Chemical Property Changes and Thermal Analysis during the Carbonizing Process of the Pollen Grains of Typha
Abstract
:1. Introduction
2. Results and Discussion
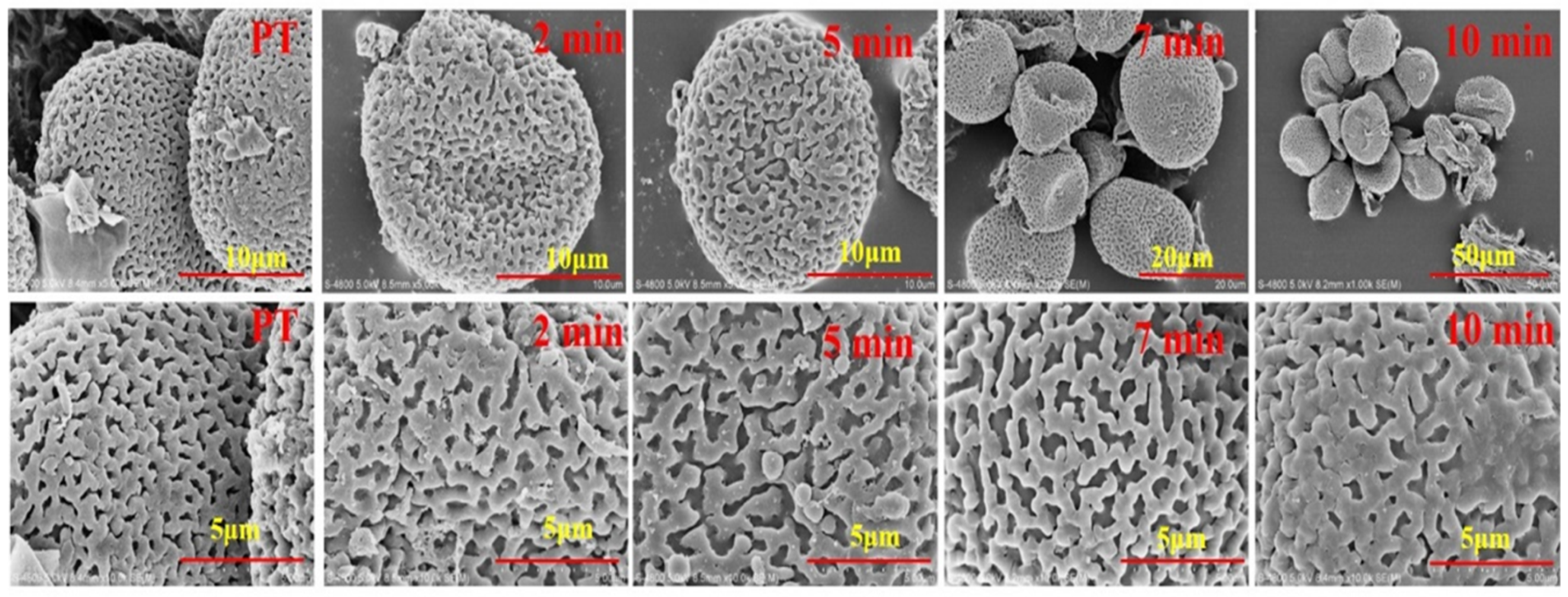
2.1. Pollen Grains Observed by SEM during Heating Process
2.2. Color Measurement
2.3. FTIR Diversity of PT
2.4. Thermal Analysis
2.4.1. Thermogravimetric Analysis of PT
2.4.2. Kinetic Analysis Using Iso-Conversional Models.
2.5. Flavonoids Content Changes during Heating Process
3. Materials and Methods
3.1. Materials and Reagents
3.2. Sample Preparation
3.3. Pollen Grains Observed by SEM during Heating Process
3.4. Color Measurement
3.5. FTIR Diversity of PT
3.6. Thermogravimetric Analysis
Mathematical Background
3.7. Determination of the Compounds during Heating Process
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Y.; Kong, H.; Liu, X.; Luo, J.; Xiong, W.; Zhu, Y.; Zhang, Y.; Zhang, Y.; Zhao, Y.; Qu, H. Novel Carbon Dots Derived from Cirsii Japonici Herba Carbonisata and Their Haemostatic Effect. J. Biomed. Nanotechnol. 2018, 14, 1635–1644. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Y.; Yan, X.; Zhang, M.; Zhang, Y.; Cheng, J.; Lu, F.; Qu, H.; Wang, Q.; Zhao, Y. Novel Phellodendri Cortex (Huang Bo)-derived carbon dots and their hemostatic effect. Nanomedicine (Lond. Engl.) 2018, 13, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.L.; Zhong, Y.; Xia, H.M.; Zhou, Q.; Lv, J. Chemical Constituents in Charred Sanguisorbae Radix. Chin. Herb. Med. 2013, 5, 1–4. [Google Scholar] [CrossRef]
- Chai, X.; Wang, Y.; Su, Y.; Bah, A.J.; Hu, L.; Gao, Y.; Gao, X. A rapid ultra performance liquid chromatography–tandem mass spectrometric method for the qualitative and quantitative analysis of ten compounds in Eucommia ulmodies Oliv. J. Pharm. Biomed. Anal. 2012, 57, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Zhao, Y.; Luo, J.; Xiong, W.; Liu, X.; Cheng, J.; Wang, Y.; Zhang, M.; Qu, H. Hemostatic bioactivity of novel Pollen Typhae Carbonisata-derived carbon quantum dots. J. Nanobiotechnol. 2017, 15, 60. [Google Scholar] [CrossRef] [PubMed]
- Akkol, E.K.; Süntar, I.; Keles, H.; Yesilada, E. The potential role of female flowers inflorescence of Typha domingensis Pers. In wound management. J. Ethnopharmacol. 2011, 133, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.D.; Yan, H.; Ding, A.W. Pharmaceutical screening of active parts of Pollen Typhae. Nanjing Zhongyiyao Daxue Xuebao 2006, 22, 302–303. [Google Scholar] [CrossRef]
- Ma, C.Z.; Chen, P.D.; Zhang, L.; Li, X.; Ding, A.W. Research of effects of Carbonized Typhae Pollen on blood coagulation system. Nanjing Zhongyiyao Daxue Xuebao 2010, 26, 42–43. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, H.; Wu, H.; Pan, Y.; Wang, K.; Jin, Y.; Zhang, C. Characterization and Quantification by LC-MS/MS of the Chemical Components of the Heating Products of the Flavonoids Extract in Pollen Typhae for Transformation Rule Exploration. Molecules 2015, 20, 18352–18366. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.D.; Kong, X.P.; Li, F.; Ding, A.W. Spectrum-active relation research on Typha angustifolia before and after carbonized. Zhongguo Yiyao Gongye Zazhi 2012, 35, 1221–1224. [Google Scholar]
- Alain, F.P.; José, M.; Fernández, J.L. Application of thermal analysis techniques in soil science. Geoderma 2009, 153, 1–10. [Google Scholar] [CrossRef]
- Meng, X.; He, M.; Guo, R.; Duan, R.; Huo, F.; Lv, C.; Wang, B.; Zhang, S. Investigation of the Effect of the Degree of Processing of Radix Rehmanniae Preparata (Shu Dihuang) on Shu Dihuangtan Carbonization Preparation Technology. Molecules 2017, 22, 1193. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Meng, X.; Guo, X.; Lan, Y.; Zhang, S. Thermal analysis during partial carbonizing process of rhubarb, moutan and burnet. PLoS ONE 2017, 21, e0173946. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.X.; Sun, S.Q.; Lv, G.H.; Chan, K.K. Study on Angelica and its different extracts by Fourier transform infrared spectroscopy and two-dimensional correlation IR spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2006, 64, 321–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hameed, I.H.; Sosa, A.A.; Bagi, S.H. Analysis of bioactive chemical compounds of Euphorbia lathyrus using gas chromatography-mass spectrometry and Fourier-transform infrared spectroscopy. J. Pharmacogn. Phytother. 2016, 7, 132–163. [Google Scholar] [CrossRef]
- Li, B.; Lu, Y.R. Observation on Pollen Morphology of six species of Typha (Puhuang) by SEM. J. Chin. Med. Mater. 1997, 20, 497–499. [Google Scholar] [CrossRef]
- Hamdi, S.M.M.; Assadi, M.; Ebadi, M.J.A. Revision of Study of Typha Genus: Three New Records Species of the Genus Typha (Typhaceae) in Iran and Their Micromorphological Pollen and Capsule Studies. Asian J. Plant Sci. 2009, 8, 455–464. [Google Scholar] [CrossRef]
- Arguelles, P.; Reinhard, K.; Shin, D.H. Forensic Palynological Analysis of Intestinal Contents of a Korean Mummy. Anat. Rec. 2015, 298, 1182–1190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, M.; Yang, S.L.; Peng, W.; Liu, Y.J.; Xie, D.S.; Li, X.Y.; Wu, C.J. A Novel Method for the Discrimination of Semen Arecae and Its Processed Products by Using Computer Vision, Electronic Nose, and Electronic Tongue. Evid. Based Complement. Altern. Med. 2015, 2015, 753942. [Google Scholar] [CrossRef] [PubMed]
- Schweiggert, U.; Mix, K.; Schieber, A.; Carle, R. An innovative process for the production of spices through immediate thermal treatment of the plant material. Innov. Food Sci. Emerg. Technol. 2005, 6, 143–153. [Google Scholar] [CrossRef]
- Leon, K.; Mery, D.; Pedreschi, F.; Leon, J. Color measurement in L*a*b* units from RGB digital images. Food Res. Int. 2006, 39, 1084–1091. [Google Scholar] [CrossRef]
- Yadav, R.A.; Rani, P.; Kumar, M.; Singh, R.; Singh, P.; Singh, N.P. Experimental IR and Raman spectra and quantum chemical studies of molecular structures, conformers and vibrational characteristics of L-ascorbic acid and its anion and cation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 84, 6–21. [Google Scholar] [CrossRef]
- Baranović, G. Intramolecular resonance-assisted hydrogen bonds: A theoretical description by means of atomic charges and charge fluxes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 117, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Mkukuma, L.D.; Skakle, J.M.S.; Gibson, I.R.; Imrie, C.T.; Aspden, R.M.; Hukins, D.W.L. Effect of the Proportion of Organic Material in Bone on Thermal Decomposition of Bone Mineral: An Investigation of a Variety of Bones from Different Species Using Thermogravimetric Analysis Coupled to Mass Spectrometry, High-Temperature X-ray Diffraction, and Fourier Transform Infrared Spectroscopy. Calcif. Tissue Int. 2004, 75, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Zwielly, A.; Portnov, A.; Levi, C.; Rosenwaks, S.; Bar, I. Rovibrational spectroscopy and intramolecular dynamics of 1,2-trans-d(2)-ethene in the first C-Hstretch overtone region. J. Chem. Phys. 2008, 128, 114305. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.Y. Application of Attenuated Total Reflectance Infrared Spectroscopy (ATR-FTIR) for Amber Identification and Research. Guang Pu Xue Yu Guang Pu Fen Xi 2016, 36, 2066–2070. [Google Scholar] [CrossRef] [PubMed]
- Katsumoto, Y.; Tanaka, T.; Ozaki, Y. Molecular interpretation for the solvation of poly(acrylamide)s. I. Solvent-dependent changes in the C=O stretching band region of poly(N,N-dialkylacrylamide)s. J. Phys. Chem. B 2005, 109, 20690–20696. [Google Scholar] [CrossRef]
- Beattie, J.R.; Bell, S.E.; Moss, B.W. A critical evaluation of Raman spectroscopy for the analysis of lipids: Fatty acid methyl esters. Lipids 2004, 39, 407–419. [Google Scholar] [CrossRef]
- Ke, J.; Chen, S.L. Thermal decomposition of lignin structural modification in termite digested softwood (II). Fuel 2013, 104, 781–787. [Google Scholar] [CrossRef]
- Zeng, J.J.; Singh, D.; Chen, S.L. Thermal decomposition kinetics of wheat straw treated by Phanerochaete chrysosporium. Int. Biodeterior. Biodegrad. 2011, 65, 410–414. [Google Scholar] [CrossRef]
- Mishra, R.K.; Mohanty, K. Pyrolysis kinetics and thermal behavior of waste sawdust biomass using thermogravimetric analysis. Bioresour. Technol. 2018, 251, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhao, W.; Meng, B.; Liu, C.; Zhu, Q.; Zhao, G. Kinetic study of corn straw pyrolysis: Comparison of two different three-pseudocomponent models. Bioresour. Technol. 2008, 99, 7616–7622. [Google Scholar] [CrossRef] [PubMed]
- Manyà, J.J.; Velo, E.; Puigjaner, L.J.I. Kinetics of Biomass Pyrolysis: A Reformulated Three-Parallel-Reactions Model. Ind. Eng. Chem. Res. 2003, 42, 434–441. [Google Scholar] [CrossRef]
- Siti Alwani, M.; Abdul Khalil, H.P.S.; Sulaiman, O.; Islam, M.N.; Dungani, R.J.B. An approach to using agricultural waste fibres in biocomposites application: Thermogravimetric analysis and activation energy study. Bioresources 2013, 9, 218–230. [Google Scholar]
- Brown, M.E.; Maciejewski, M.; Vyazovkin, S.; Nomen, R.; Sempere, J.; Burnham, A.; Opfermann, J.; Strey, R.; Anderson, H.L.; Kemmler, A.; et al. Computational aspects of kinetic analysis: Part A: The ICTAC kinetics project-data, methods and results. Thermochim. Acta 2000, 355, 125–143. [Google Scholar] [CrossRef]
- Genieva, S.D.; Vlaev, L.T.; Atanassov, A.N. Study of the thermooxidative degradation kinetics of poly(tetrafluoroethene) using iso-conversional calculation procedure. J. Therm. Anal. Calorim. 2010, 99, 551–561. [Google Scholar] [CrossRef]
- Chebli, Y.; Kaneda, M.; Zerzour, R.; Geitmann, A. The Cell Wall of the Arabidopsis Pollen Tube—Spatial Distribution, Recycling, and Network Formation of Polysaccharides. Plant Physiol. 2012, 160, 1940–1955. [Google Scholar] [CrossRef]
- Yan, H.; Chen, P.D.; Ding, A.W. Standardization of processing method for Pollen Typhae Carbonisatus. Chin. Tradit. Herb. Drugs 2006, 37, 1796–1798. [Google Scholar]
- Mert, C.; Soylu, A. Morphology and anatomy of pollen grains from male-fertile and male-sterile cultivars of chestnut (Castanea sativa Mill.). J. Hortic. Sci. Biotechnol. 2007, 82, 474–480. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, K.B.; Kim, W.C.; Kim, H.Y.; Kim, J.H. Evaluation of the color reproducibility of allceramic restorations fabricated by the digital veneering method. J. Adv. Prosthodont. 2014, 6, 71–78. [Google Scholar] [CrossRef]
- Anghel, M.; Vlase, G.; Bilanin, M.; Vlase, T.; Albu, P.; Fuliaş, A.; Tolan, I.; Doca, N. Comparative study on the thermal behavior of two similar triterpenes from birch. J. Therm. Anal. Calorim. 2013, 113, 1379–1385. [Google Scholar] [CrossRef]
- Sadhukhan, A.K.; Gupta, P.; Saha, R.K. Modelling of pyrolysis of large wood particles. Bioresour. Technol. 2009, 100, 3134–3139. [Google Scholar] [CrossRef] [PubMed]
- Santos, K.G.; Lobato, F.S.; Lira, T.S.; Murataa, V.V.; Barrozo, M.A.S. Sensitivity analysis applied to independent parallel reaction model for pyrolysis of bagasse. Chem. Eng. Res. Des. 2012, 90, 1989–1996. [Google Scholar] [CrossRef]
- Yao, F.; Wu, Q.L.; Lei, Y.; Guo, W.H.; Xu, Y.J. Thermal decomposition kinetics of natural fibers: Activation energy with dynamic thermogravimetric analysis. Polym. Degrad. Stab. 2008, 93, 90–98. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
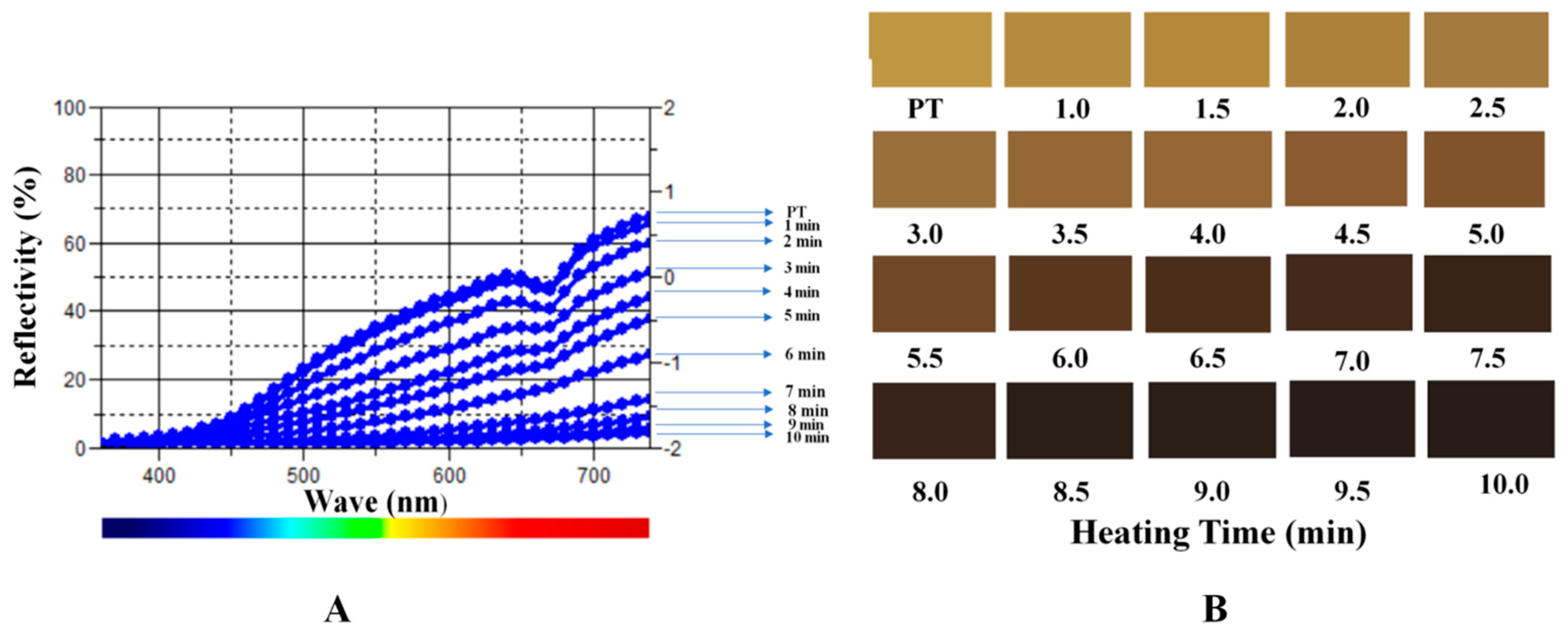




| Sample | L* | a* | b* | dL* | da* | db* | dE*ab |
|---|---|---|---|---|---|---|---|
| PT | 65.26 | 5.42 | 45.96 | −9.77 | 3.85 | 27.09 | 29.06 |
| 0.5 min | 64.19 | 5.55 | 44.91 | −10.83 | 3.98 | 26.04 | 28.48 |
| 1.0 min | 64.19 | 5.68 | 44.45 | −10.84 | 4.1 | 25.58 | 28.09 |
| 1.5 min | 61.1 | 6.36 | 42.8 | −13.93 | 4.78 | 23.93 | 28.1 |
| 2.0 min | 59.76 | 6.93 | 42.53 | −15.26 | 5.36 | 23.66 | 28.66 |
| 2.5 min | 57.19 | 7.64 | 39.26 | −17.83 | 6.07 | 20.39 | 27.76 |
| 3.0 min | 53.93 | 8.09 | 35.31 | −21.09 | 6.52 | 16.44 | 27.53 |
| 3.5 min | 49.86 | 9.08 | 32.18 | −25.17 | 7.51 | 13.32 | 29.44 |
| 4.0 min | 47.3 | 10.03 | 30.67 | −27.73 | 8.46 | 11.8 | 31.3 |
| 4.5 min | 47.12 | 10.22 | 30.69 | −27.9 | 8.65 | 11.82 | 31.51 |
| 5.0 min | 42.18 | 10.57 | 27.95 | −32.84 | 9.0 | 9.08 | 35.25 |
| 5.5 min | 38.54 | 10.59 | 25.54 | −36.48 | 9.02 | 6.67 | 38.17 |
| 6.0 min | 34.32 | 10.35 | 23.18 | −40.7 | 8.78 | 4.31 | 41.86 |
| 6.5 min | 26.86 | 9.03 | 17.24 | −48.16 | 7.45 | −1.63 | 48.76 |
| 7.0 min | 23.28 | 8.09 | 14.21 | −51.74 | 6.51 | −4.66 | 52.36 |
| 7.5 min | 21.48 | 7.0 | 11.28 | −53.54 | 5.43 | −7.59 | 54.35 |
| 8.0 min | 18.37 | 5.88 | 8.97 | −56.65 | 4.31 | −9.9 | 57.67 |
| 8.5 min | 18.24 | 5.61 | 8.25 | −56.79 | 4.03 | 10.62 | 57.91 |
| 9.0 min | 15.25 | 4.25 | 6.21 | −59.77 | 2.68 | 12.66 | 61.16 |
| 9.5 min | 15.08 | 4.26 | 6.16 | −59.94 | 2.69 | −12.7 | 61.34 |
| 10.0 min | 13.89 | 3.15 | 4.19 | −61.13 | 1.58 | 14.68 | 62.89 |
| Sample | β (°C·min−1) | Tv (°C) | Tm (°C) | Tf (°C) | DTGmax (%·min−1) | Volatiles (%) |
|---|---|---|---|---|---|---|
| PT | 5 | 150 | 283 | 348 | 3.15 | 39.30 |
| 10 | 141 | 297 | 365 | 6.71 | 45.25 | |
| 15 | 170 | 303 | 367 | 9.03 | 42.90 | |
| 20 | 172 | 291 | 376 | 10.93 | 34.55 |
| Conversion (α) | Activation Energy Friedman model (kJ·mol−1) | R2 | Activation EnergyKAS Model (kJ·mol−1) | R2 | Activation Energy OFW Model (kJ·mol−1) | R2 |
|---|---|---|---|---|---|---|
| 0.05 | −24.58 | 0.9419 | −19.72 | 0.8059 | −12.98 | 0.6691 |
| 0.10 | −15.96 | 0.0500 | −15.87 | 0.0417 | −8.01 | 0.0121 |
| 0.15 | 47.60 | 0.0980 | 49.48 | 0.0916 | 54.72 | 0.1202 |
| 0.20 | 131.72 | 0.5184 | 137.59 | 0.4983 | 138.83 | 0.5282 |
| 0.25 | 166.99 | 0.7681 | 174.65 | 0.7437 | 174.26 | 0.7618 |
| 0.30 | 168.57 | 0.8300 | 175.18 | 0.8116 | 174.94 | 0.8263 |
| 0.35 | 164.68 | 0.8604 | 170.79 | 0.8442 | 170.92 | 0.8574 |
| 0.40 | 154.01 | 0.8721 | 158.73 | 0.8555 | 159.62 | 0.8690 |
| 0.45 | 143.38 | 0.8730 | 147.36 | 0.8581 | 148.96 | 0.8726 |
| 0.50 | 129.49 | 0.8563 | 132.44 | 0.8420 | 134.95 | 0.8598 |
| 0.55 | 107.32 | 0.8112 | 108.87 | 0.7924 | 112.75 | 0.8194 |
| 0.60 | 85.59 | 0.7768 | 86.11 | 0.7518 | 91.42 | 0.7908 |
| 0.65 | 75.21 | 0.7814 | 74.72 | 0.7507 | 80.98 | 0.7966 |
| 0.70 | 80.95 | 0.8272 | 79.88 | 0.7965 | 86.28 | 0.8350 |
| 0.75 | 87.31 | 0.8650 | 85.88 | 0.8372 | 92.34 | 0.8683 |
| 0.80 | 64.88 | 0.8353 | 62.91 | 0.8009 | 70.97 | 0.8503 |
| 0.85 | 72.88 | 0.7266 | 69.07 | 0.6774 | 77.34 | 0.7444 |
| 0.90 | 142.02 | 0.9338 | 138.81 | 0.9317 | 144.01 | 0.9267 |
| Compound | Content (mg) | Detected (mg) | Added (mg) | Recovery (%) | Average Recovery (%) | RSD (%) |
|---|---|---|---|---|---|---|
| Typhaneoside | 0.1806 | 0.3601 | 0.1828 | 98.21 | 96.67 | 1.70 |
| 0.1841 | 0.3609 | 0.1828 | 96.74 | |||
| 0.1822 | 0.3534 | 0.1828 | 93.65 | |||
| 0.1832 | 0.3630 | 0.1828 | 98.37 | |||
| 0.1818 | 0.3581 | 0.1828 | 96.43 | |||
| 0.1810 | 0.3576 | 0.1828 | 96.63 | |||
| Isorhamnetin-3-O-neohespeidoside | 0.1466 | 0.2931 | 0.1445 | 101.40 | 101.84 | 1.77 |
| 0.1495 | 0.2963 | 0.1445 | 101.57 | |||
| 0.1443 | 0.2897 | 0.1445 | 100.62 | |||
| 0.1451 | 0.2946 | 0.1445 | 103.47 | |||
| 0.1440 | 0.2879 | 0.1445 | 99.63 | |||
| 0.1433 | 0.2941 | 0.1445 | 104.35 | |||
| Isorhamnetin | 0.002949 | 0.005846 | 0.002846 | 101.81 | 100.88 | 1.86 |
| 0.002903 | 0.005770 | 0.002846 | 100.73 | |||
| 0.002931 | 0.005698 | 0.002846 | 97.25 | |||
| 0.002953 | 0.005842 | 0.002846 | 101.53 | |||
| 0.002868 | 0.005782 | 0.002846 | 102.39 | |||
| 0.002886 | 0.005777 | 0.002846 | 101.57 |
| Method | Expression | Plots |
|---|---|---|
| Friedman | against 1/T | |
| KAS | against 1/ | |
| FWO | against 1/T |
| Time(min) | A (%) | B (%) |
|---|---|---|
| 0.00 | 95.00 | 5.00 |
| 5.00 | 86.00 | 14.00 |
| 10.00 | 68.50 | 31.50 |
| 15.00 | 68.50 | 31.50 |
| 40.00 | 55.00 | 45.00 |
| 43.00 | 95.00 | 5.00 |
| 46.00 | 95.00 | 5.00 |
| Component | Calibration Curves | r2 | Linear (μg) | LOQ (ng) | LOD (ng) |
|---|---|---|---|---|---|
| Typhaneoside | y = 11665x − 3.0371 | 0.9997 | 0.007516–0.4810 | 0.6413 | 0.4276 |
| Isorhamnetin-3-O-neohespeidoside | y = 14729x − 2.4082 | 0.9997 | 0.004838–0.3096 | 1.032 | 0.4128 |
| Isorhamnetin | y = 33789x − 1.1673 | 0.9997 | 0.002021–0.03234 | 1.0870 | 0.3623 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, M.; Bao, B.; Cao, Y.; Shan, M.; Cheng, F.; Jiang, M.; Chen, P.; Zhang, L. Chemical Property Changes and Thermal Analysis during the Carbonizing Process of the Pollen Grains of Typha. Molecules 2019, 24, 128. https://doi.org/10.3390/molecules24010128
Gao M, Bao B, Cao Y, Shan M, Cheng F, Jiang M, Chen P, Zhang L. Chemical Property Changes and Thermal Analysis during the Carbonizing Process of the Pollen Grains of Typha. Molecules. 2019; 24(1):128. https://doi.org/10.3390/molecules24010128
Chicago/Turabian StyleGao, Mingliang, Beihua Bao, Yudan Cao, Mingqiu Shan, Fangfang Cheng, Miao Jiang, Peidong Chen, and Li Zhang. 2019. "Chemical Property Changes and Thermal Analysis during the Carbonizing Process of the Pollen Grains of Typha" Molecules 24, no. 1: 128. https://doi.org/10.3390/molecules24010128
APA StyleGao, M., Bao, B., Cao, Y., Shan, M., Cheng, F., Jiang, M., Chen, P., & Zhang, L. (2019). Chemical Property Changes and Thermal Analysis during the Carbonizing Process of the Pollen Grains of Typha. Molecules, 24(1), 128. https://doi.org/10.3390/molecules24010128