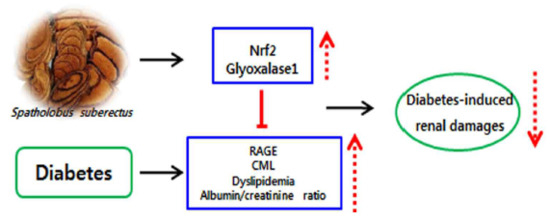
Spatholobus suberectus Ameliorates Diabetes-Induced Renal Damage by Suppressing Advanced Glycation End Products in db/db Mice
Abstract
1. Introduction
2. Results
2.1. Formononetin Concentration in S. suberectus
2.2. Effect of S. suberectus Extract on AGEs Formation and Breaking
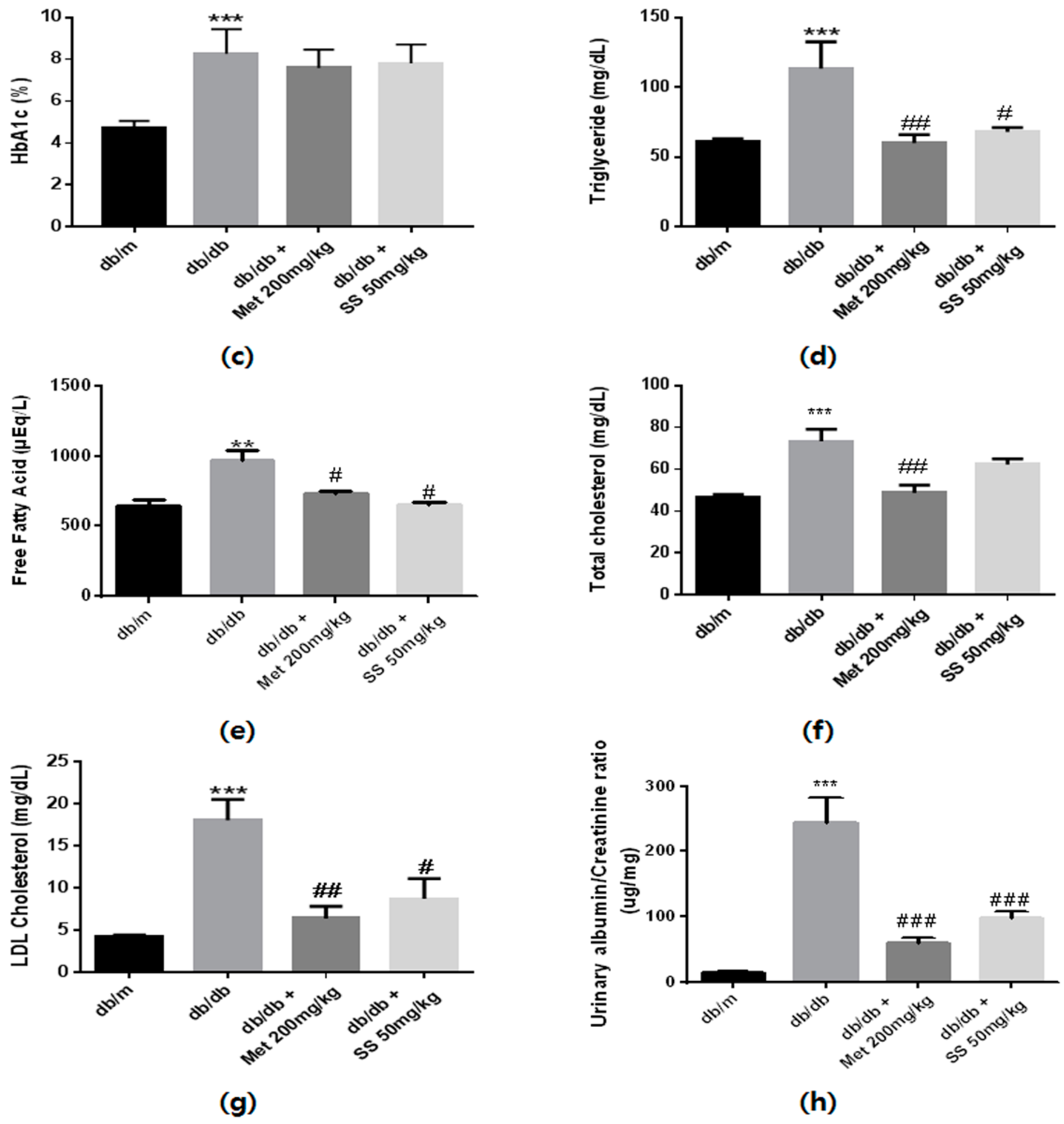
2.3. Effects of the S. suberectus Extract on the Diabetes Parameters and Lipid Profiles
2.4. Effects of the S. suberectus Extract on the Expression of CML and RAGE
2.5. Effects of the S. suberectus Extract on the Glo1 and Nrf2 Pathway
2.6. Histology and Immunohistochemistry
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Sample Preparation
4.3. HPLC analysis
4.4. Inhibitory Effects of the S. suberectus Extract on AGEs Formation
4.5. AGEs Cross-Link-Breaking Ability of the S. suberectus Extract
4.6. Animals
4.7. Blood and Urine Analysis
4.8. Western Blotting Analysis
4.9. Histology and Immunohistochemistry
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AGEs | advanced glycation end products |
| ARE | antioxidant responsive element |
| AG | aminoguanidine |
| ALT-711 | alagebrium |
| CML | Nɛ-(carboxymethyl)-lysine |
| BSA | bovine serum albumin |
| Glo1 | glyoxalase 1 |
| MET | metformin |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| NQO1 | NADPH quinine oxidoreductase 1 |
| RAGE | receptor for AGEs |
| ROS | reactive oxygen species |
| PAS | periodic acid–Schiff |
| IHC | immunohistochemistry |
References
- An, Y.; Kang, Y.; Lee, J.; Ahn, C.; Kwon, K.; Choi, C. Blood flow characteristics of diabetic patients with complications detected by optical measurement. Biomed. Eng. Online 2018, 17, 25. [Google Scholar] [CrossRef] [PubMed]
- Ghaderian, S.B.; Hayati, F.; Shayanpour, S.; Mousavi, S.S.B. Diabetes and end-stage renal disease; a review article on new concepts. J. Renal. Inj. Prev. 2015, 4, 28–33. [Google Scholar] [PubMed]
- Park, C.H.; Yokozawa, T.; Noh, J.S. Oligonol, a low-molecular-weight polyphenol derived from lychee fruit, attenuates diabetes-induced renal damage through the advanced glycation end product-related pathway in db/db mice–3. J. Nutr. 2014, 144, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharmacol. 2014, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, D.; Toyoda, M.; Yamamoto, N.; Miyauchi, M.; Katoh, M.; Kimura, M.; Maruyama, M.; Honma, M.; Umezono, T.; Yagame, M. Relationship between the expression of advanced glycation end-products (age) and the receptor for age (rage) mrna in diabetic nephropathy. Intern. Med. 2006, 45, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Rani, N.; Bharti, S.; Bhatia, J.; Nag, T.; Ray, R.; Arya, D.S. Chrysin, a ppar-γ agonist improves myocardial injury in diabetic rats through inhibiting age-rage mediated oxidative stress and inflammation. Chem. Biol. Interact. 2016, 250, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.; Reynolds, L.R.; Bruemmer, D. Intensive glycemic control and cardiovascular disease: An update. Nat. Rev. Cardiol. 2010, 7, 369. [Google Scholar] [CrossRef] [PubMed]
- Ismail-Beigi, F.; Craven, T.; Banerji, M.A.; Basile, J.; Calles, J.; Cohen, R.M.; Cuddihy, R.; Cushman, W.C.; Genuth, S.; Grimm, R.H., Jr. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: An analysis of the accord randomised trial. Lancet 2010, 376, 419–430. [Google Scholar] [CrossRef]
- Moraru, A.; Wiederstein, J.; Pfaff, D.; Fleming, T.; Miller, A.K.; Nawroth, P.; Teleman, A.A. Elevated levels of the reactive metabolite methylglyoxal recapitulate progression of type 2 diabetes. Cell Metab. 2018, 27, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Sanajou, D.; Haghjo, A.G.; Argani, H.; Aslani, S. Age-rage axis blockade in diabetic nephropathy: Current status and future directions. Eur. J. Pharmacol. 2018, 833, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.M.; Gray, S.P.; Jiaze, L.; Soro-Paavonen, A.; Wong, B.; Cooper, M.E.; Bierhaus, A.; Pickering, R.; Tikellis, C.; Tsorotes, D. Alagebrium reduces glomerular fibrogenesis and inflammation beyond preventing rage activation in diabetic apolipoprotein e knockout mice. Diabetes 2012, DB_111546. [Google Scholar] [CrossRef] [PubMed]
- Figarola, J.; Loera, S.; Weng, Y.; Shanmugam, N.; Natarajan, R.; Rahbar, S. Lr-90 prevents dyslipidaemia and diabetic nephropathy in the zucker diabetic fatty rat. Diabetologia 2008, 51, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T.; De Strihou, C.V.Y.; Imasawa, T.; Yoshino, A.; Ueda, Y.; Ogura, H.; Kominami, K.; Onogi, H.; Inagi, R.; Nangaku, M. Glyoxalase i deficiency is associated with an unusual level of advanced glycation end products in a hemodialysis patient. Kidney Int. 2001, 60, 2351–2359. [Google Scholar] [CrossRef] [PubMed]
- Rajappa, R.; Bovilla, V.; Madhunapantula, S.V. Naturally occurring nrf2 activators in the management of diabetes. Nutri. Food Sci. Int. J. 2017, 2, 555595. [Google Scholar]
- Huang, Y.; Chen, L.; Feng, L.; Guo, F.; Li, Y. Characterization of total phenolic constituents from the stems of spatholobus suberectus using lc-dad-msn and their inhibitory effect on human neutrophil elastase activity. Molecules 2013, 18, 7549–7556. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-R.; Wang, A.-Q.; Lin, L.-G.; Qiu, H.-C.; Wang, Y.-T.; Wang, Y. In vitro study on anti-hepatitis c virus activity of spatholobus suberectus dunn. Molecules 2016, 21, 1367. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.; Shim, K.-S.; An, H.; Kim, T.; Ma, J.Y. Water extract of spatholobus suberectus inhibits osteoclast differentiation and bone resorption. BMC Complement. Altern. Med. 2013, 13, 112. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Alam, M.B.; Lee, S.-h.; Kim, Y.-J.; Lee, S.; An, H.; Choi, H.-J.; Son, H.-U.; Park, C.-H.; Kim, H.-H. Spatholobus suberectus exhibits antidiabetic activity in vitro and in vivo through activation of akt-ampk pathway. Evid. Based Complement. Alternat. Med. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, L.H.; Fog-Tonnesen, M.; Nielsen Fink, L.; Norlin, J.; García de Vinuesa, A.; Hansen, T.K.; de Heer, E.; Ten Dijke, P.; Rosendahl, A. Disparate phospho-smad2 levels in advanced type 2 diabetes patients with diabetic nephropathy and early experimental db/db mouse model. Ren. Fail. 2017, 39, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-Q.; Zhang, G.-L.; Zhang, Y.; Nan, N.; Sun, X.; Yu, M.-W.; Wang, H.; Li, J.-P.; Wang, X.-M. Spatholobus suberectus column extract inhibits estrogen receptor positive breast cancer via suppressing er mapk pi3k/akt pathway. Evid. Based Complement. Alternat. Med. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jing, J.; Yu, S.; Song, M.; Tan, H.; Cui, B.; Huang, L. Advanced glycation endproducts induce apoptosis of endothelial progenitor cells by activating receptor rage and nadph oxidase/jnk signaling axis. Am. J. Translat. Res. 2016, 8, 2169. [Google Scholar]
- Hirakawa, Y.; Tanaka, T.; Nangaku, M. Mechanisms of metabolic memory and renal hypoxia as a therapeutic target in diabetic kidney disease. J. Diabetes Investig. 2017, 8, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Vlassara, H.; Striker, L.J.; Teichberg, S.; Fuh, H.; Li, Y.M.; Steffes, M. Advanced glycation end products induce glomerular sclerosis and albuminuria in normal rats. Proc. Natl. Acad. Sci. USA 1994, 91, 11704–11708. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Xu, X.-B.; Yu, S.-J. Inhibitory effect of sugarcane molasses extract on the formation of n ε-(carboxymethyl) lysine and n ε-(carboxyethyl) lysine. Food Chem. 2017, 221, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.-L.; Wan, J.-Y.; Li, P.; Qi, L.-W. Ultrasonic/microwave assisted extraction and diagnostic ion filtering strategy by liquid chromatography–quadrupole time-of-flight mass spectrometry for rapid characterization of flavonoids in spatholobus suberectus. J. Chromatogr. A 2011, 1218, 5774–5786. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.; Tian, W.; Huan, M.; Chen, J.; Fu, H. Formononetin exhibits anti-hyperglycemic activity in alloxan-induced type 1 diabetic mice. Exp. Biol. Med. (Maywood) 2017, 242, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Oza, M.J.; Kulkarni, Y.A. Formononetin treatment in type 2 diabetic rats reduces insulin resistance and hyperglycemia. Front. Pharmacol. 2018, 9, 739. [Google Scholar] [CrossRef] [PubMed]
- Skriver, M.; Borch-Johnsen, K.; Lauritzen, T.; Sandbaek, A. Hba1c as predictor of all-cause mortality in individuals at high risk of diabetes with normal glucose tolerance, identified by screening: A follow-up study of the anglo–danish–dutch study of intensive treatment in people with screen-detected diabetes in primary care (addition), Denmark. Diabetol. 2010, 53, 2328–2333. [Google Scholar]
- Sun, C.; McIntyre, K.; Saleem, A.; Haddad, P.S.; Arnason, J.T. The relationship between antiglycation activity and procyanidin and phenolic content in commercial grape seed products. Can. J. Physiol. Pharmacol. 2012, 90, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-M.; Haastert, B.; Scherbaum, W.; Hauner, H. A phytosterol-enriched spread improves the lipid profile of subjects with type 2 diabetes mellitus. Eur. J. Nutr. 2003, 42, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Toyama, T.; Shimizu, M.; Furuichi, K.; Kaneko, S.; Wada, T. Treatment and impact of dyslipidemia in diabetic nephropathy. Clin. Exp. Nephrol. 2014, 18, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Palazhy, S.; Viswanathan, V. Lipid abnormalities in type 2 diabetes mellitus patients with overt nephropathy. Diabetes Metab. J. 2017, 41, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, A.J.; Lyons, T.J.; Zheng, D.; Otvos, J.D.; Lackland, D.T.; Mcgee, D.; Garvey, W.T.; Klein, R.L.; The DCCT/EDIC Research Group. Lipoproteins in the dcct/edic cohort: Associations with diabetic nephropathy. Kidney Int. 2003, 64, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Nakamura, N.; Ojima, A.; Nishino, Y.; Yamagishi, S.-I. Sulforaphane reduces advanced glycation end products (ages)-induced inflammation in endothelial cells and rat aorta. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Fakhruddin, S.; Alanazi, W.; Jackson, K.E. Diabetes-induced reactive oxygen species: Mechanism of their generation and role in renal injury. J. Diabetes Res. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Stinghen, A.E.; Massy, Z.A.; Vlassara, H.; Striker, G.E.; Boullier, A. Uremic toxicity of advanced glycation end products in ckd. J. Am. Soc. Nephrol. 2016, 27, 354–370. [Google Scholar] [CrossRef] [PubMed]
- Spoto, B.; Pisano, A.; Zoccali, C. Insulin resistance in chronic kidney disease: A systematic review. Am. J. Physiol. Renal Physiol. 2016, 311, F1087–F1108. [Google Scholar] [CrossRef] [PubMed]
- Cremers, N.A.; Lundvig, D.; van Dalen, S.; Schelbergen, R.F.; van Lent, P.L.; Szarek, W.A.; Regan, R.F.; Carels, C.E.; Wagener, F.A. Curcumin-induced heme oxygenase-1 expression prevents H2O2-induced cell death in wild type and heme oxygenase-2 knockout adipose-derived mesenchymal stem cells. Int. J. Mol. Sci. 2014, 15, 17974–17999. [Google Scholar] [CrossRef] [PubMed]
- Mastrocola, R. Ages and neurodegeneration: The nrf2/glyoxalase-1 interaction. Oncotarget 2017, 8, 5645. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Oh, J.-G.; Kim, J.S.; Lee, K.-W. Effects of chebulic acid on advanced glycation endproducts-induced collagen cross-links. Biol. Pharm. Bull. 2014, 37, 1162–1167. [Google Scholar] [CrossRef] [PubMed]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Do, M.H.; Hur, J.; Choi, J.; Kim, Y.; Park, H.-Y.; Ha, S.K. Spatholobus suberectus Ameliorates Diabetes-Induced Renal Damage by Suppressing Advanced Glycation End Products in db/db Mice. Int. J. Mol. Sci. 2018, 19, 2774. https://doi.org/10.3390/ijms19092774
Do MH, Hur J, Choi J, Kim Y, Park H-Y, Ha SK. Spatholobus suberectus Ameliorates Diabetes-Induced Renal Damage by Suppressing Advanced Glycation End Products in db/db Mice. International Journal of Molecular Sciences. 2018; 19(9):2774. https://doi.org/10.3390/ijms19092774
Chicago/Turabian StyleDo, Moon Ho, Jinyoung Hur, Jiwon Choi, Yoonsook Kim, Ho-Young Park, and Sang Keun Ha. 2018. "Spatholobus suberectus Ameliorates Diabetes-Induced Renal Damage by Suppressing Advanced Glycation End Products in db/db Mice" International Journal of Molecular Sciences 19, no. 9: 2774. https://doi.org/10.3390/ijms19092774
APA StyleDo, M. H., Hur, J., Choi, J., Kim, Y., Park, H.-Y., & Ha, S. K. (2018). Spatholobus suberectus Ameliorates Diabetes-Induced Renal Damage by Suppressing Advanced Glycation End Products in db/db Mice. International Journal of Molecular Sciences, 19(9), 2774. https://doi.org/10.3390/ijms19092774