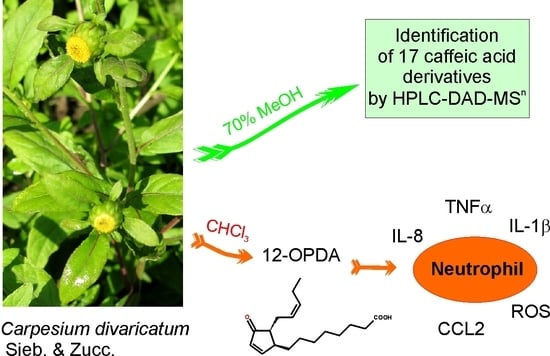
Carpesium divaricatum Sieb. & Zucc. Revisited: Newly Identified Constituents from Aerial Parts of the Plant and Their Possible Contribution to the Biological Activity of the Plant
Abstract
:1. Introduction
2. Results
2.1. Caffeic Acid Derivatives in Aerial Parts of C. divaricatum
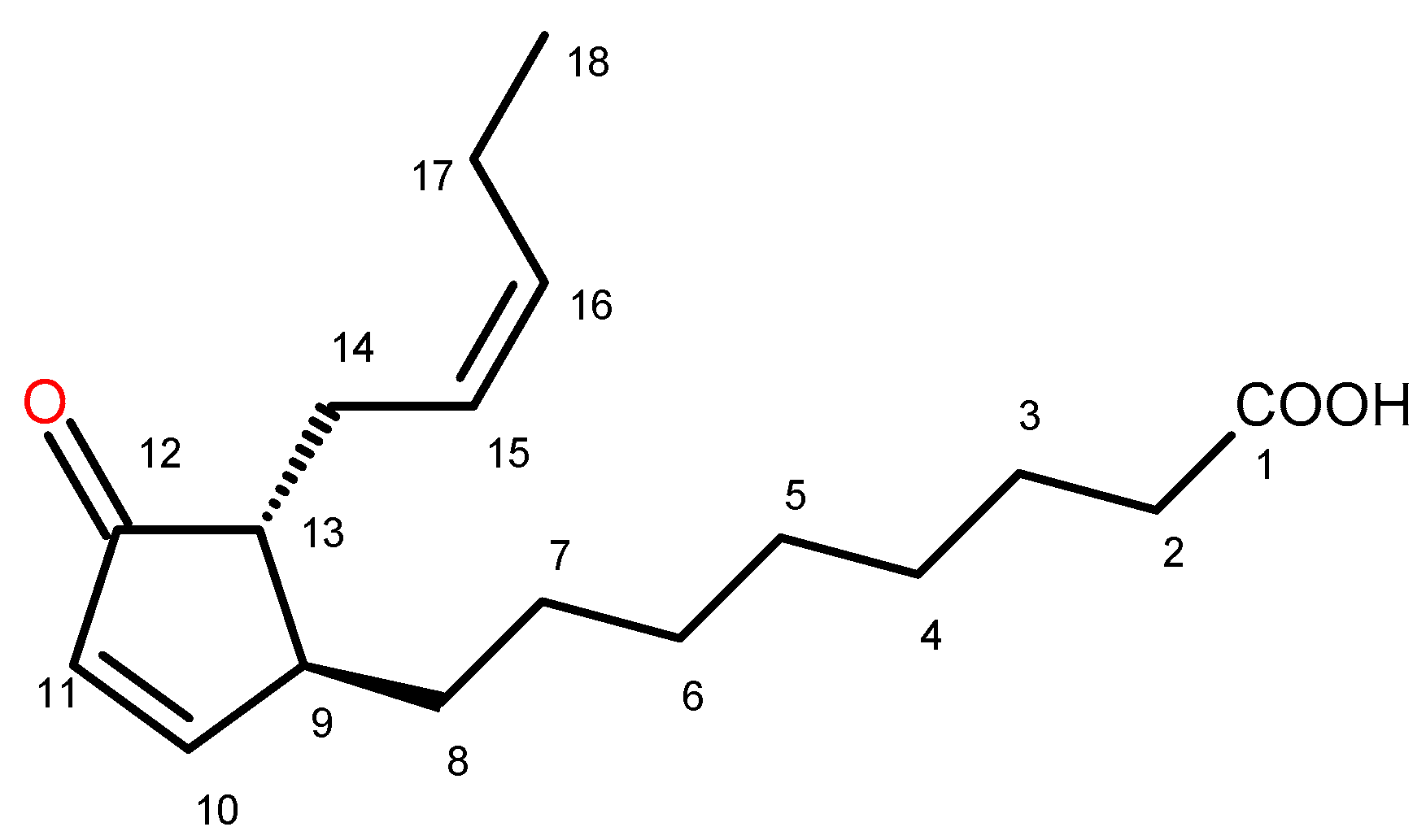
2.2. Identification of Trans-12-Oxo-phytodienoic Acid (trans-12-OPDA)
2.3. Effect of trans-12-OPDA on Lipopolysaccharide (LPS)-Stimulated Release of Pro-Inflammatory Cytokines from Human Neutrophils
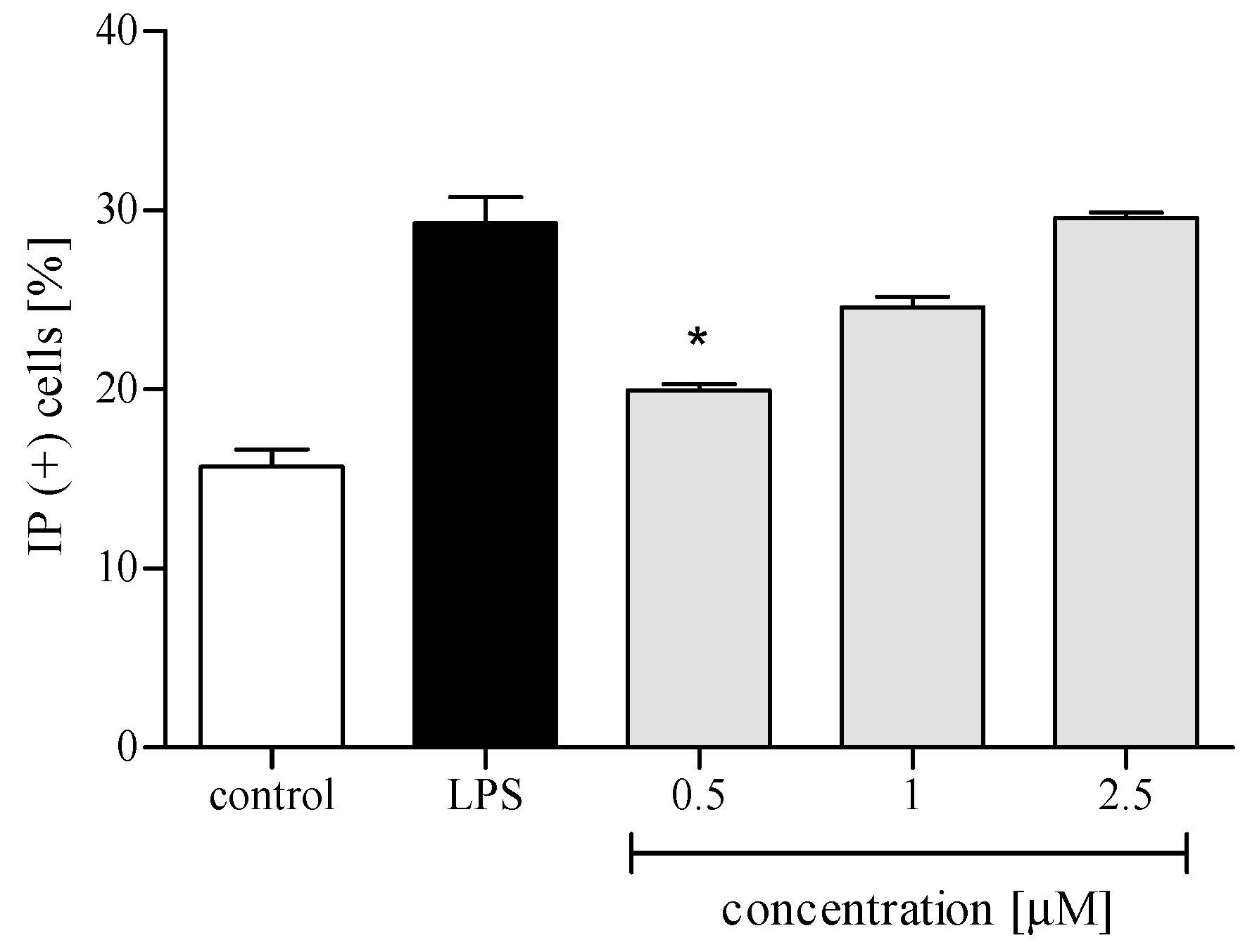
2.3.1. Cytotoxicity
2.3.2. Reactive Oxygen Species (ROS) Production
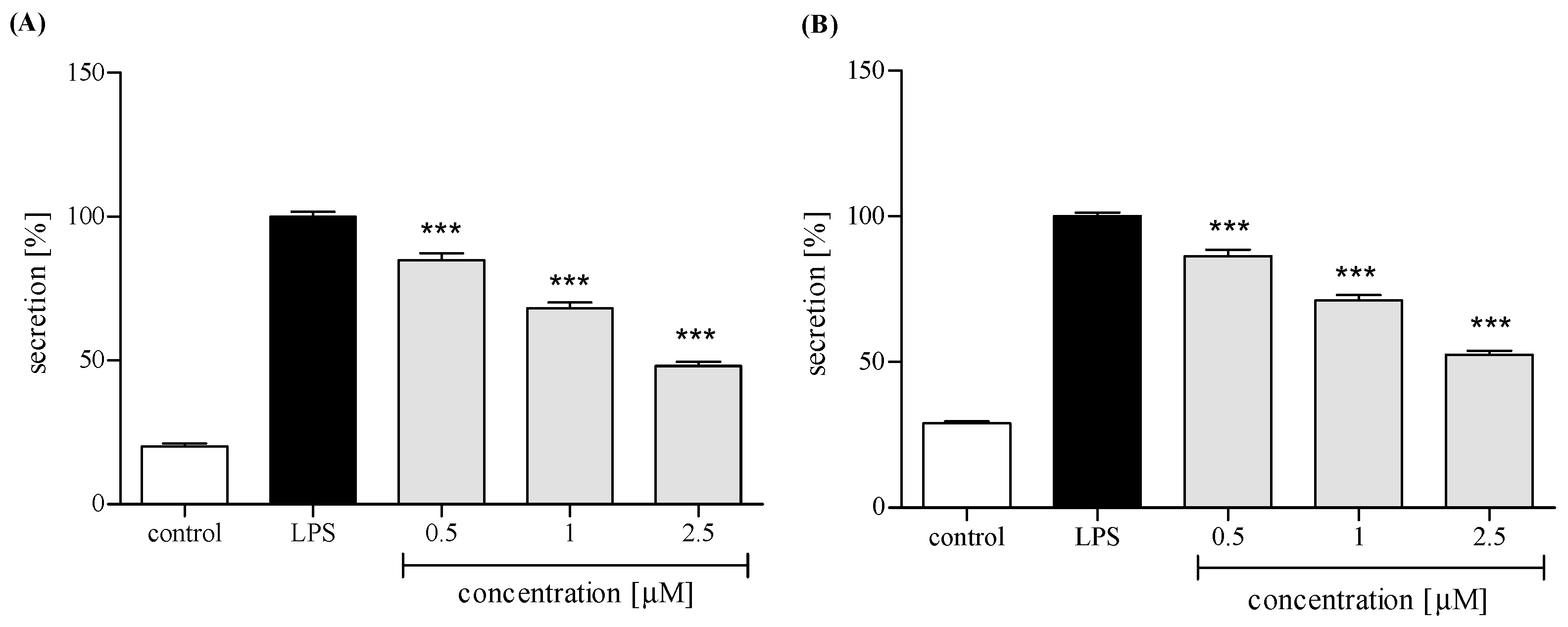
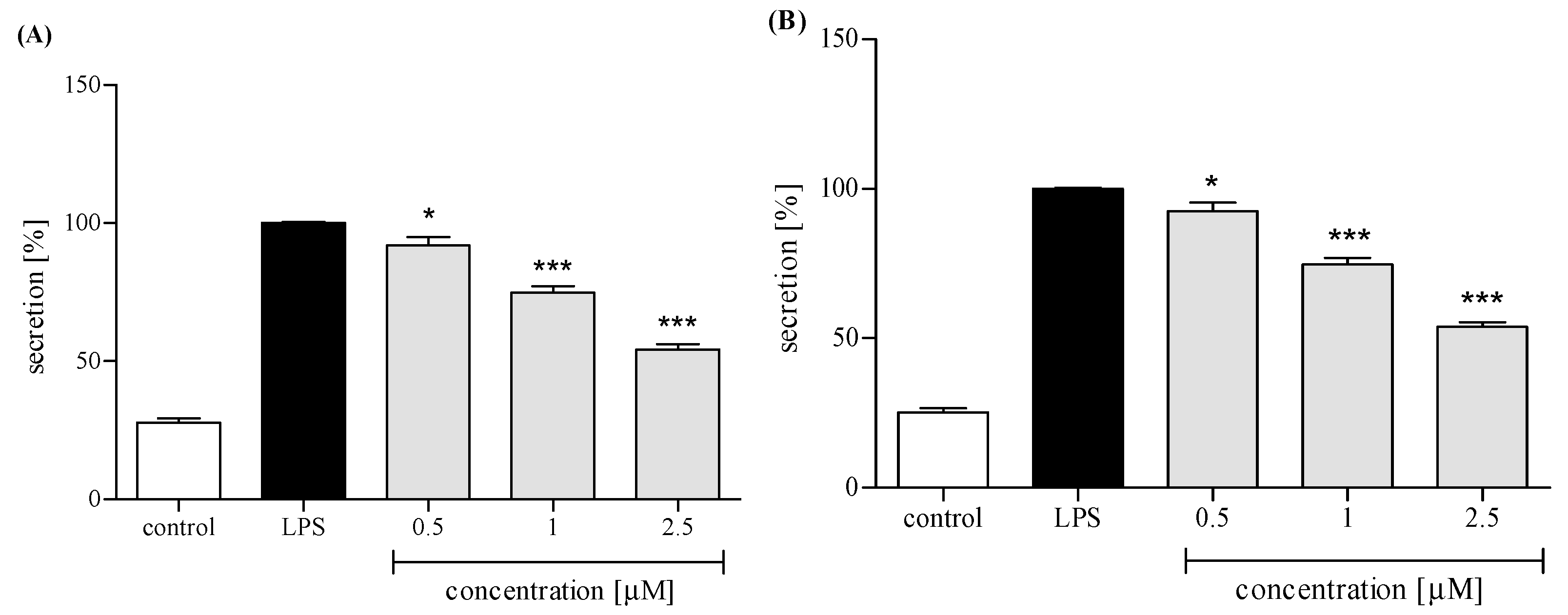
2.3.3. Release of Selected Proinflammatory Cytokines/Chemokines (IL-8, TNFα, IL-1β, CCL2)
3. Discussion
4. Materials and Methods
4.1. General Methods
4.2. Materials
4.3. Plant Material
4.4. Analysis of Caffeic Acid Derivatives
4.4.1. Extract Preparation and Characterization by the HPLC-DAD-MSn Method
4.4.2. Quantification of Chlorogenic Acid (5-CQA) and 3,5-di-O-caffeoylquinic Acid (3,5-DCQA)
4.5. Isolation of Trans-12-Oxo-Phytodienoic Acid (12-OPDA)
(+) trans-12-Oxo-phytodienoic acid: (9S,13R) OPDA
4.6. Isolation of Human Neutrophils
4.7. Assessment of the Effects of Trans-12-OPDA on Lipopolysaccharide (LPS)-Stimulated Release of Pro-Inflammatory Cytokines from Human Neutrophils
4.7.1. Cytotoxicity Measurement
4.7.2. ROS Production by Neutrophils
4.7.3. IL-8, IL-1β, CCL-2, and TNFα Production by Neutrophils
4.8. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Anderberg, A.A. Inuleae. In Systematics, Evolution, and Biogeography of Compositae; Funk, V.A., Susanna, A., Stuessy, T.F., Bayer, R.J., Eds.; International Association for Plant Taxonomy: Vienna, Austria, 2009; pp. 667–680. [Google Scholar]
- Hong, L.; Zhuo, J.; Lei, Q.; Zhou, J.; Ahmed, S.; Wang, C.; Long, Y.; Li, F.; Long, C. Ethnobotany of wild plants used for starting fermented beverages in Shui communities of southwest China. J. Ethnobiol. Ethnomed. 2015, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-P.; Wang, G.-W.; Tian, X.-H.; Yang, Y.-X.; Liu, Q.-X.; Chen, L.-P.; Li, H.-L.; Zhang, W.-D. The genus Carpesium: A review of its ethnopharmacology, phytochemistry and pharmacology. J. Ethnopharmacol. 2015, 163, 173–191. [Google Scholar] [CrossRef]
- Kim, H.; Song, M.-J. Ethnobotanical analysis for traditional knowledge of wild edible plants in North Jeolla Province (Korea). Genet. Resour. Crop Evol. 2013, 60, 1571–1585. [Google Scholar] [CrossRef]
- Geng, Y.; Zhang, Y.; Ranjitkar, S.; Huai, H.; Wang, Y. Traditional knowledge and its transmission of wild edibles used by the Naxi in Baidi Village, northwest Yunnan province. J. Ethnobiol. Ethnomed. 2016, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Rivero, A.; Quintana, J.; Eiroa, J.L.; López, M.; Triana, J.; Bermejo, J.; Estévez, F. Potent induction of apoptosis by germacranolide sesquiterpene lactones on human myeloid leukemia cells. Eur. J. Pharmacol. 2003, 482, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chen, J.-H.; Si, J.-G.; Ding, G.; Zhang, Q.-B.; Zhang, H.-W.; Jia, H.-M.; Zou, Z.-M. Isolation, structure elucidation, and absolute configuration of germacrane isomers from Carpesium divaricatum. Sci. Rep. 2018, 8, 12418. [Google Scholar] [CrossRef]
- Clifford, M.N.; Knight, S.; Kuhnert, N. Discriminating between the six isomers of dicaffeoylquinic acid by LC-MSn. J. Agric. Food Chem. 2005, 53, 3821–3832. [Google Scholar] [CrossRef]
- Willems, J.L.; Khamis, M.M.; Saeid, W.M.; Purves, R.W.; Katselis, G.; Low, N.H.; El-Aneed, A. Analysis of a series of chlorogenic acid isomers using differential ion mobility and tandem mass spectrometry. Anal. Chim. Acta 2016, 933, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Schwaiger, S.; Seger, C.; Wiesbauer, B.; Schneider, P.; Ellmerer, E.P.; Sturm, S.; Stuppner, H. Development of an HPLC-PAD-MS assay for the identification and quantification of major phenolic edelweiss (Leontopodium alpinum Cass.) constituents. Phytochem. Anal. 2006, 17, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Maas, M.; Petereit, F.; Hensel, A. Caffeic acid derivatives from Eupatorium perfoliatum L. Molecules 2009, 14, 36–45. [Google Scholar] [CrossRef]
- Cicek, S.S.; Untersulzner, C.; Schwaiger, S.; Zidorn, C. Caffeoyl-D-glucaric acid derivatives in the genus Gnaphalium (Asteraceae: Gnaphalieae). Rec. Nat. Prod. 2012, 6, 311–315. [Google Scholar]
- Bazylko, A.; Boruc, K.; Borzym, J.; Kiss, A.K. Aqueous and ethanolic extracts of Galinsoga parviflora and Galinsoga ciliata. Investigations of caffeic acid derivatives and flavonoids by HPTLC and HPLC-DAD-MS methods. Phytochem. Lett. 2015, 11, 394–398. [Google Scholar] [CrossRef]
- Stojakowska, A.; Malarz, J.; Kiss, A.K. Hydroxycinnamates from elecampane (Inula helenium L.) callus culture. Acta Physiol. Plant. 2016, 38, 41. [Google Scholar] [CrossRef]
- Heilmann, J.; Müller, E.; Merfort, I. Flavonoid glucosides and dicaffeoylquinic acids from fowerheads of Buphthalmum salicifolium. Phytochemistry 1999, 51, 713–718. [Google Scholar] [CrossRef]
- Lin, L.-Z.; Harnly, J.M. Identification of hydroxycinnamoylquinic acids of arnica flowers and burdock roots using a standardized LC-DAD-ESI/MS profiling method. J. Agric. Food Chem. 2008, 56, 10105–10114. [Google Scholar] [CrossRef]
- Schwaiger, S.; Cervellati, R.; Seger, C.; Ellmerer, E.P.; About, N.; Renimel, I.; Godenir, C.; André, P.; Gafner, F.; Stuppner, H. Leontopodic acid—A novel highly substituted glucaric acid derivative from Edelweiss (Leontopodium alpinum Cass.) and its antioxidative and DNA protecting properties. Tetrahedron 2005, 61, 4621–4630. [Google Scholar] [CrossRef]
- McDonald, P.P.; Bald, A.; Cassatella, M. Activation of NF-κB pathway by inflammatory stimuli in human neutrophils. Blood 1997, 89, 3421–3433. [Google Scholar] [PubMed]
- Tecchio, C.; Micheletti, A.; Cassatella, M.A. Neutrophil-derived cytokines: Facts beyond expression. Front. Immunol. 2014, 5, 508. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Role of chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients 2016, 8, 16. [Google Scholar] [CrossRef]
- Clifford, M.N.; Jaganath, I.B.; Ludwig, I.A.; Crozier, A. Chlorogenic acids and the acyl-quinic acids: Discovery, biosynthesis, bioavailability and bioactivity. Nat. Prod. Rep. 2017, 34, 1391–1421. [Google Scholar] [CrossRef]
- Verzelloni, E.; Pellacani, C.; Tagliazucchi, D.; Tagliaferri, S.; Calani, L.; Costa, L.G.; Brighenti, F.; Borges, G.; Crozier, A.; Conte, A.; Del Rio, D. Antiglycative and neuroprotective activity of colon-derived polyphenol catabolites. Mol. Nutr. Food Res. 2011, 55, S35–S43. [Google Scholar] [CrossRef]
- Tousch, D.; Lajoix, A.-D.; Hosy, E.; Azay-Milhau, J.; Ferrare, K.; Jahannault, C.; Cros, G.; Petit, P. Chicoric acid, a new compound able to enhance insulin release and glucose uptake. Biochem. Biophys. Res. Commun. 2008, 377, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.-B.; Wan, M.-Y.; Wang, P.-Y.; Zhang, C.-X.; Xu, D.-Y.; Liao, X.; Sun, H.-J. Chicoric acid prevents PDGF-BB-induced VSMC dedifferentiation, proliferation and migration by suppressing ROS/NFκB/mTOR/P70S6K signaling cascade. Redox Biol. 2018, 14, 656–668. [Google Scholar] [CrossRef]
- Tsai, Y.-L.; Chiu, C.-C.; Chen, J.Y.-F.; Chan, K.-C.; Lin, S.-D. Cytotoxic effects of Echinacea purpurea flower extracts and cichoric acid on human colon cancer cells through induction of apoptosis. J. Ethnopharm. 2012, 143, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.-H.; Lee, H.-K.; Kim, J.-A.; Hong, S.-I.; Kim, H.-C.; Jo, T.-H.; Park, Y.-I.; Lee, C.-K.; Kim, Y.-B.; Lee, S.-Y.; et al. Neuroprotective effects of chlorogenic acid on scopolamine-induced amnesia via anti-acetylcholinesterase and anti-oxidative activities in mice. Eur. J. Pharmacol. 2010, 649, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Miyamae, Y.; Shigemori, H.; Isoda, H. Neuroprotective effect of 3,5-di-O-caffeoylquinic acid on SH-SY5Y cells and senescence-accelerated-prone mice 8 through the up-regulation of phosphoglycerate kinase-1. Neuroscience 2010, 169, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Hu, Y.; Cao, Y.; Song, G.; Liu, Z.; Liu, X. Chicoric acid ameliorates lipopolysaccharide-induced oxidative stress via promoting the Keap1/Nrf2 transcriptional signaling pathway in BV-2 microglial cells and mouse brain. J. Agric. Food Chem. 2017, 65, 338–347. [Google Scholar] [CrossRef]
- Wang, Y.; Diao, Z.; Li, J.; Ren, B.; Zhu, D.; Liu, Q.; Liu, Z.; Liu, X. Chicoric acid supplementation ameliorates cognitive impairment induced by oxidative stress via promotion of antioxidant defence system. RSC Adv. 2017, 7, 36149–36162. [Google Scholar] [CrossRef]
- Xiao, H.; Wang, J.; Yuan, L.; Xiao, C.; Wang, Y.; Liu, X. Chicoric acid induces apoptosis in 3T3-L1 preadipocytes through ROS-mediated PI3K/Akt and MAPK signaling pathways. J. Agric. Food Chem. 2013, 61, 1509–1520. [Google Scholar] [CrossRef]
- Jaiswal, R.; Kiprotich, J.; Kuhnert, N. Determination of the hydroxycinnamate profile of 12 members of the Asteraceae family. Phytochemistry 2011, 72, 781–790. [Google Scholar] [CrossRef]
- Zheleva-Dimitrova, D.; Gevrenova, R.; Zaharieva, M.M.; Najdenski, H.; Ruseva, S.; Lozanov, V.; Balabanova, V.; Yagi, S.; Momekov, G.; Mitev, V. HPLC-UV and LC-MS analyses of acylquinic acids in Geigeria alata (DC) Oliv. & Hiern. and their contribution to antioxidant and antimicrobial capacity. Phytochem. Anal. 2016, 28, 176–184. [Google Scholar] [PubMed]
- Boukhris, M.A.; Destandau, E.; El Hakmaoui, A.; El Rhaffari, L.; Elfakir, C. A dereplication strategy for the identification of new phenolic compounds from Anvillea radiata (Coss. & Durieu). C.R. Chim. 2016, 19, 1124–1132. [Google Scholar]
- Takenaka, M.; Yan, X.; Ono, H.; Yoshida, M.; Nagata, T.; Nakanishi, T. Caffeic acid derivatives in the roots of yacon (Smallanthus sonchifolius). J. Nat. Prod. 2003, 51, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Kim, Y.; Kim, J. Acylated flavonol glycosides from the flower of Inula britannica. J. Nat. Prod. 2000, 63, 34–36. [Google Scholar] [CrossRef] [PubMed]
- Stojakowska, A.; Malarz, J.; Żylewski, M.; Kisiel, W. Acylated hydroxycinnamic acid glucosides from flowers of Telekia speciosa. Phytochem. Lett. 2015, 12, 257–261. [Google Scholar] [CrossRef]
- Stojakowska, A.; Michalska, K.; Kłeczek, N.; Malarz, J.; Beharav, A. Phenolics and terpenoids from a wild edible plant Lactuca orientalis (Boiss.) Boiss.: A preliminary study. J. Food Comp. Anal. 2018, 69, 20–24. [Google Scholar] [CrossRef]
- Maynard, D.; Gröger, H.; Dierks, T.; Dietz, K.-J. The function of the oxylipin 12-oxophytodienoic acid in cell signaling, stress acclimation, and development. J. Exp. Bot. 2018, 69, 5341–5354. [Google Scholar] [CrossRef]
- Baertschi, S.W.; Ingram, C.D.; Harris, T.M.; Brash, A.R. Absolute configuration of cis-12-oxophytodienoic acid of flaxseed: Implications for the mechanism of biosynthesis from the 13(S)-hydroperoxide of linolenic acid. Biochemistry 1988, 27, 18–24. [Google Scholar] [CrossRef]
- Schaller, F.; Hennig, P.; Weiler, E.W. 12-Oxophytodienoate-10,11-reductase: Occurrence of two isoenzymes of different specificity against stereoisomers of 12-oxophytodienoic acid. Plant Physiol. 1998, 118, 1345–1351. [Google Scholar] [CrossRef]
- Taki-Nakano, N.; Ohzeki, H.; Kotera, J.; Ohta, H. Cytoprotective effects of 12-oxo phytodienoic acid, a plant-derived oxylipin jasmonate, on oxidative stress-induced toxicity in human neuroblastoma SH-SY5Y cells. Biochim. Biophys. Acta 2014, 1840, 3413–3422. [Google Scholar] [CrossRef] [Green Version]
- Taki-Nakano, N.; Kotera, J.; Ohta, H. 12-oxo-phytodienoic acid, a plant-derived oxylipin, attenuates lipopolysaccharide-induced inflammation in microglia. Biochem. Biophys. Res. Commun. 2016, 473, 1288–1294. [Google Scholar] [CrossRef] [Green Version]
- Altiok, N.; Mezzadra, H.; Patel, P.; Koyuturk, M.; Altiok, S. A plant oxylipin, 12-oxo-phytodienoic acid, inhibits proliferation of human breast cancer cells by targeting cyclin D1. Breast Cancer Res. Treat. 2008, 109, 315–323. [Google Scholar] [CrossRef]
- Maldonado, E.M.; Salamanca, E.; Giménez, A.; Sterner, O. Antileishmanial metabolites from Lantana balansae. Rev. Bras. Farmacogn. 2016, 26, 180–183. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |


| Compound | Retention Time (min) | UV (nm) | [M − H]− | Product Ions Main Peaks 1 | |
|---|---|---|---|---|---|
| 1 | 3-O-caffeoylquinic acid | 3.2 | 325 | 353 | 191, 179 |
| 2 | 5-O-caffeoylquinic acid | 5.1 | 325 | 353 | 191 |
| 3 | Dicaffeoylhexaric acid (I) | 7.9 | 324 | 533 | 371, 209 |
| 4 | 3,4-di-O-caffeoylquinic acid | 12.6 | 325 | 515 | 353, 335, 299, 255, 203, 191, 179, 173 |
| 5 | 1,5-di-O-caffeolyquinic acid | 12.8 | 328 | 515 | 353, 335, 191 |
| 6 | 3,5-di-O-caffeoylquinic acid | 13.0 | 327 | 515 | 353, 191 |
| 7 | tricaffeoylhexaric acid (I) | 13.5 | 327 | 695 | 533, 371, 209 |
| 8 | 4,5-di-O-caffeoylquinic acid | 13.8 | 327 | 515 | 353, 317, 299, 255, 203, 173 |
| 9 | tricaffeoylhexaric acid (II) | 14.8 | 328 | 695 | 533, 371, 209 |
| 10 | isobutyryl-dicaffeoylquinic acid | 19.0 | 326 | 585 | 497, 335, 317, 299, 255, 179 |
| 11 | isobutyryl-dicaffeoylquinic acid | 19.8 | 328 | 585 | 497, 423, 335, 179 |
| 12 | tri-O-caffeoylquinic acid | 20.1 | 327 | 677 | 515, 353 |
| 13 | 2-methylbutyryl/isovaleryl-dicaffeoylquinic acid | 22.9 | 326 | 599 | 497, 335, 299, 179 |
| 14 | 2-methylbutyryl/isovaleryl-dicaffeoylquinic acid | 23.8 | 328 | 599 | 497, 437, 335, 179 |
| 15 | isobutyryl-tricaffeoylhexaric acid | 24.7 | 328 | 765 | 603, 441 |
| 16 | 2-methylbutyryl/isovaleryl-tricaffeoylhexaric acid | 29.7 | 327 | 779 | 617, 455 |
| 17 | 2-methylbutyryl/isovaleryl-tricaffeoylhexaric acid | 30.2 | 327 | 779 | 617, 455 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kłeczek, N.; Michalak, B.; Malarz, J.; Kiss, A.K.; Stojakowska, A. Carpesium divaricatum Sieb. & Zucc. Revisited: Newly Identified Constituents from Aerial Parts of the Plant and Their Possible Contribution to the Biological Activity of the Plant. Molecules 2019, 24, 1614. https://doi.org/10.3390/molecules24081614
Kłeczek N, Michalak B, Malarz J, Kiss AK, Stojakowska A. Carpesium divaricatum Sieb. & Zucc. Revisited: Newly Identified Constituents from Aerial Parts of the Plant and Their Possible Contribution to the Biological Activity of the Plant. Molecules. 2019; 24(8):1614. https://doi.org/10.3390/molecules24081614
Chicago/Turabian StyleKłeczek, Natalia, Barbara Michalak, Janusz Malarz, Anna Karolina Kiss, and Anna Stojakowska. 2019. "Carpesium divaricatum Sieb. & Zucc. Revisited: Newly Identified Constituents from Aerial Parts of the Plant and Their Possible Contribution to the Biological Activity of the Plant" Molecules 24, no. 8: 1614. https://doi.org/10.3390/molecules24081614
APA StyleKłeczek, N., Michalak, B., Malarz, J., Kiss, A. K., & Stojakowska, A. (2019). Carpesium divaricatum Sieb. & Zucc. Revisited: Newly Identified Constituents from Aerial Parts of the Plant and Their Possible Contribution to the Biological Activity of the Plant. Molecules, 24(8), 1614. https://doi.org/10.3390/molecules24081614