Abstract
Irradiation of 1-substituted benzotriazole arylhydrazones 3a–c, 4a,b and 5a,b with a 16 W low pressure mercury arc-lamp (254 nm) for 24 h gave phenanthridin-6-yl-2-phenyldiazines 9a–c, phenanthridin-6(5H)-ones 10a–c, 1-anilinobenzimidazoles 11a–c, 2-aryl-1H-benzimidazoles 12a–c, 1-arylamino-1H-benzimidazol-2-carboxylic acid ethyl esters 14a,b, 1-aryl-1H, 9H-benzo [4,5][1,2,3] triazolo[1,2-a]tetrazole-3-carboxylic acid ethyl esters 16a,b, 1-arylamino-2-benzoylbenzimidazoles 18a,b and 2-benzoylbenzoxazole 21.
1. Introduction
The behavior of benzotriazole and its derivatives under pyrolytic and photolytic conditions has already received considerable attention. In particular, 1-substituted-1H-benzotriazoles pyrolyze via elimination of N2 to give a 1,3-diradical intermediate which can interact with aromatic or unsaturated substituents to give cyclic and rearranged products [1,2,3,4,5,6,7,8,9,10]. In the earlier paper in this series, we have reported the photolysis of N1-vinylsubstituted benzotriazole derivatives 1 into 2-acyl-3-dimethyl-aminoindoles 2 [11].
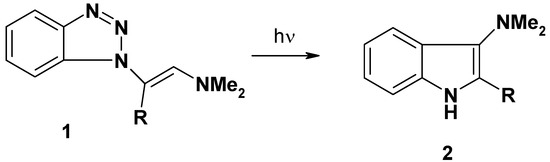
Scheme 1.
Photolysis of N1-vinylsubstituted benzotriazoles 1 into 2-acylindoles 2.
Scheme 1.
Photolysis of N1-vinylsubstituted benzotriazoles 1 into 2-acylindoles 2.
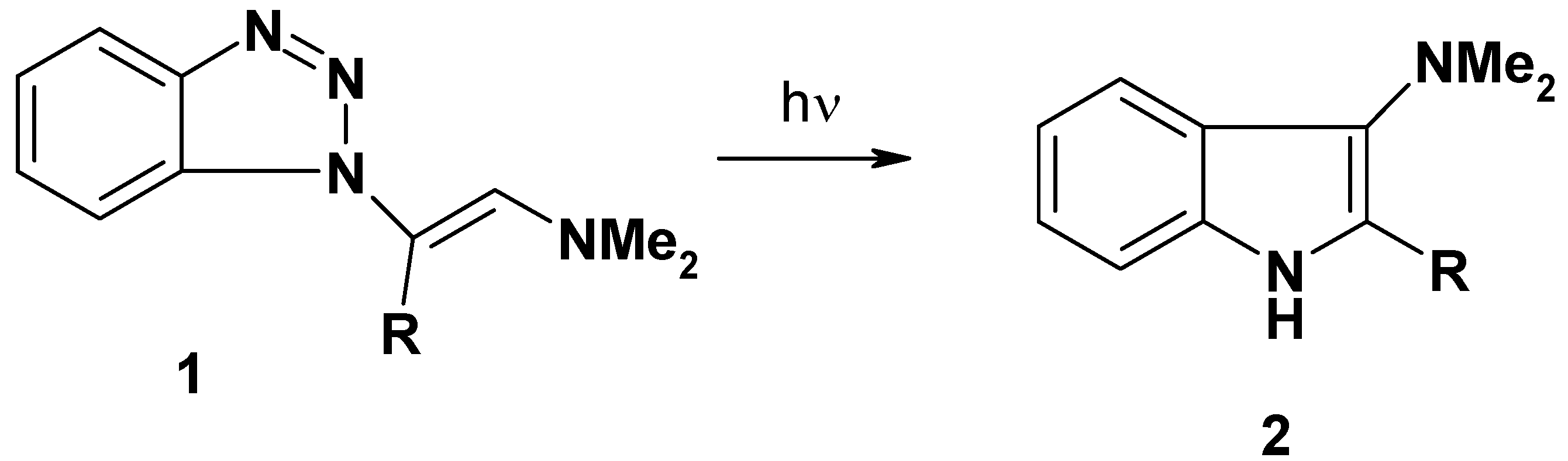
This investigation is now extended to include seven 1-substituted benzotriazole arylhydrzones 3a–c, 4a,b and 5a,b (Figure 1).
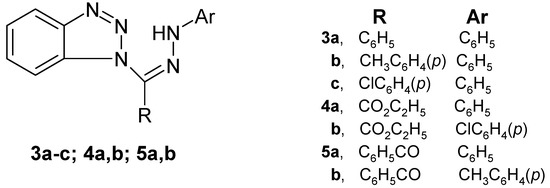
Figure 1.
1-substituted benzotriazole arylhydrzones 3a–c, 4a,b and 5a,b.
Figure 1.
1-substituted benzotriazole arylhydrzones 3a–c, 4a,b and 5a,b.
2. Results and Discussion
Compounds 3a–c and 5a,b were obtained by the procedures described earlier and were fully characterized [12,13,14]. The UV spectra of these compounds display two absorption maxima in the region 243–392 nm wavelength regions.
Irradiation of compounds 3a–c in a quartz tube with a 16 W low pressure mercury arc-lamp (254 nm) in acetonitrile for 24 hours at room temperature produced phenanthridin-6-yl-2-phenyldiazines 9a–c (48–51%), phenanthridin-6(5H)-ones 10a–c (15–18%), 1-anilino-2-arylbenzimidazoles 11a–c (10–14%) and 2-aryl-1H-benzimidazoles 12a–c (8–10%). The formation of these products can be explained by a mechanistic pathway presented in Scheme 2, involving initial photo-extrusion of N2 to form the corresponding diradical intermediate 6, which cyclizes to diradical 7 and is then converted to 8, and the latter is photooxidized to 9a–c. Partial hydrolysis of 9a–c in the reaction media produces 10a–c. Concurrently, cyclization of the tautomer of diradical intermediate 6 affords compounds 11a–c which are further converted to 12a–c. To confirm that 9a–c and 11a–c are the likely precursors of 10a–c and 12a–c respectively, 9a and 11a were isolated and then subjected to irradiation for 24 hours. In each case the corresponding reaction products 10a and 12a were obtained in quantitative yield. The structures of all isolated products were confirmed based on their full 1H-NMR, 13C-NMR, and mass spectral data. Moreover, an X-ray crystal structure of compound 9a was obtained (Figure 2 and Table 1).
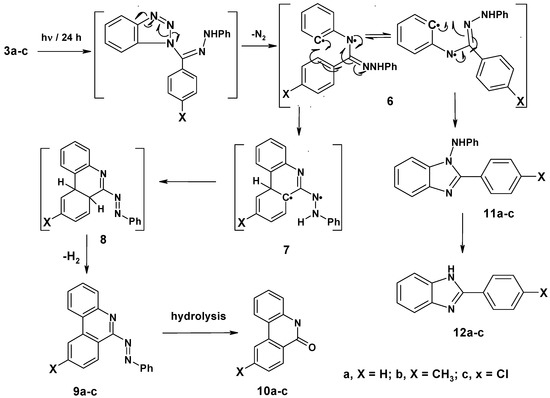
Scheme 2.
Mechanism of photolysis of N-(benzotriazol-1-yl-phenylmethylene)-N′-arylhydrazines 3a–c.
Scheme 2.
Mechanism of photolysis of N-(benzotriazol-1-yl-phenylmethylene)-N′-arylhydrazines 3a–c.
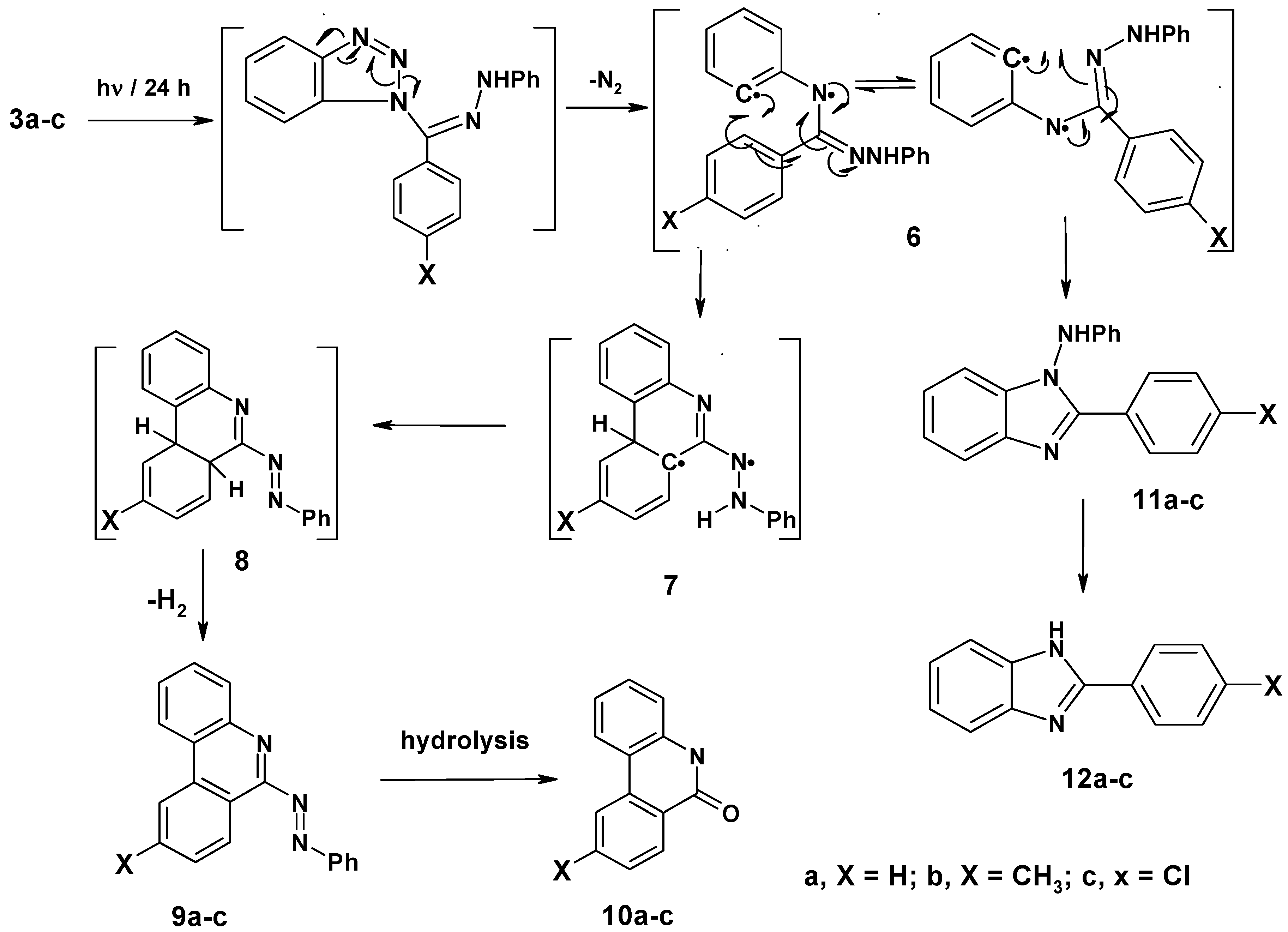
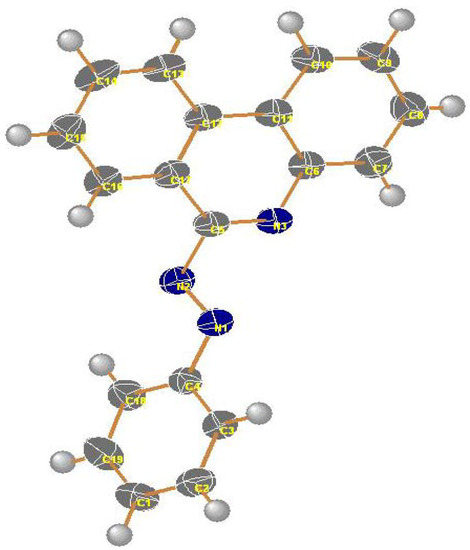
Figure 2.
X-ray structure of compound 9a (thermal ellipsoids).
Figure 2.
X-ray structure of compound 9a (thermal ellipsoids).

Table 1.
Selected bond lengths and bond angles for compound 9a.
| Bond | Bond lengths (Å) | Bond | Bond angles (Å) |
|---|---|---|---|
| N1-N2 | 1.2462 (13) | N1-N2-C5 | 112.58 (9) |
| N2-C5 | 1.4368 (14) | N3-C5-N2 | 117.90 (10) |
| N3-C6 | 1.3857 (15) | N2-C5-N17 | 116.62 (10) |
| N3-C5 | 1.2949 (15) | C5-N3-C6 | 117.99 (10) |
| C5-C17 | 1.4400 (16) | C6-C11-C12 | 118.27 (11) |
| N1-C4 | 1.4286 (14) | C10-C11-C12 | 124.11 (11) |
| C11-C12 | 1.4450 (18) | N3-C6-C11 | 122.68 (11) |
Benzotriazol-1-yl-(2-arylhydrazono) acetic acid ethyl esters 4a,b were prepared in 75−77% yield by stirring 1,2,3-benzotriazole with ethyl 2-chloro-2-(2-arylhydrazono)acetate in DCM/TEA for 24 hours at room temperature. Irradiation of 4a,b in acetonitrile for 24 hour afforded 1-arylamino-1H-benzimidazol-2-carboxylic acid ethyl esters 14a,b (45−48% yield) and 1-aryl-1H,9H-benzo[4,5][1,2,3]triazolo[1,2-a]tetrazole-3-carboxylic acid ethyl esters 16a,b (35−36% yield). It is assumed that initial N2 extrusion affords the biradical intermediate 13 which then cyclized into 14a,b. On the other hand, formation of photoproducts 16a,b may be explained by assuming a intramolecular 1,6-H shift (Scheme 3). Single crystal X-ray structure analysis (Figure 3 and Figure 4, Table 2) confirmed the structures of new compounds 4b and 14b.
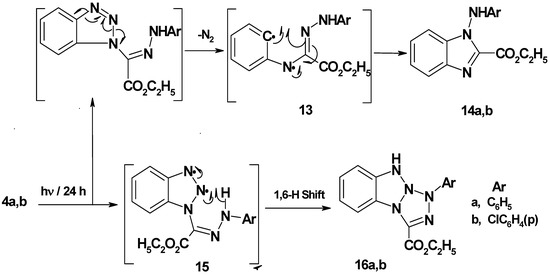
Scheme 3.
Mechanism of photolysis of benzotriazol-1-yl-(2-arylhydrazono)-acetic acid ethyl esters 4a,b.
Scheme 3.
Mechanism of photolysis of benzotriazol-1-yl-(2-arylhydrazono)-acetic acid ethyl esters 4a,b.
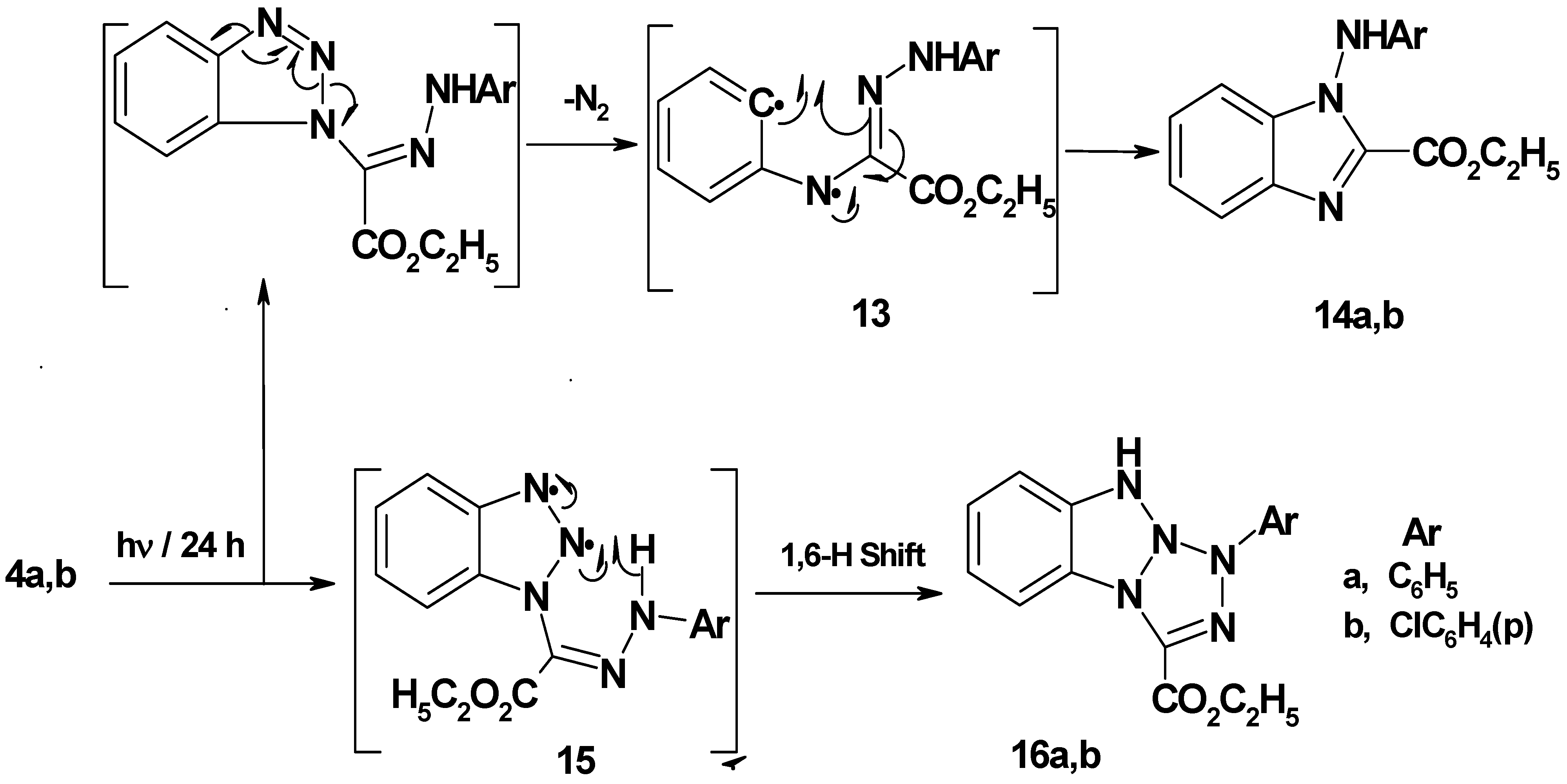
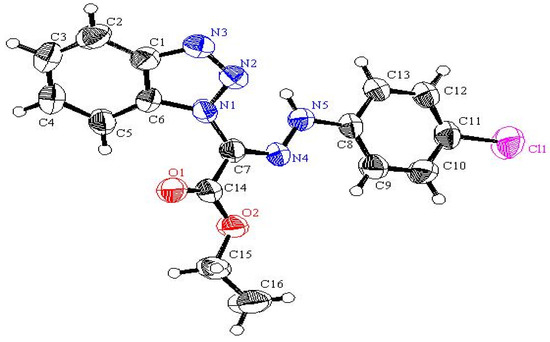
Figure 3.
ORTEP drawing of 4b.
Figure 3.
ORTEP drawing of 4b.
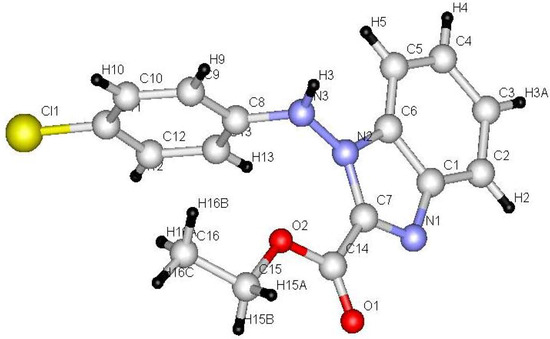
Figure 4.
Ball and stick drawing of 14b.
Figure 4.
Ball and stick drawing of 14b.
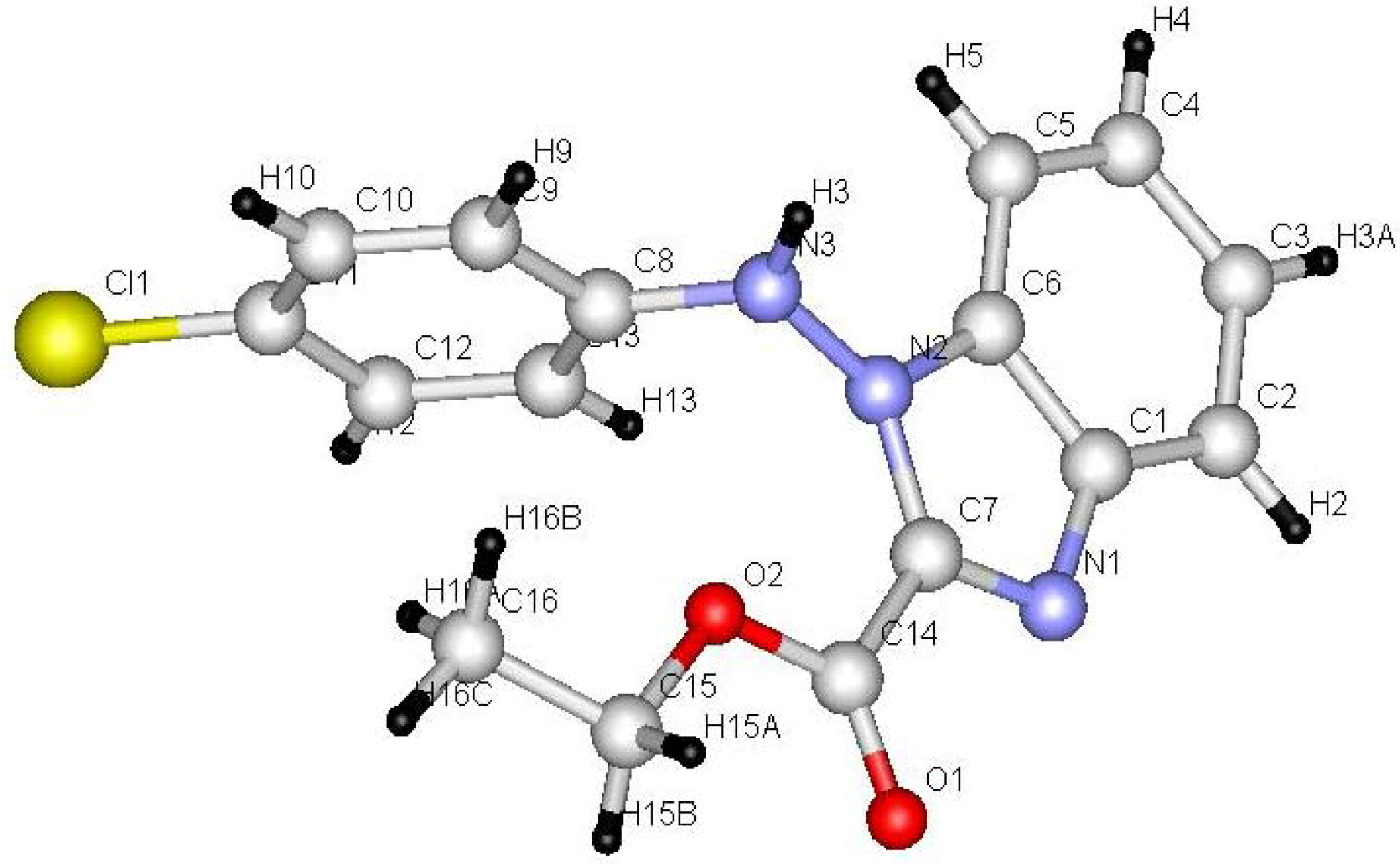

Table 2.
Selected bond lengths and bond angles for compounds 4b and 14b.
| 4b Bond | Bond lengths (Å) | Bond | Bond angles (°) | 14b Bond | Bond lengths (Å) | Bond | Bond angles (°) |
|---|---|---|---|---|---|---|---|
| N1-C6 | 1.375 (6) | N1-C6-C1 | 104.8 (4) | N1-C1 | 1.380 (10) | C1-N1-C7 | 104.4 (6) |
| N1-C7 | 1.409 (6) | N1-C7-C14 | 117.1 (4) | N1-C7 | 1.318 (10) | N1-C7-N2 | 114.1 (7) |
| N4-C7 | 1.301 (6) | N1-C7-N4 | 125.3 (4) | N2-C7 | 1.375 (9) | N3-N2-C7 | 130.6 (6) |
| C7-C14 | 1.477 (7) | N1-N2-N3 | 108.5 (4) | N2-N3 | 1.372 (9) | N2-C6-C5 | 131.6 (8) |
| N5-C8 | 1.408 (6) | N2-N1-C7 | 120.3 (4) | N2-C6 | 1.405 (10) | N2-C7-C14 | 126.8 (7) |
| N4-N5 | 1.328 (6) | N5-C8-C13 | 118.2 (5) | C7-C14 | 1.495 (11) | N1-C1-C6 | 111.1 (7) |
| N3-C1 | 1.386 (7) | N3-C1-C6 | 109.2 (4) | N3-C8 | 1.409 (10) | O2-C14-C7 | 112.7 (7) |
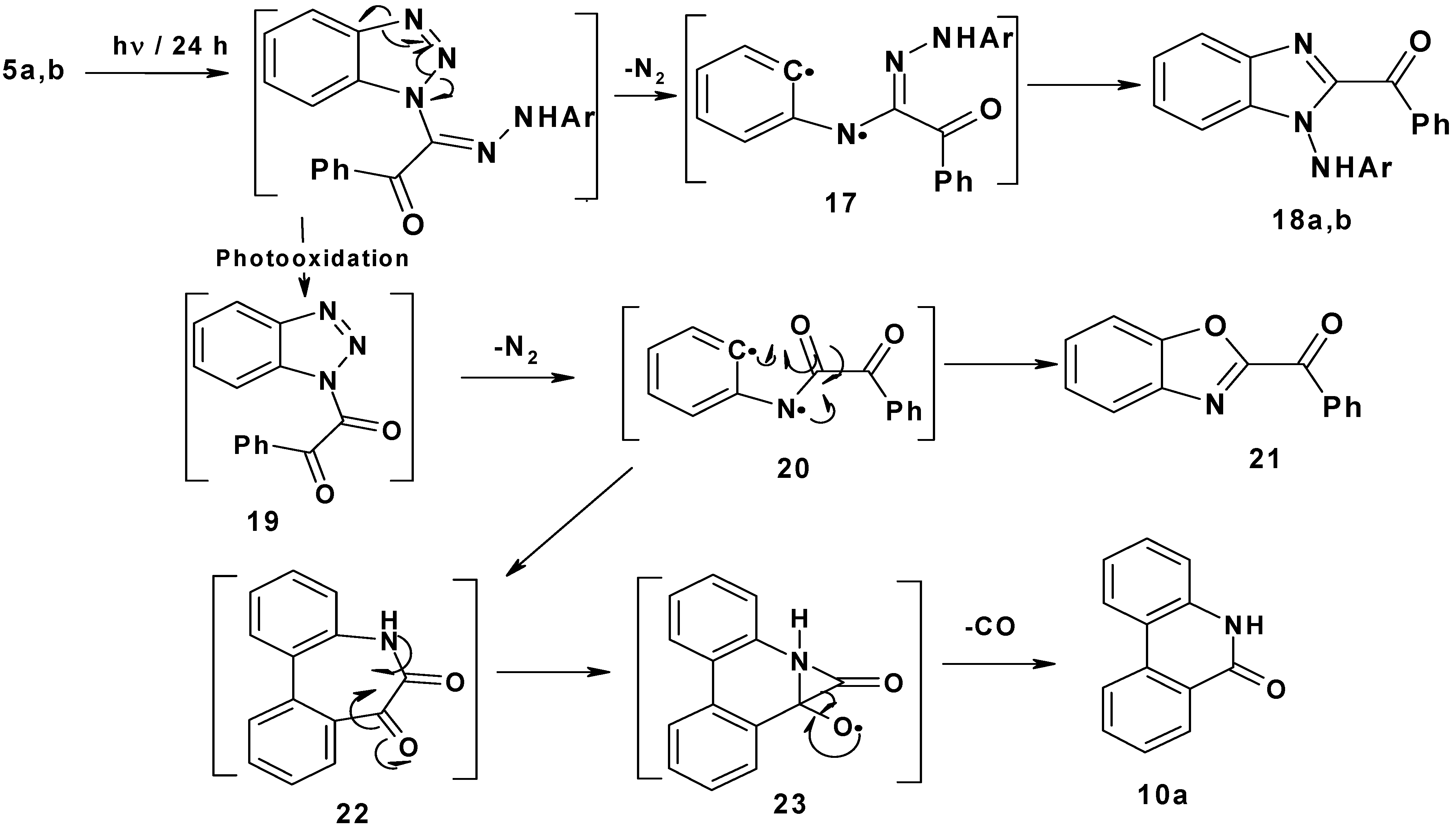
Finally, irradiation of 2-arylhydrazono-2-(benzotriazol-1-yl)-1-phenylethanones 5a,b afforded 1-arylamino-2-benzoylbenzimidazoles 18a,b (25–30%), 2-benzoylbenzoxazole 21 (24–27%) and phenantheridin-6(5H)-one 10a (15–17%). The suggested mechanism proposed for this photoreaction is shown in Scheme 4. Initial photo-extrusion of N2 forms the corresponding diradical intermediate 17, followed by cyclization to yield 18a,b. On the other hand, photooxidation of 5a,b afforded 1-benzotriazole-2-phenylethan-1,2-dione 19, which upon elimination of N2 formed diradical 20, which either cyclizes to yield 2-benzoylbenzoxazole 21 in (24–27%) or cyclizes to 22, which spontaneously loses CO through intermediate 23 to produce phenanthradin-6(5H)-one 10a in 15–17% yield.
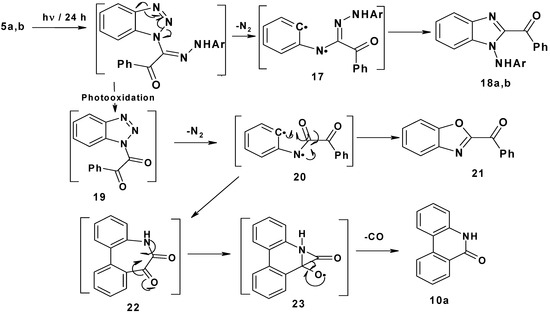
Scheme 4.
Mechanism of photolysis of 2-arylhydrazono-2-(benzotriazol-1-y)-1-phenylethanones 5a,b.
Scheme 4.
Mechanism of photolysis of 2-arylhydrazono-2-(benzotriazol-1-y)-1-phenylethanones 5a,b.
The structure of all new compounds were assigned by spectroscopic and analytical methods. The structure of 21 is readily assigned based on 2D-NMR results. The 1H- and 13C-NMR signal assignments and the H-C correlation from the HMBC 2-D experiments are displayed in Figure 5. Table 3 summarizes the absorption maxima (λmax) and the photoproducts of substrates 3a–c, 4a,b and 5a,b. The fact that the substituents R affected the nature of the products much more than the substituted Ar may be attributed to the strong influence of the substituents on the formed biradicals.
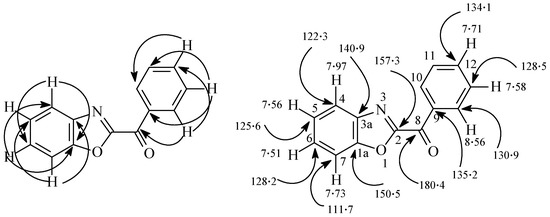
Figure 5.
H-C Correlations in the HMBC 2-D experimental of compound 21.
Figure 5.
H-C Correlations in the HMBC 2-D experimental of compound 21.
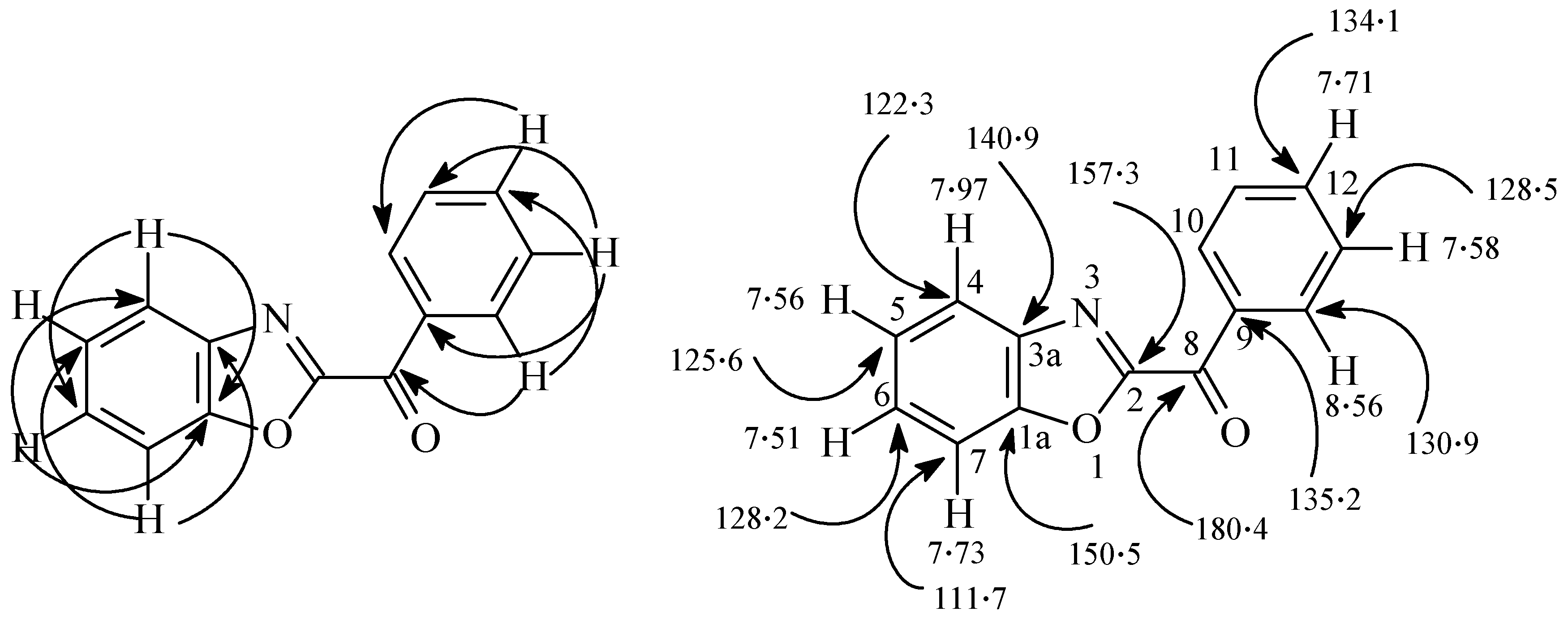

Table 3.
Photoproducts formed by irradiation of compounds 3a–c, 4a,b and 5a,b and yield.
| Comp | R | Ar | λmax | Photo-products and yields (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 9 | 10 | 11 | 12 | 14 | 16 | 18 | 21 | ||||
| 3a | C6H5 | C6H5 | 244, 338 | 51 | 16 | 12 | 10 | - | - | - | - |
| 3b | CH3C6H4(p) | C6H5 | 247, 347 | 48 | 18 | 14 | 8 | - | - | - | - |
| 3c | ClC6H4(p) | C6H5 | 243, 392 | 50 | 15 | 10 | 9 | - | - | - | - |
| 4a | CO2C2H5 | C6H5 | 244, 370 | - | - | - | - | 48 | 36 | - | - |
| 4b | CO2C2H5 | ClC6H4(p) | 245, 355 | - | - | - | - | 45 | 35 | - | - |
| 5a | COC6H5 | C6H5 | 253, 345 | - | 15 | - | - | - | - | 25 | 27 |
| 5b | COC6H5 | CH3C6H4(p) | 256, 379 | - | 17 | - | - | - | 30 | 24 | |
3. Experimental
3.1. General
All melting points were recorded on a Gallenkamp apparatus. IR spectra were recorded using KBr pellets on a Perkin-Elmer System 2000 FT-IR spectrophotometer. 1H- and 13C-NMR spectra were recorded on Bruker DPX 400 MHz or AvanceII 600 MHz super-conducting NMR spectrometers with proton spectra measured at 400, 600 MHz and carbon spectra at 100 and 150 MHz, respectively. Mass spectra were measured on a VG Auto-spec-Q (high resolution, high performance, tri-sector GC/MS/MS) and with LCMS using Agilent 1100 series LC/MSD with an API-ES/APCI ionization mode. Microanalyses were performed on LECO CH NS-932 Elemental Analyzer. The UV/VIS absorption spectra were recorded using a Varian Cary 5 instrument in the wave length range 200–450 nm using dry clean quartz cuvette of 1.0 cm path length. X-Ray analysis were performed using a Rigaku Rapid II diffractometer.
3.2. Synthesis of Starting Compounds 4a,b
To a solution of 1,2,3-benzotriazole (3.57 g, 30 mmol), in DCM (50 mL), TEA (5 drops) and ethyl 2-chloro-2-(2-arylhydrazono)acetate (30 mmol) were added [15]. The mixture was stirred at room temperature for 24 hours, and then diluted with CH2Cl2 (150 mL), washed successively with dil. HCl (6 M, 20 mL), satd. aq. NaHCO3 (150 mL), and water and then dried over anhydrous Na2SO4. The solvent was then evaporated in vacuo, and the residue was crystallized from ethanol to give 4a,b.
Benzotriazol-1-yl-phenylhydrazono-acetic acid ethyl ester (4a). Colorless crystals, yield 7.2 g (77%), m.p. 183–184 °C. MS: m/z (%) = 309 (M+, 15), 281 (35), 208 (80). IR (KBr, cm−1): 3060, 2982, 1683, 1598, 1540, 1501, 1414, 1312, 1235, 1187, 1019, 759. 1H-NMR (400 MHz, CDCl3): δ 9.69 (br, 1H, NH), 8.01 (d, 1H, J = 8.4 Hz), 7.59 (t, 1H, J = 8.0 Hz), 7.49 (d, 1H, J = 8.4 Hz), 7.43 (t, 1H, J = 8.0 Hz), 7.38–7.32 (m, 4H), 7.09 (t, 1H, J = 8.4 Hz), 4.41 (q, 2H, J = 7.0 Hz), 1.40 ppm (t, 3H, J = 7.0 Hz). 13C-NMR (100 MHz, CDCl3): δ 160.3, 144.9, 141.6, 132.5, 129.4, 128.9, 125.0, 123.8, 119.9, 118.5, 115.0, 111.2, 62.3, 14.3 ppm. Anal. Calcd. for C16H15N5O2 (309.3): C, 62.13; H, 4.89; N, 22.64. Found: C, 62.10; H, 4.80; N, 22.55.
Benzotriazol-1-yl-p-chlorophenylhydrazono-acetic acid ethyl ester (4b). Pale yellow crystals, yield 7.80 g (75%), m.p. 156–158 °C. MS: m/z (%) = 343 (M+, 10), 315 (30), 286 (35). IR (KBr, cm−1): 3094, 2986, 1686, 1571, 1547, 1489, 1401, 1282, 1235, 1172, 1148, 1092, 1061, 832. 1H-NMR (400 MHz, CDCl3): δ 9.77 (br, 1H, NH), 8.03 (d, 1H, J = 8.4 Hz), 7.60 (dt, 1H, J = 7.8, 1.2 Hz), 7.49 (d, 1H, J = 8.4 Hz), 7.44 (dt, 1H, J = 7.8, 1.2 Hz), 7.32 (d, 2H, J = 8.4 Hz), 7.24 (d, 2H, J = 8.4 Hz), 4.40 (q, 2H, J = 6.8 Hz), 1.24 ppm (t, 3H, J = 6.8 Hz). 13C-NMR (100 MHz, CDCl3): δ 160.1, 145.0, 140.4, 132.4, 129.5, 129.1, 128.7, 125.2, 120.0, 119.1, 116.2, 111.3, 62.4, 14.3 ppm. (HRMS = 343.0831, requires C16H14ClN5O2 343.0830).
3.3. Irradiation Using a Low Pressure Mercury Arc-Lamp
Each of the substrates 3a–c, 4a,b and 5a,b (10.0 mmol) was dissolved in acetonitrile (250 mL) in a number of quartz tubes (10 × 25 mL) and introduced to irradiate for 24 hours at room temperature (RT). The progress of each reaction was monitored by using TLC. The solvent was removed in vacuo and the resulting residue was subjected to column chromatography on silica gel using ethyl acetate/ petroleum ether (b.p. 60–80 °C) as the eluent to give the corresponding products.
Phenanthridin-6-yl-2-phenyldiazine (9a). Red crystals from ethanol, m.p. 158–160 °C. MS: m/z (%) = 283 (M+, 10), 254 (100), 178 (70). IR (KBr, cm−1): 3061, 3004, 2957, 1611, 1562, 1527, 1484, 1349, 1193, 925, 763. 1H-NMR (600 MHz, DMSO-d6): δ 8.98 (d, 1H, J = 7.8 Hz), 8.88 (d, 1H, J = 8.4 Hz), 8.55 (d, 1H, J = 8.0 Hz), 8.18 (d, 1H, J = 8.0 Hz), 8.15 (dd, 2H, J = 7.8, 1.2 Hz), 8.07 (t, 1H, J = 7.6 Hz), 7.88 (t, 1H, J = 7.8 Hz), 7.84 (t, 1H, J = 7.6 Hz), 7.81 (t, 1H, J = 7.6 Hz), 7.73–7.70 ppm (m, 3H). 13C-NMR (150 MHz, DMSO-d6): δ 159.6, 152.5, 142.4, 133.8, 133.0, 131.9, 130.5, 129.7, 129.6, 128.3, 127.9, 125.7, 124.5, 123.4, 123.0, 122.9 122.1 ppm. Anal. Calc. for C19H13N3 (283.3): C, 80.54; H, 4.62; N, 14.83. Found: C, 80.50; H, 4.60; N, 14.79.
9-Methylphenanthridin-6-yl-2-phenyldiazine (9b). Red crystals from ethanol, m.p. 140–142 °C. MS: m/z (%) = 297 (M+, 10), 268 (100), 192 (40). IR (KBr, cm−1): 3056, 2918, 1617, 1509, 1483, 1373, 1308, 1189, 1022, 822, 760. 1H-NMR (400 MHz, CDCl3): δ 8.62 (dd, 2H, J = 8.4, 2.0 Hz), 8.50 (s, 1H), 8.31 (d, 1H, J = 8.0 Hz), 8.21 (dd, 2H, J = 8.4, 2.0 Hz), 7.76 (t, 1H, J = 7.8 Hz), 7.70 (t, 1H, J = 7.8 Hz), 7.63–7.58 (m, 4H), 2.70 ppm (s, 3H, CH3). 13C-NMR (150 MHz, DMSO-d6): δ 159.5, 153.3, 143.3, 141.9, 134.9, 132.4, 131.6, 129.4, 129.2, 129.1, 127.4, 126.3, 125.0, 124.0, 122.1, 121.9, 121.7, 22.5 ppm. Anal. Calc. for C20H15N3 (297.4): C, 80.78; H, 5.08; N, 14.13. Found: C, 80.70; H, 5.00; N, 14.10.
9-Chlorophenanthridin-6-yl-2-phenyldiazine (9c). Red crystals from ethanol, m.p. 136–138 °C. MS: m/z (%) = 319 (M+2, 10), 317 (M+, 20), 268 (100). IR (KBr, cm−1): 3062, 3007, 2957, 1605, 1512, 1486, 1380, 1309, 1143, 1015, 910, 761. 1H-NMR (400 MHz, CDCl3): δ 8.70 (d, 1H, J = 8.4 Hz), 8.66 (s, 1H), 8.53 (d, 1H, J = 8.0 Hz), 8.33 (d, 1H, J = 7.8 Hz), 8.19 (dd, 2H, J = 7.8, 1.2 Hz), 7.81 (t, 1H, J = 7.8 Hz), 7.73 (t, 1H, J = 8.0 Hz), 7.70 (dd, 1H, J = 8.0, 1.2 Hz), 7.62-7.58 ppm (m, 3H). 13C-NMR (100 MHz, CDCl3): δ 158.9, 153.3, 143.6, 138.0, 135.9, 132.7, 131.7, 129.9, 129.2, 128.3, 128.2, 127.9, 127.3, 124.1, 124.0, 122.2, 122.0 ppm. Anal. Calc. for C19H12ClN3 (317.8): C, 71.81; H, 3.81; N, 13.22. Found: C, 71.75; H, 3.80; N, 13.17.
Phenanthridin-6(5H)-one (10a). White crystals, mp. 289–290 °C (lit. [13] m.p. 290–292 °C). MS: m/z (%) = 195 (M+, 100), 167 (20), 139 (15). 1H-NMR (400 MHz, DMSO-d6): δ 11.70 (br, 1H, NH), 8.52 (d, 1H, J = 8.0 Hz), 8.40 (d, 1H, J = 8.0 Hz), 8.33 (dd, 1H, J = 8.0, 1.2 Hz), 7.82 (dt, 1H, J = 8.0, 1.2 Hz), 7.65 (t, 1H, J = 8.0 Hz), 7.49 (dt, 1H, J = 8.0, 1.4 Hz), 7.36 (dd, 1H, J = 8.0, 1.4 Hz), 7.27 ppm (dt, 1H, J = 8.0, 1.4 Hz). 13C-NMR (100 MHz, DMSO-d6): δ 160.9, 136.6, 134.3, 132.9, 129.6, 128.0, 127.5, 125.7, 123.3, 122.7, 122.3, 117.6, 116.1 ppm.
9-Methylphenanthridin-6(5H)-one (10b). Colorless crystals, m.p. 251–253 °C (lit. [16] m.p. 250–251 °C). MS: m/z (%) = 209 (M+, 100), 180 (25). 1H-NMR (600 MHz, DMSO-d6): δ 11.58 (br, 1H, NH), 8.36 (d, 1H, J = 7.8 Hz), 8.32 (s, 1H), 8.19 (d, 1H, J = 8.0 Hz), 7.46 (dt, 2H, J = 8.4,1.6 Hz), 7.34 (dd, 1H, J = 8.0,1.4 Hz), 7.25 (dt, 1H, J = 8.4,1.2 Hz), 2.51 ppm (s, 3H, CH3). 13C-NMR (150 MHz, DMSO-d6): δ 160.8, 143.0, 136.7, 134.2, 129.4, 129.1, 127.5, 123.4, 123.2, 122.5, 122.1, 117.5, 116.1, 21.5 ppm.
9-Chlorophenanthridin-6(5H)-one (10c). Colorless crystals, m.p. 268–270 °C. LCMS: m/z = 232 (M + 3), 230 (M + 1). 1H NMR (400 MHz, CDCl3): δ 11.53 (br, 1H, NH), 8.43 (d, 1H, J = 8.0 Hz), 8.25 (d, 1H, J = 8.0 Hz), 8.14 (d, 1H, J = 8.4 Hz), 7.80 (t, 1H, J = 7.8 Hz), 7.64 (t, 1H, J = 7.8 Hz), 7.45 (d, 1H, J = 8.0 Hz), 7.38 ppm (t, 1H, J = 7.8 Hz) [13].
1-Anilino-2-phenylbenzimidazole (11a). Colorless crystals, m.p. 211–212 °C (lit. [14] m.p. 210–212 °C). LCMS: m/z = 286 (M + 1). 1H-NMR (400 MHz, CDCl3): δ 8.07 (m, 2H), 7.86 (d, 1H, J = 8.0 Hz), 7.46–7.43 (m, 3H), 7.34 (t, 1H, J = 7.8 Hz), 7.32–7.24 (m, 3H), 7.21 (t, 1H, J = 8.4 Hz), 7.00 (t, 1H, J = 7.6 Hz), 6.81 (br, 1H, NH), 7.72 ppm (d, 2H,J = 7.8 Hz).
1-Anilino-2-p-tolylbenzimidazole (11b). Colorless crystals, m.p. 233–235 °C (lit. [14] m.p. 234–236 °C). LCMS: m/z = 300 (M + 1). 1H-NMR (CDCl3): δ 8.05 (d, 2H, J = 8.0 Hz), 7.82 (d, 1H, J = 8.0 Hz), 7.44 (d, 2H, J = 8.4 Hz), 7.33 (t, 1H, J = 7.8 Hz), 7.28 (m, 2H), 7.17 (d, 1H, J = 7.8 Hz), 7.02 (t, 2H, J = 7.8 Hz), 6.81 (br, 1H), 6.67 (d, 2H, J = 8.0 Hz), 2.40 ppm (s, 3H, CH3).
1-Anilino-2-p-chlorophenylbenzimidazole (11c). Colorless crystals, m.p. 230–233 °C (lit. [14] m.p. 232–234 °C). LCMS: m/z = 321 (M + 2), 320 (M + 1). 1H-NMR (400 MHz, CDCl3): δ 8.05 (dd, 2H, J = 8.4, 1.6 Hz), 7.86 (d, 1H, J = 8.4 Hz), 7.43 (dd, 2H, J = 8.4, 1.6 Hz), 7.35 (t, 1H, J = 8.0 Hz), 7.30–7.25 (m, 3H), 7.17 (d, 1H, J = 8.0 Hz), 7.02 (t, 1H, J = 7.8 Hz), 6.81 (br, 1H, NH), 6.67 ppm (d, 2H, J = 8.0 Hz).
2-Phenyl-1H-benzimidazole (12a). Colorless crystals, m.p. 290–292 °C (lit. [14] m.p. 289–290 °C). LCMS: m/z = 195 (M + 1). 1H-NMR (400 MHz, CDCl3): δ 8.12 (m, 2H), 7.68 (m, 2H), 7.45 (m, 3H), 7.29 ppm (m, 3H, J 7.8 Hz).
2-p-Tolyl-1H-benzimidazole (12b). Colorless crystals, m.p. 271–272 °C (lit. [14] m.p. 269–272 °C). LCMS: m/z = 209 (M + 1). 1H-NMR (400 MHz, DMSO-d6): δ 12.78 (br, 1H, NH), 8.06 (d, 2H, J = 8.0 Hz), 7.56 (m, 2H), 7.32 (d, 2H, J = 8.0 Hz), 7.16 (m, 2H), 2.33 ppm (s, 3H, CH3).
2-p-Chlorophenyl-1H-benzimidazole (12c). Colorless crystals, m.p. 291–292 °C (lit. [14] m.p. 289–291 °C). LCMS: m/z = 231 (M + 3), 229 (M + 1). 1H-NMR (400 MHz, DMSO-d6): δ 12.98 (br, 1H, NH), 8.18 (d, 2H, J = 8.4 Hz), 7.58 (d, 2H, J = 8.4 Hz), 7.56 (m, 2H), 7.18 ppm (m, 2H).
1-Anilino-1H-benzimidazole-2-carboxylic acid ethyl ester (14a). Colorless crystals from ethanol, m.p. 142–144 °C. MS: m/z (%) = 281 (M+, 70), 253 (35), 208 (80). IR (KBr, cm−1): 3244, 3054, 2973, 1683, 1600, 1556, 1490, 1392, 1240, 1157, 1053, 734. 1H-NMR (600 MHz, CDCl3): δ 7.95 (d, 1H, J = 8.4 Hz), 7.76 (br, 1H, NH), 7.56 (dd, 1H, J = 8.4, 1.2 Hz), 7.45 (d, 1H, J = 8.4 Hz), 7.36 (dt, 1H, J = 7.8, 1.4 Hz), 7.12 (t, 2H, J = 8.4 Hz), 6.98 (t, 1H, J = 7.8 Hz), 6.54 (d, 2H, J = 8.0 Hz), 4.43 (q, 2H, J = 6.8 Hz), 1.42 ppm (t, 3H, J = 6.8 Hz). 13C-NMR (150 MHz, CDCl3): δ 159.8, 144.7, 141.2, 132.1, 128.9, 128.4, 124.5, 123.3, 119.6, 118.1, 114.5, 110.7, 61.7, 13.7 ppm. Anal. Calcd. for C16H15N3O2 (281.3): C, 68.30; H, 5.37; N, 14.94. Found: C, 68.25; H, 5.35; N, 14.89.
1-p-Chloroanilino-1H-benzimidazole-2-carboxylic acid ethyl ester (14b). Colorless crystals from ethanol, m.p. 146–148 °C. MS: m/z (%) = 315 (M+, 100), 242 (65), 149 (80). IR (KBr, cm−1): 3224, 3032, 2979, 1723, 1599, 824. 1H-NMR (600 MHz, CDCl3): δ 7.97 (d, 1H, J = 8.4 Hz), 7.76 (br, 1H, NH), 7.56 (dd, 1H, J = 8.4,1.4 Hz), 7.53 (dt, 1H, J = 8.4, 1.4 Hz), 7.45 (dt, 1H, J = 7.8, 1.4 Hz), 7.18 (d, 2H, J = 8.4 Hz), 6.48 (d, 2H, J = 8.4 Hz), 4.43 (q, 2H, J = 6.8 Hz), 1.42 ppm (t, 3H, J = 6.8 Hz). 13C-NMR (150 MHz, CDCl3): δ 159.2, 145.6, 138.9, 138.8, 135.5, 129.5, 127.6, 126.9, 124.7, 122.1, 115.1, 110.8, 62.7, 14.1 ppm. (HRMS = 315.0769; requires 315.0768).
1-Phenyl-1H,9H-benzo[4,5][1,2,3]triazolo[1,2-a]tetrazole-3-carboxylic acid ethyl ester (16a). Pale yellow crystals from ethanol, m.p. 152–154 °C. MS: m/z (%) = 309 (M+, 25), 295 (10), 281 (50). IR (KBr, cm−1): 3036, 2982, 1683, 1598, 1540, 1501, 1458, 1312, 1235, 1187, 1063, 1019, 759. 1H-NMR (CDCl3): δ 12.44 (s, 1H, NH), 8.16 (d, 1H, J = 8.4 Hz), 7.60–7.54 (m, 2H), 7.45 (tt, 1H, J = 8.4, 1.6 Hz), 7.37 (t, 2H, J = 8.0 Hz), 7.28 (d, 2H, J = 8.4 Hz), 7.12 (t, 1H, J = 7.8 Hz), 4.31 (q, 2H, J = 7.2 Hz), 1.22 ppm (t, 3H, J = 7.2 Hz). 13C-NMR (100 MHz, CDCl3): δ 161.9, 145.8, 142.3, 134.6, 130.1, 130.0, 128.83, 124.80, 124.76, 120.6, 115.5, 110.8, 62.9, 14.5 ppm. Anal. Calcd. for C16H15N5O2 (309.3): C, 62.13; H, 4.89; N, 22.64. Found: C, 62.07; H, 4.82; N, 22.65.
1-p-Chlorophenyl-1H,9H-benzo[4,5][1,2,3]triazolo[1,2-a]tetrazole-3-carboxylic acid ethyl ester (16b). Yellow crystals from ethanol, m.p. 156–158 °C. MS: m/z (%) = 343 (M+, 15), 315 (30), 126 (100). IR (KBr, cm−1): 3094, 2986, 1680, 1571, 1547, 1489, 1401, 1282, 1235, 1172, 1148, 1062, 832. 1H-NMR (400 MHz, CDCl3): δ 12.43 (s, 1H, NH), 8.15 (d, 1H, J = 8.0 Hz), 7.58–7.55 (m, 2H), 7.48–7.44 (m, 1H), 7.32 (d, 2H, J = 8.4 Hz), 7.22 (d, 2H, J = 8.4 Hz), 4.31 (q, 2H, J = 7.2 Hz), 1.23 ppm (t, 3H, J = 7.2 Hz). 13C-NMR (100 MHz, CDCl3): δ 161.3, 145.2, 140.3, 133.9, 129.6, 129.2, 128.3, 124.2, 120.1, 119.2, 116.1, 110.1, 62.4, 13.9 ppm. (HRMS = 343.0830; requires C16H14ClN5O2 343.0831).
1-Anilino-2-benzoylbenzimidazole (18a). Yellow crystals, m.p. 216–218. °C. MS: m/z (%) = 313 (M+, 60), 279 (20), 167 (40), 149 (100). 1H-NMR (400 MHz, CDCl3): δ 8.33 (dd, 2H, J = 8.4, 1.6 Hz), 8.12 (br, 1H, NH), 7.96 (d, 1H, J = 8.0 Hz), 7.70 (dt, 1H, J = 8.4, 1.6 Hz), 7.60 (dt, 1H, J = 8.0, 1.4 Hz), 7.53–7.42 (m, 3H), 7.34 (t, 1H, J = 7.8 Hz), 7.16 (t, 2H, J = 8.0 Hz), 6.90 (t, 1H, J = 8.2 Hz), 6.54 ppm (d, 2H, J = 7.8 Hz). 13C-NMR (100 MHz, CDCl3): δ 185.7, 147.2, 144.7, 139.1, 135.7, 134.0, 131.2, 129.4, 128.3, 127.6, 126.9, 124.4, 122.4, 122.3, 113.8, 111.1 ppm. (HRMS = 313.1209; requires C20H15N3O 313.1207).
2-Benzoyl-1-p-toluidinobenzimidazole (18b). Pale yellow crystals, m.p. 226–228 °C. MS: m/z (%) = 327 (M+, 100), 223 (40), 195 (100). IR (KBr, cm−1): 3331, 3061, 1648, 730. 1H-NMR (400 MHz, CDCl3): δ 8.34 (dd, 2H, J = 8.4, 1.6 Hz), 8.12 (br, 1H, NH), 7.99 (d, 1H, J = 8.0 Hz), 7.73 (d, 1H, J = 8.4 Hz), 7.64–7.50 (m, 3H), 7.34 (t, 1H, J = 7.8 H), 7.06 (t, 1H, J = 8.0 Hz), 6.99 (d, 2H, J = 8.8 Hz), 6.49 (d, 2H, J = 8.8 Hz), 2.22 ppm (s, 3H, CH3). 13C-NMR (100 MHz, CDCl3): δ 185.4, 144.5, 137.8, 136.2, 134.6, 132.1, 131.5, 130.0, 129.8, 129.3, 128.9, 127.4, 126.1, 122.3, 114.1, 111.5, 20.6 ppm. (HRMS = 327.1366, requires C21H17N3O 327.1366).
2-Benzoylbenzoxazole (21). Colorless crystals from ethanol, m.p. 136–138 (lit. [17] m.p. 139 °C). MS: m/z (%) = 223 (M+, 75), 195 (40), 105 (100). 1H-NMR (600 MHz, CDCl3): δ 8.56 (dd, 2H, J = 8.4, 1.6 Hz), 7.97 (d, 1H, J = 8.4 Hz), 7.73 (d, 1H, J = 8.4 Hz), 7.71 (t, 1H, J = 8.0 Hz), 7.58 (t, 2H, J = 7.8 Hz), 7.56 (t, 1H, J = 7.8 Hz), 7.51 ppm (t, 1H, J = 7.8 Hz). 13C-NMR (150 MHz, CDCl3): δ 180.4 (C), 157.3 (C), 150.5 (C), 140.9 (C), 135.2 (C), 134.1 (CH), 130.9 (2CH), 128.5 (2CH), 128.2 (CH), 125.6 (CH), 122.3 (CH), 111.7 (CH) ppm. Anal. Calcd. for C14H9NO2 (223.3): C, 75.33; H, 4.06; N, 6.27. Found: C, 75.23; H, 4.05; N, 6.29.
4. Conclusions
The present study offers a new route for the synthesis of some new heterocyclic phenanthridin-6-yl-2-phenyldiazines. Some of these photoproducts 10a–c have been shown to have efficient compelexation properties with transition metals and exhibit interesting photo-emission and fluorescence properties [18,19]. It also shows that 1-substituted benzotriazole arylhydrazones behave photochemically in a different manner than in flash vacuum pyrolysis (FVP) or static pyrolysis (STP) reactions [12,13,14].
Acknowledgements
The support of the University of Kuwait received through research grant # SC 04/08 and the facilities of ANALAB/SAF (grants no. GS01/01, GS02/01, GS03/08) are gratefully acknowledged.
References and Notes
- Hubert, A.J. Photochemistry of benzotriazole. J. Chem. Soc. C 1969, 10, 1334–1336. [Google Scholar] [CrossRef]
- Kazuo, T.; Mamoru, O.; Teijiro, Y. Thermal and photochemical decomposition of 4,5,6,7-tetrahydro-1,2,3-benzotriazole analogs. Bull. Chem. Soc. Jpn. 1973, 46, 3605–3607. [Google Scholar] [CrossRef]
- Kazuo, T.; Mamoru, O.; Teijiro, Y. Photochemical decomposition of benzotriazole. Bull. Chem. Soc. Jpn. 1972, 45, 515–519. [Google Scholar] [CrossRef]
- Crow, W.D.; Wentrup, C. Cyanocyclopentadienes by pyrolysis of isatain and 1H-benzotriazole. Chem. Commun. 1968, 17, 1026–1027. [Google Scholar]
- Mäerky, M.; Doppler, Th.; Hansen, H.; Schmid, H. Photoreactions of 1-alkylbenzotriazoles with aromatic compounds. Chimia 1969, 23, 230–231. [Google Scholar]
- Mäerky, M.; Schmid, H.; Hansen, H. Photoreactions of 1-alkylbenzotriazoles. J. Helv. Chim. Acta 1979, 62, 2129–2153. [Google Scholar] [CrossRef]
- Booker-Milburn, K.I.; Wood, P.M.; Dainty, R.F.; Urquhart, M.W.; White, A.J.; Lyon, H.J.; Charmant, J.P. Photochemistry of benzotriazole: Unprecedented tatumer-selective interamolecular [2 + 2] photocycloddition. Org. Lett. 2002, 4, 1487–1489. [Google Scholar]
- Wander, P.A.; Cooper, C.B. The photochemistry of 1-alkenybenzotriazoles: Methodology for the synthesis of indoles. Tetrahedron 1986, 42, 2985–2991. [Google Scholar] [CrossRef]
- Androsov, D.A.; Neckers, D.C. Photochemical study of tris(benzotriazol-1-yl)methane. J. Org. Chem. 2007, 72, 1148–1152. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Lan, X.; Yang, J.; Denisko, O. Properties and synthesis utility of N-substituted benzotriazoles. Chem. Rev. 1998, 98, 409–548. [Google Scholar]
- Al-Jalal, N.; Al-Awadi, N.A.; Ibrahim, M.R.; Elnagdi, M.H. The photochemistry of 1-alkenyl-substituted-1,2,3-benzotriazoles leading to formation of indole and fused indole derivatives. ARKIVOC 2011, x, 288–297. [Google Scholar]
- Dib, H.H.; Al-Awadi, N.A.; Ibrahim, Y.A.; El-Dusouqui, O.M. Gas-phase thermolysis of benzotriazole derivatives. Part 2: Synthesis of benzimidazo[1,2-b] cinnolines, a novel heterocyclic ring system, by pyrolysis of benzotriazole derivatives, kinetics and mechanistic study. Tetrahedron 2003, 59, 9455–9464. [Google Scholar]
- Al-Awadi, N.A.; George, B.; Dib, H.H.; Ibrahim, M.R.; Ibrahim, Y.A.; El-Dusouqui, O.M. Gas-phase thermolysis of benzotriazole derivatives. Part 3: Kinetic and mechanistic evidence for biradical intermediates in pyrolysis of aroylbenzotriazoles and related compounds. Tetrahedron 2005, 61, 8257–8263. [Google Scholar] [CrossRef]
- Al-Awadi, H.; Ibrahim, M.R.; Ibrahim, Y.A.; Al-Awadi, N.A. Gas-phase thermolysis of benzotriazole derivatives. Part 4: Pyrolysis of 1-acylbenzotriazole phenylhydrazones. Interesting direct route towards N-aminobenzimidazole. J. Heterocycl. Chem. 2008, 45, 723–727. [Google Scholar] [CrossRef]
- Shawali, A.S.; Eweiss, N.F.; Hassaneen, H.M.; Sami, M. Synthesis and rearrangement of ethyl aryloxyglyoxalatearylhydrazones. Bull. Chem. Soc. Jpn. 1975, 48, 365–366. [Google Scholar] [CrossRef]
- Dubost, E.; Magnelli, R.; Cailly, T.; Legay, R.; Fabis, F.; Rault, S. General methods for the synthesis of substituted phennthradin-6(5H)-ones using a KOH-mediated anionic ring closure as the key step. Tetrahedron 2010, 66, 5008–5016. [Google Scholar] [CrossRef]
- Xiao-Feng, W.; Pazhamala, A.; Helfried, N.; Matthias, B. Palladium-catalyzed carbonylative C-H activation of heteroarenes. Angew. Chem. Int. Ed. 2010, 49, 7316–7319. [Google Scholar] [CrossRef]
- Oliver, M.; Christian, L.; Evelyn, F.; Klaus, K.; Nicolle, L.; Christian, S.; Jens, R.; Gerhard, W.; Soichi, W. Transition metal cyclometalated complexes with chelating bidentate N-heterocyclic carben-heterocycle ligands as light-emitting materials for organic light-emitting devices. WO 2008-EP64064 20081017, 2008. (Chem. Abstr. 2009, 150, 472908).. [Google Scholar]
- Morgan, A.R.; Severini, A.; James, M.N.G. Methods for assaying proteinases and proteinase inhibitors. WO 1992-CA18 19920115, 1992. (Chem. Abstr. 1992, 117, 187290).. [Google Scholar]
- Crystallographic data of (excluding structure factors) for the structures in this paper have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication nos. CCDC 852370 (9a), CCDC 852374 (4b) and CCDC 852389 (14b). Copies of the data can be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK, (fax: +44-(0)1223-336033 or e-mail: deposit@ccdc.cam.ac.uk.
- Sample Availability: Samples of the compounds 4, 9, 14, 18 and 21 are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
