Metamaterial Sensing of Cyanobacteria Using THz Thermal Curve Analysis
Abstract
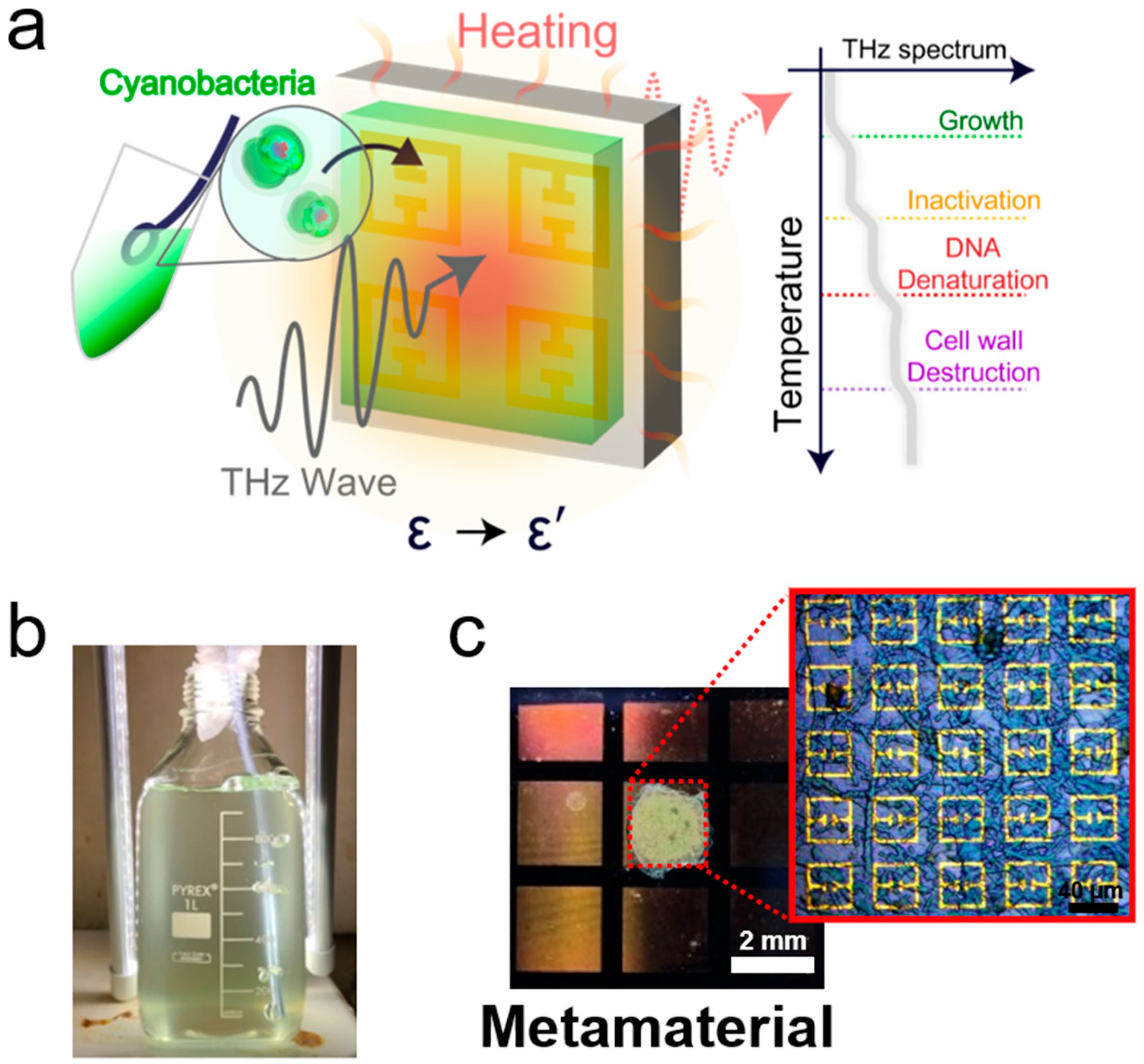
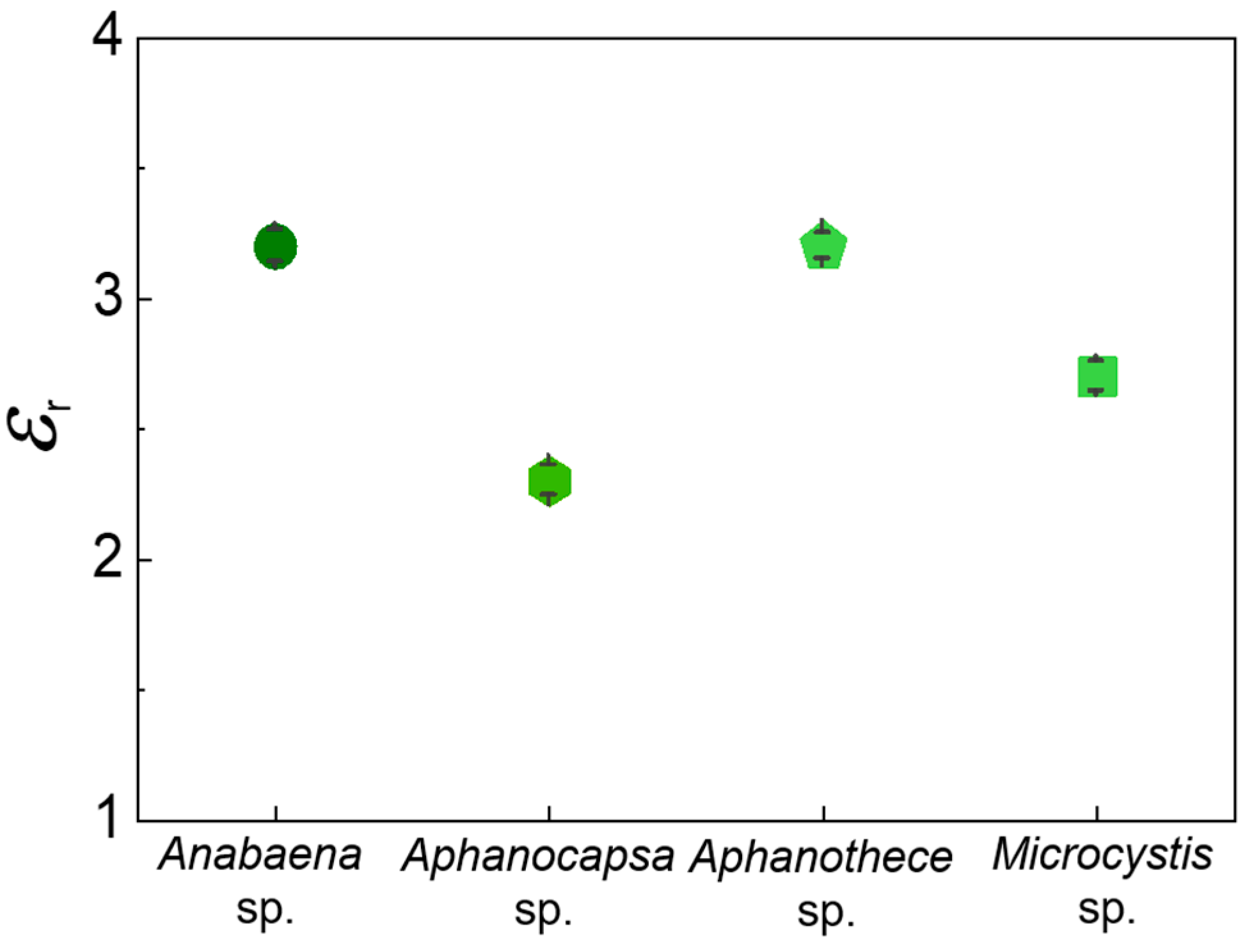
:1. Introduction
2. Experimental Setup
| Microorganism | Growth | Inactivation | DNA Denaturation | Cell Wall Destruction | Refs. |
|---|---|---|---|---|---|
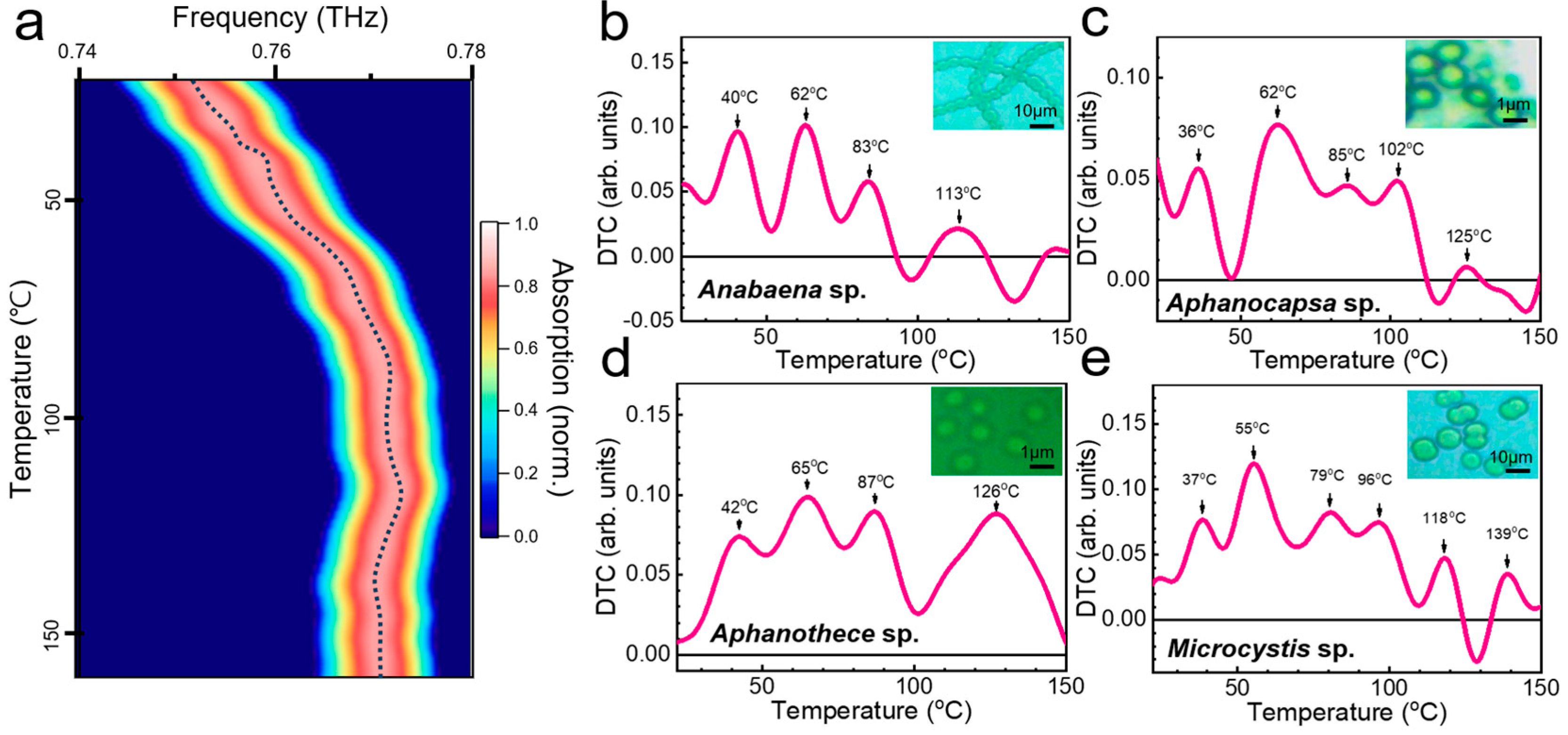
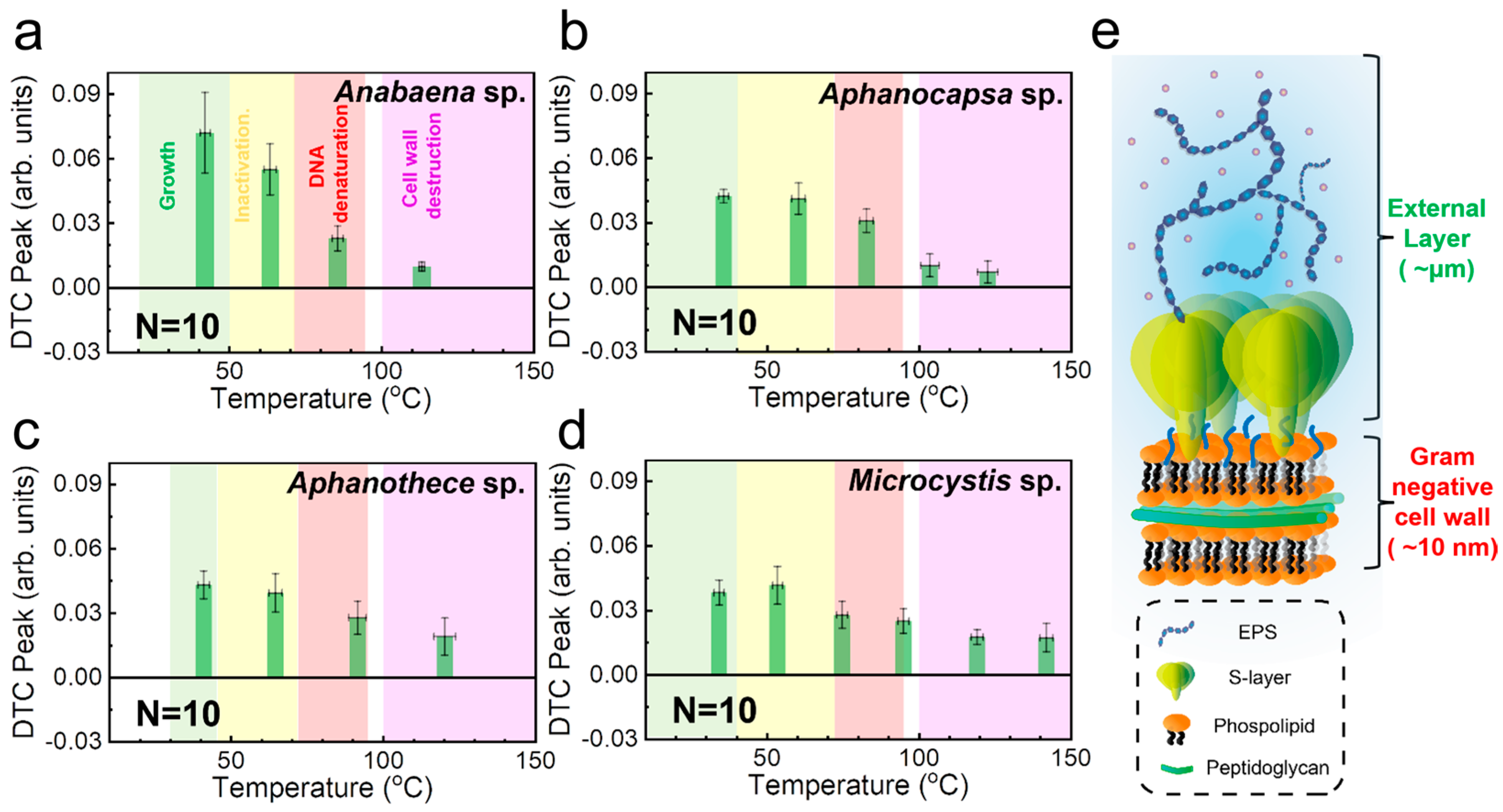
| Anabaena sp. | 42 °C (20–50 °C) | 64 °C (>50 °C) | 86 °C (72–94 °C) | 114 °C (>100 °C) | [61,62,63,64,65,66,67] |
| Aphanocapsa sp. | 36 °C (15–40 °C) | 60 °C (>40 °C) | 83 °C (72–94 °C) | 104, 123 °C (>100 °C) | [61,62,66,67,68,69] |
| Aphanothece sp. | 41 °C (30–45 °C) | 65 °C (>45 °C) | 92 °C (72–94 °C) | 121 °C (>100 °C) | [61,62,66,67,70] |
| Microcystis sp. | 34 °C (10–40 °C) | 54 °C (>40 °C) | 75, 95 °C (72–94 °C) | 119, 142 °C (>100 °C) | [61,62,66,67,71,72,73] |
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qian, K.; Chen, Y.; Song, X. Long-term development of phytoplankton dominant species related to eutrophicarion in Lake Taihu. Ecol. Sci. 2008, 27, 65–70. [Google Scholar]
- Padisak, J. Cylindrospermopsis raciborskii (Woloszynska) Seenayya et Subba Raju, an expanding, highly adaptive cyanobacterium: Worldwide distribution and review of its ecology. Arch. Hydrobiol. Suppl. 1997, 107, 563–593. [Google Scholar]
- Paerl, H.W.; Huisman, J. Blooms like it hot. Science 2008, 320, 57–58. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Visser, P.M.; Verspagen, J.M.; Sandrini, G.; Stal, L.J.; Matthijs, H.C.; Davis, T.W.; Paerl, H.W.; Huisman, J. How rising CO2 and global warming may stimulate harmful cyanobacterial blooms. Harmful Algae 2016, 54, 145–159. [Google Scholar] [CrossRef]
- Mudaliar, A.; Pandya, U. Assessment of Cyanobacterial Chlorophyll A as an Indicator of Water Quality in Two Wetlands Using Multi-Temporal Sentinel-2 Images. Environ. Sci. Proc. 2023, 25, 68. [Google Scholar] [CrossRef]
- Tiwari, P.; Misra, A.; Venturino, E. The role of algae in agriculture: A mathematical study. J. Biol. Phys. 2017, 43, 297–314. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, J.; Xiao, Y.; Zhang, Y.; Yu, Y.; Zheng, Z.; Liu, Y.; Li, Q. The impact of cyanobacteria blooms on the aquatic environment and human health. Toxins 2022, 14, 658. [Google Scholar] [CrossRef]
- Kunlasak, K.; Chitmanat, C.; Whangchai, N.; Promya, J.; Lebel, L. Relationships of dissolved oxygen with chlorophyll-a and phytoplankton composition in tilapia ponds. J. Geosci. 2013, 4, 46. [Google Scholar] [CrossRef]
- Lu, W.; Evans, E.H.; McColl, S.M.; Saunders, V.A. Identification of cyanobacteria by polymorphisms of PCR-amplified ribosomal DNA spacer region. FEMS Microbiol. Lett. 1997, 153, 141–149. [Google Scholar] [CrossRef]
- Rasmussen, U.; Svenning, M.M. Fingerprinting of cyanobacteria based on PCR with primers derived from short and long tandemly repeated repetitive sequences. Appl. Environ. Microbiol. 1998, 64, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Nübel, U.; Garcia-Pichel, F.; Muyzer, G. PCR primers to amplify 16S rRNA genes from cyanobacteria. Appl. Environ. Microbiol. 1997, 63, 3327–3332. [Google Scholar] [CrossRef] [PubMed]
- Ouellette, A.J.; Handy, S.M.; Wilhelm, S.W. Toxic Microcystis is widespread in Lake Erie: PCR detection of toxin genes and molecular characterization of associated cyanobacterial communities. Microb. Ecol. 2006, 51, 154–165. [Google Scholar] [CrossRef]
- Mariné, M.H.; Clavero, E.; Roldán, M. Microscopy methods applied to research on cyanobacteria. Limnetica 2004, 23, 179–186. [Google Scholar] [CrossRef]
- MacKeigan, P.W.; Garner, R.E.; Monchamp, M.-E.; Walsh, D.A.; Onana, V.E.; Kraemer, S.A.; Pick, F.R.; Beisner, B.E.; Agbeti, M.D.; da Costa, N.B. Comparing microscopy and DNA metabarcoding techniques for identifying cyanobacteria assemblages across hundreds of lakes. Harmful Algae 2022, 113, 102187. [Google Scholar] [CrossRef]
- Vuorio, K.; Mäki, A.; Salmi, P.; Aalto, S.L.; Tiirola, M. Consistency of targeted metatranscriptomics and morphological characterization of phytoplankton communities. Front. Microbiol. 2020, 11, 96. [Google Scholar] [CrossRef]
- Bauer, D.; Muüller, H.; Reich, J.; Riedel, H.; Ahrenkiel, V.; Warthoe, P.; Strauss, M. Identification of differentially expressed mRNA species by an improved disply technique (DDRT-PCR). Nucleic Acids Res. 1993, 21, 4272–4280. [Google Scholar] [CrossRef]
- Arya, M.; Shergill, I.S.; Williamson, M.; Gommersall, L.; Arya, N.; Patel, H.R. Basic principles of real-time quantitative PCR. Expert Rev. Mol. Diagn. 2005, 5, 209–219. [Google Scholar] [CrossRef]
- Kuno, G. Universal diagnostic RT-PCR protocol for arboviruses. J. Virol. Methods 1998, 72, 27–41. [Google Scholar] [CrossRef]
- Cheng, W.-C.; Horn, T.; Zayats, M.; Rizk, G.; Major, S.; Zhu, H.; Russell, J.; Xu, Z.; Rothman, R.E.; Celedon, A. Ultra-sensitive and rapid detection of nucleic acids and microorganisms in body fluids using single-molecule tethering. Nat. Commun. 2020, 11, 4774. [Google Scholar] [CrossRef]
- Park, S.; Cha, S.; Shin, G.; Ahn, Y. Sensing viruses using terahertz nano-gap metamaterials. Biomed. Opt. Express 2017, 8, 3551–3558. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Hong, J.; Choi, S.; Kim, H.; Park, W.; Han, S.; Park, J.; Lee, S.; Kim, D.; Ahn, Y. Detection of microorganisms using terahertz metamaterials. Sci. Rep. 2014, 4, 4988. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Son, B.; Choi, S.; Kim, H.; Ahn, Y. Sensitive detection of yeast using terahertz slot antennas. Opt. Express 2014, 22, 30467–30472. [Google Scholar] [CrossRef]
- Kim, H.S.; Cha, S.H.; Roy, B.; Kim, S.; Ahn, Y. Humidity sensing using THz metamaterial with silk protein fibroin. Opt. Express 2018, 26, 33575–33581. [Google Scholar] [CrossRef]
- Hong, J.; Jun, S.; Cha, S.; Park, J.; Lee, S.; Shin, G.; Ahn, Y. Enhanced sensitivity in THz plasmonic sensors with silver nanowires. Sci. Rep. 2018, 8, 15536. [Google Scholar] [CrossRef]
- Beruete, M.; Jáuregui-López, I. Terahertz sensing based on metasurfaces. Adv. Opt. Mater. 2020, 8, 1900721. [Google Scholar] [CrossRef]
- Nagel, M.; Haring Bolivar, P.; Brucherseifer, M.; Kurz, H.; Bosserhoff, A.; Büttner, R. Integrated THz technology for label-free genetic diagnostics. Appl. Phys. Lett. 2002, 80, 154–156. [Google Scholar] [CrossRef]
- Al-Naib, I.A.I.; Jansen, C.; Koch, M. Thin-film sensing with planar asymmetric metamaterial resonators. Appl. Phys. Lett. 2008, 93, 083507. [Google Scholar] [CrossRef]
- Chen, H.-T.; Padilla, W.J.; Zide, J.M.; Gossard, A.C.; Taylor, A.J.; Averitt, R.D. Active terahertz metamaterial devices. Nature 2006, 444, 597–600. [Google Scholar] [CrossRef]
- Tao, H.; Chieffo, L.R.; Brenckle, M.A.; Siebert, S.M.; Liu, M.; Strikwerda, A.C.; Fan, K.; Kaplan, D.L.; Zhang, X.; Averitt, R.D. Metamaterials on paper as a sensing platform. Adv. Mater. 2011, 23, 3197–3201. [Google Scholar] [CrossRef]
- O’Hara, J.F.; Withayachumnankul, W.; Al-Naib, I. A review on thin-film sensing with terahertz waves. J. Infrared Millim. Terahertz Waves 2012, 33, 245–291. [Google Scholar] [CrossRef]
- O’Hara, J.F.; Singh, R.; Brener, I.; Smirnova, E.; Han, J.; Taylor, A.J.; Zhang, W. Thin-film sensing with planar terahertz metamaterials: Sensitivity and limitations. Opt. Express 2008, 16, 1786–1795. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Cha, S.; Jun, S.; Park, S.; Park, J.-Y.; Lee, S.; Kim, H.; Ahn, Y. Identifying different types of microorganisms with terahertz spectroscopy. Biomed. Opt. Express 2020, 11, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.; Ahn, Y. Terahertz thermal curve analysis for label-free identification of pathogens. Nat. Commun. 2022, 13, 3470. [Google Scholar] [CrossRef]
- Novick, A. Growth of bacteria. Annu. Rev. Microbiol. 1955, 9, 97–110. [Google Scholar] [CrossRef]
- Smelt, J.; Brul, S. Thermal inactivation of microorganisms. Crit. Rev. Food Sci. Nutr. 2014, 54, 1371–1385. [Google Scholar] [CrossRef]
- Khandelwal, G.; Bhyravabhotla, J. A phenomenological model for predicting melting temperatures of DNA sequences. PLoS ONE 2010, 5, e12433. [Google Scholar] [CrossRef]
- Park, S.; Yoon, S.; Ahn, Y. Dielectric constant measurements of thin films and liquids using terahertz metamaterials. RSC Adv. 2016, 6, 69381–69386. [Google Scholar] [CrossRef]
- Park, S.; Jun, S.; Kim, A.; Ahn, Y. Terahertz metamaterial sensing on polystyrene microbeads: Shape dependence. Opt. Mater. Express 2015, 5, 2150–2155. [Google Scholar] [CrossRef]
- Hong, J.; Park, D.; Yim, J.; Park, J.; Park, J.-Y.; Lee, S.; Ahn, Y. Dielectric constant engineering of single-walled carbon nanotube films for metamaterials and plasmonic devices. J. Phys. Chem. Lett. 2013, 4, 3950–3957. [Google Scholar] [CrossRef]
- Park, D.; Hong, J.; Park, J.; Choi, S.; Son, B.; Rotermund, F.; Lee, S.; Ahn, K.; Kim, D.; Ahn, Y. Resonant transmission of terahertz waves through metallic slot antennas on various dielectric substrates. Curr. Appl. Phys. 2013, 13, 753–757. [Google Scholar] [CrossRef]
- Park, S.J.; Ahn, Y.H. Accurate measurement of THz dielectric constant using metamaterials on a quartz substrate. Curr. Opt. Photon. 2017, 1, 637–641. [Google Scholar]
- Siegel, J.A.; Saukko, P.J. Encyclopedia of Forensic Sciences; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Engqvist, M.K. Correlating enzyme annotations with a large set of microbial growth temperatures reveals metabolic adaptations to growth at diverse temperatures. BMC Microbiol. 2018, 18, 177. [Google Scholar] [CrossRef] [PubMed]
- Dewachter, L.; Verstraeten, N.; Fauvart, M.; Michiels, J. An integrative view of cell cycle control in Escherichia coli. FEMS Microbiol. Rev. 2018, 42, 116–136. [Google Scholar] [CrossRef] [PubMed]
- Jun, S. Polymer physics for understanding bacterial chromosomes. In Bacterial Chromatin; Springer: Dordrecht, The Netherlands, 2010; pp. 97–116. [Google Scholar]
- Russell, A. Lethal effects of heat on bacterial physiology and structure. Sci. Prog. 2003, 86, 115–137. [Google Scholar] [CrossRef]
- Jeong, H.; Bjorn, P.; Hong, S.; Cheon, S.; Oh, K. Irreversible denaturation of DNA: A method to precisely control the optical and thermo-optic properties of DNA thin solid films. Photonics Res. 2018, 6, 918–924. [Google Scholar] [CrossRef]
- Martínez de la Escalera, G.; Segura, A.M.; Kruk, C.; Ghattas, B.; Piccini, C. Genotyping and functional regression trees reveals environmental preferences of toxic cyanobacteria (Microcystis aeruginosa complex) along a wide spatial gradient. bioRxiv 2019. [Google Scholar] [CrossRef]
- Yu, L.; Wu, X.; Yu, Y.; Shi, L.; Zhang, M. Recruitment of cyanobacteria by reverse transcription quantitative real-time PCR based on expression of Microcystis gene. PeerJ 2019, 7, e7188. [Google Scholar] [CrossRef]
- Slade, L.; Levine, H. Non-equilibrium behavior of small carbohydrate-water systems. Pure Appl. Chem. 1988, 60, 1841–1864. [Google Scholar] [CrossRef]
- Thalla, M.; Gangasani, J.; Saha, P.; Ponneganti, S.; Borkar, R.M.; Naidu, V.; Murty, U.; Banerjee, S. Synthesis, characterizations, and use of o-stearoyl mannose ligand-engineered lipid nanoarchitectonics for alveolar macrophage targeting. Assay Drug Dev. Technol. 2020, 18, 249–260. [Google Scholar] [CrossRef]
- Mikami, K.; Lonnecker, A.T.; Gustafson, T.P.; Zinnel, N.F.; Pai, P.-J.; Russell, D.H.; Wooley, K.L. Polycarbonates derived from glucose via an organocatalytic approach. J. Am. Chem. Soc. 2013, 135, 6826–6829. [Google Scholar] [CrossRef] [PubMed]
- Mehta, K.K.; Evitt, N.H.; Swartz, J.R. Chemical lysis of cyanobacteria. J. Biol. Eng. 2015, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Laroche, C. Exopolysaccharides from microalgae and cyanobacteria: Diversity of strains, production strategies, and applications. Mar. Drugs 2022, 20, 336. [Google Scholar] [CrossRef]
- Rachel, R.; Pum, D.; Šmarda, J.; Šmajs, D.; Komrska, J.; Krzyzánek, V.; Rieger, G.; Stetter, K.O., II. Fine structure of S-layers. FEMS Microbiol. Rev. 1997, 20, 13–23. [Google Scholar] [CrossRef]
- Leak, L.V. Fine structure of the mucilaginous sheath of Anabaena sp. J. Ultrastruct. Res. 1967, 21, 61–74. [Google Scholar] [CrossRef]
- Kumar, D.; Kaštánek, P.; Adhikary, S.P. Exopolysaccharides from cyanobacteria and microalgae and their commercial application. Curr. Sci. 2018, 115, 234–241. [Google Scholar] [CrossRef]
- Ginzberg, A.; Korin, E.; Arad, S. Effect of drying on the biological activities of a red microalgal polysaccharide. Biotechnol. Bioeng. 2008, 99, 411–420. [Google Scholar] [CrossRef]
- Hoiczyk, E.; Hansel, A. Cyanobacterial cell walls: News from an unusual prokaryotic envelope. J. Bacteriol. 2000, 182, 1191–1199. [Google Scholar] [CrossRef]
- Zhang, D.; Dechatiwongse, P.; del Rio-Chanona, E.; Maitland, G.; Hellgardt, K.; Vassiliadis, V. Modelling of light and temperature influences on cyanobacterial growth and biohydrogen production. Algal Res. 2015, 9, 263–274. [Google Scholar] [CrossRef]
- Winder, L.; Phillips, C.; Richards, N.; Ochoa-Corona, F.; Hardwick, S.; Vink, C.J.; Goldson, S. Evaluation of DNA melting analysis as a tool for species identification. Methods Ecol. Evol. 2011, 2, 312–320. [Google Scholar] [CrossRef]
- Gallon, J.R.; Pederson, D.M.; Smith, G.D. The effect of temperature on the sensitivity of nitrogenase to oxygen in the cyanobacteria Anabaena cylindrica (Lemmermann) and Gloeothece (Nägeli). New Phytol. 1993, 124, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Riego, A.M.; Mata-Cabana, A.; Galmozzi, C.V.; Florencio, F.J. NADPH-thioredoxin reductase C mediates the response to oxidative stress and thermotolerance in the cyanobacterium Anabaena sp. PCC7120. Front. Microbiol. 2016, 7, 1283. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sivonen, K.; Rouhiainen, L.; Fewer, D.P.; Lyra, C.; Rantala-Ylinen, A.; Vestola, J.; Jokela, J.; Rantasärkkä, K.; Li, Z. Genome-derived insights into the biology of the hepatotoxic bloom-forming cyanobacterium Anabaena sp. strain 90. BMC Genom. 2012, 13, 613. [Google Scholar] [CrossRef] [PubMed]
- Kaplan Can, H.; Gurbuz, F.; Odabaşı, M. Partial characterization of cyanobacterial extracellular polymeric substances for aquatic ecosystems. Aquat. Ecol. 2019, 53, 431–440. [Google Scholar] [CrossRef]
- Supeng, L.; Guirong, B.; Hua, W.; Fashe, L.; Yizhe, L. TG-DSC-FTIR analysis of cyanobacteria pyrolysis. Phys. Procedia 2012, 33, 657–662. [Google Scholar] [CrossRef]
- Loza, V.; Perona, E.; Mateo, P. Molecular fingerprinting of cyanobacteria from river biofilms as a water quality monitoring tool. Appl. Environ. Microbiol. 2013, 79, 1459–1472. [Google Scholar] [CrossRef]
- Shyam Kumar, R.; Thajuddin, N. Influence of temperature and light intensity on growth of symbiotic cyanobacteria isolated from cyanolichens. Res. Rev. Biosci. 2009, 3, 179–182. [Google Scholar]
- Arif, I. ALGAL DISTRIBUTIONS IN A HOT-SPRING OF SAUDI-ARABIA. Arab Gulf J. Sci. Res. B 1989, 7, 145–154. [Google Scholar]
- Guo, Y.; Meng, H.; Zhao, S.; Wang, Z.; Zhu, L.; Deng, D.; Liu, J.; He, H.; Xie, W.; Wang, G. How does Microcystis aeruginosa respond to elevated temperature? Sci. Total Environ. 2023, 889, 164277. [Google Scholar] [CrossRef]
- Celeste, C.M.M.; Lorena, R.; Oswaldo, A.J.; Sandro, G.; Daniela, S.; Dario, A.; Leda, G. Mathematical modeling of Microcystis aeruginosa growth and [D-Leu1] microcystin-LR production in culture media at different temperatures. Harmful Algae 2017, 67, 13–25. [Google Scholar] [CrossRef]
- Bittencourt-Oliveira, M.d.C.; Piccin-Santos, V.; Gouvêa-Barros, S. Microcystin-producing genotypes from cyanobacteria in Brazilian reservoirs. Environ. Toxicol. 2012, 27, 461–471. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, T.H.; Jun, S.W.; Ahn, Y.H. Metamaterial Sensing of Cyanobacteria Using THz Thermal Curve Analysis. Biosensors 2024, 14, 519. https://doi.org/10.3390/bios14110519
Jeong TH, Jun SW, Ahn YH. Metamaterial Sensing of Cyanobacteria Using THz Thermal Curve Analysis. Biosensors. 2024; 14(11):519. https://doi.org/10.3390/bios14110519
Chicago/Turabian StyleJeong, Tae Hee, Seung Won Jun, and Yeong Hwan Ahn. 2024. "Metamaterial Sensing of Cyanobacteria Using THz Thermal Curve Analysis" Biosensors 14, no. 11: 519. https://doi.org/10.3390/bios14110519
APA StyleJeong, T. H., Jun, S. W., & Ahn, Y. H. (2024). Metamaterial Sensing of Cyanobacteria Using THz Thermal Curve Analysis. Biosensors, 14(11), 519. https://doi.org/10.3390/bios14110519





