The Effect of Dissolved Organic Matter (DOM) on the Release and Distribution of Endocrine-Disrupting Chemicals (Edcs) from Sediment under Hydrodynamic Forces, A Case Study of Bisphenol A (BPA) and Nonylphenol (NP)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Characterization
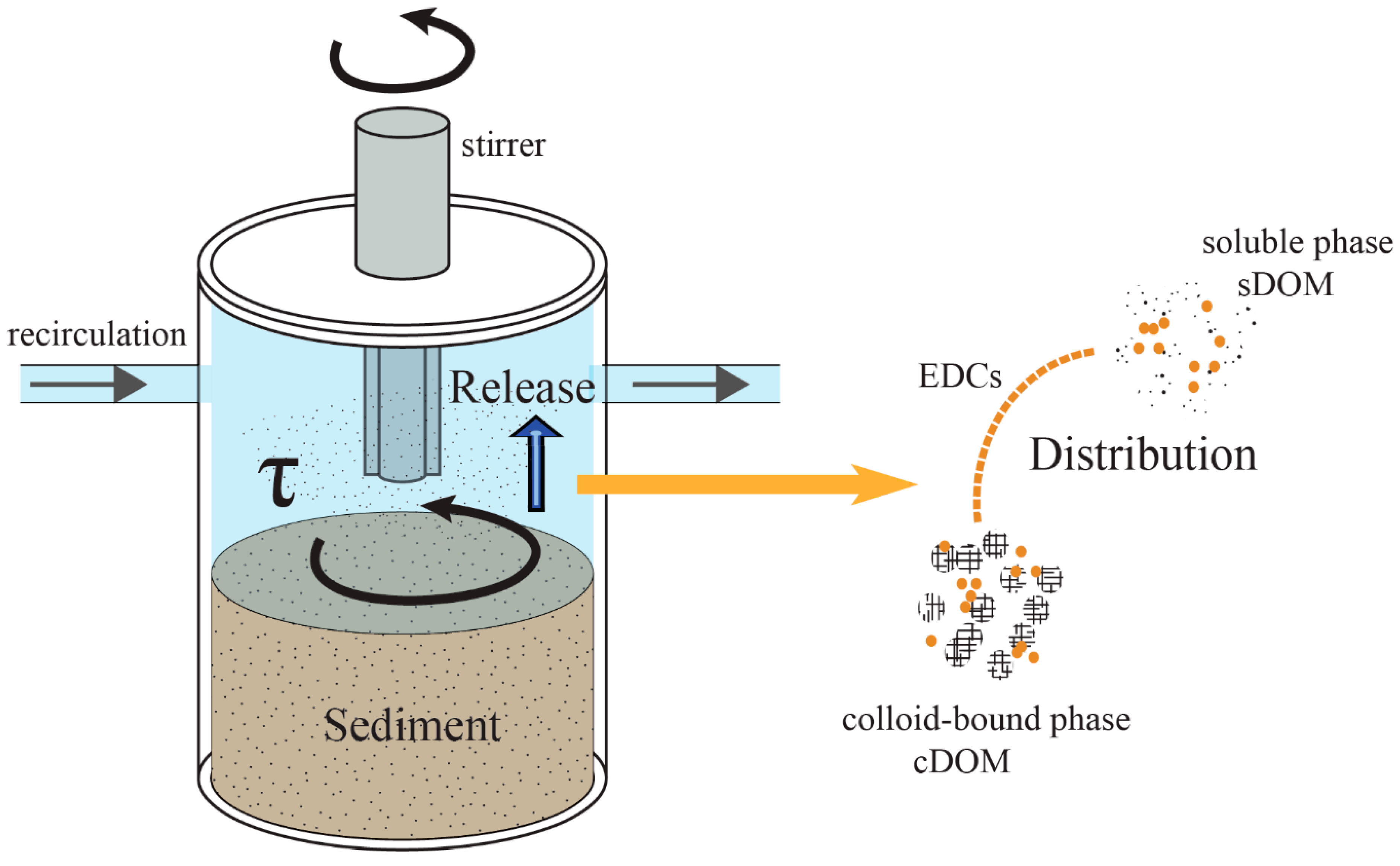
2.2. Experimental Setup
2.3. Hydrodynamic Conditions
2.4. Experimental Procedure and Sampling
2.5. Sample Analysis
2.5.1. Sample Fractionation
2.5.2. Extraction of EDCs from Water Samples
2.5.3. EDCs Extraction of Sediment Sample
2.5.4. GC-MS Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Physiochemical Properties of the Water Column
3.2. The Release EDCs in an Aquatic System under Hydrodynamic Forces
3.2.1. Distributions of EDCs in Overlying Water
3.2.2. The Correlation of Hydrodynamic Intensity and EDCs Content
3.2.3. Mass Balance of the Influx and Efflux of EDCs within the Aquatic System
3.3. The Effect of DOM for EDCs Release under Hydrodynamic Force
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huang, Y.; Li, W.; Qin, L.; Xie, X.; Gao, B.; Sun, J.; Li, A. Distribution of endocrine-disrupting chemicals in colloidal and soluble phases in municipal secondary effluents and their removal by different advanced treatment processes. Chemosphere 2019, 219, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, J.; Zhang, R.; Wei, D.; Long, Q.; Huang, Y. Comparison of different advanced treatment processes in removing endocrine disruption effects from municipal wastewater secondary effluent. Chemosphere 2017, 168, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sadmani, A.H.M.A.; Andrews, R.C.; Bagley, D.M. Influence of naturally occurring dissolved organic matter, colloids, and cations on nanofiltration of pharmaceutically active and endocrine disrupting compounds. Chemosphere 2014, 117, 170–177. [Google Scholar] [CrossRef]
- Sadmani, A.H.M.A.; Andrews, R.C.; Bagley, D.M. Rejection of pharmaceutically active and endocrine disrupting compounds by nanofiltration as a function of source water humic substances. J. Water Process Eng. 2014, 2, 63–70. [Google Scholar]
- Sadmani, A.H.M.A.; Andrews, R.C.; Bagley, D.M. Nanofiltration of pharmaceutically active and endocrine disrupting compounds as a function of compound interactions with DOM fractions and cations in natural water. Sep. Purif. Technol. 2014, 122, 462–471. [Google Scholar] [CrossRef]
- Chen, W.; Pan, S.; Cheng, H.; Sweetman, A.J.; Zhang, H.; Jones, K.C. Diffusive gradients in thin-films (DGT) for in situ sampling of selected endocrine disrupting chemicals (EDCs) in waters. Water Res. 2018, 137, 211–219. [Google Scholar] [CrossRef]
- Cheng, H.; Hua, Z. Effects of hydrodynamic disturbances and resuspension characteristics on the release of tetrabromobisphenol A from sediment. Environ. Pollut. 2016, 219, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Hua, Z. Distribution, release and removal behaviors of tetrabromobisphenol A in water-sediment systems under prolonged hydrodynamic disturbances. Sci. Total Environ. 2018, 636, 402–410. [Google Scholar] [CrossRef]
- Wu, D.; Hua, Z. The effect of vegetation on sediment resuspension and phosphorus release under hydrodynamic disturbance in shallow lakes. Ecol. Eng. 2014, 69, 55–62. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, Q.; Gao, S. Relationship between bed shear stress and suspended sediment concentration: Annular flume experiments. Int. J. Sediment Res. 2012, 26, 513–523. [Google Scholar] [CrossRef]
- He, W.; Chen, M.; Schlautman, M.A.; Hur, J. Dynamic exchanges between DOM and POM pools in coastal and inland aquatic ecosystems: A review. Sci. Total Environ. 2016, 551–552, 415–428. [Google Scholar] [CrossRef]
- He, W.; Jung, H.; Lee, J.; Hur, J. Differences in spectroscopic characteristics between dissolved and particulate organic matters in sediments: Insight into distribution behavior of sediment organic matter. Sci. Total Environ. 2016, 547, 1–8. [Google Scholar] [CrossRef]
- Jiang, T.; Bravo, A.G.; Skyllberg, U.; Björn, E.; Wang, D.; Yan, H.; Green, N.W. Influence of dissolved organic matter (DOM) characteristics on dissolved mercury (Hg) species composition in sediment porewater of lakes from southwest China. Water Res. 2018, 146, 146–158. [Google Scholar] [CrossRef]
- Galletti, Y.; Gonnelli, M.; Brogi, S.R.; Vestri, S.; Santinelli, C. DOM dynamics in open waters of the Mediterranean Sea: New insights from optical properties. Deep-Sea Res. Part I 2019, 144, 95–114. [Google Scholar] [CrossRef]
- Si, X.; Hu, Z.; Ding, D.; Fu, X. Effects of effluent organic matters on endocrine disrupting chemical removal by ultrafiltration and ozonation in synthetic secondary effluent. J. Environ. Sci. 2018, 76, 57–64. [Google Scholar] [CrossRef]
- Xie, M.; Wang, N.; Gaillard, J.F.; Packman, A.I. Hydrodynamic Forcing Mobilizes Cu in Low-Permeability Estuarine Sediments. Environ. Sci. Technol. 2016, 50, 4615–4623. [Google Scholar] [CrossRef]
- Mehta, A.J.; Hwang, K.; Khare, Y.P. Critical shear stress for mass erosion of organic-rich fi ne sediments. Estuar. Coast. Shelf Sci. 2015, 165, 97–103. [Google Scholar] [CrossRef]
- Xie, M.; Jarrett, B.A.; Da Silva-Cadoux, C.; Fetters, K.J.; Burton, G.A., Jr.; Gaillard, J.F.; Packman, A.I. Coupled effects of hydrodynamics and biogeochemistry on Zn mobility and speciation in highly contaminated sediments. Environ. Sci. Technol. 2015, 49, 5346–5353. [Google Scholar] [CrossRef]
- Huang, J.; Xi, B.; Xu, Q.; Wang, X. Experiment study of the effects of hydrodynamic disturbance on the interaction between the cyanobacterial growth and the nutrients. J. Hydrodyn. 2016, 28, 411–422. [Google Scholar] [CrossRef]
- Jia, Y.; Hammers-Wirtz, M.; Crawford, S.E.; Chen, Q.; Seiler, T.B.; Schäffer, A.; Hollert, H. Effect-based and chemical analyses of agonistic and antagonistic endocrine disruptors in multiple matrices of eutrophic freshwaters. Sci. Total Environ. 2019, 651, 1096–1104. [Google Scholar] [CrossRef]
- Xu, J.; Wu, L.; Chen, W.; Chang, A.C. Simultaneous determination of pharmaceuticals, endocrine disrupting compounds and hormone in soils by gas chromatography—Mass spectrometry. J. Chromatogr. A 2008, 1202, 189–195. [Google Scholar] [CrossRef]
- Wang, B.; Zeng, D.; Chen, Y.; Belzile, N.; Bai, Y.; Zhu, J. Adsorption behaviors of phenanthrene and bisphenol A in purple paddy soils amended with straw-derived DOM in the West Sichuan Plain of China. Ecotoxicol. Environ. Saf. 2019, 169, 737–746. [Google Scholar] [CrossRef]
- Liu, D.; Wu, S.; Xu, H.; Zhang, Q.; Zhang, S.; Shi, L.; Yao, C.; Liu, Y.; Cheng, J. Distribution and bioaccumulation of endocrine disrupting chemicals in water, sediment and fishes in a shallow chinese freshwater lake: Implications for ecological and human health risks. Ecotoxicol. Environ. Saf. 2017, 140, 222–229. [Google Scholar]
- Li, L.S.; Yang, X.X.; Wang, L. Determination of Bisphenol A in bottle water by high performance liquid chromatography. Pract. Prev. Med. 2006, 13, 429–430. [Google Scholar]
- Ma, X.Y.; Gao, N.Y.; Li, Q.S.; Xu, B.; Le, L.S.; Wu, J.M. Investigation of several endocrine disrupting chemicals in Huangpu River and water treatment units of a waterworks. China Water Wastewater 2006, 22, 1–4. [Google Scholar]
- Ma, L.; Yates, S.R. Dissolved organic matter and estrogen interactions regulate estrogen removal in the aqueous environment: A review. Sci. Total Environ. 2018, 641, 529–542. [Google Scholar] [CrossRef]
- Karickhoff, S.W. Semi-empirical estimation of sorption of hydrophobic pollutants on natural sediments and soils. Chemosphere 1981, 10, 833–846. [Google Scholar] [CrossRef]
- Yamamoto, H.; Liljestrand, H.M.; Shimizu, Y.; Morita, M. Effects of physical−chemmical characteristics on the sorption of selected endocrine disruptors by dissolved organic matter surrogates. Environ. Sci. Technol. 2003, 37, 2646–2657. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.X.; Wilding, A.; Hibberd, A.; Zhou, J.L. Partition of endocrine-disrupting chemicals between colloids and dissolved phase as determined by cross-flow ultrafiltration. Environ. Sci. Technol. 2005, 39, 2753–2761. [Google Scholar] [CrossRef]
- Zhu, D.; Hyun, S.; Pignatello, J.J.; Lee, L.S. Evidence for π−π electron donor−accepptor interactions between π-donor aromatic compounds and π-acceptor sites in soil organic matter through pH effects on sorption. Environ. Sci. Technol. 2004, 38, 4361–4368. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Ran, Y.; Zhang, D.; Chen, D.; Li, H.; Huang, Y. Vertical profiles and distributions of aqueous endocrine-disrupting chemicals in different matrices from the Pearl River Delta and the influence of environmental factors. Environ. Pollut. 2019, 246, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Bertoldi, C.; Rodrigues, A.G.; Fernandes, A.N. Engineering Removal of endocrine disrupters in water under artificial light: The effect of organic matter. J. Water Process 2019, 27, 126–133. [Google Scholar] [CrossRef]
- Xie, F.; Dai, Z.; Zhu, Y.; Li, G.; Li, H.; He, Z.; Geng, S. Adsorption of phosphate by sediments in a eutrophic lake: Isotherms, kinetics, thermodynamics and the influence of dissolved organic matter. Colloids Surf. A 2019, 562, 16–25. [Google Scholar] [CrossRef]





| Series | θ * | Shear Stress (Pa) |
|---|---|---|
| G1 | 0.05 | 0.0011 |
| G2 | 0.20 | 0.0046 |
| G3 | 0.50 | 0.0114 |
| G4 | 0.80 | 0.0182 |
| Series | DO * (mg/L) | pH | pE (mV) | Temperature (°C) |
|---|---|---|---|---|
| G1 | 8.2 | 8.44 | 174 | 24.3 |
| G2 | 8.5 | 8.36 | 173 | 24.5 |
| G3 | 8.8 | 8.33 | 171 | 24.4 |
| G4 | 9.3 | 8.28 | 169 | 24.3 |
| Phases | EDCs | k1 | k2 | R2 |
|---|---|---|---|---|
| colloid-bound | BPA | 48.73 | 1.29 | 0.992 |
| NP | 41.06 | 1.70 | 0.995 | |
| soluble | BPA | 68.89 | 1.78 | 0.985 |
| NP | 41.52 | 1.07 | 0.987 |
| Series | BPA | NP | ||||
|---|---|---|---|---|---|---|
| SEF * (ng) | WIF ** (ng) | WIF/SEF *** | SEF (ng) | SEF (ng) | WIF/SEF | |
| G1 | 550 | 541 | 0.98 | 418 | 439 | 1.05 |
| G2 | 836 | 829 | 0.99 | 572 | 516 | 0.90 |
| G3 | 1408 | 1387 | 0.99 | 946 | 880 | 0.93 |
| G4 | 2134 | 2080 | 0.97 | 1364 | 1278 | 0.94 |
| Phase | EDCs | k3 | k4 | R2 |
|---|---|---|---|---|
| colloid-bound | BPA | 7.985 | 15.86 | 0.978 |
| NP | 10.930 | −6.656 | 0.988 | |
| soluble | BPA | 26.89 | −0.902 | 0.989 |
| NP | 6.678 | 27.07 | 0.965 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, J.; Cheng, Y.; Hua, Z.; Yuan, C.; Wang, X. The Effect of Dissolved Organic Matter (DOM) on the Release and Distribution of Endocrine-Disrupting Chemicals (Edcs) from Sediment under Hydrodynamic Forces, A Case Study of Bisphenol A (BPA) and Nonylphenol (NP). Int. J. Environ. Res. Public Health 2019, 16, 1724. https://doi.org/10.3390/ijerph16101724
Ding J, Cheng Y, Hua Z, Yuan C, Wang X. The Effect of Dissolved Organic Matter (DOM) on the Release and Distribution of Endocrine-Disrupting Chemicals (Edcs) from Sediment under Hydrodynamic Forces, A Case Study of Bisphenol A (BPA) and Nonylphenol (NP). International Journal of Environmental Research and Public Health. 2019; 16(10):1724. https://doi.org/10.3390/ijerph16101724
Chicago/Turabian StyleDing, Jue, Yu Cheng, Zulin Hua, Cong Yuan, and Xiaoju Wang. 2019. "The Effect of Dissolved Organic Matter (DOM) on the Release and Distribution of Endocrine-Disrupting Chemicals (Edcs) from Sediment under Hydrodynamic Forces, A Case Study of Bisphenol A (BPA) and Nonylphenol (NP)" International Journal of Environmental Research and Public Health 16, no. 10: 1724. https://doi.org/10.3390/ijerph16101724
APA StyleDing, J., Cheng, Y., Hua, Z., Yuan, C., & Wang, X. (2019). The Effect of Dissolved Organic Matter (DOM) on the Release and Distribution of Endocrine-Disrupting Chemicals (Edcs) from Sediment under Hydrodynamic Forces, A Case Study of Bisphenol A (BPA) and Nonylphenol (NP). International Journal of Environmental Research and Public Health, 16(10), 1724. https://doi.org/10.3390/ijerph16101724





