Evaluation of Antioxidant and Free Radical Scavenging Capacities of Polyphenolics from Pods of Caesalpinia pulcherrima
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation of Phenolic Compounds of C. pulcherrima
2.2. Antioxidant Activities
2.2.1. DPPH Radical-Scavenging Effect
2.2.2. Hydroxyl Radical-scavenging Activity
2.2.3. Peroxynitrite Radical Detection
3. Experimental Section
3.1. Chemicals and Reagents
3.2. General Chemical Experiment
3.3. Plant Material
3.4. Extraction and Isolation
3.5. Characterization Data
3.6. DPPH Radical-Scavenging Effect
3.7. Hydroxyl Radical-Scavenging Activity
3.8. Peroxynitrite (PON) Radical Detection
3.8.1. Synthesis of PON
3.8.2. Assay of PON-Mediated Oxidation of Dihydrorhodamine (DHR) 123
4. Conclusions
Acknowledgments
References
- McDermott, J.H. Antioxidant nutrients: Current dietary recommendations and research update. J. Am. Pharm. Assoc 2000, 40, 785–799. [Google Scholar]
- Castro, L.; Freeman, B.A. Reactive oxygen species in human health and disease. Nutrition 2001, 17, 163–165. [Google Scholar]
- Gulcin, I.; Buyukokuroglu, M.E.; Oktay, M.; Kufreviouglu, O.I. On the in vitro antioxidative properties of melatonin. J. Pineal. Res 2002, 33, 167–171. [Google Scholar]
- Jackson, M.J.; Papa, S.; Bolanos, J.; Bruckdorfer, R.; Carlsen, H.; Elliott, R.M. Antioxidants, reactive oxygen and nitrogen species, gene induction and mitochondrial function. Mol. Aspects. Med 2002, 23, 209–285. [Google Scholar]
- Fylaktakidou, K.C.; Hadjipavlou-Litina, D.J.; Litinas, K.E.; Nicolaides, D.N. Natural and synthetic coumarin derivatives with anti-inflammatory/antioxidant activities. Curr. Pharm. Des 2004, 10, 3813–3833. [Google Scholar]
- Stanczyk, M.; Gromadzinska, J.; Wasowicz, W. Roles of reactive oxygen species and selected antioxidants in regulation of cellular metabolism. Int. J. Occup. Med. Env 2005, 18, 15–26. [Google Scholar]
- Alonso-García, A.; Cancho-Grande, B.; Simal-Gándara, J. Development of a rapid method based on solid-phase extraction and liquid chromatography with ultraviolet detection for the determination of polyphenols in alcohol-free beers. J. Chromatogr. A 2004, 1054, 175–180. [Google Scholar]
- Pérez-Lamela, C.; García-Falcon, M.S.; Simal-Gándara, J.; Orriols-Fernández, I. Influence of grape variety, vine system and enological treatments on the colour stability of young red wines. Food Chem 2007, 101, 601–606. [Google Scholar]
- Alén-Ruiz, F.; Pérez-Gregorio, M.R.; Martínez-Carballo, E.; García-Falcón, M.S.; Simal-Gándara, J. Influence of polyphenols on colour and antioxidant value in plant foods. Electron. J. Environ. Agric. Food Chem 2008, 7, 3171–3176. [Google Scholar]
- Pérez-Gregorio, M.R.; García-Falcon, M.S.; Simal-Gándara, J.; Rodrigues, A.S.; Almeida, D.P.F. Identification and quantification of flavonoids in traditional cultivars of red and white onions at harvest. J. Food Compos. Anal 2010, 23, 592–598. [Google Scholar]
- Rodrigues, A.S.; Pérez-Gregorio, M.R.; García-Falcon, M.S.; Simal-Gándara, J. Effect of curing and cooking on flavonols and anthocyanins in traditional varieties of onion bulbs. Food Res. Int 2009, 42, 1331–1336. [Google Scholar]
- Rodrigues, A.S.; Pérez-Gregorio, M.R.; García-Falcon, M.S.; Simal-Gándara, J. Effect of post-harvest practices on flavonoid content of red and white onion cultivars. Food Control 2010, 21, 878–884. [Google Scholar]
- Rodrigues, A.S.; Pérez-Gregorio, M.R.; García-Falcon, M.S.; Simal-Gándara, J.; Almeida, D.P.F. Effect of meteorological conditions on antioxidant flavonoids in Portuguese cultivars of white and red onions. Food Chem 2011, 124, 303–308. [Google Scholar]
- Pérez-Gregorio, M.R.; García-Falcon, M.S.; Simal-Gándara, J. Flavonoids changes in fresh-cut onions during storage in different packaging systems. Food Chem 2011, 124, 652–658. [Google Scholar]
- Pérez-Gregorio, M.R.; Regueiro, J.; González-Barreiro, C.; Rial-Oterol, R.; Simal-Gándara, J. Changes in antioxidant flavonoids during freeze-drying of red onions and subsequent storage. Food Control 2011, 22, 1108–1113. [Google Scholar]
- Pérez-Gregorio, M.R.; González-Barreiro, C.; Rial-Oterol, R.; Simal-Gándara, J. Comparison of sanitizing technologies on the quality appearance and antioxidant levels in onion slices. Food Control 2011, 22, 2052–2058. [Google Scholar]
- Chanda, S.; Baravalia, Y. Brine shrimp cytotoxicity of Caesalpinia pulcherrima aerial parts, antimicrobial activity and characterisation of isolated active fractions. Nat. Prod. Res 2011, 25, 1955–1964. [Google Scholar]
- Promsawan, N.; Kittakoop, P.; Boonphong, S.; Nongkunsarn, P. Antitubercular cassane furanoditerpenoids from the roots of Caesalpinia pulcherrima. Planta Med 2003, 69, 776–777. [Google Scholar]
- Chiang, L.C.; Chiang, W.; Liu, M.C.; Lin, C.C. In vitro antiviral activities of Caesalpinia pulcherrima and its related flavonoids. J. Antimicrob. Chemother 2003, 52, 194–198. [Google Scholar]
- Sharma, V.; Rajani, G.P. Evaluation of Caesalpinia pulcherrima Linn. for anti-inflammatory and antiulcer activities. Indian J. Pharmacol 2011, 43, 168–171. [Google Scholar]
- Rao, Y.K.; Fang, S.H.; Tzeng, Y.M. Anti-inflammatory activities of flavonoids isolated from Caesalpinia pulcherrima. J. Ethnopharmacol 2005, 100, 249–253. [Google Scholar]
- Pawar, C.R.; Mutha, R.E.; Landge, A.D.; Jadhav, R.B.; Surana, S.J. Antioxidant and cytotoxic activities of Caesalpinia pulcherrima wood. Indian J. Biochem. Biophys 2009, 46, 198–200. [Google Scholar]
- Chew, Y.L.; ling Chan, E.W.; Tan, P.L.; Lim, Y.Y.; Stanslas, J.; Goh, J.K. Assessment of phytochemical content, polyphenolic composition, antioxidant and antibacterial activities of leguminosae medicinal plants in Peninsular Malysia. BMC Complem. Altern. M 2011, 11. [Google Scholar] [CrossRef] [Green Version]
- Che, C.T.; McPherson, D.D.; Cordell, G.A.; Fong, H.H.S. Pulcherralpin, a new diterpene ester from Caesalpinia pulcherrima. J. Nat. Prod 1986, 49, 561–569. [Google Scholar]
- Patel, A.D.; Freyer, A.J.; Webb, R.L.; Zuber, G.; Reichwein, R.; Bean, M.F.; Faucette, L.; Johnson, R.K. Pulcherrimins A-D, diterpene dibenzoates from Caesalpinia pulcherrima with selective activity against DNA repair-deficient yeast mutants. Tetrahedron 1997, 53, 1583–1592. [Google Scholar]
- Ragasa, C.Y.; Hofilena, J.G.; Rideout, J.A. New furanoid diterpenes from Caesalpinia pulcherrima. J. Nat. Prod 2002, 65, 1107–1110. [Google Scholar]
- Roach, J.S.; McLean, S.; Reynolds, W.F.; Tinto, W.F. Cassane diterpenoids of Caesalpinia pulcherrima. J. Nat. Prod 2003, 66, 1378–1381. [Google Scholar]
- Pranithanchai, W.; Karalai, C.; Ponglimanont, C.; Subhadhirasakul, S.; Chantrapromma, K. Cassane diterpenoids from the stem of Caesalpinia pulcherrima. Phytochemistry 2009, 70, 300–304. [Google Scholar]
- Yodsaoue, O.; Karalai, C.; Ponglimanont, C.; Tewtrakul, S.; Chantrapromma, S. Pulcherrins D-R, potential anti-inflammatory diterpenoids from the roots of Caesalpinia pulcherrima. Tetrahedron 2011, 67, 6838–6846. [Google Scholar]
- McPherson, D.D.; Cordell, G.A.; Soejarto, D.D.; Pezzuto, J.M.; Fong, H.H.S. Peltogynoids and homoisoflavonoids from Caesalpinia pulcherrima. Phytochemistry 1983, 22, 2835–2838. [Google Scholar]
- Srinivas, K.V.N.S.; Rao, Y.K.; Mahender, I.; Das, B.; Rama Krishna, K.V.S.; Harakishore, K.; Murty, U.S.N. Flavonoids from Caesalpinia pulcherrima. Phytochemistry 2003, 63, 789–793. [Google Scholar]
- Maheswara, M.; Siddaiah, V.; Venkata Rao, C. Two new homoisoflavonoids from Caesalpinia pulcherrima. Chem. Pharm. Bull 2006, 54, 1193–1195. [Google Scholar]
- Gottlieb, H.E.; Kumar, S.; Sahai, M.; Ray, A.B. Ethyl brevifolin carboxylate from Flueggea microcarpa. Phytochemistry 1991, 30, 2435–2438. [Google Scholar]
- Nishioka, T.; Kawabata, J.; Aoyama, Y. Baicalein, an α-glucosidase inhibitor from Scutellaria baicalensis. J. Nat. Prod 1998, 61, 1413–1415. [Google Scholar]
- Kashiwada, Y.; Nonaka, G.; Nishioka, I. Tannins and related compounds. XXIII. Rhubarb(4): Isolation and structure of new classes of gallotannins. Chem. Pharm. Bull 1984, 32, 3461–3470. [Google Scholar]
- Nawwar, M.A.M.; Hussein, S.A.M. Gall polyphenolics of Tamarix aphylla. Phytochemistry 1994, 36, 1035–1037. [Google Scholar]
- Tanaka, T.; Nonaka, G.; Nishioka, I. Tannins and related compounds. XVI. Isolation and characterization of six methyl glucoside gallate and a gallic acid glucoside gallate from Sanguisorba officinalis L. Chem. Pharm. Bull 1984, 32, 117–121. [Google Scholar]
- Ishimaru, K.; Nonaka, G.; Nishioka, I. Phenol glucoside gallate from Quercus mongolica. Phytochemistry 1987, 26, 1147–1152. [Google Scholar]
- Saijo, R.; Nonaka, G.I.; Nishioka, I. Phenol glucoside gallate from Mallotus japonicus. Phytochemistry 1989, 28, 2443–2446. [Google Scholar]
- Foo, L.Y.; Lu, Y.; Molar, A.L.; Woodfield, D.R.; McNabb, W.C. The phenols and prodelphinidins of white clove flowers. Phytochemistry 2000, 54, 539–548. [Google Scholar]
- Nonaka, G.I.; Nishioka, I.; Nagasawa, T.; Oura, H. Tannins and related compounds I. Rhubarb. Chem. Pharm. Bull 1981, 29, 2862–2870. [Google Scholar]
- Fossen, T.; Larsen, A.; Kiremire, B.T.; Andersen, O.M. Flavonoids from blue flowers of Nymphaea caerulea. Phytochemistry 1999, 51, 1133–1137. [Google Scholar]
- Lundgern, L.N.; Theande, O. Cis- and trans-dihydroquercetin glucosides from needless of Pinus sylvestris. Phytochemistry 1988, 27, 829–832. [Google Scholar]
- Yokozawa, T.; Chen, C.P.; Dong, E.; Tanaka, T.; Nonaka, G.; Nishioka, I. Study on the Inhibitory Effect of Tannins and Flavonoids against the 1,1-Diphenyl-2-picrylhydrazyl Radical. Biochem. Pharm 1998, 56, 213–222. [Google Scholar]
- Pfundstein, B.; Desouky, S.K.E.; Hull, W.E.; Haubner, R.; Erben, G.; Owen, R.W. Polyphenolic compounds in the fruits of Egyptian medicinal plants (Terminalia bellerica, Terminalia chebula and Terminalia horrida): Characterization, quantitation and determination of antioxidant capacities. Phytochemistry 2010, 71, 1132–1148. [Google Scholar]
- Gao, D.F.; Zhang, Y.J.; Yang, C.R.; Chen, K.K.; Jiang, H.J. Phenolic Antioxidants from Green Tea Produced from Camellia taliensis. J. Agric. Food Chem 2008, 56, 7517–7521. [Google Scholar]
- Hsieh, C.Y.; Chang, S.T. Antioxidant Activities and Xanthine Oxidase Inhibitory Effects of Phenolic Phytochemicals from Acacia confusa Twigs and Branches. J. Agric. Food Chem 2010, 58, 1578–1583. [Google Scholar]
- Galli, F.; Piroddi, M.; Annetti, C.; Aisa, C.; Floridi, E.; Floridi, A. Oxidative stress and reactive oxygen species. Contrib. Nephrol 2005, 149, 240–260. [Google Scholar]
- Moharram, F.A.; Marzouk, M.S.; Ibrahim, M.T.; Mabry, T.J. Antioxidant galloylated flavonol glycosides from Calliandra haematocephala. Nat. Prod. Res 2006, 20, 927–934. [Google Scholar]
- Ye, J.; Guan, Y.; Zeng, S.; Liu, D. Ampelopsin prevents apoptosis induced by H2O2 in MT-4 lymphocytes. Planta Med 2008, 74, 252–257. [Google Scholar]
- Beckman, J.S.; Beckman, T.W.; Chen, J.; Marshall, P.A.; Freeman, B.A. Apparent hydroxyl radical production by peroxynitrite: Implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. USA 1990, 87, 1620–1624. [Google Scholar]
- Yokozawa, T.; Kashiwada, Y.; Hattori, M.; Chung, H.Y. Study on the components of luobuma with peroxynitrite-scavenging activity. Biol. Pharm. Bull 2002, 25, 748–752. [Google Scholar]
- Ketsawatsakul, U.; Whiteman, M.; Halliwell, B.A. Reevaluation of the Peroxynitrite Scavenging Activity of Some Dietary Phenolics. Biochem. Biophys. Res. Commun 2000, 279, 692–699. [Google Scholar]
- Pryor, W.A.; Cueto, R.; Jin, X.; Ngu-Schwemlein, M.; Squadrito, G.L.; Uppu, P.L.; Uppu, R.M. A practical method for preparing peroxynitrite solutions of low ionic strength and free of hydrogen peroxide. Free Radic. Biol. Med 1995, 18, 75–83. [Google Scholar]
- Lin, T.C.; Hsu, F.L. Tannins and related compounds from Terminalia catappa and Terminalia parviflora. J. Chin. Chem. Soc 1999, 46, 613–618. [Google Scholar]
- Hatano, T.; Edamatsu, R.; Hiramatsu, M.; Mori, A.; Fujita, Y.; Yasuhara, T.; Yoshida, T.; Okuda, T. Effects of the interaction of tannins with co-existing substances. VI. Effects of tannins and related polyphenols on superoxide anion radical, and on 1,1-diphenyl-2-picrylhydrazyl radical. Chem. Pharm. Bull 1989, 37, 2016–2021. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M.C.; Aruoma, O.I. The deoxyribose method: A simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal. Biochem 1987, 165, 215–219. [Google Scholar]
- Kooy, N.W.; Royall, J.A.; Ischiropoulos, H.; Beckman, J.S. Peroxynitrite mediated oxidation of dihydrorhodamine 123. Free Radic. Biol. Med 1994, 16, 149–156. [Google Scholar]
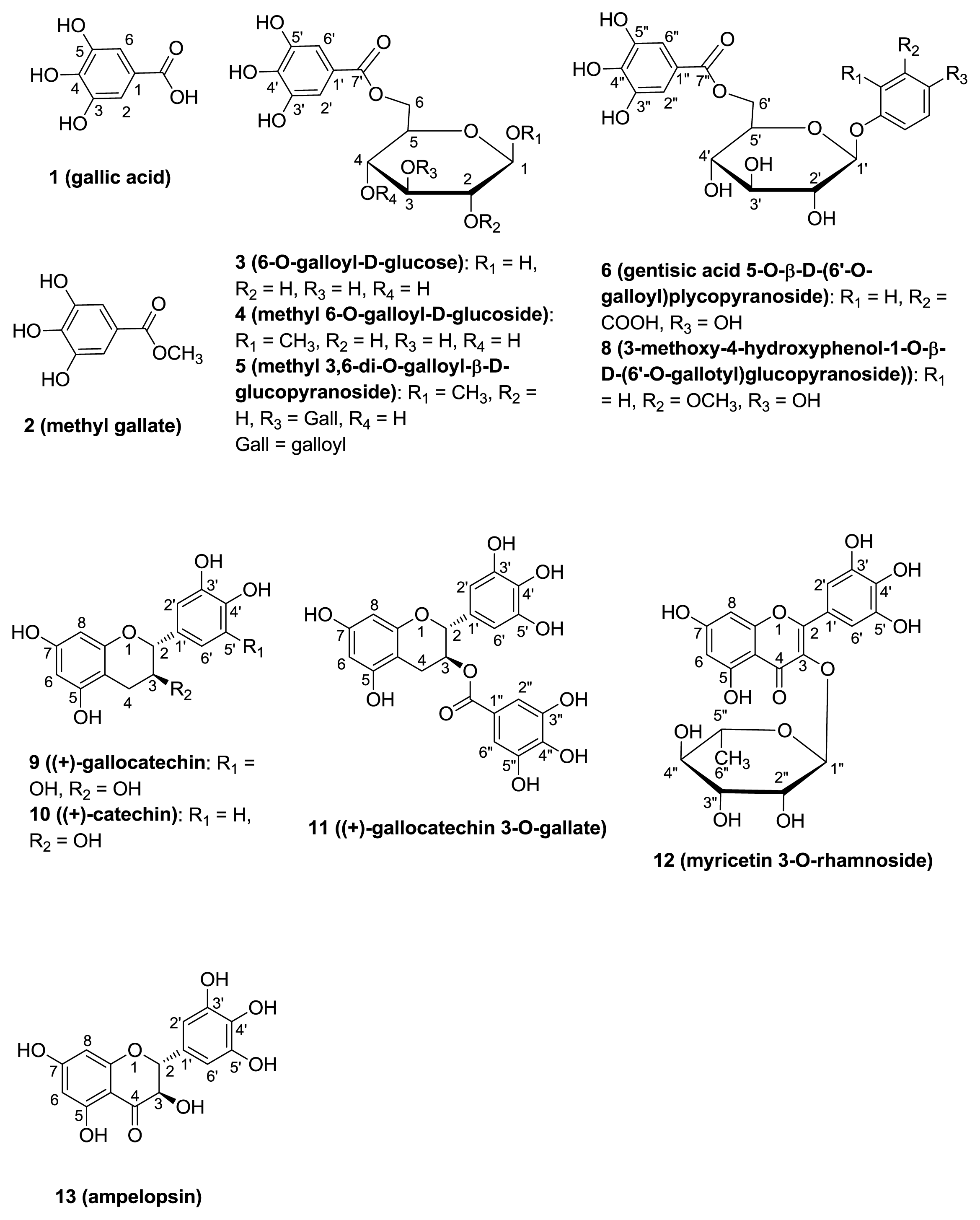
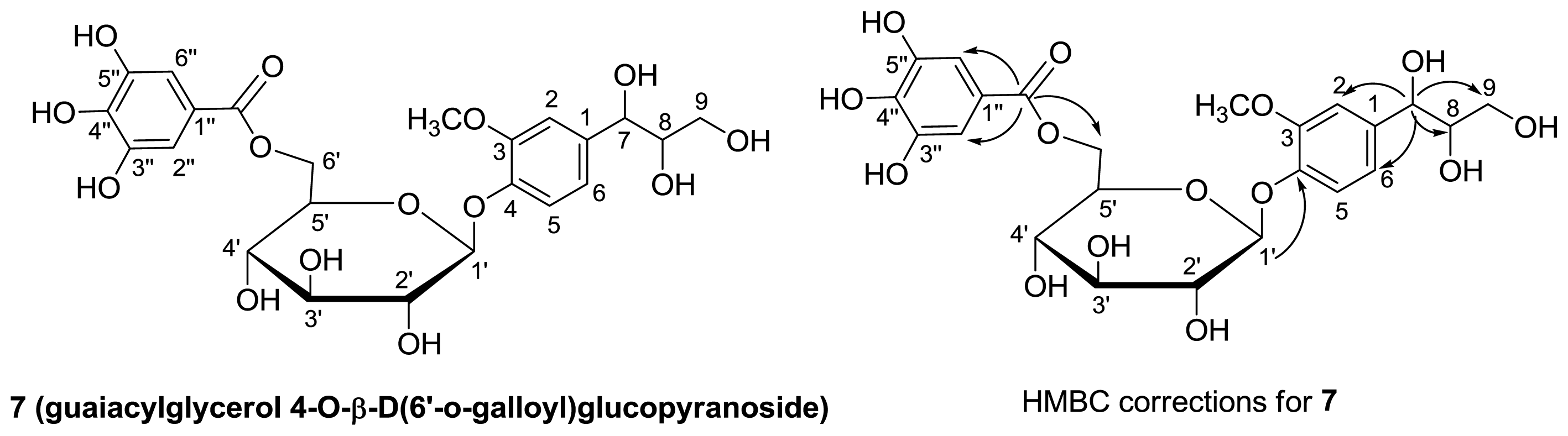
| 6 | 7 | 8 | ||||
|---|---|---|---|---|---|---|
| Position | δC | δH (mult) (J in Hz) | δC | δH (mult) (J in Hz) | δC | δH (mult) (J in Hz) |
| 1 | 119.3 | 138.0 | 140.9 | |||
| 2 | 156.6 | 111.9 | 7.05 d (1.8) | 101.7 | 6.46 d (2.7) | |
| 3 | 117.2 | 6.64 d (8.8) | 149.9 | 151.7 | ||
| 4 | 123.3 | 7.00 dd (3.1, 8.8) | 146.8 | 154.5 | ||
| 5 | 150.0 | 116.8 | 7.10 d (8.4) | 120.4 | 7.01 d (8.5) | |
| 6 | 116.2 | 7.66 d (3) | 120.2 | 6.85 dd (8.4, 1.8) | 107.3 | 6.27 dd (2.7, 8.5) |
| 7 | 74.4 | 4.57 d (5.8) | ||||
| 8 | 76.9 | 3.55 ddd (11.2, 5.8, 4.0) | ||||
| 9 | 63.9 | 3.38 dd (11.2, 6.2); 3.47 dd (11.2, 4.0) | ||||
| OCH3 | 56.3 | 3.80 (3H, s) | 56.3 | 3.75 (3H, s) | ||
| COOH | 174.4 | |||||
| Glucose | ||||||
| 1′ | 101.6 | 4.90 d (7.8) | 102.3 | 4.92 d (7.1) | 104.3 | 4.70 d (7.6) |
| 2′ | 73.6 | 3.44 dd (8.0, 9.1) | 74.5 | 3.53 m | 74.8 | 3.51 m |
| 3′ | 76.1 | 3.57 t (9.2) | 77.7 | 3.53 m | 77.6 | 3.51 m |
| 4′ | 70.7 | 3.32 t (9.5) | 71.3 | 3.53 m | 71.2 | 3.51 m |
| 5′ | 74.5 | 3.89 t (9.2) | 74.6 | 3.81 m | 75.1 | 3.70 m |
| 6′ | 65.4 | 4.00 dd (8.8, 11.8); 4.63 d (11.6) | 64.5 | 4.32 d (11.8); 4.63 dd (11.8, 1.8) | 64.4 | 4.38 dd (6.1, 11.6); 4.56 dd (2.1, 11.6 ) |
| Galloyl | ||||||
| 1″ | 119.9 | 121.6 | 121.8 | |||
| 2″, 6″ | 109.6 | 7.23 (2H, s) | 110.0 | 7.13 (2H, s) | 109.9 | 7.15 (2H, s) |
| 3″, 5″ | 145.7 | 146.1 | 146.0 | |||
| 4″ | 138.9 | 138.9 | 138.8 | |||
| 7″ | 167.8 | 166.7 | 166.7 | |||
| Compound | IC50 of DPPH radicals | IC50 of hydroxyl radicals | IC50 of peroxynitrite |
|---|---|---|---|
| BHT | 16.57 | 0.69 | 0.16 |
| Phenol compounds | |||
| 1 | 7.59 | 8.47 | 77.23 |
| 2 | 4.62 | 7.45 | 23.42 |
| Gallotannins | |||
| 3 | 7.14 | 3.90 | 121.75 |
| 4 | 5.38 | 5.55 | 101.50 |
| 5 | 3.51 | 2.41 | 84.38 |
| Phenol glycosides | |||
| 6 | 6.53 | 1.88 | 76.47 |
| 7 | 5.76 | 0.89 | 42.84 |
| 8 | 6.06 | 1.74 | 55.68 |
| Flavan-3-ols | |||
| 9 | 12.81 | 5.26 | 144.71 |
| 10 | 6.38 | 14.79 | 33.10 |
| 11 | 3.30 | 1.27 | 43.69 |
| Flavonol | |||
| 12 | 5.14 | 0.78 | 57.55 |
| Dihydroflavonol | |||
| 13 | 4.94 | 5.56 | 73.50 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hsu, F.-L.; Huang, W.-J.; Wu, T.-H.; Lee, M.-H.; Chen, L.-C.; Lu, H.-J.; Hou, W.-C.; Lin, M.-H. Evaluation of Antioxidant and Free Radical Scavenging Capacities of Polyphenolics from Pods of Caesalpinia pulcherrima. Int. J. Mol. Sci. 2012, 13, 6073-6088. https://doi.org/10.3390/ijms13056073
Hsu F-L, Huang W-J, Wu T-H, Lee M-H, Chen L-C, Lu H-J, Hou W-C, Lin M-H. Evaluation of Antioxidant and Free Radical Scavenging Capacities of Polyphenolics from Pods of Caesalpinia pulcherrima. International Journal of Molecular Sciences. 2012; 13(5):6073-6088. https://doi.org/10.3390/ijms13056073
Chicago/Turabian StyleHsu, Feng-Lin, Wei-Jan Huang, Tzu-Hua Wu, Mei-Hsien Lee, Lih-Chi Chen, Hsiao-Jen Lu, Wen-Chi Hou, and Mei-Hsiang Lin. 2012. "Evaluation of Antioxidant and Free Radical Scavenging Capacities of Polyphenolics from Pods of Caesalpinia pulcherrima" International Journal of Molecular Sciences 13, no. 5: 6073-6088. https://doi.org/10.3390/ijms13056073
APA StyleHsu, F.-L., Huang, W.-J., Wu, T.-H., Lee, M.-H., Chen, L.-C., Lu, H.-J., Hou, W.-C., & Lin, M.-H. (2012). Evaluation of Antioxidant and Free Radical Scavenging Capacities of Polyphenolics from Pods of Caesalpinia pulcherrima. International Journal of Molecular Sciences, 13(5), 6073-6088. https://doi.org/10.3390/ijms13056073




