Molecular and Cellular Mechanisms Underlying Somatostatin-Based Signaling in Two Model Neural Networks, the Retina and the Hippocampus
Abstract
:1. Introduction
2. An Overview of Retinal and Hippocampal Circuitries
2.1. Retina
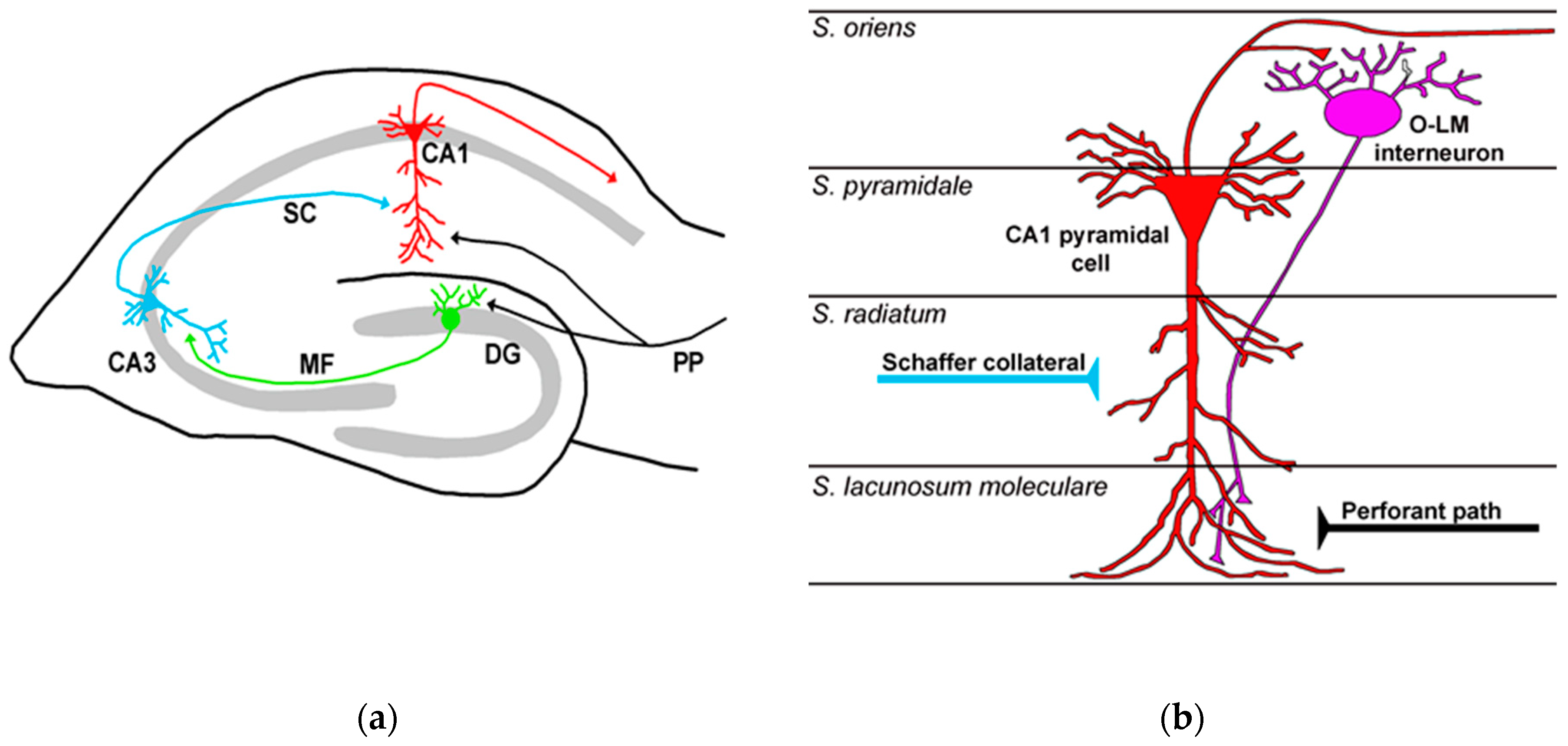
2.2. Hippocampus
3. The Somatostatinergic System in the Retina and in the Hippocampus
3.1. Retina
3.2. Hippocampus
3.3. Signal Transduction Mediating Somatostatin Action
3.4. The Complexity of Somatostatin-Based Signaling: Autocrine, Paracrine, and Synaptic Communication
4. Molecular and Cellular Physiology of Somatostatin Action: Modulation of Membrane Excitability and Neurotransmission
4.1. Retina
4.2. Hippocampus
5. Retina and Hippocampus: Common Themes
5.1. Co-Transmission of GABA and Somatostatin
5.2. Modulation of Ion Channels
5.3. The Logic of Somatostatin-Based Signaling
6. The Role of Somatostatin in Network Functioning: Implications for Health and Disease
7. Conclusions and Future Perspectives
Acknowledgments
Conflicts of Interest
Abbreviations
| 4-AP | 4-amino pyridine |
| AC | Adenylyl cyclase |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| BiC | Bistratified cell |
| cAMP | Cyclic adenosine monophosphate |
| CA | Cornu ammonis |
| CaV(L) | Voltage-gated L-type calcium channel |
| CaV(N) | Voltage-gated N-type calcium channel |
| CCK | Cholecystokinin |
| CNS | Central nervous system |
| DA | Dopamine |
| DG | Dentate gyrus |
| EC | Entorhinal cortex |
| EPSC | Excitatory post-synaptic current |
| EPSP | Excitatory post-synaptic potential |
| ERG | Electroretinogram |
| GABA | γ-aminobutyric acid |
| GC | Ganglion cell |
| GCL | Ganglion cell layer |
| GIRK | G-protein activated, inwardly rectifying potassium channel |
| GPCR | G-protein coupled receptor |
| INL | Inner nuclear layer |
| IPL | Inner plexiform layer |
| IPSC | Inhibitory post-synaptic current |
| KCa | Voltage-gated and Ca2+-dependent potassium channel |
| KO | Knock-out |
| KV | Voltage-gated potassium channel |
| LTP | Long-term potentiation |
| M1 ipRGCs | Melanopsin-containing, intrinsically photosensitive retinal ganglion cells |
| NaV | Voltage-gated sodium channel |
| NMDA | N-methyl-D-aspartate |
| NPY | Neuropeptide Y |
| O-LM | Oriens lacunosum-moleculare interneuron |
| ONL | Outer nuclear layer |
| OPL | Outer plexiform layer |
| PKA | Protein kinase A |
| PKC | Protein kinase C |
| PP | Perforant pathway |
| PTX | Pertussis toxin |
| RBC | Rod bipolar cell |
| RP | Rod photoreceptor |
| s.l.m. | Stratum lacumosum-moleculare |
| s.o. | Stratum oriens |
| s.p. | Stratum pyramidale |
| s.r. | Stratum radiatum |
| SRIF | Somatotropin release-inhibiting factor |
| SST | Somatostatin receptor |
| TLE | Temporal lobe epilepsy |
| VIP | Vasoactive intestinal peptide |
References
- Brazeau, P.; Vale, W.; Burgus, R.; Ling, N.; Butcher, M.; Rivier, J.; Guillemin, R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science 1973, 179, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Günther, T.; Tulipano, G.; Dournaud, P.; Bousquet, C.; Csaba, Z.; Kreienkamp, H.J.; Lupp, A.; Korbonits, M.; Castaño, J.P.; Wester, H.J.; et al. International Union of Basic and Clinical Pharmacology. CV. Somatostatin Receptors: Structure, Function, Ligands, and New Nomenclature. Pharmacol. Rev. 2018, 70, 763–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferguson, K.A.; Huh, C.Y.; Amilhon, B.; Manseau, F.; Williams, S.; Skinner, F.K. Network models provide insights into how oriens-lacunosum-moleculare and bistratified cell interactions influence the power of local hippocampal CA1 theta oscillations. Front. Syst. Neurosci. 2015, 9, 110. [Google Scholar] [CrossRef] [PubMed]
- Liguz-Lecznar, M.; Urban-Ciecko, J.; Kossut, M. Somatostatin and Somatostatin-Containing Neurons in Shaping Neuronal Activity and Plasticity. Front. Neural Circuits 2016, 10, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheyltjens, I.; Arckens, L. The Current Status of Somatostatin-Interneurons in Inhibitory Control of Brain Function and Plasticity. Neural Plast. 2016, 2016, 8723623. [Google Scholar] [CrossRef] [PubMed]
- Urban-Ciecko, J.; Barth, A.L. Somatostatin-expressing neurons in cortical networks. Nat. Rev. Neurosci. 2016, 17, 401–409. [Google Scholar] [CrossRef] [Green Version]
- Yavorska, I.; Wehr, M. Somatostatin-Expressing Inhibitory Interneurons in Cortical Circuits. Front. Neural Circuits 2016, 10, 76. [Google Scholar] [CrossRef]
- Fee, C.; Banasr, M.; Sibille, E. Somatostatin-Positive γ-Aminobutyric Acid Interneuron Deficits in Depression: Cortical Microcircuit and Therapeutic Perspectives. Biol. Psychiatry 2017, 82, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Artinian, J.; Lacaille, J.C. Disinhibition in learning and memory circuits: New vistas for somatostatin interneurons and long-term synaptic plasticity. Brain Res. Bull. 2018, 141, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Cardin, J.A. Inhibitory Interneurons Regulate Temporal Precision and Correlations in Cortical Circuits. Trends Neurosci. 2018, 41, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Oesch, N.W.; Kothmann, W.W.; Diamond, J.S. Illuminating synapses and circuitry in the retina. Curr. Opin. Neurobiol. 2011, 21, 238–244. [Google Scholar] [CrossRef] [Green Version]
- Diamond, J.S. Inhibitory Interneurons in the Retina: Types, Circuitry, and Function. Annu. Rev. Vis. Sci. 2017, 3, 1–24. [Google Scholar] [CrossRef]
- D’Orazi, F.D.; Suzuki, S.C.; Wong, R.O. Neuronal remodeling in retinal circuit assembly, disassembly, and reassembly. Trends Neurosci. 2014, 37, 594–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlasits, A.L.; Euler, T.; Franke, K. Function first: Classifying cell types and circuits of the retina. Curr. Opin. Neurobiol. 2019, 56, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Amaral, D.G.; Scharfman, H.E.; Lavenex, P. The dentate gyrus: Fundamental neuroanatomical organization (dentate gyrus for dummies). Prog. Brain Res. 2007, 163, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Klausberger, T.; Somogyi, P. Neuronal diversity and temporal dynamics: The unity of hippocampal circuit operations. Science 2008, 321, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Stepan, J.; Dine, J.; Eder, M. Functional optical probing of the hippocampal trisynaptic circuit in vitro: Network dynamics, filter properties, and polysynaptic induction of CA1 LTP. Front. Neurosci. 2015, 9, 160. [Google Scholar] [CrossRef]
- Senzai, Y. Function of local circuits in the hippocampal dentate gyrus-CA3 system. Neurosci. Res. 2019, 140, 43–52. [Google Scholar] [CrossRef]
- Piccolino, M.; Strettoi, E.; Laurenzi, E. Santiago Ramón Y Cajal, the retina and the neuron theory. Doc. Ophthalmol. 1989, 71, 123–141. [Google Scholar] [CrossRef]
- Dowling, J.E. The Retina—An Approachable Part of the Brain; Belknap Press of Harvard University Press: Cambridge, MA, USA, 1987. [Google Scholar]
- Wässle, H.; Boycott, B.B. Functional architecture of the mammalian retina. Physiol. Rev. 1991, 71, 447–480. [Google Scholar] [CrossRef]
- Hoon, M.; Okawa, H.; Della Santina, L.; Wong, R.O. Functional architecture of the retina: Development and disease. Prog. Retin. Eye Res. 2014, 42, 44–84. [Google Scholar] [CrossRef] [Green Version]
- Thoreson, W.B.; Mangel, S.C. Lateral interactions in the outer retina. Prog. Retin. Eye Res. 2012, 31, 407–441. [Google Scholar] [CrossRef] [Green Version]
- Pan, Z.-H.; Hu, H.-J. Voltage-dependent Na+ currents in mammalian retinal cone bipolar cells. J. Neurophysiol. 2000, 84, 2564–2571. [Google Scholar] [CrossRef]
- Zenisek, D.; Henry, D.; Studholme, K.; Yazulla, S.; Matthews, G. Voltage-dependent sodium channels are expressed in nonspiking retinal bipolar neurons. J. Neurosci. 2001, 21, 4543–4550. [Google Scholar] [CrossRef]
- Ma, Y.P.; Cui, J.; Pan, Z.H. Heterogeneous expression of voltage-dependent Na+ and K+ channels in mammalian retinal bipolar cells. Vis. Neurosci. 2005, 22, 119–133. [Google Scholar] [CrossRef]
- Cui, J.; Pan, Z.H. Two types of cone bipolar cells express voltage-gated Na+ channels in the rat retina. Vis. Neurosci. 2008, 25, 635–645. [Google Scholar] [CrossRef] [Green Version]
- Ichinose, T.; Shields, C.R.; Lukasiewicz, P.D. Sodium channels in transient retinal bipolar cells enhance visual responses in ganglion cells. J. Neurosci. 2005, 25, 1856–1865. [Google Scholar] [CrossRef]
- Saszik, S.; DeVries, S.H. A mammalian retinal bipolar cell uses both graded changes in membrane voltage and all-or-nothing Na+ spikes to encode light. J. Neurosci. 2012, 32, 297–307. [Google Scholar] [CrossRef]
- Miller, R.F. Cell communication mechanisms in the vertebrate retina the proctor lecture. Investig. Ophthalmol. Vis. Sci. 2008, 49, 5184–5198. [Google Scholar] [CrossRef]
- Connaughton, V. Glutamate and Glutamate Receptors in the Vertebrate Retina. In Webvision: The Organization of the Retina and Visual System. Available online: https://www.ncbi.nlm.nih.gov/books/NBK11526/ (accessed on 29 April 2019).
- Kolb, H. Neurotransmitters in the Retina. In Webvision: The Organization of the Retina and Visual System. Available online: https://www.ncbi.nlm.nih.gov/books/NBK11546 / (accessed on 29 April 2019).
- Brandstätter, J.H.; Koulen, P.; Wässle, H. Diversity of glutamate receptors in the mammalian retina. Vis. Res. 1998, 38, 1385–1397. [Google Scholar] [CrossRef] [Green Version]
- Kalloniatis, M.; Tomisich, G. Amino acid neurochemistry of the vertebrate retina. Prog. Retin. Eye Res. 1999, 18, 811–866. [Google Scholar] [CrossRef]
- Shen, Y.; Liu, X.L.; Yang, X.L. N-methyl-D-aspartate receptors in the retina. Mol. Neurobiol. 2006, 34, 163–179. [Google Scholar] [CrossRef]
- Kolb, H. Amacrine cells of the mammalian retina: Neurocircuitry and functional roles. Eye (Lond.) 1997, 11, 904–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; McCall, M.A. Receptor targets of amacrine cells. Vis. Neurosci. 2012, 29, 11–29. [Google Scholar] [CrossRef]
- Bartfai, T.; Iverfeldt, K.; Fisone, G.; Serfözö, P. Regulation of the release of coexisting neurotransmitters. Annu. Rev. Pharmacol. Toxicol. 1988, 28, 285–310. [Google Scholar] [CrossRef]
- Hökfelt, T. Neuropeptides in perspective: The last ten years. Neuron 1991, 7, 867–879. [Google Scholar] [CrossRef]
- Baraban, S.C.; Tallent, M.K. Interneuron Diversity series: Interneuronal neuropeptides—Endogenous regulators of neuronal excitability. Trends Neurosci. 2004, 27, 135–142. [Google Scholar] [CrossRef]
- Svensson, E.; Apergis-Schoute, J.; Burnstock, G.; Nusbaum, M.P.; Parker, D.; Schiöth, H.B. General Principles of Neuronal Co-transmission: Insights from Multiple Model Systems. Front. Neural Circuits 2019, 12, 117. [Google Scholar] [CrossRef]
- Bliss, T.V.; Lømo, T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol. 1973, 232, 331–356. [Google Scholar] [CrossRef]
- Amaral, D.G.; Witter, M.P. The three-dimensional organization of the hippocampal formation: A review of anatomical data. Neuroscience 1989, 31, 571–591. [Google Scholar] [CrossRef]
- Pelkey, K.A.; Chittajallu, R.; Craig, M.T.; Tricoire, L.; Wester, J.C.; McBain, C.J. Hippocampal GABAergic Inhibitory Interneurons. Physiol. Rev. 2017, 97, 1619–1747. [Google Scholar] [CrossRef]
- Booker, S.A.; Vida, I. Morphological diversity and connectivity of hippocampal interneurons. Cell Tissue Res. 2018, 373, 619–641. [Google Scholar] [CrossRef] [Green Version]
- Tsien, J.Z.; Huerta, P.T.; Tonegawa, S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell 1996, 87, 1327–1338. [Google Scholar] [CrossRef]
- Park, P.; Kang, H.; Sanderson, T.M.; Bortolotto, Z.A.; Georgiou, J.; Zhuo, M.; Kaang, B.K.; Collingridge, G.L. The Role of Calcium-Permeable AMPARs in Long-Term Potentiation at Principal Neurons in the Rodent Hippocampus. Front. Synaptic Neurosci. 2018, 10, 42. [Google Scholar] [CrossRef] [Green Version]
- Bettler, B.; Kaupmann, K.; Mosbacher, J.; Gassmann, M. Molecular structure and physiological functions of GABA(B) receptors. Physiol. Rev. 2004, 84, 835–867. [Google Scholar] [CrossRef] [PubMed]
- Sigel, E.; Steinmann, M.E. Structure, function, and modulation of GABA(A) receptors. J. Biol. Chem. 2012, 287, 40224–40231. [Google Scholar] [CrossRef] [PubMed]
- Caroni, P. Inhibitory microcircuit modules in hippocampal learning. Curr. Opin. Neurobiol. 2015, 35, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Freund, T.F.; Katona, I. Perisomatic inhibition. Neuron 2007, 56, 33–42. [Google Scholar] [CrossRef]
- Maccaferri, G. Stratum oriens horizontal interneurone diversity and hippocampal network dynamics. J. Physiol. 2005, 562, 73–80. [Google Scholar] [CrossRef]
- Royer, S.; Zemelman, B.V.; Losonczy, A.; Kim, J.; Chance, F.; Magee, J.C.; Buzsáki, G. Control of timing, rate and bursts of hippocampal place cells by dendritic and somatic inhibition. Nat. Neurosci. 2012, 15, 769–775. [Google Scholar] [CrossRef]
- Rorstad, O.P.; Brownstein, M.J.; Martin, J.B. Immunoreactive and biologically active somatostatin-like material in rat retina. Proc. Natl. Acad. Sci. USA 1979, 76, 3019–3023. [Google Scholar] [CrossRef]
- Yamada, T.; Marshak, D.; Basinger, S.; Walsh, J.; Morley, J.; Stell, W. Somatostatin-like immunoreactivity in the retina. Proc. Natl. Acad. Sci. USA 1980, 77, 1691–1695. [Google Scholar] [CrossRef]
- Brecha, N.; Karten, H.J.; Schenker, C. Neurotensin-like and somatostatin-like immunoreactivity within amacrine cells of the retina. Neuroscience 1981, 6, 1329–1340. [Google Scholar] [CrossRef]
- Buckerfield, M.; Oliver, J.; Chubb, I.W.; Morgan, I.G. Somatostatin-like immunoreactivity in amacrine cells of the chicken retina. Neuroscience 1981, 6, 689–695. [Google Scholar] [CrossRef]
- Rickman, D.W.; Blanks, J.C.; Brecha, N.C. Somatostatin-immunoreactive neurons in the adult rabbit retina. J. Comp. Neurol. 1996, 365, 491–503. [Google Scholar] [CrossRef]
- Johnson, J.; Rickman, D.W.; Brecha, N.C. Somatostatin and somatostatin subtype 2A expression in the mammalian retina. Microsc. Res. Tech. 2000, 50, 103–111. [Google Scholar] [CrossRef]
- Cristiani, R.; Petrucci, C.; Dal Monte, M.; Bagnoli, P. Somatostatin (SRIF) and SRIF receptors in the mouse retina. Brain Res. 2002, 936, 1–14. [Google Scholar] [CrossRef]
- Watt, C.B.; Florack, V.J. Double-label analyses of the coexistence of somatostatin with GABA and glycine in amacrine cells of the larval tiger salamander retina. Brain Res. 1993, 617, 131–137. [Google Scholar] [CrossRef]
- Chun, M.H.; Brecha, N.; Wässle, H. Light- and electron-microscopic studies of the somatostatin-immunoreactive plexus in the cat retina. Cell Tissue Res. 1992, 267, 57–66. [Google Scholar] [CrossRef]
- Casini, G.; Catalani, E.; Dal Monte, M.; Bagnoli, P. Functional aspects of the somatostatinergic system in the retina and the potential therapeutic role of somatostatin in retinal disease. Histol. Histopathol. 2005, 20, 615–632. [Google Scholar] [CrossRef]
- Ishimoto, I.; Millar, T.; Chubb, I.W.; Morgan, I.G. Somatostatin-immunoreactive amacrine cells of chicken retina: Retinal mosaic, ultrastructural features, and light-driven variations in peptide metabolism. Neuroscience 1986, 17, 1217–1233. [Google Scholar] [CrossRef]
- Feigenspan, A.; Gustincich, S.; Bean, B.P.; Raviola, E. Spontaneous activity of solitary dopaminergic cells of the retina. J. Neurosci. 1998, 18, 6776–6789. [Google Scholar] [CrossRef]
- Newkirk, G.S.; Hoon, M.; Wong, R.O.; Detwiler, P.B. Inhibitory inputs tune the light response properties of dopaminergic amacrine cells in mouse retina. J. Neurophysiol. 2013, 110, 536–552. [Google Scholar] [CrossRef]
- Vuong, H.E.; Hardi, C.N.; Barnes, S.; Brecha, N.C. Parallel Inhibition of Dopamine Amacrine Cells and Intrinsically Photosensitive Retinal Ganglion Cells in a Non-Image-Forming Visual Circuit of the Mouse Retina. J. Neurosci. 2015, 35, 15955–15970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cristiani, R.; Fontanesi, G.; Casini, G.; Petrucci, C.; Viollet, C.; Bagnoli, P. Expression of somatostatin subtype 1 receptor in the rabbit retina. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3191–3199. [Google Scholar]
- Helboe, L.; Møller, M. Immunohistochemical localization of somatostatin receptor subtypes sst1 and sst2 in the rat retina. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2376–2382. [Google Scholar] [PubMed]
- Dal Monte, M.; Petrucci, C.; Vasilaki, A.; Cervia, D.; Grouselle, D.; Epelbaum, J.; Kreienkamp, H.J.; Richter, D.; Hoyer, D.; Bagnoli, P. Genetic deletion of somatostatin receptor 1 alters somatostatinergic transmission in the mouse retina. Neuropharmacology 2003, 45, 1080–1092. [Google Scholar] [CrossRef] [Green Version]
- Mastrodimou, N.; Thermos, K. The somatostatin receptor (sst1) modulates the release of somatostatin in rat retina. Neurosci. Lett. 2004, 356, 13–16. [Google Scholar] [CrossRef]
- Thermos, K.; Bagnoli, P.; Epelbaum, J.; Hoyer, D. The somatostatin sst1 receptor: An autoreceptor for somatostatin in brain and retina? Pharmacol. Ther. 2006, 110, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; Wong, H.; Walsh, J.H.; Brecha, N.C. Expression of the somatostatin subtype 2A receptor in the rabbit retina. J. Comp. Neurol. 1998, 393, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J.; Wu, V.; Wong, H.; Walsh, J.H.; Brecha, N.C. Somatostatin receptor subtype 2A expression in the rat retina. Neuroscience 1999, 94, 675–683. [Google Scholar] [CrossRef]
- van Hagen, P.M.; Baarsma, G.S.; Mooy, C.M.; Ercoskan, E.M.; ter Averst, E.; Hofland, L.J.; Lamberts, S.W.; Kuijpers, R.W. Somatostatin and somatostatin receptors in retinal diseases. Eur. J. Endocrinol. 2000, 143, S43–S51. [Google Scholar] [CrossRef] [PubMed]
- Vasilaki, A.; Gardette, R.; Epelbaum, J.; Thermos, K. NADPH-diaphorase colocalization with somatostatin receptor subtypes sst2A and sst2B in the retina. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1600–1609. [Google Scholar]
- Cervia, D.; Casini, G.; Bagnoli, P. Physiology and pathology of somatostatin in the mammalian retina: A current view. Mol. Cell Endocrinol. 2008, 286, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Grigoryan, E.N.; Vasilaki, A.; Mastrodimou, N.; Thermos, K. Somatostatin receptor immunoreactivity in the eye of the adult newt (Pleurodeles waltlii Michan). Neurosci. Lett. 2003, 337, 143–146. [Google Scholar] [CrossRef]
- Händel, M.; Schulz, S.; Stanarius, A.; Schreff, M.; Erdtmann-Vourliotis, M.; Schmidt, H.; Wolf, G.; Höllt, V. Selective targeting of somatostatin receptor 3 to neuronal cilia. Neuroscience 1999, 89, 909–926. [Google Scholar] [CrossRef]
- Stroukov, W.; Rösch, A.; Schwan, C.; Jeney, A.; Römer, W.; Thuenauer, R. Synchronizing Protein Traffic to the Primary Cilium. Front. Genet. 2019, 10, 163. [Google Scholar] [CrossRef] [PubMed]
- Farrell, S.R.; Raymond, I.D.; Foote, M.; Brecha, N.C.; Barnes, S. Modulation of voltage-gated ion channels in rat retinal ganglion cells mediated by somatostatin receptor subtype 4. J. Neurophysiol. 2010, 104, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Ke, J.B.; Zhong, Y.M. Expression of somatostatin receptor subtype 5 in rat retinal amacrine cells. Neuroscience 2007, 144, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.H.; Deng, Q.Q.; Jiang, S.X.; Yang, X.L.; Zhong, Y.M. Distribution of somatostatin receptor 5 in mouse and bullfrog retinas. Peptides 2012, 33, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.-Q.; Sheng, W.L.; Zhang, G.; Weng, S.J.; Yang, X.L.; Zhong, Y.M. Signaling mechanism for somatostatin receptor 5-mediated suppression of AMPA responses in rat retinal ganglion cells. Neuropharmacology 2016, 107, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Brownstein, M.; Arimura, A.; Sato, H.; Schally, A.V.; Kizer, J.S. The regional distribution of somatostatin in the rat brain. Endocrinology 1975, 96, 1456–1461. [Google Scholar] [CrossRef] [PubMed]
- Petrusz, P.; Sar, M.; Grossman, G.H.; Kizer, J.S. Synaptic terminals with somatostatin-like immunoreactivity in the rat brain. Brain Res. 1977, 137, 181–187. [Google Scholar] [CrossRef]
- Köhler, C.; Chan-Palay, V. Somatostatin-like immunoreactive neurons in the hippocampus: An immunocytochemical study in the rat. Neurosci. Lett. 1982, 34, 259–264. [Google Scholar] [CrossRef]
- Johansson, O.; Hökfelt, T.; Elde, R.P. Immunohistochemical distribution of somatostatin-like immunoreactivity in the central nervous system of the adult rat. Neuroscience 1984, 13, 265–339. [Google Scholar] [CrossRef]
- Buckmaster, P.S.; Kunkel, D.D.; Robbins, R.J.; Schwartzkroin, P.A. Somatostatin-immunoreactivity in the hippocampus of mouse, rat, guinea pig, and rabbit. Hippocampus 1994, 4, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, T.; Wu, J.Y.; Benoit, R. GABAergic neurons containing somatostatin-like immunoreactivity in the rat hippocampus and dentate gyrus. Exp. Brain Res. 1988, 71, 388–398. [Google Scholar] [CrossRef]
- Esclapez, M.; Houser, C.R. Somatostatin neurons are a subpopulation of GABA neurons in the rat dentate gyrus: Evidence from colocalization of pre-prosomatostatin and glutamate decarboxylase messenger RNAs. Neuroscience 1995, 64, 339–355. [Google Scholar] [CrossRef]
- Goldin, M.; Epsztein, J.; Jorquera, I.; Represa, A.; Ben-Ari, Y.; Crépel, V.; Cossart, R. Synaptic kainate receptors tune oriens-lacunosum moleculare interneurons to operate at theta frequency. J. Neurosci. 2007, 27, 9560–9572. [Google Scholar] [CrossRef]
- McBain, C.J. Hippocampal inhibitory neuron activity in the elevated potassium model of epilepsy. J. Neurophysiol. 1994, 72, 2853–2863, republished in J. Neurophysiol. 1995, 73, 2853–2863. [Google Scholar] [CrossRef] [PubMed]
- Maccaferri, G.; McBain, C.J. The hyperpolarization-activated current (Ih) and its contribution to pacemaker activity in rat CA1 hippocampal stratum oriens-alveus interneurones. J. Physiol. 1996, 497, 119–130. [Google Scholar] [CrossRef]
- Freund, T.F.; Buzsáki, G. Interneurons of the hippocampus. Hippocampus 1996, 6, 347–470. [Google Scholar] [CrossRef]
- Binaschi, A.; Bregola, G.; Simonato, M. On the role of somatostatin in seizure control: Clues from the hippocampus. Rev. Neurosci. 2003, 14, 285–301. [Google Scholar] [CrossRef]
- Thoss, V.S.; Pérez, J.; Probst, A.; Hoyer, D. Expression of five somatostatin receptor mRNAs in the human brain and pituitary. Naunyn Schmiedebergs Arch. Pharmacol. 1996, 354, 411–419. [Google Scholar] [CrossRef]
- Selmer, I.; Schindler, M.; Allen, J.P.; Humphrey, P.P.; Emson, P.C. Advances in understanding neuronal somatostatin receptors. Regul. Pept. 2000, 90, 1–18. [Google Scholar] [CrossRef]
- Cammalleri, M.; Martini, D.; Timperio, A.M.; Bagnoli, P. Functional effects of somatostatin receptor 1 activation on synaptic transmission in the mouse hippocampus. J. Neurochem. 2009, 111, 1466–1477. [Google Scholar] [CrossRef]
- De Bundel, D.; Aourz, N.; Kiagiadaki, F.; Clinckers, R.; Hoyer, D.; Kastellakis, A.; Michotte, Y.; Thermos, K.; Smolders, I. Hippocampal sst1 receptors are autoreceptors and do not affect seizures in rats. Neuroreport 2010, 21, 254–258. [Google Scholar] [CrossRef]
- Dournaud, P.; Gu, Y.Z.; Schonbrunn, A.; Mazella, J.; Tannenbaum, G.S.; Beaudet, A. Localization of the somatostatin receptor SST2A in rat brain using a specific anti-peptide. J. Neurosci. 1996, 16, 4468–4478. [Google Scholar] [CrossRef]
- Schulz, S.; Händel, M.; Schreff, M.; Schmidt, H.; Höllt, V. Localization of five somatostatin receptors in the rat central nervous system using subtype-specific antibodies. J. Physiol. Paris 2000, 94, 259–264. [Google Scholar] [CrossRef]
- Berbari, N.F.; Bishop, G.A.; Askwith, C.C.; Lewis, J.S.; Mykytyn, K. Hippocampal neurons possess primary cilia in culture. J. Neurosci. Res. 2007, 85, 1095–1100. [Google Scholar] [CrossRef]
- Einstein, E.B.; Patterson, C.A.; Hon, B.J.; Regan, K.A.; Reddi, J.; Melnikoff, D.E.; Mateer, M.J.; Schulz, S.; Johnson, B.N.; Tallent, M.K. Somatostatin signaling in neuronal cilia is critical for object recognition memory. J. Neurosci. 2010, 30, 4306–4314. [Google Scholar] [CrossRef]
- Schreff, M.; Schulz, S.; Händel, M.; Keilhoff, G.; Braun, H.; Pereira, G.; Klutzny, M.; Schmidt, H.; Wolf, G.; Höllt, V. Distribution, targeting, and internalization of the sst4 somatostatin receptor in rat brain. J. Neurosci. 2000, 20, 3785–3797. [Google Scholar] [CrossRef]
- Qiu, C.; Zeyda, T.; Johnson, B.; Hochgeschwender, U.; de Lecea, L.; Tallent, M.K. Somatostatin receptor subtype 4 couples to the M-current to regulate seizures. J. Neurosci. 2008, 28, 3567–3576. [Google Scholar] [CrossRef]
- Izquierdo-Claros, R.M.; Boyano-Adánez Mdel, C.; Arilla-Ferreiro, E. Activity of the hippocampal somatostatinergic system following daily administration of melatonin. Brain Res. Mol. Brain Res. 2004, 126, 107–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ristori, C.; Cammalleri, M.; Martini, D.; Pavan, B.; Liu, Y.; Casini, G.; Dal Monte, M.; Bagnoli, P. Involvement of the cAMP-dependent pathway in the reduction of epileptiform bursting caused by somatostatin in the mouse hippocampus. Naunyn Schmiedebergs Arch. Pharmacol. 2008, 378, 563–577. [Google Scholar] [CrossRef] [Green Version]
- Ristori, C.; Ferretti, M.E.; Pavan, B.; Cervellati, F.; Casini, G.; Catalani, E.; Dal Monte, M.; Biondi, C. Adenylyl cyclase/cAMP system involvement in the antiangiogenic effect of somatostatin in the retina. Results Transgenic mice. Neurochem. Res. 2008, 33, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Pavan, B.; Fiorini, S.; Dal Monte, M.; Lunghi, L.; Biondi, C.; Bagnoli, P.; Cervia, D. Somatostatin coupling to adenylyl cyclase activity in the mouse retina. Naunyn Schmiedebergs Arch. Pharmacol. 2004, 370, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Lucas, S.J.; Armstrong, D.L. Protein phosphatase modulation of somatostatin receptor signaling in the mouse hippocampus. Neuropharmacology 2015, 99, 232–241. [Google Scholar] [CrossRef] [Green Version]
- Farrell, S.R.; Rankin, D.R.; Brecha, N.C.; Barnes, S. Somatostatin receptor subtype 4 modulates L-type calcium channels via Gβγ and PKC signaling in rat retinal ganglion cells. Channels 2014, 8, 519–527, erratum in Channels 2015, 9, 56–57. [Google Scholar] [CrossRef] [PubMed]
- Young Shim, E.; Jung Kim, H.; Kim, M.-J.; Rhie, D.-J.; Jo, Y.-H.; Kim, M.-S.; Hahn, S.J.; Lee, M.-Y.; Yoon, S.H. Desensitization of somatostatin-induced inhibition of low extracellular magnesium concentration-induced calcium spikes in cultured rat hippocampal neurons. Brain Res. 2006, 1111, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Ristori, C.; Cammalleri, M.; Martini, D.; Pavan, B.; Casini, G.; Cervia, D.; Bagnoli, P. The cyclooxygenase-2/prostaglandin E2 pathway is involved in the somatostatin-induced decrease of epileptiform bursting in the mouse hippocampus. Neuropharmacology 2008, 54, 874–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cervia, D.; Bagnoli, P. An update on somatostatin receptor signaling in native systems and new insights on their pathophysiology. Pharmacol. Ther. 2007, 116, 322–341. [Google Scholar] [CrossRef] [Green Version]
- Bigiani, A.; Petrucci, C.; Ghiaroni, V.; Dal Monte, M.; Cozzi, A.; Kreienkamp, H.-J.; Richter, D.; Bagnoli, P. Functional correlates of somatostatin receptor 2 overexpression in the retina of mice with genetic deletion of somatostatin receptor 1. Brain Res. 2004, 1025, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Cammalleri, M.; Cervia, D.; Langenegger, D.; Liu, Y.; Dal Monte, M.; Hoyer, D.; Bagnoli, P. Somatostatin receptors differentially affect spontaneous epileptiform activity in mouse hippocampal slices. Eur. J. Neurosci. 2004, 20, 2711–2721. [Google Scholar] [CrossRef] [Green Version]
- Iversen, L.L.; Iversen, S.D.; Bloom, F.; Douglas, C.; Brown, M.; Vale, W. Calcium-dependent release of somatostatin and neurotensin from rat brain in vitro. Nature 1978, 273, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Mathé, A.A.; Nomikos, G.G.; Svensson, T.H. In vivo release of somatostatin from rat hippocampus and striatum. Neurosci. Lett. 1993, 149, 201–204. [Google Scholar] [CrossRef]
- Vezzani, A.; Ruiz, R.; Monno, A.; Rizzi, M.; Lindefors, N.; Samanin, R.; Brodin, E. Extracellular somatostatin measured by microdialysis in the hippocampus of freely moving rats: Evidence for neuronal release. J. Neurochem. 1993, 60, 671–677. [Google Scholar] [CrossRef]
- Van den Pol, A.N. Neuropeptide transmission in brain circuits. Neuron 2012, 76, 98–115. [Google Scholar] [CrossRef]
- Weber, S.J.; Louis, R.B.; Trombley, L.; Bissette, G.; Davies, P.; Davis, T.P. Metabolic half-life of somatostatin and peptidase activities are altered in Alzheimer’s disease. J. Gerontol. 1992, 47, B18–B25. [Google Scholar] [CrossRef]
- Barnes, K.; Doherty, S.; Turner, A.J. Endopeptidase-24.11 is the integral membrane peptidase initiating degradation of somatostatin in the hippocampus. J. Neurochem. 1995, 64, 1826–1832. [Google Scholar] [CrossRef]
- Zalutsky, R.A.; Miller, R.F. The physiology of somatostatin in the rabbit retina. J. Neurosci. 1990, 10, 383–393. [Google Scholar] [CrossRef]
- Hirasawa, H.; Contini, M.; Raviola, E. Extrasynaptic release of GABA and dopamine by retinal dopaminergic neurons. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140186. [Google Scholar] [CrossRef]
- Murase, K.; Nedeljkov, V.; Randić, M. The actions of neuropeptides on dorsal horn neurons in the rat spinal cord slice preparation: An intracellular study. Brain Res. 1982, 234, 170–176. [Google Scholar] [CrossRef]
- Twery, M.J.; Moss, R.L. Sensitivity of rat forebrain neurons to growth hormone-releasing hormone. Peptides 1985, 6, 609–613. [Google Scholar] [CrossRef]
- Hamill, O.P.; Marty, A.; Neher, E.; Sakmann, B.; Sigworth, F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981, 391, 85–100. [Google Scholar] [CrossRef]
- Tsien, R.Y. A non-disruptive technique for loading calcium buffers and indicators into cells. Nature 1981, 290, 527–528. [Google Scholar] [CrossRef]
- Kamiyama, Y.; Ogura, T.; Usui, S. Ionic current model of the vertebrate rod photoreceptor. Vis. Res. 1996, 36, 4059–4068. [Google Scholar] [CrossRef] [Green Version]
- Usui, S.; Ishihara, A.; Kamiyama, Y.; Ishii, H. Ionic current model of bipolar cells in the lower vertebrate retina. Vision Res. 1996, 36, 4069–4076. [Google Scholar] [CrossRef] [Green Version]
- Akopian, A.; Johnson, J.; Gabriel, R.; Brecha, N.; Witkovsky, P. Somatostatin modulates voltage-gated K+ and Ca2+ currents in rod and cone photoreceptors of the salamander retina. J. Neurosci. 2000, 20, 929–936. [Google Scholar] [CrossRef]
- Jian, K.; Barhoumi, R.; Ko, M.L.; Ko, G.Y. Inhibitory effect of somatostatin-14 on L-type voltage-gated calcium channels in cultured cone photoreceptors requires intracellular calcium. J. Neurophysiol. 2009, 102, 1801–1810. [Google Scholar] [CrossRef]
- Ayoub, G.S.; Matthews, G. Substance P modulates calcium current in retinal bipolar cells. Vis. Neurosci. 1992, 8, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; Caravelli, M.L.; Brecha, N.C. Somatostatin inhibits calcium influx into rat rod bipolar cell axonal terminals. Vis. Neurosci. 2001, 18, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Petrucci, C.; Resta, V.; Fieni, F.; Bigiani, A.; Bagnoli, P. Modulation of potassium current and calcium influx by somatostatin in rod bipolar cells isolated from the rabbit retina via sst2 receptors. Naunyn Schmiedebergs Arch. Pharmacol. 2001, 363, 680–694. [Google Scholar] [CrossRef]
- Masland, R.H. The neuronal organization of the retina. Neuron 2012, 76, 266–280. [Google Scholar] [CrossRef]
- Pangrsic, T.; Singer, J.H.; Koschak, A. Voltage-Gated Calcium Channels: Key Players in Sensory Coding in the Retina and the Inner Ear. Physiol. Rev. 2018, 98, 2063–2096. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, M.; Okada, T. Release of endogenous excitatory amino acids from ON-type bipolar cells isolated from the goldfish retina. J. Neurosci. 1991, 11, 2199–2208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitz, Y.; Witkovsky, P. Dependence of photoreceptor glutamate release on a dihydropyridine-sensitive calcium channel. Neuroscience 1997, 78, 1209–1216. [Google Scholar] [CrossRef]
- Witkovsky, P.; Schmitz, Y.; Akopian, A.; Krizaj, D.; Tranchina, D. Gain of rod to horizontal cell synaptic transfer: Relation to glutamate release and a dihydropyridine-sensitive calcium current. J. Neurosci. 1997, 17, 7297–7306. [Google Scholar] [CrossRef]
- Dal Monte, M.; Petrucci, C.; Cozzi, A.; Allen, J.P.; Bagnoli, P. Somatostatin inhibits potassium-evoked glutamate release by activation of the sst2 somatostatin receptor in the mouse retina. Naunyn Schmiedebergs Arch. Pharmacol. 2003, 367, 188–192. [Google Scholar] [CrossRef]
- Pan, Z.H.; Lipton, S.A. Multiple GABA receptor subtypes mediate inhibition of calcium influx at rat retinal bipolar cell terminals. J. Neurosci. 1995, 15, 2668–2679. [Google Scholar] [CrossRef]
- Protti, D.A.; Llano, I. Calcium currents and calcium signaling in rod bipolar cells of rat retinal slices. J. Neurosci. 1998, 18, 3715–3724. [Google Scholar] [CrossRef]
- Hartveit, E. Reciprocal synaptic interactions between rod bipolar cells and amacrine cells in the rat retina. J. Neurophysiol. 1999, 81, 2923–2936. [Google Scholar] [CrossRef]
- Zhang, D.Q.; Wong, K.Y.; Sollars, P.J.; Berson, D.M.; Pickard, G.E.; McMahon, D.G. Intraretinal signaling by ganglion cell photoreceptors to dopaminergic amacrine neurons. Proc. Natl. Acad. Sci. USA 2008, 105, 14181–14186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dreosti, E.; Esposti, F.; Baden, T.; Lagnado, L. In vivo evidence that retinal bipolar cells generate spikes modulated by light. Nat. Neurosci. 2011, 14, 951–952. [Google Scholar] [CrossRef] [Green Version]
- Palmer, M.J. Modulation of Ca2+-activated K+ currents and Ca2+-dependent action potentials by exocytosis in goldfish bipolar cell terminals. J. Physiol. 2006, 572, 747–762. [Google Scholar] [CrossRef]
- Jacoby, R.A.; Wu, S.M. AMPA-preferring receptors mediate excitatory non-NMDA responses of primate retinal ganglion cells. Vis. Neurosci. 2001, 18, 703–710. [Google Scholar] [CrossRef]
- Feigenspan, A.; Bormann, J. Facilitation of GABAergic signaling in the retina by receptors stimulating adenylate cyclase. Proc. Natl. Acad. Sci. USA 1994, 91, 10893–10897. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ke, J.B.; Wu, H.J.; Miao, Y.; Li, F.; Yang, X.L.; Wang, Z. Somatostatin receptor-mediated suppression of gabaergic synaptic transmission in cultured rat retinal amacrine cells. Neuroscience 2014, 273, 118–127. [Google Scholar] [CrossRef]
- Dodd, J.; Kelly, S. Is somatostatin an excitatory transmitter in the hippocampus? Nature 1978, 273, 674–675. [Google Scholar] [CrossRef]
- Pittman, Q.J.; Siggins, G.R. Somatostatin hyperpolarizes hippocampal pyramidal cells in vitro. Brain Res. 1981, 221, 402–408. [Google Scholar] [CrossRef]
- Mueller, A.L.; Kunkel, D.D.; Schwartzkroin, P.A. Electrophysiological actions of somatostatin (SRIF) in hippocampus: An in vitro study. Cell Mol. Neurobiol. 1986, 6, 363–379. [Google Scholar] [CrossRef]
- Mancillas, J.R.; Siggins, G.R.; Bloom, F.E. Somatostatin selectively enhances acetylcholine-induced excitations in rat hippocampus and cortex. Proc. Natl. Acad. Sci. USA 1986, 83, 7518–7521. [Google Scholar] [CrossRef] [PubMed]
- Watson, T.W.; Pittman, Q.J. Somatostatin(14) and -(28) but not somatostatin(1-12) hyperpolarize CA1 pyramidal neurons in vitro. Brain Res. 1988, 448, 40–55. [Google Scholar] [CrossRef]
- Hille, B. Ion Channels of Excitable Membranes, 3rd ed.; Sinauer Associates: Sunderland, MA, USA, 2001; pp. 131–167. [Google Scholar]
- Storm, J.F. Potassium currents in hippocampal pyramidal cells. Prog. Brain Res. 1990, 83, 161–187. [Google Scholar] [CrossRef]
- Moore, S.D.; Madamba, S.G.; Joëls, M.; Siggins, G.R. Somatostatin augments the M-current in hippocampal neurons. Science 1988, 239, 278–280. [Google Scholar] [CrossRef] [PubMed]
- Watson, T.W.; Pittman, Q.J. Pharmacological evidence that somatostatin activates the m-current in hippocampal pyramidal neurons. Neurosci. Lett. 1988, 91, 172–176. [Google Scholar] [CrossRef]
- Schweitzer, P.; Madamba, S.; Siggins, G.R. Arachidonic acid metabolites as mediators of somatostatin-induced increase of neuronal M-current. Nature 1990, 346, 464–467. [Google Scholar] [CrossRef]
- Schweitzer, P.; Madamba, S.G.; Siggins, G.R. Somatostatin increases a voltage-insensitive K+ conductance in rat CA1 hippocampal neurons. J. Neurophysiol. 1998, 79, 1230–1238. [Google Scholar] [CrossRef]
- Schweitzer, P.; Madamba, S.; Champagnat, J.; Siggins, G.R. Somatostatin inhibition of hippocampal CA1 pyramidal neurons: Mediation by arachidonic acid and its metabolites. J. Neurosci. 1993, 13, 2033–2049. [Google Scholar] [CrossRef]
- Leaney, J.L. Contribution of Kir3.1, Kir3.2A and Kir3.2C subunits to native G protein-gated inwardly rectifying potassium currents in cultured hippocampal neurons. Eur. J. Neurosci. 2003, 18, 2110–2118. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.L.; Chen, X. Diversity of potassium channels in neuronal dendrites. Prog. Neurobiol. 2006, 78, 374–389. [Google Scholar] [CrossRef]
- Ishibashi, H.; Akaike, N. Somatostatin modulates high-voltage-activated Ca2+ channels in freshly dissociated rat hippocampal neurons. J. Neurophysiol. 1995, 74, 1028–1036. [Google Scholar] [CrossRef]
- Tallent, M.K.; Siggins, G.R. Somatostatin depresses excitatory but not inhibitory neurotransmission in rat CA1 hippocampus. J. Neurophysiol. 1997, 78, 3008–3018. [Google Scholar] [CrossRef]
- Cammalleri, M.; Cervia, D.; Dal Monte, M.; Martini, D.; Langenegger, D.; Fehlmann, D.; Feuerbach, D.; Pavan, B.; Hoyer, D.; Bagnoli, P. Compensatory changes in the hippocampus of somatostatin knockout mice: Upregulation of somatostatin receptor 2 and its function in the control of bursting activity and synaptic transmission. Eur. J. Neurosci. 2006, 23, 2404–2422. [Google Scholar] [CrossRef]
- Boehm, S.; Betz, H. Somatostatin inhibits excitatory transmission at rat hippocampal synapses via presynaptic receptors. J. Neurosci. 1997, 17, 4066–4075. [Google Scholar] [CrossRef]
- Kozhemyakin, M.; Rajasekaran, K.; Todorovic, M.S.; Kowalski, S.L.; Balint, C.; Kapur, J. Somatostatin type-2 receptor activation inhibits glutamate release and prevents status epilepticus. Neurobiol. Dis. 2013, 54, 94–104. [Google Scholar] [CrossRef] [Green Version]
- Baratta, M.V.; Lamp, T.; Tallent, M.K. Somatostatin depresses long-term potentiation and Ca2+ signaling in mouse dentate gyrus. J. Neurophysiol. 2002, 88, 3078–3086. [Google Scholar] [CrossRef] [PubMed]
- Takagi, H. Roles of ion channels in EPSP integration at neuronal dendrites. Neurosci. Res. 2000, 37, 167–171. [Google Scholar] [CrossRef]
- Bloodgood, B.L.; Sabatini, B.L. Ca2+ signaling in dendritic spines. Curr. Opin. Neurobiol. 2007, 17, 345–351. [Google Scholar] [CrossRef]
- Kitamura, K.; Kano, M. Dendritic calcium signaling in cerebellar Purkinje cell. Neural Netw. 2013, 47, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Tallent, M.K.; Siggins, G.R. Somatostatin acts in CA1 and CA3 to reduce hippocampal epileptiform activity. J. Neurophysiol. 1999, 81, 1626–1635. [Google Scholar] [CrossRef]
- Martin, J.L.; Chesselet, M.F.; Raynor, K.; Gonzales, C.; Reisine, T. Differential distribution of somatostatin receptor subtypes in rat brain revealed by newly developed somatostatin analogs. Neuroscience 1991, 41, 581–593. [Google Scholar] [CrossRef]
- Leroux, P.; Weissmann, D.; Pujol, J.F.; Vaudry, H. Quantitative autoradiography of somatostatin receptors in the rat limbic system. J. Comp. Neurol. 1993, 331, 389–401. [Google Scholar] [CrossRef]
- Matsuoka, N.; Kaneko, S.; Satoh, M. Somatostatin augments long-term potentiation of the mossy fiber-CA3 system in guinea-pig hippocampal slices. Brain Res. 1991, 553, 188–194. [Google Scholar] [CrossRef]
- Myers, C.E.; Klein, B.E.; Meuer, S.M.; Swift, M.K.; Chandler, C.S.; Huang, Y.; Gangaputra, S.; Pak, J.W.; Danis, R.P.; Klein, R. Retinal thickness measured by spectral-domain optical coherence tomography in eyes without retinal abnormalities: The Beaver Dam Eye Study. Am. J. Ophthalmol. 2015, 159, 445–456. [Google Scholar] [CrossRef]
- Nusbaum, M.P.; Blitz, D.M.; Marder, E. Functional consequences of neuropeptide and small-molecule co-transmission. Nat. Rev. Neurosci. 2017, 18, 389–403. [Google Scholar] [CrossRef] [Green Version]
- Pongs, O. Regulation of excitability by potassium channels. Results Probl. Cell Differ. 2008, 44, 145–161. [Google Scholar] [CrossRef]
- Dolphin, A.C. Voltage-gated calcium channels: Their discovery, function and importance as drug targets. Brain Neurosci. Adv. 2018, 2, 1–8. [Google Scholar] [CrossRef]
- Kaneda, M. Signal processing in the mammalian retina. J. Nippon Med. Sch. 2013, 80, 16–24. [Google Scholar] [CrossRef]
- Mauss, A.S.; Vlasits, A.; Borst, A.; Feller, M. Visual Circuits for Direction Selectivity. Annu. Rev. Neurosci. 2017, 40, 211–230. [Google Scholar] [CrossRef]
- Jacoby, J.; Schwartz, G.W. Typology and Circuitry of Suppressed-by-Contrast Retinal Ganglion Cells. Front. Cell Neurosci. 2018, 12, 269. [Google Scholar] [CrossRef]
- Whitlock, J.R.; Heynen, A.J.; Shuler, M.G.; Bear, M.F. Learning induces long-term potentiation in the hippocampus. Science 2006, 313, 1093–1097. [Google Scholar] [CrossRef]
- Van Strien, N.M.; Cappaert, N.L.; Witter, M.P. The anatomy of memory: An interactive overview of the parahippocampal-hippocampal network. Nat. Rev. Neurosci. 2009, 10, 272–282. [Google Scholar] [CrossRef]
- Ramirez, S.; Tonegawa, S.; Liu, X. Identification and optogenetic manipulation of memory engrams in the hippocampus. Front. Behav. Neurosci. 2014, 7, 226. [Google Scholar] [CrossRef] [Green Version]
- Mehta, M.R. From synaptic plasticity to spatial maps and sequence learning. Hippocampus 2015, 25, 756–762, erratum in Hippocampus 2015, 25, 771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eichenbaum, H. Memory: Organization and Control. Annu. Rev. Psychol. 2017, 68, 19–45. [Google Scholar] [CrossRef] [Green Version]
- Dal Monte, M.; Latina, V.; Cupisti, E.; Bagnoli, P. Protective role of somatostatin receptor 2 against retinal degeneration in response to hypoxia. Naunyn Schmiedebergs Arch. Pharmacol. 2012, 385, 481–494. [Google Scholar] [CrossRef]
- Wachtmeister, L. Oscillatory potentials in the retina: What do they reveal. Prog. Retin. Eye Res. 1998, 17, 485–521. [Google Scholar] [CrossRef]
- Tallent, M.K.; Qiu, C. Somatostatin: An endogenous antiepileptic. Mol. Cell Endocrinol. 2008, 286, 96–103. [Google Scholar] [CrossRef] [Green Version]
- Franke, K.; Baden, T. General features of inhibition in the inner retina. J. Physiol. 2017, 595, 5507–5515. [Google Scholar] [CrossRef] [Green Version]
- Jinno, S.; Kosaka, T. Cellular architecture of the mouse hippocampus: A quantitative aspect of chemically defined GABAergic neurons with stereology. Neurosci. Res. 2006, 56, 229–245. [Google Scholar] [CrossRef] [PubMed]
- Gábriel, R. Neuropeptides and diabetic retinopathy. Br. J. Clin. Pharmacol. 2013, 75, 1189–1201. [Google Scholar] [CrossRef] [PubMed]
- Chamberland, S.; Topolnik, L. Inhibitory control of hippocampal inhibitory neurons. Front. Neurosci. 2012, 6, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colás, B.; Arilla, E.; Prieto, J.P. Somatostatin binding to a fresh rat astrocyte-enriched suspension. Neuropeptides 1992, 23, 1–7. [Google Scholar] [CrossRef]
- Feindt, J.; Becker, I.; Blömer, U.; Hugo, H.H.; Mehdorn, H.M.; Krisch, B.; Mentlein, R. Expression of somatostatin receptor subtypes in cultured astrocytes and gliomas. J. Neurochem. 1995, 65, 1997–2005. [Google Scholar] [CrossRef]
- Viollet, C.; Lanneau, C.; Faivre-Bauman, A.; Zhang, J.; Djordjijevic, D.; Loudes, C.; Gardette, R.; Kordon, C.; Epelbaum, J. Distinct patterns of expression and physiological effects of sst1 and sst2 receptor subtypes in mouse hypothalamic neurons and astrocytes in culture. J. Neurochem. 1997, 68, 2273–2280. [Google Scholar] [CrossRef] [PubMed]
- Masmoudi, O.; Gandolfo, P.; Tokay, T.; Leprince, J.; Ravni, A.; Vaudry, H.; Tonon, M.C. Somatostatin down-regulates the expression and release of endozepines from cultured rat astrocytes via distinct receptor subtypes. J. Neurochem. 2005, 94, 561–571. [Google Scholar] [CrossRef]
- Mariotti, L.; Losi, G.; Lia, A.; Melone, M.; Chiavegato, A.; Gómez-Gonzalo, M.; Sessolo, M.; Bovetti, S.; Forli, A.; Zonta, M.; et al. Interneuron-specific signaling evokes distinctive somatostatin-mediated responses in adult cortical astrocytes. Nat. Commun. 2018, 9, 82. [Google Scholar] [CrossRef] [PubMed]
- Vecino, E.; Rodriguez, F.D.; Ruzafa, N.; Pereiro, X.; Sharma, S.C. Glia-neuron interactions in the mammalian retina. Prog. Retin. Eye Res. 2016, 51, 1–40. [Google Scholar] [CrossRef]
- Seifert, G.; Steinhäuser, C. Heterogeneity and function of hippocampal macroglia. Cell Tissue Res. 2018, 373, 653–670. [Google Scholar] [CrossRef]
- García de la Torre, N.; Wass, J.A.; Turner, H.E. Antiangiogenic effects of somatostatin analogues. Clin. Endocrinol. 2002, 57, 425–441. [Google Scholar] [CrossRef] [Green Version]
- Dasgupta, P. Somatostatin analogues: Multiple roles in cellular proliferation, neoplasia, and angiogenesis. Pharmacol. Ther. 2004, 102, 61–85. [Google Scholar] [CrossRef]
- Dal Monte, M.; Cammalleri, M.; Martini, D.; Casini, G.; Bagnoli, P. Antiangiogenic role of somatostatin receptor 2 in a model of hypoxia-induced neovascularization in the retina: Results from transgenic mice. Investig. Ophthalmol. Vis. Sci. 2007, 48, 3480–3489. [Google Scholar] [CrossRef] [PubMed]
- Dal Monte, M.; Ristori, C.; Cammalleri, M.; Bagnoli, P. Effects of somatostatin analogues on retinal angiogenesis in a mouse model of oxygen-induced retinopathy: Involvement of the somatostatin receptor subtype 2. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3596–3606. [Google Scholar] [CrossRef] [PubMed]
- Mei, S.; Cammalleri, M.; Azara, D.; Casini, G.; Bagnoli, P.; Dal Monte, M. Mechanisms underlying somatostatin receptor 2 down-regulation of vascular endothelial growth factor expression in response to hypoxia in mouse retinal explants. J. Pathol. 2012, 226, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Simó, R.; Carrasco, E.; García-Ramírez, M.; Hernández, C. Angiogenic and antiangiogenic factors in proliferative diabetic retinopathy. Curr. Diabetes Rev. 2006, 2, 71–98. [Google Scholar] [CrossRef] [PubMed]
- Santos-Carvalho, A.; Ambrósio, A.F.; Cavadas, C. Neuropeptide Y system in the retina: From localization to function. Prog. Retin. Eye Res. 2015, 47, 19–37. [Google Scholar] [CrossRef] [PubMed]
- Sperk, G.; Hamilton, T.; Colmers, W.F. Neuropeptide Y in the dentate gyrus. Prog. Brain Res. 2007, 163, 285–297. [Google Scholar] [CrossRef]
- Borbély, E.; Scheich, B.; Helyes, Z. Neuropeptides in learning and memory. Neuropeptides 2013, 47, 439–450, erratum in Neuropeptides 2014, 48, 107. [Google Scholar] [CrossRef]
- Bagnoli, P.; Dal Monte, M.; Casini, G. Expression of neuropeptides and their receptors in the developing retina of mammals. Histol. Histopathol. 2003, 18, 1219–1242. [Google Scholar] [CrossRef]
- Gonzalez-Suarez, A.D.; Nitabach, M.N. Peptide-Mediated Neurotransmission Takes Center Stage. Trends Neurosci. 2018, 41, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Vezzani, A.; Hoyer, D. Brain somatostatin: A candidate inhibitory role in seizures and epileptogenesis. Eur. J. Neurosci. 1999, 11, 3767–3776. [Google Scholar] [CrossRef]
- Burgos-Ramos, E.; Hervás-Aguilar, A.; Aguado-Llera, D.; Puebla-Jiménez, L.; Hernández-Pinto, A.M.; Barrios, V.; Arilla-Ferreiro, E. Somatostatin and Alzheimer’s disease. Mol. Cell Endocrinol. 2008, 286, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Viollet, C.; Lepousez, G.; Loudes, C.; Videau, C.; Simon, A.; Epelbaum, J. Somatostatinergic systems in brain: Networks and functions. Mol. Cell Endocrinol. 2008, 286, 75–87. [Google Scholar] [CrossRef] [Green Version]
- Simó-Servat, O.; Hernández, C.; Simó, R. Somatostatin and diabetic retinopathy: An evolving story. Endocrine 2018, 60, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Catalani, E.; Cervia, D.; Martini, D.; Bagnoli, P.; Simonetti, E.; Timperio, A.M.; Casini, G. Changes in neuronal response to ischemia in retinas with genetic alterations of somatostatin receptor expression. Eur. J. Neurosci. 2007, 25, 1447–1459. [Google Scholar] [CrossRef] [Green Version]
- Cervia, D.; Martini, D.; Ristori, C.; Catalani, E.; Timperio, A.M.; Bagnoli, P.; Casini, G. Modulation of the neuronal response to ischaemia by somatostatin analogues in wild-type and knock-out mouse retinas. J. Neurochem. 2008, 106, 2224–2235. [Google Scholar] [CrossRef]
- Hernández, C.; García-Ramírez, M.; Corraliza, L.; Fernández-Carneado, J.; Farrera-Sinfreu, J.; Ponsati, B.; González-Rodríguez, A.; Valverde, A.M.; Simó, R. Topical administration of somatostatin prevents retinal neurodegeneration in experimental diabetes. Diabetes 2013, 62, 2569–2578. [Google Scholar] [CrossRef]
- Simó, R.; Hernández, C.; Porta, M.; Bandello, F.; Grauslund, J.; Harding, S.P.; Aldington, S.J.; Egan, C.; Frydkjaer-Olsen, U.; García-Arumí, J.; et al. Effects of Topically Administered Neuroprotective Drugs in Early Stages of Diabetic Retinopathy: Results of the EUROCONDOR Clinical Trial. Diabetes 2019, 68, 457–463. [Google Scholar] [CrossRef]
- Houser, C.R. Do structural changes in GABA neurons give rise to the epileptic state? Adv. Exp. Med. Biol. 2014, 813, 151–160. [Google Scholar] [CrossRef]
- de Lanerolle, N.C.; Kim, J.H.; Robbins, R.J.; Spencer, D.D. Hippocampal interneuron loss and plasticity in human temporal lobe epilepsy. Brain Res. 1989, 495, 387–395. [Google Scholar] [CrossRef]
- Robbins, R.J.; Brines, M.L.; Kim, J.H.; Adrian, T.; de Lanerolle, N.; Welsh, S.; Spencer, D.D. A selective loss of somatostatin in the hippocampus of patients with temporal lobe epilepsy. Ann. Neurol. 1991, 29, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Moneta, D.; Richichi, C.; Aliprandi, M.; Dournaud, P.; Dutar, P.; Billard, J.M.; Carlo, A.S.; Viollet, C.; Hannon, J.P.; Fehlmann, D.; et al. Somatostatin receptor subtypes 2 and 4 affect seizure susceptibility and hippocampal excitatory neurotransmission in mice. Eur. J. Neurosci. 2002, 16, 843–849. [Google Scholar] [CrossRef]
- Stragier, B.; Clinckers, R.; Meurs, A.; De Bundel, D.; Sarre, S.; Ebinger, G.; Michotte, Y.; Smolders, I. Involvement of the somatostatin-2 receptor in the anti-convulsant effect of angiotensin IV against pilocarpine-induced limbic seizures in rats. J. Neurochem. 2006, 98, 1100–1113. [Google Scholar] [CrossRef] [PubMed]
- Aourz, N.; De Bundel, D.; Stragier, B.; Clinckers, R.; Portelli, J.; Michotte, Y.; Smolders, I. Rat hippocampal somatostatin sst3 and sst4 receptors mediate anticonvulsive effects in vivo: Indications of functional interactions with sst2 receptors. Neuropharmacology 2011, 61, 1327–1333. [Google Scholar] [CrossRef]
- Dobolyi, A.; Kékesi, K.A.; Juhász, G.; Székely, A.D.; Lovas, G.; Kovács, Z. Receptors of peptides as therapeutic targets in epilepsy research. Curr. Med. Chem. 2014, 21, 764–787. [Google Scholar] [CrossRef] [PubMed]







| Ion Channels | Rod Photoreceptor | Rod Bipolar Cell | Ganglion Cell |
|---|---|---|---|
| NaV | * | * | = |
| CaV(L) | ↓ | ↓ | ↓ |
| KV | ↑ | = | ↑ |
| KCa | = | ↓ | = |
| Ion Channels | DG Granule Cells | CA3 Pyramidal Neurons | CA1 Pyramidal Neurons |
|---|---|---|---|
| NaV | = | = | = |
| CaV(N) | ↓ | ↓ | |
| KM, KL, GIRK | = | ↑ * | ↑ |
| Retina | Hippocampus | Significance | ||
|---|---|---|---|---|
| SRIF interneuron | Co-transmitter | GABA | GABA | Fast synaptic inhibition in microcircuits |
| Autoreceptor | SST1 | SST1 | Control of somatostatin secretion | |
| SRIF receptors | Distribution | Wide | Wide | Diffuse (over several μm) and global action |
| Presynaptic neurons | SST2 | SST1/SST2 | Targets calcium channels | |
| Output neurons * | SST4 | SST4 | Targets potassium channels | |
| SRIF action on glutamatergic neurotransmission | Presynaptic site | Ca2+ channels (↓) | Ca2+ channels (↓) | ↓ neurotransmitter release |
| Postsynaptic site | K+ channels (↑) | K+ channels (↑) | ↓ firing rate | |
| SRIF signaling | Global action | Inhibitory | Inhibitory | Network stabilizer |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cammalleri, M.; Bagnoli, P.; Bigiani, A. Molecular and Cellular Mechanisms Underlying Somatostatin-Based Signaling in Two Model Neural Networks, the Retina and the Hippocampus. Int. J. Mol. Sci. 2019, 20, 2506. https://doi.org/10.3390/ijms20102506
Cammalleri M, Bagnoli P, Bigiani A. Molecular and Cellular Mechanisms Underlying Somatostatin-Based Signaling in Two Model Neural Networks, the Retina and the Hippocampus. International Journal of Molecular Sciences. 2019; 20(10):2506. https://doi.org/10.3390/ijms20102506
Chicago/Turabian StyleCammalleri, Maurizio, Paola Bagnoli, and Albertino Bigiani. 2019. "Molecular and Cellular Mechanisms Underlying Somatostatin-Based Signaling in Two Model Neural Networks, the Retina and the Hippocampus" International Journal of Molecular Sciences 20, no. 10: 2506. https://doi.org/10.3390/ijms20102506
APA StyleCammalleri, M., Bagnoli, P., & Bigiani, A. (2019). Molecular and Cellular Mechanisms Underlying Somatostatin-Based Signaling in Two Model Neural Networks, the Retina and the Hippocampus. International Journal of Molecular Sciences, 20(10), 2506. https://doi.org/10.3390/ijms20102506







