Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation
Abstract
:1. Introduction
2. A Brief Overview of Glutamine Metabolism
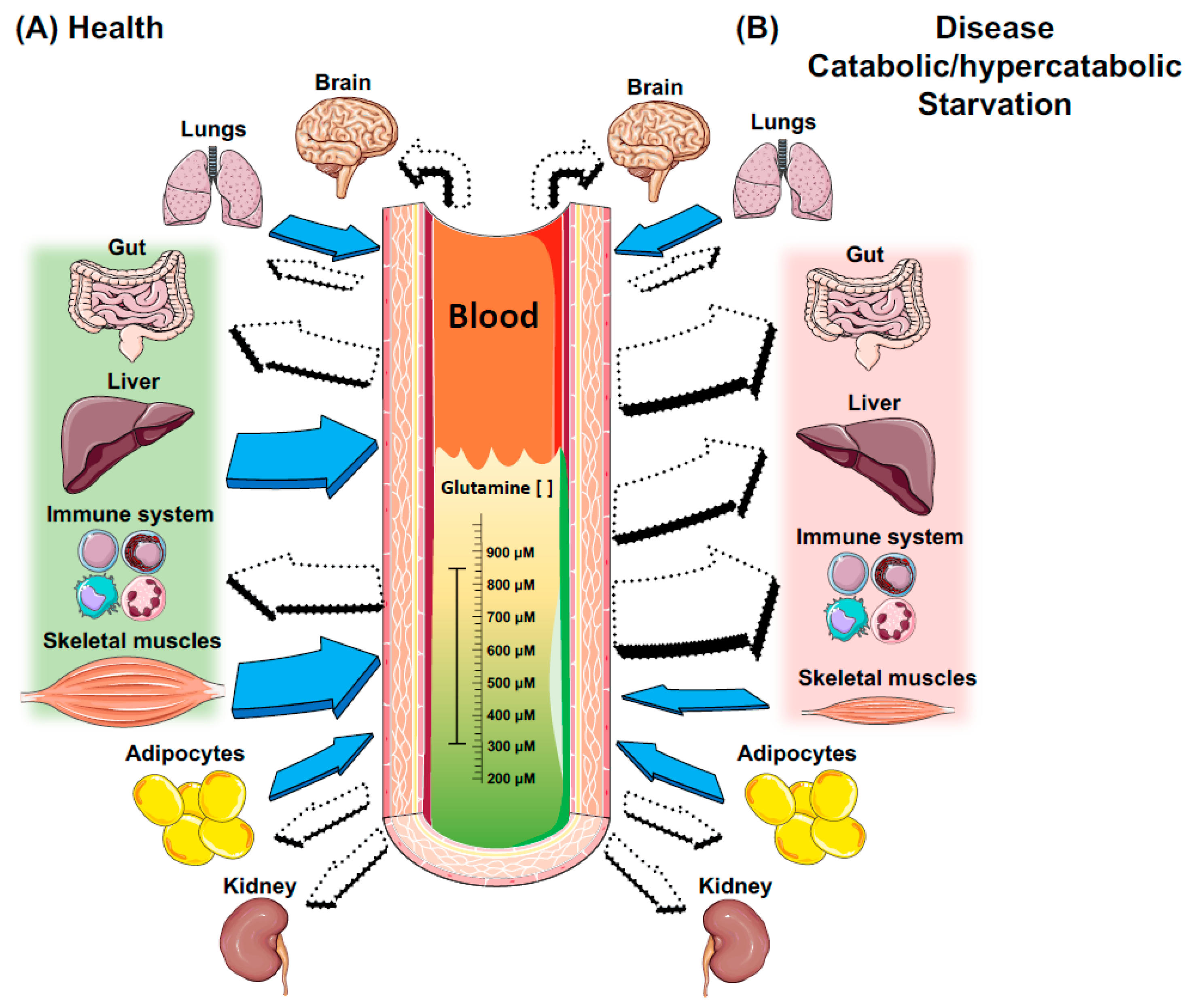
3. Key Metabolic Organs in Glutamine Homeostasis
3.1. The Gut
3.2. Skeletal Muscles
3.3. The Liver
4. Glutamine and Immune Cell Function
4.1. Neutrophils
4.2. Macrophages
4.3. Lymphocytes
5. Immunomodulatory Properties of Glutamine Supplementation
5.1. Glutamine-GSH Axis and the Redox State of the Cell
5.2. Heat Shock Protein Response
6. Clinical Translation of Glutamine Delivery
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Grohmann, U.; Mondanelli, G.; Belladonna, M.L.; Orabona, C.; Pallotta, M.T.; Iacono, A.; Puccetti, P.; Volpi, C. Amino-acid sensing and degrading pathways in immune regulation. Cytokine Growth Factor Rev. 2017, 35, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Curi, R.; Lagranha, C.J.; Doi, S.Q.; Sellitti, D.F.; Procopio, J.; Pithon-Curi, T.C.; Corless, M.; Newsholme, P. Molecular mechanisms of glutamine action. J. Cell. Physiol. 2005, 204, 392–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curi, R.; Newsholme, P.; Marzuca-Nassr, G.N.; Takahashi, H.K.; Hirabara, S.M.; Cruzat, V.; Krause, M.; de Bittencourt, P.I.H., Jr. Regulatory principles in metabolism-then and now. Biochem. J. 2016, 473, 1845–1857. [Google Scholar] [CrossRef] [PubMed]
- Cruzat, V.F.; Pantaleao, L.C.; Donato, J., Jr.; de Bittencourt, P.I.H., Jr.; Tirapegui, J. Oral supplementations with free and dipeptide forms of l-glutamine in endotoxemic mice: Effects on muscle glutamine-glutathione axis and heat shock proteins. J. Nutr. Biochem. 2014, 25, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, P. Why is l-glutamine metabolism important to cells of the immune system in health, postinjury, surgery or infection? J. Nutr. 2001, 131, 2514S–2523S. [Google Scholar] [CrossRef] [PubMed]
- Cruzat, V.F.; Krause, M.; Newsholme, P. Amino acid supplementation and impact on immune function in the context of exercise. J. Int. Soc. Sports Nutr. 2014, 11, 61. [Google Scholar] [CrossRef] [PubMed]
- Ardawi, M.S.M.; Newsholme, E.A. Maximum activities of some enzymes of glycolysis, the tricarboxylic acid cycle and ketone-body and glutamine utilization pathways in lymphocytes of the rat. Biochem. J. 1982, 208, 743–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flaring, U.B.; Rooyackers, O.E.; Wernerman, J.; Hammarqvist, F. Glutamine attenuates post-traumatic glutathione depletion in human muscle. Clin. Sci. 2003, 104, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Roth, E. Nonnutritive effects of glutamine. J. Nutr. 2008, 138, 2025S–2031S. [Google Scholar] [CrossRef] [PubMed]
- Rodas, P.C.; Rooyackers, O.; Hebert, C.; Norberg, A.; Wernerman, J. Glutamine and glutathione at icu admission in relation to outcome. Clin. Sci. 2012, 122, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, E.A.; Parry-Billings, M. Properties of glutamine release from muscle and its importance for the immune system. J. Parenter. Enter. Nutr. 1990, 14, 63S–67S. [Google Scholar] [CrossRef] [PubMed]
- Wernerman, J. Clinical use of glutamine supplementation. J. Nutr. 2008, 138, 2040S–2044S. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.; Norberg, A.; Martling, C.R.; Gamrin, L.; Rooyackers, O.; Wernerman, J. Glutamine kinetics during intravenous glutamine supplementation in icu patients on continuous renal replacement therapy. Intensive Care Med. 2007, 33, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Labow, B.I.; Souba, W.W.; Abcouwer, S.F. Mechanisms governing the expression of the enzymes of glutamine metabolism—Glutaminase and glutamine synthetase. J. Nutr. 2001, 131, 2467S–2486S. [Google Scholar] [CrossRef] [PubMed]
- Cruzat, V.F.; Newsholme, P. An introduction to glutamine metabolism. In Glutamine; CRC Press: Boca Raton, FL, USA, 2017; pp. 1–18. [Google Scholar]
- Cooney, G.; Curi, R.; Mitchelson, A.; Newsholme, P.; Simpson, M.; Newsholme, E.A. Activities of some key enzymes of carbohydrate, ketone-body, adenosine and glutamine-metabolism in liver, and brown and white adipose tissues of the rat. Biochem. Biophys. Res. Commun. 1986, 138, 687–692. [Google Scholar] [CrossRef]
- Tan, H.W.S.; Sim, A.Y.L.; Long, Y.C. Glutamine metabolism regulates autophagy-dependent mtorc1 reactivation during amino acid starvation. Nat. Commun. 2017, 8, 338. [Google Scholar] [CrossRef] [PubMed]
- Ardawi, M.S. Glutamine metabolism in the lungs of glucocorticoid-treated rats. Clin. Sci. 1991, 81, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Parry-Billings, M.; Dimitriadis, G.D.; Leighton, B.; Bond, J.; Bevan, S.J.; Opara, E.; Newsholme, E.A. Effects of hyperthyroidism and hypothyroidism on glutamine metabolism by skeletal muscle of the rat. Biochem. J. 1990, 272, 319–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parry-Billings, M.; Dimitriadis, G.; Leighton, B.; Dunger, D.; Newsholme, E. The effects of growth hormone administration in vivo on skeletal muscle glutamine metabolism of the rat. Horm. Metab. Res. 1993, 25, 292–293. [Google Scholar] [CrossRef] [PubMed]
- Cruzat, V.F.; Keane, K.N.; Scheinpflug, A.L.; Cordeiro, R.; Soares, M.J.; Newsholme, P. Alanyl-glutamine improves pancreatic beta-cell function following ex vivo inflammatory challenge. J. Endocrinol. 2015, 224, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Krebs, H.A. Metabolism of amino-acids: The synthesis of glutamine from glutamic acid and ammonia, and the enzymic hydrolysis of glutamine in animal tissues. Biochem. J. 1935, 29, 1951–1969. [Google Scholar] [CrossRef] [PubMed]
- Neu, J.; Shenoy, V.; Chakrabarti, R. Glutamine nutrition and metabolism: Where do we go from here? FASEB J. 1996, 10, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Holecek, M. Branched-chain amino acids in health and disease: Metabolism, alterations in blood plasma, and as supplements. Nutr. Metab. 2018, 15, 33. [Google Scholar] [CrossRef] [PubMed]
- Altman, B.J.; Stine, Z.E.; Dang, C.V. From krebs to clinic: Glutamine metabolism to cancer therapy. Nat. Rev. Cancer 2016, 16, 619–634. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.; Hsu, J.; Bandi, V.; Jahoor, F. Alterations in glutamine metabolism and its conversion to citrulline in sepsis. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E1359–E1364. [Google Scholar] [CrossRef] [PubMed]
- Rogero, M.M.; Borges, M.C.; Pires, I.S.D.; Borelli, P.; Tirapegui, J. Ffect of glutamine supplementation and in vivo infection with mycobacterium bovis (bacillus calmette-guerin) in the function of peritoneal macrophages in early weaned mice. Ann. Nutr. Metab. 2007, 51, 173–174. [Google Scholar]
- Karinch, A.M.; Pan, M.; Lin, C.M.; Strange, R.; Souba, W.W. Glutamine metabolism in sepsis and infection. J. Nutr. 2001, 131, 2531S–2550S. [Google Scholar] [CrossRef] [PubMed]
- Leite, J.S.; Raizel, R.; Hypolito, T.M.; Rosa, T.D.; Cruzat, V.F.; Tirapegui, J. l-glutamine and l-alanine supplementation increase glutamine-glutathione axis and muscle hsp-27 in rats trained using a progressive high-intensity resistance exercise. Appl. Physiol. Nutr. Metab. 2016, 41, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Cruzat, V.F.; Rogero, M.M.; Tirapegui, J. Effects of supplementation with free glutamine and the dipeptide alanyl-glutamine on parameters of muscle damage and inflammation in rats submitted to prolonged exercise. Cell Biochem. Funct. 2010, 28, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Curi, R.; Lagranha, C.J.; Doi, S.Q.; Sellitti, D.F.; Procopio, J.; Pithon-Curi, T.C. Glutamine-dependent changes in gene expression and protein activity. Cell Biochem. Funct. 2005, 23, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Djoko, K.Y.; Phan, M.D.; Peters, K.M.; Walker, M.J.; Schembri, M.A.; McEwan, A.G. Interplay between tolerance mechanisms to copper and acid stress in Escherichia coli. Proc. Nat. Acad. Sci. USA 2017, 114, 6818–6823. [Google Scholar] [CrossRef] [PubMed]
- Wernerman, J. Feeding the gut: How, when and with what—The metabolic issue. Curr. Opin. Crit. Care 2014, 20, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Beutheu, S.; Ouelaa, W.; Guerin, C.; Belmonte, L.; Aziz, M.; Tennoune, N.; Bole-Feysot, C.; Galas, L.; Dechelotte, P.; Coeffier, M. Glutamine supplementation, but not combined glutamine and arginine supplementation, improves gut barrier function during chemotherapy-induced intestinal mucositis in rats. Clin. Nutr. 2014, 33, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Souba, W.W.; Smith, R.J.; Wilmore, D.W. Glutamine metabolism by the intestinal tract. J. Parenter. Enter. Nutr. 1985, 9, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Holecek, M. Side effects of long-term glutamine supplementation. J. Parenter. Enter. Nutr. 2013, 37, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Kim, H. The roles of glutamine in the intestine and its implication in intestinal diseases. Int. J. Mol. Sci. 2017, 18, 1051. [Google Scholar] [CrossRef] [PubMed]
- Souba, W.W.; Herskowitz, K.; Salloum, R.M.; Chen, M.K.; Austgen, T.R. Gut glutamine metabolism. J. Parenter. Enter. Nutr. 1990, 14, 45S–50S. [Google Scholar] [CrossRef] [PubMed]
- Cruzat, V.F.; Bittencourt, A.; Scomazzon, S.P.; Leite, J.S.; de Bittencourt, P.I.H.; Tirapegui, J. Oral free and dipeptide forms of glutamine supplementation attenuate oxidative stress and inflammation induced by endotoxemia. Nutrition 2014, 30, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Aosasa, S.; Wells-Byrum, D.; Alexander, J.W.; Ogle, C.K. Influence of glutamine-supplemented caco-2 cells on cytokine production of mononuclear cells. J. Parenter. Enter. Nutr. 2003, 27, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Coeffier, M.; Claeyssens, S.; Hecketsweiler, B.; Lavoinne, A.; Ducrotte, P.; Dechelotte, P. Enteral glutamine stimulates protein synthesis and decreases ubiquitin mRNA level in human gut mucosa. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 285, G266–G273. [Google Scholar] [CrossRef] [PubMed]
- Jobin, C.; Hellerbrand, C.; Licato, L.L.; Brenner, D.A.; Sartor, R.B. Mediation by nf-kappa b of cytokine induced expression of intercellular adhesion molecule 1 (icam-1) in an intestinal epithelial cell line, a process blocked by proteasome inhibitors. Gut 1998, 42, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Coeffier, M.; Miralles-Barrachina, O.; Le Pessot, F.; Lalaude, O.; Daveau, M.; Lavoinne, A.; Lerebours, E.; Dechelotte, P. Influence of glutamine on cytokine production by human gut in vitro. Cytokine 2001, 13, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Tirapegui, J.; Cruzat, V. Glutamine and skeletal muscle. In Glutamine in Clinical Nutrition; Rajendram, R., Preedy, V.R., Patel, V.B., Eds.; Springer: New York, NY, USA, 2015; pp. 499–511. [Google Scholar]
- Cruzat, V.F.; Tirapegui, J. Effects of oral supplementation with glutamine and alanyl-glutamine on glutamine, glutamate, and glutathione status in trained rats and subjected to long-duration exercise. Nutrition 2009, 25, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Walsh, N.P.; Blannin, A.K.; Robson, P.J.; Gleeson, M. Glutamine, exercise and immune function. Links and possible mechanisms. Sports Med. 1998, 26, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Rowbottom, D.G.; Keast, D.; Morton, A.R. The emerging role of glutamine as an indicator of exercise stress and overtraining. Sports Med. 1996, 21, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Curi, R.; Newsholme, P.; Procopio, J.; Lagranha, C.; Gorjao, R.; Pithon-Curi, T.C. Glutamine, gene expression, and cell function. Front. Biosci. 2007, 12, 344–357. [Google Scholar] [CrossRef] [PubMed]
- Rogero, M.M.; Tirapegui, J.; Pedrosa, R.G.; de Castro, I.A.; Pires, I.S.D. Effect of alanyl-glutamine supplementation on plasma and tissue glutamine concentrations in rats submitted to exhaustive exercise. Nutrition 2006, 22, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Rogero, M.M.; Tirapegui, J.; Pedrosa, R.G.; Pires, I.S.D.; de Castro, I.A. Plasma and tissue glutamine response to acute and chronic supplementation with l-glutamine and l-alanyl-l-glutamine in rats. Nutr. Res. 2004, 24, 261–270. [Google Scholar] [CrossRef]
- Wagenmakers, A.J. Muscle amino acid metabolism at rest and during exercise: Role in human physiology and metabolism. Exerc. Sport Sci. Rev. 1998, 26, 287–314. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, A.L.; Chang, T.W. Regulation and significance of amino acid metabolism in skeletal muscle. Fed. Proc. 1978, 37, 2301–2307. [Google Scholar] [PubMed]
- Petry, E.R.; Cruzat, V.F.; Heck, T.G.; Leite, J.S.; Homem de Bittencourt, P.I.H.; Tirapegui, J. Alanyl-glutamine and glutamine plus alanine supplements improve skeletal redox status in trained rats: Involvement of heat shock protein pathways. Life Sci. 2014, 94, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Pedersen, B.K. Exercise and immune function. Recent developments. Sports Med. 1999, 27, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.M.; Broderius, M.A.; Fong, K.C.; Tsui, K.N.; Chew, S.F.; Ip, Y.K. Glutamine synthetase expression in liver, muscle, stomach and intestine of bostrichthys sinensis in response to exposure to a high exogenous ammonia concentration. J. Exp. Biol. 2002, 205, 2053–2065. [Google Scholar] [PubMed]
- Austgen, T.R.; Chakrabarti, R.; Chen, M.K.; Souba, W.W. Adaptive regulation in skeletal muscle glutamine metabolism in endotoxin-treated rats. J. Trauma 1992, 32, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Labow, B.I.; Souba, W.W.; Abcouwer, S.F. Glutamine synthetase expression in muscle is regulated by transcriptional and posttranscriptional mechanisms. Am. J. Physiol. 1999, 276, E1136–E1145. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Wen, H.Y.; Young, M.E.; Guthrie, P.H.; Taegtmeyer, H.; Kellems, R.E. Mammalian target of rapamycin and protein kinase a signaling mediate the cardiac transcriptional response to glutamine. J. Biolog. Chem. 2003, 278, 13143–13150. [Google Scholar] [CrossRef] [PubMed]
- Galley, H.F. Oxidative stress and mitochondrial dysfunction in sepsis. Br. J. Anaesth. 2011, 107, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Bode, B.P. Recent molecular advances in mammalian glutamine transport. J. Nutr. 2001, 131, 2475S–2486S. [Google Scholar] [CrossRef] [PubMed]
- Haussinger, D.; Schliess, F. Glutamine metabolism and signaling in the liver. Front. Biosci. 2007, 12, 371–391. [Google Scholar] [CrossRef] [PubMed]
- McGivan, J.D.; Bradford, N.M. Characteristics of the activation of glutaminase by ammonia in sonicated rat liver mitochondria. Biochim. Biophys. Acta 1983, 759, 296–302. [Google Scholar] [CrossRef]
- Hoek, J.B.; Charles, R.; De Haan, E.J.; Tager, J.M. Glutamate oxidation in rat-liver homogenate. Biochim. Biophys. Acta 1969, 172, 407–416. [Google Scholar] [CrossRef]
- Halestrap, A.P. The regulation of the matrix volume of mammalian mitochondria in vivo and in vitro and its role in the control of mitochondrial metabolism. Biochim. Biophys. Acta 1989, 973, 355–382. [Google Scholar] [CrossRef]
- Brosnan, J.T.; Brosnan, M.E. Hepatic glutaminase—A special role in urea synthesis? Nutrition 2002, 18, 455–457. [Google Scholar] [CrossRef]
- Meijer, A.J.; Verhoeven, A.J. Regulation of hepatic glutamine metabolism. Biochem. Soc. Trans. 1986, 14, 1001–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watford, M.; Smith, E.M. Distribution of hepatic glutaminase activity and mRNA in perivenous and periportal rat hepatocytes. Biochem. J. 1990, 267, 265–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moorman, A.F.; de Boer, P.A.; Watford, M.; Dingemanse, M.A.; Lamers, W.H. Hepatic glutaminase mRNA is confined to part of the urea cycle domain in the adult rodent liver lobule. FEBS Lett. 1994, 356, 76–80. [Google Scholar] [CrossRef] [Green Version]
- Gebhardt, R.; Mecke, D. Heterogeneous distribution of glutamine synthetase among rat liver parenchymal cells in situ and in primary culture. EMBO J. 1983, 2, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Häussinger, D.; Soboll, S.; Meijer, A.J.; Gerok, W.; Tager, J.M.; Sies, H. Role of plasma membrane transport in hepatic glutamine metabolism. Eur. J. Biochem. 1985, 152, 597–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenzen, C.; Soboll, S.; Sies, H.; Haussinger, D. Ph control of hepatic glutamine degradation. Role of transport. Eur. J. Biochem. 1987, 166, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Häussinger, D.; Hallbrucker, C.; Saha, N.; Lang, F.; Gerok, W. Cell volume and bile acid excretion. Biochem. J. 1992, 288, 681–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haussinger, D.; Lang, F. Cell volume in the regulation of hepatic function: A mechanism for metabolic control. Biochim. Biophys. Acta 1991, 1071, 331–350. [Google Scholar] [CrossRef]
- Gustafson, L.A.; Jumelle-Laclau, M.N.; van Woerkom, G.M.; van Kuilenburg, A.B.P.; Meijer, A.J. Cell swelling and glycogen metabolism in hepatocytes from fasted rats. Biochim. Biophys. Acta 1997, 1318, 184–190. [Google Scholar] [CrossRef]
- Baquet, A.; Gaussin, V.; Bollen, M.; Stalmans, W.; Hue, L. Mechanism of activation of liver acetyl-coa carboxylase by cell swelling. Eur. J. Biochem. 1993, 217, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Vom Dahl, S.; Dombrowski, F.; Schmitt, M.; Schliess, F.; Pfeifer, U.; Häussinger, D. Cell hydration controls autophagosome formation in rat liver in a microtubule-dependent way downstream from p38mapk activation. Biochem. J. 2001, 354, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Vom Dahl, S.; Haussinger, D. Nutritional state and the swelling-induced inhibition of proteolysis in perfused rat liver. J. Nutr. 1996, 126, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Häussinger, D.; Kubitz, R.; Reinehr, R.; Bode, J.G.; Schliess, F. Molecular aspects of medicine: From experimental to clinical hepatology. Mol. Asp. Med. 2004, 25, 221–360. [Google Scholar] [CrossRef] [PubMed]
- Jansen, L.T.; Adams, J.; Johnson, E.C.; Kavouras, S.A. Effects of cellular dehydration on glucose regulation in healthy males—A pilot study. FASEB J. 2017, 31, 1014-2. [Google Scholar]
- Friedman, S.L. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J. Biol. Chem. 2000, 275, 2247–2250. [Google Scholar] [CrossRef] [PubMed]
- Ghazwani, M.; Zhang, Y.; Gao, X.; Fan, J.; Li, J.; Li, S. Anti-fibrotic effect of thymoquinone on hepatic stellate cells. Phytomedicine 2014, 21, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ghazwani, M.; Liu, K.; Huang, Y.; Chang, N.; Fan, J.; He, F.; Li, L.; Bu, S.; Xie, W.; et al. Regulation of hepatic stellate cell proliferation and activation by glutamine metabolism. PLoS ONE 2017, 12, e0182679. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Cai, F.; Lin, N.; Ye, J.; Zheng, Q.; Ding, G. Effects of glutamine on oxidative stress and nuclear factor-κb expression in the livers of rats with nonalcoholic fatty liver disease. Exp. Ther. Med. 2014, 7, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Sellmann, C.; Baumann, A.; Brandt, A.; Jin, C.J.; Nier, A.; Bergheim, I. Oral supplementation of glutamine attenuates the progression of nonalcoholic steatohepatitis in c57bl/6j mice. J. Nutr. 2017, 147, 2041–2049. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, C.R.; Malafaia, O.; Torres, O.J.; Moreira, L.B.; Tefil, S.C.; Pinherio Mda, R.; Harada, B.A. Liver regeneration with l-glutamine supplemented diet: Experimental study in rats. Rev. Col. Bras. Cir. 2014, 41, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Helling, G.; Wahlin, S.; Smedberg, M.; Pettersson, L.; Tjäder, I.; Norberg, Å.; Rooyackers, O.; Wernerman, J. Plasma glutamine concentrations in liver failure. PLoS ONE 2016, 11, e0150440. [Google Scholar] [CrossRef] [PubMed]
- Eagle, H.; Oyama, V.I.; Levy, M.; Horton, C.L.; Fleischman, R. Growth response of mammalian cells in tissue culture to l-glutamine and l-glutamic acid. J. Biol. Chem. 1956, 218, 607–616. [Google Scholar] [PubMed]
- Newsholme, P.; Curi, R.; Gordon, S.; Newsholme, E.A. Metabolism of glucose, glutamine, long-chain fatty-acids and ketone-bodies by murine macrophages. Biochem. J. 1986, 239, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, E.A.; Newsholme, P.; Curi, R. The role of the citric acid cycle in cells of the immune system and its importance in sepsis, trauma and burns. Biochem. Soc. Symp. 1987, 54, 145–162. [Google Scholar] [PubMed]
- Curi, R.; Newsholme, P.; Newsholme, E.A. Intracellular-distribution of some enzymes of the glutamine utilization pathway in rat lymphocytes. Biochem. Biophys. Res. Commun. 1986, 138, 318–322. [Google Scholar] [CrossRef]
- Curi, T.C.P.; de Melo, M.P.; de Azevedo, R.B.; Curi, R. Glutamine utilisation by rat neutrophils. Biochem. Soc. Trans. 1997, 25, 249S. [Google Scholar] [CrossRef] [PubMed]
- Curi, T.C.P.; DeMelo, M.P.; DeAzevedo, R.B.; Zorn, T.M.T.; Curi, R. Glutamine utilization by rat neutrophils: Presence of phosphate-dependent glutaminase. Am. J. Physiol. Cell Physiol. 1997, 273, C1124–C1129. [Google Scholar] [CrossRef]
- Oudemans-van Straaten, H.M.; Bosman, R.J.; Treskes, M.; van der Spoel, H.J.; Zandstra, D.F. Plasma glutamine depletion and patient outcome in acute icu admissions. Intensiv. Care Med. 2001, 27, 84–90. [Google Scholar] [CrossRef]
- Leite, J.S.M.; Cruzat, V.F.; Krause, M.; Homem de Bittencourt, P.I. Physiological regulation of the heat shock response by glutamine: Implications for chronic low-grade inflammatory diseases in age-related conditions. Nutrire 2016, 41, 17. [Google Scholar] [CrossRef]
- Roth, E.; Oehler, R.; Manhart, N.; Exner, R.; Wessner, B.; Strasser, E.; Spittler, A. Regulative potential of glutamine—Relation to glutathione metabolism. Nutrition 2002, 18, 217–221. [Google Scholar] [CrossRef]
- Hiscock, N.; Petersen, E.W.; Krzywkowski, K.; Boza, J.; Halkjaer-Kristensen, J.; Pedersen, B.K. Glutamine supplementation further enhances exercise-induced plasma il-6. J. Appl. Physiol. 2003, 95, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.L.; Kelly, B.; O’Neill, L.A.J. Mitochondria are the powerhouses of immunity. Nat. Immunol. 2017, 18, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Pithon-Curi, T.C.; De Melo, M.P.; Curi, R. Glucose and glutamine utilization by rat lymphocytes, monocytes and neutrophils in culture: A comparative study. Cell Biochem. Funct. 2004, 22, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Pithon-Curi, T.C.; Trezena, A.G.; Tavares-Lima, W.; Curi, R. Evidence that glutamine is involved in neutrophil function. Cell Biochem. Funct. 2002, 20, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Branzk, N.; Lubojemska, A.; Hardison, S.E.; Wang, Q.; Gutierrez, M.G.; Brown, G.D.; Papayannopoulos, V. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat. Immunol. 2014, 15, 1017–1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pithon-Curi, T.C.; Levada, A.C.; Lopes, L.R.; Doi, S.Q.; Curi, R. Glutamine plays a role in superoxide production and the expression of p47(phox), p22(phox) and gp91(phox) in rat neutrophils. Clin. Sci. 2002, 103, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.; Pithon-Curi, T.C.; de Lourdes Firmano, M.; Pires de Melo, M.; Newsholme, P.; Curi, R. Effects of adrenaline on glucose and glutamine metabolism and superoxide production by rat neutrophils. Clin. Sci. 1999, 96, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, P.; Costa Rosa, L.F.; Newsholme, E.A.; Curi, R. The importance of fuel metabolism to macrophage function. Cell Biochem. Funct. 1996, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Peres, C.M.; Procopio, J.; Costa, M.; Curi, R. Thioglycolate-elicited rat macrophages exhibit alterations in incorporation and oxidation of fatty acids. Lipids 1999, 34, 1193–1197. [Google Scholar] [CrossRef] [PubMed]
- Costa Rosa, L.F.; Safi, D.A.; Curi, R. Effect of thioglycollate and bcg stimuli on glucose and glutamine metabolism in rat macrophages. J. Leukoc. Biol. 1994, 56, 10–14. [Google Scholar] [PubMed]
- Nagy, C.; Haschemi, A. Time and demand are two critical dimensions of immunometabolism: The process of macrophage activation and the pentose phosphate pathway. Front. Immunol. 2015, 6, 164. [Google Scholar] [CrossRef] [PubMed]
- Langston, P.K.; Shibata, M.; Horng, T. Metabolism supports macrophage activation. Front. Immunol. 2017, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Vergadi, E.; Ieronymaki, E.; Lyroni, K.; Vaporidi, K.; Tsatsanis, C. Akt signaling pathway in macrophage activation and m1/m2 polarization. J. Immunol. 2017, 198, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Sica, A.; Mantovani, A.; Locati, M. Macrophage activation and polarization. Front. Biosci. 2008, 13, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Taylor, P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005, 5, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Martinez, F.O. Alternative activation of macrophages: Mechanism and functions. Immunity 2010, 32, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A.; Pearce, E.J. Immunometabolism governs dendritic cell and macrophage function. J. Exp. Med. 2016, 213, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Namgaladze, D.; Brune, B. Fatty acid oxidation is dispensable for human macrophage il-4-induced polarization. Biochim. Biophys. Acta 2014, 1841, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A. A broken krebs cycle in macrophages. Immunity 2015, 42, 393–394. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O.; Wind, F.; Negelein, E. The metabolism of tumors in the body. J. Gen. Physiol. 1927, 8, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Palsson-McDermott, E.M.; Curtis, A.M.; Goel, G.; Lauterbach, M.A.; Sheedy, F.J.; Gleeson, L.E.; van den Bosch, M.W.; Quinn, S.R.; Domingo-Fernandez, R.; Johnston, D.G.; et al. Pyruvate kinase M2 regulates hif-1alpha activity and il-1beta induction and is a critical determinant of the warburg effect in lps-activated macrophages. Cell Metab. 2015, 21, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Oren, R.; Farnham, A.E.; Saito, K.; Milofsky, E.; Karnovsky, M.L. Metabolic patterns in three types of phagocytizing cells. J. Cell Biol. 1963, 17, 487–501. [Google Scholar] [CrossRef] [PubMed]
- Tannahill, G.M.; Curtis, A.M.; Adamik, J.; Palsson-McDermott, E.M.; McGettrick, A.F.; Goel, G.; Frezza, C.; Bernard, N.J.; Kelly, B.; Foley, N.H.; et al. Succinate is an inflammatory signal that induces il-1β through hif-1α. Nature 2013, 496, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.K.; Huang, S.C.; Sergushichev, A.; Lampropoulou, V.; Ivanova, Y.; Loginicheva, E.; Chmielewski, K.; Stewart, K.M.; Ashall, J.; Everts, B.; et al. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity 2015, 42, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.C.; Rice, C.M.; Palmieri, E.M.; Taylor, P.R.; Kuhns, D.B.; McVicar, D.W. Peritoneal tissue-resident macrophages are metabolically poised to engage microbes using tissue-niche fuels. Nat. Commun. 2017, 8, 2074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.S.; Wang, H.; Li, X.; Chao, T.; Teav, T.; Christen, S.; Di Conza, G.; Cheng, W.C.; Chou, C.H.; Vavakova, M.; et al. Alpha-ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat. Immunol. 2017, 18, 985–994. [Google Scholar] [PubMed]
- Nelson, V.L.; Nguyen, H.C.B.; Garcia-Canaveras, J.C.; Briggs, E.R.; Ho, W.Y.; DiSpirito, J.R.; Marinis, J.M.; Hill, D.A.; Lazar, M.A. Ppargamma is a nexus controlling alternative activation of macrophages via glutamine metabolism. Gen. Dev. 2018, 32, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Greiner, E.F.; Guppy, M.; Brand, K. Glucose is essential for proliferation and the glycolytic enzyme induction that provokes a transition to glycolytic energy production. J. Biol. Chem. 1994, 269, 31484–31490. [Google Scholar] [PubMed]
- Newsholme, E.A.; Crabtree, B.; Ardawi, M.S. Glutamine metabolism in lymphocytes: Its biochemical, physiological and clinical importance. Q. J. Exp. Physiol. 1985, 70, 473–489. [Google Scholar] [CrossRef] [PubMed]
- Curi, R.; Newsholme, P.; Newsholme, E.A. Metabolism of pyruvate by isolated rat mesenteric lymphocytes, lymphocyte mitochondria and isolated mouse macrophages. Biochem. J. 1988, 250, 383–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maciolek, J.A.; Pasternak, J.A.; Wilson, H.L. Metabolism of activated t lymphocytes. Curr. Opin. Immunol. 2014, 27, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Tripmacher, R.; Gaber, T.; Dziurla, R.; Haupl, T.; Erekul, K.; Grutzkau, A.; Tschirschmann, M.; Scheffold, A.; Radbruch, A.; Burmester, G.R.; et al. Human cd4(+) T cells maintain specific functions even under conditions of extremely restricted ATP production. Eur. J. Immunol. 2008, 38, 1631–1642. [Google Scholar] [CrossRef] [PubMed]
- Wieman, H.L.; Wofford, J.A.; Rathmell, J.C. Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/akt regulation of glut1 activity and trafficking. Mol. Biol. Cell 2007, 18, 1437–1446. [Google Scholar] [CrossRef] [PubMed]
- Delgoffe, G.M.; Kole, T.P.; Zheng, Y.; Zarek, P.E.; Matthews, K.L.; Xiao, B.; Worley, P.F.; Kozma, S.C.; Powell, J.D. The mtor kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity 2009, 30, 832–844. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Gudapati, P.; Dragovic, S.; Spencer, C.; Joyce, S.; Killeen, N.; Magnuson, M.A.; Boothby, M. Mammalian target of rapamycin protein complex 2 regulates differentiation of th1 and th2 cell subsets via distinct signaling pathways. Immunity 2010, 32, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Michalek, R.D.; Gerriets, V.A.; Jacobs, S.R.; Macintyre, A.N.; MacIver, N.J.; Mason, E.F.; Sullivan, S.A.; Nichols, A.G.; Rathmell, J.C. Cutting edge: Distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory cd4+ T cell subsets. J. Immunol. 2011, 186, 3299–3303. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Hawley, S.A.; Scott, J.W. Amp-activated protein kinas—Development of the energy sensor concept. J. Physiol. 2006, 574, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Crawford, J.; Cohen, H.J. The essential role of l-glutamine in lymphocyte differentiation in vitro. J. Cell. Physiol. 1985, 124, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Matarese, G.; Colamatteo, A.; De Rosa, V. Metabolic fuelling of proper t cell functions. Immunol. Lett. 2014, 161, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Curtis, J.D.; Maggi, L.B., Jr.; Faubert, B.; Villarino, A.V.; O’Sullivan, D.; Huang, S.C.; van der Windt, G.J.; Blagih, J.; Qiu, J.; et al. Posttranscriptional control of t cell effector function by aerobic glycolysis. Cell 2013, 153, 1239–1251. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Delgoffe, G.M.; Meyer, C.F.; Chan, W.; Powell, J.D. Anergic T cells are metabolically anergic. J. Immunol. 2009, 183, 6095–6101. [Google Scholar] [CrossRef] [PubMed]
- Buck, M.D.; O’Sullivan, D.; Klein Geltink, R.I.; Curtis, J.D.; Chang, C.H.; Sanin, D.E.; Qiu, J.; Kretz, O.; Braas, D.; van der Windt, G.J.; et al. Mitochondrial dynamics controls T cell fate through metabolic programming. Cell 2016, 166, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, S.E.; O’Neill, L.A. Hif1alpha and metabolic reprogramming in inflammation. J. Clin. Investig. 2016, 126, 3699–3707. [Google Scholar] [CrossRef] [PubMed]
- Araujo, L.; Khim, P.; Mkhikian, H.; Mortales, C.L.; Demetriou, M. Glycolysis and glutaminolysis cooperatively control T cell function by limiting metabolite supply to n-glycosylation. eLife 2017, 6, e21330. [Google Scholar] [CrossRef] [PubMed]
- Hesterberg, R.S.; Cleveland, J.L.; Epling-Burnette, P.K. Role of polyamines in immune cell functions. Med. Sci. 2018, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Yaqoob, P. Glutamine and the immune system. Amino Acids 1999, 17, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Wilmore, D.W.; Shabert, J.K. Role of glutamine in immunologic responses. Nutrition 1998, 14, 618–626. [Google Scholar] [CrossRef]
- Lagranha, C.J.; Hirabara, S.M.; Curi, R.; Pithon-Curi, T.C. Glutamine supplementation prevents exercise-induced neutrophil apoptosis and reduces p38 mapk and jnk phosphorylation and p53 and caspase 3 expression. Cell Biochem. Funct. 2007, 25, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Young, V.R.; Ajami, A.M. Glutamine: The emperor or his clothes? J. Nutr. 2001, 131, 2447S–2486S. [Google Scholar] [CrossRef] [PubMed]
- Meister, A.; Anderson, M.E. Glutathione. Ann. Rev. Biochem. 1983, 52, 711–760. [Google Scholar] [CrossRef] [PubMed]
- Gaucher, C.; Boudier, A.; Bonetti, J.; Clarot, I.; Leroy, P.; Parent, M. Glutathione: Antioxidant properties dedicated to nanotechnologies. Antioxidants 2018, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Ma, X.; Luo, X.; Zhang, Y.; He, Y.; Dai, Z.; Yang, Y.; Wu, G.; Wu, Z. l-glutamine attenuates apoptosis in porcine enterocytes by regulating glutathione-related redox homeostasis. J. Nutr. 2018, 148, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Lima, F.; Rogero, M.M.; Ramos, M.C.; Borelli, P.; Fock, R.A. Modulation of the nuclear factor-kappa b (nf-kappab) signalling pathway by glutamine in peritoneal macrophages of a murine model of protein malnutrition. Eur. J. Nutr. 2013, 52, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Smedberg, M.; Wernerman, J. Is the glutamine story over? Crit. Care 2016, 20, 361. [Google Scholar] [CrossRef] [PubMed]
- Heck, T.G.; Scholer, C.M.; de Bittencourt, P.I. Hsp70 expression: Does it a novel fatigue signalling factor from immune system to the brain? Cell Biochem. Funct. 2011, 29, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Singleton, K.D.; Wischmeyer, P.E. Glutamine’s protection against sepsis and lung injury is dependent on heat shock protein 70 expression. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R1839–R1845. [Google Scholar] [CrossRef] [PubMed]
- Jordan, I.; Balaguer, M.; Esteban, M.E.; Cambra, F.J.; Felipe, A.; Hernandez, L.; Alsina, L.; Molero, M.; Villaronga, M.; Esteban, E. Glutamine effects on heat shock protein 70 and interleukines 6 and 10: Randomized trial of glutamine supplementation versus standard parenteral nutrition in critically ill children. Clin. Nutr. 2016, 35, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Meriin, A.B.; Gabai, V.L.; Christians, E.; Benjamin, I.; Wilson, A.; Wolozin, B.; Sherman, M.Y. The heat shock transcription factor hsf1 is downregulated in DNA damage-associated senescence, contributing to the maintenance of senescence phenotype. Aging Cell 2012, 11, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Gabai, V.L.; Meng, L.; Kim, G.; Mills, T.A.; Benjamin, I.J.; Sherman, M.Y. Heat shock transcription factor hsf1 is involved in tumor progression via regulation of hypoxia-inducible factor 1 and RNA-binding protein HUR. Mol. Cell. Biol. 2012, 32, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Dokladny, K.; Zuhl, M.N.; Mandell, M.; Bhattacharya, D.; Schneider, S.; Deretic, V.; Moseley, P.L. Regulatory coordination between two major intracellular homeostatic systems: Heat shock response and autophagy. J. Biolog. Chem. 2013, 288, 14959–14972. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.R.; Dias, T.B.; Natov, P.S.; Zachara, N.E. Stress-induced o-glcnacylation: An adaptive process of injured cells. Biochem. Soc. Trans. 2017, 45, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Lafontaine-Lacasse, M.; Dore, G.; Picard, F. Hexosamines stimulate apoptosis by altering sirt1 action and levels in rodent pancreatic beta-cells. J. Endoc. 2011, 208, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, Z.; Chang, H.; Haserodt, S.; McKen, C.; Zachara, N.E. O-linked beta-n-acetylglucosamine (o-glcnac) regulates stress-induced heat shock protein expression in a gsk-3beta-dependent manner. J. Biol. Chem. 2010, 285, 39096–39107. [Google Scholar] [CrossRef] [PubMed]
- Hamiel, C.R.; Pinto, S.; Hau, A.; Wischmeyer, P.E. Glutamine enhances heat shock protein 70 expression via increased hexosamine biosynthetic pathway activity. Am. J. Physiol. Cell Physiol. 2009, 297, C1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Singleton, K.D.; Serkova, N.; Beckey, V.E.; Wischmeyer, P.E. Glutamine attenuates lung injury and improves survival after sepsis: Role of enhanced heat shock protein expression. Crit. Care Med. 2005, 33, 1206–1213. [Google Scholar] [CrossRef] [PubMed]
- Raizel, R.; Leite, J.S.; Hypolito, T.M.; Coqueiro, A.Y.; Newsholme, P.; Cruzat, V.F.; Tirapegui, J. Determination of the anti-inflammatory and cytoprotective effects of l-glutamine and l-alanine, or dipeptide, supplementation in rats submitted to resistance exercise. Br. J. Nutr. 2016, 116, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Smolka, M.B.; Zoppi, C.C.; Alves, A.A.; Silveira, L.R.; Marangoni, S.; Pereira-Da-Silva, L.; Novello, J.C.; Macedo, D.V. Hsp72 as a complementary protection against oxidative stress induced by exercise in the soleus muscle of rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, R1539–R1545. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Cooper, Z.A.; Tulapurkar, M.E.; Potla, R.; Maity, T.; Hasday, J.D.; Singh, I.S. Toll-like receptor agonists and febrile range hyperthermia synergize to induce heat shock protein 70 expression and extracellular release. J. Biolog. Chem. 2013, 288, 2756–2766. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.; Keane, K.; Rodrigues-Krause, J.; Crognale, D.; Egan, B.; De Vito, G.; Murphy, C.; Newsholme, P. Elevated levels of extracellular heat-shock protein 72 (ehsp72) are positively correlated with insulin resistance in vivo and cause pancreatic beta-cell dysfunction and death in vitro. Clin. Sci. 2014, 126, 739–752. [Google Scholar] [CrossRef] [PubMed]
- Lenders, C.M.; Liu, S.; Wilmore, D.W.; Sampson, L.; Dougherty, L.W.; Spiegelman, D.; Willett, W.C. Evaluation of a novel food composition database that includes glutamine and other amino acids derived from gene sequencing data. Eur. J. Clin. Nutr. 2009, 63, 1433–1439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermans, G.; Van den Berghe, G. Clinical review: Intensive care unit acquired weakness. Crit. Care 2015, 19, 274. [Google Scholar] [CrossRef] [PubMed]
- Stehle, P.; Ellger, B.; Kojic, D.; Feuersenger, A.; Schneid, C.; Stover, J.; Scheiner, D.; Westphal, M. Glutamine dipeptide-supplemented parenteral nutrition improves the clinical outcomes of critically ill patients: A systematic evaluation of randomised controlled trials. Clin. Nutr. ESPEN 2017, 17, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Gunst, J.; Vanhorebeek, I.; Thiessen, S.E.; Van den Berghe, G. Amino acid supplements in critically ill patients. Pharmacol. Res. 2018, 130, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Furst, P.; Alteheld, B.; Stehle, P. Why should a single nutrient—Glutamine—Improve outcome? The remarkable story of glutamine dipeptides. Clin. Nutr. Suppl. 2004, 1, 3–15. [Google Scholar] [CrossRef]
- Grau, T.; Bonet, A.; Minambres, E.; Pineiro, L.; Irles, J.A.; Robles, A.; Acosta, J.; Herrero, I.; Palacios, V.; Lopez, J.; et al. The effect of l-alanyl-l-glutamine dipeptide supplemented total parenteral nutrition on infectious morbidity and insulin sensitivity in critically ill patients. Crit. Care Med. 2011, 39, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Estivariz, C.F.; Griffith, D.P.; Luo, M.; Szeszycki, E.E.; Bazargan, N.; Dave, N.; Daignault, N.M.; Bergman, G.F.; McNally, T.; Battey, C.H.; et al. Efficacy of parenteral nutrition supplemented with glutamine dipeptide to decrease hospital infections in critically ill surgical patients. J. Parenter. Enter. Nutr. 2008, 32, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, Z.M.; Nolan, M.T.; Jiang, H.; Han, H.R.; Yu, K.; Li, H.L.; Jie, B.; Liang, X.K. The impact of glutamine dipeptide-supplemented parenteral nutrition on outcomes of surgical patients: A meta-analysis of randomized clinical trials. J. Parenter. Enter. Nutr. 2010, 34, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Bollhalder, L.; Pfeil, A.M.; Tomonaga, Y.; Schwenkglenks, M. A systematic literature review and meta-analysis of randomized clinical trials of parenteral glutamine supplementation. Clin. Nutr. 2013, 32, 213–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dechelotte, P.; Hasselmann, M.; Cynober, L.; Allaouchiche, B.; Coeffier, M.; Hecketsweiler, B.; Merle, V.; Mazerolles, M.; Samba, D.; Guillou, Y.M.; et al. l-alanyl-l-glutamine dipeptide-supplemented total parenteral nutrition reduces infectious complications and glucose intolerance in critically ill patients: The french controlled, randomized, double-blind, multicenter study. Crit. Care Med. 2006, 34, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Weitzel, L.R.; Wischmeyer, P.E. Glutamine in critical illness: The time has come, the time is now. Crit. Care Clin. 2010, 26, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Klassen, P.; Mazariegos, M.; Solomons, N.W.; Furst, P. The pharmacokinetic responses of humans to 20 g of alanyl-glutamine dipeptide differ with the dosing protocol but not with gastric acidity or in patients with acute dengue fever. J. Nutr. 2000, 130, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Melis, G.C.; Boelens, P.G.; van der Sijp, J.R.; Popovici, T.; De Bandt, J.P.; Cynober, L.; van Leeuwen, P.A. The feeding route (enteral or parenteral) affects the plasma response of the dipetide ala-gln and the amino acids glutamine, citrulline and arginine, with the administration of ala-gln in preoperative patients. Br. J. Nutr. 2005, 94, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.S.; de Bittencourt, P.I.H.J. Type 1 diabetes: Can exercise impair the autoimmune event? The l-arginine/glutamine coupling hypothesis. Cell Biochem. Funct. 2008, 26, 406–433. [Google Scholar] [CrossRef] [PubMed]
- Adibi, S.A. Regulation of expression of the intestinal oligopeptide transporter (pept-1) in health and disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 285, G779–G788. [Google Scholar] [CrossRef] [PubMed]
- Broer, S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol. Rev. 2008, 88, 249–286. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, E.R.; Wong, E.A.; Webb, K.E. Board-invited review: Peptide absorption and utilization: Implications for animal nutrition and health. J. Anim. Sci. 2008, 86, 2135–2155. [Google Scholar] [CrossRef] [PubMed]
- Petry, E.R.; Cruzat, V.F.; Heck, T.G.; de Bittencourt, P.I.H.; Tirapegui, J. l-glutamine supplementations enhance liver glutamine-glutathione axis and heat shock factor-1 expression in endurance-exercise trained rats. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Alba-Loureiro, T.C.; Ribeiro, R.F.; Zorn, T.M.; Lagranha, C.J. Effects of glutamine supplementation on kidney of diabetic rat. Amino Acids 2010, 38, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Sudderth, J.; Yang, C.; Mullen, A.R.; Jin, E.S.; Mates, J.M.; DeBerardinis, R.J. Pyruvate carboxylase is required for glutamine-independent growth of tumor cells. Proc. Natl. Acad Sci. USA 2011, 108, 8674–8679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hensley, C.T.; Wasti, A.T.; DeBerardinis, R.J. Glutamine and cancer: Cell biology, physiology, and clinical opportunities. J. Clin. Investig. 2013, 123, 3678–3684. [Google Scholar] [CrossRef] [PubMed]
- Hensley, C.T.; Faubert, B.; Yuan, Q.; Lev-Cohain, N.; Jin, E.; Kim, J.; Jiang, L.; Ko, B.; Skelton, R.; Loudat, L.; et al. Metabolic heterogeneity in human lung tumors. Cell 2016, 164, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.M.; Papagiannakopoulos, T.; Olenchock, B.A.; Heyman, J.E.; Keibler, M.A.; Luengo, A.; Bauer, M.R.; Jha, A.K.; O’Brien, J.P.; Pierce, K.A.; et al. Environment impacts the metabolic dependencies of ras-driven non-small cell lung cancer. Cell Metab. 2016, 23, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.; Ganji, S.; Hulsey, K.; Madan, A.; Kovacs, Z.; Dimitrov, I.; Zhang, S.; Pichumani, K.; Mendelsohn, D.; Mickey, B.; et al. A comparative study of short- and long-te (1) h mrs at 3 t for in vivo detection of 2-hydroxyglutarate in brain tumors. NMR Biomed. 2013, 26, 1242–1250. [Google Scholar] [CrossRef] [PubMed]
- Tardito, S.; Oudin, A.; Ahmed, S.U.; Fack, F.; Keunen, O.; Zheng, L.; Miletic, H.; Sakariassen, P.O.; Weinstock, A.; Wagner, A.; et al. Glutamine synthetase activity fuels nucleotide biosynthesis and supports growth of glutamine-restricted glioblastoma. Nat. Cell Biol. 2015, 17, 1556–1568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deep, G.; Schlaepfer, I.R. Aberrant lipid metabolism promotes prostate cancer: Role in cell survival under hypoxia and extracellular vesicles biogenesis. Int. J. Mol. Sci. 2016, 17, 1061. [Google Scholar] [CrossRef] [PubMed]
- White, M.A.; Lin, C.; Rajapakshe, K.; Dong, J.; Shi, Y.; Tsouko, E.; Mukhopadhyay, R.; Jasso, D.; Dawood, W.; Coarfa, C.; et al. Glutamine transporters are targets of multiple oncogenic signaling pathways in prostate cancer. Mol. Cancer Res. 2017, 15, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Marian, M.J. Dietary supplements commonly used by cancer survivors: Are there any benefits? Nutr. Clin. Pract. 2017, 32, 607–627. [Google Scholar] [CrossRef] [PubMed]
- Sayles, C.; Hickerson, S.C.; Bhat, R.R.; Hall, J.; Garey, K.W.; Trivedi, M.V. Oral glutamine in preventing treatment-related mucositis in adult patients with cancer: A systematic review. Nutr. Clin. Pract. 2016, 31, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Daniele, B.; Perrone, F.; Gallo, C.; Pignata, S.; De Martino, S.; De Vivo, R.; Barletta, E.; Tambaro, R.; Abbiati, R.; D’Agostino, L. Oral glutamine in the prevention of fluorouracil induced intestinal toxicity: A double blind, placebo controlled, randomised trial. Gut 2001, 48, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Hammarqvist, F.; Wernerman, J.; Ali, R.; von der Decken, A.; Vinnars, E. Addition of glutamine to total parenteral nutrition after elective abdominal surgery spares free glutamine in muscle, counteracts the fall in muscle protein synthesis, and improves nitrogen balance. Ann. Surg. 1989, 209, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Souba, W.W.; Herskowitz, K.; Klimberg, V.S.; Salloum, R.M.; Plumley, D.A.; Flynn, T.C.; Copeland, E.M. The effects of sepsis and endotoxemia on gut glutamine metabolism. Ann. Surg. 1990, 211, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Bode, B.P.; Fuchs, B.C.; Hurley, B.P.; Conroy, J.L.; Suetterlin, J.E.; Tanabe, K.K.; Rhoads, D.B.; Abcouwer, S.F.; Souba, W.W. Molecular and functional analysis of glutamine uptake in human hepatoma and liver-derived cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 283, G1062–G1073. [Google Scholar] [CrossRef] [PubMed]
- Rogero, M.M.; Borelli, P.; Fock, R.A.; Borges, M.C.; Vinolo, M.A.R.; Curi, R.; Nakajima, K.; Crisma, A.R.; Ramos, A.D.; Tirapegui, J. Effects of glutamine on the nuclear factor-kappab signaling pathway of murine peritoneal macrophages. Amino Acids 2010, 39, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Parry-Billings, M.; Evans, J.; Calder, P.C.; Newsholme, E.A. Does glutamine contribute to immunosuppression after major burns? Lancet 1990, 336, 523–525. [Google Scholar] [CrossRef]
- Roth, E.; Funovics, J.; Muhlbacher, F.; Schemper, M.; Mauritz, W.; Sporn, P.; Fritsch, A. Metabolic disorders in severe abdominal sepsis: Glutamine deficiency in skeletal muscle. Clin. Nutr. 1982, 1, 25–41. [Google Scholar] [CrossRef]



| g/100g Food | Beef | Skim Milk | White Rice | Corn | Tofu | Egg |
|---|---|---|---|---|---|---|
| Total protein | 25.9 | 3.4 | 2.7 | 2.5 | 6.6 | 12.6 |
| Glutamine | 1.2 | 0.3 | 0.3 | 0.4 | 0.6 | 0.6 |
| Glutamate | 2.7 | 0.4 | 0.2 | 0.05 | 0.7 | 1.0 |
| Leucine | 2.2 | 0.4 | 0.2 | 0.4 | 0.5 | 0.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruzat, V.; Macedo Rogero, M.; Noel Keane, K.; Curi, R.; Newsholme, P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients 2018, 10, 1564. https://doi.org/10.3390/nu10111564
Cruzat V, Macedo Rogero M, Noel Keane K, Curi R, Newsholme P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients. 2018; 10(11):1564. https://doi.org/10.3390/nu10111564
Chicago/Turabian StyleCruzat, Vinicius, Marcelo Macedo Rogero, Kevin Noel Keane, Rui Curi, and Philip Newsholme. 2018. "Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation" Nutrients 10, no. 11: 1564. https://doi.org/10.3390/nu10111564
APA StyleCruzat, V., Macedo Rogero, M., Noel Keane, K., Curi, R., & Newsholme, P. (2018). Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients, 10(11), 1564. https://doi.org/10.3390/nu10111564






