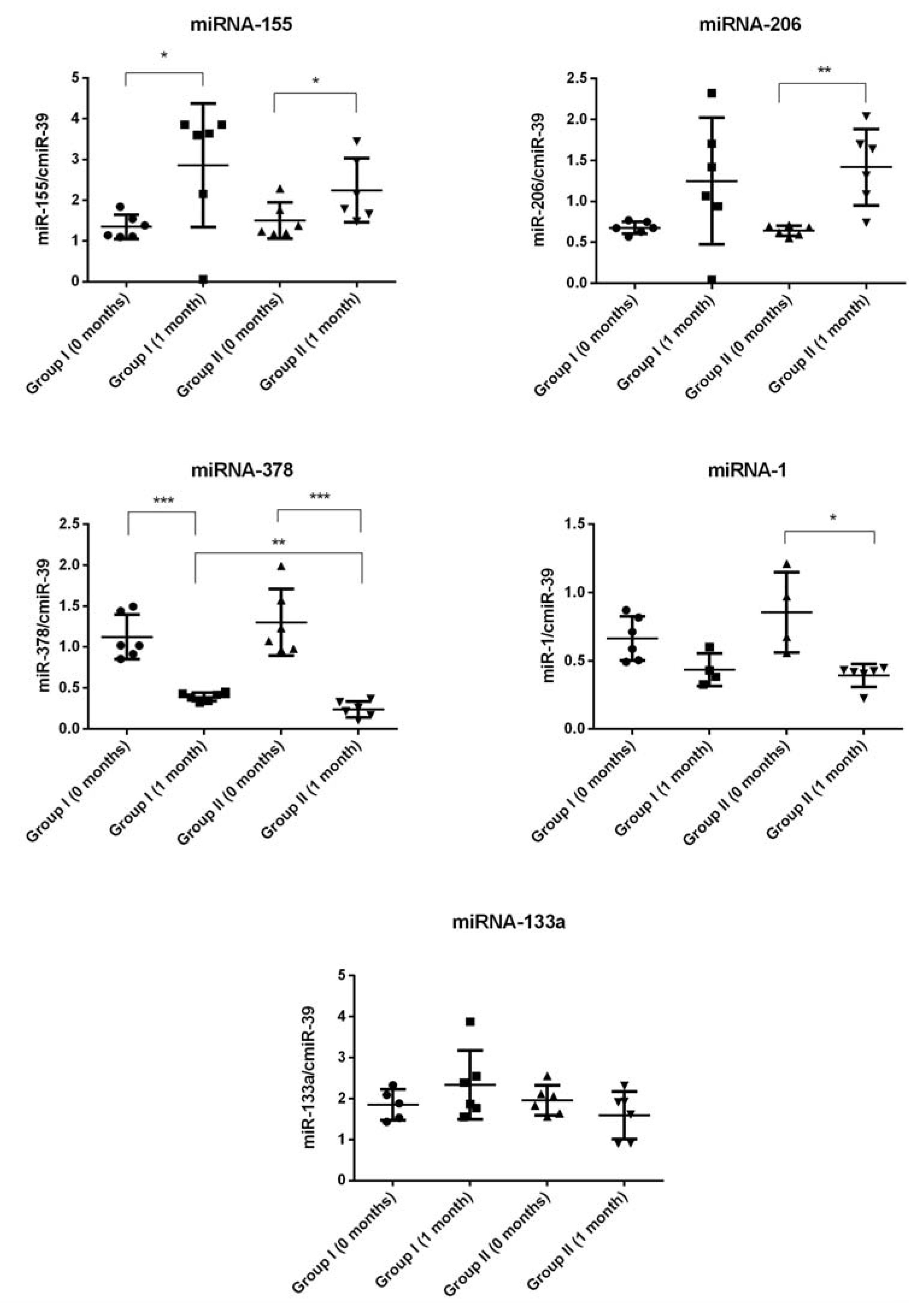
2.4. miRNA Expression
3. Discussion
Potential Study Limitations
4. Materials and Methods
4.1. Patients
4.2. Assessment
4.3. Cells
4.4. Administration Procedure
4.5. Safety Endpoints
4.6. Molecular Analysis
4.6.1. Multiplex Assay
4.6.2. qRT-PCR
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| ALS | amyotrophic lateral sclerosis |
| ALSFRS | the amyotrophic lateral sclerosis functional rating scale |
| ANGP2 | angiopoietin 2 |
| BBB | blood-brain barrier |
| BDNF | brain-derived neurotrophic factor |
| BM | bone marrow |
| CNS | central nervous system |
| CRP | C-reactive protein |
| CSF | cerebrospinal fluid |
| GDNF | glial cell line-derived neurotrophic factor |
| HSC | hematopoietic stem cell |
| HSPC | hematopoietic stem/progenitor cell |
| LIN- | lineage-negative cells |
| NGF | nerve growth factor |
| NT | neurotrophin |
| NTF | neurotrophic factor |
| PBS | phosphate buffered saline |
| PSC | pluripotent stem cell |
| qRT-PCR | quantitative reverse transcription polymerase chain reaction |
| SC | stem cell |
| SPC | stem/progenitor cell |
| Tx | transplantation |
| VEGF | vascular endothelial growth factor |
References
- Kiernan, M.C.; Vucic, S.; Cheah, B.C.; Turner, M.R.; Eisen, A.; Hardiman, O.; Burrell, J.R.; Zoing, M.C. Amyotrophic lateral sclerosis. Lancet 2011, 377, 942–955. [Google Scholar] [CrossRef]
- Turner, M.R.; Hardiman, O.; Benatar, M.; Brooks, B.R.; Chio, A.; de Carvalho, M.; Ince, P.G.; Lin, C.; Miller, R.G.; Mitsumoto, H.; et al. Controversies and priorities in amyotrophic lateral sclerosis. Lancet Neurol. 2013, 12, 310–322. [Google Scholar] [CrossRef]
- Pronto-Laborinho, A.C.; Pinto, S.; de Carvalho, M. Roles of vascular endothelial growth factor in amyotrophic lateral sclerosis. BioMed Res. Int. 2014, 2014, 947513. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.; Gomes, C.; de Carvalho, M. Diagnosis, pathogenesis and therapeutic targets in amyotrophic lateral sclerosis. CNS Neurol. Disord. Drug Targets 2010, 9, 764–778. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J. Amyotrophic lateral sclerosis: Clinical management and research update. Curr. Neurol. Neurosci. Rep. 2009, 9, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, F. Role of neuroinflammation in amyotrophic lateral sclerosis: Cellular mechanisms and therapeutic implications. Front. Immunol. 2017, 8, 1005. [Google Scholar] [CrossRef] [PubMed]
- Fellner, A.; Barhum, Y.; Angel, A.; Perets, N.; Steiner, I.; Offen, D.; Lev, N. Toll-like receptor-4 inhibitor tak-242 attenuates motor dysfunction and spinal cord pathology in an amyotrophic lateral sclerosis mouse model. Int. J. Mol. Sci. 2017, 18, 1666. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.H.; Allen, K.; Oei, F.; Leoni, E.; Kuhle, J.; Tree, T.; Fratta, P.; Sharma, N.; Sidle, K.; Howard, R.; et al. Systemic inflammatory response and neuromuscular involvement in amyotrophic lateral sclerosis. Neurol. Neuroimmunol. Neuroinflammation 2016, 3, e244. [Google Scholar] [CrossRef] [PubMed]
- Ganesalingam, J.; An, J.; Shaw, C.E.; Shaw, G.; Lacomis, D.; Bowser, R. Combination of neurofilament heavy chain and complement C3 as CSF biomarkers for ALS. J. Neurochem. 2011, 117, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.G.; Mitchell, J.D.; Lyon, M.; Moore, D.H. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Cochrane Database Syst. Rev. 2007, Cd001447. [Google Scholar] [CrossRef]
- Rothstein, J.D. Edaravone: A new drug approved for als. Cell 2017, 171, 725. [Google Scholar] [CrossRef] [PubMed]
- Sadan, O.; Shemesh, N.; Barzilay, R.; Bahat-Stromza, M.; Melamed, E.; Cohen, Y.; Offen, D. Migration of neurotrophic factors-secreting mesenchymal stem cells toward a quinolinic acid lesion as viewed by magnetic resonance imaging. Stem Cells 2008, 26, 2542–2551. [Google Scholar] [CrossRef] [PubMed]
- Machalinska, A.; Kawa, M.; Pius-Sadowska, E.; Stepniewski, J.; Nowak, W.; Roginska, D.; Kaczynska, K.; Baumert, B.; Wiszniewska, B.; Jozkowicz, A.; et al. Long-term neuroprotective effects of NT-4-engineered mesenchymal stem cells injected intravitreally in a mouse model of acute retinal injury. Investig. Ophthalmol. Vis. Sci. 2013, 54, 8292–8305. [Google Scholar] [CrossRef] [PubMed]
- Paczkowska, E.; Piecyk, K.; Luczkowska, K.; Kotowski, M.; Roginska, D.; Pius-Sadowska, E.; Oronowicz, K.; Ostrowski, M.; Machalinski, B. Expression of neurotrophins and their receptors in human CD34+ bone marrow cells. J. Physiol. Pharmacol. 2016, 67, 151–159. [Google Scholar] [PubMed]
- Paczkowska, E.; Kaczynska, K.; Pius-Sadowska, E.; Roginska, D.; Kawa, M.; Ustianowski, P.; Safranow, K.; Celewicz, Z.; Machalinski, B. Humoral activity of cord blood-derived stem/progenitor cells: Implications for stem cell-based adjuvant therapy of neurodegenerative disorders. PLoS ONE 2013, 8, e83833. [Google Scholar] [CrossRef] [PubMed]
- Hata, T.; Sakata, N.; Yoshimatsu, G.; Tsuchiya, H.; Fukase, M.; Ishida, M.; Aoki, T.; Katayose, Y.; Egawa, S.; Unno, M. Nerve growth factor improves survival and function of transplanted islets via trka-mediated beta cell proliferation and revascularization. Transplantation 2015, 99, 1132–1143. [Google Scholar] [CrossRef] [PubMed]
- Ciesler, J.; Sari, Y. Neurotrophic peptides: Potential drugs for treatment of amyotrophic lateral sclerosis and alzheimer’s disease. Open J. Neurosci. 2013, 3, 2. [Google Scholar] [PubMed]
- Gould, T.W.; Oppenheim, R.W. Motor neuron trophic factors: Therapeutic use in ALS? Brain Res. Rev. 2011, 67, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, H.T.; Yun, S.; Kim, I.S.; Lee, J.; Lee, I.S.; Park, K.I. Growth factor-expressing human neural progenitor cell grafts protect motor neurons but do not ameliorate motor performance and survival in ALS mice. Exp. Mol. Med. 2009, 41, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Xia, R.; Jiang, Y.; Wang, L.; Gao, F. Vascular endothelial growth factors enhance the permeability of the mouse blood-brain barrier. PLoS ONE 2014, 9, e86407. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xu, W.; Luo, C.; Gozal, D.; Liu, R. Vegf-induced activation of the PI3-K/Akt pathway reduces mutant SOD1-mediated motor neuron cell death. Brain Res. Mol. Brain Res. 2003, 111, 155–164. [Google Scholar] [CrossRef]
- Lunn, J.S.; Sakowski, S.A.; Kim, B.; Rosenberg, A.A.; Feldman, E.L. Vascular endothelial growth factor prevents G93A-SOD1-induced motor neuron degeneration. Dev. Neurobiol. 2009, 69, 871–884. [Google Scholar] [CrossRef] [PubMed]
- Bogaert, E.; Van Damme, P.; Poesen, K.; Dhondt, J.; Hersmus, N.; Kiraly, D.; Scheveneels, W.; Robberecht, W.; Van Den Bosch, L. VEGF protects motor neurons against excitotoxicity by upregulation of GLUR2. Neurobiol. Aging 2010, 31, 2185–2191. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Skold, M.K.; Li, J.; Nennesmo, I.; Fadeel, B.; Henter, J.I. VEGF reduces astrogliosis and preserves neuromuscular junctions in als transgenic mice. Biochem. Biophys. Res. Commun. 2007, 363, 989–993. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.H.; Lee, H.J.; Park, I.H.; Seok, J.I.; Kim, B.G.; Joo, I.S.; Kim, S.U. Intrathecal transplantation of human neural stem cells overexpressing VEGF provide behavioral improvement, disease onset delay and survival extension in transgenic als mice. Gene Ther. 2009, 16, 1234–1244. [Google Scholar] [CrossRef] [PubMed]
- Dai, R.; Ahmed, S.A. MicroRNA, a new paradigm for understanding immunoregulation, inflammation, and autoimmune diseases. Transl. Res. 2011, 157, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Engels, B.M.; Hutvagner, G. Principles and effects of microRNA-mediated post-transcriptional gene regulation. Oncogene 2006, 25, 6163–6169. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, S.; Weber, J.; Baxter, D.; Galas, D.J. Export of microRNAs and microRNA-protective protein by mammalian cells. Nucleic Acids Res. 2010, 38, 7248–7259. [Google Scholar] [CrossRef] [PubMed]
- Ponomarev, E.D.; Veremeyko, T.; Barteneva, N.; Krichevsky, A.M.; Weiner, H.L. MicroRNA-124 promotes microglia quiescence and suppresses eae by deactivating macrophages via the C/EBP-α-PU.1 pathway. Nat. Med. 2011, 17, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Diniz, G.P.; Wang, D.Z. Regulation of skeletal muscle by microRNAs. Compr. Physiol. 2016, 6, 1279–1294. [Google Scholar] [PubMed]
- Kroesen, B.J.; Teteloshvili, N.; Smigielska-Czepiel, K.; Brouwer, E.; Boots, A.M.; van den Berg, A.; Kluiver, J. Immuno-mirs: Critical regulators of T-cell development, function and ageing. Immunology 2015, 144, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Butovsky, O.; Jedrychowski, M.P.; Cialic, R.; Krasemann, S.; Murugaiyan, G.; Fanek, Z.; Greco, D.J.; Wu, P.M.; Doykan, C.E.; Kiner, O.; et al. Targeting miR-155 restores abnormal microglia and attenuates disease in SOD1 mice. Ann. Neurol. 2015, 77, 75–99. [Google Scholar] [CrossRef] [PubMed]
- Krist, B.; Florczyk, U.; Pietraszek-Gremplewicz, K.; Jozkowicz, A.; Dulak, J. The role of miR-378a in metabolism, angiogenesis, and muscle biology. Int. J. Endocrinol. 2015, 2015, 281756. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.J.; Watson, J.J.; Shoemark, D.K.; Barua, N.U.; Patel, N.K. GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol. Ther. 2013, 138, 155–175. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Kim, H.S.; Hwang, D.Y. Stem cells as promising therapeutic options for neurological disorders. J. Cell. Biochem. 2013, 114, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Czarzasta, J.; Habich, A.; Siwek, T.; Czaplinski, A.; Maksymowicz, W.; Wojtkiewicz, J. Stem cells for als: An overview of possible therapeutic approaches. Int. J. Dev. Neurosci. 2017, 57, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Y.; Liang, Z.-H.; Han, C.; Wei, W.-J.; Song, C.-L.; Zhou, L.-N.; Liu, Y.; Li, Y.; Ji, X.-F.; Liu, J. Transplantation of autologous peripheral blood mononuclear cells in the subarachnoid space for amyotrophic lateral sclerosis: A safety analysis of 14 patients. Neural Regen. Res. 2017, 12, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Corti, S.; Strazzer, S.; Del Bo, R.; Salani, S.; Bossolasco, P.; Fortunato, F.; Locatelli, F.; Soligo, D.; Moggio, M.; Ciscato, P.; et al. A subpopulation of murine bone marrow cells fully differentiates along the myogenic pathway and participates in muscle repair in the MDX dystrophic mouse. Exp. Cell Res. 2002, 277, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Machalinski, B.; Paczkowska, E.; Koziarska, D.; Ratajczak, M.Z. Mobilization of human hematopoietic stem/progenitor-enriched CD34+ cells into peripheral blood during stress related to ischemic stroke. Folia Histochem. Cytobiol. 2006, 44, 97–101. [Google Scholar] [PubMed]
- Mitchell, R.M.; Freeman, W.M.; Randazzo, W.T.; Stephens, H.E.; Beard, J.L.; Simmons, Z.; Connor, J.R. A CSF biomarker panel for identification of patients with amyotrophic lateral sclerosis. Neurology 2009, 72, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Kuhle, J.; Lindberg, R.L.; Regeniter, A.; Mehling, M.; Steck, A.J.; Kappos, L.; Czaplinski, A. Increased levels of inflammatory chemokines in amyotrophic lateral sclerosis. Eur. J. Neurol. 2009, 16, 771–774. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, P.; Volpi, N. High levels of C3C in the cerebrospinal fluid from amyotrophic lateral sclerosis patients. Acta Neurol. Scand. 1985, 72, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Goldknopf, I.L.; Sheta, E.A.; Bryson, J.; Folsom, B.; Wilson, C.; Duty, J.; Yen, A.A.; Appel, S.H. Complement C3C and related protein biomarkers in amyotrophic lateral sclerosis and parkinson’s disease. Biochem. Biophys. Res. Commun. 2006, 342, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Sta, M.; Sylva-Steenland, R.M.; Casula, M.; de Jong, J.M.; Troost, D.; Aronica, E.; Baas, F. Innate and adaptive immunity in amyotrophic lateral sclerosis: Evidence of complement activation. Neurobiol. Dis. 2011, 42, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Chiu, I.M.; Phatnani, H.; Kuligowski, M.; Tapia, J.C.; Carrasco, M.A.; Zhang, M.; Maniatis, T.; Carroll, M.C. Activation of innate and humoral immunity in the peripheral nervous system of als transgenic mice. Proc. Natl. Acad. Sci. USA 2009, 106, 20960–20965. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.; Ferluga, J.; Tsolaki, A.G.; Kishore, U. The non-classical functions of the classical complement pathway recognition subcomponent C1Q. Immunol. Lett. 2010, 131, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Veerhuis, R.; Nielsen, H.M.; Tenner, A.J. Complement in the brain. Mol. Immunol. 2011, 48, 1592–1603. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.J.; Anderson, A.J.; Barnum, S.R.; Stevens, B.; Tenner, A.J. The complement cascade: Yin-yang in neuroinflammation-neuro-protection and -degeneration. J. Neurochem. 2008, 107, 1169–1187. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.D.; Kamaruzaman, N.A.; Fung, J.N.; Taylor, S.M.; Turner, B.J.; Atkin, J.D.; Woodruff, T.M.; Noakes, P.G. Dysregulation of the complement cascade in the HSOD1G93A transgenic mouse model of amyotrophic lateral sclerosis. J. Neuroinflamm. 2013, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.D.; Kumar, V.; Fung, J.N.; Ruitenberg, M.J.; Noakes, P.G.; Woodruff, T.M. Pharmacological inhibition of complement C5A-C5A1 receptor signalling ameliorates disease pathology in the HSOD1(G93A) mouse model of amyotrophic lateral sclerosis. Br. J. Pharmacol. 2017, 174, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Roginska, D.; Kawa, M.P.; Pius-Sadowska, E.; Lejkowska, R.; Luczkowska, K.; Wiszniewska, B.; Kaarniranta, K.; Paterno, J.J.; Schmidt, C.A.; Machalinski, B.; et al. Depletion of the third complement component ameliorates age-dependent oxidative stress and positively modulates autophagic activity in aged retinas in a mouse model. Oxid. Med. Cell. Longev. 2017, 2017, 5306790. [Google Scholar] [CrossRef] [PubMed]
- Petrou, P.; Gothelf, Y.; Argov, Z.; Gotkine, M.; Levy, Y.S.; Kassis, I.; Vaknin-Dembinsky, A.; Ben-Hur, T.; Offen, D.; Abramsky, O.; et al. Safety and clinical effects of mesenchymal stem cells secreting neurotrophic factor transplantation in patients with amyotrophic lateral sclerosis: Results of phase 1/2 and 2a clinical trials. JAMA Neurol. 2016, 73, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Beck, M.; Flachenecker, P.; Magnus, T.; Giess, R.; Reiners, K.; Toyka, K.V.; Naumann, M. Autonomic dysfunction in als: A preliminary study on the effects of intrathecal bdnf. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2005, 6, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Bartus, R.T.; Johnson, E.M., Jr. Clinical tests of neurotrophic factors for human neurodegenerative diseases, part 1: Where have we been and what have we learned? Neurobiol. Dis. 2016, 97, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.M.; Behrstock, S.; McHugh, J.; Hoffmann, K.; Wallace, K.; Suzuki, M.; Aebischer, P.; Svendsen, C.N. GDNF delivery using human neural progenitor cells in a rat model of als. Hum. Gene Ther. 2005, 16, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Cedarbaum, J.M.; Stambler, N. Disease status and use of ventilatory support by als patients. BDNF study group. Amyotroph. Lateral Scler. 2001, 2, 19–22. [Google Scholar]
- Paczkowska, E.; Luczkowska, K.; Piecyk, K.; Roginska, D.; Pius-Sadowska, E.; Ustianowski, P.; Cecerska, E.; Dolegowska, B.; Celewicz, Z.; Machalinski, B. The influence of bdnf on human umbilical cord blood stem/progenitor cells: Implications for stem cell-based therapy of neurodegenerative disorders. Acta Neurobiol. Exp. 2015, 75, 172–191. [Google Scholar]
- Stuerenburg, H.J.; Kunze, K. Tissue nerve growth factor concentrations in neuromuscular diseases. Eur. J. Neurol. 1998, 5, 487–490. [Google Scholar] [CrossRef] [PubMed]
- Ferraiuolo, L.; Higginbottom, A.; Heath, P.R.; Barber, S.; Greenald, D.; Kirby, J.; Shaw, P.J. Dysregulation of astrocyte-motoneuron cross-talk in mutant superoxide dismutase 1-related amyotrophic lateral sclerosis. Brain 2011, 134, 2627–2641. [Google Scholar] [CrossRef] [PubMed]
- Ngo, S.T.; Steyn, F.J.; Huang, L.; Mantovani, S.; Pfluger, C.M.; Woodruff, T.M.; O’Sullivan, J.D.; Henderson, R.D.; McCombe, P.A. Altered expression of metabolic proteins and adipokines in patients with amyotrophic lateral sclerosis. J. Neurol. Sci. 2015, 357, 22–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakay, M.; Wang, Z.; Melcon, G.; Schiltz, L.; Xuan, J.; Zhao, P.; Sartorelli, V.; Seo, J.; Pegoraro, E.; Angelini, C.; et al. Nuclear envelope dystrophies show a transcriptional fingerprint suggesting disruption of RB-MYOD pathways in muscle regeneration. Brain 2006, 129, 996–1013. [Google Scholar] [CrossRef] [PubMed]
- Litwińska, Z.; Machaliński, B. MiRNAs in chronic myeloid leukemia: Small molecules, essential function. Leuk. Lymphoma 2017, 58, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Kawa, M.; Machalińska, A. The role of microRNA in the pathogenesis of age-related macular degeneration: Its pathophysiology and potential pharmacological aspects. J. Biochem. Pharmacol. Res. 2014, 2, 21–32. [Google Scholar]
- Pegoraro, V.; Merico, A.; Angelini, C. Micro-RNAs in als muscle: Differences in gender, age at onset and disease duration. J. Neurol. Sci. 2017, 380, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.H.; Valdez, G.; Moresi, V.; Qi, X.; McAnally, J.; Elliott, J.L.; Bassel-Duby, R.; Sanes, J.R.; Olson, E.N. MicroRNA-206 delays als progression and promotes regeneration of neuromuscular synapses in mice. Science 2009, 326, 1549–1554. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A.L.; Guedes, J.R.; Pereira de Almeida, L.; Pedroso de Lima, M.C. MiR-155 modulates microglia-mediated immune response by down-regulating SOCS-1 and promoting cytokine and nitric oxide production. Immunology 2012, 135, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Parisi, C.; Arisi, I.; D’Ambrosi, N.; Storti, A.E.; Brandi, R.; D’Onofrio, M.; Volonte, C. Dysregulated microRNAs in amyotrophic lateral sclerosis microglia modulate genes linked to neuroinflammation. Cell Death Dis. 2013, 4, e959. [Google Scholar] [CrossRef] [PubMed]
- Cardona, A.E.; Pioro, E.P.; Sasse, M.E.; Kostenko, V.; Cardona, S.M.; Dijkstra, I.M.; Huang, D.; Kidd, G.; Dombrowski, S.; Dutta, R.; et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat. Neurosci. 2006, 9, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kong, D.; Chen, H.; Liu, S.; Hu, H.; Wu, T.; Wang, J.; Chen, W.; Ning, Y.; Li, Y.; et al. MiR-155 acts as an anti-inflammatory factor in atherosclerosis-associated foam cell formation by repressing calcium-regulated heat stable protein 1. Sci. Rep. 2016, 6, 21789. [Google Scholar] [CrossRef] [PubMed]
- Gerin, I.; Bommer, G.T.; McCoin, C.S.; Sousa, K.M.; Krishnan, V.; MacDougald, O.A. Roles for miRNA-378/378* in adipocyte gene expression and lipogenesis. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E198–E206. [Google Scholar] [CrossRef] [PubMed]
- Kulyte, A.; Lorente-Cebrian, S.; Gao, H.; Mejhert, N.; Agustsson, T.; Arner, P.; Ryden, M.; Dahlman, I. MicroRNA profiling links miR-378 to enhanced adipocyte lipolysis in human cancer cachexia. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E267–E274. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Gu, Y.; Zhang, Q.; Han, Y.; Hou, J.; Lin, L.; Wu, C.; Bao, Y.; Su, X.; Jiang, M.; et al. Identification of resting and type I IFN-activated human NK cell mirnomes reveals microRNA-378 and microRNA-30E as negative regulators of NK cell cytotoxicity. J. Immunol. 2012, 189, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Xue, M.; Fu, Z.; Ji, C.; Guo, X.; Zhu, L.; Xu, L.; Pang, L.; Xu, M.; Qu, H. Insight into the effects of adipose tissue inflammation factors on miR-378 expression and the underlying mechanism. Cell. Physiol. Biochem. 2014, 33, 1778–1788. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.R.; Miller, R.G.; Swash, M.; Munsat, T.L. El escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2000, 1, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Mareschi, K.; Ferrero, I.; Rustichelli, D.; Aschero, S.; Gammaitoni, L.; Aglietta, M.; Madon, E.; Fagioli, F. Expansion of mesenchymal stem cells isolated from pediatric and adult donor bone marrow. J. Cell. Biochem. 2006, 97, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Cymbaluk-Ploska, A.; Chudecka-Glaz, A.; Pius-Sadowska, E.; Sompolska-Rzechula, A.; Chudecka, K.; Bulsa, M.; Machalinski, B.; Menkiszak, J. Clinical relevance of NGAL/MMP-9 pathway in patients with endometrial cancer. Dis. Markers 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Jazwa, A.; Kasper, L.; Bak, M.; Sobczak, M.; Szade, K.; Jozkowicz, A.; Sladek, K.; Dulak, J. Differential inflammatory microRNA and cytokine expression in pulmonary sarcoidosis. Arch. Immunol. Ther. Exp. 2015, 63, 139–146. [Google Scholar] [CrossRef] [PubMed]



| Characteristic | Group I (n = 6) | Group II (n = 6) | p-Value | |
|---|---|---|---|---|
| Age (mean ± SD, years) | 48.7 ± 15.5 | 50.7 ± 10 | p = 0.8133 | |
| Age at disease onset (mean ± SD, years) | 45.5 ± 17 | 47.3 ± 10.4 | p = 0.8141 | |
| Sex (male/female) | 4/2 | 4/2 | p = 1 | |
| Symptom duration (mean ± SD, months) | 39.3 ± 27.39 | 37.3 ± 32.14 | p = 0.9178 | |
| Number of Lin- cells administered (mean ± SD) | 11.95 ± 5.76 × 106 | 4.53 ± 3.19 × 106 | p = 0.0365 | |
| ALSFRS score (mean ± SD) | Before Lin- Tx | 26.3 ± 2.8 | 15.5 ± 3.15 | p = 0.0002 |
| 3 months after Lin- Tx | 25.5 ± 3.6 | 12.6 ± 1.7 | p = 0.0002 | |
| 6 months after Lin- Tx | 23 ± 6.4 | 10.5 ± 1.5 | p = 0.0491 | |
| Norris scale score (mean ± SD) | Before Lin− Tx | 84.3 ± 4.4 | 58 ± 6.4 | p < 0.0001 |
| 3 months after Lin- Tx | 86 ± 5.9 | 50.3 ± 3.7 | p < 0.0001 | |
| 6 months after Lin- Tx | 81.6 ± 12.2 | 38 ± 6.6 | p = 0.0007 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobuś, A.; Baumert, B.; Litwińska, Z.; Gołąb-Janowska, M.; Stępniewski, J.; Kotowski, M.; Pius-Sadowska, E.; Kawa, M.P.; Gródecka-Szwajkiewicz, D.; Peregud-Pogorzelski, J.; et al. Safety and Feasibility of Lin- Cells Administration to ALS Patients: A Novel View on Humoral Factors and miRNA Profiles. Int. J. Mol. Sci. 2018, 19, 1312. https://doi.org/10.3390/ijms19051312
Sobuś A, Baumert B, Litwińska Z, Gołąb-Janowska M, Stępniewski J, Kotowski M, Pius-Sadowska E, Kawa MP, Gródecka-Szwajkiewicz D, Peregud-Pogorzelski J, et al. Safety and Feasibility of Lin- Cells Administration to ALS Patients: A Novel View on Humoral Factors and miRNA Profiles. International Journal of Molecular Sciences. 2018; 19(5):1312. https://doi.org/10.3390/ijms19051312
Chicago/Turabian StyleSobuś, Anna, Bartłomiej Baumert, Zofia Litwińska, Monika Gołąb-Janowska, Jacek Stępniewski, Maciej Kotowski, Ewa Pius-Sadowska, Miłosz P. Kawa, Dorota Gródecka-Szwajkiewicz, Jarosław Peregud-Pogorzelski, and et al. 2018. "Safety and Feasibility of Lin- Cells Administration to ALS Patients: A Novel View on Humoral Factors and miRNA Profiles" International Journal of Molecular Sciences 19, no. 5: 1312. https://doi.org/10.3390/ijms19051312
APA StyleSobuś, A., Baumert, B., Litwińska, Z., Gołąb-Janowska, M., Stępniewski, J., Kotowski, M., Pius-Sadowska, E., Kawa, M. P., Gródecka-Szwajkiewicz, D., Peregud-Pogorzelski, J., Dulak, J., Nowacki, P., & Machaliński, B. (2018). Safety and Feasibility of Lin- Cells Administration to ALS Patients: A Novel View on Humoral Factors and miRNA Profiles. International Journal of Molecular Sciences, 19(5), 1312. https://doi.org/10.3390/ijms19051312





