Antiadipogenic Effects of Loganic Acid in 3T3-L1 Preadipocytes and Ovariectomized Mice
Abstract
:1. Introduction
2. Results and Discussion
2.1. Loganic Acid was Isolated and Identified from the 30% Ethanol Extract of GL Root
2.2. Loganic Acid Decreased Adipocyte Differentiation in 3T3-L1 Cells
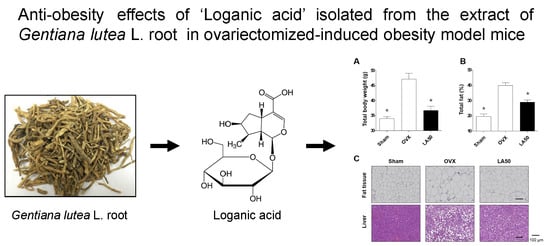
2.3. Loganic Acid Reduced Obesity-Related Phenotypes in Ovariectomy (OVX)-Induced Mice
3. Experimental Section
3.1. Fractionation, Isolation, and Structure Elucidation of the Bioactive Component
3.2. Cell Culture and Adipogenesis Induction of Preadipocytes
3.3. Water-Soluble Tetrazolium Salt (WST) Assay
3.4. Oil Red O Staining of Adipocytes
3.5. Quantitative Reverse-Transcription PCR (qRT-PCR)
3.6. In Vivo Experiments in Obesity Model Mouse
3.7. Hematoxylin and Eosin (H&E) Staining
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kang, S.I.; Ko, H.C.; Shin, H.S.; Kim, H.M.; Hong, Y.S.; Lee, N.H.; Kim, S.J. Fucoxanthin exerts differing effects on 3T3-L1 cells according to differentiation stage and inhibits glucose uptake in mature adipocytes. Biochem. Biophys. Res. Commun. 2011, 409, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, F.T.; Clee, S.M.; Meyre, D. Obesity genetics in mouse and human: Back and forth, and back again. PeerJ 2015, 3, e856. [Google Scholar] [CrossRef] [PubMed]
- Bleich, S.; Cutler, D.; Murray, C.; Adams, A. Why is the developed world obese? Annu. Rev. Public Health 2008, 29, 273–295. [Google Scholar] [CrossRef] [PubMed]
- Yanovski, S.Z.; Yanovski, J.A. Long-term drug treatment for obesity: A systematic and clinical review. JAMA 2014, 311, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.G.; Park, C.Y. Anti-Obesity Drugs: A Review about Their Effects and Safety. Diabetes Metab. J. 2012, 36, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.; McLachlan, A.J.; Sherwin, C.M.; Enioutina, E.Y. Herbal medicines: Challenges in the modern world. Part 1. Australia and New Zealand. Expert Rev. Clin. Pharmacol. 2016, 9, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Sammons, H.M.; Gubarev, M.I.; Krepkova, L.V.; Bortnikova, V.V.; Corrick, F.; Job, K.M.; Sherwin, C.M.; Enioutina, E.Y. Herbal medicines: Challenges in the modern world. Part 2. European Union and Russia. Expert Rev. Clin. Pharmacol. 2016, 9, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Baik, O.D.; Choi, Y.J.; Kim, S.M. Pretreatments for the efficient extraction of bioactive compounds from plant-based biomaterials. Crit. Rev. Food Sci. Nutr. 2014, 54, 1283–1297. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.M.; Yoga Latha, L. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wolfle, U.; Haarhaus, B.; Seiwerth, J.; Cawelius, A.; Schwabe, K.; Quirin, K.W.; Schempp, C.M. The Herbal Bitter Drug Gentiana lutea Modulates Lipid Synthesis in Human Keratinocytes In Vitro and In Vivo. Int. J. Mol. Sci. 2017, 18, 1814. [Google Scholar] [CrossRef] [PubMed]
- Mennella, I.; Fogliano, V.; Ferracane, R.; Arlorio, M.; Pattarino, F.; Vitaglione, P. Microencapsulated bitter compounds (from Gentiana lutea) reduce daily energy intakes in humans. Br. J. Nutr. 2016, 116, 1841–1850. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.M.; Caprioli, G.; Ricciutelli, M.; Maggi, F.; Marin, R.; Vittori, S.; Sagratini, G. Comparative HPLC/ESI-MS and HPLC/DAD study of different populations of cultivated, wild and commercial Gentiana lutea L. Food Chem. 2015, 174, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Jia, N.; Chu, W.; Li, Y.; Ding, L.; Duan, J.; Cui, J.; Cao, S.; Zhao, C.; Wu, Y.; Wen, A. Iridoid glycosides from the flowers of Gentiana macrophylla Pall. ameliorate collagen-induced arthritis in rats. J. Ethnopharmacol. 2016, 189, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Su, X.-T.; Xia, Q.; Wang, J.-G. Gentiana macrophylla Pall (Gentianaceae) extract exerts protective effects against osteoporosis in mice. Trop. J. Pharm. Res. 2018, 17, 429–434. [Google Scholar] [CrossRef]
- Kesavan, R.; Chandel, S.; Upadhyay, S.; Bendre, R.; Ganugula, R.; Potunuru, U.R.; Giri, H.; Sahu, G.; Kumar, P.U.; Reddy, G.B.; et al. Gentiana lutea exerts anti-atherosclerotic effects by preventing endothelial inflammation and smooth muscle cell migration. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Schmieder, A.; Schwaiger, S.; Csordas, A.; Backovic, A.; Messner, B.; Wick, G.; Stuppner, H.; Bernhard, D. Isogentisin—A novel compound for the prevention of smoking-caused endothelial injury. Atherosclerosis 2007, 194, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Ning, Y.; Zhao, Y.; Sun, W.; Thorimbert, S.; Dechoux, L.; Sollogoub, M.; Zhang, Y. Research Progress of Natural Product Gentiopicroside—A Secoiridoid Compound. Mini Rev. Med. Chem. 2017, 17, 62–77. [Google Scholar] [CrossRef] [PubMed]
- Di, L.; Li, N.; Zu, L.; Wang, K.; Zhao, Y.; Wang, Z. Three new iridoid glucosides from the roots of Patrinia scabra. Bull. Korean Chem. Soc. 2011, 32, 3251–3254. [Google Scholar] [CrossRef]
- Aberham, A.; Schwaiger, S.; Stuppner, H.; Ganzera, M. Quantitative analysis of iridoids, secoiridoids, xanthones and xanthone glycosides in Gentiana lutea L. roots by RP-HPLC and LC-MS. J. Pharm. Biomed. Anal. 2007, 45, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Recio, M.C.; Giner, R.M.; Manez, S.; Rios, J.L. Structural considerations on the iridoids as anti-inflammatory agents. Planta Med. 1994, 60, 232–234. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Zhang, C.; Li, J.; Yang, H.; Shen, J.; Yang, Z. A new iridoid glycoside from the roots of Dipsacus asper. Molecules 2012, 17, 1419–1424. [Google Scholar] [CrossRef] [PubMed]
- Sozanski, T.; Kucharska, A.Z.; Rapak, A.; Szumny, D.; Trocha, M.; Merwid-Lad, A.; Dzimira, S.; Piasecki, T.; Piorecki, N.; Magdalan, J.; et al. Iridoid-loganic acid versus anthocyanins from the Cornus mas fruits (cornelian cherry): Common and different effects on diet-induced atherosclerosis, PPARs expression and inflammation. Atherosclerosis 2016, 254, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Gregoire, F.M.; Smas, C.M.; Sul, H.S. Understanding adipocyte differentiation. Physiol. Rev. 1998, 78, 783–809. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Yanagawa, Y.; Kim, S.; Whang, W.K.; Park, T. Artemisia iwayomogi Extract Attenuates High-Fat Diet-Induced Obesity by Decreasing the Expression of Genes Associated with Adipogenesis in Mice. Evid. Based Complement. Alternat. Med. 2013, 2013, 915953. [Google Scholar] [CrossRef] [PubMed]
- Reed, B.C.; Lane, M.D. Insulin receptor synthesis and turnover in differentiating 3T3-L1 preadipocytes. Proc. Natl. Acad. Sci. USA 1980, 77, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Kamon, J.; Minokoshi, Y.; Ito, Y.; Waki, H.; Uchida, S.; Yamashita, S.; Noda, M.; Kita, S.; Ueki, K.; et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002, 8, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Lara-Castro, C.; Fu, Y.; Chung, B.H.; Garvey, W.T. Adiponectin and the metabolic syndrome: Mechanisms mediating risk for metabolic and cardiovascular disease. Curr. Opin. Lipidol. 2007, 18, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Oger, F.; Dubois-Chevalier, J.; Gheeraert, C.; Avner, S.; Durand, E.; Froguel, P.; Salbert, G.; Staels, B.; Lefebvre, P.; Eeckhoute, J. Peroxisome proliferator-activated receptor gamma regulates genes involved in insulin/insulin-like growth factor signaling and lipid metabolism during adipogenesis through functionally distinct enhancer classes. J. Biol. Chem. 2014, 289, 708–722. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, A.M.; Orlando, R.A. Role of adipocyte-derived lipoprotein lipase in adipocyte hypertrophy. Nutr. Metab. (Lond.) 2007, 4, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padilla-Benavides, T.; Velez-delValle, C.; Marsch-Moreno, M.; Castro-Munozledo, F.; Kuri-Harcuch, W. Lipogenic Enzymes Complexes and Cytoplasmic Lipid Droplet Formation during Adipogenesis. J. Cell. Biochem. 2016, 117, 2315–2326. [Google Scholar] [CrossRef] [PubMed]
- Iso, T.; Sunaga, H.; Matsui, H.; Kasama, S.; Oshima, N.; Haruyama, H.; Furukawa, N.; Nakajima, K.; Machida, T.; Murakami, M.; et al. Serum levels of fatty acid binding protein 4 and fat metabolic markers in relation to catecholamines following exercise. Clin. Biochem. 2017, 50, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, P.R.; Gnudi, L.; Tozzo, E.; Yang, H.; Leach, F.; Kahn, B.B. Adipose cell hyperplasia and enhanced glucose disposal in transgenic mice overexpressing GLUT4 selectively in adipose tissue. J. Biol. Chem. 1993, 268, 22243–22246. [Google Scholar] [PubMed]
- Clarke, S.L.; Robinson, C.E.; Gimble, J.M. CAAT/enhancer binding proteins directly modulate transcription from the peroxisome proliferator-activated receptor gamma 2 promoter. Biochem. Biophys. Res. Commun. 1997, 240, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Kraus, N.A.; Ehebauer, F.; Zapp, B.; Rudolphi, B.; Kraus, B.J.; Kraus, D. Quantitative assessment of adipocyte differentiation in cell culture. Adipocyte 2016, 5, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Kendig, S. Implications for Policy to Support Healthy Weight for Women. J. Obstet. Gynecol. Neonatal. Nurs. 2015, 44, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Ekici, H.; Sontas, B.H.; Toydemir, T.S.; Senmevsim, O.; Kabasakal, L.; Imre, Y. Effect of prepubertal ovariohysterectomy on bone mineral density and bone mineral content in puppies. Acta Vet. Hung. 2005, 53, 469–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Not available. |




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, E.; Kim, J.; Yeo, S.; Kim, G.; Ko, E.-H.; Lee, S.W.; Li, W.Y.; Choi, C.W.; Jeong, S.-Y. Antiadipogenic Effects of Loganic Acid in 3T3-L1 Preadipocytes and Ovariectomized Mice. Molecules 2018, 23, 1663. https://doi.org/10.3390/molecules23071663
Park E, Kim J, Yeo S, Kim G, Ko E-H, Lee SW, Li WY, Choi CW, Jeong S-Y. Antiadipogenic Effects of Loganic Acid in 3T3-L1 Preadipocytes and Ovariectomized Mice. Molecules. 2018; 23(7):1663. https://doi.org/10.3390/molecules23071663
Chicago/Turabian StylePark, Eunkuk, Jeonghyun Kim, Subin Yeo, Gijeong Kim, Eun-Hee Ko, Sang Woo Lee, Wan Yi Li, Chun Whan Choi, and Seon-Yong Jeong. 2018. "Antiadipogenic Effects of Loganic Acid in 3T3-L1 Preadipocytes and Ovariectomized Mice" Molecules 23, no. 7: 1663. https://doi.org/10.3390/molecules23071663
APA StylePark, E., Kim, J., Yeo, S., Kim, G., Ko, E.-H., Lee, S. W., Li, W. Y., Choi, C. W., & Jeong, S.-Y. (2018). Antiadipogenic Effects of Loganic Acid in 3T3-L1 Preadipocytes and Ovariectomized Mice. Molecules, 23(7), 1663. https://doi.org/10.3390/molecules23071663