Co3O4/g-C3N4 Hybrids for Gas-Phase Hg0 Removal at Low Temperature
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of Co3O4/g-C3N4 Hybrids
2.2. Characterization of Co3O4/g-C3N4 Hybrids
2.3. Mercury Oxidation
3. Results and discussion
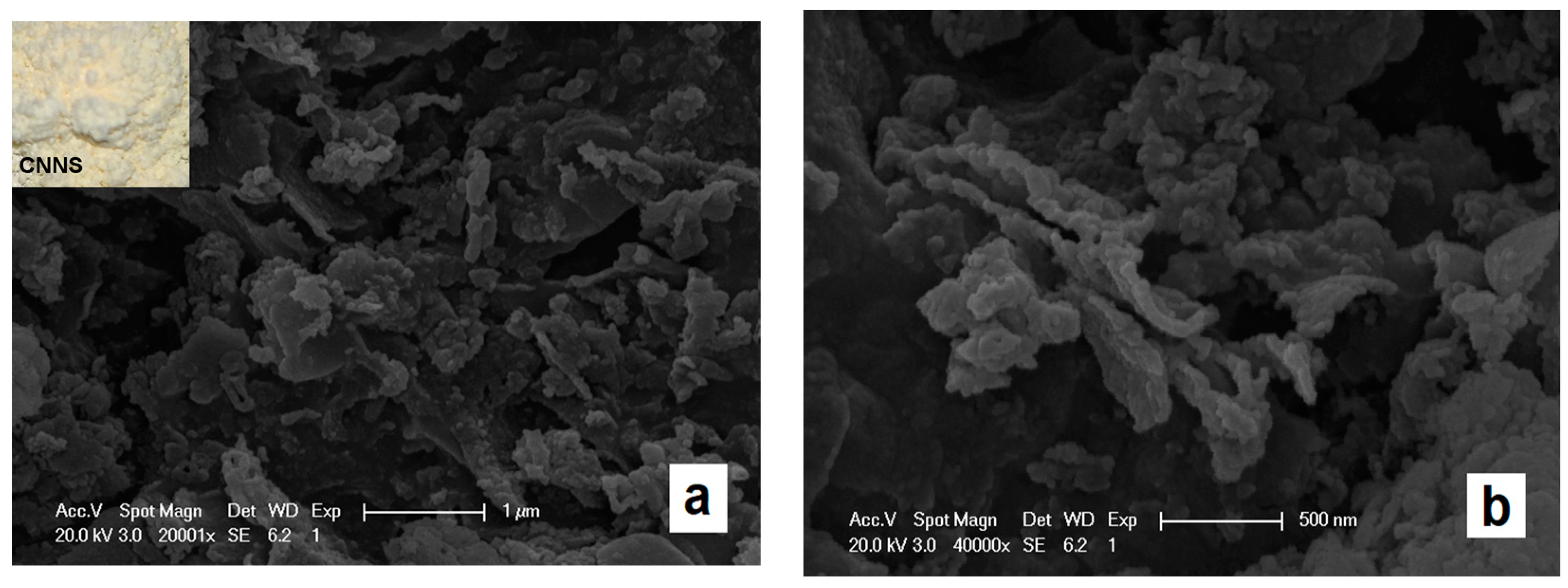
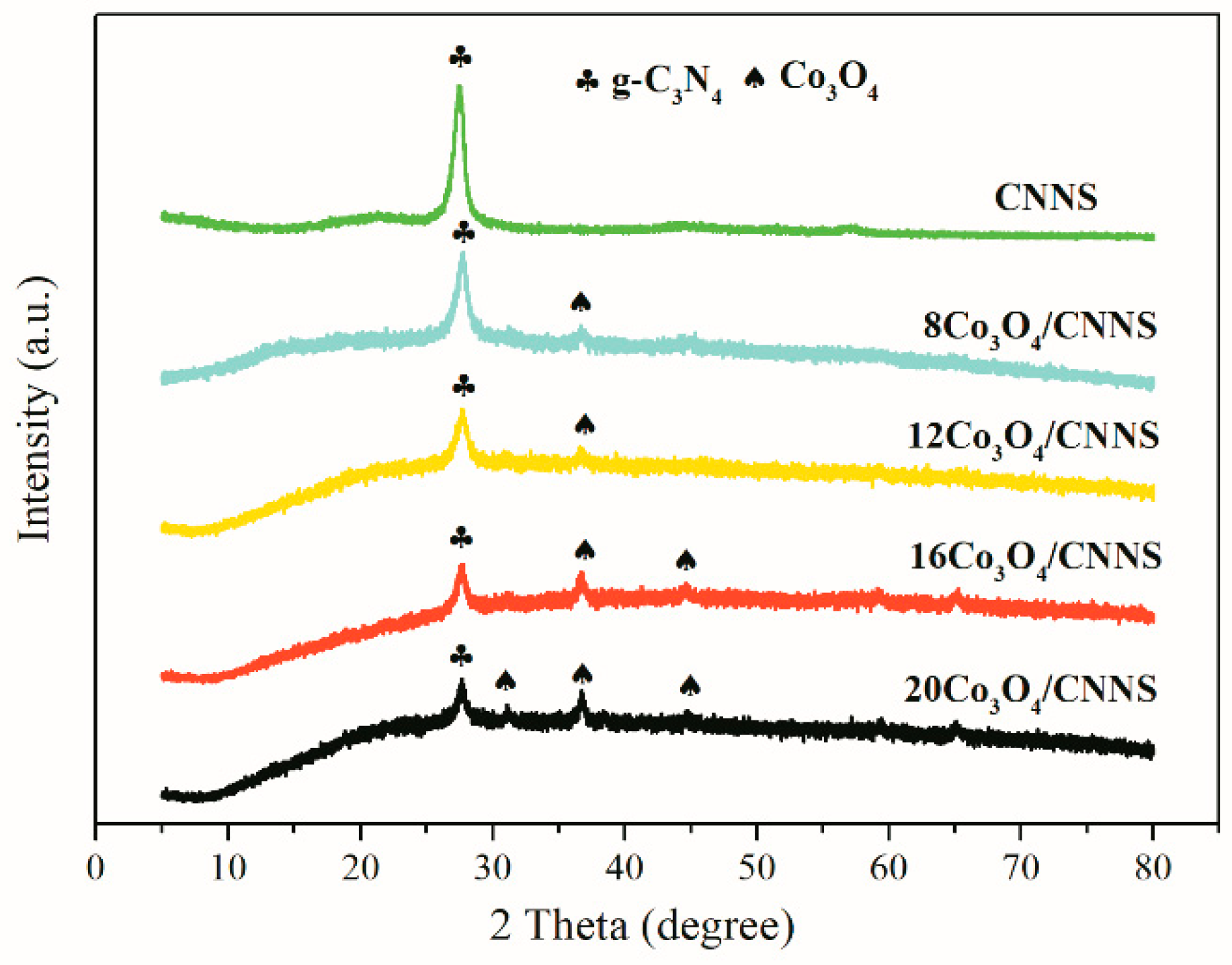
3.1. Characterization Analysis
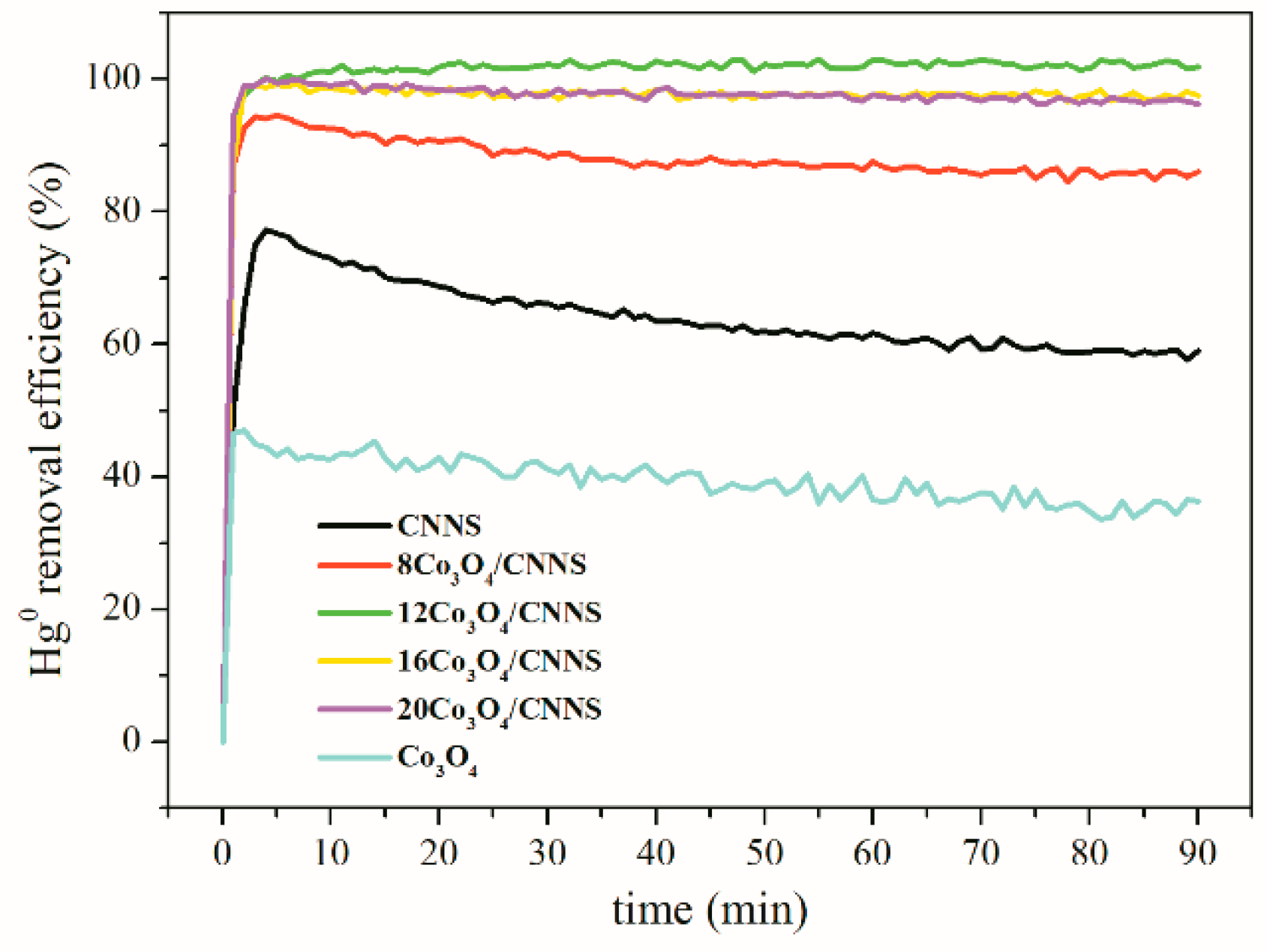
3.2. Impact of Loading Value
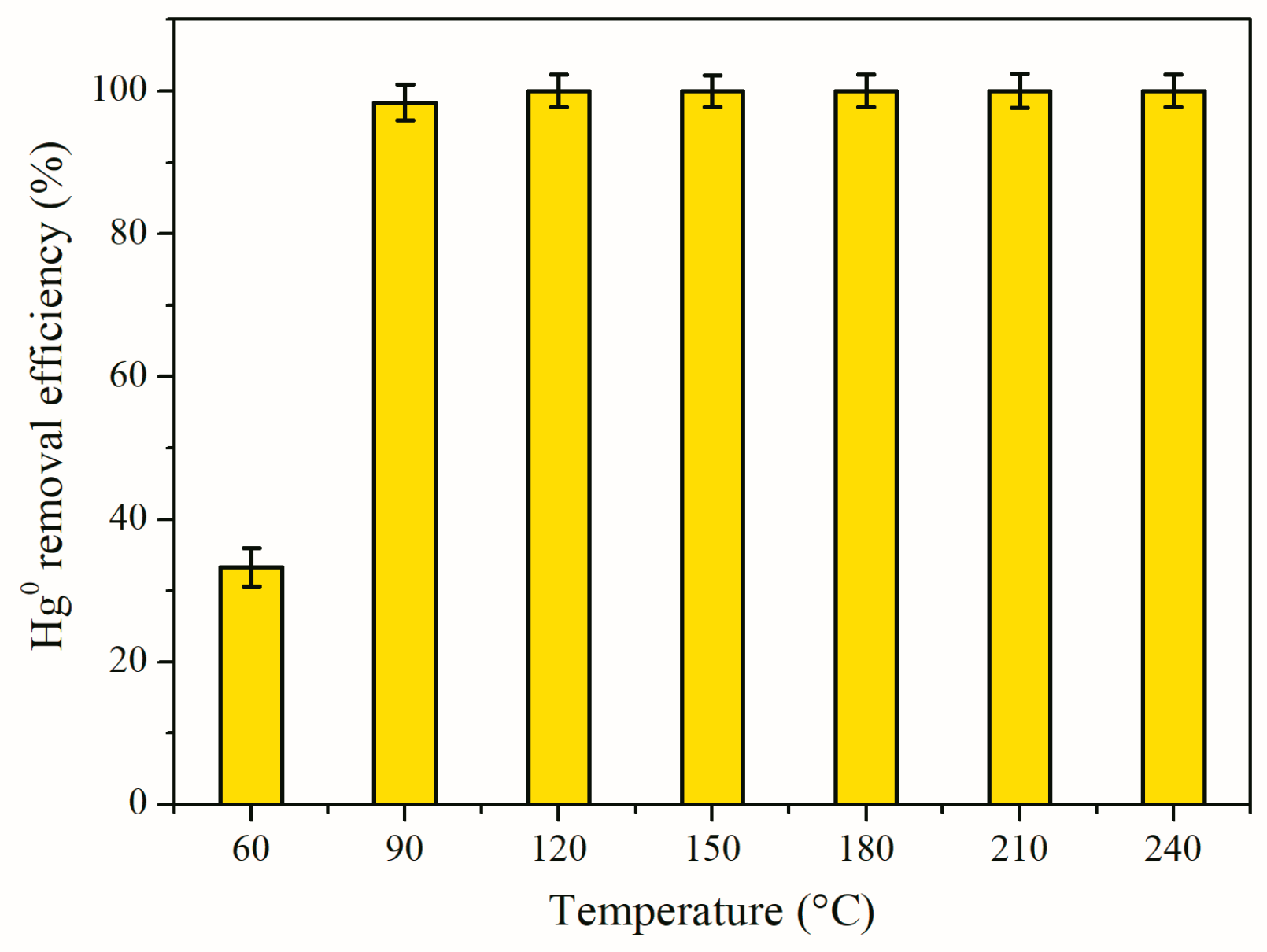
3.3. Impact of Reaction Temperature
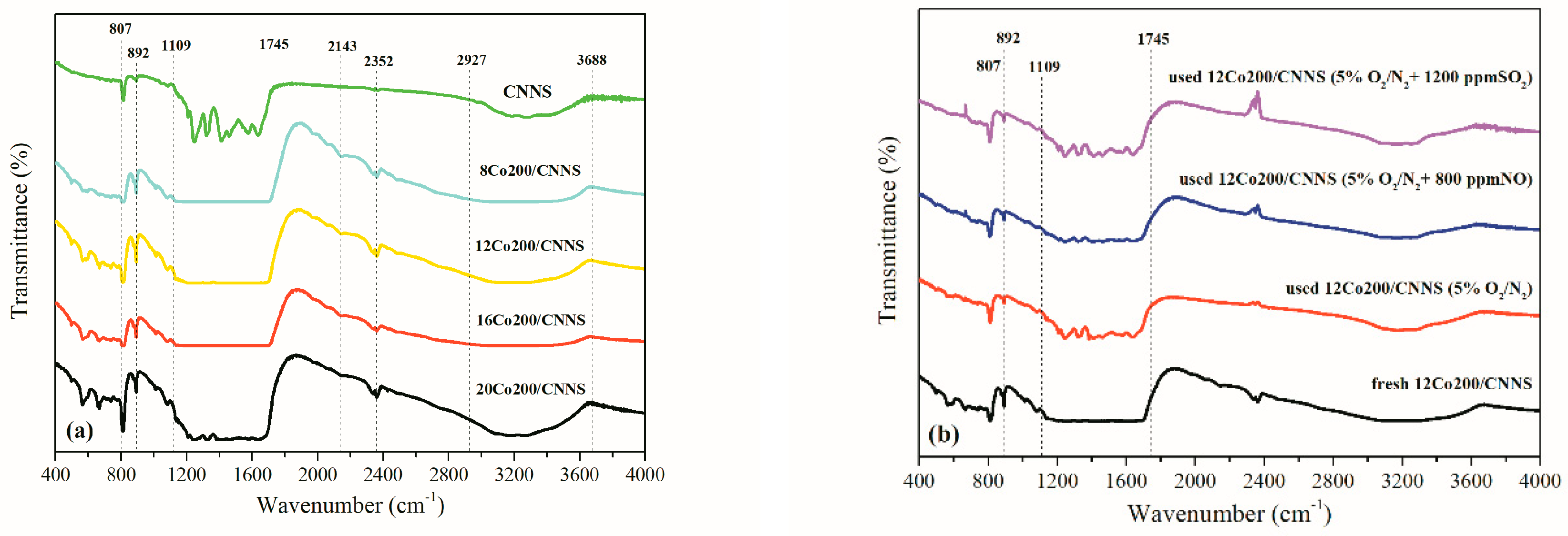
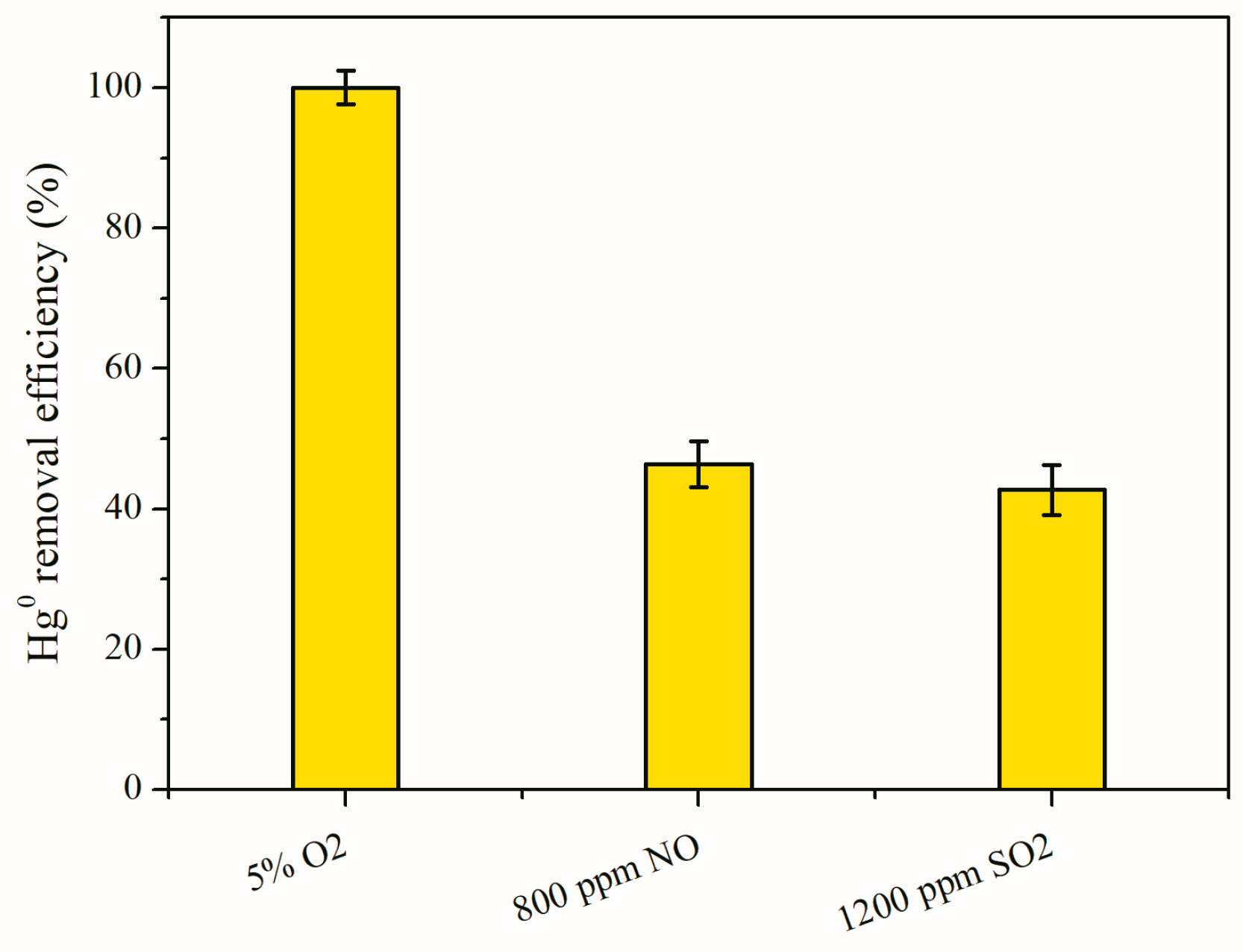
3.4. Impact of Flue Gas
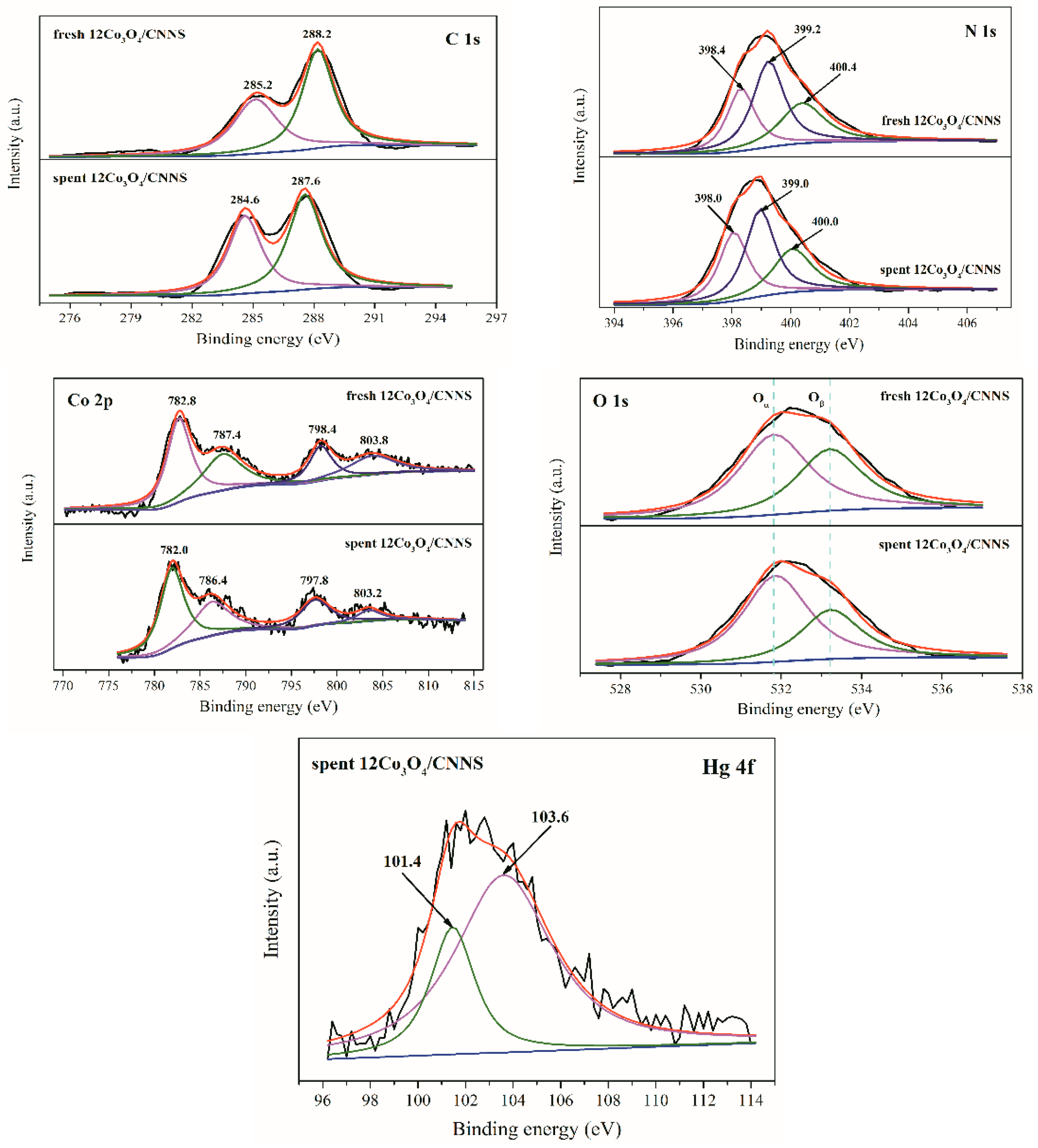
3.5. Mercury Capture Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Driscoll, C.T.; Mason, R.P.; Chan, H.M.; Jacob, D.J.; Pirrone, N. Mercury as a global pollutant: Sources, pathways, and effects. Environ. Sci. Technol. 2013, 47, 4967–4983. [Google Scholar] [CrossRef]
- Schofield, K. Mercury emission control from coal combustion systems: A modified air preheater solution. Combust. Flame 2012, 159, 1741–1747. [Google Scholar] [CrossRef]
- Xie, J.; Yan, N.; Yang, S.; Qu, Z.; Chen, W.; Zhang, W.; Li, K.; Liu, P.; Jia, J. Synthesis and characterization of nano-sized Mn–TiO2 catalysts and their application to removal of gaseous elemental mercury. Res. Chem. Intermed. 2012, 38, 2511–2522. [Google Scholar] [CrossRef]
- Galbreath, K.C.; Zygarlicke, C.J. Mercury transformations in coal combustion flue gas. Fuel Process. Technol. 2000, 65–66, 289–310. [Google Scholar] [CrossRef]
- Jampaiah, D.; Ippolito, S.J.; Sabri, Y.M.; Tardio, J.; Selvakannan, P.R.; Nafady, A.; Reddy, B.M.; Bhargava, S.K. Ceria-zirconia modified mnox catalysts for gaseous elemental mercury oxidation and adsorption. Catal. Sci. Technol. 2016, 6, 1792–1803. [Google Scholar] [CrossRef]
- Xu, H.; Jia, J.; Guo, Y.; Qu, Z.; Liao, Y.; Xie, J.; Shangguan, W.; Yan, N. Design of 3D MnO2/Carbon sphere composite for the catalytic oxidation and adsorption of elemental mercury. J. Hazard. Mater. 2018, 342, 69–76. [Google Scholar] [CrossRef]
- Sjostrom, S.; Durham, M.; Bustard, C.J.; Martin, C. Activated carbon injection for mercury control: Overview. Fuel 2010, 89, 1320–1322. [Google Scholar] [CrossRef]
- Luo, G.; Yao, H.; Xu, M.; Cui, X.; Chen, W.; Gupta, R.; Xu, Z. Carbon nanotube-silver composite for mercury capture and analysis. Energy Fuels 2010, 24, 419–426. [Google Scholar] [CrossRef]
- Xu, W.; Adewuyi, Y.G.; Liu, Y.; Wang, Y. Removal of elemental mercury from flue gas using CuOx and CeO2 modified rice straw chars enhanced by ultrasound. Fuel Process. Technol. 2018, 170, 21–31. [Google Scholar] [CrossRef]
- Xu, H.; Qu, Z.; Zhao, S.; Mei, J.; Quan, F.; Yan, N. Different crystal-forms of one-dimensional MnO2 nanomaterials for the catalytic oxidation and adsorption of elemental mercury. J. Hazard. Mater. 2015, 299, 86–93. [Google Scholar] [CrossRef]
- Jampaiah, D.; Chalkidis, A.; Sabri, Y.M.; Edwin, L.H.; Mayes, E.L.H.; Reddy, B.M.; Bhargava, S.K. Low-temperature elemental mercury removal over TiO2 nanorods-supported MnOx-FeOx-CrOx. Catal. Today 2019, 324, 174–182. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Wang, H.; Wu, Z. Catalytic oxidation of gas-phase mercury over Co/TiO2 catalysts prepared by sol-gel method. Catal. Commun. 2011, 12, 1291–1294. [Google Scholar] [CrossRef]
- Jampaiah, D.; Chalkidis, A.; Sabri, Y.M.; Bhargava, M.K. Role of ceria in the design of composite materials for elemental mercury removal. Chem. Rec. 2018, 18, 1–14. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, X.; Zhou, Z.; Shao, H.; Xu, M. Catalytic oxidation of elemental mercury by Mn-Mo/CNT low temperature. Chem. Eng. J. 2016, 284, 1233–1241. [Google Scholar] [CrossRef]
- Samanta, S.; Martha, S.; Parida, K. Facile synthesis of Au/g-C3N4 nanocomposites: An inorganic/organic hybrid plasmonic photocatalyst with enhanced hydrogen gas evolution under visible-light irradiation. ChemCatChem 2014, 6, 1453–1462. [Google Scholar]
- Xu, J.; Antonietti, M. The performance of nanoparticulate graphitic carbon nitride as an amphiphile. J. Am. Chem. Soc. 2017, 139, 6026–6029. [Google Scholar] [CrossRef]
- Xun, H.; Zhang, Z.; Yu, A.; Yi, J. Remarkably enhanced hydrogen sensing of highly-ordered SnO2-decorated TiO2 nanotubes. Sens. Actuators B Chem. 2018, 273, 983–990. [Google Scholar] [CrossRef]
- Wu, J.; Sheng, P.; Xu, W.; Zhou, X.; Lu, C.; Ji, Z.; Xu, K.; Zhu, L.; Zhang, X.; Feng, W. Constructing interfacial contact for enhanced photocatalytic activity through BiOIO3/g-C3N4 nanoflake heterostructure. Catal. Commun. 2018, 109, 55–59. [Google Scholar] [CrossRef]
- Idrees, F.; Dillert, R.; Bahnemann, D.; Butt, F.K.; Tahir, M. In-situ synthesis of Nb2O5/g-C3N4 heterostructures as highly efficient photocatalysts for molecular H2 elution under solar illumination. Catalysts 2019, 9, 169. [Google Scholar] [CrossRef]
- Tahir, M.; Mahmood, N.; Zhang, X.; Mahmood, T.; Butt, F.K.; Aslam, I.; Tanveer, M.; Idrees, F.; Khalid, S.; Shakir, I.; et al. Bifunctional catalysts of Co3O4@GCN tubular nanostructured (TNS) hybrids for oxygen and hydrogen evolution reactions. Nano Res. 2015, 8, 3725–3736. [Google Scholar] [CrossRef]
- Zhu, H.L.; Zheng, Y.Q. Mesoporous Co3O4 anchored on the graphitic carbon nitride for enhanced performance supercapacitor. Electrochim. Acta 2018, 265, 372–378. [Google Scholar] [CrossRef]
- Xiao, J.; Rabeah, J.; Yang, J.; Xie, Y.; Cao, H.; Brückner, A. Fast electron transfer and OH formation: Key features for high activity in visible-light-driven ozonation with C3N4 catalyst. ACS Catal. 2017, 7, 6198–6206. [Google Scholar] [CrossRef]
- Liu, D.J.; Zhang, Z.; Wu, J. Elemental mercury removal by MnO2 nanoparticle decorated carbon nitride nanosheet. Energy Fuels 2019, 33, 3089–3097. [Google Scholar] [CrossRef]
- Liu, D.J.; Lu, C.; Wu, J. Gaseous mercury capture by copper-activated nanoporous carbon nitride. Energy Fuels 2018, 32, 8287–8295. [Google Scholar] [CrossRef]
- Liu, D.J.; Zhou, W.G.; Wu, J. Effect of Ce and La on the activity of CuO/ZSM-5 and MnOx/ZSM-5 composites for elemental mercury removal at low temperature. Fuel 2017, 194, 115–122. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Wang, J.; Uchaker, E.; Zhang, Q.; Sun, S.; Huang, Y.; Li, J.; Cao, G. Titanium alkoxide induced BiOBr-Bi2WO6 mesoporous nanosheet composites with much enhanced photocatalytic activity. J. Mater. Chem. A 2013, 1, 7949–7956. [Google Scholar] [CrossRef]
- Liu, D.J.; Lu, C.; Wu, J. CuO/g-C3N4 nanocomposite for elemental mercury capture at low temperature. J. Nanopart. Res. 2018, 20, 227. [Google Scholar] [CrossRef]
- Hu, H.; Cai, S.; Li, H.; Huang, L.; Shi, L.; Zhang, D. Mechanistic aspects of deNOx processing over TiO2 supported Co-Mn oxide catalysts: Structure-activity relationships and in situ DRIFTs analysis. ACS Catal. 2015, 5, 6069–6077. [Google Scholar] [CrossRef]
- Xiao, J.; Xie, Y.; Nawaz, F.; Wang, Y.; Du, P.; Cao, H. Dramatic coupling of visible light with ozone on honeycomb-like porous g-C3N4 towards superior oxidation of water pollutants. Appl. Catal. B Environ. 2016, 183, 417–425. [Google Scholar] [CrossRef]
- Shen, B.; Yao, Y.; Ma, H.; Liu, T. Ceria modified MnOx/TiO2-pillared clays catalysts for the selective catalytic reduction of NO with NH3 at low temperature. Chin. J. Catal. 2011, 32, 1803–1811. [Google Scholar] [CrossRef]
- Bing, W.; Chen, Z.; Sun, H.; Shi, P.; Gao, N.; Ren, J.; Qu, X. Visible-light-driven enhanced antibacterial and biofilm elimination activity of graphitic carbon nitride by embedded ag nanoparticles. Nano Res. 2015, 8, 1648–1658. [Google Scholar] [CrossRef]
- Dong, F.; Wu, L.; Sun, Y.; Fu, M.; Wu, Z.; Lee, S.C. Efficient synthesis of polymeric g-C3N4 layered materials as novel efficient visible light driven photocatalysts. J. Mater. Chem. 2011, 21, 15171–15174. [Google Scholar] [CrossRef]
- Yuan, B.; Chu, Z.; Li, G.; Jiang, Z.; Hu, T.; Wang, Q.; Wang, C. Water-soluble ribbonlike graphitic carbon nitride (g-C3N4): Green synthesis, self-assembly and unique optical properties. J. Mater. Chem. C 2014, 2, 8212–8215. [Google Scholar] [CrossRef]
- Liu, D.J.; Zhou, W.G.; Wu, J. CeO2-MnOx/ZSM-5 sorbents for H2S removal at high temperature. Chem. Eng. J. 2016, 284, 862–871. [Google Scholar] [CrossRef]
- Chen, Z.; Sun, P.; Fan, B.; Liu, Q.; Zhang, Z.; Fang, X. Textural and electronic structure engineering of carbon nitride via doping with π-deficient aromatic pyridine ring for improving photocatalytic activity. Appl. Catal. B Environ. 2015, 170–171, 10–16. [Google Scholar] [CrossRef]
- Vinu, A. Two-dimensional hexagonally-ordered mesoporous carbon nitrides with tunable pore diameter, surface area and nitrogen content. Adv. Funct. Mater. 2008, 18, 816–827. [Google Scholar] [CrossRef]
- Teng, Z.; Lv, H.; Wang, C.; Xue, H.; Pang, H.; Wang, G. Bandgap engineering of ultrathin graphene-like carbon nitride nanosheets with controllable oxygenous functionalization. Carbon 2017, 113, 63–75. [Google Scholar] [CrossRef]
- Tang, X.; Gao, F.; Xiang, Y.; Yi, H.; Zhao, S. Low temperature catalytic oxidation of nitric oxide over the Mn-CoOx catalyst modified by nonthermal plasma. Catal. Commun. 2015, 64, 12–17. [Google Scholar] [CrossRef]
- Yang, W.; Liu, Y.; Wang, Q.; Pan, J. Removal of elemental mercury from flue gas using wheat straw chars modified by Mn-Ce mixed oxides with ultrasonic-assisted impregnation. Chem. Eng. J. 2017, 326, 169–181. [Google Scholar] [CrossRef]
- Sasmaz, E.; Kirchofer, A.; Jew, A.D.; Saha, A.; Abram, D.; Jaramillo, T.F.; Wilcox, J. Mercury chemistry on brominated activated carbon. Fuel 2012, 99, 188–196. [Google Scholar] [CrossRef]
- Hutson, N.D.; Attwood, B.C.; Scheckel, K.G. XAS and XPS characterization of mercury binding on brominated activated carbon. Environ. Sci. Technol. 2007, 41, 1747–1752. [Google Scholar] [CrossRef]
- Kijlstra, W.S.; Biervliet, M.; Poels, E.K.; Bliek, A. Deactivation by SO2 of MnOx/Al2O3 catalysts used for the selective catalytic reduction of NO with NH3 at low temperatures. Appl. Catal. B Environ. 1998, 16, 327–337. [Google Scholar] [CrossRef]
- Yang, S.; Guo, Y.; Yan, N.; Wu, D.; He, H.; Qu, Z.; Jia, J. Elemental mercury capture from flue gas by magnetic Mn-Fe spinel: Effect of chemical heterogeneity. Ind. Eng. Chem. Res. 2011, 50, 9650–9656. [Google Scholar] [CrossRef]




| Samples | BET Surface Area (m2/g) | Total Pore Volume (cm3/g) | Micropore Volume (cm3/g) | Mesopore Volume (cm3/g) | Pore Diameter (nm) |
|---|---|---|---|---|---|
| CNNS | 109 | 0.456 | 0.011 | 0.445 | 19 |
| 8Co3O4/CNNS | 42 | 0.206 | 0.004 | 0.202 | 10 |
| 12Co3O4/CNNS | 42 | 0.211 | 0.003 | 0.208 | 11 |
| 16Co3O4/CNNS | 33 | 0.175 | 0.003 | 0.172 | 12 |
| 20Co3O4/CNNS | 27 | 0.125 | 0.002 | 0.123 | 10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Wu, J.; Liu, D. Co3O4/g-C3N4 Hybrids for Gas-Phase Hg0 Removal at Low Temperature. Processes 2019, 7, 279. https://doi.org/10.3390/pr7050279
Zhang Z, Wu J, Liu D. Co3O4/g-C3N4 Hybrids for Gas-Phase Hg0 Removal at Low Temperature. Processes. 2019; 7(5):279. https://doi.org/10.3390/pr7050279
Chicago/Turabian StyleZhang, Zhen, Jiang Wu, and Dongjing Liu. 2019. "Co3O4/g-C3N4 Hybrids for Gas-Phase Hg0 Removal at Low Temperature" Processes 7, no. 5: 279. https://doi.org/10.3390/pr7050279
APA StyleZhang, Z., Wu, J., & Liu, D. (2019). Co3O4/g-C3N4 Hybrids for Gas-Phase Hg0 Removal at Low Temperature. Processes, 7(5), 279. https://doi.org/10.3390/pr7050279






