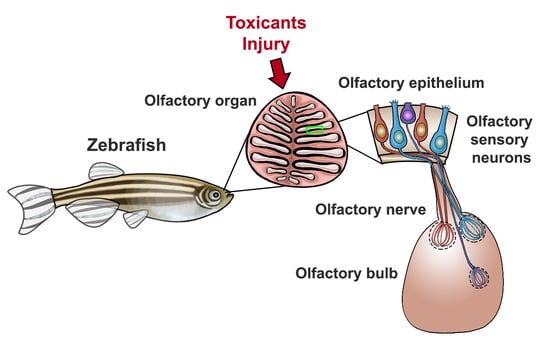
The Olfactory System of Zebrafish as a Model for the Study of Neurotoxicity and Injury: Implications for Neuroplasticity and Disease
Abstract
:1. Introduction
2. The Olfactory System of Zebrafish: Anatomical, Morphological, and Functional Organization
2.1. The General Organization of the Olfactory System of Zebrafish
2.2. The Olfactory Organ of Zebrafish
2.3. The Olfactory Bulb of Zebrafish
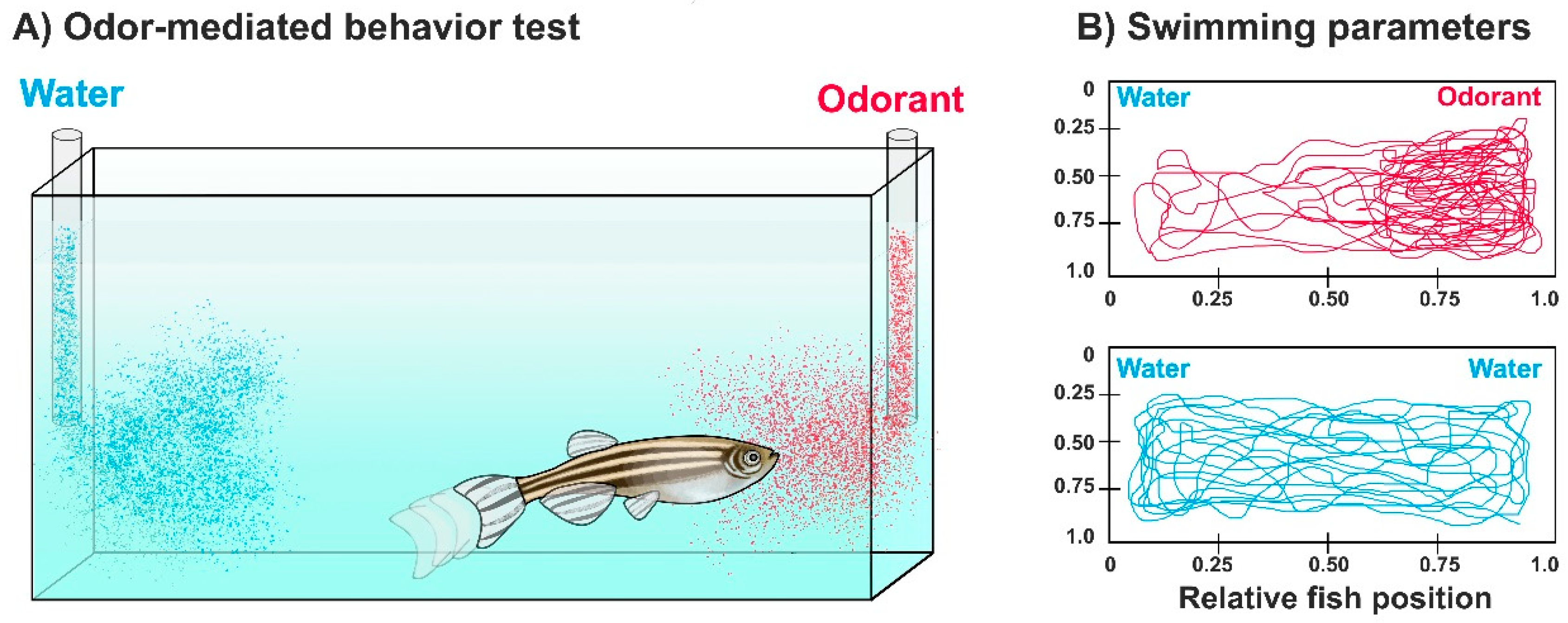
3. Assessing Olfaction and Olfactory Impairment in Zebrafish by Odor-Mediated Behavioral Tasks
4. The Olfactory System of Zebrafish as a Model of Neuroplasticity (and Disease) Following Neurotoxicant Exposure and Injury
4.1. Effects of Heavy Metal Exposure on the Zebrafish Olfactory Epithelium
4.2. Effects of Diverse Toxicants and Physical Lesioning on the Zebrafish Olfactory Epithelium
4.3. Effects of Olfactory Toxicants and Injury on the Olfactory Bulb of Zebrafish
4.4. Regeneration and Repair of the Zebrafish Olfactory System Following Damage
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Firestein, S. How the olfactory system makes sense of scents. Nature 2001, 413, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Kermen, F.; Franco, L.M.; Wyatt, C.; Yaksi, E. Neural circuits mediating olfactory-driven behavior in fish. Front. Neural Circuits 2013, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, K.E. The sense of scents: Olfactory behaviors in the zebrafish. Zebrafish 2006, 3, 203–213. [Google Scholar] [CrossRef]
- Marks, C.A.; Cheng, K.; Cummings, D.M.; Belluscio, L. Activity-dependent plasticity in the olfactory intrabulbar map. J. Neurosci. 2006, 26, 11257–11266. [Google Scholar] [CrossRef] [PubMed]
- Cummings, D.M.; Belluscio, L. Continuous neural plasticity in the olfactory intrabulbar circuitry. J. Neurosci. 2010, 30, 9172–9180. [Google Scholar] [CrossRef]
- Tierney, K.B.; Baldwin, D.H.; Hara, T.J.; Ross, P.S.; Scholz, N.L.; Kennedy, C.J. Olfactory toxicity in fishes. Aquat. Toxicol. 2010, 96, 2–26. [Google Scholar] [CrossRef]
- Hildebrand, J.G.; Shepherd, G.M. Mechanisms of olfactory discrimination: Converging evidence for common principles across phyla. Annu. Rev. Neurosci. 1997, 20, 595–631. [Google Scholar] [CrossRef] [PubMed]
- Parent, J.M. Maps of odorant molecular features in the Mammalian olfactory bulb. Neurobiol. Dis. 2006, 86, 409–433. [Google Scholar] [CrossRef]
- Friedrich, R.W.; Jacobson, G.A.; Zhu, P. Circuit neuroscience in zebrafish. Curr. Biol. 2010, 20, R371–R381. [Google Scholar] [CrossRef]
- Byrd, C.A.; Brunjes, P.C. Organization of the olfactory system in the adult zebrafish: Histological, immunohistochemical, and quantitative analysis. J. Comp. Neurol. 1995, 358, 247–259. [Google Scholar] [CrossRef]
- Saraiva, L.R.; Ahuja, G.; Ivandic, I.; Syed, A.S.; Marioni, J.C.; Korsching, S.I.; Logan, D.W. Molecular and neuronal homology between the olfactory systems of zebrafish and mouse. Sci. Rep. 2015, 5, 11487. [Google Scholar] [CrossRef] [PubMed]
- Hamdani, H.; Døving, K.B. The functional organization of the fish olfactory system. Prog. Neurobiol. 2007, 82, 80–86. [Google Scholar] [CrossRef]
- Hansen, A.; Zielinski, B.S. Diversity in the olfactory epithelium of bony fishes: Development, lamellar arrangement, sensory neuron cell types and transduction components. J. Neurocytol. 2005, 34, 183–208. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [Green Version]
- Sakai, C.; Ijaz, S.; Hoffman, E.J. Zebrafish Models of Neurodevelopmental Disorders: Past, Present, and Future. Front. Mol. Neurosci. 2018, 11, 294. [Google Scholar] [CrossRef] [PubMed]
- Ganzen, L.; Venkatraman, P.; Pang, C.P.; Leung, Y.F.; Zhang, M. Utilizing Zebrafish Visual Behaviors in Drug Screening for Retinal Degeneration. Int. J. Mol. Sci. 2017, 18, 1185. [Google Scholar] [CrossRef] [PubMed]
- Baier, H.; Korsching, S. Olfactory glomeruli in the zebrafish form an invariant pattern and are identifiable across animals. J. Neurosci. 1994, 14, 219–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iqbal, T.; Byrd-Jacobs, C. Rapid degeneration and regeneration of the zebrafish olfactory epithelium after triton X-100 application. Chem. Senses 2010, 35, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Hentig, J.T.; Byrd-Jacobs, C.A. Exposure to Zinc Sulfate Results in Differential Effects on Olfactory Sensory Neuron Subtypes in Adult Zebrafish. Int. J. Mol. Sci. 2016, 17, 1445. [Google Scholar] [CrossRef]
- Paskin, T.R.; Iqbal, T.R.; Byrd-Jacobs, C.A. Olfactory bulb recovery following reversible deafferentation with repeated detergent application in the adult zebrafish. Neuroscience 2011, 196, 276–284. [Google Scholar] [CrossRef]
- Paskin, T.R.; Byrd-Jacobs, C.A. Reversible deafferentation of the adult zebrafish olfactory bulb affects glomerular distribution and olfactory-mediated behavior. Behav. Brain Res. 2012, 235, 293–301. [Google Scholar] [CrossRef]
- White, E.J.; Kounelis, S.K.; Byrd-Jacobs, C.A. Plasticity of glomeruli and olfactory-mediated behavior in zebrafish following detergent lesioning of the olfactory epithelium. Neuroscience 2015, 284, 622–631. [Google Scholar] [CrossRef]
- Kroehne, V.; Freudenreich, D.; Hans, S.; Kaslin, J.; Brand, M. Regeneration of the adult zebrafish brain from neurogenic radial glia-type progenitors. Development 2011, 138, 4831–4841. [Google Scholar] [CrossRef] [Green Version]
- Kizil, C.; Kaslin, J.; Kroehne, V.; Brand, M. Adult neurogenesis and brain regeneration in zebrafish. Dev. Neurobiol. 2012, 72, 429–461. [Google Scholar] [CrossRef] [Green Version]
- Kyritsis, N.; Kizil, C.; Zocher, S.; Kroehne, V.; Kaslin, J.; Freudenreich, D.; Iltzsche, A.; Brand, M. Acute inflammation initiates the regenerative response in the adult zebrafish brain. Science 2012, 338, 1353–1356. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, R.W.; Genoud, C.; Wanner, A.A. Analyzing the structure and function of neuronal circuits in zebrafish. Front. Neural Circuits 2013, 7, 71. [Google Scholar] [CrossRef] [PubMed]
- Byrd, C.A.; Jones, J.T.; Quattro, J.M.; Rogers, M.E.; Brunjes, P.C.; Vogt, R.G. Ontogeny of odorant receptor gene expression in zebrafish, Danio rerio. J. Neurobiol. 1996, 29, 445–458. [Google Scholar] [CrossRef]
- Korsching, S.I.; Argo, S.; Campenhausen, H.; Friedrich, R.W.; Rummrich, A.; Weth, F. Olfaction in zebrafish: What does a tiny teleost tell us? Semin. Cell Dev. Biol. 1997, 8, 181–187. [Google Scholar] [CrossRef]
- Miyasaka, N.; Wanner, A.A.; Li, J.; Mack-Bucher, J.; Genoud, C.; Yoshihara, Y.; Friedrich, R.W. Functional development of the olfactory system in zebrafish. Mech. Dev. 2013, 130, 336–346. [Google Scholar] [CrossRef]
- Laurent, G. Dynamic optimization of odor representations by slow temporal patterning of mitral cell activity. J. Neurophysiol. 2001, 291, 889–894. [Google Scholar] [CrossRef]
- Braubach, O.R.; Fine, A.; Croll, R.P. Distribution and functional organization of glomeruli in the olfactory bulbs of zebrafish (Danio rerio). J. Comp. Neurol. 2012, 520, 2317–2339. [Google Scholar] [CrossRef] [PubMed]
- Pozzuto, J.M.; Fuller, C.L.; Byrd-Jacobs, C.A. Deafferentation-induced alterations in mitral cell dendritic morphology in the adult zebrafish olfactory bulb. J. Bioenerg. Biomembr. 2018, 51, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Fuller, C.L.; Yettaw, H.K.; Byrd, C.A. Mitral cells in the olfactory bulb of adult zebrafish (Danio rerio): Morphology and distribution. J. Comp. Neurol. 2006, 499, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Miyasaka, N.; Morimoto, K.; Tsubokawa, T.; Higashijima, S.; Okamoto, H.; Yoshihara, Y. From the olfactory bulb to higher brain centers: Genetic visualization of secondary olfactory pathways in zebrafish. J. Neurosci. 2009, 29, 4756–4767. [Google Scholar] [CrossRef]
- Miyasaka, N.; Arganda-Carreras, I.; Wakisaka, N.; Masuda, M.; Sümbül, U.; Seung, H.S.; Yoshihara, Y. Olfactory projectome in the zebrafish forebrain revealed by genetic single-neuron labelling. Nat. Commun. 2014, 5, 3639. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, S.; Mathuru, A.S.; Kibat, C.; Rahman, M.; Lupton, C.E.; Stewart, J.; Claridge-Chang, A.; Yen, S.C.; Jesuthasan, S. The right dorsal habenula limits attraction to an odor in zebrafish. Curr. Biol. 2014, 24, 1167–1175. [Google Scholar] [CrossRef]
- Nikonov, A.A.; Finger, T.E.; Caprio, J. Beyond the olfactory bulb: An odotopic map in the forebrain. Proc. Natl. Acad. Sci. USA 2005, 102, 18688–18693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayramli, X.; Kocagöz, Y.; Sakizli, U.; Fuss, S.H. Patterned Arrangements of Olfactory Receptor Gene Expression in Zebrafish are Established by Radial Movement of Specified Olfactory Sensory Neurons. Sci. Rep. 2017, 7, 5572. [Google Scholar] [CrossRef] [PubMed]
- Byrd, C.A.; Brunjes, P.C. Addition of new cells to the olfactory bulb of adult zebrafish. Ann. N. Y. Acad. Sci. 1998, 855, 274–276. [Google Scholar] [CrossRef]
- Byrd, C.A.; Brunjes, P.C. Neurogenesis in the olfactory bulb of adult zebrafish. Neuroscience 2001, 105, 793–801. [Google Scholar] [CrossRef]
- Villanueva, R.; Byrd-Jacobs, C.A. Peripheral sensory deafferentation affects olfactory bulb neurogenesis in zebrafish. Brain Res. 2009, 1269, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Trimpe, D.M.; Byrd-Jacobs, C.A. Patterns of olfactory bulb neurogenesis in the adult zebrafish are altered following reversible deafferentation. Neuroscience 2016, 331, 134–147. [Google Scholar] [CrossRef]
- Hansen, A.; Zeiske, E. The peripheral olfactory organ of the zebrafish, Danio rerio: An ultrastructural study. Chem. Senses 1998, 23, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Barth, A.L.; Justice, N.J.; Ngai, J. Asynchronous onset of odorant receptor expression in the developing zebrafish olfactory system. Neuron 1996, 16, 23–34. [Google Scholar] [CrossRef]
- Ahuja, G.; Ivandic, I.; Saltürk, M.; Oka, Y.; Nadler, W.; Korsching, S.I. Zebrafish crypt neurons project to a single, identified mediodorsal glomerulus. Sci. Rep. 2013, 3, 2063. [Google Scholar] [CrossRef]
- Ahuja, G.; Bozorg Nia, S.; Zapilko, V.; Shiriagin, V.; Kowatschew, D.; Oka, Y.; Korsching, S.I. Kappe neurons, a novel population of olfactory sensory neurons. Sci. Rep. 2014, 4, 4037. [Google Scholar] [CrossRef] [PubMed]
- Oka, Y.; Saraiva, L.R.; Korsching, S.I. Crypt neurons express a single V1R-related ora gene. Chem. Senses 2012, 37, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Biechl, D.; Tietje, K.; Ryu, S.; Grothe, B.; Gerlach, G.; Wullimann, M.F. Identification of accessory olfactory system and medial amygdala in the zebrafish. Sci. Rep. 2017, 7, 44295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, A.; Finger, T.E. Phyletic distribution of crypt-type olfactory receptor neurons in fishes. Brain Behav. Evol. 2000, 55, 100–110. [Google Scholar] [CrossRef]
- Wakisaka, N.; Miyasaka, N.; Koide, T.; Masuda, M.; Hiraki-Kajiyama, T.; Yoshihara, Y. An Adenosine Receptor for Olfaction in Fish. Curr. Biol. 2017, 27, 1437–1447. [Google Scholar] [CrossRef]
- Weth, F.; Nadler, W.; Korsching, S. Nested expression domains for odorant receptors in zebrafish olfactory epithelium. Proc. Natl. Acad. Sci. USA 1996, 93, 13321–13326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gayoso, J.; Castro, A.; Anadón, R.; Manso, M.J. Differential bulbar and extrabulbar projections of diverse olfactory receptor neuron populations in the adult zebrafish (Danio rerio). J. Comp. Neurol. 2011, 519, 247–276. [Google Scholar] [CrossRef] [PubMed]
- Barth, A.L.; Dugas, J.C.; Ngai, J. Noncoordinate expression of odorant receptor genes tightly linked in the zebrafish genome. Neuron 1997, 19, 359–369. [Google Scholar] [CrossRef]
- Sato, Y.; Miyasaka, N.; Yoshihara, Y. Hierarchical regulation of odorant receptor gene choice and subsequent axonal projection of olfactory sensory neurons in zebrafish. J. Neurosci. 2007, 27, 1606–1615. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Lakhina, V.; Dang, P.; Cheng, R.P.; Marcaccio, C.L.; Raper, J.A. Olfactory sensory axons target specific protoglomeruli in the olfactory bulb of zebrafish. Neural Dev. 2017, 12, 18. [Google Scholar] [CrossRef]
- Sato, Y.; Miyasaka, N.; Yoshihara, Y. Mutually exclusive glomerular innervation by two distinct types of olfactory sensory neurons revealed in transgenic zebrafish. J. Neurosci. 2005, 25, 4889–4897. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, R.W.; Korsching, S.I. Combinatorial and chemotopic odorant coding in the zebrafish olfactory bulb visualized by optical imaging. Neuron 1997, 18, 737–752. [Google Scholar] [CrossRef]
- Oka, Y.; Katada, S.; Omura, M.; Suwa, M.; Yoshihara, Y.; Touhara, K. Odorant receptor map in the mouse olfactory bulb: In vivo sensitivity and specificity of receptor-defined glomeruli. Neuron 2006, 52, 857–869. [Google Scholar] [CrossRef]
- Alioto, T.S.; Ngai, J. The repertoire of olfactory C family G protein-coupled receptors in zebrafish: Candidate chemosensory receptors for amino acids. BMC Genom. 2006, 7, 309. [Google Scholar] [CrossRef]
- Ma, L.; Michel, W.C. Drugs affecting phospholipase C-mediated signal transduction block the olfactory cyclic nucleotide-gated current of adult zebrafish. J. Neurophysiol. 1998, 79, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Liberles, S.D.; Buck, L.B. A second class of chemosensory receptors in the olfactory epithelium. Nature 2006, 442, 645–650. [Google Scholar] [CrossRef]
- Hashiguchi, Y.; Nishida, M. Evolution of trace amine associated receptor (TAAR) gene family in vertebrates: Lineage-specific expansions and degradations of a second class of vertebrate chemosensory receptors expressed in the olfactory epithelium. Mol. Biol. Evol. 2007, 24, 2099–2107. [Google Scholar] [CrossRef]
- Hussain, A.; Saraiva, L.R.; Korsching, S.I. Positive Darwinian selection and the birth of an olfactory receptor clade in teleosts. Proc. Natl. Acad. Sci. USA 2009, 106, 4313–4318. [Google Scholar] [CrossRef] [Green Version]
- Liberles, S.D. Trace amine-associated receptors are olfactory receptors in vertebrates. Ann. N. Y. Acad. Sci. 2009, 1170, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, L.R.; Korsching, S.I. A novel olfactory receptor gene family in teleost fish. Genome Res. 2007, 17, 1448–1457. [Google Scholar] [CrossRef] [Green Version]
- Michel, W.C.; Sanderson, M.J.; Olson, J.K.; Lipschitz, D.L. Evidence of a novel transduction pathway mediating detection of polyamines by the zebrafish olfactory system. J. Exp. Biol. 2003, 206, 1697–1706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsunami, H.; Buck, L.B. A multigene family encoding a diverse array of putative pheromone receptors in mammals. Cell 1997, 90, 775–784. [Google Scholar] [CrossRef]
- Dulac, C.; Axel, R. A novel family of genes encoding putative pheromone receptors in mammals. Cell 1995, 83, 195–206. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Oh, B.C.; Stryer, L. Cloning and localization of two multigene receptor families in goldfish olfactory epithelium. Proc. Natl. Acad. Sci. USA 1998, 95, 11987–11992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfister, P.; Rodriguez, I. Olfactory expression of a single and highly variable V1r pheromone receptor-like gene in fish species. Proc. Natl. Acad. Sci. USA 2005, 102, 5489–5494. [Google Scholar] [CrossRef] [Green Version]
- Michel, W.C.; Derbidge, D.S. Evidence of distinct amino acid and bile salt receptors in the olfactory system of the zebrafish, Danio rerio. Brain Res. 1997, 764, 179–187. [Google Scholar] [CrossRef]
- DeMaria, S.; Berke, A.P.; Van Name, E.; Heravian, A.; Ferreira, T.; Ngai, J. Role of a ubiquitously expressed receptor in the vertebrate olfactory system. J. Neurosci. 2013, 33, 15235–15247. [Google Scholar] [CrossRef]
- Oka, Y.; Korsching, S.I. Shared and unique G alpha proteins in the zebrafish versus mammalian senses of taste and smell. Chem. Senses 2011, 36, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Behrens, M.; Frank, O.; Rawel, H.; Ahuja, G.; Potting, C.; Hofmann, T.; Meyerhof, W.; Korsching, S. ORA1, a zebrafish olfactory receptor ancestral to all mammalian V1R genes, recognizes 4-hydroxyphenylacetic acid, a putative reproductive pheromone. J. Biol. Chem. 2014, 289, 19778–19788. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, G.; Korsching, S. Zebrafish olfactory receptor ORA1 recognizes a putative reproductive pheromone. Commun. Integr. Biol. 2014, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhainazarov, A.B.; Ache, B.W. Odor-induced currents in Xenopus olfactory receptor cells measured with perforated-patch recording. J. Neurophysiol. 1995, 74, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Zak, J.D.; Grimaud, J.; Li, R.C.; Lin, C.C.; Murthy, V.N. Calcium-activated chloride channels clamp odor-evoked spike activity in olfactory receptor neurons. Sci. Rep. 2018, 8, 10600. [Google Scholar] [CrossRef] [PubMed]
- Fuller, C.L.; Byrd, C.A. Ruffed cells identified in the adult zebrafish olfactory bulb. Neurosci. Lett. 2005, 379, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.G.; Michel, W.C. Odor-stimulated glutamatergic neurotransmission in the zebrafish olfactory bulb. J. Comp. Neurol. 2002, 454, 294–309. [Google Scholar] [CrossRef] [PubMed]
- Bundschuh, S.T.; Zhu, P.; Schärer, Y.P.; Friedrich, R.W. Dopaminergic modulation of mitral cells and odor responses in the zebrafish olfactory bulb. J. Neurosci. 2012, 32, 6830–6840. [Google Scholar] [CrossRef]
- Mack-Bucher, J.A.; Li, J.; Friedrich, R.W. Early functional development of interneurons in the zebrafish olfactory bulb. Eur. J. Neurosci. 2007, 25, 460–470. [Google Scholar] [CrossRef]
- Sorensen, P.W.; Hara, T.J.; Stacey, N.E. Sex pheromones selectively stimulate the medial olfactory tracts of male goldfish. Brain Res. 1991, 558, 343–347. [Google Scholar] [CrossRef]
- Stacey, N.E.; Kyle, A.L. Effects of olfactory tract lesions on sexual and feeding behavior in the goldfish. Physiol. Behav. 1983, 30, 621–628. [Google Scholar] [CrossRef]
- Braubach, O.; Wyeth, R.; Murray, A.; Fine, A.; Roger, P.C. A Simple and Effective Method to Condition Olfactory Behaviors in Groups of Zebrafish. In Zebrafish Neurobehavioral Protocols; Kalueff, A.V., Ed.; Springer Science Business Media: Berlin/Heidelberg, Germany, 2011; Volume 51, pp. 85–97. [Google Scholar]
- Namekawa, I.; Moenig, N.R.; Friedrich, R.W. Rapid olfactory discrimination learning in adult zebrafish. Exp. Brain Res. 2018, 236, 2959–2969. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Gebhardt, M.; Stewart, A.M.; Cachat, J.M.; Brimmer, M.; Chawla, J.S.; Craddock, C.; Kyzar, E.J.; Roth, A.; Landsman, S.; et al. Towards a comprehensive catalog of zebrafish behavior 1.0 and beyond. Zebrafish 2013, 10, 70–86. [Google Scholar] [CrossRef]
- Saverino, C.; Gerlai, R. The social zebrafish: Behavioral responses to conspecific, heterospecific, and computer animated fish. Behav. Brain Res. 2008, 191, 77–87. [Google Scholar] [CrossRef] [Green Version]
- Heffern, K.; Tierney, K.; Gallagher, E.P. Comparative effects of cadmium, zinc, arsenic and chromium on olfactory-mediated neurobehavior and gene expression in larval zebrafish (Danio rerio). Aquat. Toxicol. 2018, 201, 83–90. [Google Scholar] [CrossRef]
- Spence, R.; Gerlach, G.; Lawrence, C.; Smith, C. The behaviour and ecology of the zebrafish, Danio rerio. Biol. Rev. Camb. Philos. Soc. 2008, 83, 13–34. [Google Scholar] [CrossRef] [PubMed]
- Valentincic, T. Taste and olfactory stimuli and behavior in fishes. In The Senses of Fish; Emde, G., Mogdans, J., Kapoor, B.G., Eds.; Kluwer Academic Publishers: Boston, MA, USA, 2004. [Google Scholar]
- Koide, T.; Miyasaka, N.; Morimoto, K.; Asakawa, K.; Urasaki, A.; Kawakami, K.; Yoshihara, Y. Olfactory neural circuitry for attraction to amino acids revealed by transposon-mediated gene trap approach in zebrafish. Proc. Natl. Acad. Sci. USA 2009, 106, 9884–9889. [Google Scholar] [CrossRef] [Green Version]
- Braubach, O.R.; Wood, H.D.; Gadbois, S.; Fine, A.; Croll, R.P. Olfactory conditioning in the zebrafish (Danio rerio). Behav. Brain Res. 2009, 198, 190–198. [Google Scholar] [CrossRef]
- Miklavc, P.; Valentinčič, T. Chemotopy of amino acids on the olfactory bulb predicts olfactory discrimination capabilities of zebrafish Danio rerio. Chem. Senses 2012, 37, 65–75. [Google Scholar] [CrossRef]
- Miller, N.; Gerlai, R. From schooling to shoaling: Patterns of collective motion in zebrafish (Danio rerio). PLoS ONE 2012, 7, e48865. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, G.; Hodgins-Davis, A.; Avolio, C.; Schunter, C. Kin recognition in zebrafish: A 24-hour window for olfactory imprinting. Proc. Biol. Sci. 2008, 275, 2165–2170. [Google Scholar] [CrossRef]
- Darrow, K.O.; Harris, W.A. Characterization and development of courtship in zebrafish, Danio rerio. Zebrafish 2004, 1, 40–45. [Google Scholar] [CrossRef]
- Bloom, H.D.; Perlmutter, A. A sexual aggregating pheromone system in the zebrafish, Brachydanio rerio (Hamilton-Buchanan). J. Exp. Zool. 1977, 199, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Yabuki, Y.; Koide, T.; Miyasaka, N.; Wakisaka, N.; Masuda, M.; Ohkura, M.; Nakai, J.; Tsuge, K.; Tsuchiya, S.; Sugimoto, Y.; et al. Olfactory receptor for prostaglandin F2α mediates male fish courtship behavior. Nat. Neurosci. 2016, 19, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Maximino, C.; Meinerz, D.L.; Fontana, B.D.; Mezzomo, N.J.; Stefanello, F.V.; de Prestes, A.; Batista, C.B.; Rubin, M.A.; Barbosa, N.V.; Rocha, J.B.T.; et al. Extending the analysis of zebrafish behavioral endophenotypes for modeling psychiatric disorders: Fear conditioning to conspecific alarm response. Behav. Process. 2018, 149, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Speedie, N.; Gerlai, R. Alarm substance induced behavioral responses in zebrafish (Danio rerio). Behav. Brain Res. 2008, 188, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Quadros, V.A.; Silveira, A.; Giuliani, G.S.; Didonet, F.; Silveira, A.S.; Nunes, M.E.; Silva, T.O.; Loro, V.L.; Rosemberg, D.B. Strain and context-dependent behavioural responses of acute alarm substance exposure in zebrafish. Behav. Process. 2016, 122, 1–11. [Google Scholar] [CrossRef]
- Jesuthasan, S.J.; Mathuru, A.S. The alarm response in zebrafish: Innate fear in a vertebrate genetic model. J. Neurogenet. 2008, 22, 211–228. [Google Scholar] [CrossRef]
- Cachat, J.; Canavello, P.R.; Elkhayat, S.I.; Bartels, B.; Hart, P.; Elegante, M.F.; Beeson, E.C.; Laffoon, A.; Haymore, W.M.; Tien, D.H.; et al. Video-Aided Analysis of Zebrafish Locomotion and Anxiety-Related Behavioral Responses. In Zebrafish Neurobehavioral Protocols; Kalueff, A.V., Ed.; Humana Press: New York, NY, USA, 2011; Volume 51, pp. 1–14. [Google Scholar]
- Fangmeier, M.L.; Noble, D.W.A.; O’Dea, R.E.; Usui, T.; Lagisz, M.; Hesselson, D.; Nakagawa, S. Computer Animation Technology in Behavioral Sciences: A Sequential, Automatic, and High-Throughput Approach to Quantifying Personality in Zebrafish (Danio rerio). Zebrafish 2018, 15, 206–210. [Google Scholar] [CrossRef]
- Blaser, R.; Gerlai, R. Behavioral phenotyping in zebrafish: Comparison of three behavioral quantification methods. Behav. Res. Methods 2006, 38, 456–469. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Weng, W. Catadioptric stereo-vision system for the real-time monitoring of 3D behavior in aquatic animals. Physiol. Behav. 2007, 91, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.M.; Grieco, F.; Tegelenbosch, R.A.; Kyzar, E.J.; Nguyen, M.; Kaluyeva, A.; Song, C.; Noldus, L.P.; Kalueff, A.V. A novel 3D method of locomotor analysis in adult zebrafish: Implications for automated detection of CNS drug-evoked phenotypes. J. Neurosci. Methods 2015, 255, 66–74. [Google Scholar] [CrossRef]
- Kim, C.; Ruberto, T.; Phamduy, P.; Porfiri, M. Closed-loop control of zebrafish behaviour in three dimensions using a robotic stimulus. Sci. Rep. 2018, 8, 657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cachat, J.; Stewart, A.; Utterback, E.; Hart, P.; Gaikwad, S.; Wong, K.; Kyzar, E.; Wu, N.; Kalueff, A.V. Three-dimensional neurophenotyping of adult zebrafish behavior. PLoS ONE 2011, 6, e17597. [Google Scholar] [CrossRef] [PubMed]
- Elinder, F.; Arhem, P. Metal ion effects on ion channel gating. Q. Rev. Biophys. 2003, 36, 373–427. [Google Scholar] [CrossRef]
- Vijverberg, H.P.; Oortgiesen, M.; Leinders, T.; van Kleef, R.G. Metal interactions with voltage- and receptor-activated ion channels. Environ. Health Perspect. 1994, 102 (Suppl. 3), 153–158. [Google Scholar] [CrossRef]
- Block, E.; Batista, V.S.; Matsunami, H.; Zhuang, H.; Ahmed, L. The role of metals in mammalian olfaction of low molecular weight organosulfur compounds. Nat. Prod. Rep. 2017, 34, 529–557. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Luthey-Schulten, Z.A.; Suslick, K.S. Is the olfactory receptor a metalloprotein? Proc. Natl. Acad. Sci. USA 2003, 100, 3035–3039. [Google Scholar] [CrossRef]
- Matz, C.J.; Krone, P.H. Cell death, stress-responsive transgene activation, and deficits in the olfactory system of larval zebrafish following cadmium exposure. Environ. Sci. Technol. 2007, 41, 5143–5148. [Google Scholar] [CrossRef]
- Blechinger, S.R.; Kusch, R.C.; Haugo, K.; Matz, C.; Chivers, D.P.; Krone, P.H. Brief embryonic cadmium exposure induces a stress response and cell death in the developing olfactory system followed by long-term olfactory deficits in juvenile zebrafish. Toxicol. Appl. Pharm. 2007, 224, 72–80. [Google Scholar] [CrossRef]
- Monaco, A.; Capriello, T.; Grimaldi, M.C.; Schiano, V.; Ferrandino, I. Neurodegeneration in zebrafish embryos and adults after cadmium exposure. Eur. J. Histochem. 2017, 61, 2833. [Google Scholar] [CrossRef]
- Monaco, A.; Grimaldi, M.C.; Ferrandino, I. Neuroglial alterations in the zebrafish brain exposed to cadmium chloride. J. Appl. Toxicol. 2016, 36, 1629–1638. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gallagher, E.P. Role of Nrf2 antioxidant defense in mitigating cadmium-induced oxidative stress in the olfactory system of zebrafish. Toxicol. Appl. Pharm. 2013, 266, 177–186. [Google Scholar] [CrossRef]
- Kusch, R.C.; Krone, P.H.; Chivers, D.P. Chronic exposure to low concentrations of waterborne cadmium during embryonic and larval development results in the long-term hindrance of antipredator behavior in zebrafish. Environ. Toxicol. Chem. 2008, 27, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Bammler, T.K.; Beyer, R.P.; Gallagher, E.P. Copper-induced deregulation of microRNA expression in the zebrafish olfactory system. Environ. Sci. Technol. 2013, 47, 7466–7474. [Google Scholar] [CrossRef] [Green Version]
- Tilton, F.; Tilton, S.C.; Bammler, T.K.; Beyer, R.; Farin, F.; Stapleton, P.L.; Gallagher, E.P. Transcriptional biomarkers and mechanisms of copper-induced olfactory injury in zebrafish. Environ. Sci. Technol. 2008, 42, 9404–9411. [Google Scholar] [CrossRef]
- Dew, W.A.; Azizishirazi, A.; Pyle, G.G. Contaminant-specific targeting of olfactory sensory neuron classes: Connecting neuron class impairment with behavioural deficits. Chemosphere 2014, 112, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Lazzari, M.; Bettini, S.; Milani, L.; Maurizii, M.G.; Franceschini, V. Differential response of olfactory sensory neuron populations to copper ion exposure in zebrafish. Aquat. Toxicol. 2017, 183, 54–62. [Google Scholar] [CrossRef]
- Ma, E.Y.; Heffern, K.; Cheresh, J.; Gallagher, E.P. Differential copper-induced death and regeneration of olfactory sensory neuron populations and neurobehavioral function in larval zebrafish. Neurotoxicology 2018, 69, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Hussainzada, N.; Lewis, J.A.; Baer, C.E.; Ippolito, D.L.; Jackson, D.A.; Stallings, J.D. Whole adult organism transcriptional profiling of acute metal exposures in male zebrafish. BMC Pharm. Toxicol. 2014, 15, 15. [Google Scholar] [CrossRef]
- Cai, G.; Zhu, J.; Shen, C.; Cui, Y.; Du, J.; Chen, X. The effects of cobalt on the development, oxidative stress, and apoptosis in zebrafish embryos. Biol. Trace Elem. Res. 2012, 150, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Lazzari, M.; Bettini, S.; Milani, L.; Maurizii, M.G.; Franceschini, V. Differential nickel-induced responses of olfactory sensory neuron populations in zebrafish. Aquat. Toxicol. 2019, 206, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Faucher, K.; Floriani, M.; Gilbin, R.; Adam-Guillermin, C. Uranium-induced sensory alterations in the zebrafish Danio rerio. Aquat. Toxicol. 2012, 124–125, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Lerebours, A.; Bourdineaud, J.P.; van der Ven, K.; Vandenbrouck, T.; Gonzalez, P.; Camilleri, V.; Floriani, M.; Garnier-Laplace, J.; Adam-Guillermin, C. Sublethal effects of waterborne uranium exposures on the zebrafish brain: Transcriptional responses and alterations of the olfactory bulb ultrastructure. Environ. Sci. Technol. 2010, 44, 1438–1443. [Google Scholar] [CrossRef]
- Lerebours, A.; Gonzalez, P.; Adam, C.; Camilleri, V.; Bourdineaud, J.P.; Garnier-Laplace, J. Comparative analysis of gene expression in brain, liver, skeletal muscles, and gills of zebrafish (Danio rerio) exposed to environmentally relevant waterborne uranium concentrations. Environ. Toxicol. Chem. 2009, 28, 1271–1278. [Google Scholar] [CrossRef]
- Lim, J.H.; Davis, G.E.; Wang, Z.; Li, V.; Wu, Y.; Rue, T.C.; Storm, D.R. Zicam-induced damage to mouse and human nasal tissue. PLoS ONE 2009, 4, e7647. [Google Scholar] [CrossRef]
- Alexander, T.H.; Davidson, T.M. Intranasal zinc and anosmia: The zinc-induced anosmia syndrome. Laryngoscope 2006, 116, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Abreu, M.S.; Giacomini, A.C.; Rodriguez, R.; Kalueff, A.V.; Barcellos, L.J. Effects of ZnSO. Behav. Brain Res. 2017, 320, 275–281. [Google Scholar] [CrossRef]
- Osborne, O.J.; Mukaigasa, K.; Nakajima, H.; Stolpe, B.; Romer, I.; Philips, U.; Lynch, I.; Mourabit, S.; Hirose, S.; Lead, J.R.; et al. Sensory systems and ionocytes are targets for silver nanoparticle effects in fish. Nanotoxicology 2016, 10, 1276–1286. [Google Scholar] [CrossRef] [PubMed]
- Tilton, F.A.; Tilton, S.C.; Bammler, T.K.; Beyer, R.P.; Stapleton, P.L.; Scholz, N.L.; Gallagher, E.P. Transcriptional impact of organophosphate and metal mixtures on olfaction: Copper dominates the chlorpyrifos-induced response in adult zebrafish. Aquat. Toxicol. 2011, 102, 205–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Liu, W.; Yang, J.; Wang, F.; Sima, Y.; Zhong, Z.M.; Wang, H.; Hu, L.F.; Liu, C.F. Parkinson’s disease-like motor and non-motor symptoms in rotenone-treated zebrafish. Neurotoxicology 2017, 58, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Fuller, C.L.; Villanueva, R.; Byrd, C.A. Changes in glutamate receptor subunit 4 expression in the deafferented olfactory bulb of zebrafish. Brain Res. 2005, 1044, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Bettini, S.; Lazzari, M.; Ferrando, S.; Gallus, L.; Franceschini, V. Histopathological analysis of the olfactory epithelium of zebrafish (Danio rerio) exposed to sublethal doses of urea. J. Anat. 2016, 228, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Ferrando, S.; Gallus, L.; Gambardella, C.; Marchesotti, E.; Ravera, S.; Franceschini, V.; Masini, M.A. Effects of urea on the molecules involved in the olfactory signal transduction: A preliminary study on Danio rerio. Fish Physiol. Biochem. 2014, 40, 1793–1800. [Google Scholar] [CrossRef] [PubMed]
- Scheib, J.J.; Pozzuto, J.M.; Byrd-Jacobs, C.A. Reversible deafferentation of the zebrafish olfactory bulb with wax plug insertion. J. Neurosci. Methods 2018, 311, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Byrd, C.A. Deafferentation-induced changes in the olfactory bulb of adult zebrafish. Brain Res. 2000, 866, 92–100. [Google Scholar] [CrossRef]
- Vankirk, A.M.; Byrd, C.A. Apoptosis following peripheral sensory deafferentation in the olfactory bulb of adult zebrafish. J. Comp. Neurol. 2003, 455, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Frasnelli, J.A.; Temmel, A.F.; Quint, C.; Oberbauer, R.; Hummel, T. Olfactory function in chronic renal failure. Am. J. Rhinol. 2002, 16, 275–279. [Google Scholar] [CrossRef]
- Hanson, L.R.; Frey, W.H. Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci. 2008, 9 (Suppl. 3), S5. [Google Scholar] [CrossRef] [PubMed]
- Pardeshi, C.V.; Belgamwar, V.S. Direct nose to brain drug delivery via integrated nerve pathways bypassing the blood-brain barrier: An excellent platform for brain targeting. Expert Opin. Drug Deliv. 2013, 10, 957–972. [Google Scholar] [CrossRef] [PubMed]
- Jansson, B.; Björk, E. Visualization of in vivo olfactory uptake and transfer using fluorescein dextran. J. Drug Target. 2002, 10, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Mellert, T.K.; Getchell, M.L.; Sparks, L.; Getchell, T.V. Characterization of the immune barrier in human olfactory mucosa. Otolaryngol. Head Neck Surg. 1992, 106, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Tallkvist, J.; Henriksson, J.; d’Argy, R.; Tjälve, H. Transport and subcellular distribution of nickel in the olfactory system of pikes and rats. Toxicol. Sci. 1998, 43, 196–203. [Google Scholar] [CrossRef]
- Gottofrey, J.; Tjälve, H. Axonal transport of cadmium in the olfactory nerve of the pike. Pharm. Toxicol. 1991, 69, 242–252. [Google Scholar] [CrossRef]
- Sunderman, F.W. Nasal toxicity, carcinogenicity, and olfactory uptake of metals. Ann. Clin. Lab. Sci. 2001, 31, 3–24. [Google Scholar]
- Persson, E.; Henriksson, J.; Tallkvist, J.; Rouleau, C.; Tjälve, H. Transport and subcellular distribution of intranasally administered zinc in the olfactory system of rats and pikes. Toxicology 2003, 191, 97–108. [Google Scholar] [CrossRef]
- Oehlmann, V.D.; Berger, S.; Sterner, C.; Korsching, S.I. Zebrafish beta tubulin 1 expression is limited to the nervous system throughout development, and in the adult brain is restricted to a subset of proliferative regions. Gene Expr. Patterns 2004, 4, 191–198. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Mori, K. Critical period for sensory experience-dependent survival of newly generated granule cells in the adult mouse olfactory bulb. Proc. Natl. Acad. Sci. USA 2005, 102, 9697–9702. [Google Scholar] [CrossRef] [Green Version]
- Kelsch, W.; Lin, C.W.; Mosley, C.P.; Lois, C. A critical period for activity-dependent synaptic development during olfactory bulb adult neurogenesis. J. Neurosci. 2009, 29, 11852–11858. [Google Scholar] [CrossRef] [Green Version]
- Maruniak, J.A.; Taylor, J.A.; Henegar, J.R.; Williams, M.B. Unilateral naris closure in adult mice: Atrophy of the deprived-side olfactory bulbs. Brain Res. Dev. Brain Res. 1989, 47, 27–33. [Google Scholar] [CrossRef]
- Coppola, D.M. Studies of olfactory system neural plasticity: The contribution of the unilateral naris occlusion technique. Neural Plast. 2012, 2012, 351752. [Google Scholar] [CrossRef] [PubMed]
- Graziadei, P.P.; Monti Graziadei, G.A. Neurogenesis and neuron regeneration in the olfactory system of mammals. III. Deafferentation and reinnervation of the olfactory bulb following section of the fila olfactoria in rat. J. Neurocytol. 1980, 9, 145–162. [Google Scholar] [CrossRef]
- Monti Graziadei, G.A.; Karlan, M.S.; Bernstein, J.J.; Graziadei, P.P. Reinnervation of the olfactory bulb after section of the olfactory nerve in monkey (Saimiri sciureus). Brain Res. 1980, 189, 343–354. [Google Scholar] [CrossRef]
- Cummings, D.M.; Emge, D.K.; Small, S.L.; Margolis, F.L. Pattern of olfactory bulb innervation returns after recovery from reversible peripheral deafferentation. J. Comp. Neurol. 2000, 421, 362–373. [Google Scholar] [CrossRef]
- Cheung, M.C.; Jang, W.; Schwob, J.E.; Wachowiak, M. Functional recovery of odor representations in regenerated sensory inputs to the olfactory bulb. Front. Neural Circuits 2013, 7, 207. [Google Scholar] [CrossRef] [PubMed]


| Toxicant or Injury Paradigm | Effects on OE | Effects on OB | Behavioral Effects | Recovery | Ref. |
|---|---|---|---|---|---|
| Cadmium (Cd) | OSN loss, reduced neurogenesis, ROS increase | Long-term decrease in alarm and avoidance responses | [88,114,115,116,117,118,119] | ||
| Copper (Cu) | Predominantly ciliated OSN loss, decreased OR and ionic channels transcripts | Impaired response to bile salts, reduced alarm response | OSN recovery after 72 h. Partial recovery of bile salt response. | [120,121,122,123,124] | |
| Cobalt (Co) | Acute damage to OE, apoptosis and increased ROS | Alterations in schooling behavior | [125,126] | ||
| Nickel (Ni) | OSN loss and OO anatomical disturbances | [127] | |||
| Uranium (U) | Ciliated OSNs and non-sensory cells damage and loss | Morphological damage and disruption of glomerular structure | [128,129,130] | ||
| Zinc (Zn) | Damage to the sensory epithelium with ciliated OSN loss | Impaired response predominantly to bile salts, and aminoacids. Anxiety-like behaviors and reduction in locomotion | [19,88,133] | ||
| Silver (Ag) | Oxidative stress and expression of oxidative damage genes | [134] | |||
| Chlorpyrifos | Decrease in transcripts related to olfactory sensing and neuronal repair and regeneration | [135] | |||
| Rotenone | Impaired response to amino acids | [136] | |||
| Triton X-100 | Thinning of OE, fused lamellae, inflammation and OSN loss | Deafferentation, glomerular defasciculation, reduction of size and activity. Mitral cell structural alterations | Reduced response to predominantly bile salts, and aminoacids | Following acute exposure, OE regenerates in 5 days; bulbar reinnervation observed in 7 days; functional olfactory recovery at 10 days. Following chronic exposure, OE and OB structure, activity and volume are recovered at 21 days. | [18,20,21,22,32,42,137] |
| Urea | Thinning of OE with crypt OSN loss, upregulation of Gαolf transcript | [138,139] | |||
| Chronic physical olfactory organ lesion | OE damage, inflammation and OSN loss | Bulbar deafferentation and glomerular defasciculation | OE regeneration and OB recovery observed in 7 days. Complete bulbar reinnervation at 21 days | [140] | |
| Olfactory organ removal | Reduction in size and activity. Complete deafferentation, degeneration of both olfactory nerve and glomerular layer. Increased apopotsis | [32,41,42,137,141,142] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvo-Ochoa, E.; Byrd-Jacobs, C.A. The Olfactory System of Zebrafish as a Model for the Study of Neurotoxicity and Injury: Implications for Neuroplasticity and Disease. Int. J. Mol. Sci. 2019, 20, 1639. https://doi.org/10.3390/ijms20071639
Calvo-Ochoa E, Byrd-Jacobs CA. The Olfactory System of Zebrafish as a Model for the Study of Neurotoxicity and Injury: Implications for Neuroplasticity and Disease. International Journal of Molecular Sciences. 2019; 20(7):1639. https://doi.org/10.3390/ijms20071639
Chicago/Turabian StyleCalvo-Ochoa, Erika, and Christine A. Byrd-Jacobs. 2019. "The Olfactory System of Zebrafish as a Model for the Study of Neurotoxicity and Injury: Implications for Neuroplasticity and Disease" International Journal of Molecular Sciences 20, no. 7: 1639. https://doi.org/10.3390/ijms20071639
APA StyleCalvo-Ochoa, E., & Byrd-Jacobs, C. A. (2019). The Olfactory System of Zebrafish as a Model for the Study of Neurotoxicity and Injury: Implications for Neuroplasticity and Disease. International Journal of Molecular Sciences, 20(7), 1639. https://doi.org/10.3390/ijms20071639