Mpox and Lessons Learned in the Light of the Recent Outbreak: A Narrative Review
Abstract
1. Introduction
2. Taxonomy and Virology
3. Origin and Epidemiology
3.1. West and Central Africa
3.2. 2022 Global Outbreak
3.3. 2023–2024 Outbreak
4. Risk Factors
Mpox in People Living with HIV
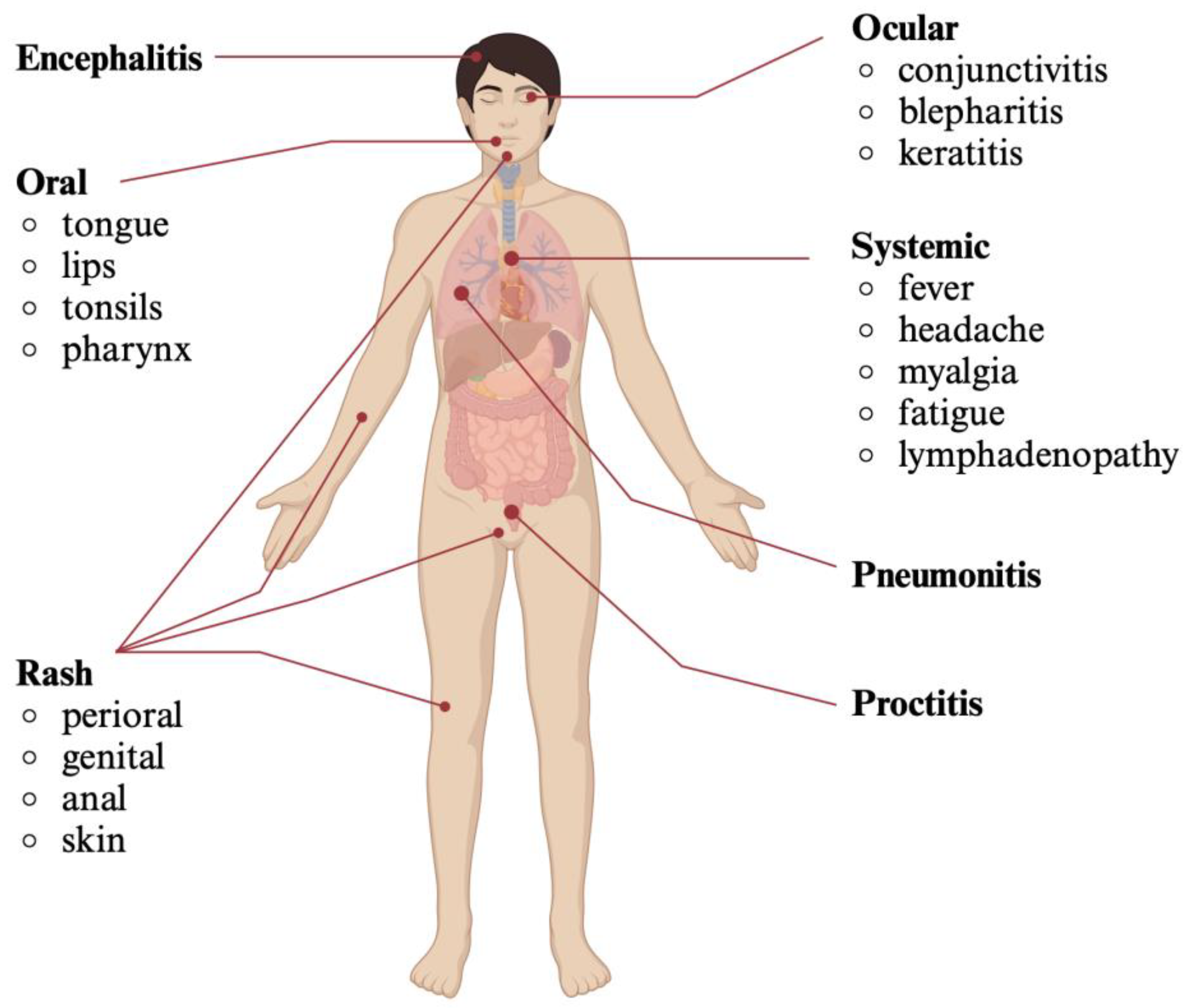
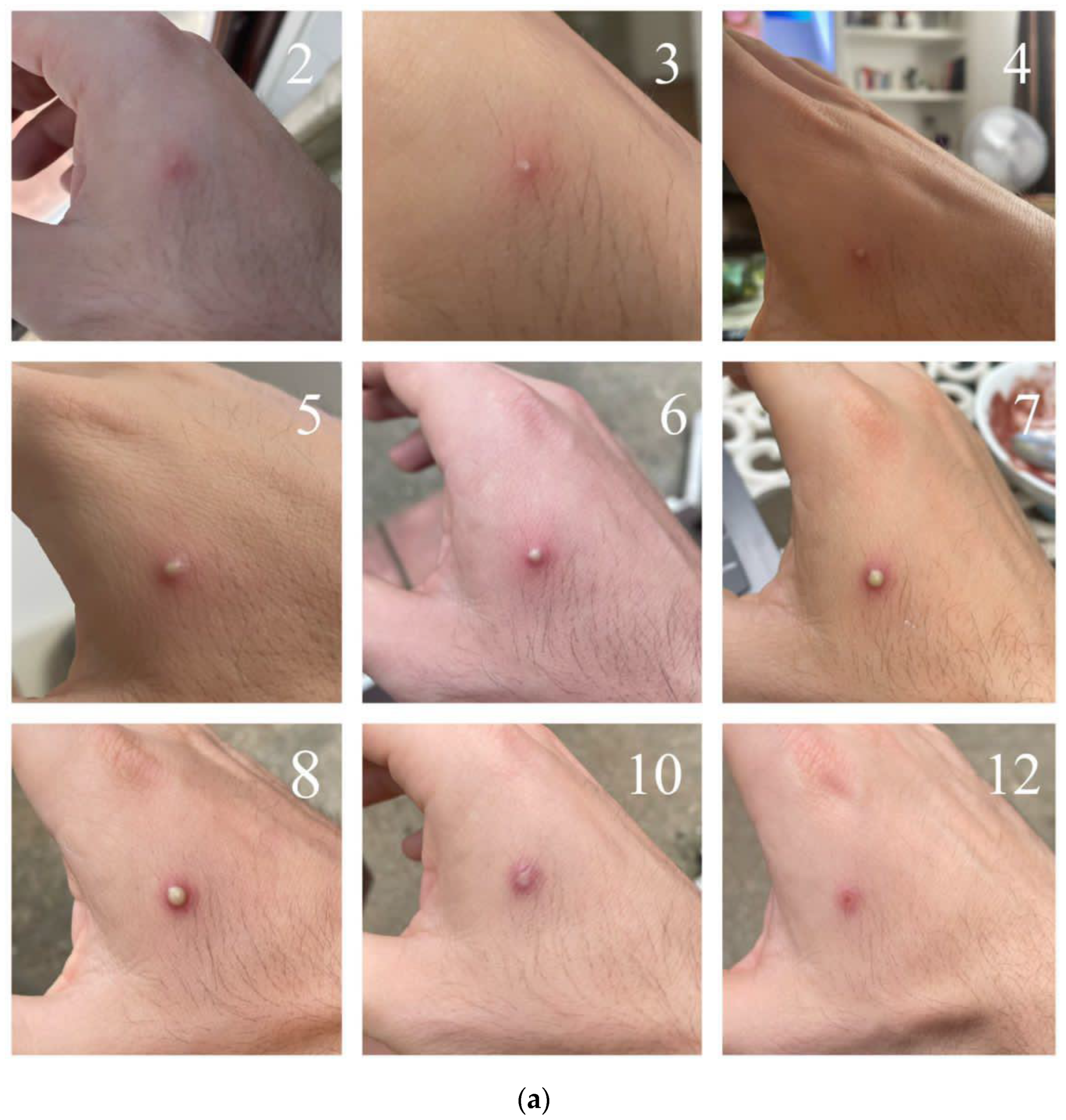
5. Clinical Presentation
6. Diagnosis
7. Treatment
8. Prevention
8.1. Contact Precautions
8.2. Vaccination
9. Reinfection and Post-Vaccination Infection
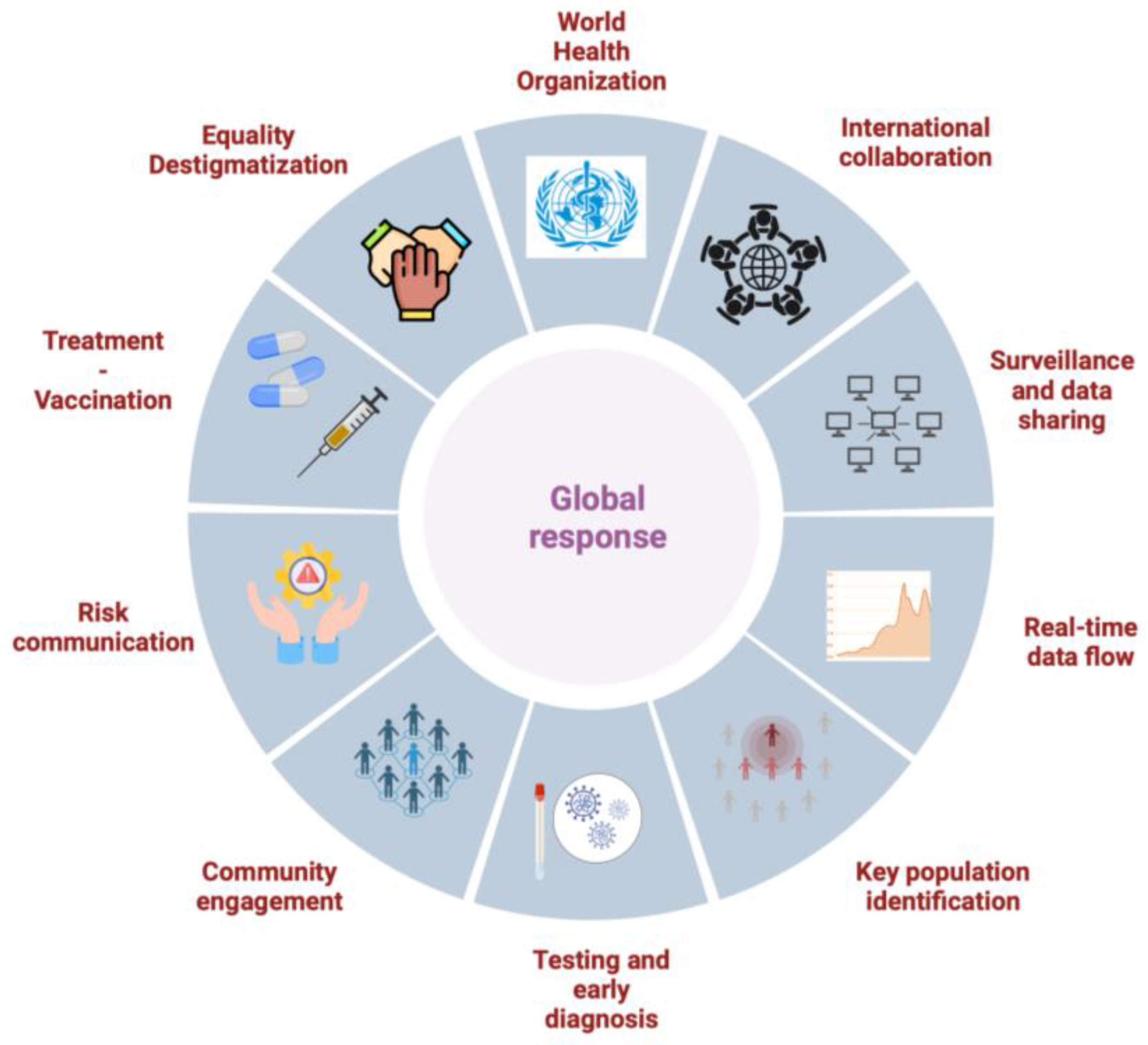
10. Global and Community Responses
11. Challenges and Future Perspectives
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antunes, F.; Cordeiro, R.; Virgolino, A. Monkeypox: From A Neglected Tropical Disease to a Public Health Threat. Infect. Dis. Rep. 2022, 14, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Mitjà, O.; Alemany, A.; Marks, M.; Lezama Mora, J.I.; Rodríguez-Aldama, J.C.; Torres Silva, M.S.; Corral Herrera, E.A.; Crabtree-Ramirez, B.; Blanco, J.L.; Girometti, N.; et al. Mpox in People with Advanced HIV Infection: A Global Case Series. Lancet 2023, 401, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-S.; Mandal, S.; Mohammed, H.; Turner, C.; Florence, I.; Walker, J.; Niyomsri, S.; Amirthalingam, G.; Ramsay, M.; Charlett, A.; et al. Transmission Dynamics and Effect of Control Measures on the 2022 Outbreak of Mpox among Gay, Bisexual, and Other Men Who Have Sex with Men in England: A Mathematical Modelling Study. Lancet Infect. Dis. 2024, 24, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Brand, S.P.C.; Cavallaro, M.; Cumming, F.; Turner, C.; Florence, I.; Blomquist, P.; Hilton, J.; Guzman-Rincon, L.M.; House, T.; Nokes, D.J.; et al. The Role of Vaccination and Public Awareness in Forecasts of Mpox Incidence in the United Kingdom. Nat. Commun. 2023, 14, 4100. [Google Scholar] [CrossRef] [PubMed]
- van Ewijk, C.E.; Miura, F.; van Rijckevorsel, G.; de Vries, H.J.; Welkers, M.R.; van den Berg, O.E.; Friesema, I.H.; van den Berg, P.R.; Dalhuisen, T.; Wallinga, J.; et al. Mpox Outbreak in the Netherlands, 2022: Public Health Response, Characteristics of the First 1,000 Cases and Protection of the First-Generation Smallpox Vaccine. Eurosurveillance 2023, 28, 2200772. [Google Scholar] [CrossRef]
- McQuiston, J.H.; Braden, C.R.; Bowen, M.D.; McCollum, A.M.; McDonald, R.; Carnes, N.; Carter, R.J.; Christie, A.; Doty, J.B.; Ellington, S.; et al. The CDC Domestic Mpox Response—United States, 2022–2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 547–552. [Google Scholar] [CrossRef]
- Walker, P.J.; Siddell, S.G.; Lefkowitz, E.J.; Mushegian, A.R.; Adriaenssens, E.M.; Alfenas-Zerbini, P.; Dempsey, D.M.; Dutilh, B.E.; García, M.L.; Curtis Hendrickson, R.; et al. Recent Changes to Virus Taxonomy Ratified by the International Committee on Taxonomy of Viruses (2022). Arch. Virol. 2022, 167, 2429–2440. [Google Scholar] [CrossRef]
- Gessain, A.; Nakoune, E.; Yazdanpanah, Y. Monkeypox. N. Engl. J. Med. 2022, 387, 1783–1793. [Google Scholar] [CrossRef]
- Curaudeau, M.; Besombes, C.; Nakouné, E.; Fontanet, A.; Gessain, A.; Hassanin, A. Identifying the Most Probable Mammal Reservoir Hosts for Monkeypox Virus Based on Ecological Niche Comparisons. Viruses 2023, 15, 727. [Google Scholar] [CrossRef]
- World Health Organization. Monkeypox: Experts Give Virus Variants New Names. Available online: https://www.who.int/news/item/12-08-2022-monkeypox--experts-give-virus-variants-new-names (accessed on 19 September 2024).
- Happi, C.; Adetifa, I.; Mbala, P.; Njouom, R.; Nakoune, E.; Happi, A.; Ndodo, N.; Ayansola, O.; Mboowa, G.; Bedford, T.; et al. Urgent Need for a Non-Discriminatory and Non-Stigmatizing Nomenclature for Monkeypox Virus. PLoS Biol. 2022, 20, e3001769. [Google Scholar] [CrossRef]
- Ulaeto, D.; Agafonov, A.; Burchfield, J.; Carter, L.; Happi, C.; Jakob, R.; Krpelanova, E.; Kuppalli, K.; Lefkowitz, E.J.; Mauldin, M.R.; et al. New Nomenclature for Mpox (Monkeypox) and Monkeypox Virus Clades. Lancet Infect. Dis. 2023, 23, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Vakaniaki, E.H.; Kacita, C.; Kinganda-Lusamaki, E.; O’Toole, Á.; Wawina-Bokalanga, T.; Mukadi-Bamuleka, D.; Amuri-Aziza, A.; Malyamungu-Bubala, N.; Mweshi-Kumbana, F.; Mutimbwa-Mambo, L.; et al. Sustained Human Outbreak of a New MPXV Clade I Lineage in Eastern Democratic Republic of the Congo. Nat. Med. 2024. [Google Scholar] [CrossRef] [PubMed]
- Shchelkunov, S.N.; Totmenin, A.V.; Safronov, P.F.; Mikheev, M.V.; Gutorov, V.V.; Ryazankina, O.I.; Petrov, N.A.; Babkin, I.V.; Uvarova, E.A.; Sandakhchiev, L.S.; et al. Analysis of the Monkeypox Virus Genome. Virology 2002, 297, 172–194. [Google Scholar] [CrossRef]
- Sklenovská, N. Monkeypox Virus. In Animal-Origin Viral Zoonoses; Springer: Singapore, 2020; pp. 39–68. [Google Scholar]
- Condit, R.C.; Moussatche, N.; Traktman, P. In a Nutshell: Structure and Assembly of the Vaccinia Virion. Adv. Virus Res. 2006, 66, 31–124. [Google Scholar] [CrossRef]
- Begum, J.P.S.; Ngangom, L.; Semwal, P.; Painuli, S.; Sharma, R.; Gupta, A. Emergence of Monkeypox: A Worldwide Public Health Crisis. Hum. Cell 2023, 36, 877–893. [Google Scholar] [CrossRef]
- Kaler, J.; Hussain, A.; Flores, G.; Kheiri, S.; Desrosiers, D. Monkeypox: A Comprehensive Review of Transmission, Pathogenesis, and Manifestation. Cureus 2022, 14, e26531. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.B.; McFadden, G. Poxvirus Immunomodulatory Strategies: Current Perspectives. J. Virol. 2003, 77, 6093–6100. [Google Scholar] [CrossRef]
- Stanford, M.M.; McFadden, G.; Karupiah, G.; Chaudhri, G. Immunopathogenesis of Poxvirus Infections: Forecasting the Impending Storm. Immunol. Cell Biol. 2007, 85, 93–102. [Google Scholar] [CrossRef]
- Kataria, R.; Kaur, S.; Kaundal, R. Deciphering the Complete Human-Monkeypox Virus Interactome: Identifying Immune Responses and Potential Drug Targets. Front. Immunol. 2023, 14, 1116988. [Google Scholar] [CrossRef]
- Stilpeanu, R.I.; Stercu, A.M.; Stancu, A.L.; Tanca, A.; Bucur, O. Monkeypox: A Global Health Emergency. Front. Microbiol. 2023, 14, 1094794. [Google Scholar] [CrossRef]
- Chen, N.; Li, G.; Liszewski, M.K.; Atkinson, J.P.; Jahrling, P.B.; Feng, Z.; Schriewer, J.; Buck, C.; Wang, C.; Lefkowitz, E.J.; et al. Virulence Differences between Monkeypox Virus Isolates from West Africa and the Congo Basin. Virology 2005, 340, 46–63. [Google Scholar] [CrossRef] [PubMed]
- Liszewski, M.K.; Leung, M.K.; Hauhart, R.; Buller, R.M.L.; Bertram, P.; Wang, X.; Rosengard, A.M.; Kotwal, G.J.; Atkinson, J.P. Structure and Regulatory Profile of the Monkeypox Inhibitor of Complement: Comparison to Homologs in Vaccinia and Variola and Evidence for Dimer Formation. J. Immunol. 2006, 176, 3725–3734. [Google Scholar] [CrossRef] [PubMed]
- Harapan, H.; Ophinni, Y.; Megawati, D.; Frediansyah, A.; Mamada, S.S.; Salampe, M.; Bin Emran, T.; Winardi, W.; Fathima, R.; Sirinam, S.; et al. Monkeypox: A Comprehensive Review. Viruses 2022, 14, 2155. [Google Scholar] [CrossRef]
- Martínez-Fernández, D.E.; Fernández-Quezada, D.; Casillas-Muñoz, F.A.G.; Carrillo-Ballesteros, F.J.; Ortega-Prieto, A.M.; Jimenez-Guardeño, J.M.; Regla-Nava, J.A. Human Monkeypox: A Comprehensive Overview of Epidemiology, Pathogenesis, Diagnosis, Treatment, and Prevention Strategies. Pathogens 2023, 12, 947. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, Á.; Neher, R.A.; Ndodo, N.; Borges, V.; Gannon, B.; Gomes, J.P.; Groves, N.; King, D.J.; Maloney, D.; Lemey, P.; et al. APOBEC3 Deaminase Editing in Mpox Virus as Evidence for Sustained Human Transmission since at Least 2016. Science 2023, 382, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, S.; Ross, S.R. APOBEC3 Proteins in Viral Immunity. J. Immunol. 2015, 195, 4565–4570. [Google Scholar] [CrossRef]
- Suspène, R.; Raymond, K.A.; Boutin, L.; Guillier, S.; Lemoine, F.; Ferraris, O.; Tournier, J.-N.; Iseni, F.; Simon-Lorière, E.; Vartanian, J.-P. APOBEC3F Is a Mutational Driver of the Human Monkeypox Virus Identified in the 2022 Outbreak. J. Infect. Dis. 2023, 228, 1421–1429. [Google Scholar] [CrossRef]
- Gigante, C.M.; Korber, B.; Seabolt, M.H.; Wilkins, K.; Davidson, W.; Rao, A.K.; Zhao, H.; Smith, T.G.; Hughes, C.M.; Minhaj, F.; et al. Multiple Lineages of Monkeypox Virus Detected in the United States, 2021–2022. Science 2022, 378, 560–565. [Google Scholar] [CrossRef]
- Arita, I.; Henderson, D.A. Smallpox and Monkeypox in Non-Human Primates. Bull. World Health Organ. 1968, 39, 277–283. [Google Scholar]
- Alakunle, E.; Moens, U.; Nchinda, G.; Okeke, M.I. Monkeypox Virus in Nigeria: Infection Biology, Epidemiology, and Evolution. Viruses 2020, 12, 1257. [Google Scholar] [CrossRef]
- Ladnyj, I.D.; Ziegler, P.; Kima, E. A Human Infection Caused by Monkeypox Virus in Basankusu Territory, Democratic Republic of the Congo. Bull. World Health Organ. 1972, 46, 593–597. [Google Scholar] [PubMed]
- Hutin, Y.J.; Williams, R.J.; Malfait, P.; Pebody, R.; Loparev, V.N.; Ropp, S.L.; Rodriguez, M.; Knight, J.C.; Tshioko, F.K.; Khan, A.S.; et al. Outbreak of Human Monkeypox, Democratic Republic of Congo, 1996 to 1997. Emerg. Infect. Dis. 2001, 7, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Lum, F.-M.; Torres-Ruesta, A.; Tay, M.Z.; Lin, R.T.P.; Lye, D.C.; Rénia, L.; Ng, L.F.P. Monkeypox: Disease Epidemiology, Host Immunity and Clinical Interventions. Nat. Rev. Immunol. 2022, 22, 597–613. [Google Scholar] [CrossRef]
- Zahmatyar, M.; Fazlollahi, A.; Motamedi, A.; Zolfi, M.; Seyedi, F.; Nejadghaderi, S.A.; Sullman, M.J.M.; Mohammadinasab, R.; Kolahi, A.-A.; Arshi, S.; et al. Human Monkeypox: History, Presentations, Transmission, Epidemiology, Diagnosis, Treatment, and Prevention. Front. Med. 2023, 10, 1157670. [Google Scholar] [CrossRef]
- Reed, K.D.; Melski, J.W.; Graham, M.B.; Regnery, R.L.; Sotir, M.J.; Wegner, M.V.; Kazmierczak, J.J.; Stratman, E.J.; Li, Y.; Fairley, J.A.; et al. The Detection of Monkeypox in Humans in the Western Hemisphere. N. Engl. J. Med. 2004, 350, 342–350. [Google Scholar] [CrossRef]
- Sklenovská, N.; Van Ranst, M. Emergence of Monkeypox as the Most Important Orthopoxvirus Infection in Humans. Front. Public Health 2018, 6, 241. [Google Scholar] [CrossRef]
- Rimoin, A.W.; Mulembakani, P.M.; Johnston, S.C.; Lloyd Smith, J.O.; Kisalu, N.K.; Kinkela, T.L.; Blumberg, S.; Thomassen, H.A.; Pike, B.L.; Fair, J.N.; et al. Major Increase in Human Monkeypox Incidence 30 Years after Smallpox Vaccination Campaigns Cease in the Democratic Republic of Congo. Proc. Natl. Acad. Sci. USA 2010, 107, 16262–16267. [Google Scholar] [CrossRef] [PubMed]
- Bunge, E.M.; Hoet, B.; Chen, L.; Lienert, F.; Weidenthaler, H.; Baer, L.R.; Steffen, R. The Changing Epidemiology of Human Monkeypox-A Potential Threat? A Systematic Review. PLoS Negl. Trop. Dis. 2022, 16, e0010141. [Google Scholar] [CrossRef]
- Mauldin, M.R.; McCollum, A.M.; Nakazawa, Y.J.; Mandra, A.; Whitehouse, E.R.; Davidson, W.; Zhao, H.; Gao, J.; Li, Y.; Doty, J.; et al. Exportation of Monkeypox Virus From the African Continent. J. Infect. Dis. 2022, 225, 1367–1376. [Google Scholar] [CrossRef]
- Ogoina, D.; Izibewule, J.H.; Ogunleye, A.; Ederiane, E.; Anebonam, U.; Neni, A.; Oyeyemi, A.; Etebu, E.N.; Ihekweazu, C. The 2017 Human Monkeypox Outbreak in Nigeria-Report of Outbreak Experience and Response in the Niger Delta University Teaching Hospital, Bayelsa State, Nigeria. PLoS ONE 2019, 14, e0214229. [Google Scholar] [CrossRef]
- Isidro, J.; Borges, V.; Pinto, M.; Sobral, D.; Santos, J.D.; Nunes, A.; Mixão, V.; Ferreira, R.; Santos, D.; Duarte, S.; et al. Phylogenomic Characterization and Signs of Microevolution in the 2022 Multi-Country Outbreak of Monkeypox Virus. Nat. Med. 2022, 28, 1569–1572. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Multi-Country Monkeypox Outbreak in Non-Endemic Countries. Geneva. 2022. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2022-don385 (accessed on 3 March 2024).
- Nuzzo, J.B.; Borio, L.L.; Gostin, L.O. The WHO Declaration of Monkeypox as a Global Public Health Emergency. JAMA 2022, 328, 615–617. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). 2022–2023 Mpox Outbreak Global Map. Data as of 7 February 2024. Available online: https://archive.cdc.gov/\#/details?url=https://www.cdc.gov/poxvirus/mpox/response/2022/world-map.html (accessed on 5 March 2024).
- Mitjà, O.; Ogoina, D.; Titanji, B.K.; Galvan, C.; Muyembe, J.-J.; Marks, M.; Orkin, C.M. Monkeypox. Lancet 2023, 401, 60–74. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. 2022–24 Mpox (Monkeypox) Outbreak: Global Trends. Geneva. 11 October 2024. Available online: https://worldhealthorg.shinyapps.io/mpx_global/ (accessed on 11 October 2024).
- World Health Organization (WHO). Fifth Meeting of the International Health Regulations (2005) (IHR) Emergency Committee on the Multi-Country Outbreak of Monkeypox (Mpox). Geneva. 11 May 2023. Available online: https://www.who.int/news/item/11-05-2023-fifth-meeting-of-the-international-health-regulations-(2005)-(ihr)-emergency-committee-on-the-multi-country-outbreak-of-monkeypox-(mpox) (accessed on 11 October 2024).
- World Health Organization. WHO Director-General Declares Mpox Outbreak a Public Health Emergency of International Concern. Geneva. 14 August 2024. Available online: https://www.who.int/news/item/14-08-2024-who-director-general-declares-mpox-outbreak-a-public-health-emergency-of-international-concern (accessed on 17 September 2024).
- Africa Centres for Disease Control and Prevention. Mpox Technical Factsheet. 22 August 2024. Available online: https://africacdc.org/download/mpox-technical-factsheet/ (accessed on 17 September 2024).
- Africa Centres for Disease Control and Prevention. Africa CDC Epidemic Intelligence Weekly Report. August 2024. Available online: https://africacdc.org/download/africa-cdc-weekly-event-based-surveillance-report-august-2024/ (accessed on 17 September 2024).
- Africa Centres for Disease Control and Prevention. Outbreak Report, 30 July 2024: Mpox Situation in Africa. Available online: https://africacdc.org/disease-outbreak/mpox-situation-in-africa/ (accessed on 17 September 2024).
- European Centre for Disease Prevention and Control. Risk Assessment for the EU/EEA of the Mpox Epidemic Caused by Monkeypox Virus Clade I in Affected African Countries. 16 August 2024. Available online: https://www.ecdc.europa.eu/en/publications-data/risk-assessment-mpox-epidemic-monkeypox-virus-clade-i-africa (accessed on 17 September 2024).
- European Centre for Disease Prevention and Control. Epidemiological Update—Week 35/2024: Mpox Due to Monkeypox Virus Clade I. 2 September 2024. Available online: hhttps://www.ecdc.europa.eu/en/news-events/mpox-epidemiological-update-monkeypox-2-september-2024 (accessed on 17 September 2024).
- European Centre for Disease Prevention and Control. ECDC Recommends Enhancing Preparedness as More Imported Cases of Clade I Mpox Highly Likely. 16 August 2024. Available online: https://static.poder360.com.br/2024/08/ECDC-recommends-enhancing-preparedness-as-more-imported-cases-of-clade-I-mpox-highly-likely.pdf (accessed on 17 September 2024).
- Ullah, M.; Li, Y.; Munib, K.; Zhang, Z. Epidemiology, Host Range, and Associated Risk Factors of Monkeypox: An Emerging Global Public Health Threat. Front. Microbiol. 2023, 14, 1160984. [Google Scholar] [CrossRef]
- Seang, S.; Burrel, S.; Todesco, E.; Leducq, V.; Monsel, G.; Le Pluart, D.; Cordevant, C.; Pourcher, V.; Palich, R. Evidence of Human-to-Dog Transmission of Monkeypox Virus. Lancet 2022, 400, 658–659. [Google Scholar] [CrossRef]
- Islam, M.M.; Dutta, P.; Rashid, R.; Jaffery, S.S.; Islam, A.; Farag, E.; Zughaier, S.M.; Bansal, D.; Hassan, M.M. Pathogenicity and Virulence of Monkeypox at the Human-Animal-Ecology Interface. Virulence 2023, 14, 2186357. [Google Scholar] [CrossRef]
- Chen, J.-M.; Chen, R.-X.; Gong, H.-Y.; Zhao, M.-M.; Ji, Y.-F.; Sun, M.-H.; Li, G.-H.; Tan, S.-M.; Zhang, G.-H.; Chen, J.-W. Epidemiology-Based Analysis of the Risks and Elimination Strategies of the Monkeypox Outbreak in 2022. Front. Vet. Sci. 2022, 9, 1064766. [Google Scholar] [CrossRef] [PubMed]
- Fahrni, M.L.; Priyanka; Choudhary, O.P. Possibility of Vertical Transmission of the Human Monkeypox Virus. Int. J. Surg. 2022, 105, 106832. [Google Scholar] [CrossRef]
- Nakoune, E.; Lampaert, E.; Ndjapou, S.G.; Janssens, C.; Zuniga, I.; Van Herp, M.; Fongbia, J.P.; Koyazegbe, T.D.; Selekon, B.; Komoyo, G.F.; et al. A Nosocomial Outbreak of Human Monkeypox in the Central African Republic. Open Forum Infect. Dis. 2017, 4, ofx168. [Google Scholar] [CrossRef]
- Quiner, C.A.; Moses, C.; Monroe, B.P.; Nakazawa, Y.; Doty, J.B.; Hughes, C.M.; McCollum, A.M.; Ibata, S.; Malekani, J.; Okitolonda, E.; et al. Presumptive Risk Factors for Monkeypox in Rural Communities in the Democratic Republic of the Congo. PLoS ONE 2017, 12, e0168664. [Google Scholar] [CrossRef]
- Thornhill, J.P.; Barkati, S.; Walmsley, S.; Rockstroh, J.; Antinori, A.; Harrison, L.B.; Palich, R.; Nori, A.; Reeves, I.; Habibi, M.S.; et al. Monkeypox Virus Infection in Humans across 16 Countries—April–June 2022. N. Engl. J. Med. 2022, 387, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Guzzetta, G.; Mammone, A.; Ferraro, F.; Caraglia, A.; Rapiti, A.; Marziano, V.; Poletti, P.; Cereda, D.; Vairo, F.; Mattei, G.; et al. Early Estimates of Monkeypox Incubation Period, Generation Time, and Reproduction Number, Italy, May–June 2022. Emerg. Infect. Dis. 2022, 28, 2078–2081. [Google Scholar] [CrossRef] [PubMed]
- Huhn, G.D.; Bauer, A.M.; Yorita, K.; Graham, M.B.; Sejvar, J.; Likos, A.; Damon, I.K.; Reynolds, M.G.; Kuehnert, M.J. Clinical Characteristics of Human Monkeypox, and Risk Factors for Severe Disease. Clin. Infect. Dis. 2005, 41, 1742–1751. [Google Scholar] [CrossRef] [PubMed]
- Yinka-Ogunleye, A.; Dalhat, M.; Akinpelu, A.; Aruna, O.; Garba, F.; Ahmad, A.; Adeleye, A.; Botson, I.; Oluwafemi, B.; Ogunbode, O.; et al. Mpox (Monkeypox) Risk and Mortality Associated with HIV Infection: A National Case-Control Study in Nigeria. BMJ Glob. Health 2023, 8, e013126. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, A.P.; Padhi, B.K.; Sandeep, M.; Shamim, M.A.; Suvvari, T.K.; Satapathy, P.; Siddiq, A.; Sah, R.; Rustagi, S.; Al-Qaim, Z.H.; et al. Monkeypox Patients Living with HIV: A Systematic Review and Meta-Analysis of Geographic and Temporal Variations. Epidemiologia 2023, 4, 352–369. [Google Scholar] [CrossRef]
- Iroezindu, M.O.; Crowell, T.A.; Ogoina, D.; Yinka-Ogunleye, A. Human Mpox in People Living with HIV: Epidemiologic and Clinical Perspectives from Nigeria. AIDS Res. Hum. Retroviruses 2023, 39, 593–600. [Google Scholar] [CrossRef]
- Adam, P.C.G.; Op de Coul, E.L.M.; Zantkuijl, P.; Xiridou, M.; Bos, H.; Blom, C.; Ketsuwan, I.; Te Wierik, M.J.M.; David, S.; de Wit, J.B.F. A Survey-Based Assessment of Rates and Covariates of Mpox Diagnosis and Vaccination Provides Evidence to Refine Eligibility Criteria for Mpox Vaccination among Gay, Bisexual and Other Men Who Have Sex with Men in the Netherlands. Front. Public Health 2024, 12, 1194844. [Google Scholar] [CrossRef]
- Betancort-Plata, C.; Lopez-Delgado, L.; Jaén-Sanchez, N.; Tosco-Nuñez, T.; Suarez-Hormiga, L.; Lavilla-Salgado, C.; Pisos-Álamo, E.; Hernández-Betancor, A.; Hernández-Cabrera, M.; Carranza-Rodríguez, C.; et al. Monkeypox and HIV in the Canary Islands: A Different Pattern in a Mobile Population. Trop. Med. Infect. Dis. 2022, 7, 318. [Google Scholar] [CrossRef]
- Garneau, W.M.; Jones, J.L.; Dashler, G.M.; Mostafa, H.H.; Judson, S.D.; Kwon, N.; Hamill, M.M.; Gilliams, E.A.; Rudolph, D.S.; Keruly, J.C.; et al. Risk Factors for Hospitalization and Effect of Immunosuppression on Clinical Outcomes Among an Urban Cohort of Patients With Mpox. Open Forum Infect. Dis. 2023, 10, ofad533. [Google Scholar] [CrossRef]
- Vivancos-Gallego, M.J.; Sánchez-Conde, M.; Rodríguez-Domínguez, M.; Fernandez-Gonzalez, P.; Martínez-García, L.; Garcia-Mouronte, E.; Martínez-Sanz, J.; Moreno-Zamora, A.M.; Casado, J.L.; Ron, R.; et al. Human Monkeypox in People With HIV: Transmission, Clinical Features, and Outcome. Open Forum Infect. Dis. 2022, 9, ofac557. [Google Scholar] [CrossRef]
- Silva, M.S.T.; Coutinho, C.; Torres, T.S.; Peixoto, E.M.; Bastos, M.O.; Mesquita, M.B.; Tavares, I.C.F.; Andrade, H.B.; Reges, P.P.S.; Martins, P.S.; et al. Mpox Severity and Associated Hospitalizations among People with HIV and Related Immunosuppression in Brazil. AIDS 2024, 38, 105–113. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, D.; Volovetska, Y.; Nunes, D.; Lemos, C.; Borges-Costa, J.; Filipe, P. Clinical and Epidemiological Characteristics of Mpox in HIV-Infected and Uninfected Men Who Have Sex with Men: A Retrospective Study in Lisbon. Viruses 2024, 16, 225. [Google Scholar] [CrossRef]
- Pilkington, V.; Quinn, K.; Campbell, L.; Payne, L.; Brady, M.; Post, F.A. Clinical Presentation of Mpox in People With and Without HIV in the United Kingdom During the 2022 Global Outbreak. AIDS Res. Hum. Retroviruses 2023, 39, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Minhaj, F.S.; Ogale, Y.P.; Whitehill, F.; Schultz, J.; Foote, M.; Davidson, W.; Hughes, C.M.; Wilkins, K.; Bachmann, L.; Chatelain, R.; et al. Monkeypox Outbreak—Nine States, May 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Tarín-Vicente, E.J.; Alemany, A.; Agud-Dios, M.; Ubals, M.; Suñer, C.; Antón, A.; Arando, M.; Arroyo-Andrés, J.; Calderón-Lozano, L.; Casañ, C.; et al. Clinical Presentation and Virological Assessment of Confirmed Human Monkeypox Virus Cases in Spain: A Prospective Observational Cohort Study. Lancet 2022, 400, 661–669. [Google Scholar] [CrossRef]
- Miura, F.; van Ewijk, C.E.; Backer, J.A.; Xiridou, M.; Franz, E.; Op de Coul, E.; Brandwagt, D.; van Cleef, B.; van Rijckevorsel, G.; Swaan, C.; et al. Estimated Incubation Period for Monkeypox Cases Confirmed in the Netherlands, May 2022. Eurosurveillance 2022, 27, 2200448. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Mpox Cases by Age and Gender and Race and Ethnicity. Data as of 27 February 2024. Available online: https://www.cdc.gov/mpox/data-research/cases/?CDC_AAref_Val=https://www.cdc.gov/poxvirus/mpox/response/2022/demographics.html (accessed on 5 March 2024).
- Reynolds, M.G.; Davidson, W.B.; Curns, A.T.; Conover, C.S.; Huhn, G.; Davis, J.P.; Wegner, M.; Croft, D.R.; Newman, A.; Obiesie, N.N.; et al. Spectrum of Infection and Risk Factors for Human Monkeypox, United States, 2003. Emerg. Infect. Dis. 2007, 13, 1332–1339. [Google Scholar] [CrossRef]
- Patel, A.; Bilinska, J.; Tam, J.C.H.; Da Silva Fontoura, D.; Mason, C.Y.; Daunt, A.; Snell, L.B.; Murphy, J.; Potter, J.; Tuudah, C.; et al. Clinical Features and Novel Presentations of Human Monkeypox in a Central London Centre during the 2022 Outbreak: Descriptive Case Series. BMJ 2022, 378, e072410. [Google Scholar] [CrossRef]
- Català, A.; Clavo-Escribano, P.; Riera-Monroig, J.; Martín-Ezquerra, G.; Fernandez-Gonzalez, P.; Revelles-Peñas, L.; Simon-Gozalbo, A.; Rodríguez-Cuadrado, F.J.; Castells, V.G.; de la Torre Gomar, F.J.; et al. Monkeypox Outbreak in Spain: Clinical and Epidemiological Findings in a Prospective Cross-Sectional Study of 185 Cases. Br. J. Dermatol. 2022, 187, 765–772. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Clinical Recognition. Key Characteristics for Identifying Mpox. Available online: https://www.cdc.gov/mpox/hcp/clinical-signs/?CDC_AAref_Val=https://www.cdc.gov/poxvirus/mpox/clinicians/clinical-recognition.html (accessed on 5 March 2024).
- Thornhill, J.P.; Palich, R.; Ghosn, J.; Walmsley, S.; Moschese, D.; Cortes, C.P.; Galliez, R.M.; Garlin, A.B.; Nozza, S.; Mitja, O.; et al. Human Monkeypox Virus Infection in Women and Non-Binary Individuals during the 2022 Outbreaks: A Global Case Series. Lancet 2022, 400, 1953–1965. [Google Scholar] [CrossRef]
- Iñigo Martínez, J.; Gil Montalbán, E.; Jiménez Bueno, S.; Martín Martínez, F.; Nieto Juliá, A.; Sánchez Díaz, J.; García Marín, N.; Córdoba Deorador, E.; Nunziata Forte, A.; Alonso García, M.; et al. Monkeypox Outbreak Predominantly Affecting Men Who Have Sex with Men, Madrid, Spain, 26 April to 16 June 2022. Eurosurveillance 2022, 27, 2200471. [Google Scholar] [CrossRef] [PubMed]
- Oakley, L.P.; Hufstetler, K.; O’Shea, J.; Sharpe, J.D.; McArdle, C.; Neelam, V.; Roth, N.M.; Olsen, E.O.; Wolf, M.; Pao, L.Z.; et al. Mpox Cases Among Cisgender Women and Pregnant Persons—United States, 11 May–7 November 2022. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 9–14. [Google Scholar] [CrossRef]
- Cash-Goldwasser, S.; Labuda, S.M.; McCormick, D.W.; Rao, A.K.; McCollum, A.M.; Petersen, B.W.; Chodosh, J.; Brown, C.M.; Chan-Colenbrander, S.Y.; Dugdale, C.M.; et al. Ocular Monkeypox—United States, July-September 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1343–1347. [Google Scholar] [CrossRef]
- Carrubba, S.; Geevarghese, A.; Solli, E.; Guttha, S.; Sims, J.; Sperber, L.; Meehan, S.; Ostrovsky, A. Novel Severe Oculocutaneous Manifestations of Human Monkeypox Virus Infection and Their Historical Analogues. Lancet Infect. Dis. 2023, 23, e190–e197. [Google Scholar] [CrossRef] [PubMed]
- Badenoch, J.B.; Conti, I.; Rengasamy, E.R.; Watson, C.J.; Butler, M.; Hussain, Z.; Carter, B.; Rooney, A.G.; Zandi, M.S.; Lewis, G.; et al. Neurological and Psychiatric Presentations Associated with Human Monkeypox Virus Infection: A Systematic Review and Meta-Analysis. EClinicalMedicine 2022, 52, 101644. [Google Scholar] [CrossRef] [PubMed]
- Pastula, D.M.; Copeland, M.J.; Hannan, M.C.; Rapaka, S.; Kitani, T.; Kleiner, E.; Showler, A.; Yuen, C.; Ferriman, E.M.; House, J.; et al. Two Cases of Monkeypox-Associated Encephalomyelitis—Colorado and the District of Columbia, July–August 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1212–1215. [Google Scholar] [CrossRef]
- Tan, D.H.S.; Jaeranny, S.; Li, M.; Sukhdeo, S.S.; Monge, J.C.; Callejas, M.F.; Hasso, M.; Fattouh, R.; Lalonde, S.D.; Lam, J.; et al. Atypical Clinical Presentation of Monkeypox Complicated by Myopericarditis. Open Forum Infect. Dis. 2022, 9, ofac394. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Severe Manifestations of Monkeypox among People Who Are Immunocompromised Due to HIV or Other Conditions. 29 September 2022. Available online: https://emergency.cdc.gov/han/2022/han00475.asp#:~:text=If%20you%20are%20someone%20with,monkeypox%20from%20a%20healthcare%20provider (accessed on 5 March 2024).
- World Health Organization. Mpox (Monkeypox). Geneva. 18 April 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/monkeypox (accessed on 6 March 2024).
- Fink, D.L.; Callaby, H.; Luintel, A.; Beynon, W.; Bond, H.; Lim, E.Y.; Gkrania-Klotsas, E.; Heskin, J.; Bracchi, M.; Rathish, B.; et al. Clinical Features and Management of Individuals Admitted to Hospital with Monkeypox and Associated Complications across the UK: A Retrospective Cohort Study. Lancet Infect. Dis. 2023, 23, 589–597. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Case Definitions for Use in the 2022 Mpox Response. 9 November 2023. Available online: https://www.cdc.gov/mpox/hcp/case-definitions/?CDC_AAref_Val=https://www.cdc.gov/poxvirus/mpox/clinicians/case-definition.html (accessed on 6 March 2024).
- Minhaj, F.S.; Petras, J.K.; Brown, J.A.; Mangla, A.T.; Russo, K.; Willut, C.; Lee, M.; Beverley, J.; Harold, R.; Milroy, L.; et al. Orthopoxvirus Testing Challenges for Persons in Populations at Low Risk or Without Known Epidemiologic Link to Monkeypox—United States, 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1155–1158. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Guidelines for Collecting and Handling Specimens for Mpox Testing. 20 September 2022. Available online: https://www.cdc.gov/mpox/hcp/diagnosis-testing/collecting-specimens.html?CDC_AAref_Val=https://www.cdc.gov/poxvirus/mpox/clinicians/prep-collection-specimens.html (accessed on 6 March 2024).
- Karem, K.L.; Reynolds, M.; Braden, Z.; Lou, G.; Bernard, N.; Patton, J.; Damon, I.K. Characterization of Acute-Phase Humoral Immunity to Monkeypox: Use of Immunoglobulin M Enzyme-Linked Immunosorbent Assay for Detection of Monkeypox Infection during the 2003 North American Outbreak. Clin. Diagn. Lab. Immunol. 2005, 12, 867–872. [Google Scholar] [CrossRef]
- da Silva, S.J.R.; Kohl, A.; Pena, L.; Pardee, K. Clinical and Laboratory Diagnosis of Monkeypox (Mpox): Current Status and Future Directions. iScience 2023, 26, 106759. [Google Scholar] [CrossRef] [PubMed]
- Bayer-Garner, I.B. Monkeypox Virus: Histologic, Immunohistochemical and Electron-Microscopic Findings. J. Cutan. Pathol. 2005, 32, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Long, B.; Liang, S.Y.; Carius, B.M.; Chavez, S.; Gottlieb, M.; Koyfman, A.; Brady, W.J. Mimics of Monkeypox: Considerations for the Emergency Medicine Clinician. Am. J. Emerg. Med. 2023, 65, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Kaler, J.; Lau, G.; Maxwell, T. Clinical Conundrums: Differentiating Monkeypox From Similarly Presenting Infections. Cureus 2022, 14, e29929. [Google Scholar] [CrossRef] [PubMed]
- McNeil, C.J.; Barroso, L.F.; Workowski, K. Proctitis: An Approach to the Symptomatic Patient. Med. Clin. N. Am. 2024, 108, 339–354. [Google Scholar] [CrossRef]
- Herrera, K.; Lyang, J.; Holly, T.; Faherty, E.A.; Luc, C.; Korban, C.; Kern, D.; Tabidze, I. Extragenital Gonorrhoea, Chlamydia, and HIV Co-Infection in People with Mpox. Lancet Infect. Dis. 2023, 23, e334–e336. [Google Scholar] [CrossRef]
- Rizk, J.G.; Lippi, G.; Henry, B.M.; Forthal, D.N.; Rizk, Y. Prevention and Treatment of Monkeypox. Drugs 2022, 82, 957–963. [Google Scholar] [CrossRef]
- Huang, Y.; Mu, L.; Wang, W. Monkeypox: Epidemiology, Pathogenesis, Treatment and Prevention. Signal Transduct. Target. Ther. 2022, 7, 373. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Mpox. Treatment Information for Healthcare Professionals. 10 July 2023. Available online: >https://www.cdc.gov/mpox/?CDC_AAref_Val=https://www.cdc.gov/poxvirus/mpox/clinicians/treatment.html#anchor_1655488137245 (accessed on 20 March 2024).
- Centers for Disease Control and Prevention (CDC). Clinical Considerations for Mpox in People Who Are Pregnant or Breastfeeding. 27 March 2023. Available online: https://www.cdc.gov/mpox/hcp/clinical-care/pregnancy.html?CDC_AAref_Val=https://www.cdc.gov/poxvirus/mpox/clinicians/pregnancy.html (accessed on 20 March 2024).
- Centers for Disease Control and Prevention (CDC). Clinical Considerations for Mpox in Children and Adolescents. 1 September 2023. Available online: https://www.cdc.gov/mpox/hcp/clinical-care/pediatric.html?CDC_AAref_Val=https://www.cdc.gov/poxvirus/mpox/clinicians/pediatric.html (accessed on 20 March 2024).
- Beeson, A.M.; Haston, J.; McCormick, D.W.; Reynolds, M.; Chatham-Stephens, K.; McCollum, A.M.; Godfred-Cato, S. Mpox in Children and Adolescents: Epidemiology, Clinical Features, Diagnosis, and Management. Pediatrics 2023, 151, 060179. [Google Scholar] [CrossRef]
- Patel, M.; Adnan, M.; Aldarhami, A.; Bazaid, A.S.; Saeedi, N.H.; Alkayyal, A.A.; Saleh, F.M.; Awadh, I.B.; Saeed, A.; Alshaghdali, K. Current Insights into Diagnosis, Prevention Strategies, Treatment, Therapeutic Targets, and Challenges of Monkeypox (Mpox) Infections in Human Populations. Life 2023, 13, 249. [Google Scholar] [CrossRef]
- Fox, T.; Gould, S.; Princy, N.; Rowland, T.; Lutje, V.; Kuehn, R. Therapeutics for Treating Mpox in Humans. Cochrane Database Syst. Rev. 2023, 3, CD015769. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, L.E.; Jadoo, A.; Kirsner, R.S. Human Monkeypox Virus Infection in an Immunocompromised Man: Trial with Tecovirimat. Lancet 2022, 400, e8. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.N.; Thompson, G.R.; Neumeister, S.M.; Arutyunova, A.M.; Trigg, K.; Cohen, S.H. Compassionate Use of Tecovirimat for the Treatment of Monkeypox Infection. JAMA 2022, 328, 1348–1350. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health (NIH) National Institutes of Health (NIH). The Antiviral Tecovirimat Is Safe but Did Not Improve Clade I Mpox Resolution in Democratic Republic of the Congo. 15 August 2024. Available online: https://www.nih.gov/news-events/news-releases/antiviral-tecovirimat-safe-did-not-improve-clade-i-mpox-resolution-democratic-republic-congo (accessed on 19 September 2024).
- Grosenbach, D.W.; Honeychurch, K.; Rose, E.A.; Chinsangaram, J.; Frimm, A.; Maiti, B.; Lovejoy, C.; Meara, I.; Long, P.; Hruby, D.E. Oral Tecovirimat for the Treatment of Smallpox. N. Engl. J. Med. 2018, 379, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Sherwat, A.; Brooks, J.T.; Birnkrant, D.; Kim, P. Tecovirimat and the Treatment of Monkeypox—Past, Present, and Future Considerations. N. Engl. J. Med. 2022, 387, 579–581. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Guidance for Tecovirimat Use. 7 June 2023. Available online: https://www.cdc.gov/mpox/?CDC_AAref_Val=https://www.cdc.gov/poxvirus/mpox/clinicians/tecovirimat.html (accessed on 20 March 2024).
- Mertes, H.; Rezende, A.M.; Brosius, I.; Naesens, R.; Michiels, J.; deBlock, T.; Coppens, J.; Van Dijck, C.; Bomans, P.; Bottieau, E.; et al. Tecovirimat Resistance in an Immunocompromised Patient With Mpox and Prolonged Viral Shedding. Ann. Intern. Med. 2023, 176, 1141–1143. [Google Scholar] [CrossRef]
- Smith, T.G.; Gigante, C.M.; Wynn, N.T.; Matheny, A.; Davidson, W.; Yang, Y.; Condori, R.E.; O’Connell, K.; Kovar, L.; Williams, T.L.; et al. Tecovirimat Resistance in Mpox Patients, United States, 2022–2023. Emerg. Infect. Dis. 2023, 29, 2426–2432. [Google Scholar] [CrossRef]
- Hutson, C.L.; Kondas, A.V.; Mauldin, M.R.; Doty, J.B.; Grossi, I.M.; Morgan, C.N.; Ostergaard, S.D.; Hughes, C.M.; Nakazawa, Y.; Kling, C.; et al. Pharmacokinetics and Efficacy of a Potential Smallpox Therapeutic, Brincidofovir, in a Lethal Monkeypox Virus Animal Model. mSphere 2021, 6, e00927-20. [Google Scholar] [CrossRef]
- Rice, A.D.; Adams, M.M.; Wallace, G.; Burrage, A.M.; Lindsey, S.F.; Smith, A.J.; Swetnam, D.; Manning, B.R.; Gray, S.A.; Lampert, B.; et al. Efficacy of CMX001 as a Post Exposure Antiviral in New Zealand White Rabbits Infected with Rabbitpox Virus, a Model for Orthopoxvirus Infections of Humans. Viruses 2011, 3, 47–62. [Google Scholar] [CrossRef]
- Parker, S.; Chen, N.G.; Foster, S.; Hartzler, H.; Hembrador, E.; Hruby, D.; Jordan, R.; Lanier, R.; Painter, G.; Painter, W.; et al. Evaluation of Disease and Viral Biomarkers as Triggers for Therapeutic Intervention in Respiratory Mousepox—An Animal Model of Smallpox. Antivir. Res. 2012, 94, 44–53. [Google Scholar] [CrossRef]
- Baker, R.O.; Bray, M.; Huggins, J.W. Potential Antiviral Therapeutics for Smallpox, Monkeypox and Other Orthopoxvirus Infections. Antivir. Res. 2003, 57, 13–23. [Google Scholar] [CrossRef]
- Stittelaar, K.J.; Neyts, J.; Naesens, L.; van Amerongen, G.; van Lavieren, R.F.; Holý, A.; De Clercq, E.; Niesters, H.G.M.; Fries, E.; Maas, C.; et al. Antiviral Treatment Is More Effective than Smallpox Vaccination upon Lethal Monkeypox Virus Infection. Nature 2006, 439, 745–748. [Google Scholar] [CrossRef]
- Food and Drug Administration. FDA Approves Drug to Treat Smallpox. 6 April 2021. Available online: https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-drug-treat-smallpox (accessed on 20 March 2024).
- Semba, R.D. The Ocular Complications of Smallpox and Smallpox Immunization. Arch. Ophthalmol. 2003, 121, 715. [Google Scholar] [CrossRef] [PubMed]
- Simmons, W.F.; Chan, J.D.; Budak, J.Z.; Dhanireddy, S.; Green, M.L.; Jain, R.; Neme, S.; Rietberg, K.; Roxby, A.C.; Lynch, J.B.; et al. Antibiotic Prescribing Patterns for Bacterial Superinfection of Mpox: A Retrospective Cohort Study in an Urban Center. Antimicrob. Steward. Healthc. Epidemiol. ASHE 2023, 3, e108. [Google Scholar] [CrossRef]
- World Health Organization. Clinical Management and Infection Prevention and Control for Monkeypox. Interim Rapid Response Guidance. Geneva. 10 June 2022. Available online: https://iris.who.int/bitstream/handle/10665/355798/who-mpx-clinical_and_ipc-2022.1-eng.pdf?sequence=1 (accessed on 30 May 2024).
- Centers for Disease Control and Prevention (CDC). Mpox. Infection Control in Healthcare Settings. Available online: https://www.cdc.gov/mpox/hcp/infection-control/healthcare-settings.html?CDC_AAref_Val=https://www.cdc.gov/poxvirus/mpox/clinicians/infection-control-healthcare.html (accessed on 30 May 2024).
- Simpson, K.; Heymann, D.; Brown, C.S.; Edmunds, W.J.; Elsgaard, J.; Fine, P.; Hochrein, H.; Hoff, N.A.; Green, A.; Ihekweazu, C.; et al. Human Monkeypox—After 40 Years, an Unintended Consequence of Smallpox Eradication. Vaccine 2020, 38, 5077–5081. [Google Scholar] [CrossRef] [PubMed]
- Monath, T.P.; Caldwell, J.R.; Mundt, W.; Fusco, J.; Johnson, C.S.; Buller, M.; Liu, J.; Gardner, B.; Downing, G.; Blum, P.S.; et al. ACAM2000 Clonal Vero Cell Culture Vaccinia Virus (New York City Board of Health Strain)—A Second-Generation Smallpox Vaccine for Biological Defense. Int. J. Infect. Dis. 2004, 8 (Suppl. S2), S31–S44. [Google Scholar] [CrossRef]
- Reina, J.; Iglesias, C. Vaccines against Monkeypox. Med. Clin. (Engl. Ed.) 2023, 160, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Mpox. JYNNEOS Vaccine Additional Considerations for Intradermal Administration. Available online: https://www.cdc.gov/mpox/?CDC_AAref_Val=https://www.cdc.gov/poxvirus/mpox/interim-considerations/jynneos-vaccine.html (accessed on 30 May 2024).
- Pittman, P.R.; Hahn, M.; Lee, H.S.; Koca, C.; Samy, N.; Schmidt, D.; Hornung, J.; Weidenthaler, H.; Heery, C.R.; Meyer, T.P.H.; et al. Phase 3 Efficacy Trial of Modified Vaccinia Ankara as a Vaccine against Smallpox. N. Engl. J. Med. 2019, 381, 1897–1908. [Google Scholar] [CrossRef]
- Greenberg, R.N.; Hay, C.M.; Stapleton, J.T.; Marbury, T.C.; Wagner, E.; Kreitmeir, E.; Röesch, S.; von Krempelhuber, A.; Young, P.; Nichols, R.; et al. A Randomized, Double-Blind, Placebo-Controlled Phase II Trial Investigating the Safety and Immunogenicity of Modified Vaccinia Ankara Smallpox Vaccine (MVA-BN®) in 56-80-Year-Old Subjects. PLoS ONE 2016, 11, e0157335. [Google Scholar] [CrossRef]
- Bertran, M.; Andrews, N.; Davison, C.; Dugbazah, B.; Boateng, J.; Lunt, R.; Hardstaff, J.; Green, M.; Blomquist, P.; Turner, C.; et al. Effectiveness of One Dose of MVA-BN Smallpox Vaccine against Mpox in England Using the Case-Coverage Method: An Observational Study. Lancet Infect. Dis. 2023, 23, 828–835. [Google Scholar] [CrossRef]
- Eto, A.; Saito, T.; Yokote, H.; Kurane, I.; Kanatani, Y. Recent Advances in the Study of Live Attenuated Cell-Cultured Smallpox Vaccine LC16m8. Vaccine 2015, 33, 6106–6111. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.S.; Gurwith, M.; Dekker, C.L.; Frey, S.E.; Edwards, K.M.; Kenner, J.; Lock, M.; Empig, C.; Morikawa, S.; Saijo, M.; et al. Safety and Immunogenicity of LC16m8, an Attenuated Smallpox Vaccine in Vaccinia-Naive Adults. J. Infect. Dis. 2011, 204, 1395–1402. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Vaccines and Immunization for Monkeypox: Interim Guidance, 16 November 2022. Geneva. 16 November 2022. Available online: https://www.who.int/publications/i/item/who-mpx-immunization (accessed on 30 May 2024).
- Musumeci, S.; Laflamme, J.; Kaiser, L.; Segeral, O.; Calmy, A. Characteristics of Possible Mpox Reinfection Cases: Literature Review. J. Travel. Med. 2023, 30, taad136. [Google Scholar] [CrossRef]
- Keikha, M.; Abavisani, M.; Sahebkar, A. Common Clinical Features of Cases with Mpox Reinfection. Curr. Drug Targets 2023, 24, 1151–1154. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sanz, J.; Ponce-Alonso, M.; Martínez-García, L.; de la Cueva, V.; Olavarrieta, L.; Montero, L.; Pérez-Elías, M.J.; Galán, J.C. Description of Mpox Reinfection by Whole Genome Sequencing. Int. J. Infect. Dis. 2023, 137, 111–113. [Google Scholar] [CrossRef]
- Hazra, A.; Zucker, J.; Bell, E.; Flores, J.; Gordon, L.; Mitjà, O.; Suñer, C.; Lemaignen, A.; Jamard, S.; Nozza, S.; et al. Mpox in People with Past Infection or a Complete Vaccination Course: A Global Case Series. Lancet Infect. Dis. 2024, 24, 57–64. [Google Scholar] [CrossRef]
- Jiang, W.; Hu, Y.; Yang, X.; Hou, L.; Zhang, J.; Niu, H.; Hu, C.; Lin, J. Breakthrough Infection and Reinfection in Patients with Mpox. Rev. Med. Virol. 2024, 34, e2522. [Google Scholar] [CrossRef]
- Guagliardo, S.A.J.; Kracalik, I.; Carter, R.J.; Braden, C.; Free, R.; Hamal, M.; Tuttle, A.; McCollum, A.M.; Rao, A.K. Monkeypox Virus Infections After 2 Preexposure Doses of JYNNEOS Vaccine—United States, May 2022–May 2024. MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 460–466. [Google Scholar] [CrossRef]
- Allard, R.; Leclerc, P.; Bergeron, G.; Cadieux, G. Breakthrough Cases of Mpox: One-Dose Vaccination Is Associated with Milder Clinical Manifestations. J. Infect. Public Health 2024, 17, 676–680. [Google Scholar] [CrossRef]
- de Sousa, Á.F.L.; de Sousa, A.R.; Fronteira, I. Monkeypox: Between Precision Public Health and Stigma Risk. Rev. Bras. Enferm. 2022, 75, e750501. [Google Scholar] [CrossRef]
- Byanyima, W.; Lauterbach, K.; Kavanagh, M.M. Community Pandemic Response: The Importance of Action Led by Communities and the Public Sector. Lancet 2023, 401, 253–255. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.; Barnes-Balenciaga, J.; Osmundson, J.; Smith, M.D.R.; Tran, N.K.; Diamond, N.; Makofane, K. Global North Learning from Global South: A Community-Led Response to Mpox in New York City. PLOS Glob. Public Health 2023, 3, e0002042. [Google Scholar] [CrossRef] [PubMed]
- Phillips, G.; Curtis, M.G.; Felt, D.; Davoudpour, S.; Rodriguez-Ortiz, A.E.; Cortez, A.; French, A.L.; Hosek, S.G.; Serrano, P.A. Changes in Sexual Behaviors Due to Mpox: A Cross-Sectional Study of Sexual and Gender Minority Individuals in Illinois. Prev. Sci. 2024, 25, 628–637. [Google Scholar] [CrossRef]
- Low, N.; Bachmann, L.H.; Ogoina, D.; McDonald, R.; Ipekci, A.M.; Quilter, L.A.S.; Cevik, M. Mpox Virus and Transmission through Sexual Contact: Defining the Research Agenda. PLoS Med. 2023, 20, e1004163. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Mpox. Safer Sex, Social Gatherings, and Mpox. Available online: https://www.cdc.gov/mpox/prevention/safer-sex-social-gatherings-and-mpox.html?CDC_AAref_Val=https://www.cdc.gov/poxvirus/mpox/prevention/sexual-health.html (accessed on 30 May 2024).
- Ogunkola, I.O.; Abiodun, O.E.; Bale, B.I.; Elebesunu, E.E.; Ujam, S.B.; Umeh, I.C.; Tom-James, M.; Musa, S.S.; Manirambona, E.; Evardone, S.B.; et al. Monkeypox Vaccination in the Global South: Fighting a War without a Weapon. Clin. Epidemiol. Glob. Health 2023, 22, 101313. [Google Scholar] [CrossRef] [PubMed]
- United States Agency for International Development (USAID). United States Donation of 50,000 Mpox Vaccine Doses Arrives in the Democratic Republic of the Congo. 10 September 2024. Available online: https://www.usaid.gov/news-information/press-releases/sep-10-2024-united-states-donation-50000-mpox-vaccine-doses-arrives-democratic-republic-congo (accessed on 19 September 2024).
- European Commission. Directorate-General for Health and Food Safety. Mpox: HERA to Donate over 215,000 Vaccine Doses to Africa CDC amid Urgent Outbreak. 14 August 2024. Available online: https://health.ec.europa.eu/latest-updates/mpox-hera-donate-over-215000-vaccine-doses-africa-cdc-amid-urgent-outbreak-2024-08-14_en (accessed on 19 September 2024).
- World Health Organization. WHO Prequalifies the First Vaccine against Mpox. Geneva. 2024. Available online: https://www.who.int/news/item/13-09-2024-who-prequalifies-the-First-vaccine-against-mpox (accessed on 19 September 2024).
- Dimitrov, D.; Adamson, B.; Matrajt, L. Evaluation of Mpox Vaccine Dose-Sparing Strategies. PNAS Nexus 2023, 2, pgad095. [Google Scholar] [CrossRef]
- Wolff Sagy, Y.; Zucker, R.; Hammerman, A.; Markovits, H.; Arieh, N.G.; Abu Ahmad, W.; Battat, E.; Ramot, N.; Carmeli, G.; Mark-Amir, A.; et al. Real-World Effectiveness of a Single Dose of Mpox Vaccine in Males. Nat. Med. 2023, 29, 748–752. [Google Scholar] [CrossRef]
- May, T.; Towler, L.; Smith, L.E.; Horwood, J.; Denford, S.; Rubin, G.J.; Hickman, M.; Amlôt, R.; Oliver, I.; Yardley, L. Mpox Knowledge, Behaviours and Barriers to Public Health Measures among Gay, Bisexual and Other Men Who Have Sex with Men in the UK: A Qualitative Study to Inform Public Health Guidance and Messaging. BMC Public. Health 2023, 23, 2265. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Protopapas, K.; Dimopoulou, D.; Kalesis, N.; Akinosoglou, K.; Moschopoulos, C.D. Mpox and Lessons Learned in the Light of the Recent Outbreak: A Narrative Review. Viruses 2024, 16, 1620. https://doi.org/10.3390/v16101620
Protopapas K, Dimopoulou D, Kalesis N, Akinosoglou K, Moschopoulos CD. Mpox and Lessons Learned in the Light of the Recent Outbreak: A Narrative Review. Viruses. 2024; 16(10):1620. https://doi.org/10.3390/v16101620
Chicago/Turabian StyleProtopapas, Konstantinos, Dimitra Dimopoulou, Nikolaos Kalesis, Karolina Akinosoglou, and Charalampos D. Moschopoulos. 2024. "Mpox and Lessons Learned in the Light of the Recent Outbreak: A Narrative Review" Viruses 16, no. 10: 1620. https://doi.org/10.3390/v16101620
APA StyleProtopapas, K., Dimopoulou, D., Kalesis, N., Akinosoglou, K., & Moschopoulos, C. D. (2024). Mpox and Lessons Learned in the Light of the Recent Outbreak: A Narrative Review. Viruses, 16(10), 1620. https://doi.org/10.3390/v16101620







