A Template Method Leads to Precisely Synthesize SiO2@Fe3O4 Nanoparticles at the Hundred-Nanometer Scale
Abstract
1. Introduction
2. Experimental Methods
2.1. Surface Modification of Silica
2.1.1. The Effect of Modifier Types on the Coating of Ferroferric Oxide
2.1.2. The Effect of Modifier Concentration on Nanoparticles
2.2. The Effect of Experimental Conditions
2.2.1. The Effect of Reaction Temperature
2.2.2. The Effect of Reaction Time
2.2.3. The Effect of Mechanical Stirring Speed
2.2.4. The Impact of Dispersion Methods
2.3. The Impact of Reactants
2.3.1. Feeding Process
2.3.2. The Effect of Stabilizer
2.3.3. Catalyst and Stabilizer Sequence
2.3.4. Iron Source Ratio
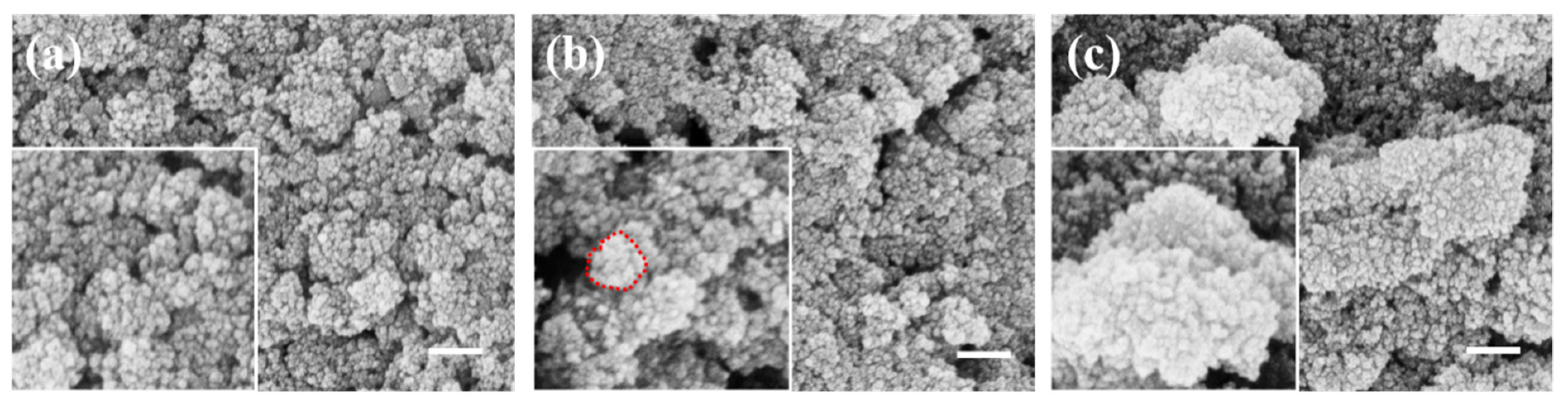
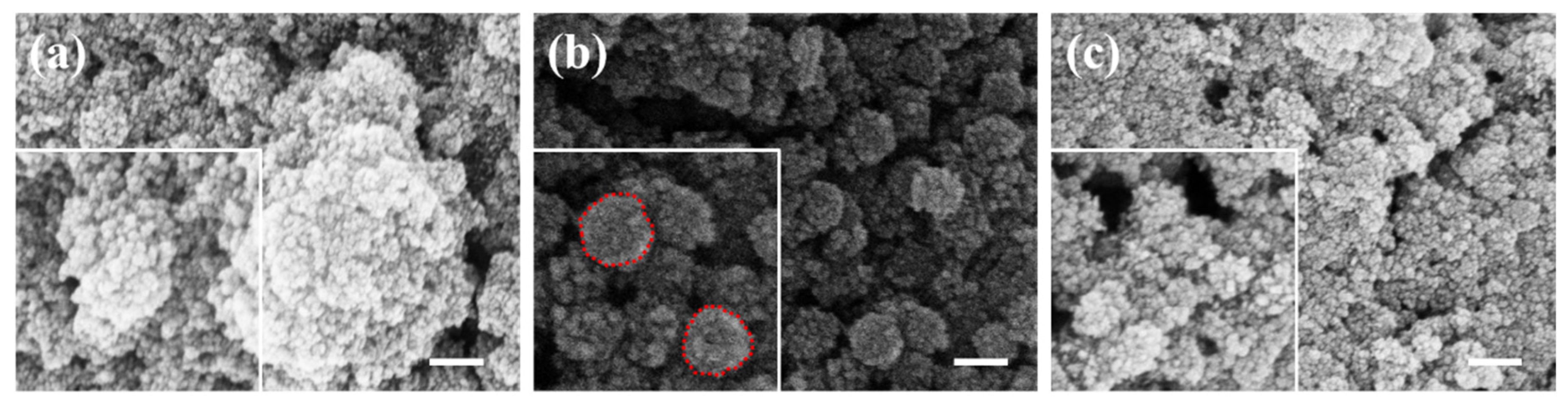
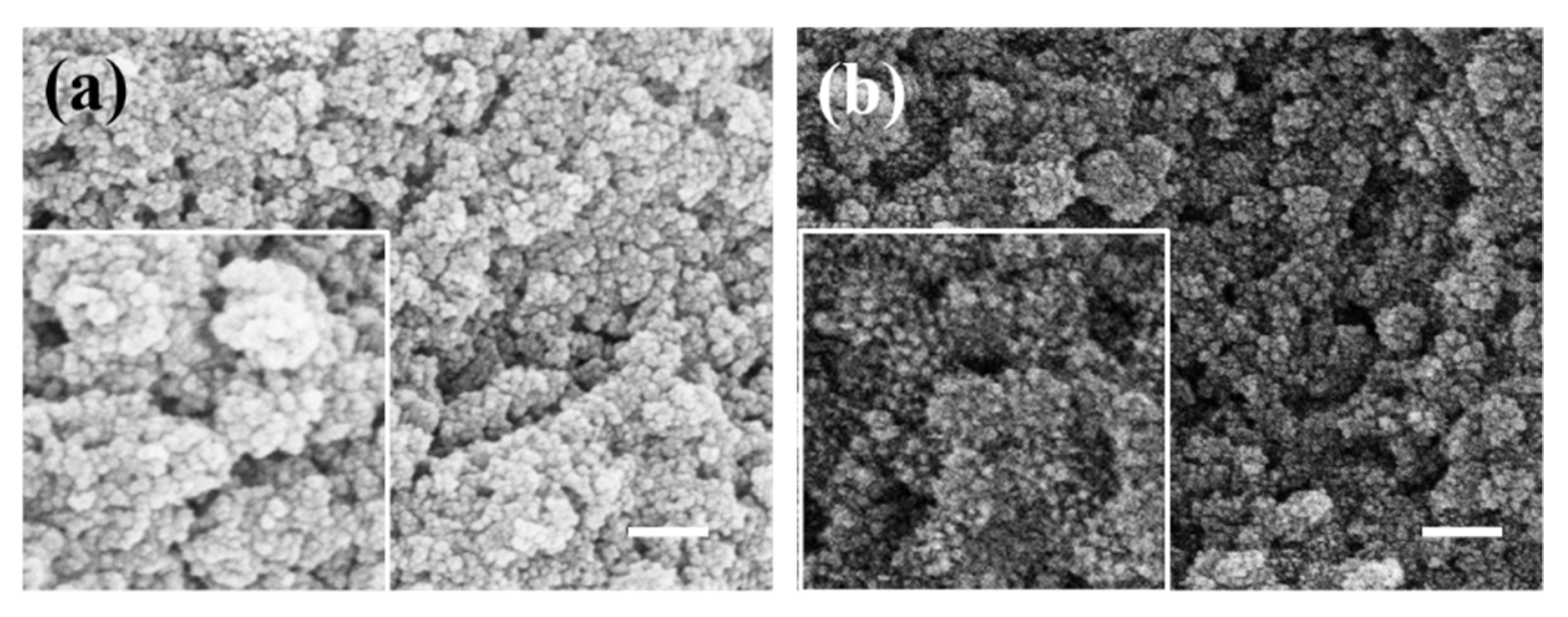
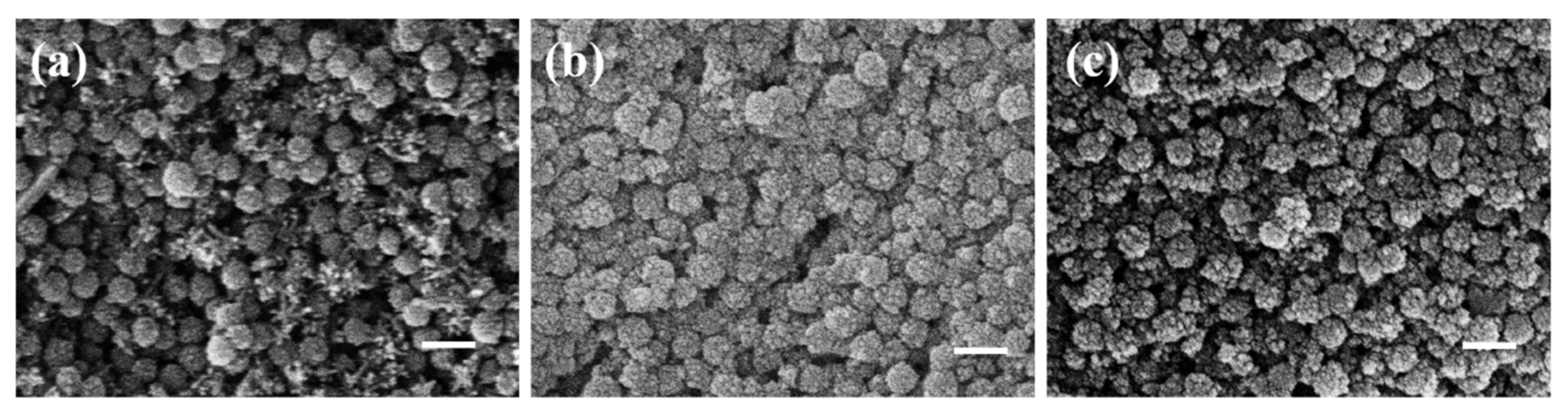
3. Results and Discussion
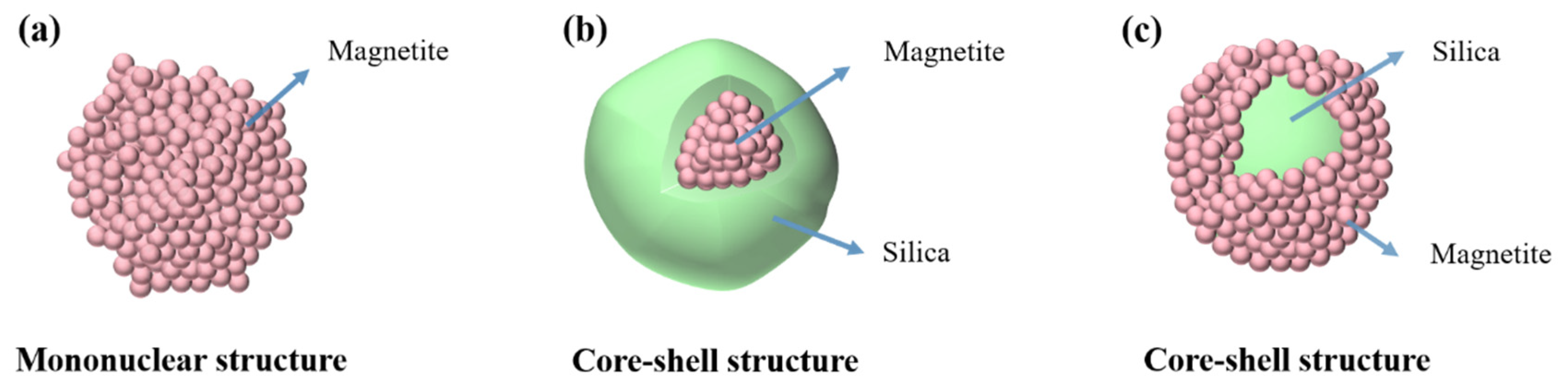
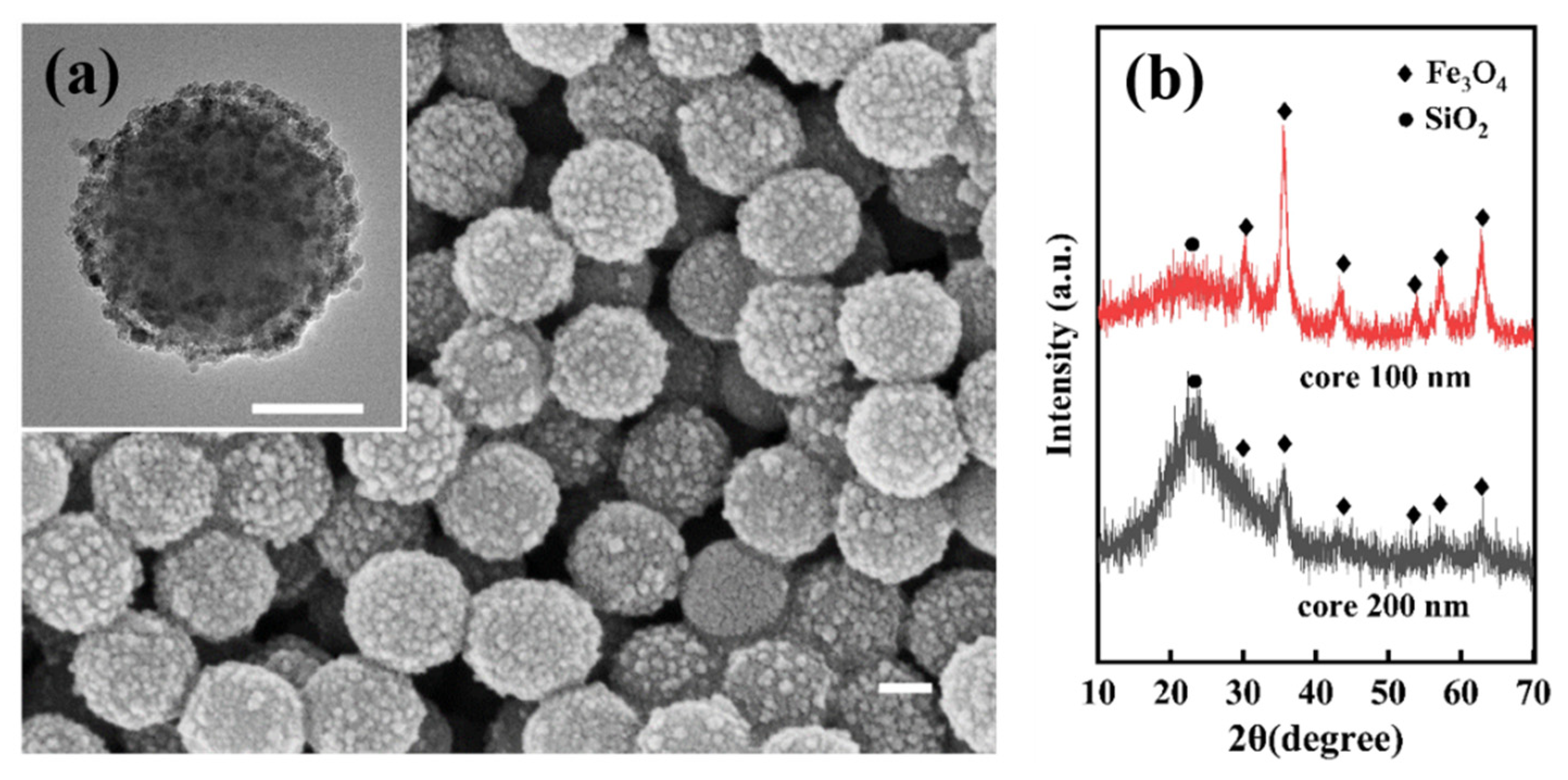
3.1. SiO2@Fe3O4 Characterization of Core-Shell Structured Nanoparticles
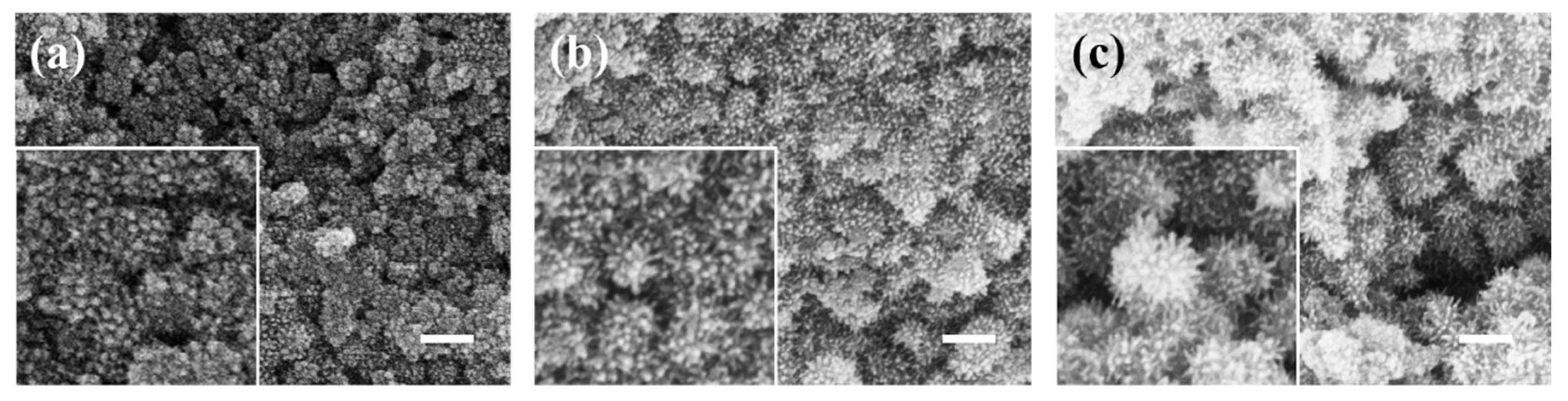
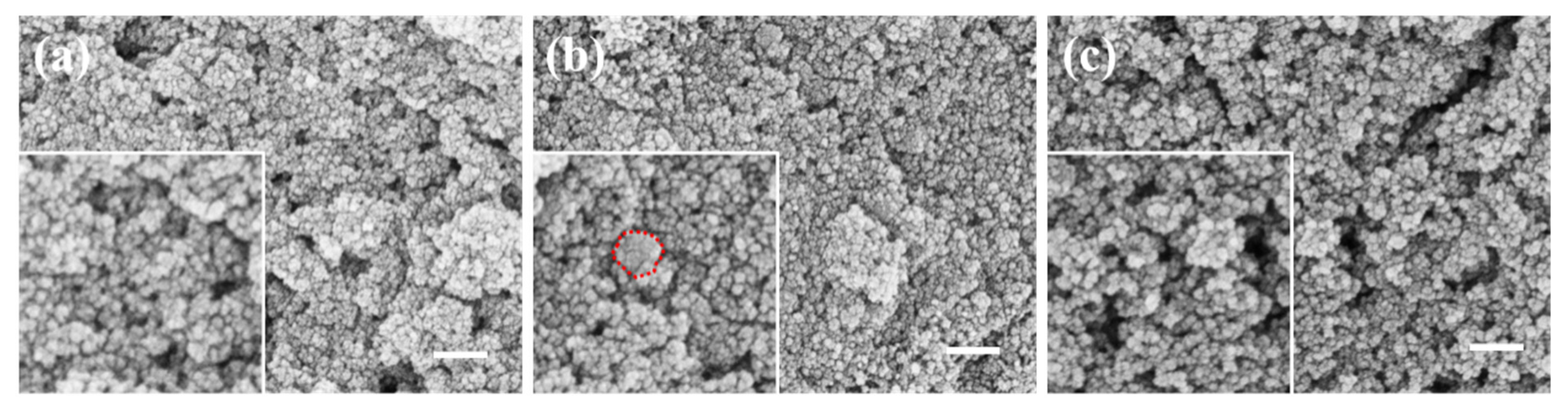
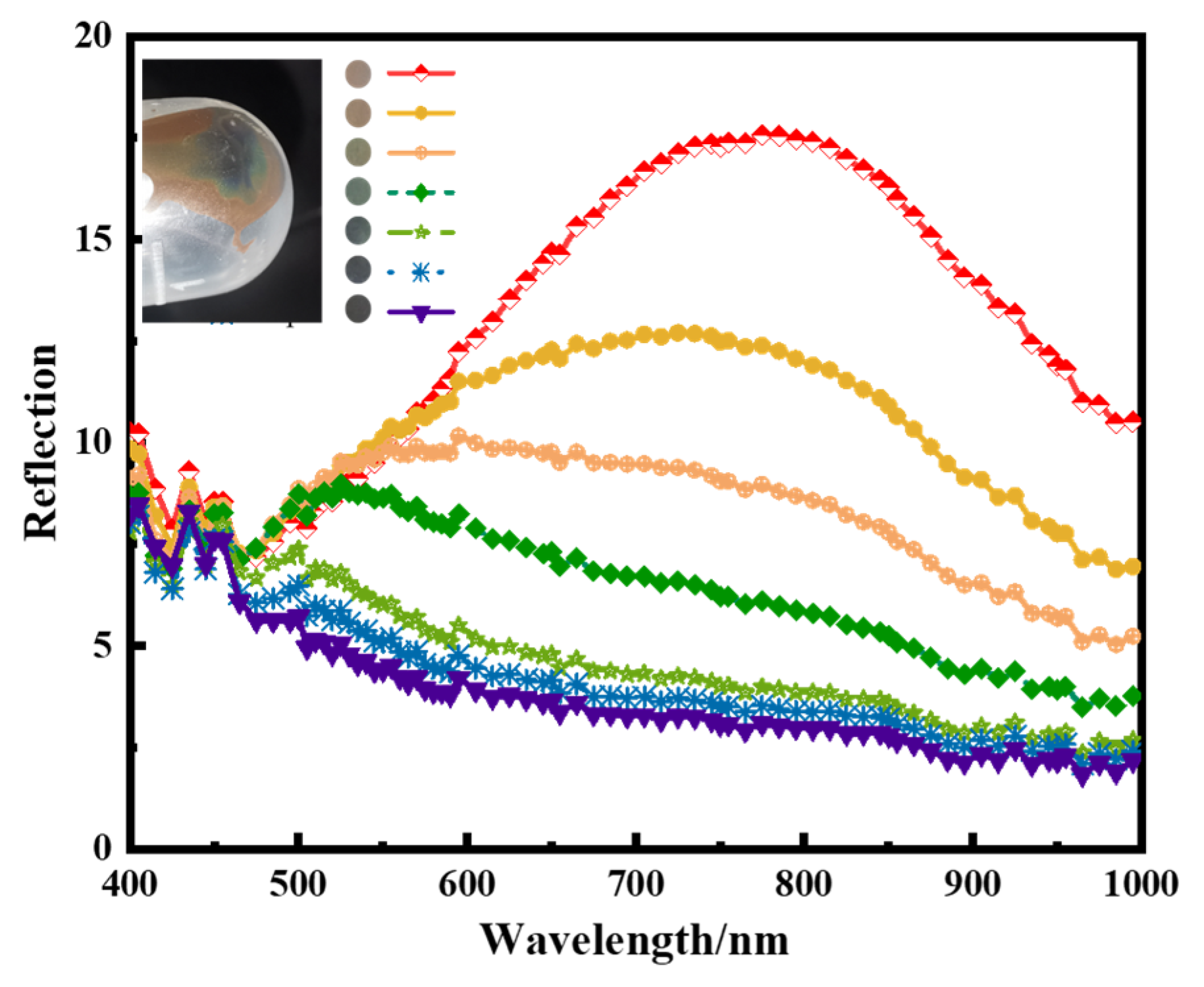
3.2. Preparation of Visible Light Photonic Crystals
4. Conclusions
5. Optimized Preparation Steps
5.1. Optimal Preparation Conditions
5.2. Surface Modification Steps of Silica
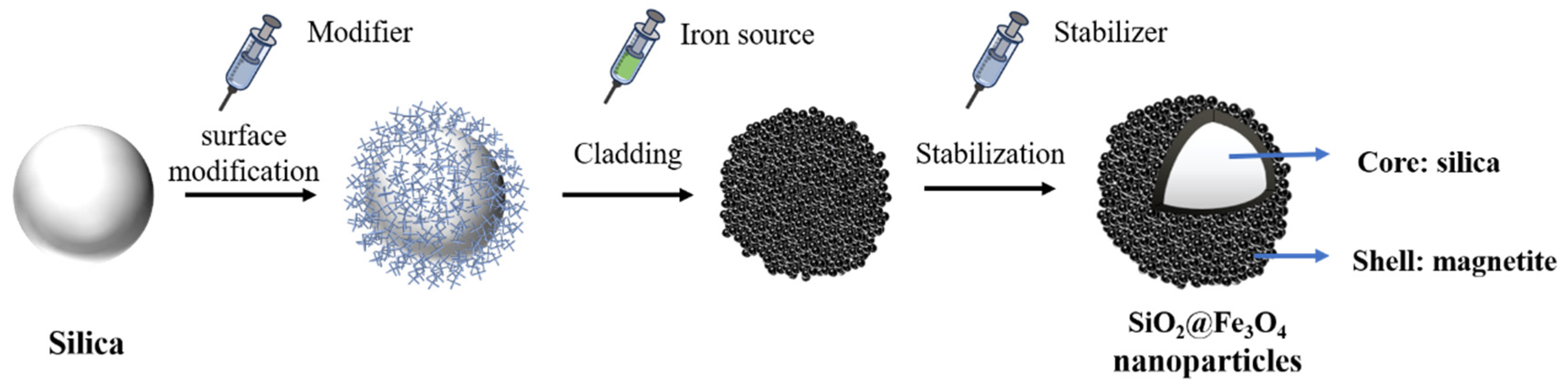
5.3. Preparation of SiO2@Fe3O4
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liang, Y.; Liang, Z.; Liu, Z.; Jun, P.; Qiu, D. Study on the transmission characteristics and band structure of 2D and 3D plasma photonic crystals. Opt. Express 2023, 31, 2776–2791. [Google Scholar] [CrossRef]
- Lv, X.; Zhong, B.; Huang, Y.; Xing, Z.; Wang, H.; Guo, W.; Zhang, Z. Research progress in preparation and application of photonic crystals. Chin. J. Mech. Eng. 2023, 36, 39. [Google Scholar] [CrossRef]
- Inoue, T.; Morita, R.; Nigo, K.; Yoshida, M.; De Zoysa, M.; Ishizaki, K.; Noda, S. Self-evolving photonic crystals for ultrafast photonics. Nat. Commun. 2023, 14, 50. [Google Scholar] [CrossRef]
- Liu, J.; Nero, M.; Jansson, K.; Willhammar, T.; Sipponen, M.H. Photonic crystals with rainbow colors by centrifugation-assisted assembly of colloidal lignin nanoparticles. Nat. Commun. 2023, 14, 3099. [Google Scholar] [CrossRef]
- Jia, R.; Xiang, S.; Wang, Y.; Chen, H.; Xiao, M. Electrically Triggered Color-Changing Materials: Mechanisms, Performances, and Applications. Adv. Opt. Mater. 2024, 12, 2302222. [Google Scholar] [CrossRef]
- Madanu, T.L.; Mouchet, S.R.; Deparis, O.; Liu, J.; Li, Y.; Su, B.L. Tuning and transferring slow photons from TiO2 photonic crystals to BiVO4 nanoparticles for unprecedented visible light photocatalysis. J. Colloid Interface Sci. 2023, 634, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Sorathiya, V.; Lavadiya, S.; Faragallah, O.S.; Eid, M.M.; Rashed, A.N.Z. D shaped dual core photonics crystal based refractive index sensor using graphene–titanium–silver materials for infrared frequency spectrum. Opt. Quantum Electron. 2022, 54, 290. [Google Scholar] [CrossRef]
- Li, H.; Wu, P.; Zhao, G.; Guo, J.; Wang, C. Fabrication of industrial-level polymer photonic crystal films at ambient temperature Based on uniform core/shell colloidal particles. J. Colloid Interface Sci. 2021, 584, 145–153. [Google Scholar] [CrossRef]
- Nikzamir, M.; Akbarzadeh, A.; Panahi, Y. An overview on nanoparticles used in biomedicine and their cytotoxicity. J. Drug Deliv. Sci. Technol. 2021, 61, 102316. [Google Scholar] [CrossRef]
- Ma, X.; Li, Y.Y.; Hussain, I.; Shen, R.; Yang, G.; Zhang, K. Core–shell structured nanoenergetic materials: Preparation and fundamental properties. Adv. Mater. 2020, 32, 2001291. [Google Scholar] [CrossRef]
- Chen, S.; Cheng, Y.; Zhao, Z.; Zhang, K.; Hao, T.; Sui, Y.; Wang, W.; Zhao, J.; Li, Y. Core–Shell-Structured Electrorheological Fluid with a Polarizability-Tunable Nanocarbon Shell for Enhanced Stimuli-Responsive Activity. ACS Appl. Mater. Interfaces 2023, 15, 35741–35749. [Google Scholar] [CrossRef]
- Wang, Z.; Valenzuela, C.; Xue, P.; Zhang, X.; Zhang, X.; Chen, Y.; Yang, Y.; Wang, L.; Xu, X. Magnetic structural color hydrogels for patterned photonic crystals and dynamic camouflage. ACS Appl. Polym. Mater. 2022, 4, 3618–3626. [Google Scholar] [CrossRef]
- Wang, D.; Li, J.; Sun, X.; Hu, J.; Tan, X.; Jia, Q.; Liu, J.; Zhang, X.; Wu, G.; Wang, X. New electric field responsive photonic crystals with remarkable yellow-to-green switch for adaptive camouflage. J. Colloid Interface Sci. 2024, 654, 581–591. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, W.; Zheng, H.; Li, C.; Zhang, B.; Wang, B.; Hao, C. The electrorheological response behavior of small coral-like H2Ti2O5@SiO2 core-shell nanoparticles. J. Taiwan Inst. Chem. Eng. 2021, 129, 327–341. [Google Scholar] [CrossRef]
- Hao, L.W.; Liu, J.D.; Li, Q.; Qing, R.K.; He, Y.Y.; Guo, J.; Li, G.; Zhu, L.; Xu, C.; Chen, S. Microfluidic-directed magnetic controlling supraballs with multi-responsive anisotropic photonic crystal structures. J. Mater. Sci. Technol. 2021, 81, 203–211. [Google Scholar] [CrossRef]
- Park, J.G.; Rogers, W.B.; Magkiriadou, S.; Kodger, T.; Kim, S.H.; Kim, Y.S.; Manoharan, V.N. Photonic-crystal hydrogels with a rapidly tunable stop band and high reflectivity across the visible. Opt. Mater. Express 2017, 7, 253–263. [Google Scholar] [CrossRef]
- Cai, Z.; Li, Z.; Ravaine, S.; He, M.; Song, Y.; Yin, Y.; Zheng, H.; Teng, J.; Zhang, A. From colloidal particles to photonic crystals: Advances in self-assembly and their emerging applications. Chem. Soc. Rev. 2021, 50, 5898–5951. [Google Scholar] [CrossRef] [PubMed]
- Jesus, A.C.B.; Jesus, J.D.; Lima, R.J.S.; Moura, K.O.; Almeida, J.M.A.; Duque, J.G.S.; Meneses, C.T. Synthesis and magnetic interaction on concentrated Fe3O4 nanoparticles obtained by the co-precipitation and hydrothermal chemical methods. Ceram. Int. 2020, 46, 11149–11153. [Google Scholar] [CrossRef]
- Imran, M.; Riaz, S.; Shah, S.M.H.; Batool, T.; Khan, H.N.; Sabri, A.N.; Naseem, S. In-vitro hemolytic activity and free radical scavenging by sol-gel synthesized Fe3O4 stabilized ZrO2 nanoparticles. Arab. J. Chem. 2020, 13, 7598–7608. [Google Scholar] [CrossRef]
- Ba-Abbad, M.M.; Benamour, A.; Ewis, D.; Mohammad, A.W.; Mahmoudi, E. Synthesis of Fe3O4 nanoparticles with different shapes through a co-precipitation method and their application. JOM 2022, 74, 3531–3539. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, Q.; Hu, T.; Chen, X.; Cao, Y. Rapid preparation of size-tunable Fe3O4@SiO2 nanoparticles to construct magnetically responsive photonic crystals. J. Nanopart. Res. 2021, 23, 232. [Google Scholar] [CrossRef]
- Wei, Y.; Han, B.; Hu, X.; Lin, Y.; Wang, X.; Deng, X. Synthesis of Fe3O4 nanoparticles and their magnetic properties. Procedia Eng. 2012, 27, 632–637. [Google Scholar] [CrossRef]
- Kulkarni, S.A.; Sawadh, P.S.; Palei, P.K.; Kokate, K.K. Effect of synthesis route on the structural, optical and magnetic properties of Fe3O4 nanoparticles. Ceram. Int. 2014, 40, 1945–1949. [Google Scholar] [CrossRef]
- Park, S.K.; Do Kim, K.; Kim, H.T. Preparation of silica nanoparticles: Determination of the optimal synthesis conditions for small and uniform particles. Colloids Surf. A 2002, 197, 7–17. [Google Scholar] [CrossRef]
- Lalegani, Z.; Ebrahimi, S.A.S.; Hamawandi, B.; La Spada, L.; Batili, H.; Toprak, M.S. Targeted dielectric coating of silver nanoparticles with silica to manipulate optical properties for metasurface applications. Mater. Chem. Phys. 2022, 287, 126250. [Google Scholar] [CrossRef]
- Lincoln, R.L.; Scarpa, F.; Ting, V.P.; Trask, R.S. Multifunctional composites: A metamaterial perspective. Multifunct. Mater. 2019, 2, 043001. [Google Scholar] [CrossRef]
- Ghimire, P.P.; Jaroniec, M. Renaissance of Stöber method for synthesis of colloidal particles: New developments and opportunities. J. Colloid Interface Sci. 2021, 584, 838–865. [Google Scholar] [CrossRef]
- Karthikeyan, B.; Vettumperumal, R. Structural and optical characterization of Mg2SiO4 and Mg2SiO4-Pr6O(11) nanocomposite for optical devices. Opt. Mater. Jan. 2022, 123, 111878. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Wang, X.; Yang, J.; Zhang, Y. A Template Method Leads to Precisely Synthesize SiO2@Fe3O4 Nanoparticles at the Hundred-Nanometer Scale. Materials 2024, 17, 4325. https://doi.org/10.3390/ma17174325
Zhang J, Wang X, Yang J, Zhang Y. A Template Method Leads to Precisely Synthesize SiO2@Fe3O4 Nanoparticles at the Hundred-Nanometer Scale. Materials. 2024; 17(17):4325. https://doi.org/10.3390/ma17174325
Chicago/Turabian StyleZhang, Jinying, Xinye Wang, Jiaxing Yang, and Yexiaotong Zhang. 2024. "A Template Method Leads to Precisely Synthesize SiO2@Fe3O4 Nanoparticles at the Hundred-Nanometer Scale" Materials 17, no. 17: 4325. https://doi.org/10.3390/ma17174325
APA StyleZhang, J., Wang, X., Yang, J., & Zhang, Y. (2024). A Template Method Leads to Precisely Synthesize SiO2@Fe3O4 Nanoparticles at the Hundred-Nanometer Scale. Materials, 17(17), 4325. https://doi.org/10.3390/ma17174325






